Abstract
Leaf rust (LR) is a devastating foliar disease that impacts common wheat (Triticum aestivum L.) globally. For optimal disease protection, wheat cultivars should possess adult plant resistance (APR) to leaf rust. In the current study, the objective was to map quantitative trait loci (QTL) related to leaf rust resistance. This was achieved by using 193 recombinant inbred line (RIL) populations which were developed from the cross between N. Strampelli and Huixianhong. Four trials were conducted in China (three in Baoding, Hebei province, and one in Zhoukou, Henan province) to assesses the leaf rust response of the RILs and parental lines. The wheat 660K SNP array and additional SSR markers were used to genotype the RIL populations. Through inclusive composite interval mapping (ICIM), three QTL related to leaf rust (LR) resistance were detected. ICIM was also employed to reevaluate previously published data in order to identify QTL with pleiotropic effects. To determine the physical positions, the flanking sequences of all SNP probes were compared against the Chinese Spring wheat reference sequence through BLAST searches. Three leaf rust resistance loci, two on chromosome 2A and 5B, were contributed by N. Strampelli. QLr.hbau-2AL.1 was detected in three leaf rust environments with phenotypic variance explained (PVE of 12.2–17%); QLr.hbau-2AL.2 was detected in two environments with 12.5–13.2% of the PVE; and QLr.hbau-5BL was detected in all leaf rust environments with phenotypic variance explained (PVE) of 17.8–19.1%. QLr.hbau-5BL exhibited potentially pleiotropic responses to multiple diseases. The QTL and the associated flanking markers discovered in this study could prove valuable for purposes such as fine mapping, the exploration of candidate genes, and marker-assisted selection (MAS).
1. Introduction
Leaf rust, which is triggered by Puccinia triticina Eriks, ranks among the most destructive diseases that impact common wheat (Triticum aestivum L.) on a global scale [1]. The main diseases of wheat include wheat rust, Fusarium head blight, powdery mildew, root rot, and others. These diseases are widely distributed globally and cause significant losses in wheat yield and quality. In China, stripe rust is particularly severe, mainly occurring in the northwest and southwest regions. Internationally, countries such as India, Pakistan, Ethiopia, and Australia also face serious threats from rust diseases. The spread and outbreak of rust diseases are closely related to climatic conditions, the disease resistance of wheat varieties, and agricultural management practices. The leaf rust pathogen mainly targets the leaf blades. However, in highly susceptible wheat cultivars, it can also infect the leaf sheath and glumes. Under epidemic circumstances, this disease has the potential to cause significant yield losses of up to 40%. These losses mainly occur due to a decrease in kernel weight and the number of kernels per spike [2]. Leaf rust seriously threatens crop growth. To effectively control plant diseases, prioritize the cultivation of disease-resistant plant varieties by combining multiple resistance genes through modern breeding techniques. Use chemical treatments with care, ensuring accurate timing and correct dosages. Implement farming practices such as crop rotation and the elimination of infected plant material to reduce the spread of diseases. Furthermore, adopt biological control methods by leveraging beneficial microorganisms to inhibit the activity of pathogens. While fungicides can be employed to manage leaf rust, the utilization of resistant cultivars represents a more efficient, cost-effective, and environmentally friendly approach [3].
Resistance to leaf rust can be generally divided into two types: race-specific and race-non-specific resistance. Race-specific resistance works against certain pathogen pathotypes but not all. Typically, it involves one or multiple genes that trigger a hypersensitive response when faced with avirulent pathotypes [4]. This form of resistance mainly persists throughout the whole growth cycle, thus being characterized as “all-stage resistance”. Race-non-specific resistance is inherited in a quantitative manner. It typically becomes evident at the later growth stages and is frequently effective against a variety of pathogens [5]. This kind of resistance is alternatively called adult plant resistance (APR) or slow-rusting resistance. A key characteristic of race non-specific resistance is its durability [6]. Currently, 83 leaf rust resistance genes in wheat have been mapped to chromosome locations and assigned gene designations [7]. While the majority of the reported genes provide race-specific resistance, only a small number of them confer race-non-specific resistance. The identification of new slow-rusting genes is important for breeding cultivars with durable resistance.
Molecular markers, including restriction fragment length polymorphisms (RFLPs), amplified fragment length polymorphisms (AFLPs), simple sequence repeats (SSRs), resistance gene analogue polymorphisms (RGAPs), and single nucleotide polymorphisms (SNPs), have been widely used in the genetic mapping of wheat disease resistance genes. Among them, high-density SNP arrays provide a superior approach for QTL mapping due to their higher accuracy and higher density compared to other markers [8,9,10].
N. Strampelli is a classic wheat variety bred by Italian agronomist Nazareno Strampelli, known for its high yield, disease resistance, and adaptability, significantly impacting modern agriculture. The Italian common wheat cultivar N. Strampelli, introduced to the Gansu Province of China in 1973 exhibits adult plant resistance to stripe rust that is controlled by three QTL, including Lr18, QYr.caas-4BL and QYr.caas-5BL.1 [11]. For example, observations of cultivar N. Strampelli in Longnan, Gansu, China showed that this cultivar was generally immune or highly resistant to leaf rust, and the disease resistance of this cultivar was stable despite environmental changes for over 30 years.
The objective of this study was to explore the genetic basis of durable leaf rust resistance in cultivar N. Strampelli. To further analyze adult leaf rust resistance genes in this cultivar and to locate the QTL associated with leaf rust resistance, 193 RIL populations of N. Strampelli × Huixianhong were monitored in Baoding, Hebei Province, China, and Zhoukou, Henan Province, China, from 2020 to 2022. QTL associated with resistance were identified based on phenotypic characters and SNP/SSR genotyping.
2. Materials and Methods
2.1. Plant Materials and Puccinia Triticina Pathotypes
The N. Strampelli (pLibero*San Pastore-14*Jacometti-49) × Huixianhong population consisted of 193 RILs. The cultivar Zhengzhou 5389 was used as the susceptible control. The development model of RILs involves selfing, and it involves 8 generations of selfing to obtain a stable RIL population, with Zheng 5389 serving as the susceptible control variety for experimental comparison (These materials are provided by the Wheat Rust Laboratory of Hebei Agricultural University, Baoding, China).
2.2. Puccinia Triticina Races in the Experimental Plots
To comprehensively understand the number and virulence characteristics of wheat leaf rust (Puccinia triticina, Pt) strains in the experimental fields, systematic strain identification was conducted in Hebei in 2020. Specifically, the researchers collected leaf samples of the Zhengzhou 5389 variety from the experimental plots, which exhibited visible uredinia. Subsequently, individual uredinia were isolated using microscopy techniques, and the spores were propagated under laboratory conditions to ensure pure strain samples. The resulting urediniospores were used to inoculate a set of 16 near-isogenic lines of wheat differentials, each designated with an additional fourth letter to distinguish their unique genetic backgrounds. Through this inoculation experiment, four major Pt pathotypes were successfully identified: THTS, THTQ, PHPS, and THTT. The virulence information of these pathotypes provided critical insights for further analysis of wheat resistance and laid foundation for subsequent disease-resistant breeding efforts. The experimental results revealed significant differences in the distribution and virulence of these pathotypes within the wheat population, offering scientific support for optimizing field disease management strategies and breeding resistant varieties. Additionally, by comparing the disease responses of susceptible and resistant lines, the researchers were able to more accurately assess the effects of resistance genes and their stability in field conditions, providing valuable references for wheat disease control and genetic improvement.
2.3. Field
The leaf rust severities of wheat RIL populations field trials evaluation was conducted in Baoding, Hebei province, China (115.47° E, 38.85° N) and in Zhoukou, Henan province, China (114.53° E, 33.80° N). Three sets of autumn-sown wheat crop seasons data were collected during the 2019/2020, 2020/2021, and 2021/2022 cropping seasons in Baoding and during the 2020/2021 cropping season in Zhoukou. Herein, the corresponding datasets are named 2020BD, 2021BD, 2022BD, and 2020HN respectively. In 2020 and 2022, experiments were carried out on N. Strampelli × Huixianhong (193 recombinant inbred lines, RILs) in Hebei and Henan. Each of the four combinations of location and year was regarded as an individual environment. The experiment followed a randomized complete block design with two replicates in each environment. Each plot comprised a single 1 m long row, spaced 50 cm apart. In every environment, the experiment was arranged in a randomized complete block design, and it was replicated twice. An individual plot consisted of a single 1 m long row with 50 cm between adjacent rows. Each plot was sown with approximately 20 seeds of RIL. A control wheat variety, Zhengzhou 5389, was placed after every 10 rows of recombinant inbred lines (RILs). Two rows of Zhengzhou 5389 perpendicular and adjacent to the plot rows were sown along each field block to serve as spreader plants of inoculums. Leaf rust infection was initiated by spraying the spreader at tillering stage with a water suspension of an equal urediniospores mixture of Pt pathotypes THTS, THTQ, PHPS, and THTT, to which a few drops of Tween 20 (0.03%) were added.
Disease severity was scored based on the percentage of leaf area covered with uredinia according to the modified Cobb scale and was assessed three times per cropping season at about 1 week intervals, with the first scoring performed 4 weeks after inoculation in each environment.
2.4. Statistical Analysis
Significant differences in phenotype and final disease severity (FDS) among trials (2020BD, 2021BD, 2022BD, and 2020HN) were identified using an analysis of variance (ANOVA), which was performed using the generalized linear model procedure (PROC MIX) of the Statistical Analysis System (Version 9.1, SAS Institute Inc., Cary, NC, USA).
2.5. Molecular Markers
Genomic DNA samples were extracted from the leaves of uninfected seedlings (both parental lines and RILs) using the CTAB method [12]. The 193 RILs and the parental lines were genotyped using Affymetrix 660K SNP arrays (660,009 markers) by Capital Bio Technology Company (Beijing, China). Monomorphic SNPs (heterozygosity > 25%), those with high deletion rates (>25% missing data), and those with distorted segregation ratios (minimum gene frequency > 5%) were removed. The remaining high-quality markers were used for the SNP analysis. Genotypic data for 21 more SSR markers were also gathered [13].
The mapping population of N. Strampelli × Huixianhong, consisting of 193 recombinant inbred lines (RILs), underwent genotyping. This was done using 323 simple sequence repeat (SSR) markers. These markers were chosen from previously documented resources available at the website (http://www.gramene.org/db/searches/ssrtool, accessed on 1 June 2022). PCR reaction system consisted of 1.0 μL 10 × PCR buffer (Mg2+) (Purchased from Baoding Chengxin Chemical Reagent and Scientific Instruments Co., Ltd., China), 5 μL 2 × Taq PCR Master Mix (Purchased from Baoding Chengxin Chemical Reagent and Scientific Instruments Co., Ltd., China), 1.0 μL forward primer (10 molμL−1) (Purchased from Beijing Tsingke Biotechnology Co., Ltd), 1.0 μL reverse primer (10 molμL−1), 2.0 μL DNA template (50 ng/μL), and 3.0 μL ddH2O. While the normal PCR cycling was performed for most of the markers, for certain markers, a touchdown program was used, which started with 94 °C for 5 min; then, 35 cycles of 94 °C denaturing for 30 s, 60 °C annealing for 30 s (touchdown with 0.5 °C each cycle), and 72 °C extending for 30 s; followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and ended with 72 °C for 10 min. Amplicons were separated in 12% denaturing polyacrylamide gels and visualized with the silver staining method. Silver staining involved the following steps: fix, wash the gel, stain with silver nitrate (Purchased from Baoding Chengxin Chemical Reagent and Scientific Instruments Co., Ltd., China), develop and stop, and then preserve. Imaging involved the following steps: use imaging system as select light source, capture, analyze with software, and save the images. The linkage map was developed with the help of Map Manager QTXb2.0 and QTL Icimapping 4.0.
2.6. Genotyping, Map Construction, and QTL Analysis
Mapping analyses to identify quantitative trait loci (QTL) linked to leaf rust final disease severity (FDS) in each trial were conducted using the inclusive composite interval mapping (ICIM) algorithm in IciMapping 4.1 [14]. QTL detection was carried out using the composite inclusive interval mapping (ICIM) method. The threshold LOD score was determined through a permutation program with 1000 replicates at a significance level of α = 0.05. Permutation analysis was applied to all experiments, and the highest LOD value obtained (2.5) was selected as a consistent threshold across all trials. The effect size of QTL was measured with the determination coefficient.
Genotypic data were filtered to include only markers with missing values in less than 5% of the recombinant inbred lines (RILs), while those with missing values exceeding 5% were excluded. After eliminating redundant markers identified using the Bin function in QTL IciMapping V4.1 software, the retained high-quality markers (each marker representing a unique locus) were grouped and ordered using JoinMap 4.0. Genetic distances (in cM) were estimated based on the Kosambi mapping function. If the distance between two adjacent markers was ≥20 cM, a linkage group was divided. Stepwise regressions were used to detect the percentage of variance explained (PVE) by individual QTLs and additive effects at LOD peaks. Linkage groups were then orientated and assigned to chromosomes through alignments of the sequences flanking SSR with a chromosomal survey sequence map. The flanking sequences of all SNP probes were aligned with the wheat reference genome (cultivar Chinese Spring; https://urgi.versailles.inra.fr/blast_iwgsc/blast.php, IWGSC2022) URL (accessed on 1 June 2022), using BLAST to determine their physical positions. Factorial ANOVAs were performed to assess the significance of interactions between stably identified resistance loci based on final disease severity (FDS).
3. Results
3.1. Resistance of N. Strampelli × Huixianhong RILs to Leaf Rust
In each of the four field experiments, the leaf rust severities of susceptible control Zhengzhou 5389 ranged from 90% to 100%. Leaf rust severity reached approximately 80% on the susceptible control wheat Huixianhong, while it remained below 10% on the resistant landrace N. Strampelli, as illustrated by the typical symptoms depicted in Figure 1. This indicates that disease pressure was adequately high for revealing resistance and susceptibility. Mean leaf rust FDS for the RILs was 30.5–45% across all the trials (Table 1). The frequency distribution of leaf rust severities in each trial showed a continuous distribution skewed towards resistance (Figure 1), indicating polygenic inheritance. Pearson correlation comparisons showed that FDS was significantly correlated between all pairs of trials, with Pearson correlation coefficients (R) of 0.7–0.8 (p < 0.05; Table 2). Correlation coefficients for among the experiments were all significant, with p < 0.05, ranging from 0.7 to 0.8 in N. Strampelli × Huixianhong. Variances due to RIL and environment were significant and predominated though the interaction variances were also significant. The ANOVAs confirmed that there was significant variation among the genotypes Ril × Environment.
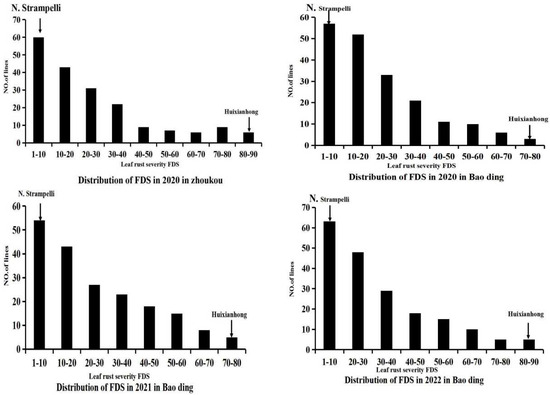
Figure 1.
Frequency distributions of N. Strampelli × Huixianhong RIL lines for final disease severity. Mean values for the parents, N. Strampelli and Huixianhong, are indicated by arrows. 2020BD, 2020HN, 2021BD, 2022BDFDS, and 2019/2020, 2020/2021, and 2021/2022 cropping seasons in Baoding, Hebei Province and Zhoukou in Henan during the 2021 cropping seasons, respectively.

Table 1.
Summary of leaf rust FDS in the N. Strampelli × Huixianhong parental and RIL populations in the four trials.

Table 2.
Pearson correlation coefficients (R) for 2-way comparisons of leaf rust FDS between trials.
3.2. SNP and SSR Genotypes
The distribution of SNP markers across chromosomes (Figure 2), in combination with its histogram, showed that the polymorphic loci controlling the FDS-associated QTL were mainly distributed on chromosomes 1B, 2A, 5B, and 7D (Figure 3).
Figure 2.
Distribution Table of Polymorphic Markers in Each Chromosome.
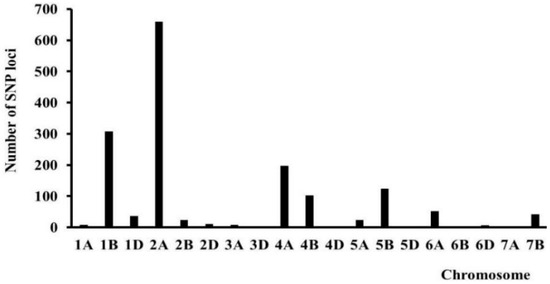
Figure 3.
SNP Exchange on chromosome in small population.
3.3. QTL Mapping for Leaf Rust Resistance
After inoculation with four Pt pathotypes, 1200 SSR markers were identified between the parental lines and the bulked RILs. Three QTL were identified on chromosomes 2A (QLr.hbau-2A.1 and QLr.hbau-2A.2), and 5B (QLr.hbau-5B).Lr.hbau-2AL.1, located between molecular markers xgwm122-xwmc294, was identified in trials 2020BD, 2021BD, and 2020HN, explaining 14.4%, 12.2%, and 17.0%, of the phenotypic variance, respectively, in these trials (Table 3, Figure 4). QLr.hbau-2AL.2 was detected in the trials 2021BD and 2022BD between SSR markers xwmc728-xgwm122 (Figure 4). QLr.hbau-2A.2 explained 13.2% and 12.5% of the phenotypic variance in trials 2021BD and 2022BD, respectively, corresponding to minor effects (Table 3). QLr.hbau-5BL was an important QTL for leaf rust resistance in all environments, consistently explaining 17–19% of the phenotypic variances across all environments (Table 3). QLr.hbau-5BL was located between SSR markers xgwm499-xwmc415. The results show that the alleles associated with leaf rust resistance within the QTL on 2AL and 5AL were inherited from N. Strampelli (Figure 4).

Table 3.
APR QTLs associated with leaf rust in wheat N. Strampelli × Huixianhong RIL populations.
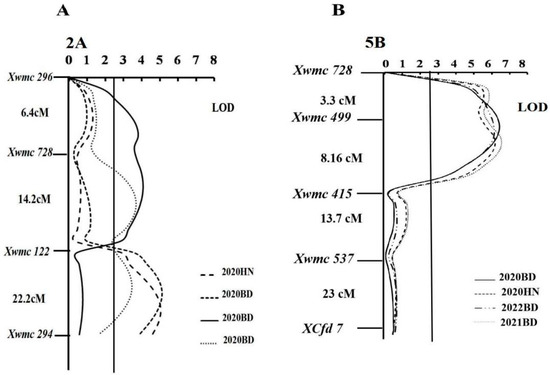
Figure 4.
Genetic maps of chromosome 2A (A) and 5B (B) for the N. Strampelli × Huixianhong RIL population, displaying the logarithm of the odds (LOD) values. Marker names and the distances (in Kosambi cM) between adjacent markers are indicated along the chromosomes, with the telomere of 2AL and the centromere of 5BL oriented to the left. Horizontal lines indicate the threshold LOD of 2.5. Each of the small triangles along the x-axis represents a marker used for QTL mapping.
4. Discussion
Comparisons with previous reports of LR QTLs.
4.1. QLr.hbau-2AL.1
To date, three APR QTLs QLr.cimmyt-2AL, QLr.sfr-2AL, and QLr.ubo-2AL [15,16] were reported on chromosome 2A in a durum population. QLr.cimmyt-2AL was located between SSR markers wpt4419-wpt8226 in the Mexican wheat cultivar Avocet and explained 5.8–7.2% of the phenotypic variation [17]. QLr.sfr-2AL was located between SSR markers cfa2263c-sfr.be590525 in the Swiss bread-wheat cultivar Forno and explained 9.5–12% of the phenotypic variation [18]. QLr.ubo-2AL was located between SSR markers wpt-386-310911 in the North American durum wheat cultivar Lloyd and explained 18.6–30% of the phenotypic variation [19]. QLr.cimmyt-2AL and QLr.sfr-2AL are located at 63 cM on chromosome 2A, while QLr.ubo-2AL is located at 143 cM at the end of chromosome 2AL [20]. Herein, QLr.hbau-2AL.1 was detected in all the environments and explained 12–17% of the phenotypic variance, and the SSR markers area xgwm122-xwmc294 is 20cM near the 2A centromere, and different from the aforementioned QTL, suggesting that it might be a new QTL.
4.2. QLr.hbau-2AL.2
QLr.hbau-2AL.2 was detected in two environments (2021BD and2022BD) and explained 12.5–13.2% of the phenotypic variance. To date, four APR genes, Lr17, Lr37, Lr45, and Lr65 were mapped on chromosome 2A. Lr37 was located at 10 cM near the centromere of 2AS chromosome [21,22]. Herein, QLr.hbau-2AL.2 was located at 9 cM near the centromere of the 2AL chromosome of cultivar N. Strampelli. Similarly, Lr37 was located at 10 cM near the centromere of 2AS chromosome. However, Lr37 is a major gene, explaining high levels of variation, while QLr.tbau-2AL.2 explained less than 15% of the variance in FDS. Therefore, QLr.tbau-2AL.2 is unlikely to correspond to Lr37 and may represent a novel site associated with leaf rust resistance and might be new.
4.3. QLr.hbau-5BL
QLr.hbau-5BL, which was located on chromosome 5B between SSR markers Xgwm499-Xwmc415 (genetic distance 8.1 cM; 477–507 Mb in the Chinese Spring reference map based on the position of the reference sequence), was strongly associated with leaf rust resistance. Four QTL (QLr.ccs-5b.4, QLr.ccs-5b.5, QLr.inra-5B, and Lr.locus-5B.1) and two genes (Lr18 and Lr52) for LR resistance have been mapped to chromosome 5B [23,24]. QLr.Ccsu-5b.4 and QLr.Ccsu-5b.5 were obtained using an inclusive composite interval mapping (ITMI) mapping population, with the SSR markers intervals Xcdo1326-Xbarc140 and Xbarc142-Xbarc69 [25,26]. QLr.Locus-5B.1 was identified between SSR markers Xgwm433-Xgwm234 in the durum wheat variety Wollaroi, which is resistant to leaf rust throughout the growth period, QLr.inra-5B was derived from a double haploid (DH) population that was the result of a hybridization between the Apache and balance varieties, while QLr.inra-5B obtained from double haploid (DH) population hybridized by Apache/balance varieties, was between the SSR markers Wmc517-Wpt7720 [25]. Hence, the identified Lr.hbau-5BL, with different marker intervals and from different genetic groups, is different from the aforementioned, and might be new.
4.4. Potentially Pleiotropic QTL Associated with Resistance to Multiple Wheat Diseases
A QTL locus (QPM) associated with adult plant resistance to powdery mildew was identified on chromosome 5BL in the F2:3 generation derived from the cross between N. Strampelli and Huixianhong [27]. The SSR markers interval was located in xbarc331-xwmc537 (608-953 Mb on the physical map). We located the QTL locus on 5BL for the F2:3 population of N. Strampelli/Mingxian 169. The SSR marker xgwm499-xwmc415 (477–507 Mb on the physical map) was similar to the stripe rust resistance gene YrN.S and is closely linked. In this study, QLr.hbau-5B was located between SSR markers xgwm499-xwmc415 (477–507 Mb on the physical map). Thus, QTL associated with resistance located on chromosome 5BL near QLr.hbau-5BL may be a pleiotropic site with multiple effects.
4.5. Interactions Among QTL for Leaf Rust Resistance in Adult Plants
Although several studies have reported major genes associated with disease resistance, few studies to date have focused on the effects of minor resistance genes because the many interactions among minor genes affect accurate QTL localization. However, it has been suggested that the effects produced by the aggregation of multiple minor-effect genes will be longer lasting than major genes. The selection pressure exerted by minor-effect resistance genes on Pt is low, and the pathogen is less likely to evolve defenses or be replaced by competitors. Thus, minor-effect resistance genes are more industrially valuable than major genes. In this study, the RIL population of N. Strampelli × Huixianhong showed good horizontal resistance in the field, and the QTL on 5BL are likely to be closely linked with the stripe-rust resistance gene YrN.S. Thus, disease resistance in the RIL population is likely to be controlled by one major gene or several minor genes, but further study is needed to explore these two alternatives [28].
The findings of this study, achieved through the use of high-density SNP markers, represent significant advancements compared to earlier research that relied solely on SSR markers. These improvements might be because we used a wheat 660K SNP chip, which has many polymorphic markers for genotyping. Previous studies used only limited SSR markers and few chromosomes for QTL mapping and analysis.
4.6. Implications of QTL Detected in the RIL Population
The Italian wheat variety N. Strampelli has demonstrated strong resistance to leaf rust, stripe rust, and powdery mildew over many years. In this research, three QTL associated with leaf rust resistance, including three potentially pleiotropic QLr loci and closely linked SNP markers, were identified in the N. Strampelli × Huixianhong recombinant inbred line (RIL) population. Among these, the novel adult plant resistance QLr.hbau-5BL on chromosome 5B conferred stable resistance to leaf rust. QLr.hbau-5BL was also associated with resistance to leaf rust and stripe rust, and this QTL was closely linked to the stripe rust resistance gene YrN.S, although this association requires further verification. In future research, the SNP markers associated with the two QTL will be analyzed using KASP assays to aid in the stacking of rust resistance loci into new wheat cultivars, as well as marker-assisted selection (MAS) in wheat breeding programs. Using marker-assisted selection, multiple rust resistance genes are pyramided into new varieties, enhancing resistance and stability for sustainable agriculture.
4.7. Comparisons with the Studies on Rust Resistance
QLr.hbau-2AL.1: Located between markers xgwm122 and xwmc294 on chromosome 2A, this QTL explained 12.2% to 17.0% of the phenotypic variance. Several QTL associated with leaf rust resistance have been previously identified on chromosome 2A, such as QLr.cimmyt-2AL, QLr.sfr-2AL and QLr.ubo-2AL. However, these QTL are located at different positions compared to QLr.hbau-2AL.1, suggesting that QLr.hbau-2AL.1 is likely a novel QTL.
QLr.hbau-2AL.2: Located between markers xwmc728 and xgwm122 on chromosome 2A, this QTL explained 12.5% to 13.2% of the phenotypic variance. The previously identified Lr37 gene is also located near the centromere of chromosome 2A. However, Lr37 is a major gene, whereas QLr.hbau-2AL.2 is a minor-effect QTL. Therefore, QLr.hbau-2AL.2 is likely a new resistance locus.
QLr.hbau-5BL: Located between markers xgwm499 and xwmc415 on chromosome 5B, this QTL explained 17.8% to 19.1% of the phenotypic variance. Previous studies have identified several QTL on chromosome 5B (e.g., QLr.ccs-5b.4, QLr.ccs-5b.5, QLr.inra-5B, and Lr.locus-5B.1), but their positions are different from QLr.hbau-5BL, indicating that QLr.hbau-5BL is likely a novel QTL. Additionally, QLr.hbau-5BL is closely linked to the stripe rust resistance gene YrN.S and may have pleiotropic effects.
These newly identified QTL not only enrich the genetic basis of leaf rust resistance in wheat but also provide important tools for future marker-assisted selection (MAS) and gene pyramiding.
5. Conclusions
Three QTL associated with leaf rust (LR) resistance were discovered. Interval composite interval mapping (ICIM) was also utilized to re-assess previously published data so as to identify QTL with pleiotropic effects. To figure out the physical positions, the flanking sequences of all SNP probes were compared with the Chinese Spring wheat reference sequence via BLAST searches.
Three leaf rust resistance loci, two located on chromosomes 2A and one on chromosome 5B, were derived from N. Strampelli. QLr.hbau-2AL.1 was identified in three leaf rust environments, accounting for a phenotypic variance explained (PVE) ranging 12.2–17%. QLr.hbau-2AL.2 was found in two environments, with a PVE of 12.5–13.2%. As for QLr.hbau-5BL, it was detected in all leaf rust environments, and the phenotypic variance explained (PVE) was between 17.8% and 19.1%. Moreover, QLr.hbau-5BL showed potentially pleiotropic responses to multiple diseases.
Author Contributions
Conceptualization, M.L.; methodology, M.L.; software, X.L. (Xue Li); validation, M.L.; formal analysis, T.G.; investigation, X.L. (Xue Li) and M.L.; resources, X.L. (Xing Li); data curation, J.Z. and M.L.; writing—original draft preparation, M.L.; writing—review and editing, M.L.; visualization, X.L. (Xue Li); supervision, Z.K. and X.L. (Xing Li); project administration, Z.K.; and funding acquisition, X.L. (Xing Li). All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (32001890), 2023 Scholarship Program for Introducing Overseas Students (C20230107).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to thank the members of the team for their help with the laboratory work and data analysis.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| 2AL | The long arm of chromosome 2A |
| 5BL | The long arm of chromosome 5B |
| ANOVA | Analysis of variance |
| APR | Adult plant resistance |
| IT | Infection type |
| LOD | Logarithm of odds |
| Lr gene | Leaf rust resistance gene |
| MAS | Marker-assisted selection |
| PVE | Phenotypic variation explained |
| QTL | Quantitative trait locus/loci |
| RIL | Recombinant inbred line |
| SNP | Single nucleotide polymorphism |
| ICIM | Inclusive composite interval mapping |
| MDS | The maximum disease severity |
References
- Yadav, J.K.; Sinha, S.; Shukla, H.; Singh, A.; Sahu, T.K.; Jha, S.K.; Kumari, J.; Verma, M.; Kumar, S.; Singh, R.; et al. Genetic dissection of leaf rust resistance in a diversity panel of tetraploid wheat (Triticum turgidum). BMC Plant Biol. 2025, 25, 406. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, C.; Zhang, Y.; Zhou, W.; Guo, Q.; Bai, B.; Shen, S.; Huang, G. Study on stripe rust (Puccinia striiformis) effect on grain filling and seed morphology building of special winter wheat germplasm Huixianhong. PLoS ONE 2019, 14, e0215066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rehman, S.U.; Qiao, L.; Shen, T.; Hua, L.; Li, H.; Ahmad, Z.; Chen, S. Exploring the Frontier of Wheat Rust Resistance: Latest Approaches, Mechanisms, and Novel Insights. Plants 2024, 13, 2502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prasad, P.; Savadi, S.; Bhardwaj, S.C.; Gupta, P.K. The progress of leaf rust research in wheat. Fungal Biol. 2020, 124, 537–550. [Google Scholar] [CrossRef]
- Ivanova, V. Seedling and adult plant resistance to leaf rust in some Bulgarian common wheat lines. Vavilovskii Zhurnal Genet. Sel. 2023, 27, 447–453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, D.; Kumar, A.; Chhokar, V.; Gangwar, O.P.; Bhardwaj, S.C.; Sivasamy, M.; Prasad, S.V.S.; Prakasha, T.L.; Khan, H.; Singh, R.; et al. Genome-wide association studies in diverse spring wheat panel for stripe, stem, and leaf rust resistance. Front. Plant Sci. 2020, 11, 748. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Bajgain, P.; Rouse, M.N.; Li, J.; Zhang, P. Mapping and characterization of the recessive leaf rust resistance gene Lr83 on wheat chromosome arm 1DS. Theor. Appl. Genet. 2023, 136, 115. [Google Scholar] [CrossRef]
- Achilli, A.L.; Kumar, N.; Fetterley, V.; Khangura, R.S.; Brar, G.S. QTL/Linkage Mapping for Rust Resistance: Step-by-Step Guide. Methods Mol. Biol. 2025, 2898, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Krishnappa, G.; Kumar, S.; Devate, N.B.; Rathan, N.D.; Kumar, S.; Mishra, C.N.; Ram, S.; Tiwari, R.; Parkash, O.; et al. Genome-wide association study identifies novel loci and candidate genes for rust resistance in wheat (Triticum aestivum L.). BMC Plant Biol. 2024, 24, 411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinto da Silva, G.B.; Zanella, C.M.; Martinelli, J.A.; Chaves, M.S.; Hiebert, C.W.; McCallum, B.D.; Boyd, L.A. Quantitative trait loci conferring leaf rust resistance in hexaploid wheat. Phytopathology 2018, 108, 1344–1354. [Google Scholar] [CrossRef]
- Lu, Y.; Lan, C.; Liang, S.; Zhou, X.; Liu, D.; Zhou, G.; Lu, Q.; Jing, J.; Wang, M.; Xia, X.; et al. QTL mapping for adult-plant resistance to stripe rust in Italian common wheat cultivars Libellula and Strampelli. Theor. Appl. Genet. 2009, 119, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Brar, G.S.; Schwessinger, B.; Jones, A. High Molecular Weight DNA Extraction from Wheat Rust Spores. Methods Mol. Biol. 2025, 2898, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.; Liang, S.; Zhou, X.; Zhou, G.; Lu, Q.; Xia, X.; He, Z. Identification of genomic regions controlling adult-plant stripe rust resistance in Chinese landrace Pingyuan 50 through bulked segregant analysis. Phytopathology 2010, 100, 313–318. [Google Scholar] [CrossRef]
- Zhou, J.; Singh, R.P.; Ren, Y.; Bai, B.; Li, Z.; Yuan, C.; Li, S.; Huerta-Espino, J.; Liu, D.; Lan, C. Identification of Two New Loci for Adult Plant Resistance to Leaf Rust and Stripe Rust in the Chinese Wheat Variety ‘Neimai 836’. Plant Dis. 2021, 105, 3705–3714. [Google Scholar] [CrossRef] [PubMed]
- Schnurbusch, T.; Paillard, S.; Fossati, D.; Messmer, M.; Schachermayr, G.; Winzeler, M.; Keller, B. Detection of QTLs for Stagonospora glume blotch resistance in Swiss winter wheat. Theor Appl Genet. 2003, 107, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Rosewarne, G.M.; Singh, R.P.; Huerta-Espino, J.; Rebetzke, G.J. Quantitative trait loci for slow-rusting resistance in wheat to leaf rust and stripe rust identified with multi-environment analysis. Theor. Appl. Genet. 2008, 116, 1027–1034. [Google Scholar] [CrossRef]
- Rosewarne, G.M.; Singh, R.P.; Huerta-Espino, J.; Herrera-Foessel, S.A.; Forrest, K.L.; Hayden, M.J.; Rebetzke, G.J. Analysis of leaf and stripe rust severities reveals pathotype changes and multiple minor QTLs associated with resistance in an Avocet × Pastor wheat population. Theor. Appl. Genet. 2012, 124, 1283–1294. [Google Scholar] [CrossRef]
- Schnurbusch, T.; Paillard, S.; Schori, A.; Messmer, M.; Schachermayr, G.; Winzeler, M.; Keller, B. Dissection of quantitative and durable leaf rust resistance in Swiss winter wheat reveals a major resistance QTL in the Lr34 chromosomal region. Theor. Appl. Genet. 2004, 108, 477–484. [Google Scholar] [CrossRef]
- Maccaferri, M.; Mantovani, P.; Tuberosa, R.; Deambrogio, E.; Giuliani, S.; Demontis, A.; Massi, A.; Sanguineti, M.C. A major QTL for durable leaf rust resistance widely exploited in durum wheat breeding programs maps on the distal region of chromosome arm 7BL. Theor. Appl. Genet. 2008, 117, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lan, C.; He, Z.; Singh, R.P.; Rosewarne, G.M.; Chen, X.; Xia, X. Overview and Application of QTL for Adult Plant Resistance to Leaf Rust and Powdery Mildew in Wheat. Crop Sci. 2014, 54, 1907. [Google Scholar] [CrossRef]
- Kloppers, F.J.; Pretorius, Z.A. Effects of combinations amongst genes Lr13, Lr34 and Lr37 on components of resistance in wheat to leaf rust. Plant Pathol. 1997, 46, 737–750. [Google Scholar] [CrossRef]
- Mohler, V.; Singh, D.; Singrün, C.; Park, R.F. Characterization and mapping of Lr65 in spelt wheat ‘Altgold Rotkorn’. Plant Breed. 2012, 131, 252–257. [Google Scholar] [CrossRef]
- Yamamori, M. An N-band marker for gene Lr18 for resistance to leaf rust in wheat. Theor. Appl. Genet. 1994, 89, 643–646. [Google Scholar] [CrossRef]
- Juliana, P.; Singh, R.P.; Singh, P.K.; Poland, J.A.; Bergstrom, G.C.; Huerta-Espino, J.; Bhavani, S.; Crossa, J.; Sorrells, M.E. Genome-wide association mapping for resistance to leaf rust, stripe rust and tan spot in wheat reveals potential candidate genes. Theor. Appl. Genet. 2018, 131, 1405–1422. [Google Scholar] [CrossRef]
- Azzimonti, G.; Marcel, T.C.; Robert, O.; Paillard, S.; Lannou, C.; Goyeau, H. Diversity, specificity and impacts on field epidemics of QTLs involved in components of quantitative resistance in the wheat leaf rust pathosystem. Mol. Breed. 2014, 34, 549–567. [Google Scholar] [CrossRef]
- Aoun, M.; Breiland, M.; Kathryn Turner, M.; Loladze, A.; Chao, S.; Xu, S.S.; Ammar, K.; Anderson, J.A.; Kolmer, J.A.; Acevedo, M. Genome-wide association mapping of leaf rust response in a durum wheat worldwide germplasm collection. Plant Genome 2016, 9. [Google Scholar] [CrossRef]
- Azeem, A.M.; Bai, B.; Lan, C.; Yan, J.; Xia, C.; Zhang, Y.; He, Z. QTL Mapping for adult plant resistance to powdery mildew in Italian wheat cv. Strampelli. J. Integr. Agric. 2013, 5, 756–764. [Google Scholar] [CrossRef]
- Qureshi, N.; Singh, R.P.; Bhavani, S. Genetic Dissection of Triple Rust Resistance (Leaf, Yellow, and Stem Rust) in Kenyan Wheat Cultivar, “Kasuku”. Plants 2025, 14, 1007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).