Abstract
Commercial production of the button mushroom, Agaricus bisporus (Lange) Imbach, is threatened by various pests and mycopathogenic microorganisms. Sciarid flies (Sciaridae) of the genus Lycoriella are considered as major pests, while major pathogens include the fungi Lecanicillium fungicola (Preuss), Zare and Gams, Hypomyces perniciosus Magnus, Cladobotryum spp., and Trichoderma aggressivum Samuels & W. Gams, the causative agents of dry bubble, wet bubble, cobweb, and green mold diseases, respectively. Control of mushroom pests and diseases has long relied on synthetic chemical pesticides. Pesticide resistance and various health and environmental issues have created a need for sustainable and eco-friendly alternatives to the use of synthetic chemical pesticides for mushroom pest and disease control. The concept of bioprotection, which involves using biological control agents (BCAs) and biopesticide products, offers a viable alternative. The entomopathogenic nematode Steinernema feltiae (Filipjev) and predatory mite Stratiolaelaps scimitus (Womersley) are the most important invertebrate BCAs, while the bacteria Bacillus thuringiensis Berliner, B. amyloliquefaciens, and B. velezensis stand out as the most widely used microbial BCAs/biopesticides. Azadirachtin- and pyrethrum-based products are the most important biochemical biopesticides. Bioprotection agents require inclusion in the integrated pest and disease management (IPDM) programs in order to achieve their full effectiveness.
1. Introduction
Global mushroom production in the 21st century had reached 50 MT by the end of 2023 [1]. The button mushroom, Agaricus bisporus (Lange) Imbach, is a species of basidiomycete fungi belonging to the family Agaricaceae. Currently, A. bisporus is among the four most widely grown edible mushroom species, accounting for 11% of worldwide mushroom production [2].
Commercial cultivation of A. bisporus is practiced under controlled environmental conditions regarding temperature, humidity, CO2 content, and ventilation (Figure 1). Cultivation requires compost previously prepared out of mixture of wheat straw, chicken/horse manure, and gypsum. Composting process involves two phases: phase I (aerobic fermentation), which includes changes in and mineralization of organic material under thermophilic conditions (14 days), and phase II (pasteurization), where partially composted substrate is transferred indoors for seven days of controlled heat treatment. Phase III (compost spawning) includes the seeding of compost with A. bisporus mycelium and subsequent incubation for 15 days at 25 °C and 90–100% humidity, high CO2 content, and low ventilation. This is followed by a post-incubation one-week step, in which a layer of casing soil (usually black peat) is placed onto the compost to allow the formation of A. bisporus fruiting bodies. The next phase is fructification, where the environmental conditions are modified at the beginning (17 °C, 85–95% humidity, low CO2 content, and strong ventilation) [3,4,5,6]. The whole button mushroom production cycle lasts approximately two months. with two to three flushes (harvest).
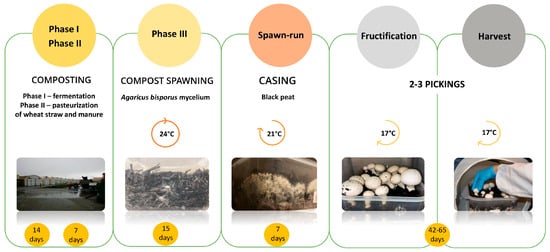
Figure 1.
Button mushroom crop production cycle—from composting to harvest.
The controlled environment in mushroom growing rooms provides optimal conditions for growth of A. bisporus, but also facilitates the growth and augmentation of various pests and pathogens that decrease the yield and quality of the mushroom fruiting bodies, thus causing great economic losses [7,8,9]. Commercial mushroom production is threatened by pests (insects, mites, and nematodes) and mycopathogenic microorganisms such as fungi, bacteria, and viruses (Table 1).

Table 1.
The major pests and pathogens of the button mushroom.
2. Pests and Diseases of the Button Mushroom
Mushroom flies (Diptera) of the families Sciaridae (sciarid flies, dark-winged fungus gnats), Phoridae (phorid flies, scuttle flies), and Cecidomyiidae (cecid flies, gall midges) are the most important pests of A. bisporus. Among them, sciarid flies of the genus Lycoriella (Frey) have traditionally been considered as the major pests [9,10]. The predominant species of this genus are Lycoriella ingenua (Dufour) [=L. mali, L. solani] and L. castanescens (Lengersdorf) [=L. auripila]. Other important sciarids belong to the genus Bradysia Winnertz. Sciarids cause mushroom yield losses directly by larval feeding on the compost, mycelium and sporophore primordia, or by larval tunneling into the stipes of mature sporophores. They are indirectly harmful by acting as mechanical vectors of T. aggressivum and other mushroom pathogens, as well as mite pests [9,11,12]. The economic threshold for sciarids is virtually zero: for example, according to an estimation, even one larva of L. castanescens per 125 g of a casing sample causes economic yield loss [10].
Phorid flies are usually considered as minor pests of A. bisporus, although their predominance over sciarids has been reported in the USA and some other countries. Phorids also cause direct and indirect yield losses in mushrooms. The most important phorid fly is Megaselia halterata (Wood), but it has a 12 times higher economic threshold than L. ingenua. Although M. halterata larvae can cause direct yield loss by feeding on the mushroom mycelium, this species is potentially more harmful indirectly as a vector of mushroom pathogens and mites [9,11,13,14]. Cecid flies (Mycophila spp. and Heteropeza spp.) are minor pests of the button mushroom that occasionally cause direct yield loss [9].
The most important mite pests of button mushrooms are the pygmephoroid species Microdispus (Brennandania) lambi (Krczal) (Acari: Microdispidae), Pediculaster mesembrinae (Canestrini), and Pediculaster fletchmanni Wicht (Acari: Pygmephoridae), as well as the mold mite, Tyrophagous putrescentiae (Schrank) (Acari: Acaridae). In addition to the direct damage that they are able to cause by feeding on the mycelium, these mites are also vectors of mushroom pathogens [11,15,16,17,18]. Mushroom nematodes that can affect commercial production are both myceliophagous (Ditylenchus myceliophagus, Aphelenchus spp., Aphelenchoides spp.) and saprophagous (Rhabditida). The former reduce A. bisporus yield by feeding, while the latter affect mushroom quality by secreting some enzymes and toxins that increase the pH of compost and hampers spawn run [9,19].
The most devastating pathogens of button mushroom include the fungi Lecanicillium fungicola (Preuss), Zare and Gams [formerly Verticillium fungicola (Preuss), Hassebrauk], Hypomyces perniciosus Magnus [formerly Mycogone perniciosa (Magnus) Delacr.], Cladobotryum spp. [formerly Dactylium spp.], and Trichoderma aggressivum f. europaeum and f. aggressivum Samuels & W. Gams, the causative agents of dry bubble, wet bubble, cobweb, and compost green mold diseases, respectively [20,21,22,23]. Unlike their fungal counterparts, bacteria occur less frequently and involve mostly Pseudomonas tolaasii, the causative agent of brown blotch disease, and Pseudomonas agarici, the causative agent of drippy gill disease [7,24], while the relevant viruses include the mushroom virus X complex, which is assumed to cause the largest losses [25].
Over the past two decades, Trichoderma species have become one of the biggest problems in button mushroom farms, reducing yield by between 60 and 100% [26,27]. The causal agents of compost green mold disease, T. aggressivum f. europaeum in Europe and T. aggressivum f. aggressivum in North America, are considered to be the most devastating. All of these diseases exhibit diverse symptoms while decreasing the yield and quality of mushroom crops [28,29,30,31]. There are also several less aggressive Trichoderma species, found mainly in casing soil, such as T. harzianum Rifai, T. longibrachiatum Rifai, T. atroviride Bissett, T. virens (J.H. Mill., Giddens & A.A. Foster) Arx, T. koningii Oudem., T. guishouense Q.R. Li, McKenzie & Yong Wang, T. simonsii P. Chaverri, F.B. Rocha, Samuels, Degenkolb & Jaklitsch, T. atrobrunneum F.B. Rocha, P. Chaverri & Jaklitsch, and T. afroharzianum P. Chaverri, and F.B. Rocha Degenkolb & Druzhin [23,32].
Considering the losses that can be caused by mushroom flies, mycopathogenic fungi, and other harmful organisms, commercial production of A. bisporus is unsustainable without appropriate pest and disease management measures. They include preventive (prophylactic) measures, aimed at preventing the introduction and spread of pests and pathogens, and curative (reactive) measures, taken to control introduced pests and pathogens by chemical and/or biological means.
3. Chemical Control of Mushroom Pests and Diseases
Control of mushroom flies as major pests in mushroom production has long relied on the use of synthetic chemical insecticides. Historically, insecticides from several generations have been used for chemical control: after broad-spectrum neurotoxic compounds (organochlorines, organophosphates and carbamates, pyrethroids), various insect growth regulators (methoprene, diflubenzuron, triflumuron, and cyromazine) were introduced, followed by newer neurotoxic compounds (neonicotinoids and fipronil). Although chemical treatments usually provided effective pest control, negative effects (phytotoxicity to A. bisporus mycelium, insecticide residues in harvested mushrooms) have also been observed [10,14]. Additionally, instances of resistance in sciarid flies to organophosphates and pyrethroids have been reported [33,34]. The current list of chemical insecticides approved for the control of mushroom flies is not long, as many compounds have been withdrawn from the market due to risks to the environment and human health. The list varies from country to country, with a prospect of further application restrictions and reductions in the number of available compounds [10,14]. In the European Union, for example, no chemical insecticides are approved for the use in commercial button mushroom production [35].
Several organophosphorus and carbamate compounds, as well as diflubenzuron and some other compounds, were used in the past against nematodes, incorporated in the compost. However, no current chemical pesticides are recommended for nematode control in the button mushroom. There are also no recommendations for the control of mushroom mites with chemical pesticides [9,19].
Over the years, mushroom diseases caused by the mycopathogenic fungi have been controlled using several groups of synthetic chemical fungicides with various modes of action. These have included dithiocarbamates, chloronitriles (chlorothalonil), methyl benzimidazole carbamates (MBC), and demethylation inhibitors (DMI). However, as a consequence of their intensive utilization, multiple cases of fungicide resistance have been reported [36]. Resistant strains of L. fungicola [37,38], Cladobotryum spp. [28,39,40], and T. aggressivum [41,42] to MBC fungicides have been reported. Reduced susceptibility of L. fungicola and Cladobotryum spp. to the DMI fungicide prochloraz–manganese was also noted [28,43]. Chlorothalonil was found ineffective against Trichoderma either in toxicity tests in the laboratory or in efficacy trials carried out in A. bisporus growing rooms [44].
On the other hand, there have been studies showing the susceptibility of various fungal isolates/strains to fungicides. For example, some Hypomices spp. isolates have shown high susceptibility to the DMI fungicide prochloraz–manganese [45,46]. Trichoderma spp. strains from North Macedonia, Croatia, Serbia, and Hungary were susceptible to prochloraz. Additionally, some T. aggressivum f. europaeum strains showed high susceptibility to chlorothalonil and carbendazim [29]. Studies carried out in China [47] reveal that several DMI and MBC fungicides are still useful against H. perniciosus. Recent studies have shown that introduction of a novel fungicide, metrafenone, proved promising against the fungal pathogens Cladobotryum spp. and L. fungicola [48,49,50]. Nevertheless, evolution of metrafenone resistant strains has also been noted recently, after a study by Clarke et al. [31] reported Cladobotryum strains tolerant to metrafenone.
Similar to the insecticides intended for use against mushroom flies, the number of synthetic chemical fungicides currently available for controlling mushroom diseases is very limited. In addition to the problem of resistance, this situation is also the result of withdrawal from the market of certain fungicides or entire groups, due to environmental and health risks [36,51]. For example, metrafenone remained the only fungicide approved for use against mushroom pathogenic fungi after 2021 [31].
4. Bioprotection in the Management of Mushroom Pests and Diseases
Resistance problems and other negative effects, along with environmental and health issues, have created a need for sustainable and eco-friendly alternatives to the use of synthetic chemical pesticides in mushroom pest and disease control. Generally speaking, the concept of biological control and the application of biopesticides are often recommended as biologically based alternatives to chemical control. Although both alternatives have been known for decades, their practical importance has grown considerably in recent times due to increasingly stringent requirements of pesticide regulation and global endorsement of integrated pest and disease management as a new paradigm for crop protection [52,53,54,55].
The term biological control (or biocontrol) in scientific literature is usually defined as the exploitation of living organisms to suppress pests and pathogens directly or indirectly. Living organisms act as biological control agents (BCAs) by mechanisms of predation, parasitism, pathogenicity, antibiosis, competition, and resistance induction. This definition excludes non-living agents (e.g., substances of biological origin that are not produced in situ) and living organisms employing mechanisms that do not constitute biological control (e.g., plant growth promoting microorganisms). The emphasis on living organisms and corresponding mechanisms aims to harmonize the terminology used by entomologists, plant pathologists, and other scientists and experts working in the field of biological control [53,54,56,57]. The biological control vocabulary employed in official publications from the European Union, USA, United Nations, and affiliated institutions also includes only living organisms in the corresponding definitions [57]. Living organisms used as BCAs belong to invertebrates (insects, mites, nematodes) and microorganisms (bacteria, fungi, viruses), occurring naturally or commercially available. The latter are mass-produced in biofactories and applied in large numbers or concentrations as part of augmentative biological control of pests and diseases [53,54,58].
There is no globally accepted definition of the term biopesticide. The Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) define biopesticides as natural pest control products applied in a manner similar to chemical pesticides. This definition includes microbial biopesticides (microorganisms: bacteria, fungi, viruses, etc.), botanical biopesticides (plant extracts, essential oils and their components), and semiochemicals (allelochemicals and pheromones) [59]. The Environmental Protection Agency of the United States (EPA) defines microbial biopesticides in the same way as the FAO and WHO, but distinguishes biochemical pesticides (biochemicals that control pests and pathogens by non-toxic action: growth regulators, semiochemicals, induced resistance promoters, etc.) and plant-incorporated protectants (pesticidal substances produced by genetically modified plants) as the other two biopesticide classes [60]. On the other hand, the European Union legislation does not treat biopesticides as a specific category among plant protection products [61]. According to a widely accepted definition [52], also adopted in this review, biopesticides are commercial pest and disease control products manufactured from living microorganisms or natural substances that fall into three main categories: microbial biopesticides, semiochemicals, and biochemicals. Microbial biopesticides, which account for around 60% of the global biopesticide market, are dominated by products manufactured from the entomopathogenic bacterium Bacillus thuringiensis Berliner. Biochemical biopesticides, which include substances of microbial, plant, and animal origin that control pests and pathogens by toxic action, are the second most widely used biopesticide category. Among them, the most important are substances (compounds) derived from plants, such as azadirachtin, pyrethrum, and various essential oils, or obtained by fermentation from soil actinobacteria (mostly Streptomyces spp.). Semiochemicals are products of minor importance [52,62,63].
Obviously, the definitions of the terms biological control [53,56] and biopesticide [52] overlap, as both include living microorganisms. Commercially available living microorganisms, defined as BCAs, are applied as formulated biopesticide products in a manner similar to chemical pesticides. In order to avoid terminological problems, the broader term bioprotection was promoted [53,54,57]. This term encompasses protection provided by either living organisms used as BCAs or other nature-based protective agents. In this review, we adopted the terms bioprotection and bioprotection agents (Figure 2), which include both BCAs and biopesticides, bearing in mind that microorganisms can be viewed as microbial biopesticides used in biological control. In addition to BCAs and biopesticides, the bioprotection agents also include plant-growth-promoting organisms and substances, priming compounds, RNA-based products, mineral-based substances, etc. An overview of properties and uses of bioprotection agents for the management of A. bisporus pests and diseases follows further below.
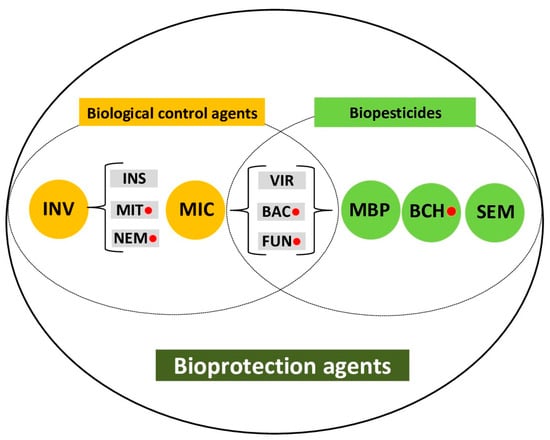
Figure 2.
Biological control agents (BCAs) and biopesticides within the concept of bioprotection. INV = invertebrates [INS—insects, predators and parasitoids; MIT—predatory mites; NEM—entomopathogenic nematodes]; MIC/MBP = microorganisms/microbial biopesticides [VIR—viruses; BAC—bacteria; FUN—fungi]; BCH = biochemical biopesticides; SEM = semiochemicals; ● indicates bioprotection agents used for the management of mushroom pests and diseases (the bioprotection agents also include plant-growth-promoting organisms and substances, priming compounds, RNA-based products, mineral-based substances).
4.1. Management of Mushroom Pests Using Bioprotection Agents
4.1.1. Invertebrates
Two groups of invertebrates are important as BCAs in mushroom production: entomopathogenic nematodes and predatory mites (Figure 2). Strains of several species of these BCAs, commercially available on the global market, have been used in augmentative biological control of mushroom flies [10,14,58,64].
Entomopathogenic nematodes (EPNs) from the families Steinernematidae and Heterorhabditidae have been used as effective BCAs against a number of insect pests. These EPNs are parasites that kill their insect hosts by the aid of mutualistic bacteria of the genera Xenorhabdus (for steinernematids) and Photorhabdus (for heterorhabditids), carried in the alimentary canal. The free-living, third-stage EPN infective juveniles (IJs) actively seek out and attack a host entering through natural openings (or through the cuticle in some cases). After establishing in the hemocoel, the IJs molt and release the bacteria that produce toxins causing septicemia and death of the host. The nematodes feed on host tissues digested by bacteria, grow and reproduce over one to three generations, and eventually exit as IJs to infect new hosts. At least 13 species from the genera Steinernema Travassos and Heterorhabditis Poinar have been commercialized for pest control [65,66,67].
The efficacy of EPNs is dependent on the longevity of IJs and recycling of populations in host cadavers. In addition to biotic factors (innate virulence and longevity of EPN species and strains, presence of nematophagous organisms), various abiotic factors may also affect the persistence and efficacy of EPNs. Avoidance of UV radiation, and moderate substrate moisture and temperature are the essential conditions for effective pest control [66,68].
The most extensively used EPN to control mushroom flies is Steinernema feltiae (Filipjev) (Table 2). Products containing various S. feltiae strains have demonstrated effective biological control of sciarid larvae in trials conducted under commercial mushroom-growing conditions, when applied at the end or a few days after casing (summarized in Table 3). Rinker et al. [69] found that the application of S. feltiae (a laboratory-reared strain) in small-scale trials at the casing time at concentrations ranging from 0.28 to 1.12 × 106 IJs m−2 caused an 86–100% reduction in L. ingenua population, introduced at the beginning of the spawn run. Scheepmaker et al. [70] applied a commercial product of S. feltiae against L. castanescens in mushroom-growing rooms 1 day before and 1 day after the casing time at 1 × 106 IJs m−2 and achieved a 97% reduction in the F1 generation, while application 7 days after the casing time caused a 95% reduction in the F2 generation. Jess and Kilpatrick [71] applied another commercial product of the nematode at 3 × 106 IJs m−2 immediately after the casing time and reduced L. ingenua population by 82%; the flies were introduced at the beginning of the spawn run. Jess and Bingham [72] applied the same commercial product at 3 × 106 IJs m−2 in a small-scale trial two days after the introduction of L. ingenua to spawned compost and achieved ca. 85% reduction in adult sciarid emergence. Navarro and Gea [73] applied a commercial product of the nematode at 1 × 106 IJs m−2 against L. castanescens 10 days after its introduction (and one day after the casing time) and found a 63% reduction.

Table 2.
Commercial bioprotection agents for mushroom pest and disease management.
Table 2.
Commercial bioprotection agents for mushroom pest and disease management.
| Bioprotection Agents | Description | Examples of Products | Targets |
|---|---|---|---|
| Invertebrates | |||
| Steinernema feltiae (Filipjev) | Nematode | Scia-Rid (Koppert) | Sciaridae, Phoridae |
| Nemasys-M (BASF) | |||
| Nemycel (E-nema) | |||
| Stratiolaelaps scimitus (Womersley) | Mite | Entomite-M (Koppert) | Sciaridae |
| Microorganisms/microbial biopesticides | |||
| Bacillus thuringiensis Berliner | Bacterium | VectoBac WDG (Valent Biosciences) | Sciaridae |
| subsp. israelensis | |||
| Bacillus velezensis QST 713 | Bacterium | Serenade Max (Bayer CropScience) | T. harzianum |
| T. aggresivum | |||
| Bacillus amyloliquefaciens | Bacterium | Amylo-X (Certis) | T. aggresivum |
| subsp. plantarum D747 | |||
| Bacillus amyloliquefaciens MBI 600 | Bacterium | Serifel (BASF) | T. harzianum |
| T. aggresivum | |||
| Streptomyces griseoviridis K61 | Bacterium | Mycostop (Danstar Lallemand) | L. fungicola |
| T. aggressivum | |||
| Streptomyces lydicus WYEC 108 | Bacterium | Actinovate (Novozymes) | L. fungicola |
| T. aggressivum | |||
| Clonostachys rosea J1446 | Fungus | Prestop (Danstar Lallemand) | L. fungicola |
| Biochemical biopesticides | |||
| Azadirachtin | Plant-derived compound | AzaGuard (BioSafe Systems LLC) Azatin XL (Certis) | Sciaridae, Phoridae |
| NeemAzal T/S 1.2 EC (EID Parry) | Nematodes | ||
| Pyrethrum | Plant-derived compound | Pyganic (MGK) Biogard Pyrethrum Dust (Certis | Sciaridae, Phoridae |
| Rosemary oil + geraniol + peppermint oil | Plant-derived compound | Ecotrol Plus (KeyPlex) | Sciaridae, Phoridae |
| Abamectin | Microbial-derived compound | Sorcerer 36 (Adama) | Mites Nematodes |
Based on data from EC [35], Karamouna et al. [61], Ortiz and Sansinenea [74], EPA [75], and a number of manufacturer websites.
The control of phorids by S. feltiae is usually less effective. However, some authors reported levels of efficacy comparable to those achieved in the control of sciarids. In a study mentioned before [70] in which a 95–97% reduction in L. castanescens population was achieved, variable M. halterata control was observed as well. The maximum observed reduction in the latter (75%) was explained by the presence of new S. feltiae IJs recycled in L. castanescens larvae of the F2 generation. Jess and Bingham [72] achieved >90% reduction in M. halterata emergence by applying S. feltiae two days after the inoculation of flies to spawned compost, but this reduction resulted from application of an unusually high concentration of 2.4 × 104 IJs m−2. Erler et al. [76] also carried out a trial with artificial introduction, but they applied a different commercial product of the nematode as casing drench, using 3 × 106 IJs m−2 at the time when M. halterata larvae began to eclose and found a 71% reduction. In the same trial, the organophosphorus insecticide chlorpyrifos-ethyl achieved a 72% reduction.
Some other EPNs, such as Steinernema carpocapsae Weiser, Heterorhabditis bacteriophora Poinar, and H. indica Poinar, have also been evaluated against mushroom flies and have shown acceptable effects [66,69,77]. However, these species have not been commercialized for the use in mushroom crops. In addition to improvements made in formulation technology, novel commercial EPN products could also emerge as a result of research aimed at finding and evaluating new strains of S. feltiae, as well as new effective EPN species [78,79,80,81].
Soil-dwelling predatory mites (Acari: Laelapidae, Macrochelidae, Parasitidae) are generalist predators that feed on various edaphic arthropod pests. This group of mites includes several species that are capable of controlling sciarid larvae. Among them, the most commercially important species is the laelapid Stratiolaelaps scimitus (Womersley) [=Hypoaspis miles], which has been used in greenhouse vegetables and ornamentals, as well as in mushroom cultivation (Table 2). Other commercially important laelapids are the species Gaeolaelaps aculeifer (Canestrini) and Gaeolaelaps gillespiei Beaulieu. As generalists, the laelapids can survive periods of prey scarcity by feeding on alternative prey or food, which facilitates their establishment [82,83].
Evaluation of the effectiveness of laelapids in the control of mushroom flies has mainly been focused on S. scimitus (summarized in Table 3). Ali et al. [84] evaluated this species against L. ingenua in large-scale trials carried out on a mushroom farm. The predatory mite, obtained from a laboratory culture, was applied either once (at spawning time) or three times (once at spawning time and twice at casing time), at 700 mites m−2, each time 5 days after artificial introduction of sciarids. Applied once, S. scimitus reduced L. ingenua population by 51–77%, while the reduction was 69–80% when the mite was applied three times. In the study in which the flies were introduced at the beginning of the spawn run, followed by immediate application of S. scimitus (a commercial product, ca. 710 mites m−2), the emergence of L. ingenua was reduced by 87%, while a later introduction of the mite (at the beginning of casing period) caused a reduction of 77% [71]. In another small-scale trial, a commercial product of S. scimitus (the same as in the previous study) was applied (700 mites m−2) at different time intervals after the introduction of L. ingenua to spawned compost. The highest reduction (ca. 80%) was achieved with the application 2 days after sciarid introduction, while applications 9 and 16 days after the introduction resulted in considerably lower efficacy [72]. In addition to the evaluation against L. ingenua, promising results have also been reported with a Brazilian population of S. scimitus in controlling Bradysia matogrossensis (Lane) in commercial-scale trials [85]. There are very few data on the evaluation of S. scimitus in controlling phorid flies. A rare example is the mentioned study by Jess and Bingham [72], in which the commercial product was applied at a rate of 700 mites m−2 after artificial introduction of M. halterata to spawned compost. Application 2 days after the introduction caused a reduction of ca. 85%, while application 4 days later resulted in lower efficacy.

Table 3.
Efficacy of commercial invertebrate bioprotection agents against sciarid flies evaluated under mushroom growing conditions.
Table 3.
Efficacy of commercial invertebrate bioprotection agents against sciarid flies evaluated under mushroom growing conditions.
| Bioprotection Agent | Application Time | Concentration/Rate | Target | Efficacy (%) | References |
|---|---|---|---|---|---|
| Steinernema | At the casing time | 0.28–1.12 × 106 IJs m−2 | L. ingenua | 86–100 | [69] |
| feltiae | 1 d before + 1 d after the casing time | 1 × 106 IJs m−2 | L. castanescens | 97 | [70] |
| 7 d after the casing time | 95 | ||||
| At the casing time | 3 × 106 IJs m−2 | L. ingenua | 82 | [71] | |
| At the spawn run | 3 × 106 IJs m−2 | L. ingenua | 85 | [72] | |
| 1 d after the casing time | 1 × 106 IJs m−2 | L. castanescens | 63 | [73] | |
| Stratiolaelaps | At the spawn run | 700 mites m−2 | L. ingenua | 51–77 | [84] |
| scimitus | At the spawn run + at the casing time | 69–80 | |||
| At the spawn run | 710 mites m−2 | L. ingenua | 87 | [71] | |
| At the casing time | 77 | ||||
| At the spawn run | 700 mites m−2 | L. ingenua | 80 | [72] |
Potential new BCAs that could be used against sciarid flies in mushrooms are soil predatory mites of the family Macrochelidae, primarily Macrocheles robustulus (Berlese), a commercialized species. This species was proven to be more effective in controlling sciarids in ornamentals than G. aculeifer [86], but there are currently no data on its evaluation in mushrooms. Another promising BCA belongs to the family Parasitidae. Al-Amidi and Downes [87] and Al-Amidi et al. [88] presented data showing Parasitus bituberosus Karg as effective against sciarid and cecidomyiid flies, while Szafranek et al. [89] provided further data on its effectiveness in controlling sciarids in mushrooms, as well as in controlling rhabditid nematodes.
4.1.2. Microorganisms/Microbial Biopesticides
Several subspecies of B. thuringiensis have been commercially developed for the control of insect pests and vectors in the orders Lepidoptera, Coleoptera, and Diptera, making this bacterium the world’s best-selling microbial bioagent. The bioinsecticidal effect of B. thuringiensis, a Gram-positive, spore-forming bacterium, relies on δ-endotoxins, which are insecticidal secondary metabolites formed as crystalline (Cry) proteins. After ingestion, solubilized and activated toxins in larval body bind to the cell membrane of epithelial midgut cells, causing disruption in ion channel function, leakage of gut content, and cell lysis. One of the bacterium’s subspecies, B. thuringiensis subsp. israelensis (Bti), highly effective against mosquitoes and other dipteran vectors [74,90], has also been used in the control of mushroom flies (Table 2).
There have been contrasting results in assessing the effects of Bti on sciarid and phorid larvae. Keil [91] conducted six commercial-scale field trials in which a Bti strain showed efficacy in controlling L. ingenua and M. halterata equal or better than the insect-growth regulators methoprene and diflubenzuron, applied at 147–215 mL m−2 6–10 days after the beginning of the spawn run, and immediately after casing. Erler et al. [76] demonstrated a 77% reduction in M. halterata adult emergence caused by a Bti commercial product applied at casing drench. On the other hand, Jess and Kilpatrick [71] observed the ineffectiveness of another Bti product applied at 15.4 mg a.i. m−2 against L. solani at the beginning of the spawn run or at the casing time. In laboratory bioassays, Cloyd and Dickinson [92] evaluated the efficacy of the same Bti product used by Erler et al. [76]. The bioinsecticide, applied as a drench to the growing medium at 0.29–1.15 × 106 Itu L−1, was not effective against the second and third instars of B. coprophila larvae. Shamshad et al. [93] incorporated a Bti product into the growing medium and found its ineffectiveness against third instar larvae of L. ingenua. Later instars are less susceptible to Bti because they have to consume large amounts of the product to be affected. These results indicate that it is essential to apply Bti products before the sciarid population build up. However, their short residual activity requires several applications, which is probably not cost-effective [10,93].
Among microorganisms/microbial biopesticides, mycoinsecticides constitute a large group of products, manufactured from spores and other living propagules of entomopathogenic fungi. Their pathogenic action against insects and mites is based on penetration of the host integument (by mechanical force and cuticle-degrading hydrolytic enzymes), colonization, and production of toxic metabolites, leading to mycosis and death. The majority of mycoinsecticide products are based on two fungal species, Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) [65,74]. The effectiveness of these products in controlling mushroom flies has been evaluated in few studies. Andreadis et al. [94,95] assessed the efficacy of a commercial product of B. bassiana (strain GHA) that was permitted for use against sciarid and phorid flies on mushroom farms in Pennsylvania. The product was ineffective in controlling L. ingenua population, incorporated into compost at spawning at the label rate (1.8 × 108 conidia mL−1) following artificial introduction of sciarids. In laboratory bioassays, directly treated eggs and larvae were not susceptible to the product, while pupae showed a corrected mortality of 21%. Exposure of adult females to the treated surface for one hour resulted in 100% mortality within 8 days [94]. Laboratory bioassays with M. halterata also showed that eggs and larvae were not susceptible, but pupal mortality was significantly higher (59%), and it took twice as long (16 days) to achieve 100% female mortality [95]. These results indicate that the product could be effective only if used as a premise spray for the control of L. ingenua adults. On the other hand, Ajvad et al. [96] applied an isolate of M. anisopliae against L. castanescens larvae in a small-scale trial at the casing stage immediately following inoculation and found that a concentration of 1 × 108 conidia mL−1 caused 62% mortality. Considering that both M. anisopliae and B. bassiana are saprophytic fungi and can grow over the mushroom media/spawn, further research is needed to show the real possibilities for using mycoinsecticide products in controlling mushroom flies.
4.1.3. Biochemical Biopesticides
Commercial products manufactured from the Indian neem tree, Azadirachta indica (Meliaceae), and Dalmatian daisy, Tanacetum cinerariifolium (Asteraceae), have been the most widely used biochemical biopesticides of plant origin or botanicals. The seeds of A. indica are a source of neem oil that contains tetranortriterpenoid (limonoid) azadirachtin, and a number of other bioactive compounds. Azadirachtin is an insect and mite growth regulator and a potent feeding and oviposition deterrent. The flowers of T. cinerariifolium contain oleoresin pyrethrum, a mixture of six related neurotoxic insecticidal and acaricidal esters, the most important of which are pyrethrin I and pyrethrin II [63,97]. In recent decades, various products based on essential oils—complex mixtures of compounds obtained from aromatic plants belonging to the families Lamiaceae, Myrtaceae, Rutaceae, and some others—have become increasingly important in the market of botanicals. These products are manufactured from either a single oil/constituent or a mixture of oils/constituents. The main constituents of essential oils are terpenoids (such as cineole, citronellol, geraniol, menhol), volatile compounds that act rapidly on insects and mites by direct contact or vapor phase [63,98].
Used as broad-spectrum insect growth regulators, azadirachtin/neem-based products are also the most important botanicals for controlling sciarid larvae. Although a number of these products have been commercialized for use against mushroom flies (e.g., Table 2), there are few data in the scientific literature on their effectiveness (summarized in Table 4). In test of Lycoriella sp. control, Rovesti et al. [99] demonstrated 62% efficacy of an azadirachtin-based commercial product, applied 9 days after casing at 2 g m−2. Erler et al. [100] evaluated two commercial products, containing 1% and 0.3% azadirachtin, against M. halterata in a small-scale trial. Applied as casing drench at a concentration of 5 mL L−1, the products achieved a 63–72% reduction in artificially inoculated population, and this reduction was at the same level as that achieved by chlorpyrifos-ethyl, an organophosphate. Drobnjaković et al. [101] applied another commercial product (containing 1% azadirachtin) at 0.5 mL L−1 in four treatments after casing at 7-day intervals and found that the product was superior in controlling L. ingenua population to the organophosphorus insecticide malathion. A neem cake-based product, added to casing soil at a rate of 2.5%, reduced the number of L. ingenua adults by 84% [102]. Azadirachtin/neem-based products have also shown potential to be used against nematodes [19].

Table 4.
Efficacy of commercial azadirachtin/neem-based bioprotection agents against mushroom flies evaluated under mushroom growing conditions.
Pyrethrum-based products have been used in the control of a wide range of insect and mite pests on agricultural crops, ornamentals, and stored products, as well as in public health and for structural pest control. Recent considerable research interests in essential oils have resulted in a moderate commercial success. The area of use of essential oil-based products is similar to that of pyrethrum [63,97]. Various pyrethrum-based products (some of them are mixtures of pyrethrum and plant oils), as well as some products manufactured from essential oils and/or their components, are recommended for use in controlling mushroom flies (e.g., Table 2). However, there are no studies evaluating their effectiveness in mushroom-growing environments.
Compounds obtained by fermentation from soil actinobacteria have been the main source for mass production of biochemical biopesticides of microbial origin. Abamectin (a mixture of avermectins B1a and B1b, derived from Streptomyces avermitilis) and spinosad (a mixture of spinosyns A and D, derived from Saccharopolyspora spinosa) are very well-known examples of globally successful microbial insecticide. Both products have a neurotoxic mode of action and have been used against a wide range of insect pests; abamectin has also been used against mites and nematodes [103,104]. However, few data are available on their effectiveness against mushroom pests. Shamshad et al. [93] found that abamectin was ineffective against the third instar of L. ingenua larvae in a laboratory dose–response bioassay. On the other hand, Erler et al. [76] applied spinosad as soil drench at the recommended label rate against M. halterata and observed a 72% reduction in adult emergence. Babar et al. [105,106] evaluated spinosad against M. halterata and L. castanescens and found an 84–86% reduction in adult emergence. There are no recommendations for the use of abamectin and/or spinosad to control mushroom flies. Some abamectin-based products (e.g., Table 2) are labeled for use against mushroom mites (P. mesembrinae, M. lambi) and soil-borne nematodes.
4.2. Management of Mushroom Diseases Using Bioprotection Agents
4.2.1. Microorganisms/Microbial Biopesticides
Far behind B. thuringiensis-based products, the second group of microorganisms or microbial biopesticides, considering global market share, includes biofungicide products based on Bacillus subtilis, Bacillus amyloliquefaciens, Bacillus velezensis, Bacillus pumilus, and Bacillus licheniformis, mostly oriented towards control of plant and mycopathogens [107,108,109,110]. Regarding commercial Bacillus-based biofungicides aimed at the control of plant pathogens, the list of products currently available on the market is long, although only a few are registered for application in button mushroom production. One of them is B. velezensis strain QST 713, formerly described as B. subtillis QST 713, B. amyloliquefaciens strain QST 713, and B. amyloliquefaciens ssp. plantarum strain QST 713, eventually reclassified based on recent phylogenetic analysis [5]. Of all fungal pathogens in the Agaricus production system, B. velezensis QST 713 has shown the best control results against T. aggressivum f. europaeum, acting as an excellent competitor for nutrients and space [5].
The use of this strain against T. aggressivum started in France in 2008. Since pilot trials on the prevention of green mold disease in mushrooms were carried out by Vedie and Rousseau [111], proving the efficiency of a B. velezensis QST 713-based product (Table 2), several studies have been conducted with a purpose to evaluate its efficacy in disease control and impact on mushroom yield (summarized in Table 5). In a study by Milijašević-Marčić et al. [4], carried out under conditions of artificial inoculation with T. agressivum and T. harzianum, the efficacy of the biofungicide B. velezensis QST 713 against T. aggressivum f. europaeum was 48% (without statistical difference from prochloraz–Mn efficacy of 49%), while the yield was not negatively affected by either treatment. Similarly, Stanojević et al. [110] reported its satisfactory efficacy (52–57% in two experiments), also under high disease incidence conditions; the highest yield was noted in inoculated treated plots. In addition, Pandin et al. [5] also reported mushroom yield improvement of up to 54% after treatment of compost inoculated with T. aggressivum in comparison with untreated inoculated control. Investigating the impact of B. velezensis QST 713 spawn grain treatment on compost green mold disease, Potočnik et al. [112] reported that prochloraz–Mn was the most efficient treatment, achieving 70% efficacy against T. aggressivum f. europaeum T77, whereas B. velezensis QST 713 showed 50% efficacy. This strain was also evaluated against T. harzianum, the causal agent of casing green mold. Kosanović et al. [32] observed 44% efficacy, while Milijašević-Marčić et al. [4] reported efficacy values of 56% and 58% for the strain QST 713 and prochloraz–Mn, respectively.

Table 5.
Efficacy of commercial B. velezensis QST 713 based bioprotection agents against mushroom diseases evaluated under mushroom growing conditions.
The ability of B. velezensis QST713 to control fungal pathogens is linked with its multiple modes of action: competition with pathogens for space and nutrients, production and delivery of lipopeptides and different proteases, induction of plant systemic resistance, and stimulation of plant growth. Additionally, B. velezensis QST 713 forms spatially organized biofilms, enabling its superior colonization and persistence in various environments [113]. Metagenomic studies by Pandin et al. [5] on compost microbiota dynamics in the presence of B. velezensis QST713 have proved that this strain influenced the structure of microbiome only during the early stages of Agaricus production (up to 15 days after treatment), while colonizing the substrate. Also, sequence analysis of the whole genome of B. velezensis QST713 strain unveiled that this strain bears various antimicrobial clusters and a significant arsenal to form complex 3D biofilms [5]. Additionally, Pandin et al. [113,114] revealed the overexpression of several genes of the strain involved in biofilm formation in the compost in comparison with their expression in broth compost extract either in the exponential growth phase (epsC, blsA, and tapA genes) or in the stationary growth phase (tapA gene). Also, the overexpression of B. velezensis QST713 genes related to surfactin (srfAA) and fengycin (fenA) production in the presence of the fungal pathogen in compost was reported [114].
Although most evaluations of B. velezensis QST713 have been conducted against Trichoderma species, several more recent studies aimed to prove its efficacy against other mushroom pathogens. Stanojević et al. [110] reported that the strain achieved the efficacy of 42% and 62% against L. fungicola in repeated trials. The trials were conducted under conditions of artificial inoculation with the same inoculum rate. However, these differences in efficacy could be attributed to different compost quality. Also, the total number of mushrooms was notably higher in the first trial regardless of lower efficacy of the tested biocontrol strain [110]. The investigation of in vitro response of L. fungicola to B. velezensis QST 713, carried out by Clarke et al. [115], reveals that the strain induced inhibition of L. fungicola plate cultures, reduced mycelium growth in liquid cultures, and damaged the morphology and structure of hyphae. In addition, proteomic analysis demonstrates that the strain was able to reduce the growth of L. fungicola and induce a stress response in this pathogen.
Clarke et al. [31] reported that B. velezensis QST 713 did not prevent significant yield reductions or disease incidence caused by C. mycophilum. Similarly, gathering results from six trials that included this strain and a commercial biofungicide based on B. amyloliquefaciens subsp. plantarum strain D747 (Table 1), Navarro et al. [30] concluded that neither of the two Bacillus treatments was effective against H. perniciosus in mushroom crops. Moreover, apart from the fact that the trials included different levels of inoculum, even in the case of the lowest inoculum rate with negligible disease incidence levels, the effectiveness of Bacillus-based treatments was low, rendering them nonapplicable for H. perniciosus control in button mushroom [30]. The product based on B. amyloliquefaciens subsp. plantarum D747 was recently registered against T. agressivum as a compost treatment, while yet another product, based on B. amyloliquefaciens strain MBI 600, was registered against both T. harzianum and T. agressivum (Table 2).
Taking into account that only a few Bacillus-based products present on the market have been registered for the control of mycopathogens in Agaricus production systems, several recent studies have evaluated the efficacy of newly isolated strains against fungal pathogens of mushrooms that could potentially lead to their commercialization. Milijašević-Marčić et al. [4] reported that native B. subtilis strain B-38 caused 48% and 52% in vitro growth inhibition of T. harzianum T54 and T. aggressivum f. europaeum T77, respectively. However, under mushroom-growing room conditions, this strain showed 50% efficacy against T. harzianum while the efficacy in controlling T. aggressivum f. europaeum was only 36%. Liu et al. [116] tested B. velezensis strain B154, isolated from button mushroom compost in China, against several fungal pathogens, including T. harzianum. The authors indicated that treatment with this strain significantly increased the yield of white button mushroom. In a large-scale test, Stanojević et al. [109] evaluated indigenous Bacillus spp. strains isolated from different compost phases, against several Trichoderma species (T. aggressivum f. europaeum, T. harzianum, T. atroviride) and L. fungicola. Strains with the highest antagonistic activity in vitro, B. amyloliquefaciens B-241, B. pumilus B-138, and B. subtilis B-233, were selected for in vivo evaluation against T. aggressivum f. europaeum and L. fungicola. The in vivo study showed that strain B. amyloliquefaciens B-241 was a good candidate for further trials at a commercial scale, as it achieved the highest efficacy in two trials against compost green mold (52% and 68%), and dry bubble disease (45% and 58%). The efficacy of this strain was similar to that of the biofungicide B. velezensis QST 713 [110].
In a pilot study by Büchner et al. [117], B. velezensis strain SZMC 25,431 was selected for further investigation, based on its antagonistic activity in confrontation assays against several Trichoderma species, as well as L. fungicola. The authors noted that the strain had a positive effect on mushroom yield, whether applied alone or in combination with synthetic fungicides. Also, when the bacterial suspension of this strain was mixed in water tanks and applied during regular irrigation in industrial-scale experiments, the mushroom yield increased, rendering it a potentially good candidate for the control of these mycopathogens [117]. Recent studies have demonstrated that B. velezensis Kos strain could inhibit in vitro the green mold disease agent, T. aggressivium [118] and cobweb disease agent, C. mycophilum [115]. Additionally, Clarke et al. [115] demonstrated biocontrol potentials of B. velezensis Kos strain through in vitro growth inhibition of L. fungicola, as well as an ability of that strain to induce a stress response in the pathogen, similar to B. velezensis QST 713 strain. Later on, Clarke et al. [31] demonstrated a moderate control ability of C. mycophilum in two trials, reaching 30–40% efficacy.
Jafaripour et al. [119] tested the effectiveness of several potential antagonistic bacteria in the reduction in the severity of the bacterial blotch disease caused by P. tolaasii. The examined isolates showed a significant effect on the fresh weight and the number of mushrooms in comparison to the control treatment. Potential biocontrol agents were identified as B. velezensis, Bacillus wiedmannii Bacillus altitudinis, and Kocuria rhizophila.
Besides Bacillus species, the actinomycetes Streptomyces griseoviridis strain K61 and Streptomyces lydicus strain WYEC 108 have also been registered for use in button mushroom crops as biofungicides (Table 2). Beyer et al. [120] reported satisfactory efficacy of S. griseoviridis K61 against L. fungicola when it was applied either 7, 9, and 15 days after casing time at label rate, or 9 days after casing at the double rate. Its efficacy was not significantly different from that of the chemical fungicides thiabendazole (applied 7 days after casing) or chlorothalonil (applied 9 days after casing). Furthermore, this study also showcased that the strain S. lydicus WYEC 108, albeit registered for both L. fungicola and T. aggressivum in mushrooms, was not as effective against the latter, when applied on mushroom spawn grains (mycelium) as commercially prepared synthetic spawn or spawn treated with B. velezensis QST 713 [120].
Regarding potential new products for the control of fungal pathogens in Agaricus production, an indigenous Streptomyces flavovirens strain A06, isolated from mushroom substrate during composting process, has been shown to achieve 71% efficacy against T. aggressivum, while its efficacy in the control of T. harzianum was only 17%. Elevation of mushroom yield by this strain was also noted [121]. Some actinobacteria have also been tested as potential biobactericides. Sahin [122] reported in vitro antimicrobial activity of actinomycetes from the Streptomyces cluster group (S. rochei, S. lydicus, and S. antibioticus) against the bacterial blotch pathogen, P. tolaasii. After conducting fermentation, and preliminary extraction and isolation of bioactive components, the author concluded that β-lactam was the bioactive compound structurally related to penicillin.
While fungi acting as microbial biofungicides are widely used in plant protection, they are of minor importance in the control of fungal diseases of A. bisporus. Only Clonostachys rosea (formerly Gliocladium catenulatum) strain J1446 has been registered for use in button mushrooms (Table 1). Evaluating several commercially available biofungicides for the control of L. fungicola, Beyer et al. [120] found a lower efficacy of C. rosea J1446, compared to S. griseoviridis K61, but total fresh mushroom yield was higher in the former treatment.
4.2.2. Biochemical Biopesticides
Regarding biochemical biopesticides aimed against mycopathogens, there are no available commercial products for use in button mushrooms. Those of microbial origin, such as streptomycin, kasugamycin, and other compounds obtained from actinobacteria, have been well-known for a long time, but their use in mushrooms is not allowed. On the other hand, there is a plethora of recent studies that have searched for potential new biochemical biopesticides of plant origin. Gea et al. [36] gave an up-to-date and detailed review of recent research on botanicals as alternatives for control of mycopathogenic fungi of cultivated mushrooms. Although the research on the possibilities of their use has been extensive, somehow they failed to find a way to commercialization. Also, only a few studies were conducted with biofungicides that are currently available on the market. Among the rare ones tested in a mushroom crop is the biofungicide based on tea tree oil, an essential oil obtained from Melaleuca alternifolia (Myrtaceae). This product showed a potential in the cobweb disease control, suppressing C. dendroides in vitro and inducing significant disease reduction after drench application in crop trials [123].
5. Combined and Integrated Use of Bioprotection Agents in Mushrooms
More efficient management of mushroom pests and diseases could be achieved by combining various bioprotection agents. Therefore, it is necessary to evaluate the compatibility of various bioprotection agents and their impact when applied in combination. For example, Thoeming and Poehling [124] showed that soil application of azadirachtin was not harmful to G. aculeifer, while Duarte et al. [125] observed that S. scimitus was not significantly affected by exposure to the residues of that bioinsecticide in laboratory. Saito and Brownbridge [126] found different reactions of S. scimitus and G. gillespiei to B. bassiana- and M. aniospliae-based mycoinsecticides and S. feltiae, while S. scimitus was not significantly affected by either mycoinsecticide or the nematode, and G. gillespiei was adversely affected by both. Based on the results obtained in concentration–mortality bioassays, Laznik and Trdan [127] and Petrikowszki et al. [128] concluded that azadirachtin was compatible with S. feltiae.
Combined applications of bioprotective agents in controlling mushroom pests and diseases have been evaluated in several studies. Navarro and Gea [73] evaluated the efficacy of combined application of S. feltiae and S. carpocapsae against L. castanescens at reduced rates (0.5 × 106 Ijs m−2), and observed a 50% reduction, while single application of S. feltiae achieved a 63% reduction. Ajvad et al. [96] combined a M. anisopliae isolate and G. aculeifer against L. castanescens applied immediately after inoculation, and reported increased efficacy, compared to single application of the fungus. Milijašević-Marčić et al. [129] showed an additive interaction between the indigenous strains B. amyloliquefaciens B-241 and S. flavovirens A06 against T. aggressivum, while synergistic interaction between the two microorganisms was observed regarding their impact on mushroom yield.
So far, most studies examining combinations of BCAs or different plant protection products have aimed to test potential synergistic effects in the control of the same pest or pathogen. A recent study by Potočnik et al. [6] evaluated interactions between the commercial population of S. feltiae and the indigenous strains B. amyloliquefaciens B-241 or S. flavovirens A06, in an attempt to simultaneously control both T. aggressivum and L. ingenua in Agaricus production. Additive relationships in efficacy against T. aggressivum were observed between S. feltiae and each microorganism. Considering the efficacy against L. ingenua, a mild antagonistic relationship between the nematode and each microorganism was observed in plots without T. aggressivum, and additive in plots inoculated with the pathogenic fungus, although high efficacy was achieved in all combinations (>80%). As for the impact on yield, synergistic interactions were indicated [6].
Bioprotection agents require inclusion in integrated pest and disease management (IPDM) programs to create conditions for full effectiveness. The IPDM programs for mushrooms are based on exclusion, cultural practices, monitoring, and control [9,21]. Exclusion refers to minimizing the risk of introduction and spread of pests and pathogens by applying various techniques and procedures, such as establishing physical barriers, while cultural practices (pasteurization, temperature and humidity control, crop cycle shortening, sanitation) manipulate growing environments to favor the mushrooms and discourage pests and pathogens. Monitoring provides crucial information for defining management tactics and their evaluation [9,19,36,130].
Control measures play a central role in IPDM programs. Judicious application of synthetic chemical pesticides assumes their use as a last resort [51,131], as well as their compatibility with bioprotection agents [32,132,133] in combined applications. On the other hand, there has been a trend towards a reduction in the number of available chemical pesticides [10,36,51]. Bioprotection agents can be at least equally efficacious as synthetic chemical pesticides, as shown by comparisons of S. feltiae or azadirachtin and organophosphorus insecticides [76,100,101], or the B. velezensis QST 713-based biofungicide and prochloraz–Mn [4]. Providing acceptable efficacy levels, and integrated preventive and other measures within IPDM programs, bioprotection agents can offer sustainable alternatives to chemical pesticides in the management of A. bisporus pests and diseases.
6. Conclusions
- Bioprotection agents, both biological control agents and biopesticides, are of growing importance in controlling pests and diseases of the button mushroom.
- Although the amount of research in this field has increased, there are currently not many commercialized agents intended for the use in A. bisporus cultivation.
- There are two main issues that need to be addressed in future research of bioprotection agents: (1) further evaluation of already commercialized agents, and (2) the search for novel agents.
- Further research is warranted in terms of discovering ecological interactions and compatibility between various bioprotection agents in mushroom cultivation, in order to enable their incorporation in IPDM programs.
Author Contributions
Conceptualization: D.M. and S.M.-M.; literature search, data analysis and interpretation: D.M., S.M.-M., I.P., T.D., J.L., L.Š. and N.G.; writing—original draft preparation: D.M., S.M.-M., I.P., T.D., J.L., L.Š. and N.G.; writing—review and editing: D.M., S.M.-M. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Fund of the Republic of Serbia: Green Program of Cooperation between Science and Industry #GRANT no. 3/4848 (2023-25) Microbial recipe for edible mushroom production—MICRO-MUSH, and the Ministry of Science, Technological Development and Innovation of the Republic of Serbia: contract nos. 451-03-136/2025-03/200214 and 451-03-137/2025-03/200116.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Database. Available online: http://www.fao.org/faostat/en/#data (accessed on 27 March 2025).
- Singh, M.; Kamal, S.; Sharma, V. Status and trends in world mushroom production-III-World Production of Different Mushroom Species in 21st Century. Mushroom Res. 2021, 29, 75. [Google Scholar] [CrossRef]
- Zhang, X.; Zhong, Y.; Yang, S.; Zhang, W.; Xu, M.; Ma, A.; Zhuang, G.; Chen, G.; Liu, W. Diversity and dynamics of the microbial community on decomposing wheat straw during mushroom compost production. Biores. Technol. 2014, 170, 183–195. [Google Scholar] [CrossRef]
- Milijašević-Marčić, S.; Stepanović, M.; Todorović, B.; Duduk, B.; Stepanović, J.; Rekanović, E.; Potočnik, I. Biological control of green mould on Agaricus bisporus by a native Bacillus subtilis strain from mushroom compost. Eur. J. Plant Pathol. 2017, 148, 509–519. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Deschamps, J.; Védie, R.; Rousseau, T.; Aymerich, S.; Briandet, R. Complete genome sequence of Bacillus velezensis QST713: A biocontrol agent that protects Agaricus bisporus crops against the green mould disease. J. Biotechnol. 2018, 278, 10–19. [Google Scholar] [CrossRef]
- Potočnik, I.; Šantrić, L.; Luković, J.; Grujić, N.; Anđelković, N.; Majić, I.; Drobnjaković, T.; Marčić, D.; Milijašević-Marčić, S. Discovering ecological interactions between biocontrol bacterial strains and entomopathogenic nematodes in button mushroom production. Microorganisms 2025, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.J.; Carrasco, J.; Gea, F.J. The Role of Water Content in the Casing Layer for Mushroom Crop Production and the Occurrence of Fungal Diseases. Agronomy 2021, 11, 2063. [Google Scholar] [CrossRef]
- Savoie, J.; Mata, G. Growing Agaricus bisporus as a contribution to sustainable agricultural development. In Mushroom Biotechnology, Developments and Applications; Marian, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 69–91. [Google Scholar]
- Rinker, D.L. Insect, mite, and nematode pests of commercial mushroom production. In Edible and Medicinal Mushrooms: Technology and Applications, 1st ed.; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 221–237. [Google Scholar] [CrossRef]
- Shamshad, A. The development of integrated pest management for the control of mushroom sciarid flies, Lycoriella ingenua (Dufour) and Bradysia ocellaris (Comstock), in cultivated mushrooms. Pest Manag. Sci. 2010, 66, 1063–1074. [Google Scholar] [CrossRef]
- Navarro, M.J.; Lopez-Serrano, F.R.; Escudero-Colomar, L.A. Phoretic relationship between the myceliophagous mite Microdispus lambi (Acari: Microdispiddae) and mushroom flies in Spanish crops. Ann. Appl. Biol. 2019, 174, 277–283. [Google Scholar] [CrossRef]
- Coles, P.S.; Mazin, M.; Nogin, G. The association between mushroom sciarid flies, cultural techniques, and green mold disease incidence on commercial mushroom farms. J. Econ. Entomol. 2021, 114, 555–559. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, S.R. Phorid affecting mushroom production and their management—A review. Mushroom Res. 2000, 8, 55–69. [Google Scholar]
- Navarro, M.J.; Escudero-Colomar, L.A.; Carrasco, J.; Gea, F.J. Mushroom phorid flies—A review. Agronomy 2021, 11, 1958. [Google Scholar] [CrossRef]
- Kheradmand, K.; Kamali, K.; Fathipour, Y.; Goltapeh, E.M.; Nemati, A.R. Crop loss assessment of Pediculastes fletchmanni Wich (Acari: Pygmephoridae) on button mushroom. IOBC/WPRS Bull. 2006, 29, 109–114. [Google Scholar]
- Kheradmand, K.; Kamali, K.; Fathipour, Y.; Goltapeh, E.M. Development, life table and thermal requirement of Tyrophagus putrescentiae (Astigmata: Acaridae) on mushrooms. J. Stored Prod. Res. 2007, 43, 276–281. [Google Scholar] [CrossRef]
- Gao, J.R.; Zou, P. Biology, life table and host specificity of the mushroom pest, Brennandania lambi (Acari: Pygmephoroidea). Exp. Appl. Acarol. 2001, 25, 187–202. [Google Scholar] [CrossRef]
- Szafranek, P.; Lewandowski, M. Mite community on Polish mushroom farms. Int. J. Acarol. 2017, 43, 217–222. [Google Scholar] [CrossRef]
- Keshari, N.; Kranti, K. Integrated Management of Phytopathogenic Nematodes Infesting Mushroom. In Management of Phytonematodes: Recent Advances and Future Challenges; Ansari, R.A., Rizvi, R., Mahmood, I., Eds.; Springer Nature: Singapore, 2020; pp. 141–170. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W. A revision of the Verticillium fungicola species complex and its affinity with the genus Lecanicillium. Mycol. Res. 2008, 112, 811–824. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Gaze, R.H. Mushroom Pest and Disease Control: A Colour Handbook, 1st ed.; Manson Publishing Ltd. Academic Press: San Diego, CA, USA, 2008; p. 192. [Google Scholar]
- Largeteau, M.L.; Savoie, J.-M. Microbially induced diseases of Agaricus bisporus: Biochemical mechanisms and impact on commercial mushroom production. Appl. Microbiol. Biotechnol. 2010, 86, 63–73. [Google Scholar] [CrossRef]
- Allaga, H.; Zhumakayev, A.; Büchner, R.; Kocsubé, S.; Szűcs, A.; Vágvölgyi, C.; Kredics, L.; Hatvani, L. Members of the Trichoderma harzianum species complex with mushroom pathogenic potential. Agronomy 2021, 11, 2434. [Google Scholar] [CrossRef]
- Geels, F.P.; Hessen, L.P.W.; Van Griensven, L.J.L.D. Brown discoloration of mushrooms caused by Pseudomonas agarici. J. Phytopathol. 2008, 140, 249–259. [Google Scholar] [CrossRef]
- Grogan, H.M.; Adie, B.A.T.; Gaze, R.H.; Chalen, M.P.; Mills, P.R. Double-stranded RNA elements associated with the MVX disease of Agaricus bisporus. Mycol. Res. 2003, 107, 147–154. [Google Scholar] [CrossRef]
- Hatvani, L.; Kredics, L.; Allaga, H.; Manczinger, L.; Vágvolgyi, C.; Kuti, K.; Geosel, A. First report of Trichoderma aggressivum f. aggressivum green mold on Agaricus bisporus in Europe. Plant Dis. 2017, 101, 1052. [Google Scholar] [CrossRef]
- Krupke, O.A.; Castle, A.J.; Rinker, D.L. The North American mushroom competitor, Trichoderma aggressivum f. aggressivum, produces antifungal compounds in mushroom compost that inhibit mycelial growth of the commercial mushroom Agaricus bisporus. Mycol. Res. 2003, 107, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Grogan, H.M. Fungicide control of mushroom cobweb disease caused by Cladobotryum strains with different benzimidazole resistance profiles. Pest Manag. Sci. 2006, 62, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Luković, J.; Milijašević-Marčić, S.; Hatvani, L.; Kredics, L.; Szücs, A.; Vàgvölgyi, C.; Duduk, N.; Vico, I.; Potočnik, I. Sensitivity of Trichoderma strains from edible mushrooms to the fungicides prochloraz and metrafenone. J. Environ. Sci. Health B 2021, 56, 54–63. [Google Scholar] [CrossRef]
- Navarro, M.J.; Santos, M.; Dianez, F.; Gea, F.J. Chemical and biological control of wet bubble disease (Hypomyces perniciosus) in mushroom crops. Agronomy 2023, 13, 1672. [Google Scholar] [CrossRef]
- Clarke, J.; McGuinness, B.; Fitzpatrick, D.; Kavanagh, K.; Grogan, H. Response of the mushroom pathogen Cladobotryum mycophilum to the fungicides prochloraz and metrafenone and two Bacillus-based biological control agents in mushroom crop trials. Crop Prot. 2024, 177, 106530. [Google Scholar] [CrossRef]
- Kosanović, D.; Potočnik, I.; Duduk, B.; Vukojević, J.; Stajić, M.; Rekanović, E.; Milijašević-Marčić, S. Trichoderma species on Agaricus bisporus farms in Serbia and their biocontrol. Ann. Appl. Biol. 2013, 163, 218–230. [Google Scholar] [CrossRef]
- Smith, J.E.; White, P.F. Diazinon resistance in mushroom pests. HDC Proj. News 1996, 36, 12–15. [Google Scholar]
- Bartlett, G.R.; Keil, C.B.O. Identification and characterization of a permethrin resistance mechanism in populations of the fungus gnat Lycoriella mali (Fitch) (Diptera: Sciaridae). Pestic. Biochem. Physiol. 1997, 58, 173–181. [Google Scholar] [CrossRef]
- EC (European Commission). EU Pesticide Database—Active Substances, Safeners and Synergists. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 28 January 2025).
- Gea, F.K.; Navarro, M.J.; Santos, M.; Dianez, F.; Carrasco, J. Control of fungal diseases in mushroom crops while dealing with fungicide resistance: A review. Microorganisms 2021, 9, 585. [Google Scholar] [CrossRef]
- Bonnen, A.M.; Hopkins, C. Fungicide resistance and population variation in Verticillium fungicola, a pathogen of the button mushroom, Agaricus bisporus. Mycol. Res. 1997, 101, 89–96. [Google Scholar] [CrossRef]
- Gea, F.J.; Tello, J.C.; Honrubia, M. In vitro sensitivity of Verticillium fungicola to selected fungicides. Mycopathologia 1996, 136, 133–137. [Google Scholar] [CrossRef]
- McKay, G.L.; Egan, D.; Morris, E.; Brown, A.E. Identification of benzimidazole resistance in Cladobotryum dendroides using a PCR-based method. Mycol. Res. 1998, 102, 671–676. [Google Scholar] [CrossRef]
- Grogan, H.M.; Gaze, R.H. Fungicide resistance among Cladobotryum spp.—Causal agent of cobweb disease of the edible mushroom Agaricus bisporus. Mycol. Res. 2000, 104, 357–364. [Google Scholar] [CrossRef]
- Romaine, C.P.; Royse, D.J.; Schlagnhaufer, C. Emergence of benzimidazole-resistant green mould Trichoderma aggressivum, on cultivated Agaricus bisporus in North America. In Science and Cultivation of Edible and Medicinal Fungi: Mushroom Science XVII, Proceeding of the 17th Congress of the International Society for Mushroom Science (ISMS), Cape Town, South Africa, 20–24 May 2008; International Society for Mushroom Science: Maasbree, The Netherlands, 2008; pp. 510–523. [Google Scholar]
- Romaine, C.P.; Royse, D.J.; Schlagnhaufer, B. Superpathogenic Trichoderma resistant to Topsin M found in Pennsylvania and Delaware. Mushroom News 2005, 53, 6–9. [Google Scholar]
- Gea, F.J.; Navarro, M.J.; Tello, J.C. Reduced sensitivity of the mushroom pathogen Verticillium fungicola to prochloraz-manganese in vitro. Mycol. Res. 2005, 109, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Rinker, D.L.; Alm, G. Management of casing Trichoderma using fungicides. In Science and Cultivation of Edible and Medicinal Fungi: Mushroom Science XVII, Proceeding of the 17th Congress of the International Society for Mushroom Science (ISMS), Cape Town, South Africa, 20–24 May 2008; International Society for Mushroom Science: Maasbree, The Netherlands, 2008; pp. 496–509. [Google Scholar]
- Du, Y.; Shi, N.; Ruan, H.; Miao, J.; Yan, H.; Shi, C.; Chen, F.; Liu, X. Analysis of the prochloraz-Mn resistance risk and its molecular basis in Mycogone rosea from Agaricus bisporus. Pest Manag. Sci. 2021, 77, 4680–4690. [Google Scholar] [CrossRef]
- Gea, F.J.; Tello, J.C.; Navarro, M.J. Efficacy and effects on yield of different fungicides for control of wet bubble disease of mushroom caused by the mycoparasite Mycogone perniciosa. Crop Prot. 2010, 29, 1021–1025. [Google Scholar] [CrossRef]
- Shi, N.; Ruan, H.; Jie, Y.; Chen, F.; Du, Y. Sensitivity and efficacy of fungicides against wet bubble disease of Agaricus bisporus caused by Mycogone perniciosa. Eur. J. Plant Pathol. 2020, 157, 873–885. [Google Scholar] [CrossRef]
- Pyck, N.; Sedeyn, P.; Demeulemeester, M.; Grogan, H. Evaluation of metrafenone against Verticillium and Cladobotryum spp.—Causal agents of dry bubble and cobweb disease. In Science and Cultivation of Edible and Medicinal Fungi—Mushroom Science XIX, Proceedings of the 19th International Congress on the Science and Cultivation of Edible and Medicinal Fungi, Amsterdam, The Netherlands, 30 May–2 June 2016; Wageningen University and Research Centre: Amsterdam, The Netherlands, 2016; pp. 82–85. [Google Scholar]
- Carrasco, J.; Navarro, M.J.; Santos, M.; Gea, F.J. Effect of five fungicides with different modes of action on mushroom cobweb disease (Cladobotryum mycophilum) and mushroom yield. Ann. Appl. Biol. 2017, 171, 62–69. [Google Scholar] [CrossRef]
- Carrasco, J.; Navarro, M.-J.; Gea, F.J. Cobweb, a serious pathology in mushroom crops: A review. Spanish J. Agric. Res. 2017, 15, e10R01. [Google Scholar] [CrossRef]
- Grogan, H.M. Challenges facing mushroom disease control in the 21st century. In Proceedings of the 6th International Conference on Mushroom Biology and Mushroom Products, Bonn, Germany, 29 September–3 October 2008; pp. 120–127. [Google Scholar]
- Chandler, D.; Bailey, A.S.; Tatchell, G.M.; Davidson, G.; Greaves, J.; Grant, W.P. The development, regulation and use of biopesticides for integrated pest management. Philos. Trans. R. Soc. B 2011, 366, 1987–1998. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, J.A.; Sundh, I.; Becher, P.G.; Björkman, C.; Dubey, M.; Egan, P.A.; Friberg, H.; Gil, J.F.; Dan, F.; Jensen, D.F.; et al. When is it biological control? A framework of definitions, mechanisms, and classifications. J. Pest Sci. 2021, 94, 665–676. [Google Scholar] [CrossRef]
- Collinge, D.B.; Jensen, D.F.; Rabiey, M.; Sarrocco, S.; Shaw, M.W.; Shaw, R.H. Biological control of plant diseases—What has been achieved and what is the direction? Plant Pathol. 2022, 71, 1024–1047. [Google Scholar] [CrossRef]
- Koul, O. Biopesticides: Commercial opportunities and challenges. In Development and Commercialization of Biopesticides—Costs and Benefits; Koul, O., Ed.; Academic Press: London, UK, 2023; pp. 1–23. [Google Scholar] [CrossRef]
- Eilenberg, J.; Hajek, A.; Lomer, C. Suggestions for unifying the terminology in biological control. BioControl 2001, 46, 387–400. [Google Scholar] [CrossRef]
- Galli, M.; Feldmann, F.; Vogler, U.V.; Kogel, K.H. Can biocontrol be the game-changer in integrated pest management? A review of definitions, methods and strategies. J. Plant Dis. Prot. 2024, 131, 265–291. [Google Scholar] [CrossRef]
- Van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations); WHO (World Health Organization). International Code of Conduct on Pesticide Management: Guidelines for the Registration of Miucrobial, Botanical and Semiochemical Pest Control Agents for Plant Protection and Public Health Issues; FAO & WHO: Rome, Italy, 2017; p. 76. [Google Scholar]
- EPA (United States Environmental Protection Agency). Biopesticides. Available online: https://www.epa.gov/pesticides/biopesticides (accessed on 28 January 2025).
- Karamaouna, F.; Economou, L.P.; Lykogianni, M.; Mantzoukas, S.; Eliopoulos, P.A. Biopesticides in the EU: State of play and perspectives after the Green Deal for agriculture. In Development and Commercialization of Biopesticides—Costs and Benefits; Koul, O., Ed.; Academic Press: London, UK, 2023; pp. 213–239. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides in the twenty-first century: Fulfilling the promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef]
- Van Lenteren, J.C. The state of commercial augmentative biological control: Plenty of natural enemies, but a frustrating lack of uptake. BioControl 2012, 57, 1–20. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Koppenhöfer, A.M.; Shapiro-Ilan, D.I.; Hiltpold, I. Entomopathogenic Nematodes in sustainable food production. Front. Sustain. Food Syst. 2020, 4, 125. [Google Scholar] [CrossRef]
- Tarasco, E.; Fanelli, E.; Salvemini, C.; El-Khoury, Y.; Troccoli, A.; Vovlas, A.; De Luca, F. Entomopathogenic nematodes and their symbiotic bacteria: From genes to field uses. Front. Insect Sci. 2023, 3, 1195254. [Google Scholar] [CrossRef] [PubMed]
- Matuska-Łyżwa, J.; Duda, S.; Nowak, D.; Kaca, W. Impact of abiotic and biotic environmental conditions on the development and infectivity of entomopathogenic nematodes in agricultural soils. Insects 2024, 15, 421. [Google Scholar] [CrossRef]
- Rinker, D.L.; Althof, T.H.A.; Dano, J.; Alm, G. Effect of entomopathogenic nematodes on control of a mushroom sciarid fly and on mushroom production. Biocontrol. Sci. Technol. 1995, 5, 109–119. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Geels, F.P.; Smits, P.H.; Van Griensven, L.J.L.D. Control of the mushroom pests Lycoriella auripila (Diptera: Sciaridae) and Megaselia halterata (Diptera: Phoridae) by Steinernema feltiae (Nematoda: Steinernematidae) in field experiments. Ann. Appl. Biol. 1997, 131, 359–368. [Google Scholar] [CrossRef]
- Jess, S.; Kilpatrick, M. An integrated approach to the control of Lycoriella solani (Diptera: Sciaridae) during production of the cultivated mushroom (Agaricus bisporus). Pest Manag. Sci. 2000, 56, 477–485. [Google Scholar] [CrossRef]
- Jess, S.; Bingham, J.F.W. Biological control of sciarid and phorid pests of mushrooms with predatory mites from the genus Hypoaspis (Acari: Hypoaspidae) and the entomopathogenic nematode Steinernema feltiae. Bull. Entomol. Res. 2004, 94, 159–167. [Google Scholar] [CrossRef]
- Navarro, M.J.; Gea, F.J. Entomopathogenic nematodes for the control of phorid and sciarid flies in mushroom crops. Pesqui. Agropec. Brasileira 2014, 49, 11–17. [Google Scholar] [CrossRef]
- Ortiz, A.; Sansinenea, E. Microbial-based biopesticides: Commercialization and regulatory perspectives. In Development and Commercialization of Biopesticides—Costs and Benefits; Koul, O., Ed.; Academic Press: London, UK, 2023; pp. 103–118. [Google Scholar] [CrossRef]
- EPA (United States Environmental Protection Agency). Pesticide Product and Label System. Available online: https://ordspub.epa.gov/ords/pesticides/f?p=PPLS:1 (accessed on 28 January 2025).
- Erler, F.; Polat, E.; Demir, H.; Cetin, H.; Erdemir, T. Evaluation of microbial products for the control of the mushroom phorid fly, Megaselia halterata (Wood). J. Entomol. Sci. 2009, 44, 1–9. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Geels, F.P.; Smits, P.H.; Rutjens, A.I.; Van Griensven, L.J.L.D. Comparison of the efficacy of entomopathogenic nematodes for the biological control of the mushroom pests Lycoriella auripila (Sciaridae) and Megaselia halterata (Phoridae). Biocontrol. Sci. Technol. 1998, 8, 277–288. [Google Scholar] [CrossRef]
- San-Blas, E.; Luzardo, M.; Larreal, J.; Portillo, E.; Bastidas, B. Biological control of the fungus gnats Bradysia difformis (Diptera, Mycetophilidae) in mushrooms with Heterorhabditis amazonensis in tropical conditions. Sci. Hort. 2017, 216, 120–125. [Google Scholar] [CrossRef]
- Chen, C.; Ma, H.; Ma, M.; Li, J.; Zheng, S.; Song, Q.; Gu, X.; Shapiro-Ilan, D.; Ruan, W. An innovative strategy for control of fungus gnats using entomopathogenic nematodes alone or in combination with waterlogging. J. Nematol. 2021, 52, e2020-57. [Google Scholar] [CrossRef]
- Matuska-Łyżwa, J.; Żarnowiec, P.; Kaca, W. Comparison of biological activity of field isolates of Steinernema feltiae with a commercial S. feltiae biopesticide product. Insects 2021, 12, 816. [Google Scholar] [CrossRef] [PubMed]
- Drobnjaković, T.; Grujić, N.; Luković, J.; Anđelković, N.; Potočnik, I.; Milijašević-Marčić, S.; Šantrić, L.; Popović, A.; Marčić, D. Potential of Steinernema feltiae (Nematoda: Steinernematidae) native populations in the biocontrol of Lycoriella ingenua (Diptera: Sciaridae) and their impact on mushroom production. Agriculture 2025, 15, 537. [Google Scholar] [CrossRef]
- Knapp, M.; van Houten, Y.; van Baal, E.; Groot, T. Use of predatory mites in commercial biocontrol: Current status and future prospects. Acarologia 2018, 58, 72–82. [Google Scholar] [CrossRef]
- Beretta, G.M.; Deere, J.A.; Messelink, G.J.; Muňoz-Cardenas, K.; Janssen, A. Review: Predatory soil mites as biocontrol agents of above and below-ground plant pests. Exp. Appl. Acarol. 2022, 87, 143–162. [Google Scholar] [CrossRef]
- Ali, O.; Dunne, R.; Brennan, P. Biological control of the sciarid fly, Lycoriella solani, by the predatory mite Hypoaspis miles (Acari: Laelapidae) in mushroom crops. Syst. Appl. Acarol. 1997, 2, 71–80. [Google Scholar] [CrossRef]
- Castilho, R.C.; de Moraes, G.J.; Silva, E.S.; Freire, R.A.P.; Da Eira, F.C. The predatory mite Stratiolaelaps scimitus as a control agent of the fungus gnat Bradysia matogrossensis in commercial production of the mushroom Agaricus bisporus. Int. J. Pest Manag. 2009, 55, 181–185. [Google Scholar] [CrossRef]
- Grosman, A.H.; Messelink, G.J.; de Groot, E. Combined use of a mulch layer and the soil-dwelling predatory mite Macrocheles robustulus (Berlese) enhance the biological control of sciarids in potted plants. IOBC/WPRS Bull. 2011, 68, 51–54. [Google Scholar]
- Al-Amidi, A.H.K.; Downes, M.J. Parasitus bituberosus (Acari: Parasitidae), a possible agent for biological control of Heteropeza pygmaea (Diptera: Cecidomyiidae) in mushroom compost. Exp. Appl. Acarol. 1990, 8, 13–25. [Google Scholar] [CrossRef]
- Al-Amidi, A.H.K.; Dunne, R.; Downes, M.J. Parasitus bituberosus (Acari: Parasitidae): An agent for control of Lycoriella solani (Diptera: Sciaridae) in mushroom crops. Exp. Appl. Acarol. 1991, 11, 159–166. [Google Scholar] [CrossRef]
- Szafranek, P.; Lewandowski, M.; Kozak, M. Prey preference and life tables of the predatory mite Parasitus bituberosus (Acari: Parasitidae) when offered various prey combinations. Exp. Appl. Acarol. 2013, 61, 53–67. [Google Scholar] [CrossRef]
- Sanchis, V. From microbial sprays to insect-resistant transgenic plants: History of the biospesticide Bacillus thuringiensis. A review. Agron. Sustain. Dev. 2011, 31, 217–231. [Google Scholar] [CrossRef]
- Keil, C.B. Field and laboratory evaluation of a Bacillus thuringiensis var. israelensis formulation for control of fly pests of mushrooms. J. Econ. Entomol. 1991, 84, 1180–1188. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Dickinson, A. Effect of Bacillus thuringiensis subsp. israelensis and neonicotenoid insecticides on the fungus gnat, Bradysia sp., coprophila (Lintner) (Diptera: Sciaridae). Pest Manag. Sci. 2006, 62, 171–177. [Google Scholar] [CrossRef]
- Shamshad, A.; Clift, A.D.; Mansfield, S. Toxicity of six commercially formulated insecticides and biopesticides to third instar larvae of mushroom sciarid, Lycoriella ingenua Dufour (Diptera: Sciaridae), in New South Wales, Australia. Austral. J. Entomol. 2008, 47, 256–260. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Cloonan, K.R.; Bellicanta, G.S.; Paley, K.; Pecchia, J.; Jenkins, N.E. Efficacy of Beauveria bassiana formulations against the fungus gnat Lycoriella ingenua. Biol. Control 2016, 103, 165–171. [Google Scholar] [CrossRef]
- Andreadis, S.S.; Cloonan, K.R.; Bellicanta, G.S.; Jenkins, N.E. Efficacy of BotaniGard® against the mushroom phorid fly Megaselia halterata. Biocontrol Sci. Technol. 2021, 31, 1098–1106. [Google Scholar] [CrossRef]
- Ajvad, F.T.; Madadi, H.; Michaud, J.P.; Zafari, D.; Khanjani, M. Combined applications of an entomopathogenic fungus and a predatory mite to control fungus gnats (Diptera: Sciaridae) in mushroom production. Biol. Control 2020, 141, 104101. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–425. [Google Scholar] [CrossRef]
- Rovesti, L.; Viccinelli, R.; Barbarossa, B. Biological contol of sciarid flies. IOBC/WPRS Bull. 1996, 19, 20–23. [Google Scholar]
- Erler, F.; Polat, E.; Demir, H.; Cetin, H.; Erdemir, T. Control of the mushroom phorid fly, Megaselia halterata (Wood), with plant extracts. Pest Manag. Sci. 2009, 65, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Drobnjaković, T.; Marčić, D.; Potočnik, I.; Rekanović, E.; Prijović, M.; Milijašević-Marčić, S.; Stepanović, M. Control of mushroom sciarid fly Lycoriella ingenua (Dufour) with an azadirachtin-based insecticide. Pestic. Phytomed. 2019, 34, 111–121. [Google Scholar] [CrossRef]
- Drobnjaković, T.; Luković, J.; Milijašević-Marčić, S.; Todorović, B.; Stepanović, M.; Potočnik, I.; Rekanović, E. Impact of neem cake amendment in the casing soil on control of Trichoderma aggressivum Samuels & W. Gams and Lycoriella ingenua (Dufour) and mushroom yield. Pestic. Phytomed. 2023, 38, 111–121. [Google Scholar] [CrossRef]
- Copping, L.G.; Menn, J.J. Biopesticides: A review of their action, applications and efficacy. Pest Manag. Sci. 2000, 56, 651–676. [Google Scholar] [CrossRef]
- Copping, L.G.; Duke, S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007, 63, 524–554. [Google Scholar] [CrossRef]
- Babbar, M.H.; Ashfaq, M.; Afzal, M.; Bashir, M.H.; Ali, M.A. Efficacy of different insecticides against mushroom phorid fly, Megaselia halterata (Wood) in Punjab, Pakistan. Int. J. Biodvers. Conserv. 2012, 4, 183–188. Available online: http://www.academicjournals.org/IJBC (accessed on 28 January 2025).
- Babbar, M.H.; Ashfaq, M.; Afzal, M.; Bashir, M.H.; Ali, M.A. Efficacy of different insecticides against mushroom sciarid fly (Lycoriella auripila) in Punjab, Pakistan. Pak. J. Nutr. 2014, 13, 50–55. [Google Scholar] [CrossRef]
- Cawoy, H.; Bettiol, W.; Fickers, P.; Ongena, M. Bacillus-based biological control of plant diseases. In Pesticides in the Modern World—Pesticides Use and Management; Stoytcheva, M., Ed.; InTech: Rijeka, Croatia, 2011; pp. 273–302. [Google Scholar]
- Kumar, S.; Singh, A. Biopesticides: Present Status and the Future Prospects. J. Fertil. Pestic. 2015, 6, e129. [Google Scholar] [CrossRef]
- Stanojević, O.; Milijašević-Marčić, S.; Potočnik, I.; Stepanović, M.; Dimkić, I.; Stanković, S.; Berić, T. Isolation and identification of Bacillus spp. from compost material, compost and mushroom casing soil active against Trichoderma spp. Arch. Biol. Sci. 2016, 68, 845–852. [Google Scholar] [CrossRef]
- Stanojević, O.; Berić, T.; Potočnik, I.; Rekanović, E.; Stanković, S.; Milijašević-Marčić, S. Biological control of green mould and dry bubble diseases of cultivated mushroom (Agaricus bisporus L.) by Bacillus spp. Crop Prot. 2019, 126, 104944. [Google Scholar] [CrossRef]
- Védie, R.; Rousseau, T. Biofungicide Serenade: A major innovation in disease control against Trichoderma aggressivum, agent of compost green mold of button mushroom in France. Lett. CTC 2008, 21, 1–2. [Google Scholar]
- Potočnik, I.; Todorović, B.; Rekanović, E.; Luković, J.; Paunović, D.; Milijašević-Marčić, S. Impact of Bacillus subtilis QST713 mushroom grain spawn treatment on yield and green mould control. Pestic. Phytomed. 2018, 33, 205–211. [Google Scholar] [CrossRef]
- Pandin, C.; Le Coq, D.; Canette, A.; Aymerich, S.; Briandet, R. Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb. Biotechnol. 2017, 10, 719–734. [Google Scholar] [CrossRef]
- Pandin, C.; Darsonval, M.; Mayeur, C.; Le Coq, D.; Aymerich, S.; Briandet, R. Biofilm formation and synthesis of antimicrobial compounds by the biocontrol agent Bacillus velezensis QST713 in an Agaricus bisporus compost micromodel. Appl. Environ. Microbiol. 2019, 85, e00327-19. [Google Scholar] [CrossRef]
- Clarke, J.; Grogan, H.; Fitzpatrick, D.; Kavanagh, K. Characterizing the proteomic response of mushroom pathogen Lecanicillium fungicola to Bacillus velezensis QST 713 and Kos biocontrol agents. Eur. J. Plant Pathol. 2022, 163, 369–379. [Google Scholar] [CrossRef]
- Liu, C.; Sheng, J.; Chen, L.; Zheng, Y.; Lee, D.Y.W.; Yang, Y.; Xu, M.; Shen, L. Biocontrol activity of Bacillus subtilis isolated from Agaricus bisporus mushroom compost against pathogenic fungi. J. Agricul. Food Chem. 2015, 63, 6009–6018. [Google Scholar] [CrossRef]
- Büchner, R.; Vörös, M.; Allaga, H.; Varga, A.; Bartal, A.; Szekeres, A.; Varga, S.; Bajzát, J.; Bakos-Barczi, N.; Misz, A.; et al. Selection and characterization of a Bacillus strain for potential application in industrial production of white button mushroom (Agaricus bisporus). Agronomy 2022, 12, 467. [Google Scholar] [CrossRef]
- Kosanović, D.; Dyas, M.; Grogan, H.; Kavanagh, K. Differential proteomic response of Agaricus bisporus and Trichoderma aggressivum f. europaeum to Bacillus velezensis supernatant. Eur. J. Plant Pathol. 2021, 160, 397–409. [Google Scholar] [CrossRef]
- Jafaripour, E.M.; Ahmadzadeh, M.; Charkhabi, N.F.; Dousti, M.; Sadeghi, R. Bacteria antagonistic to Pseudomonas tolaasii and their control of brown blotch of the cultivated mushroom Agaricus bisporus. J. Plant Pathol. 2025, 107, 537–549. [Google Scholar] [CrossRef]
- Beyer, D.M.; Pecchia, J.A.; Paley, K. Evaluation of bio-fungicides for the control of fungal diseases on Agaricus bisporus. In Proceedings of the 19th International Society for Mushroom Science ISMS Conference, Amsterdam, The Netherlands, 30 May–2 June 2016; pp. 86–90. [Google Scholar]
- Šantrić, L.; Potočnik, I.; Radivojević, L.j.; Gajić Umiljendić, J.; Rekanović, E.; Duduk, B.; Milijašević-Marčić, S. Impact of a native Streptomyces flavovirens from mushroom compost on green mold control and yield of Agaricus bisporus. J. Environ. Sci. Health B 2018, 53, 677–684. [Google Scholar] [CrossRef]
- Sahin, N. Antimicrobial activity of Streptomyces species against mushroom blotch disease pathogen. J. Basic Microbiol. 2005, 45, 64–71. [Google Scholar] [CrossRef]
- Potočnik, I.; Vukojević, J.; Stajić, M.; Rekanović, E.; Stepanović, M.; Milijašević, S.; Todorović, B. Toxicity of biofungicide Timorex 66 EC to Cladobotryum dendroides and Agaricus bisporus. Crop Prot. 2010, 29, 290–294. [Google Scholar] [CrossRef]
- Thoeming, G.; Poehling, H.M. Integrating soil-applied azadirachtin with Amblyseius cucumeris (Acari: Phytoseiidae) and Hypoaspis aculeifer (Acari: Laelapidae) for the management of Frankliniella occidentalis (Thysanoptera: Thripidae). Environ. Entomol. 2000, 35, 746–756. [Google Scholar] [CrossRef]
- Duarte, A.D.F.; de Bastos Pazini, J.; Duarte, J.L.P.; Da Silva, L.R.; Da Cunha, U.S. Compatibility of pesticides used in strawberry crops with predatory mites Stratiolaelaps scimitus (Womersley) and Cosmolaelaps brevistilis (Karg). Ecotoxicology 2020, 29, 148–155. [Google Scholar] [CrossRef]
- Saito, T.; Brownbridge, M. Compatibility of soil-dwelling predators and microbial agents and their efficacy in controlling soil-dwelling stages of western flower thrips Frankliniella occidentalis. Biol. Control 2016, 92, 92–100. [Google Scholar] [CrossRef]
- Laznik, Ž.; Trdan, S. The influence of insecticides on the viability of entomopathogenic nematodes (Rhabditida: Steinernematidae and Heterorhabditidae) under laboratory conditions. Pest Manag. Sci. 2014, 70, 784–789. [Google Scholar] [CrossRef]
- Petrikovszki, R.; Doshi, P.; Turóczi, G.; Tóth, F.; Nagy, P. Investigating the side-effects of neem-derived pesticides on commercial entomopathogenic and slug-parasitic nematode products under laboratory conditions. Plants 2019, 8, 281. [Google Scholar] [CrossRef]
- Milijašević-Marčić, S.; Šantrić, L.; Luković, J.; Potočnik, I.; Grujić, N.; Drobnjaković, T.; Marčić, D. Altering microbial communities in substrate to stimulate the growth of healthy button mushrooms. Agriculture 2024, 14, 1152. [Google Scholar] [CrossRef]
- Khanna, P.K.; Sodhi, H.S.; Kapoor, S. Diseases of Agaricus bisporus and their management. Annu. Rev. Plant Pathol. 2003, 2, 163–205. [Google Scholar]
- Barzman, M.; Bàrberi, P.; Birch, A.N.E.; Boonekamp, P.; Dachbrodt-Saaydeh, S.; Graf, B.; Hommel, B.; Jensen, J.E.; Kiss, J.; Kudsk, P.; et al. Eight principles of integrated pest management. Agron. Sustain. Dev. 2015, 35, 1199–1215. [Google Scholar] [CrossRef]
- Sznyk-Basalyga, A.; Bodnarek, A. Effects of entomopathogenic nematodes and insecticides on Megaselia halterata mortality. IOBC/WPRS Bull. 2003, 26, 147–150. [Google Scholar]
- Sznyk-Basalyga, A.; Bodnarek, A. Integrated control of Lycoriella solani with entomopathogenic nematodes and insecticides. IOBC/WPRS Bull. 2003, 26, 189–192. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).