Abstract
The remediation of lead (Pb)-contaminated soils through eco-friendly strategies is critical for sustainable agriculture. This study investigated the role of arbuscular mycorrhizal fungi (AMF) in enhancing maize tolerance to Pb stress and modulating rhizosphere microbial communities. A pot experiment was conducted with maize (Baiyu833) under four Pb concentrations (0, 900, 1800, 2700 mg·kg−1) and three AMF treatments: non-inoculation (Non), Funneliformis mosseae (Fm), or Rhizophagus intraradices (Ri). The results demonstrated that AMF inoculation significantly increased plant biomass, boosted antioxidant enzyme activities (SOD, POD), and reduced malondialdehyde (MDA) levels, mitigating Pb-induced oxidative stress. AMF restricted Pb translocation to aerial parts, with root Pb accumulation reaching 2110.76 mg·kg−1 (Fm) and 2090.56 mg·kg−1 (Ri) under Pb2700, enhancing phytostabilization. High-throughput sequencing revealed that AMF inoculation enriched α-diversity indices and restructured bacterial communities, favoring beneficial taxa like Promicromonospora, which are linked to heavy metal resistance and plant growth promotion. Principal coordinate analysis highlighted distinct clustering of microbial communities driven by AMF, emphasizing their role in alleviating Pb toxicity. These findings underscore that AMF enhance maize resilience to Pb by regulating antioxidant defense, immobilizing Pb in roots, and recruiting stress-tolerant rhizosphere microbiomes. This study provides insights into AMF-assisted phytoremediation as a sustainable strategy for Pb-contaminated soils.
1. Introduction
The increasing accumulation of heavy metals in soil due to industrial activities presents a significant ecological and environmental challenge on a global scale. Lead (Pb), a particularly harmful form of heavy metal pollution, has garnered attention for its high toxicity and widespread use in various industries [1,2]. The absorption of Pb by plants can have detrimental effects such as stunted seedling growth, inhibition of root and shoot growth, decreased seed germination, and disruption of various biological processes [3,4]. Excessive levels of Pb not only harm plants but also pose a risk to human health, as it can enter the food chain [5,6]. Therefore, it is crucial to remediate soils contaminated with heavy metals, especially Pb.
Various physical and chemical remediation techniques have been utilized to address Pb-contaminated soils, but their extensive use has been limited due to their high cost [7]. Conversely, bioremediation, a method that integrates plants and microorganisms, is regarded as an eco-friendly and cost-efficient approach for the restoration of polluted soils [8]. In symbiotic systems between soil microbes and plants, certain microbes with growth-promoting properties can boost plant resistance to biotic and abiotic stresses [9,10]. Arbuscular mycorrhizal fungi (AMF) can establish obligate symbiotic relationships with over 80% of terrestrial plants, offering plant protection against various stresses [11]. Previous studies have demonstrated the effectiveness of AMF-assisted phytoremediation in remediating Pb-contaminated soils [12,13]. In addition, AMF play a crucial role in enhancing plant nutrient absorption through their hyphal network, thereby influencing various physiological characteristics that contribute to improved plant growth [14,15]. Plants that form associations with AMF demonstrate improved antioxidant defenses and photosynthesis efficiency, leading to increased tolerance to lead contamination and enhanced plant growth [16]. Interestingly, AMF have been observed to influence metal accumulation and transport in plants, reducing toxicity in the presence of heavy metal stress [17].
Furthermore, AMF have the ability to establish stress interaction mechanisms with rhizosphere microorganisms, ultimately enhancing the tolerance of host plants to heavy metal stress [18]. In a study conducted by Zhao et al. (2023), it was discovered that AMF can influence the composition of rhizosphere microorganism and fungal communities, promote the growth of beneficial microorganisms with heavy metal tolerance genes, facilitate the development of heavy metal stress response systems, and consequently improve plant tolerance to heavy metal stress [19]. Research has shown that AMF decreased the presence of cadmium (Cd) in rice by impacting the composition of the rhizosphere soil bacterial community [20]. However, it remains unclear how AMF enhance plant resistance and impact rhizosphere microorganisms in the presence of Pb stress. Recent studies highlight the critical role of rhizosphere bacteria in heavy metal remediation, particularly Pb immobilization and detoxification. For instance, Promicromonospora (Actinobacteria) exhibits robust Cd resistance through biosorption and extracellular precipitation mechanisms [21]. Members of the Proteobacteria phylum, such as Pseudomonas and Burkholderia, enhance Pb sequestration via biofilm formation and siderophore production [22]. Additionally, Bacillus species (Firmicutes) reduce Pb bioavailability through phosphatase-mediated mineralization [23]. These bacterial taxa often synergize with AMF, forming tripartite symbioses that amplify phytostabilization efficiency by improving soil structure and metal immobilization [24].
Maize, with its powerful biomass and well-developed root systems compared to rice, wheat, and most other crops, is considered one of the most ideal plants for phytoremediation [25]. As a Pb-accumulating species, maize demonstrates significant potential for phytostabilization due to its ability to restrict Pb translocation to edible parts (e.g., grains) under symbiotic conditions with AMF, minimizing entry into the food chain [16]. Therefore, it is essential to examine the impact of maize rhizosphere beneficial bacteria on enhancing plant resistance to Pb stress when inoculated with AMF. This study aims to elucidate the dual mechanisms by which AMF enhance maize tolerance to Pb stress: (1) restructuring the rhizosphere microbiome to immobilize Pb within the roots, and (2) reducing Pb translocation to aerial tissues through the modulation of antioxidant defense mechanisms. By integrating these findings, we seek to advance AMF-assisted phytoremediation as a sustainable strategy for managing Pb-contaminated agroecosystems, thereby ensuring both ecological safety and agricultural productivity.
2. Materials and Methods
2.1. Preparation of Materials and Plot Experiment
2.1.1. Soil Preparation and Pb Contamination
The soil used in this pot experiment was collected from the surface soil (0–30 cm) at Henan Institute of Science and Technology (Xinxiang, Henan Province, N35°, E113°). Prior to the experiment, the soil was naturally air-dried and then processed to remove hard soil, stones, residual roots, and leaves. Subsequently, the soil samples were sieved through a 3 mm sieve for uniformity. The initial soil characteristics included a pH of 8.63, organic matter (OM) content of 8.19 g·kg−1, available phosphorus (AP) level of 0.70 g·kg−1, and available potassium (AK) content of 9.89 g·kg−1. To eliminate the influence of indigenous AMF and other microorganisms, the soil samples were autoclaved at 121 °C for 2 h.
Subsequently, Pb(NO3)2 (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was dissolved in deionized water to prepare a Pb2+ solution for the study. The soils were mixed thoroughly with different concentrations of Pb(NO3)2 to create four variations of Pb-containing soils with final levels of 0, 900, 1800, and 2700 mg·kg−1, named Pb0, Pb900, Pb1800, and Pb2700 treatments, respectively. The control group, designated as Pb0, consisted of soils without additional Pb. Following this, all soils were stored and subjected to four weeks of weathering under both wet and dry conditions to stabilize the heavy metals.
2.1.2. AMF Inoculum Source and Application
Two AMF strains, Rhizophagus intraradices BEG 141 [26] and Funneliformis mosseae (formerly Glomus mosseae) BEG 167 [27], were kindly provided by Professor Fayuan Wang and Dr. Fei Wang. These strains were propagated on maize and grown in sterilized sand for a duration of eight months. These strains were selected for their documented efficacy in heavy metal tolerance and symbiosis with maize. Each soil treatment (Pb0, Pb900, Pb1800, and Pb2700) was subjected to three different AMF inoculations treatments: non-inoculation (Non), inoculation with Funneliformis mosseae (F. mosseae) alone (Fm), and inoculation with Rhizophagus intraradices (R. intraradices) alone (Ri). For the AMF inoculation treatments, each pot was uniformly mixed with 10% (350 g) of AMF inoculum. To ensure consistency, the non-inoculation treatments were supplemented with an equal amount of sterilized inoculum. Additionally, all treatments received 10 mL of FR (Fm + Ri) inoculum filtrate (1:2 w/v soil: water) to maintain a similar microflora.
2.2. Plant Cultivation and Sampling
2.2.1. Plant Growth and Biomass Measurement
The experiment utilized the seeds of the Baiyu833 maize variety cultivated by Henan Institute of Science and Technology as the material. Prior to planting, the seeds underwent surface sterilization with 2% NaClO for 30 min, followed by rinsing with sterilized distilled water to eliminate any remaining solvent. After germinating for 2 days on a petri dish, three evenly developed seedlings were planted in individual plastic pots with dimensions of top diameter 20.5 cm, bottom 10.5 cm, and height 17.5 cm, each filled with 3.5 kg of soil. After a duration of seven days, the maize seedlings were reduced in number to a single plant per pot. To ensure the healthy growth of the seedlings, they were provided with 100 mL of modified Hoagland’s nutrient solution (Coolaber Technology Co., Ltd., Beijing, China) (Table S1) on a weekly basis. This process was repeated six times for each treatment.
The Baiyu833 was planted on 10 May 2024. After 75 days, the plant height was recorded using a ruler, and the plants were harvested. The harvested plants were thoroughly washed several times with distilled water to eliminate any adhering soil. Residual soil was sieved through a 3 mm mesh to collect fragmented roots. The roots were then immersed in a 10 mM EDTA solution (pH 5.0) for 15 min to desorb metal ions bound to the root surfaces. Following this, the roots, stems, and leaves were separated, and their fresh weights were documented. The roots, stems, and leaves were dried in an oven at 105 °C for 30 min, followed by drying at 70 °C for 72 h until a constant weight was achieved. Finally, their dry weight was recorded.
2.2.2. Antioxidant Enzyme Activity and Pb Quantification
In accordance with the specific kits guidelines provided by Sangon Biotech (Shanghai, China) Co., Ltd. (https://www.sangon.com/) (accessed on 15 July 2024), we carried out detailed measurements of the levels of catalase (CAT) using product code D799597, peroxidase (POD) with product code D799591, and superoxide dismutase (SOD) with product code D799593 in the roots of maize. Subsequently, the absorbance was measured at 240 nm, 470 nm, and 560 nm using a UV-2600i Spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Additionally, dried plant tissue samples were ground to determine the concentration of the heavy metal Pb. A 0.5 g sample of powdered plant tissue was placed into Teflon vessels and digested using 10 mL of concentrated nitric acid (HNO3, 65%) and 2 mL of hydrogen peroxide (H2O2, 30%) in a microwave digestion system (Mars 6, CEM Corporation, Matthews, NC, USA). The temperature was increased to 180 °C over a period of 20 min and maintained for 30 min. After cooling, the digests were diluted to a final volume of 50 mL with ultrapure water (Milli-Q, 18.2 MΩ·cm) and filtered through 0.45 μm nylon membranes. The total concentration of lead (Pb) in the plant tissues was quantified using inductively coupled plasma mass spectrometry (ICP-MS). This process was conducted at Nanjing Ruiyuan Biotechnology Co., Ltd. (Nanjing, China).
2.3. Rhizosphere Microbiome Analysis
2.3.1. Sampling and Preparation of Rhizosphere Soil
To conduct a comprehensive investigation of the microbial community in rhizosphere soil, a meticulous procedure was employed to prepare the rhizosphere soil samples. Initially, each plant’s roots were gently shaken to dislodge any loosely attached soil. Subsequently, the remaining soil particles adhering to the root hairs were carefully collected using a sterile brush. This precise method is widely acknowledged as the collection of rhizosphere soil, which is crucial for our analysis of the soil’s microbial dynamics. This process was repeated three times for each treatment. These rhizosphere soils were sent to LC-BIO Technology Co., Ltd. in Hangzhou, China for 16S rRNA gene analysis.
2.3.2. Diversity and Structure of the Microbial Community
Raw tags were subjected to a rigorous quality control process utilizing the QIIME2, ensuring that only the most reliable sequences were retained for further analysis [28]. Following this initial screening, the tag sequences underwent a comparative analysis against a species annotation database, with the aim of identifying and eliminating chimeric sequences. The removal of chimeric sequences was crucial to preserve the integrity of the data, leading to a refined set of effective tags for subsequent processing [29].
The amplicon sequence variants (ASVs) table generated from QIIME2 facilitated the computation of various metrics, including the Chao1 richness estimator, observed species count, Shannon diversity index, Simpson index, and ACE. These metrics were visually represented through block diagrams. To assess the abundance and evenness of ASVs across samples, abundance curves were generated. Beta diversity analysis was performed utilizing the UniFrac distance metric, with results illustrated through hierarchical clustering via the unweighted pair group method with arithmetic mean (UPGMA), principal coordinate analysis (PCoA), principal component analysis (PCA), non-metric multidimensional scaling (NMDS), and unweighted arithmetic means. The analysis of similarity (ANOSIM) was employed to evaluate the significance of microbial community structure regarding inter-group differentiation. Venn diagrams were utilized to visualize the presence of shared and unique ASVs among samples or groups, independent of their relative abundance. Additionally, linear discriminant analysis effect size (LEfSe) with default settings was applied to identify classification groups exhibiting significant differences. All analyses were performed using the OmicStudio platform, accessible at https://www.omicstudio.cn/tool (accessed on 10 November 2024).
2.4. Data Analysis
This study utilized SPSS version 20.0 to conduct statistical analyses. A one-way analysis of variance (ANOVA) was employed to assess the differences in means obtained from various treatments. Significance was determined using Duncan’s multiple range test, with thresholds set at p < 0.05 for main effects (e.g., Pb concentration or AMF inoculation) and p < 0.01 for interaction effects (e.g., Pb × AMF) or stress-specific contrasts. For multivariate analyses (e.g., Adonis), significance was evaluated at p < 0.001. Results are reported as means ± standard deviation (SD) from three independent replicates.
3. Results
3.1. Plant Biomass and Physicochemical Properties
This study demonstrates that, after 75 days of growth, both the inoculation and the addition of Pb treatments significantly affected the biomass of the maize plants, with effects observed in the roots, stems, and leaves (Figure 1a–d). Specifically, the inoculation treatments markedly enhanced the biomass of the roots, stems, and leaves systems, whereas the addition of Pb resulted in a significant reduction in biomass for the roots, stems, and leaves (p < 0.01). Inoculation with AMF significantly enhanced the biomass of maize plants exposed to varying concentrations of Pb (Figure 1a). At a Pb supplementation level of 900 mg·kg−1, inoculation with Ri resulted in a maximum root dry weight of 16.78 g·plant−1 (Figure 1b). In contrast, at the highest Pb concentration, the non-inoculation plants exhibited the lowest roots, stems, and leaves biomasses, measuring 1.18 g·plant−1, 0.95 g·plant−1, and 1.69 g·plant−1, respectively.
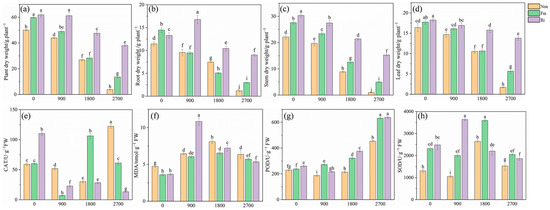
Figure 1.
Effects of AMF on plant dry weight (a), root dry weight (b), stem dry weight (c), leaf dry weight (d), CAT activity (e), MDA content (f), POD activity (g), and SOD activity (h) under different Pb concentrations. Values are the means ± standard deviation (SD) (n = 3). Different letters in the same column indicate statistically significant differences (p < 0.05).
Under the same Pb concentration treatment, the antioxidant capacity of maize roots significantly increased following inoculation, for which the activities of SOD and POD were all markedly higher in the Fm and Ri treatments compared to the non-inoculation treatments (Figure 1e–g). The highest activities of SOD and POD in maize were observed in the Ri inoculation treatment relative to the non-inoculated treatments. Conversely, the increase in CAT activity was highest under non-inoculated conditions. For instance, at a Pb supplementation level of 900 mg·kg−1, the SOD activity in the roots was 3.45 times greater in the Ri treatment than in the non-inoculated treatment. Additionally, at a Pb supplementation level of 2700 mg·kg−1, the MDA content of the roots was significantly lower in the Fm and Ri treatments compared to the non-inoculated treatment, with reductions of 10.13% and 15.77%, respectively (Figure 1h). Notably, the MDA content was lowest in the Pb0_Fm treatment when compared to the other treatment plants.
3.2. Pb Uptake and Translocation in Maize Under Different Pb Treatments
As the level of Pb supplementation increased, the Pb concentrations in the roots, stems, and leaves showed a consistent upward trend across all inoculated treatment conditions (Figure 2). Under the treatment with Fm, at the addition level of 2700 mg·kg−1, the Pb concentrations in the roots, stems, and leaves reached their maximum values of 2110.76, 160.97, and 110.46 mg·kg−1, respectively. Similarly, under the treatment with Ri, at the same addition level of 2700 mg·kg−1, the Pb concentrations in the roots, stems, and leaves peaked at 2090.56, 133.57, and 66.48 mg·kg−1, respectively. Notably, the Pb content in the roots and stems displayed a fluctuating pattern, characterized by a low–high–low trend as the Pb concentration increased under non-inoculation conditions, while the Pb content in the leaves exhibited a steady upward trend.
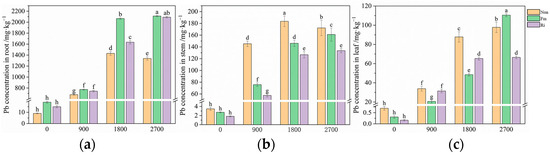
Figure 2.
Pb concentration in roots (a), stems (b), and leaves (c) of maize inoculated without or with AMF under different Pb stress levels. Values are the means ± standard deviation (SD) (n = 3). Different letters in the same column indicate statistically significant differences (p < 0.05).
Following inoculation with AMF, the concentration of Pb in the stems and leaves of the maize plants decreased, while the Pb concentration in the roots increased, reaching a significant level under the treatment condition of 900 mg·kg−1 (Figure 2). Furthermore, the Pb concentration in the roots and stems of maize plants inoculated with Ri was lower than that in the roots and stems of maize plants inoculated with Fm (Figure 2a,b). Additionally, the Pb concentration in the leaves of maize plants inoculated with Fm was significantly lower than that in the leaves of maize plants inoculated with Ri, with the exception of the addition level of 2700 mg·kg−1 (Figure 2c). These findings suggest that inoculation with AMF could effectively reduce Pb content in the above-ground parts of maize while simultaneously increasing the Pb content in the root system. This mechanism significantly decreased the transfer coefficient, thereby mitigating the transport of the Pb from the soil to the aerial parts of the maize.
3.3. Diversity of Microbial Communities in the Rhizosphere Soils
Microbial communities serve as sensitive indicators of the adverse effects resulting from both biotic and abiotic stresses, playing a crucial role in enhancing plant tolerance and resistance to the hazards posed by heavy metals in contaminated soil [30,31]. In our study, we examined the microbial communities in rhizosphere soil influenced by AMF using high-throughput sequencing of the bacterial 16S rRNA variable regions 3 and 4. We obtained a total of 2,982,083 reads and 2,507,799 valid tags from 36 soil samples, resulting in an average of 69,661 filtered sequences generated per sample (Table S2). These sequences were subsequently clustered into 15,286 ASVs. The rarefaction curves exhibited clear asymptotes (Figure S1), indicating that our sequencing coverage captured the majority of the bacterial diversity.
The α-diversity of the rhizosphere bacterial communities was assessed using several indices, including Chao1, observed species, ACE, Simpson, and Shannon (Table 1). Upon exposure to the same Pb concentration, the non-inoculation treatments exhibited the lowest values of Chao1, observed species, ACE, Shannon, and Simpson when compared to the two inoculated treatments (Fm and Ri). These findings suggest that AMF likely enhance biological richness within the maize rhizosphere. Among the 12 treatments, Pb900 exhibited the lowest values for Chao1, observed species, ACE, Shannon, and Simpson indices, while Pb0_Fm demonstrated the highest values for these same indices. Overall, Pb stress was found to reduce the microbial diversity of bacteria, whereas AMF positively influenced the microbial diversity of bacteria.

Table 1.
Alpha diversity indices of bacterial communities in maize rhizosphere soils.
Furthermore, to evaluate the β-diversity of rhizosphere bacterial communities, PCA and PCoA were performed at the ASV level (Figure 3). The results of the PCA revealed that biological replicates within each treatment group displayed significant spatial clustering in the ordination plot. This observation indicates high intra-group reproducibility and robust experimental consistency. Notably, data points from the Pb2700 treatment were distinctly separated from those of the other treatments (Figure 3a). Additionally, the PCoA results demonstrated a significant separation along the first principal component between inoculated and non-inoculated rhizosphere soil (Figure 3b). The Adonis test further demonstrated that the bacterial communities differed significantly among the 12 treatments, with an R2 value of 0.89 and a p-value of 0.001. The results indicated that the addition of Pb and AMF significantly altered the composition and structure of the soil bacterial community. This distinction was particularly pronounced in the treatments that were amended with high levels of Pb and those that included AMF alone.
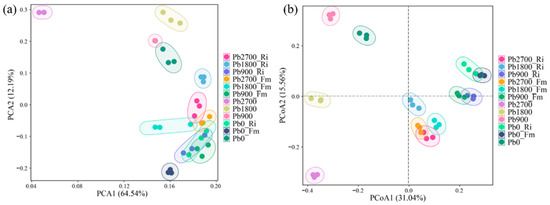
Figure 3.
PCA (a) and PCoA (b) analysis of rhizobacterial communities under different treatment conditions.
3.4. Microbial Community Composition in the Rhizosphere Soils
In this study, we identified a total of 48 bacterial phylum, among which 21 (43.75%) were common across all treatments, as illustrated in Figure S2 and Table S3. The number of specific phylum varied by treatment, with 34 identified for Pb2700_Ri, 33 for Pb1800_Ri, 31 for Pb900_Ri, 35 for Pb2700_Fm, 33 for Pb1800_Fm, 31 for Pb900_Fm, 32 for Pb2700, 28 for Pb1800, 25 for Pb900, 28for Pb0_Ri, 29 for Pb0_Fm, and 31 for Pb0. Additionally, a total of 21 bacterial phylum were identified across the various treatments, each exhibiting a relative abundance greater than 1%. These phyla include Actinobacteria, Proteobacteria, Firmicutes, Planctomycetota, Chloroflexi, Gemmatimonadota, Bacteroidota, Verrucomicrobiota, Patescibacteria, Acidobacteriota, Cyanobacteria, Myxococcota, Bdellovibrionota, Sumerlaeota, Abditibacteriota, Nitrospirota, and Desulfobacterota, as illustrated in Figure 4. Phylum with a relative abundance of less than 1% were categorized as ‘Others’. Notably, Actinobacteria, Proteobacteria, Firmicutes, Planctomycetota, and Chloroflexi emerged as the dominant bacterial phyla across all treatments. The addition of Pb, along with inoculation and their interaction, significantly influenced the relative abundances of Actinobacteria, Chloroflexi, and Gemmatimonadota, as detailed in Figure 4. Specifically, the introduction of Pb resulted in a marked decrease in the relative abundance of Actinobacteria across all inoculation treatments (Fm and Ri) while concurrently increasing the relative abundances of Planctomycetota and Chloroflexi in two inoculation treatments (Fm and Ri).
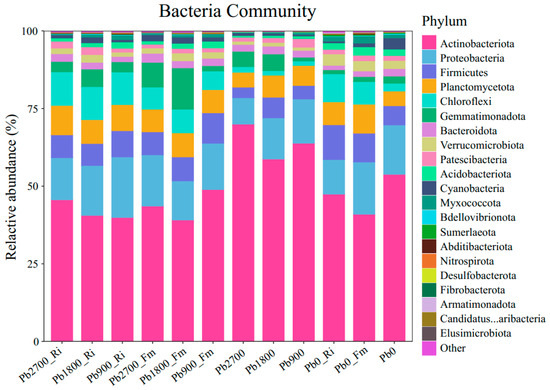
Figure 4.
Effect of AMF on bacterial phylum community composition in rhizosphere soils of maize grown in different Pb-contaminated soils.
3.5. Analysis of Differences in Microbial Community
The Sloan model was utilized to examine the construction processes of soil microbial communities and AMF under various Pb treatment conditions, as shown in Figure S3. The results indicated that in rhizosphere soil containing AMF, the assembly mechanisms of bacterial communities exhibited significantly greater randomness and increased species drift rates. This pattern suggests that the presence of AMF fosters a more chaotic and dynamic environment for the development of bacterial communities. Conversely, rhizosphere soils lacking AMF demonstrated assembly mechanisms for their bacterial communities that were characterized by lower levels of randomness and species drift rates. This observation implies that, in the absence of AMF, the development of bacterial communities is more readily influenced by predictable and deterministic factors.
Additionally, the LEfSe method was employed to evaluate the differences in taxa between the rhizosphere microbiota of non-inoculated control and the inoculated treatments (Fm or Ri) across each Pb treatment. The top 60 genera, ranked by scores for all treatment groups, are presented in Figure 5. A greater number of species exhibited significant enrichment (LDA > 3.0) in the Pb2700_Ri and Fm treatments compared to the other treatments. In contrast, the Pb1800_Fm and Pb2700 treatments showed the lowest number of significantly enriched species. The following genera were enriched in various treatments: Kribbella, Gammaproteobacteria_unclassified, and Gemmatimonas were enriched in Pb0. In Pb0_Fm, the enriched genera included Nocardioides, Arthrobacter, Lechevalieria, Marmoricola, Knoellia, Cystobacter, Haliangium, Iamia, Burkholderiales_unclassified, OM190_unclassified, Proteobacteria_unclassified, and Berkelbacteria_unclassified. In Pb0_Ri, significant enrichment was observed for Fictibacillus, Saccharothrix, KD4-96_unclassified, WD2101_soil_group_unclassified, Luteolibacter, and Actinomycetales_unclassified. The genera Pirellula, Pseudomonas, and Candidatus_Nomurabacteria_unclassified were enriched in Pb900. In Pb1800, the enriched genera comprised Saccharopolyspora, Microvirga, Microscillaceae_unclassified, Longimicrobiaceae_unclassified, Planctomycetaceae_unclassified, Mesorhizobium, and Ramlibacter. The genera Streptomyces and Amycolatopsis were enriched in Pb2700. In the Pb900_Fm treatment, enrichment was noted for Bacillus, Singulisphaera, and Glycomyces. For Pb1800_Fm, Longimicrobium and Paenarthrobacter were enriched. In Pb2700_Fm, the enriched genera included S0134_terrestrial_group_unclassified, Alphaproteobacteria_unclassified, Lysobacter, Gemmatimonadaceae_unclassified, Devosia, and Chloroplast_unclassified. The genera Azoarcus, Phycicoccus, Azospirillum, and Sphingomonas were enriched in Pb900_Ri. In Pb1800_Ri, enrichment was observed for Gitt-GS-136_unclassified, JG30-KF-CM45_unclassified, and LWQ8_unclassified. Finally, in Pb2700_Ri, the enriched genera included Planctomycetales_unclassified, AKYG1722_unclassified, Promicromonospora, AKIW781_unclassified, Gemmata, Actinobacteriota_unclassified, Nonomuraea, Acidimicrobiia_unclassified, and Mycobacterium.
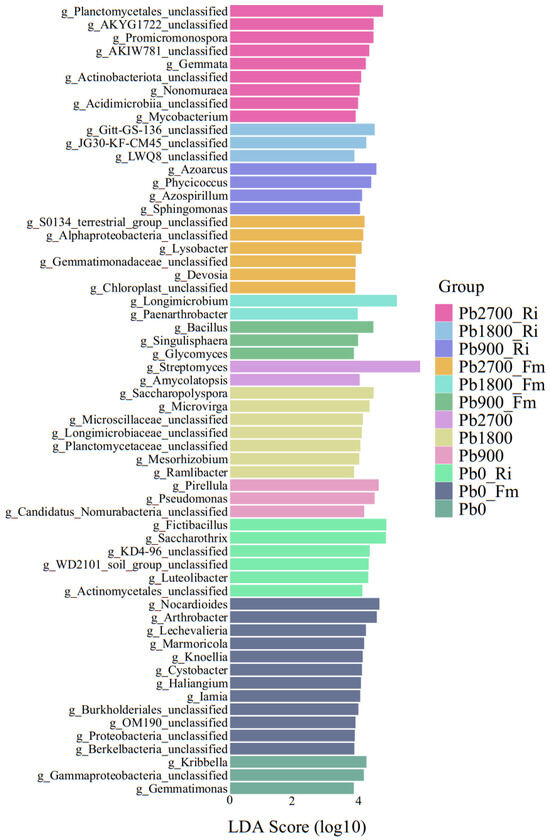
Figure 5.
LEfSe of bacterial community in the rhizosphere of maize with LDA scores higher than 3.0.
4. Discussion
4.1. Implications of AMF Inoculation for Maize Resistance to Pb Stress
In this study, treatment with Pb significantly inhibited the growth and biomass of maize plants, with a more pronounced decline observed at higher Pb application doses (Figure 1a–d). Elevated Pb concentrations are known to adversely affect plant health, a finding that aligns with results from previous research [32]. Nevertheless, AMF symbiosis plays a crucial role in restoring plant growth by alleviating stress caused by hazardous elements [33]. In comparison to non-inoculated maize plants, those inoculated with AMF exhibited significantly greater roots, stems, and leaves biomasses under varying concentrations of Pb stress (Figure 1b–d). This finding suggests that the AMF-inoculated plants possessed a heightened tolerance to Pb stress. Our results further substantiated that the AMF strains Fm and Ri demonstrated resilience to Pb stress, thereby enhancing the plants’ ability to mitigate Pb toxicity. In addition, the feasibility of a remediation strategy is largely contingent upon the bioactivity of residual heavy metals in soil and their availability to plants [34]. The uptake of Pb in the roots of maize was significantly higher in the treatments with AMF inoculation compared to the non-inoculated treatments (Figure 2). Notably, Pb predominantly accumulated in the roots, with a minimal amount translocated to the stems and leaves. This limited translocation mitigated the development of Pb-induced toxicity symptoms in the stems and leaves, thereby enhancing the effectiveness of phytostabilization [35,36]. Under Pb2700 stress, plants inoculated with Fm exhibited higher root Pb accumulation (2110.76 mg·kg−1) compared to those inoculated with Ri, which accumulated 2090.56 mg·kg−1 (Figure 2a). However, Ri-treated roots demonstrated marginally greater biomass (16.78 g·plant−1 at Pb900) than Fm (Figure 1b), suggesting that Ri may enhance root growth under moderate Pb stress. In addition, Fm-inoculated plants exhibited higher Pb concentrations in stems (160.97 mg·kg−1) and leaves (110.46 mg·kg−1) at Pb2700. In contrast, Ri significantly reduced Pb translocation, with concentrations in stems at 133.57 mg·kg−1 and leaves at 66.48 mg·kg−1 (Figure 2b,c). The lower translocation observed in Ri treatments correlates with a stronger suppression of the Pb transfer coefficient (root-to-shoot ratio), which is a critical metric for assessing phytostabilization efficacy [37].
AMF have been shown to enhance the de novo synthesis of plant enzymes [38]. Consequently, this enhancement leads to increased antioxidant enzymatic activity within plants, which serves to protect cells from damage induced by excessive reactive oxygen species (ROS) resulting from Pb stress [39]. In our study, we observed a significant increase in the activities of antioxidant enzymes, specifically POD and SOD, in the roots of maize following inoculation with AMF (Figure 1e–g). This finding further suggests mitigation of Pb stress. MDA is a byproduct of membrane lipid peroxidation, and its concentration can serve as a reliable indicator of cell membrane damage [40]. This is evidenced by the significantly elevated levels of MDA observed in maize roots exposed to Pb2700. Furthermore, our research indicates that AMF inoculation treatment results in a significant decrease in MDA content compared to the non-inoculation treatment except for the Pb900 treatment group, which correlates with the higher total biomass of maize observed in the AMF inoculation treatment (Figure 1h). Therefore, AMF inoculation can assist plants in reducing the accumulation of membrane lipid peroxidation and ROS by enhancing the activities of antioxidant enzymes under Pb stress, thereby improving plant tolerance to Pb.
4.2. Effects of AMF on Microbial Diversity and Composition in the Rhizosphere Soil of Maize
Soil microorganism diversity serves as a crucial indicator of soil health and plant fitness, as a diverse range of microorganisms is involved in vital soil functions [41]. In the present study, the addition of Pb significantly decreased the α-diversity index (Shannon index) of the bacterial community in uninoculated treatments, indicating that Pb adversely affects the rhizosphere bacterial community. This finding aligns with the report by Luo et al. (2018) [42], which noted that exogenous yttrium (Y) at concentrations of 250 mg·kg−1 and 500 mg·kg−1 negatively impacted soil bacterial diversity. Furthermore, for each Pb treatment, the AMF significantly enhanced the α-diversity indexes (Shannon and Sobs index) of the bacterial community. This result suggests that AMF may positively influence the rhizosphere microbiome of maize, thereby alleviating the toxicity of Pb stress on rhizosphere bacteria.
The results of the PCoA indicated that both the AMF and Pb treatments affected the rhizosphere soil bacterial community structure of the maize (Figure 3a,b). Moreover, the structure of the bacterial communities in the rhizosphere soils was predominantly influenced by AMF inoculation rather than Pb concentration (PC1), a finding that is consistent with the report by Zhao et al. (2023) [19]. In the AMF inoculation treatment, Pb affected the bacterial community, causing a deviation from the community structure observed in the non-Pb treatments (Figure 3b, PC2). However, a previous report indicated that only high concentrations of lanthanum (La) treatment affected the bacterial community structure in soils [43]. This discrepancy may be attributed to the higher toxicity of Pb compared to La.
The role of AMF inoculation in enhancing plant resistance is crucial for understanding the mechanisms that facilitate growth following inoculation. Community diversity is indicative of both stability and functional diversity, which can ultimately affect process rates and state variables within ecosystems [44]. In our study, the inoculation of AMF significantly enhanced bacterial diversity within the rhizosphere of maize subjected to Pb stress. This finding suggests that AMF inoculation may facilitate maize’s ability to withstand Pb stress by restoring the diversity of the bacterial community (Table 1).
The bacterial community structure was notably affected by AMF inoculation (Figure 4). At the phylum level, microbial assemblages comprise taxa exhibiting similar functional, metabolic, and morphological traits [45]. As a result, our investigation focused specifically on alterations at the phylum level within the bacterial communities. Under Pb stress, the inoculation of AMF resulted in significant increases in the relative abundances of Firmicutes, Planctomycetota, Chloroflexi, and Verrucomicrobiota, while Acidobacteria exhibited notable decline (Figure 4). Although Firmicutes were the dominant taxa observed under Pb contamination, they did not emerge as the main taxa following rhizosphere inoculation treatments. The heightened presence of Proteobacteria, including Pseudomonas, plays a role in reducing pathogen density and incidences of disease, particularly because many pathogens are linked to Bacteroidetes and Firmicutes. This behavior can be explained by increased competition for resources and interference with pathogenic organisms [35,46]. Although Bacteroidetes and Firmicutes can withstand challenging conditions like acidic environments and heavy metal contamination, they seem to lack competitiveness in healthy (non-contaminated) settings [47]. These outcomes indicate that the rhizosphere microenvironments were improved under Pb stress after inoculation.
4.3. Impact of Pb Stress and AMF on Key Rhizosphere Bacteria
To identify specific bacterial groups enriched in the various treatments, LEfSe analysis was conducted from the phylum to genus level according to distinct grouping patterns. The results of the LEfSe analysis indicated that Ri treatment promoted the enrichment of the bacteria Promicromonospora and Mycobacterium in the Pb2700 treatment (Figure 5). A previous study revealed that Promicromonospora exhibits tolerance to heavy metals and strong Cd removal ability [21]. Mycobacterium possesses numerous genes that confer resistance to heavy metals, suggesting its potential for remediating environments contaminated by such pollutants [48,49]. Additionally, Fm treatment significantly increased the abundance of the genus Lysobacter in the Pb2700 treatment (Figure 5). The genus Lysobacter encompasses several species capable of producing phytohormones, such as indole-3-acetic acid (IAA) and 1-aminocyclopropane-1-carboxylic acid (ACC), which are known to promote plant growth [50]. Notably, Fm significantly recruited two beneficial bacteria, Longimicrobium and Paenarthrobacter, during the Pb1800 treatment. These bacteria are resistant to heavy metal toxicity and are also advantageous for plant health. Longimicrobium is capable of tolerating heavy metal stress and plays a crucial role in reducing Pb accumulation in plants, thereby promoting their growth [51]. Additionally, Paenarthrobacter demonstrated remarkable tolerance to heavy metal chromium (Cr(VI)) and significantly enhanced the reduction capacity of the indigenous microflora towards Cr(VI) [52].
This research demonstrates that AMF can mitigate the detrimental effects of Pb and enhance the ability of maize to tolerate Pb exposure. The primary mechanism underlying this enhancement is the significant influence of AMF on both plant growth and the microbial communities within the rhizosphere soils. AMF specifically assist plants by decreasing the accumulation of ROS and reducing membrane lipid peroxidation through the increased activity of antioxidant enzymes during Pb stress, ultimately improving the resilience of plants against Pb. Furthermore, it is anticipated that AMF may facilitate maize growth under Pb stress by modifying the structure of bacterial communities in the rhizosphere. The findings of this study underscore the capacity of AMF to immobilize and absorb Pb by promoting the relative abundance of beneficial microorganisms, which correlates with elevated Pb levels observed in maize plants inoculated with AMF (Figure 2). In summary, the microbial community acts as a functional extension of AMF, amplifying Pb phytostabilization through nutrient mobilization, redox regulation, and direct metal immobilization. This tripartite symbiosis highlights the potential of microbiome engineering to optimize AMF-assisted phytoremediation strategies.
5. Conclusions
This study demonstrates that AMF enhance maize resilience to Pb through species-specific mechanisms, providing distinct ecological and agronomic advantages. Fm excels in Pb immobilization within roots, achieving levels of 2110.76 mg·kg−1 at Pb2700, making it particularly suitable for phytostabilization in heavily contaminated soils. In contrast, Ri effectively minimizes Pb translocation to aerial tissues, with only 66.48 mg·kg−1 detected in leaves at Pb2700, thereby reducing the risks of Pb entering the food chain. Importantly, AMF symbiosis recruits stress-adapted rhizobacteria, such as Promicromonospora and Lysobacter, which synergistically enhance metal resistance, nutrient cycling, and redox homeostasis. These findings position AMF as crucial allies in sustainable Pb remediation by balancing soil detoxification with crop safety. Future field trials should prioritize multispecies AMF consortia and microbiome engineering to optimize phytomanagement strategies while addressing real-world soil heterogeneity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15061310/s1. Figure S1: Rarefaction curves analysis (the curve was constructed using the Chao1 index of ASVs and the number of reads). Figure S2: Venn diagram of microbial species distribution under different treatments (phylum level). Figure S3: Abundance–occupancy distributions were employed to identify the core ASVs of maize-associated bacteria. The solid lines represent the best fit of Sloan’s neutral model, while the dashed lines denote the 95% confidence intervals surrounding the model fit. Points falling within the 95% confidence intervals (depicted in black) are inferred to be neutrally selected. In contrast, points located above (shown in green) and below (illustrated in red) the 95% confidence intervals are inferred to be deterministically selected. Table S1: The detailed composition of the full-strength nutrient solution. Table S2: Summary of clean reads identified in this study. Table S3: Phylum statistics.
Author Contributions
Conceptualization, X.Z. and X.W.; methodology, X.Z.; software. B.Z. and Y.Z.; formal analysis, P.W. and M.L.; writing—original draft preparation, X.Z.; writing—review and editing, X.J., X.W. and H.Z.; visualization, X.Z.; resources, S.C.; supervision, P.W. and S.C.; funding acquisition, X.Z. and S.C. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32301772), Joint Research on Agricultural Improved Seed of Henan Province (2022010204), Shennong Laboratory First-rate Research Subject (SN01-2022-02), and the Key Research and Development Project of Henan Province (241111114300).
Data Availability Statement
The data are contained within the article and its Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, D.; Yu, W.; Hao, Z.; Qiu, M.; Cui, J.; Tang, Y.; Teng, X.; Liu, Y.; Liu, H. Molecular mechanism of selenium against lead-induced apoptosis in chicken brainstem relating to heat shock protein, selenoproteins, and inflammatory cytokines. Ecotoxicol. Environ. Saf. 2024, 272, 116028. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R.S. Lead toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 35–52. [Google Scholar] [CrossRef]
- Dubey, S.; Shri, M.; Gupta, A.; Rani, V.; Chakrabarty, D. Toxicity and detoxification of heavy metals during plant growth and metabolism. Environ. Chem. Lett. 2018, 16, 1169–1192. [Google Scholar] [CrossRef]
- Soares, T.; Dias, D.; Oliveira, A.M.S.; Ribeiro, D.M.; Dias, L. Exogenous brassinosteroids increase lead stress tolerance in seed germination and seedling growth of Brassica juncea L. Ecotoxicol. Environ. Saf. 2020, 193, 110296. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Wang, C.; Liu, P.; Ma, L.; Zou, C.; Pan, G.; Shen, Y. Combined genome-wide association study and gene co-expression network analysis identified ZmAKINβγ1 involved in lead tolerance and accumulation in maize seedlings. Int. J. Biol. Macromol. 2023, 226, 1374–1386. [Google Scholar] [CrossRef]
- Kanwar, V.S.; Sharma, A.; Srivastav, A.L.; Rani, L. Phytoremediation of toxic metals present in soil and water environment: A critical review. Environ. Sci. Pollut. Res. 2020, 27, 44835–44860. [Google Scholar] [CrossRef]
- Wei, Z.; Hao, Z.; Li, X.; Guan, Z.; Cai, Y.; Liao, X. The effects of phytoremediation on soil bacterial communities in an abandoned mine site of rare earth elements. Sci. Total Environ. 2019, 670, 950–960. [Google Scholar] [CrossRef]
- Jin, Y.; Luan, Y.; Ning, Y.; Wang, L. Effects and mechanisms of microbial remediation of heavy metals in soil: A critical review. Appl. Sci. 2018, 8, 1336. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, L.; Wang, Y.; Mei, P.; Zhou, F.; Rozhkova, T.; Li, C. Effects of Streptomyces sp. HU2014 inoculation on wheat growth and rhizosphere microbial diversity under hexavalent chromium stress. Ecotoxicol. Environ. Saf. 2024, 276, 116313. [Google Scholar] [CrossRef]
- Khalid, M.; Saeed, U.-R.; Hassani, D.; Hayat, K.; Pei, Z.; Nan, H. Advances in fungal-assisted phytoremediation of heavy metals: A review. Pedosphere 2021, 31, 475–495. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Y.; Han, X.; Chiu, T.-Y.; Ghosh, A.; Chen, H.; Tang, M. The roles of arbuscular mycorrhizal fungi (AMF) in phytoremediation and tree-herb interactions in Pb contaminated soil. Sci. Rep. 2016, 6, 20469. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Sharma, S.; Monti, A. Bio-remediation of Pb and Cd polluted soils by switchgrass: A case study in India. Int. J. Phytoremediat. 2016, 18, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, Z.; Lu, S.; Wang, F. AMF Inoculation Alleviates Molybdenum Toxicity to Maize by Protecting Leaf Performance. J. Fungi 2023, 9, 479. [Google Scholar] [CrossRef]
- Wipf, D.; Krajinski, F.; van Tuinen, D.; Recorbet, G.; Courty, P.E. Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytol. 2019, 223, 1127–1142. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, B.; Xu, J.; Li, Z.; Tang, Z.; Wu, X. Heavy metal domestication enhances beneficial effects of arbuscular mycorrhizal fungi on lead (Pb) phytoremediation efficiency of Bidens parviflora through improving plant growth and root Pb accumulation. Environ. Sci. Pollut. Res. 2022, 29, 32988–33001. [Google Scholar] [CrossRef]
- Wang, H.-R.; Zhao, X.-Y.; Zhang, J.-M.; Lu, C.; Feng, F.-J. Arbuscular mycorrhizal fungus regulates cadmium accumulation, migration, transport, and tolerance in Medicago sativa. J. Hazard. Mater. 2022, 435, 129077. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Z.; Yang, X.; Sheng, L.; Mao, H.; Zhu, S. Metagenomics reveal arbuscular mycorrhizal fungi altering functional gene expression of rhizosphere microbial community to enhance Iris tectorum’s resistance to Cr stress. Sci. Total Environ. 2023, 895, 164970. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, S.; Yang, X.; Xia, G.; Wang, B.; Gu, B. Arbuscular mycorrhizal fungi alter rhizosphere bacterial community characteristics to improve Cr tolerance of Acorus calamus. Ecotoxicol. Environ. Saf. 2023, 253, 114652. [Google Scholar]
- Chen, X.W.; Wu, L.; Luo, N.; Mo, C.H.; Wong, M.H.; Li, H. Arbuscular mycorrhizal fungi and the associated bacterial community influence the uptake of cadmium in rice. Geoderma 2019, 337, 749–757. [Google Scholar] [CrossRef]
- Hamedi, J.; Dehhaghi, M.; Mohammdipanah, F. Isolation of extremely heavy metal resistant strains of rare actinomycetes from high metal content soils in Iran. Int. J. Environ. Res. 2015, 9, 475–480. [Google Scholar]
- Mitra, A.; Chatterjee, S.; Kataki, S.; Rastogi, R.P.; Gupta, D.K. Bacterial tolerance strategies against lead toxicity and their relevance in bioremediation application. Environ. Sci. Pollut. Res. 2021, 28, 14271–14284. [Google Scholar] [CrossRef] [PubMed]
- George, S.E.; Wan, Y. Microbial functionalities and immobilization of environmental lead: Biogeochemical and molecular mechanisms and implications for bioremediation. J. Hazard. Mater. 2023, 457, 131738. [Google Scholar] [CrossRef]
- Riaz, M.; Kamran, M.; Fang, Y.; Wang, Q.; Cao, H.; Yang, G.; Deng, L.; Wang, Y.; Zhou, Y.; Anastopoulos, I. Arbuscular mycorrhizal fungi-induced mitigation of heavy metal phytotoxicity in metal contaminated soils: A critical review. J. Hazard. Mater. 2021, 402, 123919. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Chen, J.; Lin, H.; Zhao, M.; Peng, H.; Liu, L.; Yuan, G.; Zhang, S.; Zhang, Z.; et al. Genome expression profile analysis reveals important transcripts in maize roots responding to the stress of heavy metal Pb. Physiol. Plant. 2013, 147, 270–282. [Google Scholar] [CrossRef]
- Wang, F.; Jiang, R.; Kertesz, M.A.; Zhang, F.; Feng, G. Arbuscular mycorrhizal fungal hyphae mediating acidification can promote phytate mineralization in the hyphosphere of maize (Zea mays L.). Soil Biol. Biochem. 2013, 65, 69–74. [Google Scholar] [CrossRef]
- Wang, F.; Jing, X.; Adams, C.A.; Shi, Z.; Sun, Y. Decreased ZnO nanoparticle phytotoxicity to maize by arbuscular mycorrhizal fungus and organic phosphorus. Environ. Sci. Pollut. Res. 2018, 25, 23736–23747. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Mu, M.; Wang, Z.; Chen, Z.; Wu, Y.; Nie, W.; Zhao, S.; Yin, X.; Teng, X. Physiological characteristics, rhizosphere soil properties, and root-related microbial communities of Trifolium repens L. in response to Pb toxicity. Sci. Total Environ. 2024, 907, 167871. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Jiang, C.; Mao, H.; Zhang, Y. Immobilization of Cd in soil and changes of soil microbial community by bioaugmentation of UV-mutated Bacillus subtilis 38 assisted by biostimulation. Eur. J. Soil Biol. 2014, 65, 62–69. [Google Scholar] [CrossRef]
- Li, Q.; Xing, Y.; Huang, B.; Chen, X.; Ji, L.; Fu, X.; Li, T.; Wang, J.; Chen, G.; Zhang, Q. Rhizospheric mechanisms of Bacillus subtilis bioaugmentation-assisted phytostabilization of cadmium-contaminated soil. Sci. Total Environ. 2022, 825, 154136. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, B.; Ma, X.; Jin, X.; Chen, S.; Wang, P.; Zhongrong, G.; Wu, X.; Zhang, H. Combining transcriptome and metabolome analyses to reveal the response of maize roots to Pb stress. Plant Physiol. Biochem. 2024, 217, 109265. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Lounes-Hadj Sahraoui, A. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef]
- Wood, J.L.; Tang, C.; Franks, A.E. Microbial associated plant growth and heavy metal accumulation to improve phytoextraction of contaminated soils. Soil Biol. Biochem. 2016, 103, 131–137. [Google Scholar] [CrossRef]
- Wang, X.; Fang, L.; Beiyuan, J.; Cui, Y.; Peng, Q.; Zhu, S.; Wang, M.; Zhang, X. Improvement of alfalfa resistance against Cd stress through rhizobia and arbuscular mycorrhiza fungi co-inoculation in Cd-contaminated soil. Environ. Pollut. 2021, 277, 116758. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, T.; Zhou, J.; Peng, X. Effects of Arbuscular Mycorrhizal Fungi on Robinia pseudoacacia L. Growing on Soils Contaminated with Heavy Metals. J. Fungi 2023, 9, 684. [Google Scholar] [CrossRef]
- Jia, Q.; Sun, J.; Gan, Q.; Shi, N.-N.; Fu, S. Zea mays cultivation, biochar, and arbuscular mycorrhizal fungal inoculation influenced lead immobilization. Microbiol. Spectr. 2024, 12, e03427-23. [Google Scholar] [CrossRef]
- Fatima, R.A.; Ahmad, M. Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci. Total Environ. 2005, 346, 256–273. [Google Scholar] [CrossRef]
- Fang, L.; Ju, W.; Yang, C.; Jin, X.; Liu, D.; Li, M.; Yu, J.; Zhao, W.; Zhang, C. Exogenous application of signaling molecules to enhance the resistance of legume-rhizobium symbiosis in Pb/Cd-contaminated soils. Environ. Pollut. 2020, 265, 114744. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Ma, X.; Jin, X.; Wu, X.; Zhang, H.; Guan, Z.; Fu, Z.; Chen, S.; Wang, P. Transcriptomics Combined with Physiology and Metabolomics Reveals the Mechanism of Tolerance to Lead Toxicity in Maize Seedling. Physiol. Plant. 2024, 176, e14547. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Yu, Z.; Shen, G.; Cheng, H.; Tao, S. Composition and diversity of soil microbial communities in the alpine wetland and alpine forest ecosystems on the Tibetan Plateau. Sci. Total Environ. 2020, 747, 141358. [Google Scholar] [CrossRef]
- Luo, C.; Deng, Y.; Liang, J.; Zhu, S.; Wei, Z.; Guo, X.; Luo, X. Exogenous rare earth element-yttrium deteriorated soil microbial community structure. J. Rare Earth. 2018, 36, 430–439. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Z.; Hao, B.; Diao, F.; Zhang, J.; Bao, Z.; Guo, W. Arbuscular mycorrhizal fungi alter microbiome structure of rhizosphere soil to enhance maize tolerance to La. Ecotoxicol. Environ. Saf. 2021, 212, 111996. [Google Scholar] [CrossRef]
- Hillebrand, H.; Matthiessen, B. Biodiversity in a complex world: Consolidation and progress in functional biodiversity research. Ecol. Lett. 2009, 12, 1405–1419. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; Van Der Heijden, M.G. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; Friman, V.-P.; Gu, S.-h.; Wang, X.f.; Eisenhauer, N.; Yang, T.j.; Ma, J.; Shen, Q.r.; Xu, Y.c. Probiotic diversity enhances rhizosphere microbiome function and plant disease suppression. mBio 2016, 7, 10–1128. [Google Scholar] [CrossRef]
- Quadros, P.D.; Zhalnina, K.; Davis-Richardson, A.G.; Drew, J.C.; Menezes, F.B.; Camargo, F.A.d.O.; Triplett, E.W. Coal mining practices reduce the microbial biomass, richness and diversity of soil. Appl. Soil Ecol. 2016, 98, 195–203. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Wei, D.-Z. Biosorption of Mycobacterium phlei to the Heavy Metal Ions Pb2+, Zn2+, Ni2+ and Cu2+. J. Northeast. Univ. Nat. Sci. 2003, 24, 91–93. [Google Scholar]
- Erardi, F.; Failla, M.; Falkinham, J., 3rd. Accumulation and transport of cadmium by tolerant and susceptible strains of Mycobacterium scrofulaceum. Antimicrob. Agents Chemother. 1989, 33, 350–355. [Google Scholar] [CrossRef]
- Laborda, P.; Ling, J.; Chen, X.; Liu, F. ACC deaminase from Lysobacter gummosus OH17 can promote root growth in Oryza sativa Nipponbare plants. J. Agric. Food Chem. 2018, 66, 3675–3682. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Guo, P.; Zhang, Z.; Cui, X.; Hao, B.; Guo, W. Arbuscular mycorrhizal fungi facilitate Astragalus adsurgens growth and stress tolerance in cadmium and lead contaminated saline soil by regulating rhizosphere bacterial community. Appl. Soil Ecol. 2023, 187, 104842. [Google Scholar] [CrossRef]
- Gan, M.; Zhou, Y.; Huang, D.; He, P.; Tang, B.; Cai, Y.; Zhu, J. The enhanced effect of key microorganisms in chromium contaminated soil in Cr(VI) reduction. Chemosphere 2024, 362, 142682. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).