Integrating Cytochrome P450-Mediated Herbicide Tolerance into Anthocyanin-Rich Maize Through Conventional Breeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Plant Establishment and Herbicide Treatments
2.3. Foliar Phenotyping
2.4. Dry-Matter Reduction Index and Absolute Growth Rate
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Dry-Matter Retention and Visual Injury
3.2. Absolute Growth Rate
3.3. Chlorophyll Fluorescence
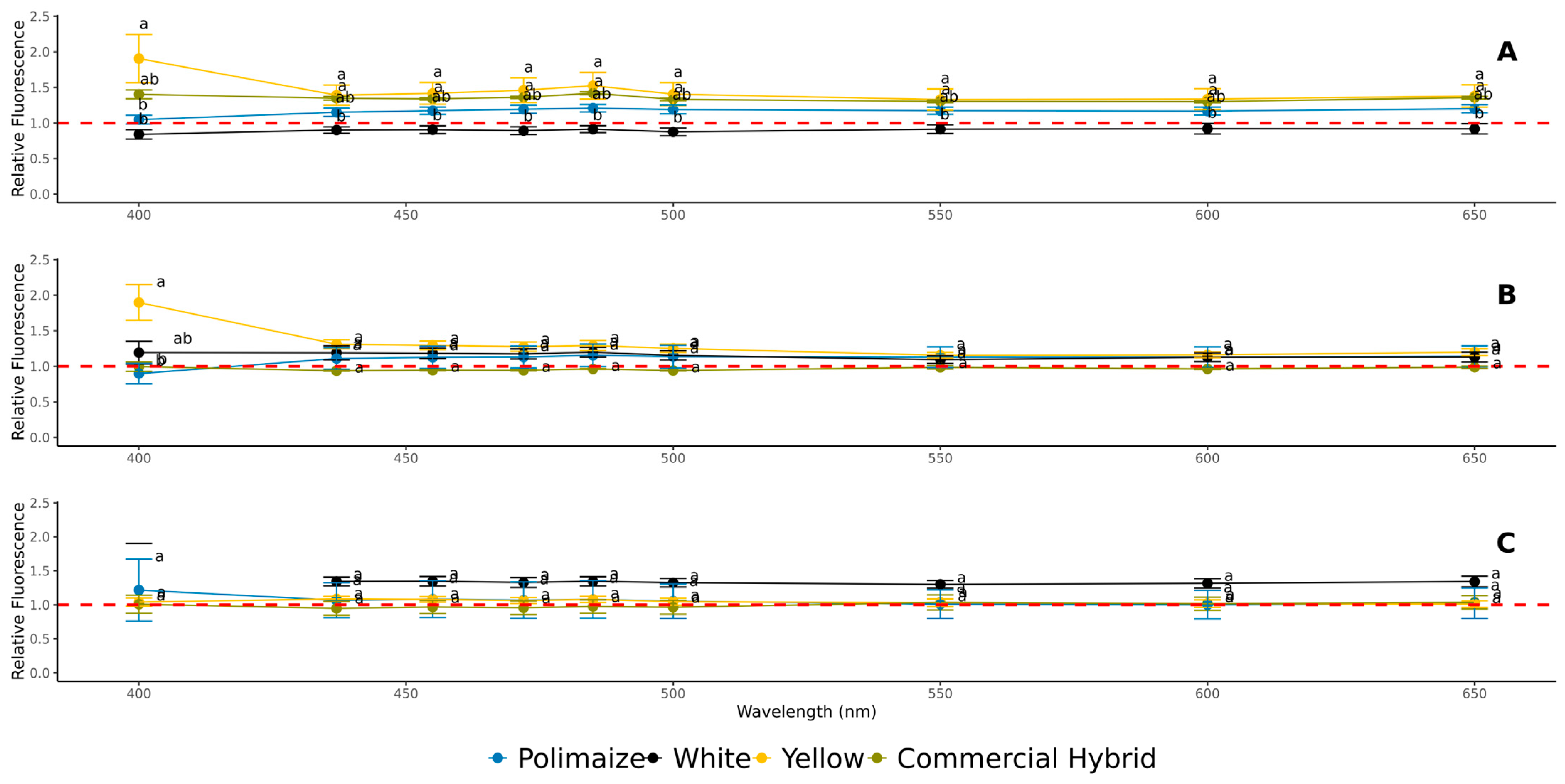
3.4. Secondary-Pigment Fluorescence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CYP450 | Cytochrome P450 |
| FD | Foliar Damage |
| DMRI | Dry Matter Reduction Index |
| AGR | Absolute Growth Rate |
| ALS | Acetolactate Synthase |
| HPPD | 4-Hydroxyphenylpyruvate Dioxygenase |
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Khoury, C.K.; Bjorkman, A.D.; Dempewolf, H.; Ramirez-Villegas, J.; Guarino, L.; Jarvis, A.; Rieseberg, L.H.; Struik, P.C. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl. Acad. Sci. USA 2014, 111, 4001–4006. [Google Scholar] [CrossRef] [PubMed]
- Zizumbo-Villarreal, D.; Colunga-GarcíaMarín, P. Origin of agriculture and plant domestication in West Mesoamerica. Genet. Resour. Crop Evol. 2010, 57, 813–825. [Google Scholar] [CrossRef]
- Villaseñor, J.L.; Ortiz, E.; Juárez, D. Transition zones and biogeographic characterization of endemism in three biogeographic provinces of central Mexico. Bot. Sci. 2021, 99, 938–954. [Google Scholar] [CrossRef]
- Martínez-Vega, A.; Oregel-Zamudio, E.; García-Ruíz, I.; Villapando-Arteaga, E.; Torres-García, J. Genetic and metabolomic differentiation of Physalis ixocarpa Brot. ex Hornem. populations in Michoacan State, Mexico. Genet. Resour. Crop Evol. 2022, 69, 1867–1877. [Google Scholar] [CrossRef]
- Arias-Martínez, S.; Oyoque-Salcedo, G.; Gutiérrez-Cárdenas, O.G.; Oregel-Zamudio, E.; Torres-García, J.R. Comparative metabolomic fingerprinting analysis of tomato fruits from physalis species in mexico’s balsas basin. Horticulturae 2024, 10, 600. [Google Scholar] [CrossRef]
- Oyoque-Salcedo, G.; Arias-Martínez, S.; Gutiérrez-Cárdenas, O.G.; Montañez-Soto, J.L.; Oregel-Zamudio, E.; Torres-García, J.R. Nutritional Enhancement of Polimaize Lines: Integrating Native Mexican Maize Alleles into High-Yield Varieties. Agronomy 2024, 14, 403. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Krikorian, R.; Shidler, M.D.; Nash, T.A.; Kalt, W.; Vinqvist-Tymchuk, M.R.; Shukitt-Hale, B.; Joseph, J.A. Blueberry supplementation improves memory in older adults. J. Agric. Food Chem. 2010, 58, 3996–4000. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, P.; Biswas, A.; Dey, S.; Bhattacharjee, T.; Chakrabarty, S. Cytochrome P450 gene families: Role in plant secondary metabolites production and plant defense. J. Xenobiotics 2023, 13, 402–423. [Google Scholar] [CrossRef] [PubMed]
- Pandian, B.A.; Sathishraj, R.; Djanaguiraman, M.; Prasad, P.V.; Jugulam, M. Role of cytochrome P450 enzymes in plant stress response. Antioxidants 2020, 9, 454. [Google Scholar] [CrossRef]
- Casey, A.; Dolan, L. Genes encoding cytochrome P450 monooxygenases and glutathione S-transferases associated with herbicide resistance evolved before the origin of land plants. PLoS ONE 2023, 18, e0273594. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Li, W.; Zhang, X.; Zhang, Y.; Dong, S.; Song, X.e.; Zhao, J.; Chen, M.; Yuan, X. Genome-wide identification and expression profiling of cytochrome P450 Monooxygenase Superfamily in Foxtail Millet. Int. J. Mol. Sci. 2023, 24, 11053. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Tsujii, H.; Ohkawa, Y. The use of cytochrome P450 genes to introduce herbicide tolerance in crops: A review. Pestic. Sci. 1999, 55, 867–874. [Google Scholar] [CrossRef]
- Williams, M., II; Pataky, J.K.; Nordby, J.N.; Riechers, D.E.; Sprague, C.L.; Masiunas, J.B. Cross-sensitivity in sweet corn to nicosulfuron and mesotrione applied postemergence. HortScience 2005, 40, 1801–1805. [Google Scholar] [CrossRef]
- Williams, M.M.; Wax, L.M.; Pataky, J.K.; Meyer, M.D. Further evidence of a genetic basis for varied levels of injury to sweet corn hybrids from cytochrome P450-metabolized herbicides applied postemergence. HortScience 2008, 43, 2093–2097. [Google Scholar] [CrossRef]
- Williams, M.M., II; Pataky, J.K. Factors affecting differential sensitivity of sweet corn to HPPD-inhibiting herbicides. Weed Sci. 2010, 58, 289–294. [Google Scholar] [CrossRef]
- Hassannejad, S.; Lotfi, R.; Ghafarbi, S.P.; Oukarroum, A.; Abbasi, A.; Kalaji, H.M.; Rastogi, A. Early identification of herbicide modes of action by the use of chlorophyll fluorescence measurements. Plants 2020, 9, 529. [Google Scholar] [CrossRef]
- O′Neill, P.M.; Shanahan, J.F.; Schepers, J.S. Use of chlorophyll fluorescence assessments to differentiate corn hybrid response to variable water conditions. Crop Sci. 2006, 46, 681–687. [Google Scholar] [CrossRef]
- McCurdy, J.D.; McElroy, J.S.; Kopsell, D.A.; Sams, C.E.; Sorochan, J.C. Effects of mesotrione on perennial ryegrass (Lolium perenne L.) carotenoid concentrations under varying environmental conditions. J. Agric. Food Chem. 2008, 56, 9133–9139. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Armel, G.R.; Mueller, T.C.; Sams, C.E.; Deyton, D.E.; McElroy, J.S.; Kopsell, D.E. Increase in nutritionally important sweet corn kernel carotenoids following mesotrione and atrazine applications. J. Agric. Food Chem. 2009, 57, 6362–6368. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.D.; Pataky, J.K.; Williams, M.M. Genetic factors influencing adverse effects of mesotrione and nicosulfuron on sweet corn yield. Agron. J. 2010, 102, 1138–1144. [Google Scholar] [CrossRef]
- Hunt, R. Growth analysis of individual plants. In Plant Growth Analysis; Studies in Biology No. 96; Edward Arnold: London, UK, 1978. [Google Scholar]
- Lavieille, D.; Ter Halle, A.; Richard, C. Understanding mesotrione photochemistry when applied on leaves. Environ. Chem. 2008, 5, 420–425. [Google Scholar] [CrossRef]
- Nordby, J.N.; Williams, M.M., II; Pataky, J.K.; Riechers, D.E.; Lutz, J.D. A common genetic basis in sweet corn inbred Cr1 for cross sensitivity to multiple cytochrome P450-metabolized herbicides. Weed Sci. 2008, 56, 376–382. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Zandstra, J.; Sikkema, P. Sweet corn (Zea mays) cultivar sensitivity to mesotrione. Weed Technol. 2002, 16, 421–425. [Google Scholar] [CrossRef]
- Liu, X.-M.; Xu, X.; Li, B.-H.; Yao, X.-X.; Zhang, H.-H.; Wang, G.-Q.; Han, Y.-J. Genomic and transcriptomic insights into cytochrome P450 monooxygenase genes involved in nicosulfuron tolerance in maize (Zea mays L.). J. Integr. Agric. 2018, 17, 1790–1799. [Google Scholar] [CrossRef]
- Schuster, C.L.; Al-Khatib, K.; Dille, J.A. Mechanism of antagonism of mesotrione on sulfonylurea herbicides. Weed Sci. 2007, 55, 429–434. [Google Scholar] [CrossRef]

| Mesotrione | Nicosulfuron | Meso + Nico | ||||
|---|---|---|---|---|---|---|
| Genotype | DMRI | FD (%) | DMRI | FD (%) | DMRI | FD (%) |
| Parents | ||||||
| Polimaize (blue landrace) | 0.75 ± 0.11 a | 0.30 ± 0.10 ab | 0.72 ± 0.18 b | 0.36 ± 0.09 a | 0.77 ± 0.17 b | 0.45 ± 0.08 a |
| Cimarrón® (white hybrid) | 0.96 ± 0.09 a | 0.07 ± 0.08 b | 0.98 ± 0.16 ab | 0.03 ± 0.07 b | 0.95 ± 0.15 b | 0.04 ± 0.07 b |
| F1 segregants | ||||||
| Yellow (blue/yellow) | 0.83 ± 0.10 a | 0.26 ± 0.09 ab | 0.97 ± 0.17 ab | 0.42 ± 0.08 a | 0.87 ± 0.15 b | 0.10 ± 0.07 b |
| White (blue/white) | 0.89 ± 0.12 a | 0.48 ± 0.09 a | 1.60 ± 0.19 a | 0.44 ± 0.09 a | 1.82 ± 0.18 a | 0.10 ± 0.08 b |
| p-value (ANOVA) | NS | ** | *** | *** | *** | * |
| HSD (α = 0.05) | 0.41 | 0.37 | 0.68 | 0.33 | 0.62 | 0.30 |
| Parents | F1 Segregants | |||
|---|---|---|---|---|
| Treatment | Polimaize (Blue Landrace) | Cimarrón® (White Hybrid) | Yellow (Blue/Yellow) | White (Blue/White) |
| Control | 7.1 ± 0.95 a | 4.5 ± 0.90 a | 4.9 ± 1.60 a | 0.5 ± 1.20 b |
| Mesotrione | 2.6 ± 0.85 ab | 3.8 ± 0.88 a | 2.3 ± 1.50 a | 0.1 ± 1.10 b |
| Nicosulfuron | 2.4 ± 1.05 b | 4.7 ± 1.02 a | 4.0 ± 1.70 a | 5.7 ± 1.90 ab |
| Meso + Nico | 3.0 ± 1.00 ab | 3.9 ± 0.94 a | 2.7 ± 1.55 a | 7.6 ± 2.10 a |
| p-value (ANOVA) | * | NS | * | NS |
| HSD (α = 0.05) | 4.6 | 4.9 | 8.6 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Martínez, S.; Peña-Vázquez, L.J.; Oregel-Zamudio, J.M.; Barajas-Chávez, J.A.; Oregel-Zamudio, E.; Torres-García, J.R. Integrating Cytochrome P450-Mediated Herbicide Tolerance into Anthocyanin-Rich Maize Through Conventional Breeding. Agronomy 2025, 15, 1308. https://doi.org/10.3390/agronomy15061308
Arias-Martínez S, Peña-Vázquez LJ, Oregel-Zamudio JM, Barajas-Chávez JA, Oregel-Zamudio E, Torres-García JR. Integrating Cytochrome P450-Mediated Herbicide Tolerance into Anthocyanin-Rich Maize Through Conventional Breeding. Agronomy. 2025; 15(6):1308. https://doi.org/10.3390/agronomy15061308
Chicago/Turabian StyleArias-Martínez, Sergio, Luis Jesús Peña-Vázquez, Jose Manuel Oregel-Zamudio, José Andrés Barajas-Chávez, Ernesto Oregel-Zamudio, and Jesús Rubén Torres-García. 2025. "Integrating Cytochrome P450-Mediated Herbicide Tolerance into Anthocyanin-Rich Maize Through Conventional Breeding" Agronomy 15, no. 6: 1308. https://doi.org/10.3390/agronomy15061308
APA StyleArias-Martínez, S., Peña-Vázquez, L. J., Oregel-Zamudio, J. M., Barajas-Chávez, J. A., Oregel-Zamudio, E., & Torres-García, J. R. (2025). Integrating Cytochrome P450-Mediated Herbicide Tolerance into Anthocyanin-Rich Maize Through Conventional Breeding. Agronomy, 15(6), 1308. https://doi.org/10.3390/agronomy15061308









