Multiple Resistance to PS II-Inhibiting and ALS-Inhibiting Herbicides in Common Lambsquarters (Chenopodium album L.) from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Whole-Plant Dose–Response Experiment
2.3. PsbA and ALS Gene Sequence Analysis
2.4. PsbA and ALS Expression Analysis
2.5. In Vitro ALS Enzyme Activity Assays
2.6. Cross- and Multiple-Resistance Patterns of Specific Populations to Other Herbicides
2.7. Data Analysis
3. Results
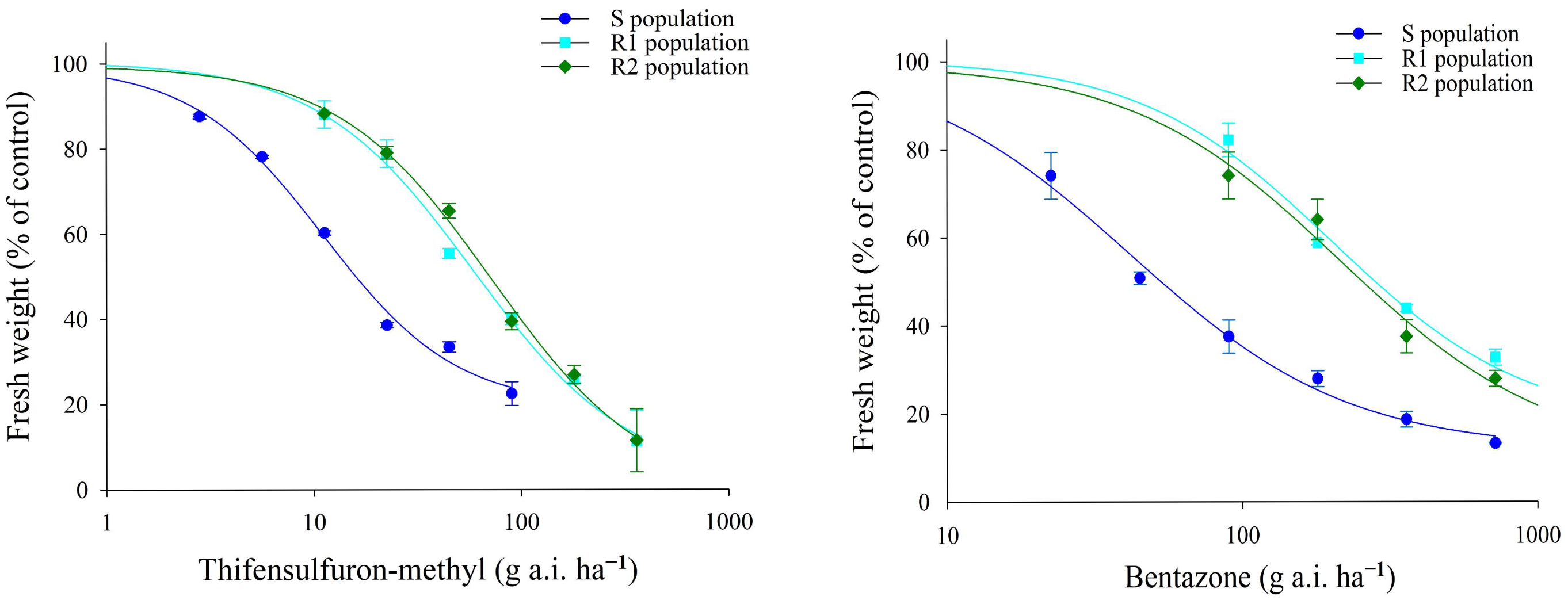
3.1. Whole-Plant Dose–Response Assays
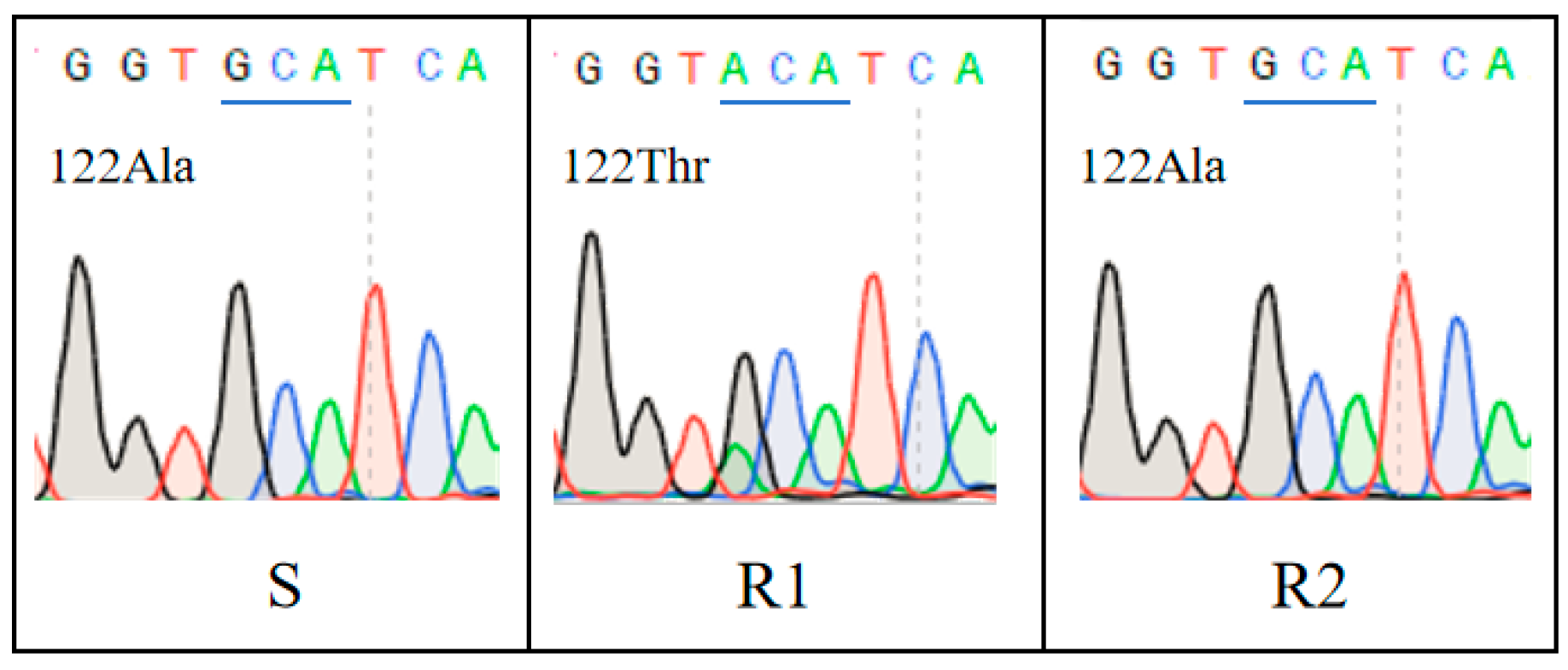
3.2. PsbA and ALS Gene Sequencing Analysis
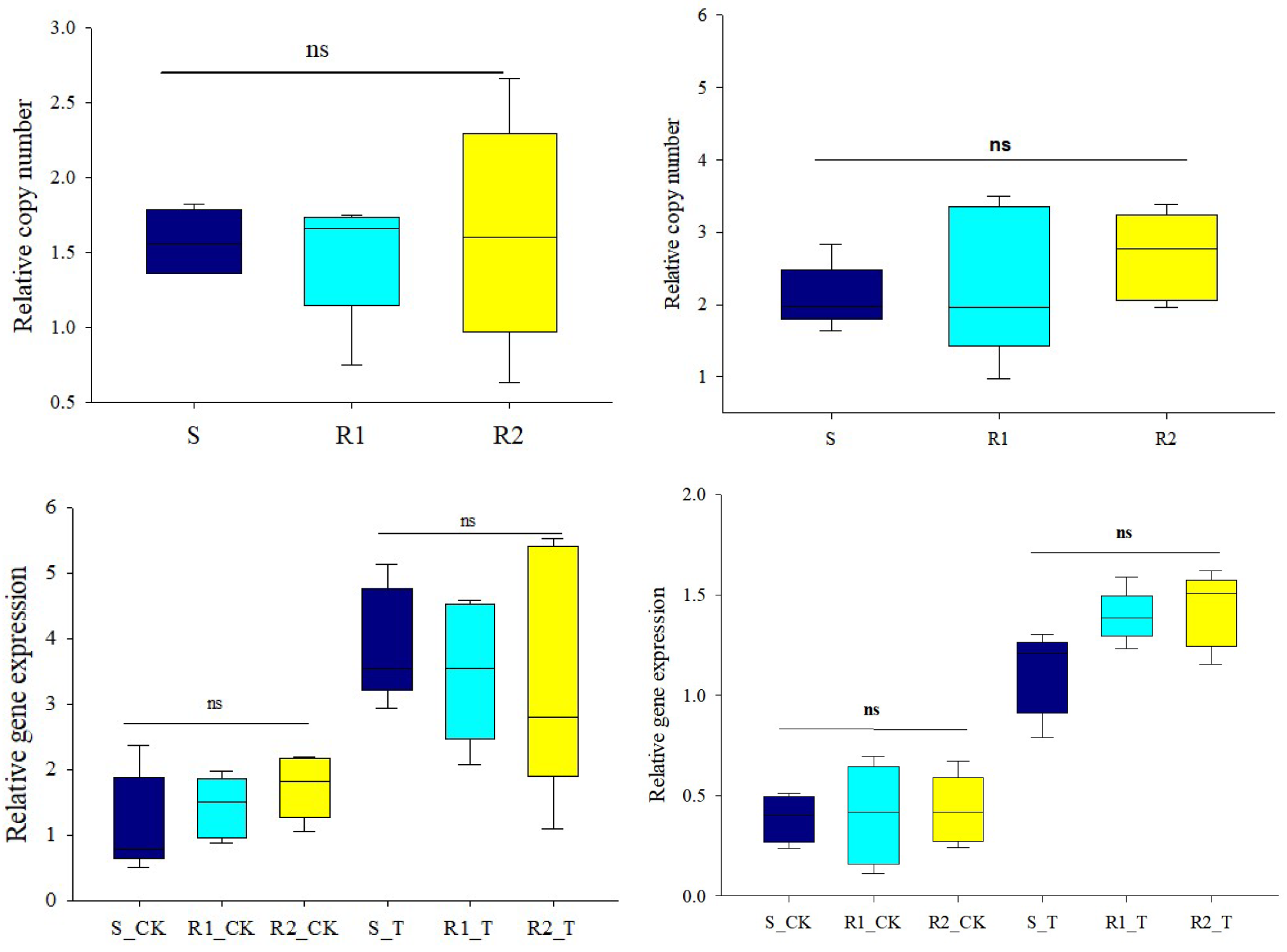
3.3. PsbA and ALS Gene Expression Quantification by qRT–PCR
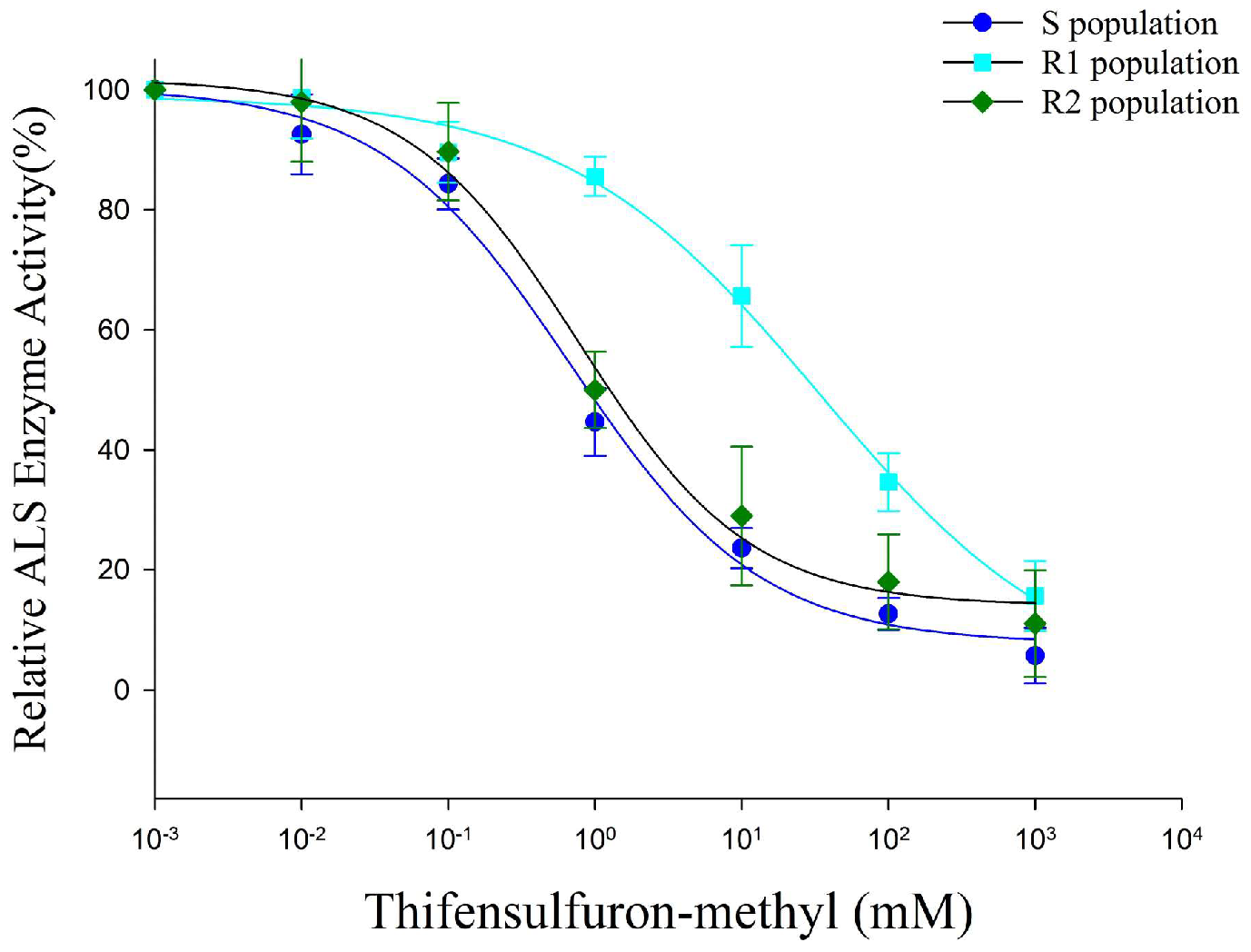
3.4. Effects of Thifensulfuron-Methyl on Vitro ALS Enzyme Activity
3.5. Cross- and Multiple-Resistance Patterns of C. album to Other Herbicides
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mazur, B.J.; Chui, C.F.; Smith, J.K. Isolation and characterization of plant genes coding for acetolactate synthase, the target enzyme for two classes of herbicides. Plant Physiol. 1987, 85, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Chaleff, R.S.; Mauvais, C.J. Acetolactate synthase is the site of action of two sulfonylurea herbicides in higher plants. Science 1984, 224, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Evans, R.R.; Dahmer, M.L.; Singh, B.K.; Shaner, D.L. Imidazolinone-tolerant crops: History, current status and future. Pest Manag. Sci. 2005, 61, 246–257. [Google Scholar] [CrossRef]
- Scarabel, L.; Varotto, S.; Sattin, M. A European biotype of Amaranthus retroflexus cross-resistant to ALS inhibitors and response to alternative herbicides. Weed Res. 2007, 47, 527–533. [Google Scholar] [CrossRef]
- Sibony, M.; Michel, A.; Haas, H.U.; Rubin, B.; Hurle, K. Sulfometuron-resistant Amaranthus retroflexus: Cross-resistance and molecular basis for resistance to acetolactate synthase-inhibiting herbicides. Weed Res. 2001, 41, 509–522. [Google Scholar] [CrossRef]
- Yu, Q.; Powles, S. Metabolism-based herbicide resistance and cross-resistance in crop weeds: A threat to herbicide sustainability and global crop production. Plant Physiol. 2014, 166, 1106–1118. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, X.Q.; Hashem, A.; Walsh, M.J.; Powles, S.B. ALS gene proline (197) mutations confer ALS herbicide resistance in eight separated wild radish (Raphanus raphanistrum) populations. Weed Sci. 2003, 51, 831–838. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Online. Available online: http://www.weedscience.org (accessed on 6 November 2024).
- Marshall, R.; Moss, S.R. Characterisation and molecular basis of ALS inhibitor resistance in the grass weed Alopecurus myosuroides. Weed Res. 2008, 48, 439–447. [Google Scholar] [CrossRef]
- Ashigh, J.; Corbett, C.A.L.; Smith, P.J.; Laplante, J.; Tardif, F.J. Characterization and diagnostic tests of resistance to acetohydroxyacid synthase inhibitors due to an Asp376Glu substitution in Amaranthus powellii. Pestic. Biochem. Physiol. 2009, 95, 38–46. [Google Scholar] [CrossRef]
- Pandolfo, C.E.; Presotto, A.; Moreno, F.; Dossou, I.; Migasso, J.P.; Sakima, E.; Cantamutto, M. Broad resistance to acetohydroxyacid-synthase-inhibiting herbicides in feral radish (Raphanus sativus L.) populations from Argentina. Pest Manag. Sci. 2016, 72, 354–361. [Google Scholar] [CrossRef]
- Mengistu, L.W.; Mueller-Warrant, G.W.; Liston, A.; Barker, R.E. PsbA mutation (valine219 to isoleucine) in Poa annua resistant to metribuzin and diuron. Pest Manag. Sci. Former. Pestic. Sci. 2000, 56, 209–217. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed]
- Qiang, S. Weed Science, 2nd ed.; China Agricultural Press: Beijing, China, 2009. [Google Scholar]
- Feng, L. Summarization of application technology of chemical herbicides in soybean field. Mod. Agric. 2019, 2, 5–6. [Google Scholar]
- Li, D. Occurrence period and control techniques of weeds in soybean field in Heihe City. Soybean Sci. Technol. 2017, 3547, 3–4. [Google Scholar]
- Li, W. Studies on Damage Investigation and Chemical Control of Soybean Weeds in the North of Heilongjiang Province. Master’s Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2014. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yu, Q.; Friesen, L.S.; Zhang, X.Q.; Powles, S.B. Tolerance to acetolactate synthase and acetyl-coenzyme A carboxylase inhibiting herbicides in Vulpia bromoides is conferred by two co-existing resistance mechanisms. Pestic. Biochem. Physiol. 2004, 78, 21–30. [Google Scholar] [CrossRef]
- Tehranchian, P.; Nandula, V.K.; Matzrafi, M.; Jasieniuk, M. Multiple herbicide resistance in California Italian ryegrass (Lolium perenne ssp. multiflorum): Characterization of ALS-inhibiting herbicide resistance. Weed Sci. 2019, 67, 273–280. [Google Scholar] [CrossRef]
- Mikulka, J.; Sen, M.K.; Košnarová, P.; Hamouz, P.; Hamouzová, K.; Sur, V.P.; Šuk, J.; Bhattacharya, S.; Soukup, J. Molecular Mechanisms of Resistance against PSII-Inhibiting Herbicides in Amaranthus retroflexus from the Czech Republic. Genes 2024, 15, 904. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R. Resistance of weeds to ALS-inhibiting herbicides: What have we learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, X.; Zhang, C.; Jiang, C.; Huang, H.; Wei, S. First report of molecular basis of resistance to imazethapyr in common lambsquarters (Chenopodium album). Weed Sci. 2020, 68, 63–68. [Google Scholar] [CrossRef]
- Délye, C. Unravelling the genetic bases of non-target-site-based resistance (NTSR) to herbicides: A major challenge for weed science in the forthcoming decade. Pest Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Zulfiqar, U.; Sadia, S.; Bhowmik, P.; Chauhan, B.S. A global perspective on the biology, impact and management of Chenopodium album and Chenopodium murale: Two troublesome agricultural and environmental weeds. Environ. Sci. Pollut. Res. 2019, 26, 5357–5371. [Google Scholar] [CrossRef] [PubMed]
- Rigon, C.A.; Gaines, T.A.; Küpper, A.; Dayan, F.E. Metabolism-based herbicide resistance, the major threat among the non-target site resistance mechanisms. Outlooks Pest Manag. 2020, 31, 162–168. [Google Scholar] [CrossRef]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of evolved herbicide resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef] [PubMed]
- Ghanizadeh, H.; Mesarich, C.H.; Harrington, K.C. Molecular characteristics of the first case of haloxyfop-resistant Poa annua. Sci. Rep. 2020, 10, 4231. [Google Scholar] [CrossRef] [PubMed]
- Ganie, Z.A.; Jhala, A.J. Glyphosate-resistant common ragweed (Ambrosia artemisiifolia) in Nebraska: Confirmation and response to postemergence corn and soybean herbicides. Weed Technol. 2017, 31, 225–237. [Google Scholar] [CrossRef]
- Liu, X.; Merchant, A.; Xiang, S. Managing herbicide resistance in China. Weed Sci. 2021, 69, 4–17. [Google Scholar] [CrossRef]
- Owen, M.D.K. World maize/soybean and herbicide resistance. In Herbicide Resistance and World Grains; CRC Press: Boca Raton, FL, USA, 2001; pp. 101–163. [Google Scholar] [CrossRef]
- Milani, A. Co-Existence of ALS-Resistant Amaranthus Species in North-Eastern Italy: How to Manage Them. Ph.D. Thesis, University of Padova, Padova, Italy, 2019. [Google Scholar]
- Vrbničanin, S.; Pavlović, D.; Božić, D. Weed resistance to herbicides. In Herbicide Resistance in Weeds and Crops; IntechOpen: London, UK, 2017; pp. 7–35. [Google Scholar] [CrossRef][Green Version]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Mechant, E. Metamitron Resistant Chenopodium album: Characterisation, Detection and Distribution in Belgian Sugar Beet. Ph.D. Thesis, Ghent University, Gent, Belgium, 2011. [Google Scholar]
| Primers | Sequence (5′-3′) | Amplicon | Tm (°C) | Purpose of |
|---|---|---|---|---|
| Length (bp) | Primers | |||
| psbA-680F | TTTCCGTCTGGGTATGCGTC | 680 | 58.1 | Amplify the psbA |
| psbA-680R | AGATGGAGCTTCGATAGCGG | |||
| psbA-182F | AACCGGAGCCGAATATGCAA | 182 | 56.3 | qRT–PCR analysis of psbA gene |
| psbA-182R | GTGGTTATACAACGGTGGTCCT | |||
| β-actin F | TCCATAATGAAGTGTGATGT | 129 | 57.5 | qRT–PCR analysis of β-actin gene |
| β-actin R | GGACCTGACTCGTCATACTC | |||
| ALS-853F | TTTGCCCCTGATGAACCC | 853 | 58.1 | Amplify the ALS |
| ALS-853R | ACAAAAAAGAGCCAACGAG | |||
| ALS-798F | AGCAGATTGTGAGGTTGATGAG | 798 | 57.2 | |
| ALS-798R | TAACAAAAAAGAGCCAACGAG | |||
| ALS-179F | GATAACCAGCACTGAAAACC | 179 | 55.7 | qRT–PCR analysis of ALS gene |
| ALS-179R | ATTGGAAGATCAATCGACC | |||
| β-tub F | CGTAAGCTTGCTGTGAATCTCATC | 133 | 56.4 | qRT–PCR analysis of β-tub gene |
| β-tub R | CTGCTCGTCAACTTCCTTTGTG |
| Target | Herbicide a | Rate Applied (g a.i. ha−1) |
|---|---|---|
| ALS | Cloransulam-methyl [Methyl 2-chloro-5-ethoxycarbonyl-6-methoxyanthranilate 85% WP] | 25.2 50.4 |
| Flumetsulam [N-(2,6-difluorophenyl)-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine-2-sulfonamide 80% WP] | 48.0 96.0 | |
| PPO | Acifluorfen [5-[2-chloro-4-(trifluoromethyl)phenoxy]-2-nitrobenzoic acid 25% SL] | 384.0 774.0 |
| Fomesafen [5-[2-chloro-4-(trifluoromethyl)phenoxy]-N-(methylsulfonyl)-2-nitrobenzamide 25% SL] | 450.0 900.0 | |
| Oxyfluorfen [2-chloro-1-(3-ethoxy-4-nitrophenoxy)-4-(trifluoromethyl)benzene 240 g/L EC] | 900.0 1800.0 |
| Population | Thifensulfuron-Methyl | ||||||
|---|---|---|---|---|---|---|---|
| GR50 a (g a.i. ha −1) | RI b | Mix | Max | Hillslope | R2 | Adjust R2 | |
| S | 11.15 ± 1.61 | - | 19.4922 | 99.4435 | −1.3612 | 0.99 | 0.99 |
| R1 | 58.95 ± 10.55 | 5.28 | 2.0991 | 92.2802 | −1.1605 | 0.99 | 0.99 |
| R2 | 72.59 ± 13.34 | 6.51 | 1.3732 | 90.4761 | −1.1666 | 0.99 | 0.99 |
| Bentazone | |||||||
| S | 41.92 ± 5.33 | - | 11.8279 | 97.6648 | −1.177 | 0.99 | 0.99 |
| R1 | 202.68 ± 25.03 | 4.83 | 17.2614 | 89.1525 | −1.3197 | 0.99 | 0.99 |
| R2 | 213.65 ± 33.38 | 5.1 | 10.1247 | 99.6071 | −1.2267 | 0.99 | 0.99 |
| Herbicide | Populations | GR50 (g a.i. ha−1) | RIs |
|---|---|---|---|
| Cloransulam-methyl | R1 | 27.82 | 1.47 |
| R2 | 21.92 | 1.16 | |
| S | 18.95 | - | |
| Flumetsulam | R1 | 21.54 | 1.30 |
| R2 | 18.24 | 1.11 | |
| S | 16.57 | - | |
| Acifluorfen | R1 | 674.12 | 2.44 |
| R2 | 604.90 | 2.19 | |
| S | 276.21 | - | |
| Fomesafen | R1 | 557.19 | 1.54 |
| R2 | 498.37 | 1.38 | |
| S | 362.11 | - | |
| Oxyfluorfen | R1 | 373.44 | 4.74 |
| R2 | 315.38 | 4.01 | |
| S | 78.82 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Huang, L.; Li, Y.; Wang, R.; Hu, S.; Huang, Z. Multiple Resistance to PS II-Inhibiting and ALS-Inhibiting Herbicides in Common Lambsquarters (Chenopodium album L.) from China. Agronomy 2025, 15, 1309. https://doi.org/10.3390/agronomy15061309
Li J, Huang L, Li Y, Wang R, Hu S, Huang Z. Multiple Resistance to PS II-Inhibiting and ALS-Inhibiting Herbicides in Common Lambsquarters (Chenopodium album L.) from China. Agronomy. 2025; 15(6):1309. https://doi.org/10.3390/agronomy15061309
Chicago/Turabian StyleLi, Jinglin, Lichun Huang, Yue Li, Ruolin Wang, Shenao Hu, and Zhaofeng Huang. 2025. "Multiple Resistance to PS II-Inhibiting and ALS-Inhibiting Herbicides in Common Lambsquarters (Chenopodium album L.) from China" Agronomy 15, no. 6: 1309. https://doi.org/10.3390/agronomy15061309
APA StyleLi, J., Huang, L., Li, Y., Wang, R., Hu, S., & Huang, Z. (2025). Multiple Resistance to PS II-Inhibiting and ALS-Inhibiting Herbicides in Common Lambsquarters (Chenopodium album L.) from China. Agronomy, 15(6), 1309. https://doi.org/10.3390/agronomy15061309





