Synergistic Effects of Compost and Biochar on Soil Health and Heavy Metal Stabilization in Contaminated Mine Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Mine Tailings Samples
2.2. Origin of Amendments
2.3. Phytotoxicity Bioassay Using Phaseolus vulgaris
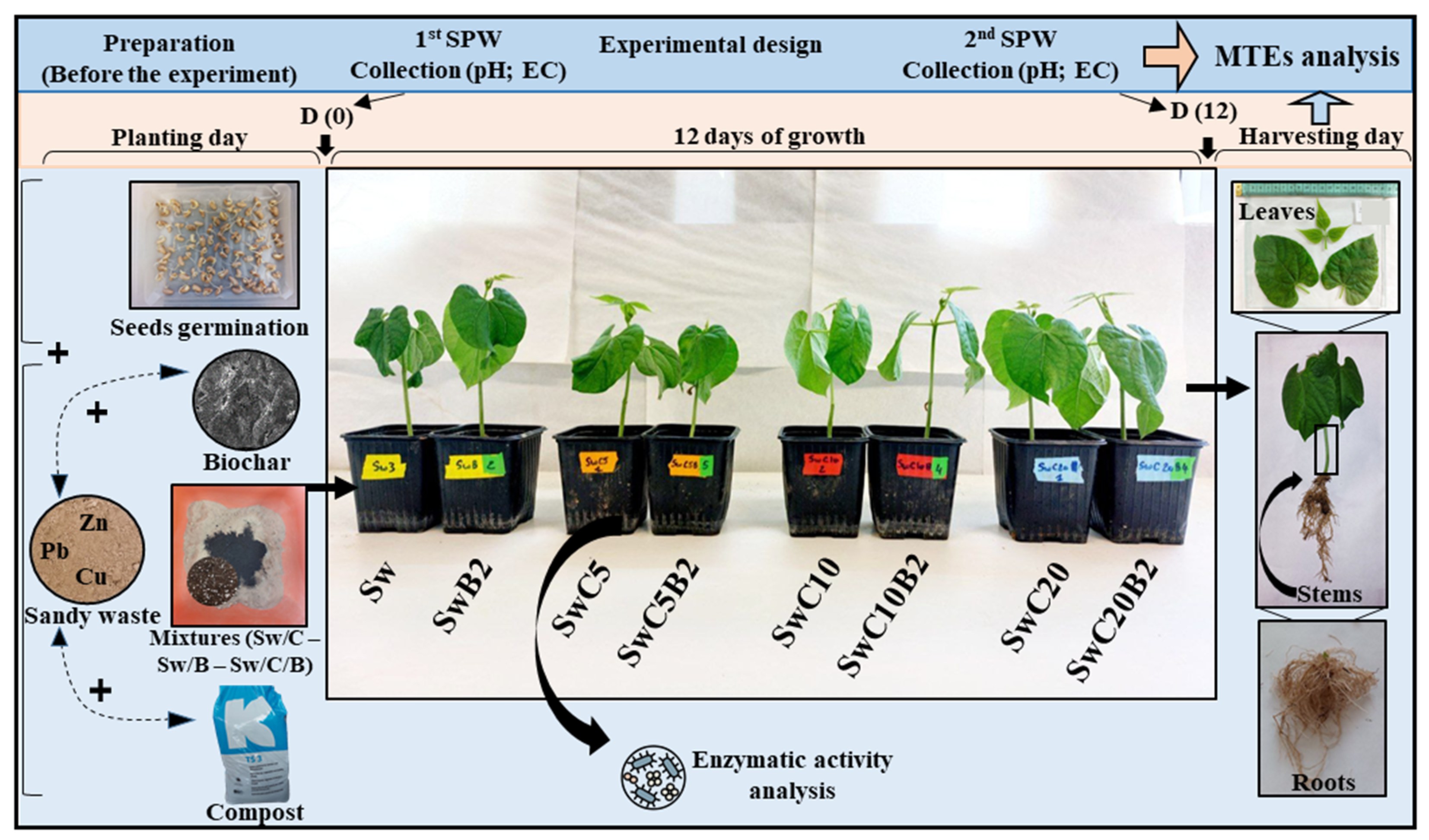
2.3.1. Soil Preparation and Treatments
2.3.2. Experimental Design
2.3.3. Soil Pore Water
2.3.4. Metals Concentration Analysis
2.3.5. Biogeochemical Index
2.3.6. Soil Enzymatic Activities
2.4. Data Analysis
3. Results and Discussion
3.1. Soil Basic Physicochemical Characteristics
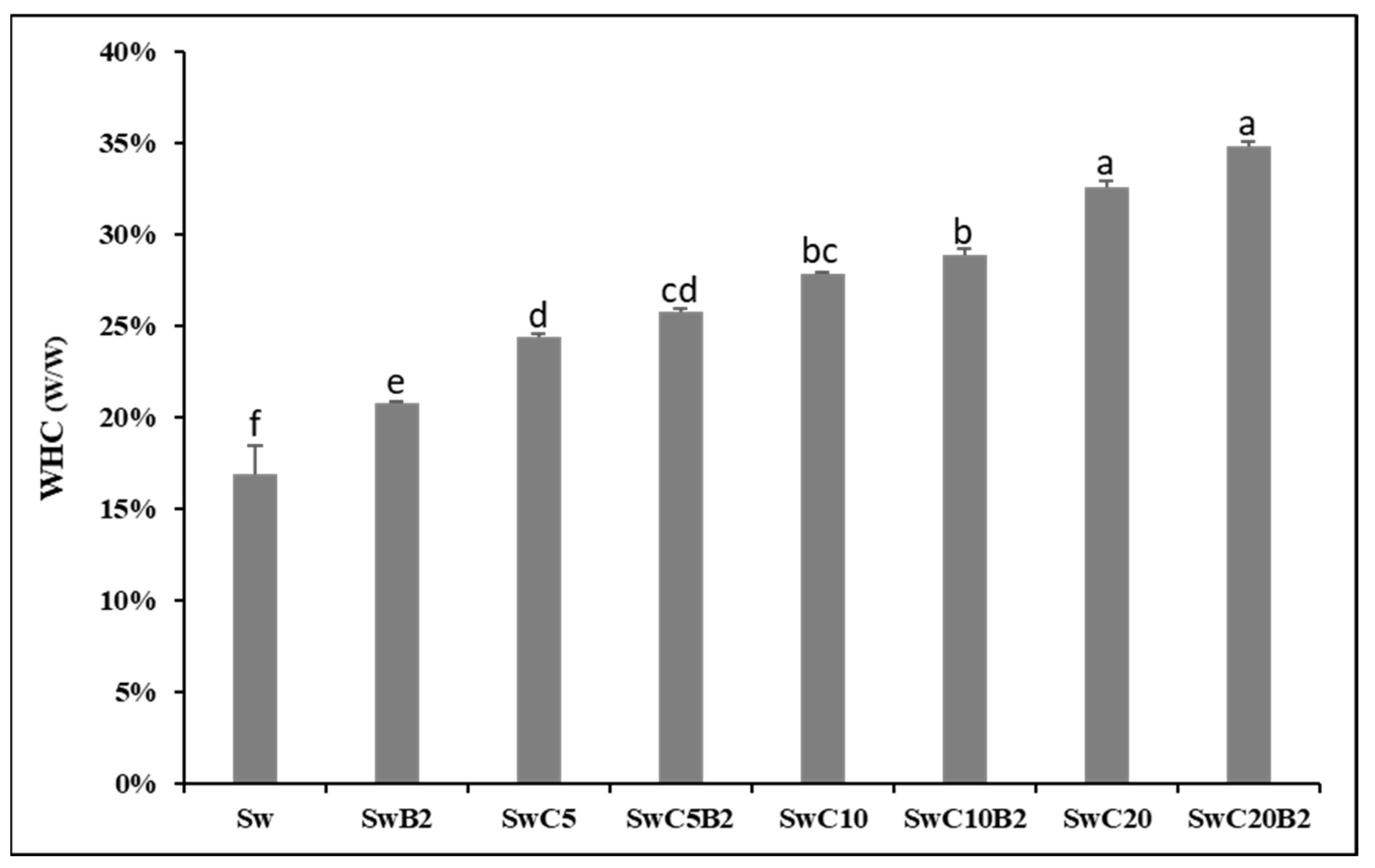
3.1.1. Substrates Water Holding Capacity (WHC)
3.1.2. Soil Pore Water (SPW) pH and Electrical Conductivity (EC)
3.1.3. SPW Metal Concentrations
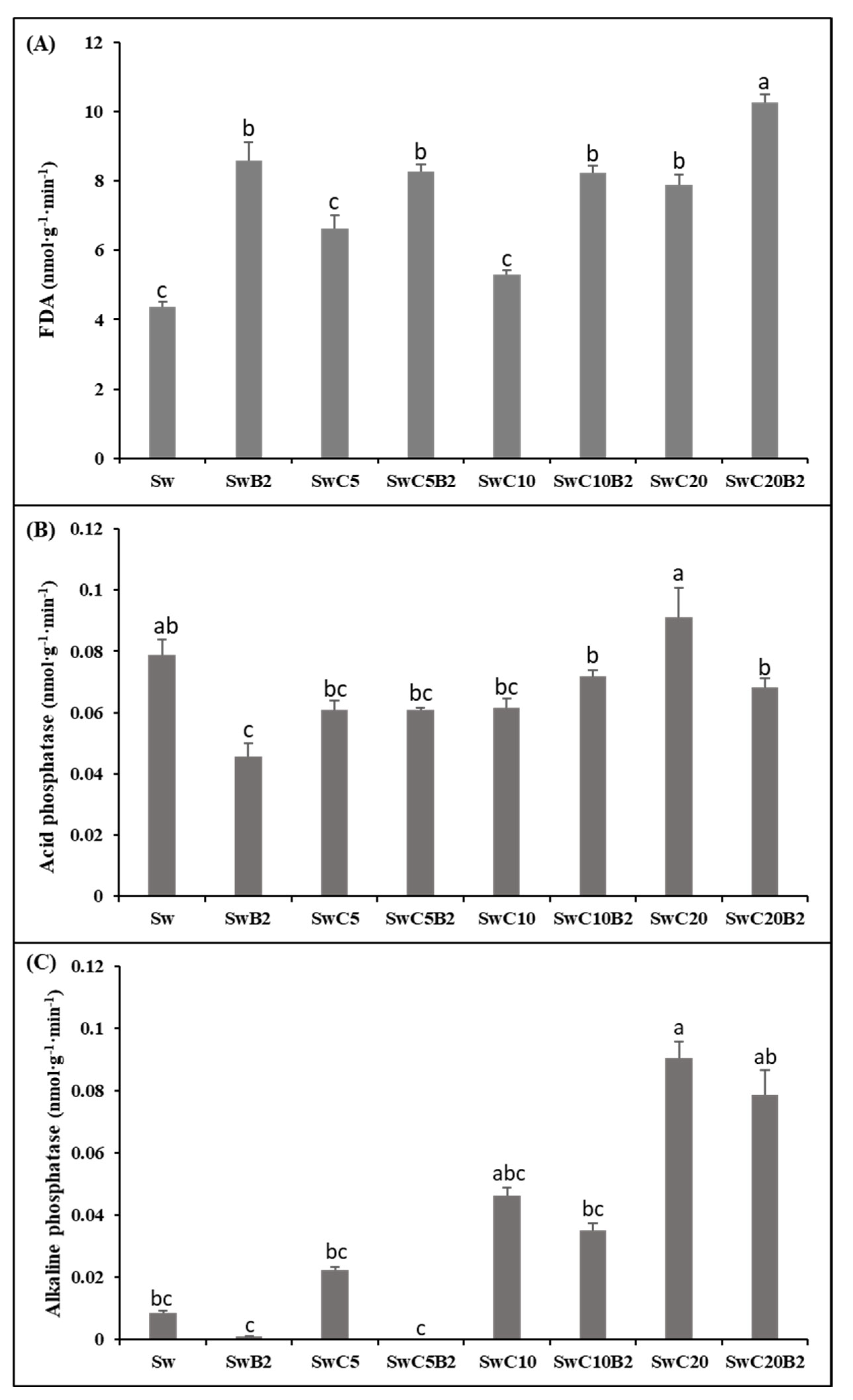
3.2. Substrates Enzymatic Activities
3.3. Plant Growth Characteristics
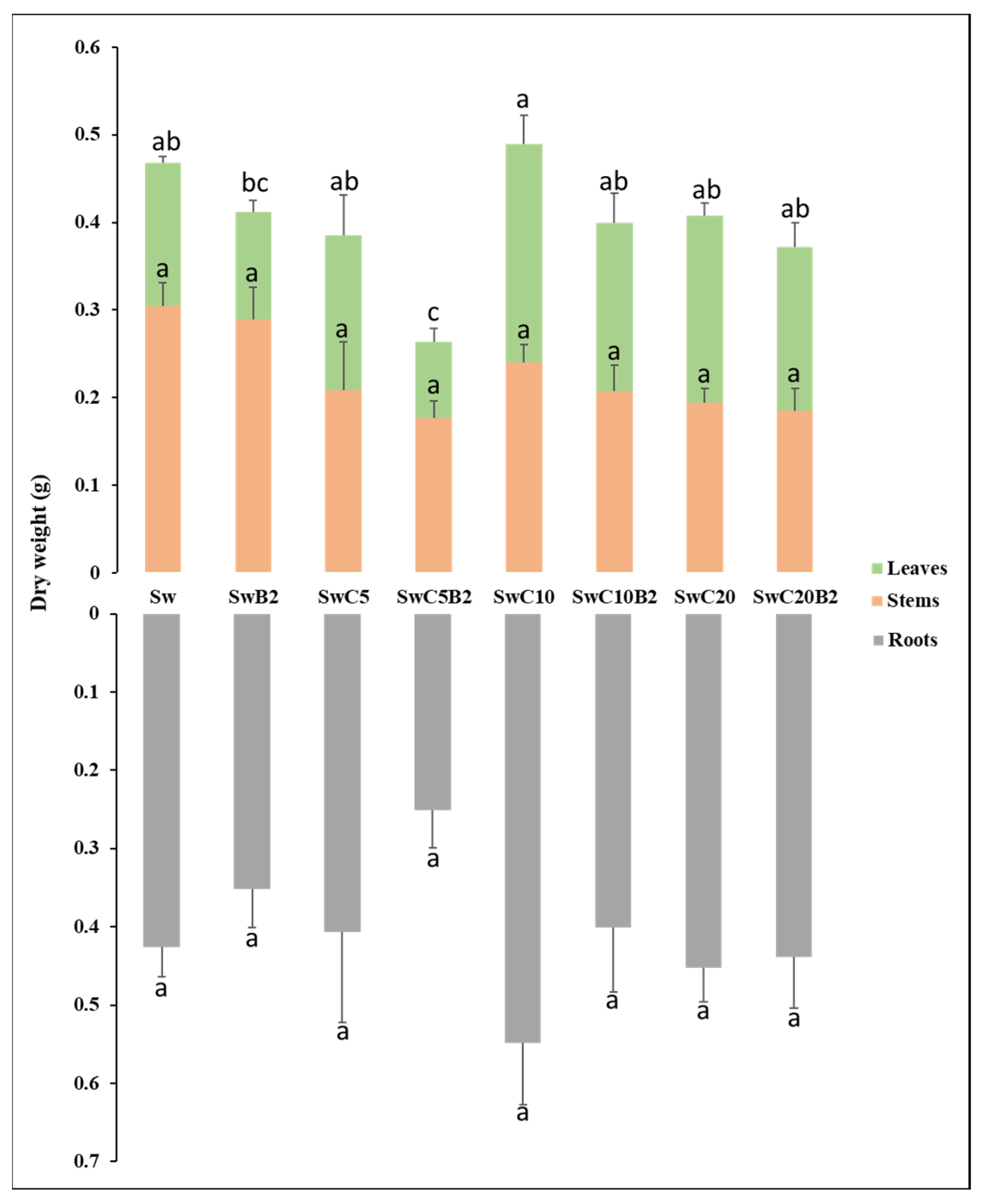
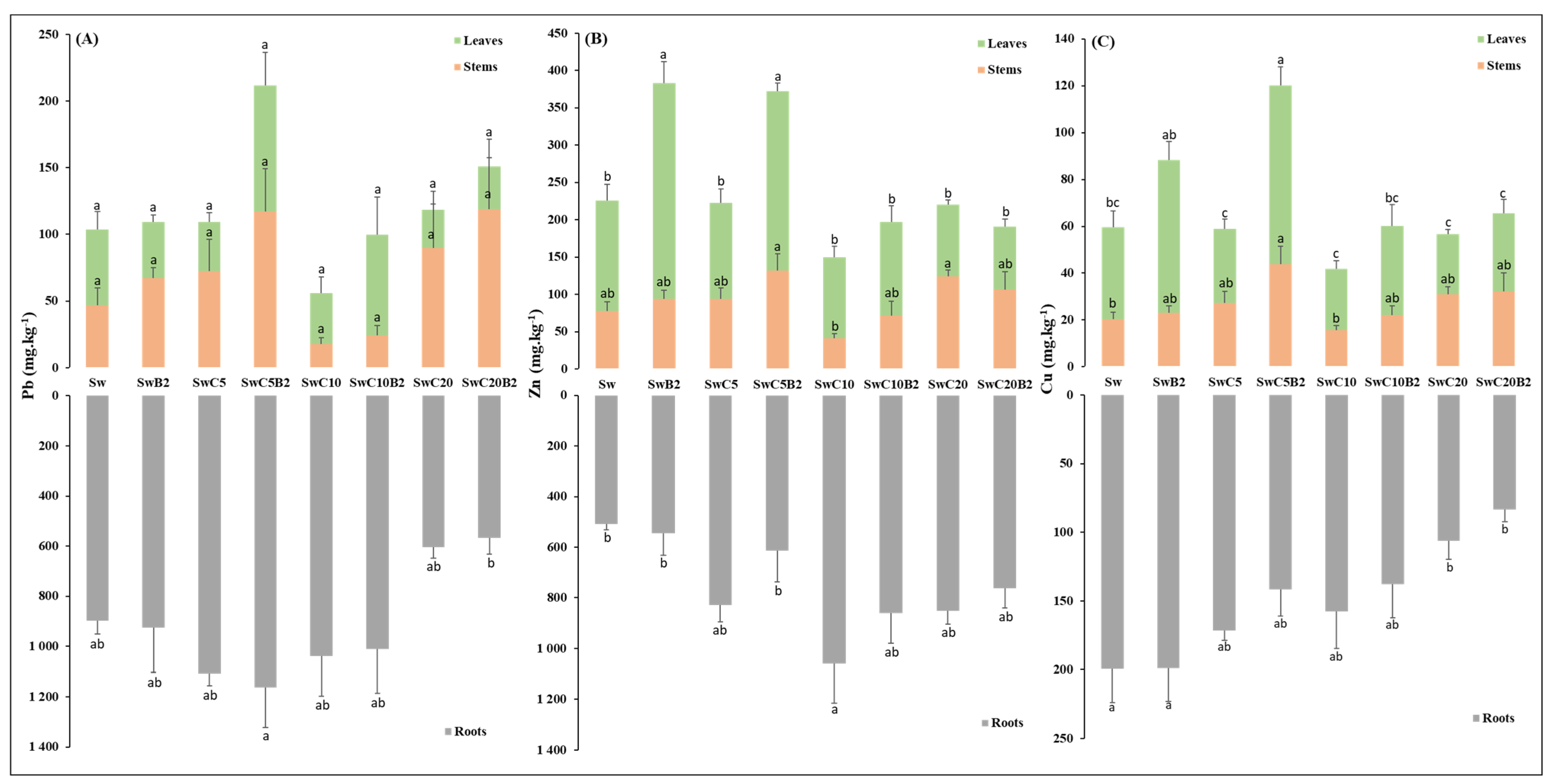
3.3.1. Dry Weight (DW) and Metal Accumulation in Phaseolus vulgaris
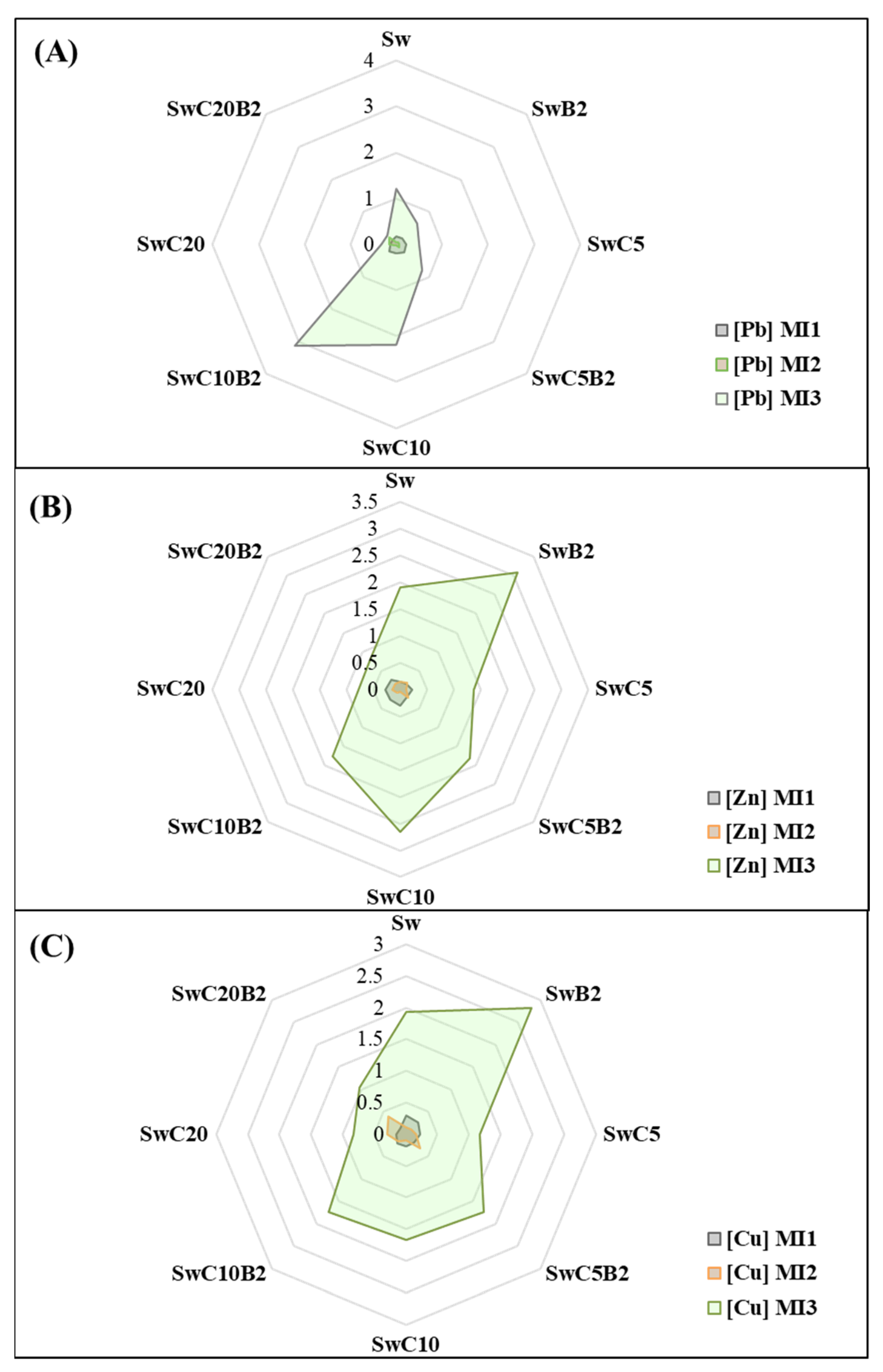
3.3.2. Mobility Index of Metals
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fayiah, M. Mining and Environmental Degradation: A Gift Brings Grief Scenario for Mining Communities in Sierra Leone. J. Min. Environ. 2020, 11, 347–361. [Google Scholar] [CrossRef]
- Tepanosyan, G.; Sahakyan, L.; Belyaeva, O.; Asmaryan, S.; Saghatelyan, A. Continuous Impact of Mining Activities on Soil Heavy Metals Levels and Human Health. Sci. Total Environ. 2018, 639, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Sincovich, A.; Gregory, T.; Wilson, A.; Brinkman, S. The Social Impacts of Mining on Local Communities in Australia. Rural. Soc. 2018, 27, 18–34. [Google Scholar] [CrossRef]
- Worlanyo, A.S.; Jiangfeng, L. Evaluating the Environmental and Economic Impact of Mining for Post-Mined Land Restoration and Land-Use: A Review. J. Environ. Manag. 2021, 279, 111623. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, P.; Jeyakumar, P.; Bolan, N.; Wang, H.; Gao, B.; Wang, S.; Wang, B. Biochar as a Potential Strategy for Remediation of Contaminated Mining Soils: Mechanisms, Applications, and Future Perspectives. J. Environ. Manag. 2022, 313, 114973. [Google Scholar] [CrossRef]
- Kan, X.; Dong, Y.; Feng, L.; Zhou, M.; Hou, H. Contamination and Health Risk Assessment of Heavy Metals in China’s Lead–Zinc Mine Tailings: A Meta–Analysis. Chemosphere 2021, 267, 128909. [Google Scholar] [CrossRef]
- Qian, L.; Lin, H.; Li, B.; Dong, Y. Physicochemical Characteristics and Microbial Communities of Rhizosphere in Complex Amendment-Assisted Soilless Revegetation of Gold Mine Tailings. Chemosphere 2023, 320, 138052. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Southam, G.; Robertson, L.; Chiu, T.H.; Cross, A.T.; Dixon, K.W.; Stevens, J.C.; Zhong, H.; Chan, T.-S.; et al. Geochemical and Mineralogical Constraints in Iron Ore Tailings Limit Soil Formation for Direct Phytostabilization. Sci. Total Environ. 2019, 651, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Madline, A.; Benidire, L.; Pereira, S.I.A.; Khalil, H.E.; Michalski, A.; Castro, P.M.L.; Charzyński, P.; Boularbah, A. Optimizing Biological and Physicochemical Properties of Acidic Mine Tailings through Combined Organo-Mineral Amendments and Topsoil Application. J. Soils Sediments 2025, 25, 609–624. [Google Scholar] [CrossRef]
- Morrison, K.F. Tailings Management Handbook: A LifeCycle Approach; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 2022; ISBN 978-0-87335-490-5. [Google Scholar]
- Araujo, F.S.M.; Taborda-Llano, I.; Nunes, E.B.; Santos, R.M. Recycling and Reuse of Mine Tailings: A Review of Advancements and Their Implications. Geosciences 2022, 12, 319. [Google Scholar] [CrossRef]
- Raffa, C.M.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Hassan, S.; Bhadwal, S.S.; Khan, M.; Sabreena; Nissa, K.-U.; Shah, R.A.; Bhat, H.M.; Bhat, S.A.; Lone, I.M.; Ganai, B.A. Revitalizing Contaminated Lands: A State-of-the-Art Review on the Remediation of Mine-Tailings Using Phytoremediation and Genomic Approaches. Chemosphere 2024, 356, 141889. [Google Scholar] [CrossRef] [PubMed]
- Gotore, O.; Watanabe, M.; Okano, K.; Miyata, N.; Katayama, T.; Yasutaka, T.; Semoto, Y.; Hamai, T. Effects of Batch and Continuous-Flow Operation on Biotreatment of Mn(II)-Containing Mine Drainage. J. Environ. Sci. 2025, 152, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Chafik, Y.; Sena-Velez, M.; Henaut, H.; Oujdi, M.; Ceriani, A.; Carpin, S.; Morabito, D.; Bourgerie, S. Phytotoxicity and Metals Mobility Assessment in Mining Wastes Amended with Various Biochars. Land 2025, 14, 372. [Google Scholar] [CrossRef]
- Jakovljević, K.; Ranđelović, D.; Mišljenović, T. Phytoremediation of Mine Waste Disposal Sites: Current State of Knowledge and Examples of Good Practice. In Biotechnology for Sustainable Environment; Springer: Berlin/Heidelberg, Germany, 2021; pp. 223–250. ISBN 978-981-16-1955-7. [Google Scholar]
- Huang, M.; Liu, Z.; Li, X. Phytoremediation of Rare Tailings-Contaminated Soil. J. Renew. Mater. 2022, 10, 3351–3372. [Google Scholar] [CrossRef]
- Peco, J.D.; Higueras, P.; Campos, J.A.; Esbrí, J.M.; Moreno, M.M.; Battaglia-Brunet, F.; Sandalio, L.M. Abandoned Mine Lands Reclamation by Plant Remediation Technologies. Sustainability 2021, 13, 6555. [Google Scholar] [CrossRef]
- Rahman, R.A.; Wintoko, J.; Prasetya, A. Comparison of Different Phytoremediation Strategies for Acid Mine Drainage (AMD). IOP Conf. Ser. Earth Environ. Sci. 2022, 963, 012040. [Google Scholar] [CrossRef]
- Xie, L.; van Zyl, D. Distinguishing Reclamation, Revegetation and Phytoremediation, and the Importance of Geochemical Processes in the Reclamation of Sulfidic Mine Tailings: A Review. Chemosphere 2020, 252, 126446. [Google Scholar] [CrossRef]
- Shackira, A.M.; Puthur, J.T. Phytostabilization of Heavy Metals: Understanding of Principles and Practices. In Plant-Metal Interactions; Srivastava, S., Srivastava, A.K., Suprasanna, P., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 263–282. ISBN 978-3-030-20732-8. [Google Scholar]
- Zine, H.; Midhat, L.; Hakkou, R.; El Adnani, M.; Ouhammou, A. Guidelines for a Phytomanagement Plan by the Phytostabilization of Mining Wastes. Sci. Afr. 2020, 10, e00654. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Mourinha, C.; Palma, P.; Alexandre, C.; Cruz, N.; Rodrigues, S.M.; Alvarenga, P. Potentially Toxic Elements’ Contamination of Soils Affected by Mining Activities in the Portuguese Sector of the Iberian Pyrite Belt and Optional Remediation Actions: A Review. Environments 2022, 9, 11. [Google Scholar] [CrossRef]
- Ramírez-Zamora, J.; Mussali-Galante, P.; Rodríguez, A.; Castrejón-Godínez, M.L.; Valencia-Cuevas, L.; Tovar-Sánchez, E. Assisted Phytostabilization of Mine-Tailings with Prosopis Laevigata (Fabaceae) and Biochar. Plants 2022, 11, 3441. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Miard, F.; Nandillon, R.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Biochar Effect Associated with Compost and Iron to Promote Pb and As Soil Stabilization and Salix Viminalis L. Growth. Chemosphere 2019, 222, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Nandillon, R.; Lebrun, M.; Miard, F.; Gaillard, M.; Sabatier, S.; Villar, M.; Bourgerie, S.; Morabito, D. Capability of Amendments (Biochar, Compost and Garden Soil) Added to a Mining Technosol Contaminated by Pb and As to Allow Poplar Seed (Populus nigra L.) Germination. Environ. Monit. Assess. 2019, 191, 465. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, X.; Tian, C.; Zhang, X.; Liu, J. Effects of Amendments and Aided Phytostabilization of an Energy Crop on the Metal Availability and Leaching in Mine Tailings Using a Pot Test. Environ. Sci. Pollut. Res. 2020, 27, 2745–2759. [Google Scholar] [CrossRef]
- Garau, M.; Castaldi, P.; Diquattro, S.; Pinna, M.V.; Senette, C.; Roggero, P.P.; Garau, G. Combining Grass and Legume Species with Compost for Assisted Phytostabilization of Contaminated Soils. Environ. Technol. Innov. 2021, 22, 101387. [Google Scholar] [CrossRef]
- Lebrun, M.; Michel, C.; Joulian, C.; Morabito, D.; Bourgerie, S. Rehabilitation of Mine Soils by Phytostabilization: Does Soil Inoculation with Microbial Consortia Stimulate Agrostis Growth and Metal(Loid) Immobilization? Sci. Total Environ. 2021, 791, 148400. [Google Scholar] [CrossRef]
- Shahrokh, V.; Martínez-Martínez, S.; Faz, Á.; Zornoza, R.; Acosta, J.A. Efficiency of Large-Scale Aided Phytostabilization in a Mining Pond. Environ. Geochem. Health 2023, 45, 4665–4677. [Google Scholar] [CrossRef]
- Altıkat, A.; Alma, M.H.; Altıkat, A.; Bilgili, M.E.; Altıkat, S. A Comprehensive Study of Biochar Yield and Quality Concerning Pyrolysis Conditions: A Multifaceted Approach. Sustainability 2024, 16, 937. [Google Scholar] [CrossRef]
- Wang, J.; Shi, L.; Zhai, L.; Zhang, H.; Wang, S.; Zou, J.; Shen, Z.; Lian, C.; Chen, Y. Analysis of the Long-Term Effectiveness of Biochar Immobilization Remediation on Heavy Metal Contaminated Soil and the Potential Environmental Factors Weakening the Remediation Effect: A Review. Ecotoxicol. Environ. Saf. 2021, 207, 111261. [Google Scholar] [CrossRef]
- Ghosh, D.; Maiti, S.K. Biochar Assisted Phytoremediation and Biomass Disposal in Heavy Metal Contaminated Mine Soils: A Review. Int. J. Phytoremediation 2021, 23, 559–576. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; An, Q.; Syafika Mohd Nasir, A.; Babin, A.; Lucero Saucedo, S.; Vallenas, A.; Li, L.; Baldwin, S.A.; Lau, A.; Bi, X. Emerging Applications of Biochar: A Review on Techno-Environmental-Economic Aspects. Bioresour. Technol. 2023, 388, 129745. [Google Scholar] [CrossRef] [PubMed]
- Bolan, N.; Sarmah, A.K.; Bordoloi, S.; Bolan, S.; Padhye, L.P.; Van Zwieten, L.; Sooriyakumar, P.; Khan, B.A.; Ahmad, M.; Solaiman, Z.M.; et al. Soil Acidification and the Liming Potential of Biochar. Environ. Pollut. 2023, 317, 120632. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cen, B.; Yu, Z.; Qiu, R.; Gao, T.; Long, X. The Key Role of Biochar in Amending Acidic Soil: Reducing Soil Acidity and Improving Soil Acid Buffering Capacity. Biochar 2025, 7, 52. [Google Scholar] [CrossRef]
- Serra-Ventura, J.; Rasero-López, S.; Romera-Miró, M.; Vidal, M.; Rigol, A. Biochar as a Sustainable Sorbent for the Removal of Lanthanides from Acid Mine Drainage. Chemosphere 2025, 380, 144448. [Google Scholar] [CrossRef]
- Chafik, Y.; Hassan, S.H.; Lebrun, M.; Sena-Velez, M.; Cagnon, B.; Carpin, S.; Boukroute, A.; Bourgerie, S.; Morabito, D. Biochar Characteristics and Pb2+/Zn2+ Sorption Capacities: The Role of Feedstock Variation. Int. J. Environ. Sci. Technol. 2024, 21, 9829–9842. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Env. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Rodriguez-Franco, C.; Page-Dumroese, D.S. Woody Biochar Potential for Abandoned Mine Land Restoration in the U.S.: A Review. Biochar 2021, 3, 7–22. [Google Scholar] [CrossRef]
- Liu, M.; Almatrafi, E.; Zhang, Y.; Xu, P.; Song, B.; Zhou, C.; Zeng, G.; Zhu, Y. A Critical Review of Biochar-Based Materials for the Remediation of Heavy Metal Contaminated Environment: Applications and Practical Evaluations. Sci. Total Environ. 2022, 806, 150531. [Google Scholar] [CrossRef]
- Du, T.; Bogush, A.; Mašek, O.; Purton, S.; Campos, L.C. Algae, Biochar and Bacteria for Acid Mine Drainage (AMD) Remediation: A Review. Chemosphere 2022, 304, 135284. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Tiwari, A.K. The Role of Modified Biochar for the Remediation of Coal Mining-Impacted Contaminated Soil: A Review. Sustainability 2023, 15, 3973. [Google Scholar] [CrossRef]
- Frimpong, K.A.; Abban-Baidoo, E.; Marschner, B. Can Combined Compost and Biochar Application Improve the Quality of a Highly Weathered Coastal Savanna Soil? Heliyon 2021, 7, e07089. [Google Scholar] [CrossRef]
- Karer, J.; Zehetner, F.; Dunst, G.; Fessl, J.; Wagner, M.; Puschenreiter, M.; Stapkēviča, M.; Friesl-Hanl, W.; Soja, G. Immobilisation of Metals in a Contaminated Soil with Biochar-Compost Mixtures and Inorganic Additives: 2-Year Greenhouse and Field Experiments. Environ. Sci. Pollut. Res. 2018, 25, 2506–2516. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical Features, Metal Availability and Enzyme Activity in Heavy Metal-Polluted Soil Remediated by Biochar and Compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef]
- Kranz, C.N.; McLaughlin, R.A.; Johnson, A.; Miller, G.; Heitman, J.L. The Effects of Compost Incorporation on Soil Physical Properties in Urban Soils—A Concise Review. J. Environ. Manag. 2020, 261, 110209. [Google Scholar] [CrossRef]
- Głąb, T.; Żabiński, A.; Sadowska, U.; Gondek, K.; Kopeć, M.; Mierzwa-Hersztek, M.; Tabor, S.; Stanek-Tarkowska, J. Fertilization Effects of Compost Produced from Maize, Sewage Sludge and Biochar on Soil Water Retention and Chemical Properties. Soil Tillage Res. 2020, 197, 104493. [Google Scholar] [CrossRef]
- Lebrun, M.; Nandillon, R.; Miard, F.; Bourgerie, S.; Morabito, D. Chapter 4—Biochar Assisted Phytoremediation for Metal(Loid) Contaminated Soils. In Assisted Phytoremediation; Pandey, V., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–130. ISBN 978-0-12-822893-7. [Google Scholar]
- Mukhopadhyay, S.; Masto, R.E.; Singh, A.K.; Singh, P.K. Impact of the Combined Application of Biochar and Compost on Mine Soil Quality and Growth of Lady’s Finger (Abelmoschus Esculentus). Bull. Environ. Contam. Toxicol. 2022, 108, 396–402. [Google Scholar] [CrossRef]
- Singh, E.; Mishra, R.; Kumar, A.; Shukla, S.K.; Lo, S.-L.; Kumar, S. Circular Economy-Based Environmental Management Using Biochar: Driving towards Sustainability. Process Saf. Environ. Prot. 2022, 163, 585–600. [Google Scholar] [CrossRef]
- Gao, S.; Medina, M.; Gonzalez-Ospina, L.; Burce, K.; Burce, K.; Melbourne, A. Boosting Soil Health and Crop Nutrients with Locally Sourced Biochar and Compost in Sacramento Urban Agriculture. Front. Sustain. Food Syst. 2025, 9, 1546426. [Google Scholar] [CrossRef]
- Li, S. Reviewing Air Pollutants Generated during the Pyrolysis of Solid Waste for Biofuel and Biochar Production: Toward Cleaner Production Practices. Sustainability 2024, 16, 1169. [Google Scholar] [CrossRef]
- El Aallaoui, A.; El Ghorfi, M.; Elghali, A.; Taha, Y.; Zine, H.; Benzaazoua, M.; Hakkou, R. Investigating the Reprocessing Potential of Abandoned Zinc-Lead Tailings Ponds: A Comprehensive Study Using Physicochemical, Mineralogical, and 3D Geometallurgical Assessments. Miner. Eng. 2024, 209, 108634. [Google Scholar] [CrossRef]
- Oujdi, M.; Chafik, Y.; Boukroute, A.; Bourgerie, S.; Sena-Velez, M.; Morabito, D.; Addi, M. Exploring Phytoremediation Potential: A Comprehensive Study of Flora Inventory and Soil Heavy Metal Contents in the Northeastern Mining Districts of Morocco. Plants 2024, 13, 1811. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Miard, F.; Hattab-Hambli, N.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Effect of Different Tissue Biochar Amendments on As and Pb Stabilization and Phytoavailability in a Contaminated Mine Technosol. Sci. Total Environ. 2020, 707, 135657. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Nandillon, R.; Hattab-Hambli, N.; Léger, J.C.; Scippa, G.S.; Morabito, D.; Bourgerie, S. Influence of Biochar Particle Size and Concentration on Pb and As Availability in Contaminated Mining Soil and Phytoremediation Potential of Poplar Assessed in a Mesocosm Experiment. Water Air Soil. Pollut. 2020, 232, 3. [Google Scholar] [CrossRef]
- Lebrun, M.; Miard, F.; Trakal, L.; Bourgerie, S.; Morabito, D. The Reduction of the As and Pb Phytotoxicity of a Former Mine Technosol Depends on the Amendment Type and Properties. Chemosphere 2022, 300, 134592. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Palmeggiani, G.; Renouard, S.; Chafik, Y.; Cagnon, B.; Bourgerie, S.; Morabito, D. Natural Ageing of Biochar Improves Its Benefits to Soil Pb Immobilization and Reduction in Soil Phytotoxicity. Environ. Geochem. Health 2023, 45, 6109–6135. [Google Scholar] [CrossRef]
- Hassan, S.H.; Chafik, Y.; Sena-Velez, M.; Lebrun, M.; Scippa, G.S.; Bourgerie, S.; Trupiano, D.; Morabito, D. Importance of Application Rates of Compost and Biochar on Soil Metal(Loid) Immobilization and Plant Growth. Plants 2023, 12, 2077. [Google Scholar] [CrossRef]
- JI, N.K.; Soni, H.; Kumar, R.N.; Bhatt, I. Hyperaccumulation and Mobility of Heavy Metals in Vegetable Crops in India. J. Agric. Environ. 2009, 10, 34–45. [Google Scholar] [CrossRef]
- Tukura, B.; Anhwange, B.; Mohammed, Y.; Usman, N. Translocation of Trace Metals in Vegetable Crops Grown on Irrigated Soil along Mada River, Nasarawa State, Nigeria. Int. J. Mod. Anal. Sep. Sci. 2012, 1, 13–22. [Google Scholar]
- Alghamdi, A.G.; Alkhasha, A.; Ibrahim, H.M. Effect of Biochar Particle Size on Water Retention and Availability in a Sandy Loam Soil. J. Saudi Chem. Soc. 2020, 24, 1042–1050. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does Biochar Improve Soil Water Retention? A Systematic Review and Meta-Analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Rivier, P.-A.; Jamniczky, D.; Nemes, A.; Makó, A.; Barna, G.; Uzinger, N.; Rékási, M.; Farkas, C. Short-Term Effects of Compost Amendments to Soil on Soil Structure, Hydraulic Properties, and Water Regime. J. Hydrol. Hydromech. 2022, 70, 74–88. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Boukirou, W.; Potra, A.; Melchiorre, E.; Bouzahzah, H.; Yans, J.; Zaid, K.; Idbaroud, M.; Poot, J.; Dekoninck, A.; et al. Origin of the Moroccan Touissit-Bou Beker and Jbel Bou Dahar Supergene Non-Sulfide Biomineralization and Its Relevance to Microbiological Activity, Late Miocene Uplift and Climate Changes. Minerals 2021, 11, 401. [Google Scholar] [CrossRef]
- Oubohssaine, M.; Dahmani, I.; Sbabou, L.; Bruneel, O.; Aurag, J. The Rhizosphere of Sulla Spinosissima Growing in Abandoned Mining Soils Is a Reservoir of Heavy Metals Tolerant Plant Growth-Promoting Rhizobacteria. Biocatal. Agric. Biotechnol. 2022, 39, 102236. [Google Scholar] [CrossRef]
- Singh, B.; MM, D.; Shen, Q.; Camps Arbestain, M. Chapter 3. Biochar pH, Electrical Conductivity and Liming Potential. In Biochar: A Guide to Analytical Methods; CSIRO: Canberra, Australia, 2017; pp. 23–38. [Google Scholar]
- Wu, S.; Zhang, Y.; Tan, Q.; Sun, X.; Wei, W.; Hu, C. Biochar Is Superior to Lime in Improving Acidic Soil Properties and Fruit Quality of Satsuma Mandarin. Sci. Total Environ. 2020, 714, 136722. [Google Scholar] [CrossRef]
- El Alaoui, A.; Raklami, A.; Bechtaoui, N.; El Gharmali, A.; Ouhammou, A.; Imziln, B.; Achouak, W.; Pajuelo, E.; Oufdou, K. Use of Native Plants and Their Associated Bacteria Rhizobiomes to Remediate-Restore Draa Sfar and Kettara Mining Sites, Morocco. Environ. Monit. Assess. 2021, 193, 232. [Google Scholar] [CrossRef]
- Pérez, I.; Romero, F.M.; Zamora, O.; Gutiérrez-Ruiz, M.E. Magnetic Susceptibility and Electrical Conductivity as a Proxy for Evaluating Soil Contaminated with Arsenic, Cadmium and Lead in a Metallurgical Area in the San Luis Potosi State, Mexico. Environ. Earth Sci. 2014, 72, 1521–1531. [Google Scholar] [CrossRef]
- Rezaee, M.; Warner, R.C.; Honaker, R.Q. Development of an Electrical Conductivity Screening Test for Mine Waste Assessments. Chemosphere 2016, 160, 13–21. [Google Scholar] [CrossRef]
- Song, D.; Tang, J.; Xi, X.; Zhang, S.; Liang, G.; Zhou, W.; Wang, X. Responses of Soil Nutrients and Microbial Activities to Additions of Maize Straw Biochar and Chemical Fertilization in a Calcareous Soil. Eur. J. Soil Biol. 2018, 84, 1–10. [Google Scholar] [CrossRef]
- Karimi, A.; Moezzi, A.; Chorom, M.; Enayatizamir, N. Application of Biochar Changed the Status of Nutrients and Biological Activity in a Calcareous Soil. J. Soil. Sci. Plant Nutr. 2020, 20, 450–459. [Google Scholar] [CrossRef]
- Bartoli, M.; Troiano, M.; Giudicianni, P.; Amato, D.; Giorcelli, M.; Solimene, R.; Tagliaferro, A. Effect of Heating Rate and Feedstock Nature on Electrical Conductivity of Biochar and Biochar-Based Composites. Appl. Energy Combust. Sci. 2022, 12, 100089. [Google Scholar] [CrossRef]
- Król, A.; Mizerna, K.; Bożym, M. An Assessment of pH-Dependent Release and Mobility of Heavy Metals from Metallurgical Slag. J. Hazard. Mater. 2020, 384, 121502. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Wang, J.; Ma, Y.; Li, J.; Zeng, X. Influence of DOM and Its Subfractions on the Mobilization of Heavy Metals in Rhizosphere Soil Solution. Sci. Rep. 2022, 12, 14082. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Uptake of Heavy Metals by Green Leafy Vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Campos, P.; De la Rosa, J. Assessing the Effects of Biochar on the Immobilization of Trace Elements and Plant Development in a Naturally Contaminated Soil. Sustainability 2020, 12, 6025. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of Phytoremediation and Biochar to Remediate Heavy Metal Polluted Soils: A Review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.P.J.; Gascó, G. Biochar from Biosolids Pyrolysis: A Review. IJERPH 2018, 15, 956. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Wang, Y.; Yeh, K.-C. Role of Root Exudates in Metal Acquisition and Tolerance. Curr. Opin. Plant Biol. 2017, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Lebrun, M.; Miard, F.; Bucci, A.; Fougère, L.; Nandillon, R.; Naclerio, G.; Scippa, G.S.; Destandeau, E.; Morabito, D.; Bourgerie, S. The Rhizosphere of Salix Viminalis Plants after a Phytostabilization Process Assisted by Biochar, Compost, and Iron Grit: Chemical and (Micro)-Biological Analyses. Environ. Sci. Pollut. Res. 2021, 28, 47447–47462. [Google Scholar] [CrossRef]
- Agarwal, P.; Vibhandik, R.; Agrahari, R.; Daverey, A.; Rani, R. Role of Root Exudates on the Soil Microbial Diversity and Biogeochemistry of Heavy Metals. Appl. Biochem. Biotechnol. 2023, 196, 2673–2693. [Google Scholar] [CrossRef]
- Lutts, S.; Zhou, M.X.; Flores-Bavestrello, A.; Hainaut, P.; Dailly, H.; Debouche, G.; Foucart, G. Season-Dependent Physiological Behavior of Miscanthus x Giganteus Growing on Heavy-Metal Contaminated Areas in Relation to Soil Properties. Heliyon 2024, 10, e25943. [Google Scholar] [CrossRef] [PubMed]
- Wahsha, M.; Nadimi-Goki, M.; Fornasier, F.; Al-Jawasreh, R.; Hussein, E.I.; Bini, C. Microbial Enzymes as an Early Warning Management Tool for Monitoring Mining Site Soils. CATENA 2017, 148, 40–45. [Google Scholar] [CrossRef]
- Venson, G.R.; Marenzi, R.C.; Almeida, T.C.M.; Deschamps-Schmidt, A.; Testolin, R.C.; Rörig, L.R.; Radetski, C.M. Restoration of Areas Degraded by Alluvial Sand Mining: Use of Soil Microbiological Activity and Plant Biomass Growth to Assess Evolution of Restored Riparian Vegetation. Environ. Monit. Assess. 2017, 189, 120. [Google Scholar] [CrossRef]
- Nurzhan, A.; Tian, H.; Nuralykyzy, B.; He, W. Soil Enzyme Activities and Enzyme Activity Indices in Long-Term Arsenic-Contaminated Soils. Eurasian Soil Sci. 2022, 55, 1425–1435. [Google Scholar] [CrossRef]
- Kompała-Bąba, A.; Bierza, W.; Sierka, E.; Błońska, A.; Besenyei, L.; Woźniak, G. The Role of Plants and Soil Properties in the Enzyme Activities of Substrates on Hard Coal Mine Spoil Heaps. Sci. Rep. 2021, 11, 5155. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Brzezińska, M.; Siwik-Ziomek, A.; Koper, J. Activity of Selected Enzymes and Phosphorus Content in Soils of Former Sulphur Mines. Sci. Total Environ. 2020, 708, 134545. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Toledo, Á.; Montes-Rocha, A.; González-Mille, D.J.; Espinosa-Reyes, G.; Torres-Dosal, A.; Mejia-Saavedra, J.J.; Ilizaliturri-Hernández, C.A. Evaluation of Enzyme Activities in Long-Term Polluted Soils with Mine Tailing Deposits of San Luis Potosí, México. J. Soils Sediments 2017, 17, 364–375. [Google Scholar] [CrossRef]
- Wang, A.; Liu, S.; Xie, J.; Ouyang, W.; He, M.; Lin, C.; Liu, X. Response of Soil Microbial Activities and Ammonia Oxidation Potential to Environmental Factors in a Typical Antimony Mining Area. J. Environ. Sci. 2023, 127, 767–779. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar Increases Soil Microbial Biomass but Has Variable Effects on Microbial Diversity: A Meta-Analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Xu, W.; Xu, H.; Delgado-Baquerizo, M.; Gundale, M.J.; Zou, X.; Ruan, H. Global Meta-Analysis Reveals Positive Effects of Biochar on Soil Microbial Diversity. Geoderma 2023, 436, 116528. [Google Scholar] [CrossRef]
- Ciarkowska, K.; Sołek-Podwika, K.; Wieczorek, J. Enzyme Activity as an Indicator of Soil-Rehabilitation Processes at a Zinc and Lead Ore Mining and Processing Area. J. Environ. Manag. 2014, 132, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar Application Increased the Growth and Yield and Reduced Cadmium in Drought Stressed Wheat Grown in an Aged Contaminated Soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Kicińska, A.; Pomykała, R.; Izquierdo-Diaz, M. Changes in Soil pH and Mobility of Heavy Metals in Contaminated Soils. Eur. J. Soil Sci. 2022, 73, e13203. [Google Scholar] [CrossRef]
- Ayyar, S.; Appavoo, S.; N, M. Role of Zinc Nutrition for Increasing Zinc Availability, Uptake, Yield, and Quality of Maize (Zea Mays L.) Grains: An Overview. Commun. Soil Sci. Plant Anal. 2020, 51, 2001–2021. [Google Scholar] [CrossRef]
- Zeng, F.; Ali, S.; Zhang, H.; Ouyang, Y.; Qiu, B.; Wu, F.; Zhang, G. The Influence of pH and Organic Matter Content in Paddy Soil on Heavy Metal Availability and Their Uptake by Rice Plants. Environ. Pollut. 2011, 159, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Yang, Z.; Tang, L.; Zeng, G.; Yu, M.; Li, X.; Wu, H.; Qian, Y.; Li, X.; Luo, Y. Changes in Heavy Metal Mobility and Availability from Contaminated Wetland Soil Remediated with Combined Biochar-Compost. Chemosphere 2017, 181, 281–288. [Google Scholar] [CrossRef]
- Huang, R.; Dong, M.; Mao, P.; Zhuang, P.; Paz-Ferreiro, J.; Li, Y.; Li, Y.; Hu, X.; Netherway, P.; Li, Z. Evaluation of Phytoremediation Potential of Five Cd (Hyper)Accumulators in Two Cd Contaminated Soils. Sci. Total Environ. 2020, 721, 137581. [Google Scholar] [CrossRef]
- He, J.; Li, C.; Tan, X.; Peng, Z.; Li, H.; Luo, X.; Tang, L.; Wei, J.; Tang, C.; Yang, W.; et al. Driving Factors for Distribution and Transformation of Heavy Metals Speciation in a Zinc Smelting Site. J. Hazard. Mater. 2024, 471, 134413. [Google Scholar] [CrossRef]
- Cheng, Y.; Bu, X.; Li, J.; Ji, Z.; Wang, C.; Xiao, X.; Li, F.; Wu, Z.; Wu, G.; Jia, P.; et al. Application of Biochar and Compost Improved Soil Properties and Enhanced Plant Growth in a Pb–Zn Mine Tailings Soil. Environ. Sci. Pollut. Res. 2023, 30, 32337–32347. [Google Scholar] [CrossRef]
- Novak, J.M.; Ippolito, J.A.; Watts, D.W.; Sigua, G.C.; Ducey, T.F.; Johnson, M.G. Biochar Compost Blends Facilitate Switchgrass Growth in Mine Soils by Reducing Cd and Zn Bioavailability. Biochar 2019, 1, 97–114. [Google Scholar] [CrossRef]
- Egerić, M.; Smičiklas, I.; Dojčinović, B.; Sikirić, B.; Jović, M.; Šljivić-Ivanović, M.; Čakmak, D. Interactions of Acidic Soil near Copper Mining and Smelting Complex and Waste-Derived Alkaline Additives. Geoderma 2019, 352, 241–250. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of Heavy Metal Contaminated Soils by Biochar: Mechanisms, Potential Risks and Applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy Metals in Agricultural Soils: Sources, Influencing Factors, and Remediation Strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, Y.; AL-Huqail, A.A.; Ding, Z.; Al-Harbi, M.S.; Ali, E.F.; Abeed, A.H.A.; Rekaby, S.A.; Eissa, M.A.; Ghoneim, A.M.; et al. Effect of Manure and Compost on the Phytostabilization Potential of Heavy Metals by the Halophytic Plant Wavy-Leaved Saltbush. Plants 2021, 10, 2176. [Google Scholar] [CrossRef]
- Meers, E.; Samson, R.; Tack, F.M.G.; Ruttens, A.; Vandegehuchte, M.; Vangronsveld, J.; Verloo, M.G. Phytoavailability Assessment of Heavy Metals in Soils by Single Extractions and Accumulation by Phaseolus vulgaris. Environ. Exp. Bot. 2007, 60, 385–396. [Google Scholar] [CrossRef]
- Aldoobie, N.F.; Beltagi, M.S. Physiological, Biochemical and Molecular Responses of Common Bean (Phaseolus Vulgaris L.) Plants to Heavy Metals Stress. Afr. J. Biotechnol. 2013, 12, 4614–4622. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K. Accumulation and Translocation of Metals in Soil and Different Parts of French Bean (Phaseolus Vulgaris L.) Amended with Sewage Sludge. Bull. Environ. Contam. Toxicol. 2014, 92, 103–108. [Google Scholar] [CrossRef]
- Silva-Gigante, M.; Hinojosa-Reyes, L.; Rosas-Castor, J.M.; Quero-Jiménez, P.C.; Pino-Sandoval, D.A.; Guzmán-Mar, J.L. Heavy Metals and Metalloids Accumulation in Common Beans (Phaseolus Vulgaris L.): A Review. Chemosphere 2023, 335, 139010. [Google Scholar] [CrossRef]
- Gutiérrez-Martínez, P.B.; Torres-Morán, M.I.; Romero-Puertas, M.C.; Casas-Solís, J.; Zarazúa-Villaseñor, P.; Sandoval-Pinto, E.; Ramírez-Hernández, B.C. Assessment of Antioxidant Enzymes in Leaves and Roots of Phaseolus Vulgaris Plants under Cadmium Stress//Evaluación de Enzimas Antioxidantes En Hojas y Raíces de Plantas Phaseolus Vulgaris Bajo Estrés de Cadmio. BIOTECNIA 2020, 22, 110–118. [Google Scholar] [CrossRef]
| Abbreviation | Sw (%) | Biochar (%) | Compost (%) |
|---|---|---|---|
| Sw | 100 | 0 | 0 |
| SwB2 | 98 | 2 | 0 |
| SwC5 | 95 | 0 | 5 |
| SwC5B2 | 93 | 2 | 5 |
| SwC10 | 90 | 0 | 10 |
| SwC10B2 | 88 | 2 | 10 |
| SwC20 | 80 | 0 | 20 |
| SwC20B2 | 78 | 2 | 20 |
| A | SPW pH | SPW EC (µs·cm−1) | SPW Pb (mg·L−1) | SPW Zn (mg·L−1) | SPW Cu (mg·L−1) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(0) | D(12) | D(0) | D(12) | D(0) | D(12) | D(0) | D(12) | D(0) | D(12) | |||||||||||
| Sw | 8.42 ± 0.06 | a | 8.24 ± 0.04 | ab | 1276 ± 94.60 | ab | 1302 ± 37.27 | ab | 0.12 ± 0.02 | d | 0.12 ± 0.01 | ab | 0.17 ± 0.02 | c | 0.99 ± 0.10 | ab | 0.09 ± 0.03 | ab | 0.24 ± 0.04 | ab |
| SwB2 | 8.39 ± 0.02 | ab | 8.17 ± 0.02 | ab | 1465 ± 93.51 | ab | 1478 ± 118.58 | a | 0.09 ± 0.01 | d | 0.16 ± 0.01 | ab | 0.15 ± 0.03 | c | 1.36 ± 0.18 | a | 0.06 ± 0.002 | b | 0.26 ± 0.02 | a |
| SwC5 | 8.16 ± 0.04 | ab | 8.17 ± 0.03 | ab | 1021 ± 38.22 | c | 1048 ± 55.94 | bc | 0.21 ± 0.02 | cd | 0.22 ± 0.07 | ab | 0.29 ± 0.02 | bc | 0.62 ± 0.10 | bc | 0.09 ± 0.01 | ab | 0.20 ± 0.06 | ab |
| SwC5B2 | 8.19 ± 0.02 | ab | 8.16 ± 0.04 | ab | 1544 ± 145.90 | a | 1422 ± 113.91 | ab | 0.71 ± 0.20 | ab | 0.39 ± 0.12 | a | 0.44 ± 0.09 | ab | 0.70 ± 0.06 | bc | 0.16 ± 0.03 | a | 0.20 ± 0.03 | ab |
| SwC10 | 8.22 ± 0.04 | ab | 8.13 ± 0.04 | b | 1068 ± 29.88 | bc | 951 ± 37.10 | c | 0.20 ± 0.03 | cd | 0.12 ± 0.01 | ab | 0.23 ± 0.03 | bc | 0.52 ± 0.04 | cd | 0.07 ± 0.003 | b | 0.11 ± 0.004 | bc |
| SwC10B2 | 8.15 ± 0.03 | b | 8.25 ± 0.03 | ab | 1330 ± 51.68 | ab | 1149 ± 51.99 | bc | 0.62 ± 0.08 | bc | 0.20 ± 0.02 | ab | 0.42 ± 0.05 | ab | 0.43 ± 0.03 | cd | 0.12 ± 0.02 | ab | 0.11 ±0.01 | bc |
| SwC20 | 8.15 ± 0.09 | b | 8.23 ± 0.03 | ab | 1275 ± 168.11 | ab | 1091 ± 76.98 | bc | 0.81 ± 0.14 | ab | 0.20 ± 0.09 | ab | 0.51 ± 0.06 | a | 0.37 ± 0.06 | cd | 0.09 ± 0.01 | ab | 0.11 ± 0.02 | bc |
| SwC20B2 | 8.26 ± 0.08 | ab | 8.32 ± 0.06 | a | 1303 ± 39.15 | ab | 922 ± 29.53 | c | 1.10 ± 0.10 | a | 0.11 ± 0.02 | b | 0.55 ± 0.05 | a | 0.20 ± 0.01 | d | 0.10 ± 0.01 | ab | 0.10 ± 0.01 | c |
| B | SPW pH | SPW EC (µs·cm−1) | SPW Pb (mg·L−1) | SPW Zn (mg·L−1) | SPW Cu (mg·L−1) | |||||||||||||||
| D(0)/D(12) | D(0)/D(12) | D(0)/D(12) | D(0)/D(12) | D(0)/D(12) | ||||||||||||||||
| Sw | — | — | — | ** | ** | |||||||||||||||
| SwB2 | ** | — | *** | ** | ** | |||||||||||||||
| SwC5 | — | — | — | * | — | |||||||||||||||
| SwC5B2 | — | — | — | * | * | |||||||||||||||
| SwC10 | — | * | * | ** | ** | |||||||||||||||
| SwC10B2 | ** | — | * | — | — | |||||||||||||||
| SwC20 | — | — | * | — | — | |||||||||||||||
| SwC20B2 | — | ** | ** | ** | — | |||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chafik, Y.; Sena-Velez, M.; Henaut, H.; Missbah El Idrissi, M.; Carpin, S.; Bourgerie, S.; Morabito, D. Synergistic Effects of Compost and Biochar on Soil Health and Heavy Metal Stabilization in Contaminated Mine Soils. Agronomy 2025, 15, 1295. https://doi.org/10.3390/agronomy15061295
Chafik Y, Sena-Velez M, Henaut H, Missbah El Idrissi M, Carpin S, Bourgerie S, Morabito D. Synergistic Effects of Compost and Biochar on Soil Health and Heavy Metal Stabilization in Contaminated Mine Soils. Agronomy. 2025; 15(6):1295. https://doi.org/10.3390/agronomy15061295
Chicago/Turabian StyleChafik, Yassine, Marta Sena-Velez, Hugo Henaut, Mustapha Missbah El Idrissi, Sabine Carpin, Sylvain Bourgerie, and Domenico Morabito. 2025. "Synergistic Effects of Compost and Biochar on Soil Health and Heavy Metal Stabilization in Contaminated Mine Soils" Agronomy 15, no. 6: 1295. https://doi.org/10.3390/agronomy15061295
APA StyleChafik, Y., Sena-Velez, M., Henaut, H., Missbah El Idrissi, M., Carpin, S., Bourgerie, S., & Morabito, D. (2025). Synergistic Effects of Compost and Biochar on Soil Health and Heavy Metal Stabilization in Contaminated Mine Soils. Agronomy, 15(6), 1295. https://doi.org/10.3390/agronomy15061295








