Salinity Stress in Strawberry Seedlings Determined with a Spectral Fusion Model
Abstract
1. Introduction
2. Materials and Methods
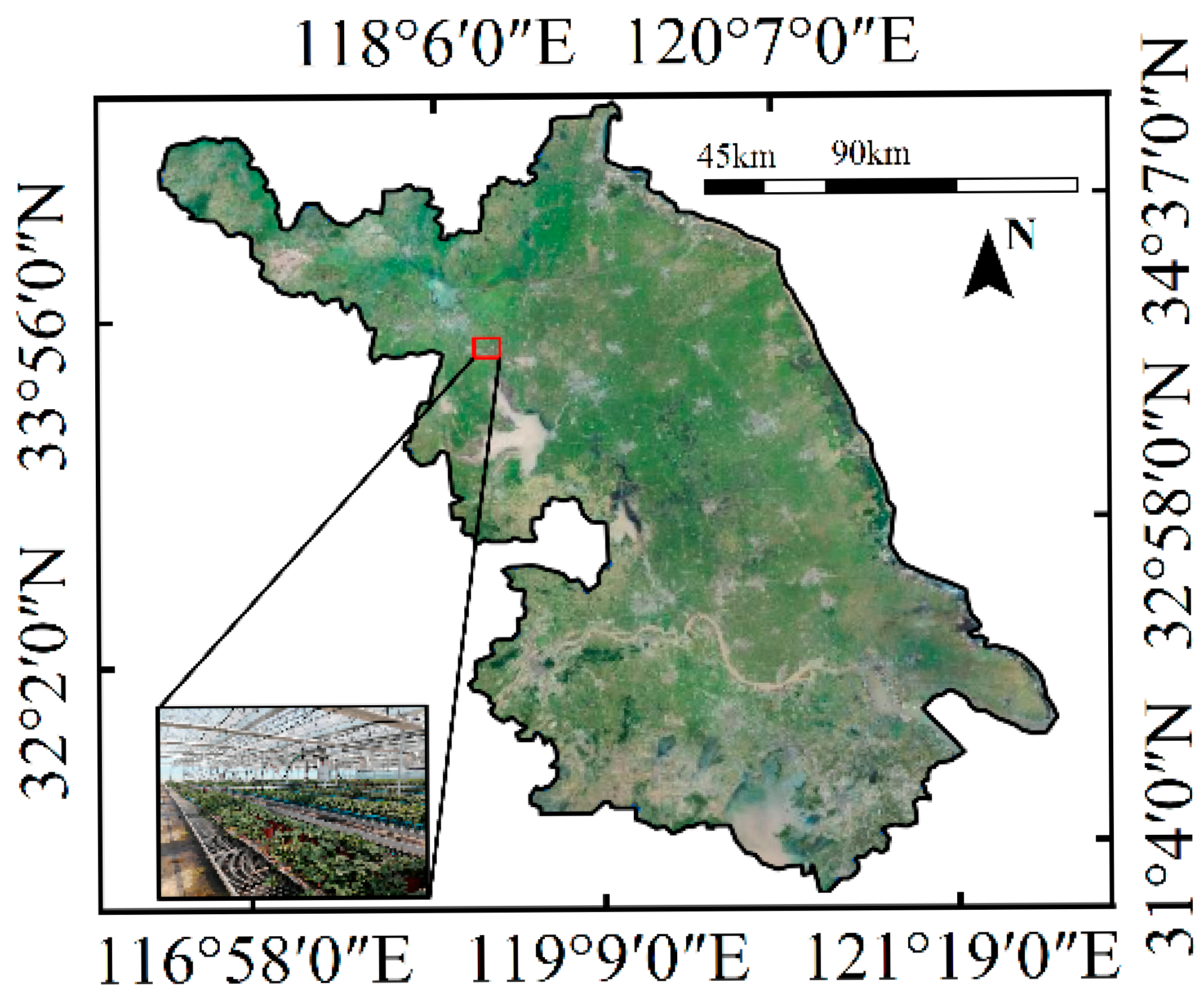
2.1. Experimental Materials
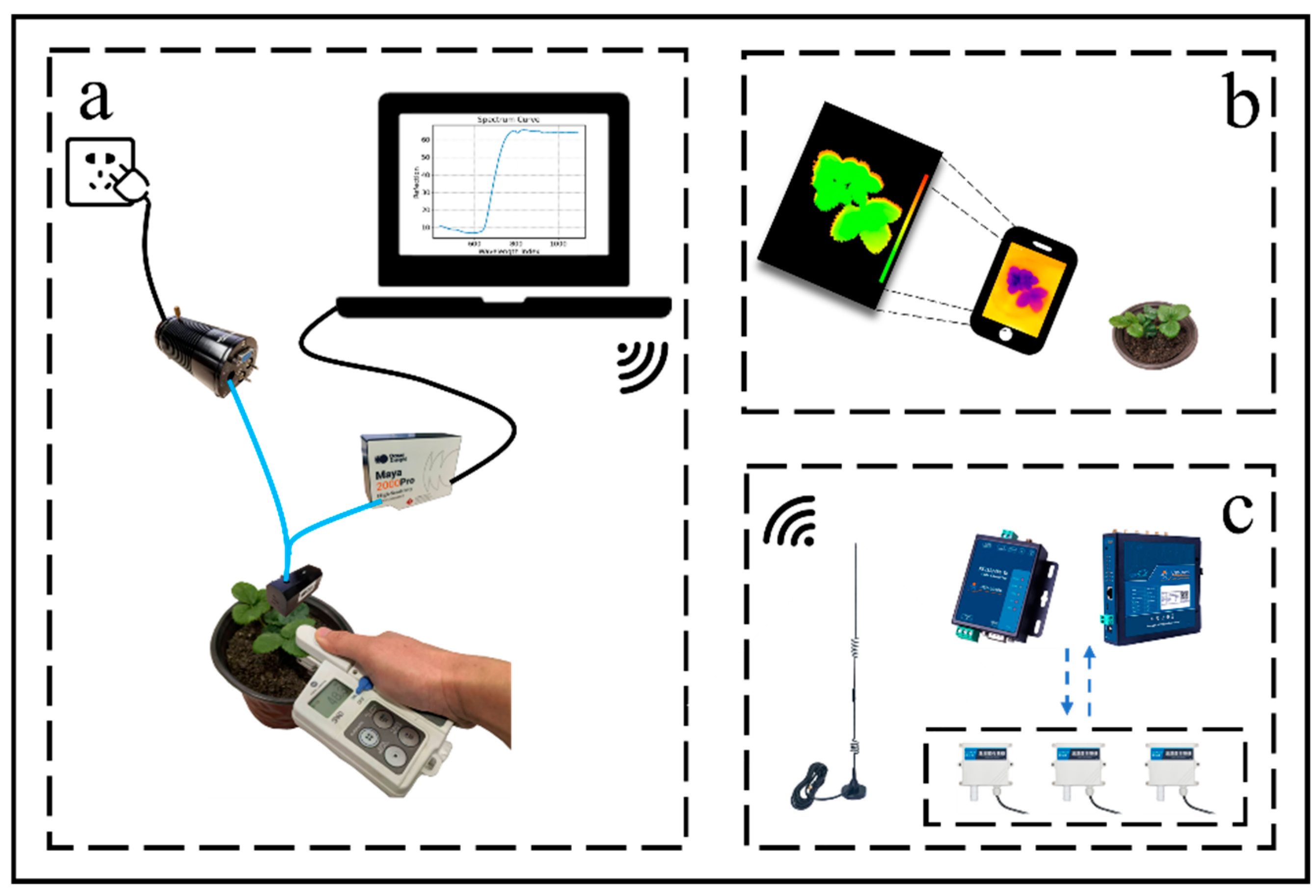
2.2. Experimental Equipment
2.3. Experimental Design
2.4. Experimental Methods
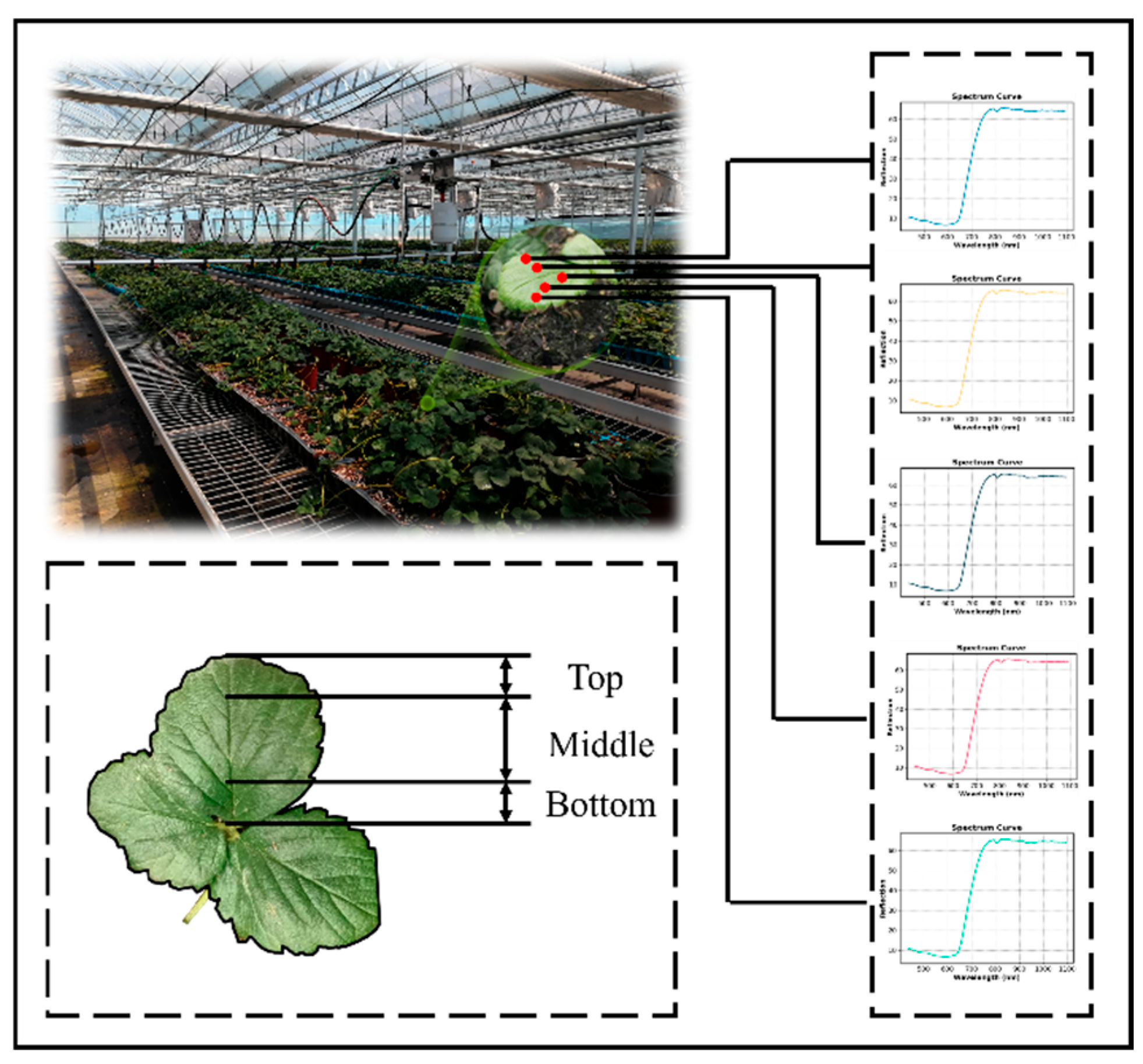
2.4.1. Spectral Data Acquisition and Calibration
2.4.2. Spectral Data Extraction
2.4.3. Relative Chlorophyll Content Collection
2.4.4. Leaf Temperature Extraction and Canopy Temperature Visualization
2.5. Data Analysis
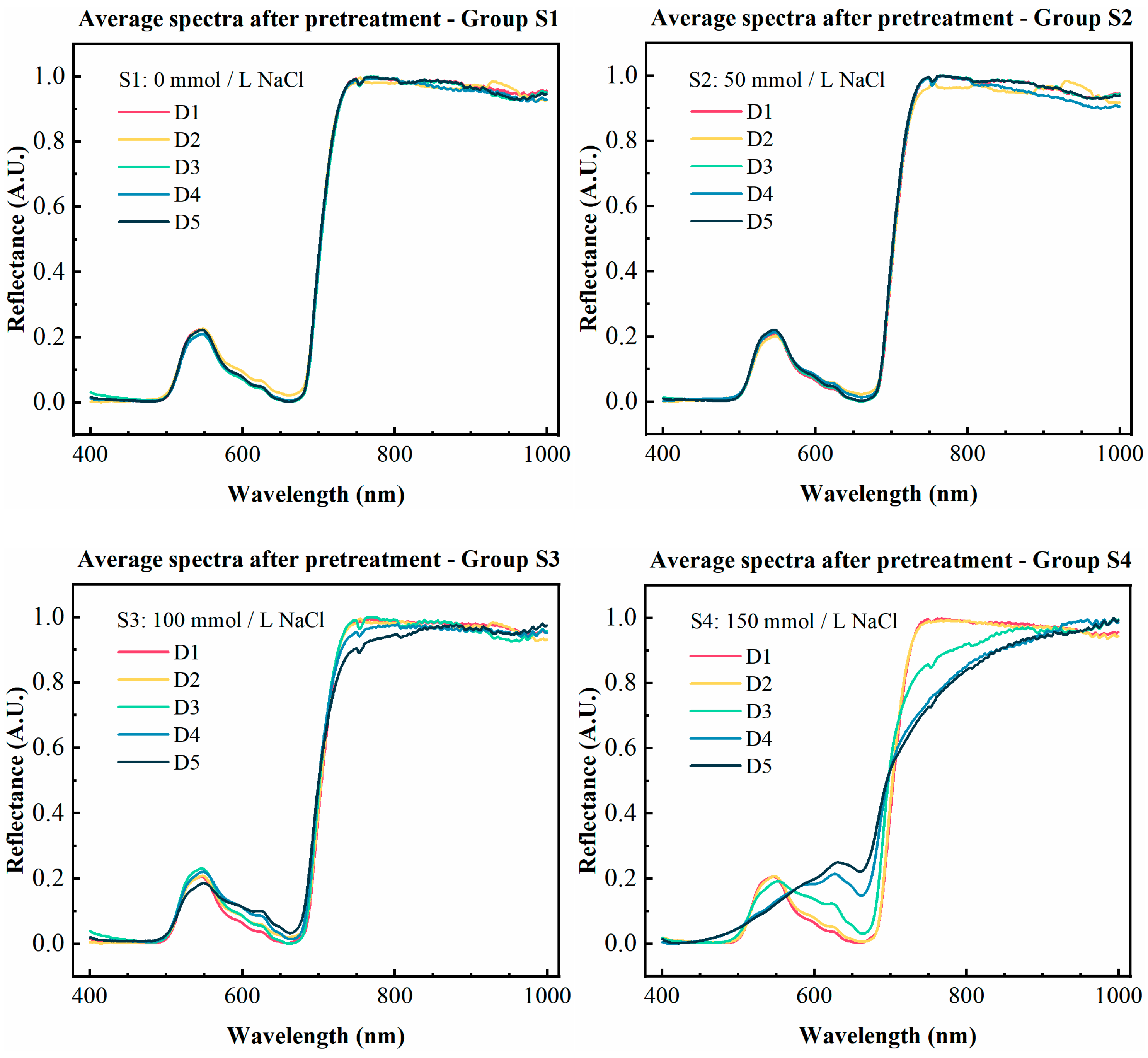
2.5.1. Spectral Data Preprocessing
2.5.2. Wavelength Screening Method
2.5.3. Model Establishment and Evaluation
3. Results
3.1. Spectral Preprocessing
3.2. Spectral Wavelength Screening
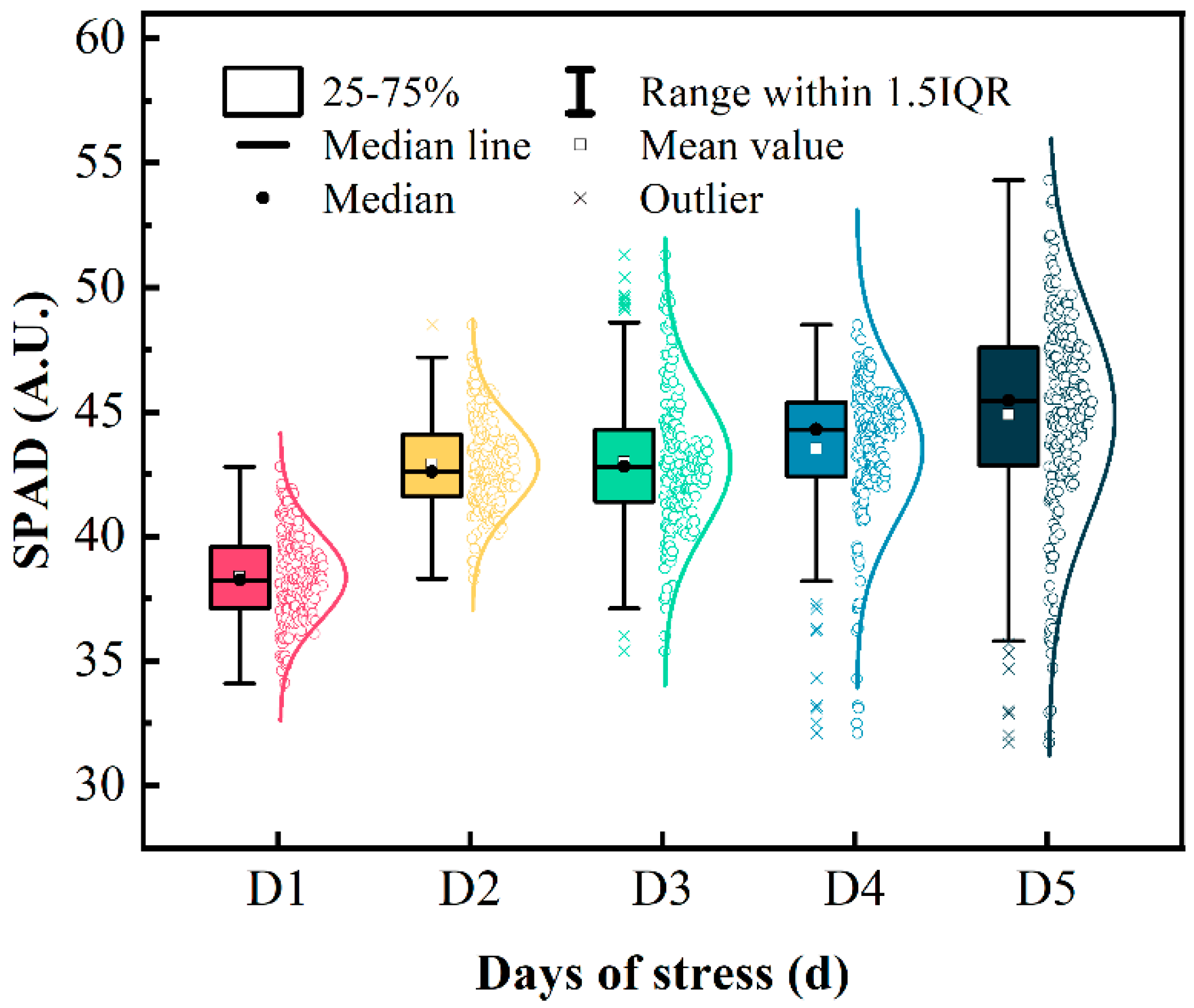
3.3. Relative Chlorophyll Content
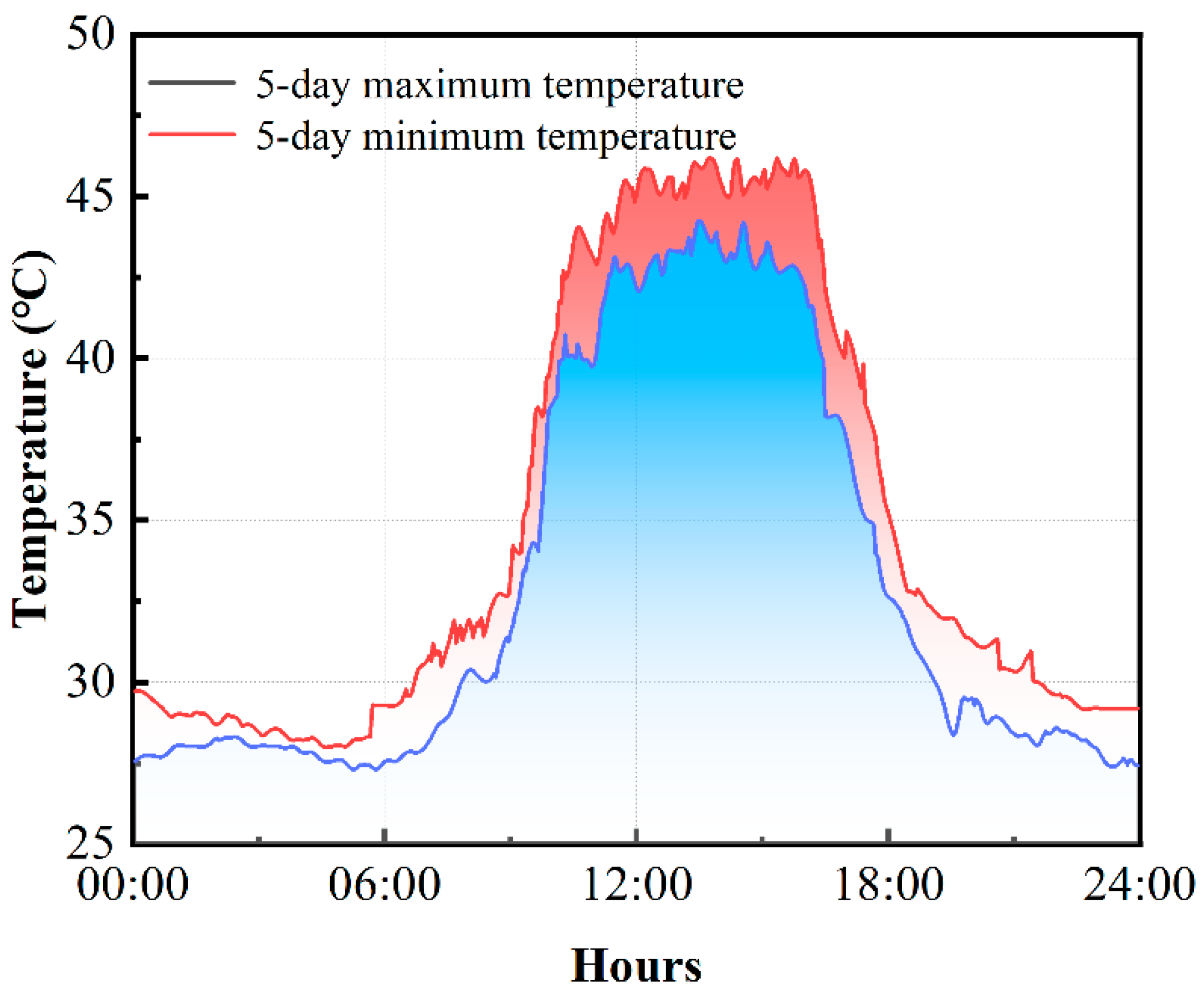
3.4. Visualization of Environmental Temperature Changes and Canopy Temperature
3.5. Establishment of Spectrum-Relative Chlorophyll Content Model
3.5.1. Classical Machine Learning Method
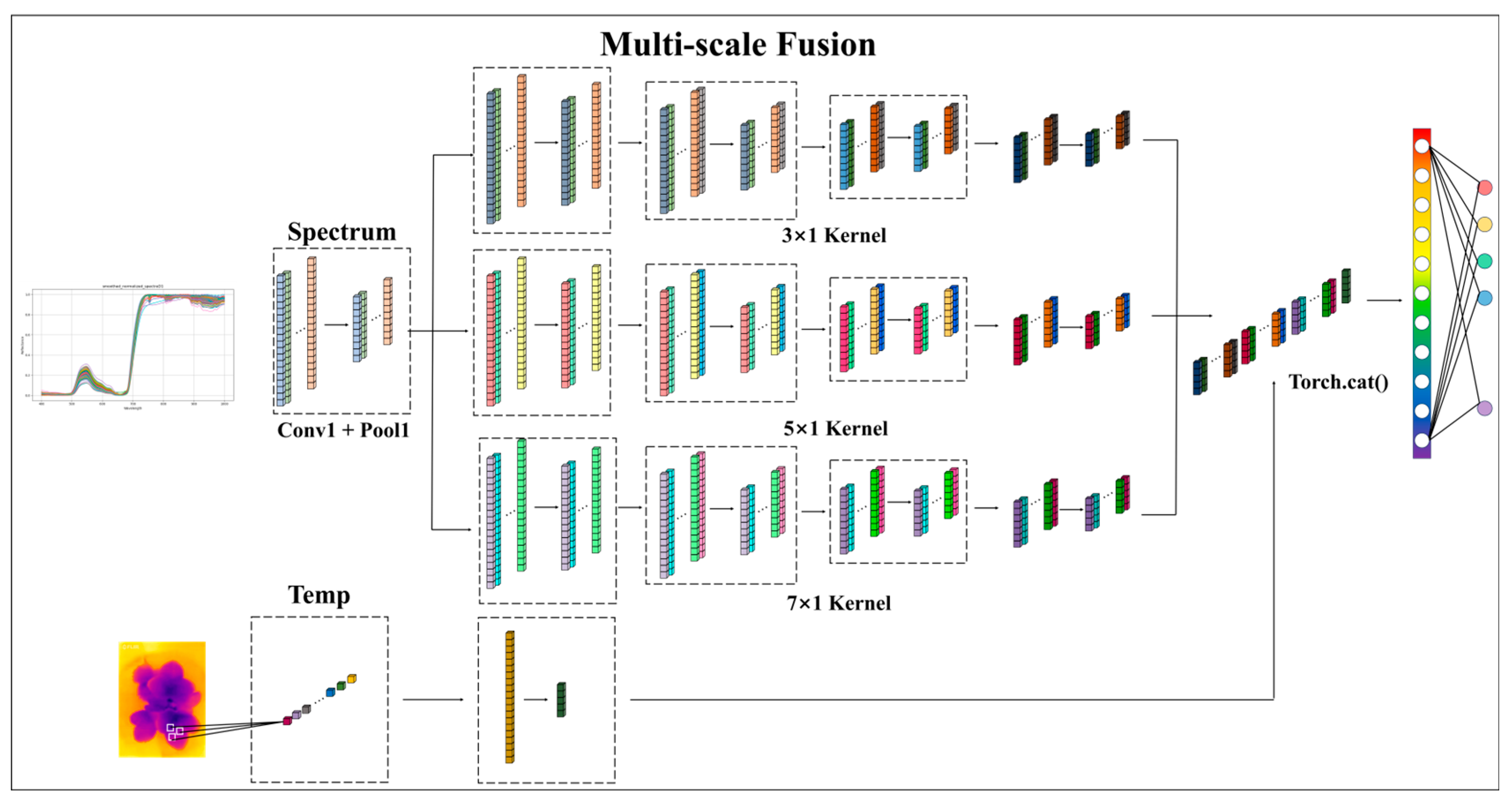
3.5.2. Building a Deep Learning Model
4. Discussion
4.1. Comparison of Preprocessing Methods
4.2. Temperature Trend
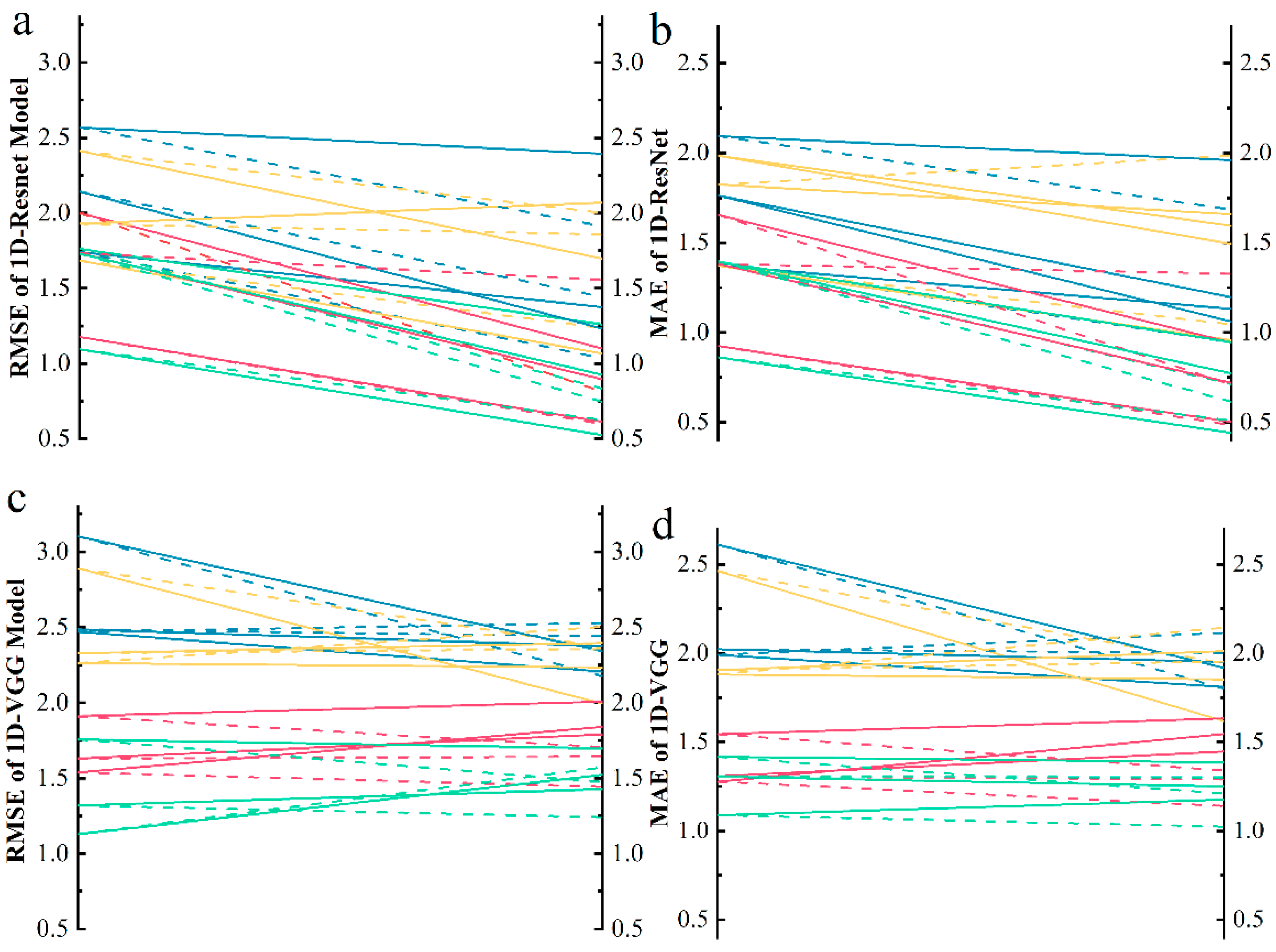
4.3. Model Comparison
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1D-CNN | One-dimensional convolutional neural network |
| ResNet | Residual network |
| MSCNN | Multi-scale convolutional neural network |
| IoT | Internet of Things |
| MSC | Multivariate scatter correction |
| SNV | Standard normal correction |
| PLSR | Partial least-squares regression |
| SVM | Support vector machine |
| SVR | Support vector regression |
| AT | Ambient temperature |
| LT | Left temperature |
| R2 | Coefficient of determination |
| RMSE | Root mean square error |
| RPD | Relative percent difference |
| MAE | Mean absolute error |
| SNR | Signal-to-noise ratio |
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT: Crops and Livestock Products. 2024. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 April 2025).
- IndexBox. World-Strawberries-Market Analysis, Forecast, Size, Trends and Insights Please mention the Source. Available online: https://www.indexbox.io/blog/strawberry-world-market-overview-2024 (accessed on 25 January 2025).
- Peng, Y.; Zhao, S.Y.; Liu, J.Z. Fused-Deep-Features Based Grape Leaf Disease Diagnosis. Agronomy 2021, 11, 2234. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Peng, Y.; Liu, J.Z.; Wu, S. Tomato Leaf Disease Diagnosis Based on Improved Convolution Neural Network by Attention Module. Agriculture 2021, 11, 651. [Google Scholar] [CrossRef]
- Francisco, A.R.; Rosario, B.P.; Juan, M.B.; José, L.C. The Strawberry Plant Defense Mechanism: A Molecular Review. Plant Cell Physiol. 2021, 52, 1873–1903. [Google Scholar] [CrossRef]
- Coatsworth, P.; Gonzalez-Macia, L.; Collins, A.S.P.; Bozkurt, T.; Güder, F. Continuous monitoring of chemical signals in plants under stress. Nat. Rev. Chem. 2023, 7, 7–25. [Google Scholar] [CrossRef]
- Guo, J.; Yang, Z.; Karkee, M.; Jiang, Q.; Feng, X.P.; He, Y. Technology progress in mechanical harvest of fresh market strawberries. Comput. Electron. Agric. 2024, 226, 109468. [Google Scholar] [CrossRef]
- Singh, T.; Rajan, R.; Vamsi, T.; Kumar, A.; Ramprasad, R.R.; Gopichand, G.B.; Chundurwar, K. A Review on the Impact of Organic and Biofertilizer Amendments on Growth, Yield, and Quality of Strawberry. Int. J. Plant Soil Sci. 2023, 35, 147–158. [Google Scholar] [CrossRef]
- Wang, R.Y.; Yang, X.J.; Chi, Y.W.; Zhang, X.; Ma, X.Z.; Zhang, D.; Zhao, T.; Ren, Y.F.; Yang, H.Y.; Ding, W.J.; et al. Regulation of hydrogen rich water on strawberry seedlings and root endophytic bacteria under salt stress. Front Plant Sci. 2024, 15, 1497362. [Google Scholar] [CrossRef]
- Katel, S.; Mandal, H.R.; Kattel, S.; Yadav, S.P.S.; Lamshal, B.S. Impacts of plant growth regulators in strawberry plant: A review. Heliyon 2022, 8, e11959. [Google Scholar] [CrossRef]
- Lu, C.X.; Li, L.Y.; Liu, X.L.; Chen, M.; Wan, S.B.; Li, G.W. Salt stress inhibits photosynthesis and destroys chloroplast structure by downregulating chloroplast development-Related genes in Bobinia pseudoacacia Seedlings. Plants 2023, 12, 1283. [Google Scholar] [CrossRef]
- Turan, S.; Tripathy, B.C. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plant. 2014, 153, 477–491. [Google Scholar] [CrossRef]
- EI-Shal, R.M.; EI-Naggar, A.H.; EI-Beshbeshy, T.R.; Mahmoud, E.K.; EI-Kader, N.I.A.; Missaui, A.M.; Du, D.L.; Ghoneim, A.M.; EI-Sharkawy, M.S. Effect of nano-fertilizers on alfalfa plants grown under different salt stresses in hydroponic system. Agriculture 2022, 12, 1113. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wang, X.C.; Zhang, X.L.; Wang, D.Z.; Xu, X. A band selection method combining spectral color characteristics for estimating chlorophyll content of rice in different backgrounds. Spectrochim. Acta Part A. 2024, 24, 1386–1425. [Google Scholar] [CrossRef]
- Muhammad, A.; Zou, X.B.; Haroon, E.T.; Hu, X.T.; Allah, R.; Sajid, B.; Zhao, H. Near-infrared spectroscopy coupled chemometric algorithms for prediction of antioxidant activity of black goji berries (Lycium ruthenicum Murr.). Food Measure. 2018, 12, 2366–2376. [Google Scholar] [CrossRef]
- Fan, S.X.; Li, J.B.; Xia, Y.; Tian, X.; Guo, Z.M.; Huang, W.Q. Long-term evaluation of soluble solids content of apples with biological variability by using near-infrared spectroscopy and calibration transfer method. Postharvest Biol. Technol. 2019, 151, 79–87. [Google Scholar] [CrossRef]
- Ge, X.; Sun, J.; Lu, B.; Chen, Q.S.; Xun, W.; Jin, Y.T. Classification of oolong tea varieties based on hyperspectral imaging technology and BOSS-Light GBM model. J. Food Process. Eng. 2019, 42, e13289. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Zhou, X.; Yao, K.S.; Tian, Y.; Nirere, A. A method of information fusion for identification of rice seed varieties based on hyperspectral imaging technology. J. Food Process. Eng. 2021, 44, e13797. [Google Scholar] [CrossRef]
- Elsayed, S.; EI-Hendawy, S.; Elsherbiny, O.; Okasha, A.M.; Elmetwalli, A.H.; Elwakeel, A.E.; Memon, M.S.; Ibrahim, M.E.M.; Ibrahim, H.H. Estimating Chlorophyll Content, Production, and Quality of Sugar Beet under Various Nitrogen Levels Using Machine Learning Models and Novel Spectral Indice. Agronomy 2023, 13, 2743. [Google Scholar] [CrossRef]
- Liu, C.H.; Yu, H.Y.; Liu, Y.C.; Zhang, L.; Li, D.; Zhang, J.H.; Li, X.K.; Sui, Y.Y. Prediction of Anthocyanin Content in Purple-Leaf Lettuce Based on Spectral Features and Optimized Extreme Learning Machine Algorithm. Agronomy 2024, 14, 2915. [Google Scholar] [CrossRef]
- Zhu, W.J.; Li, J.Y.; Li, L.; Wang, A.C.; Wei, X.H.; Mao, H.P. Nondestructive diagnostics of soluble sugar, total nitrogen and their ratio of tomato leaves in greenhouse by polarized spectra-hyperspectral data fusion. Int. J. Agric. Biol. Eng. 2020, 13, 189–197. [Google Scholar] [CrossRef]
- Zareef, M.; Arslan, M.; Hassan, M.M.; Ahmad, W.; Ali, S.; Li, H.H.; Yang, Q.O.; Wu, X.Y.; Hashim, M.M.; Chen, Q.S. Recent advances in assessing qualitative and quantitative aspects of cereals using nondestructive techniques: A review. Tends Food Sci. Technol. 2021, 116, 815–828. [Google Scholar] [CrossRef]
- Huang, Y.P.; Li, Z.A.; Bian, Z.C.; Jin, H.J.; Zheng, G.Q.; Hu, D.; Sun, Y.; Fan, C.L.; Xie, W.J.; Fang, H.M. Overview of Deep Learning and Nondestructive Detection Technology for Quality Assessment of Tomatoes. Foods 2025, 14, 286. [Google Scholar] [CrossRef]
- Xu, S.Z.; Xu, X.G.; Zhu, Q.Z.; Meng, Y.; Yang, G.J.; Feng, H.K.; Yang, M.; Zhu, Q.L.; Xue, H.Y.; Wang, B.B. Monitoring leaf nitrogen content in rice based on information fusion of multi-sensor imagery from UAV. Precis. Agric. 2023, 24, 2327–2349. [Google Scholar] [CrossRef]
- Wei, L.L.; Yang, H.S.; Niu, Y.X.; Zhang, Y.N.; Xu, L.Z.; Chai, X.Y. Wheat biomass, yield, and straw-grain ratio estimation from multi-temporal UAV-based RGB and multispectral images. Biosyst. Eng. 2023, 234, 187–205. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Owen, T.A.; Cai, W.W.; Hasan, T. Miniaturization of optical spectrometers. Science 2021, 371, eabe0722. [Google Scholar] [CrossRef]
- Li, A.; Yao, C.H.; Xia, J.F.; Wang, H.J.; Cheng, Q.X.; Penty, R.; Fainman, Y.; Pan, S. Advances in cost-effective integrated spectrometers. Light Sci. Appl. 2022, 11, 174. [Google Scholar] [CrossRef]
- Yang, M.; Kim, T.Y.; Hwang, H.C.; Yi, S.K.; Kim, D.H. Development of a palm portable mass spectrometer. J. Am. Soc. Mass. Spectrom. 2008, 19, 1442–1448. [Google Scholar] [CrossRef]
- Ang, K.L.M.; Seng, J.K.P. Big Data and Machine Learning with Hyperspectral Information in Agriculture. IEEE Access. 2021, 9, 36699–36718. [Google Scholar] [CrossRef]
- Li, L.Q.; Xie, S.M.; Ning, J.M.; Chen, Q.S.; Zhang, Z.Z. Evaluating green tea quality based on multisensor data fusion combining hyperspectral imaging and olfactory visualization systems. J. Sci. Food Agric. 2019, 99, 1787–1794. [Google Scholar] [CrossRef]
- Guo, M.Q.; Wang, K.Q.; Lin, H.; Wang, L.; Cao, L.M.; Sui, J.X. Spectral data fusion in nondestructive detection of food products: Strategies, recent applications, and future perspectives. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13301. [Google Scholar] [CrossRef]
- Dai, C.X.; Huang, X.Y.; Huang, D.M.; Lv, R.Q.; Sun, J.; Zhang, Z.C.; Ma, M.; Aheto, J.H. Detection of submerged fermentation of Tremella aurantialba using data fusion of electronic nose and tongue. J. Food Process. Eng. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Ignat, T.; Shavit, Y.; Rachmilevitch, S.; Karnieli, A. Spectral monitoring of salinity stress in tomato plants. Biosyst. Eng. 2022, 217, 26–40. [Google Scholar] [CrossRef]
- Ma, X.X.; Zeng, X.Y.; Huang, Y.R.; Liu, S.H.; Yin, J.; Yang, G.F. Visualizing plant salt stress with a NaCl-responsive fluorescent probe. Nat. Protoc. 2024, 20, 902–933. [Google Scholar] [CrossRef]
- Zahir, S.A.D.M.; Omar, A.F.; Jamlos, M.F.; Azmi, M.A.M.; Muncan, J. A review of visible and near-infrared (Vis-NIR) spectroscopy application in plant stress detection. Sens. Actuators A 2022, 338, 113468. [Google Scholar] [CrossRef]
- Calzone, A.; Cotrozzi, L.; Lorenzini, G.; Nali, C.; Pellegrini, E. Hyperspectral Detection and Monitoring of Salt Stress in Pomegranate Cultivars. Agronomy 2021, 11, 1038. [Google Scholar] [CrossRef]
- Das, A.; Bhattacharya, B.K.; Setia, R.; Jayasree, G.; Das, B.S. A novel method for detecting soil salinity using AVIRIS-NG imaging spectroscopy and ensemble machine learning. ISPRS 2023, 200, 191–212. [Google Scholar] [CrossRef]
- Dinh, T.H.; Watanabe, K.; Takaragawa, H.; Nakabaru, M.; Kawamitsu, Y. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Prod. Sci. 2017, 20, 412–422. [Google Scholar] [CrossRef]
- Liu, F.H.; Wang, X.R.; Wang, B.; Xiao, F.; He, K.Q. Physiological response and drought resistance evaluation of Gleditsia sinensis seedings under drought-rehydration state. Nature 2023, 13, 19963. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Wang, A.N.; Wang, S.H.; Tang, L.; Cao, W.X.; Zhu, Y.; Liu, L. L Modelling the effects of post-heading heat stress on biomass growth of winter wheat. Agric. For. Meteorol. 2017, 247, 476–490. [Google Scholar] [CrossRef]
- Kong, Y.; Masabni, J.; Niu, G. Temperature and Light Spectrum Differently Affect Growth, Morphology, and Leaf Mineral Content of Two Indoor-Grown Leafy Vegetables. Horticulturae 2023, 9, 331. [Google Scholar] [CrossRef]
- Santos, T.B.D.; Ribas, A.F.; Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stress 2022, 2, 113–115. [Google Scholar] [CrossRef]
- Das, B.; Manohara, K.K.; Mahajan, G.R.; Sahoo, R.N. Spectroscopy Based Novel Spectral Indices, PCA– and PLSR–coupled Machine Learning Models for Salinity Stress Phenotyping of Rice. Spectrochim. Acta Part A. 2020, 229, 117983. [Google Scholar] [CrossRef]
- Blasch, E.; Pham, T.; Chong, C.Y.; Koch, W.; Leung, H.; Braines, D. Machine Learning/Artificial Intelligence for Sensor Data Fusion—Opportunities and Challenges. IEEE Access 2021, 26, 80–93. [Google Scholar] [CrossRef]
- Ahmad, U.; Nasirahmadi, A.; Hensel, O.; Marino, S. Technology and Data Fusion Methods to Enhance Site-Specific Crop Monitoring. Agronomy 2022, 12, 555. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Sun, Z.G.; Zhao, F.H.; Yang, T.; Tian, Z.R.; Lai, J.B.; Zhu, W.X.; Long, B.J. Relating Hyperspectral Vegetation Indices with Soil Salinity at Different Depths for the Diagnosis of Winter Wheat Salt Stress. Remote Sens. 2021, 13, 250. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Yang, C.; Guardia, M.D.L.; Zhang, W.K.; Li, X.L.; He, Y. Proximal hyperspectral sensing of abiotic stresses in plants. Sci. Total Environ. 2023, 861, 160652. [Google Scholar] [CrossRef]
- Wan, L.; Li, H.; Li, C.S.; Wang, A.C.; Yang, Y.H.; Wang, P. Hyperspectral Sensing of Plant Diseases: Principle and Methods. Agronomy 2022, 12, 1451. [Google Scholar] [CrossRef]
- Rinnan, A.; Berg, F.V.D.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. TrAC. Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Jiao, Y.P.; Li, Z.C.; Chen, X.S.; Fei, S.M. Preprocessing methods for near-infrared spectrum calibration. J. Chem. 2020, 34, e3306. [Google Scholar] [CrossRef]
- Galli, V.; Messias, R.D.S.; Perin, E.C.; Borowski, J.M.; Bamberg, A.L.; Rombaldi, C.V. Mild salt stress improves strawberry fruit quality. Food Sci. Technol. 2016, 73, 693–699. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Liu, X.; Suarez, D.L. Fruit yield and survival of five commercial strawberry cultivars under field cultivation and salinity stress. Sci. Hortic. 2019, 243, 401–410. [Google Scholar] [CrossRef]
- Chen, X.J.; Jiang, Y.; Cong, Y.D.; Liu, X.F.; Yang, Q.; Xing, J.Y.; Liu, H.Y. Ascorbic acid mitigates salt stress in tomato seedlings by enhancing chlorophyll synthesis pathways. Agronomy 2024, 14, 1810. [Google Scholar] [CrossRef]
- Taibi, K.; Taibi, F.; Abderrahim, L.A.; Ennajah, A.; Belkhodja, M.; Mulet, M.B. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Yang, H.L.; Wang, L.; Zhang, X.L.; Shi, Y.Y.; Wu, Y.; Jiang, Y.; Wang, X.C. Exploring optimal soil moisture for seedling tomatoes using thermal infrared imaging and chlorophyll fluorescence techniques. Sci. Hortic. 2025, 339, 113846. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.L.; Zhang, X.; Zheng, W.G. A Review on Leaf Temperature Sensor: Measurement Methods and Application. In Proceedings of the 9th IFIP WG 5.14 International Conference, Beijing, China, 27–30 September 2015. pp. 216–230. [CrossRef]
- Hernandez-Hierro, J.M.; Cozzolino, D.; Feng, C.H.; Rato, A.E.; Nogales-Bueno, J. Editorial: Recent Advances of Near Infrared Applications in Fruits and Byproducts. Front Plant Sci. 2022, 13, 858040. [Google Scholar] [CrossRef]
- Gates, D.M. Leaf Temperature and Transpiration. Agron. J. 1964, 53, 273–277. [Google Scholar] [CrossRef]
- Zahir, S.A.D.M.; Jamlos, M.F.; Omar, A.F.; Jamlos, M.A.; Mamat, R.; Muncan, J.; Tsenkova, R. Review—Plant nutritional status analysis employing the visible and near-infrared spectroscopy spectral sensor. Spectrochim. Acta Part A. 2024, 304, 123273. [Google Scholar] [CrossRef]
- Zhong, C.; Li, L.; Wang, Y.Z. Applications of chemical fingerprints and machine learning in plant ecology: Recent progress and future perspectives. Microchem. J. 2024, 206, 111447. [Google Scholar] [CrossRef]
- Luo, X.L.; Sun, C.J.; He, Y.; Zhu, F.L.; Li, X.L. Cross-cultivar prediction of quality indicators of tea based on VIS-NIR hyperspectral imaging. Ind. Crops Prod. 2023, 202, 117009. [Google Scholar] [CrossRef]
- Ji, W.; Zhai, K.L.; Xu, B.; Wu, J.W. Green Apple Detection Method Based on Multidimensional Feature Extraction Network Model and Transformer Module. J. Food Prot. 2025, 88, 100397. [Google Scholar] [CrossRef]
- Ozaki, Y. Infrared Spectroscopy—Mid-infrared, Near-infrared, and Far-infrared/Terahertz Spectroscopy. Anal. Sci. 2021, 37, 1193–1212. [Google Scholar] [CrossRef]
- Yin, C.G.; Luo, Z.Y.; Ni, M.J.; Cen, K.F. Predicting coal ash fusion temperature with a back-propagation neural network model. Fuel 1998, 77, 1777–1782. [Google Scholar] [CrossRef]
- Wu, Z.F.; Shen, C.H.; Hengel, A.V.D. Wider or Deeper: Revisiting the ResNet Model for Visual Recognition. Patt. Reco. 2019, 90, 119–133. [Google Scholar] [CrossRef]



| Smooth-Normalized | Smooth-Normalized-SNV | Smooth-Normalized-MSC | Smooth-Normalized-MSC-SNV | Smooth-Normalized-SNV-MSC | |
|---|---|---|---|---|---|
| Smoothness | |||||
| SNR | 14.99 | 1.66 | 18.76 | 1.66 | 2.19 |
| r | 0.19 | 0.50 | 0.52 | 0.50 | 0.52 |
| Spectral Bands (nm) | |
|---|---|
| SPA | [400.0, 513.49, 639.74, 673.17, 686.36, 699.56, 721.11, 753.23, 930.94, 993.40] |
| CARS | [500.73, 562.32, 564.08, 565.84, 652.93, 673.17, 696.0, 824.93, 830.21, 930.5] |
| VIP | [736.9–740.37], [814.88–822.41] |
| S1 | S2 | S3 | S4 | |
|---|---|---|---|---|
| SD) | 1.37 a | 1.35 a | 1.32 b | 1.46 c |
| Model | Evaluation Indicator | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| PLSR | R2 | 0.741 | 0.693 | 0.747 | 0.659 |
| RMSE | 1.234 | 1.064 | 1.651 | 1.958 | |
| RPD | 1.963 | 1.804 | 1.986 | 1.712 | |
| SVR | R2 | 0.738 | 0.704 | 0.763 | 0.706 |
| RMSE | 1.239 | 1.044 | 1.597 | 1.819 | |
| RPD | 1.955 | 1.838 | 2.054 | 1.843 | |
| Kernel Ridge | R2 | 0.770 | 0.707 | 0.727 | 0.712 |
| RMSE | 1.163 | 1.038 | 1.715 | 1.800 | |
| RPD | 2.083 | 1.849 | 1.912 | 1.862 | |
| Decision_tree | R2 | 0.807 | 0.695 | 0.682 | 0.777 |
| RMSE | 1.013 | 0.987 | 1.537 | 1.371 | |
| RPD | 2.277 | 1.811 | 1.774 | 2.117 | |
| Random Forest | R2 | 0.687 | 0.637 | 0.625 | 0.651 |
| RMSE | 1.355 | 1.157 | 2.009 | 1.980 | |
| RPD | 1.788 | 1.659 | 1.632 | 1.693 |
| Model + AT | Evaluation Indicators | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| PLSR + AT | R2 | 0.744 | 0.690 | 0.738 | 0.646 |
| RMSE | 1.226 | 1.068 | 1.678 | 1.995 | |
| RPD | 1.976 | 1.796 | 1.954 | 1.680 | |
| SVR + AT | R2 | 0.727 | 0.704 | 0.757 | 0.725 |
| RMSE | 1.265 | 1.044 | 1.615 | 1.759 | |
| RPD | 1.915 | 1.838 | 2.030 | 1.906 | |
| Kernel Ridge + AT | R2 | 0.783 | 0.723 | 0.789 | 0.722 |
| RMSE | 1.129 | 1.010 | 1.506 | 1.767 | |
| RPD | 2.145 | 1.899 | 2.177 | 1.897 | |
| Decision_tree + AT | R2 | 0.793 | 0.726 | 0.676 | 0.778 |
| RMSE | 1.116 | 0.936 | 1.552 | 1.368 | |
| RPD | 2.215 | 1.910 | 1.756 | 2.122 | |
| Random Forest + AT | R2 | 0.691 | 0.651 | 0.626 | 0.630 |
| RMSE | 1.347 | 1.134 | 2.004 | 2.039 | |
| RPD | 1.798 | 1.692 | 1.636 | 1.644 |
| Model + LT | Evaluation Indicators | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| PLSR + LT | R2 | 0.767 | 0.782 | 0.768 | 0.713 |
| RMSE | 1.169 | 0.895 | 1.580 | 1.796 | |
| RPD | 2.071 | 2.143 | 2.076 | 1.867 | |
| SVR + LT | R2 | 0.757 | 0.761 | 0.739 | 0.755 |
| RMSE | 1.195 | 0.938 | 1.675 | 1.661 | |
| RPD | 2.027 | 2.047 | 1.957 | 2.018 | |
| Kernel Ridge + LT | R2 | 0.787 | 0.774 | 0.759 | 0.761 |
| RMSE | 1.119 | 0.912 | 1.609 | 1.639 | |
| RPD | 2.164 | 2.105 | 2.038 | 2.045 | |
| Decision_tree + LT | R2 | 0.789 | 0.813 | 0.899 | 0.746 |
| RMSE | 1.063 | 0.774 | 0.864 | 1.462 | |
| RPD | 2.170 | 2.310 | 3.154 | 1.986 | |
| Random Forest + LT | R2 | 0.699 | 0.609 | 0.664 | 0.721 |
| RMSE | 1.327 | 1.200 | 1.902 | 1.770 | |
| RPD | 1.825 | 1.599 | 1.724 | 1.894 |
| Model | Evaluation Indicator | S1 | S2 | S3 | S4 |
|---|---|---|---|---|---|
| 1D-MSCNN | RMSE | 2.142 | 2.427 | 2.849 | 2.856 |
| MAE | 1.683 | 1.965 | 2.108 | 2.288 | |
| 1D-MSCNN + AT | RMSE | 2.255 | 2.314 | 2.795 | 3.718 |
| MAE | 1.731 | 1.664 | 2.169 | 2.531 | |
| 1D-MSCNN + LT | RMSE | 1.922 | 2.192 | 2.482 | 2.541 |
| MAE | 1.547 | 1.686 | 1.970 | 2.010 | |
| 1D-ResNet | RMSE | 1.305 | 1.393 | 2.103 | 1.993 |
| MAE | 1.036 | 1.126 | 1.688 | 1.656 | |
| 1D-ResNet + AT | RMSE | 1.139 | 0.873 | 1.540 | 1.125 |
| MAE | 0.964 | 0.747 | 1.254 | 0.994 | |
| 1D-ResNet + LT | RMSE | 0.877 | 0.992 | 1.692 | 1.452 |
| MAE | 0.788 | 0.716 | 1.404 | 1.253 | |
| 1D-VGGNet | RMSE | 1.725 | 1.856 | 2.166 | 2.269 |
| MAE | 1.377 | 1.574 | 1.780 | 1.883 | |
| 1D-VGGNet + AT | RMSE | 1.537 | 1.769 | 2.414 | 2.382 |
| MAE | 1.240 | 1.487 | 1.974 | 2.006 | |
| 1D-VGGNet + LT | RMSE | 1.368 | 1.366 | 2.115 | 2.217 |
| MAE | 1.088 | 1.120 | 1.726 | 1.867 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, X.; Shi, Y.; Wang, L.; Chen, Y.; Wu, Z.; Lu, W.; Wang, X. Salinity Stress in Strawberry Seedlings Determined with a Spectral Fusion Model. Agronomy 2025, 15, 1275. https://doi.org/10.3390/agronomy15061275
Yang H, Zhang X, Shi Y, Wang L, Chen Y, Wu Z, Lu W, Wang X. Salinity Stress in Strawberry Seedlings Determined with a Spectral Fusion Model. Agronomy. 2025; 15(6):1275. https://doi.org/10.3390/agronomy15061275
Chicago/Turabian StyleYang, Haolin, Xiaolei Zhang, Yinyan Shi, Lei Wang, Yanyu Chen, Zhongxian Wu, Wei Lu, and Xiaochan Wang. 2025. "Salinity Stress in Strawberry Seedlings Determined with a Spectral Fusion Model" Agronomy 15, no. 6: 1275. https://doi.org/10.3390/agronomy15061275
APA StyleYang, H., Zhang, X., Shi, Y., Wang, L., Chen, Y., Wu, Z., Lu, W., & Wang, X. (2025). Salinity Stress in Strawberry Seedlings Determined with a Spectral Fusion Model. Agronomy, 15(6), 1275. https://doi.org/10.3390/agronomy15061275






