Abstract
Cadmium (Cd) contamination in alkaline soils threatens wheat safety in northern China. This study evaluates biochar’s dual role in Cd remediation and ecological trade-offs using a multi-species soil system (wheat–earthworm–soil). Biochar (Pennisetum hydridum) was applied to Cd-contaminated alkaline fluvo-aquic soils under controlled conditions. The results revealed that biochar increased soil pH (8.6–9.6) and reduced CaCl2-extractable Cd by 30–45% in the topsoil (0–20 cm), lowering shoot Cd accumulation in wheat by 42–47%. However, alkaline stress from biochar suppressed wheat biomass by 42%, while earthworm Cd concentrations rose 30–45%, correlating with reduced survival (75% vs. 85–87% in controls). Structural equation modeling identified pH-driven chemisorption as the primary Cd immobilization mechanism, yet biochar amplified ecotoxicity to soil fauna. These findings highlight the need for balanced strategies to optimize biochar’s benefits in alkaline agroecosystems.
1. Introduction
Soil cadmium (Cd) pollution is a serious environmental issue that threatens food safety. Cadmium (Cd), distinguished by its exceptional mobility compared to other heavy metals such as Cu, Ni, Pb, and Zn, exhibits pronounced bioavailability in soil–plant systems even at trace concentrations due to its chemical similarity to essential plant nutrients like Ca and Zn [1]. This unique property facilitates its rapid uptake by crops, leading to disproportionate accumulation in edible tissues—a critical concern given Cd’s potent toxicity that disrupts cellular redox balance and compromises food safety [2]. In China, the Cd-contaminated soils account for 7% of the contaminated agricultural soils (the Ministry of Environmental Protection and the Ministry of Land and Resources of the People’s Republic of China, 2014). Wheat (Triticum aestivum L.) is one of the cereals that has a long cultivation history and is one of the most widely consumed foods worldwide. Cultivated on approximately 3 × 106 km2 of arable land worldwide, wheat produces approximately 7 × 108 t of grains annually for human consumption [3]. Henan Province, contributing 25% of China’s wheat production [4], faces severe Cd contamination in agricultural soils due to sewage irrigation and industrial wastewater discharge [5,6]. Wheat grains from these regions often exceed the National Food Safety Standard (GB2762-2017) [7], with Cd accumulation rates higher than in maize [6,8]. Thus, effective remediation of Cd-contaminated soils is critical for sustainable wheat production.
Biochar, a carbon-rich material produced via biomass pyrolysis under oxygen-limited conditions [9], immobilizes metals through mechanisms such as precipitation, ion exchange, and surface complexation. Its large specific surface area (SSA), porous structure, and functional groups (e.g., -C=O and -C-OH) enhance Cd adsorption [10,11,12]. However, biochar’s efficacy depends on soil properties (e.g., pH and texture) and its own characteristics (e.g., pHZPC and ash content) [13,14]. For instance, biochar surface charge shifts with soil pH: when soil pH < pHZPC, the positively charged surface repels Cd2+, whereas at pH > pHZPC, negative charges facilitate Cd adsorption [15,16]. While biochar is widely used in acidic soils [17,18], its effects in alkaline environments remain underexplored.
Soil ecosystems are complex, with Cd bioavailability influenced by plant roots, microbial activity, and earthworm bioturbation [19,20]. Earthworms are beneficial soil animals and play an important role in improving soil fertility and the soil ecological environment. Their activities may enhance Cd availability via acidification [21] or reduce it through mucus–metal complexation [22,23]. Additionally, earthworms synthesize (non-)metallothioneins under Cd stress, binding and detoxifying metal ions [24,25,26]. To simulate these interactions, multi-species soil systems (MS·3) integrate soil, plants, microorganisms, and fauna to assess environmental chemical fate [27,28]. MS·3 systems have been used to investigate the environmental fate of various chemicals reaching arable soils [28,29,30,31,32,33,34]. Fernández et al. [35] used multi-species soil systems to evaluate the toxic effects of the nanoparticles. Adapted MS·3 systems have also been employed by researchers to assess the influence of soil biota on heavy metal mobility [36,37]. Previous MS·3 studies demonstrated that earthworms significantly influence Cd and Pb mobility [19], while biochar application in alkaline fluvo-aquic soils—common in northern China—remains poorly understood.
Although biochar has demonstrated significant efficacy in immobilizing heavy metals in acidic soils, its application in alkaline fluvo-aquic soils—characterized by distinct mineralogical composition and high pH buffering capacity—remains critically underexplored. This knowledge gap is particularly concerning in northern China, where alkaline fluvo-aquic soils dominate agricultural landscapes yet paradoxically support wheat crops with grain Cd concentrations exceeding safety thresholds despite the soils’ theoretically higher metal retention capacity [6,8]. The unique physicochemical interplay between biochar’s functional groups and alkaline soil matrices may fundamentally alter Cd speciation and bioavailability, necessitating systematic investigation within ecologically relevant multi-species systems.
This study investigates biochar’s role in Cd migration and bioaccumulation within an MS·3 system using alkaline fluvo-aquic soils. The objectives were to (1) clarify biochar’s impact on soil physicochemical properties in alkaline environments; (2) determine biochar’s effect on Cd bioavailability in a multi-species system; and (3) elucidate Cd migration mechanisms under combined biochar, wheat, and earthworm influences. By addressing these gaps, this work provides insights into biochar’s ecological risks and benefits in alkaline agricultural systems.
2. Materials and Methods
2.1. Soils, Earthworms, and Wheat Seeds
The two fluvo-aquic soils were taken from the 0–20 cm layer of two agricultural fields in Henan and Beijing, respectively, as described by Lai et al. [19]. The physicochemical characteristics of the Henan fluvo-aquic soil were a pH of 8.43, a cation exchange capacity (CEC) of 9.70 cmol kg−1, and an SOM of 1.55%, whereas those of the Beijing fluvo-aquic soil were a pH of 8.48, a CEC of 12.50 cmol kg−1, and an SOM of 1.35%. Both soils are sandy clay loam. The heavy metal concentrations in the Henan fluvo-aquic soil were 6.44 mg kg−1 of Cd (exceeding China’s Soil Environmental Quality Standard risk screening threshold of 0.6 mg kg−1 for agricultural soils, GB 15618-2018) [38], 163.98 mg kg−1 of Pb (below the national threshold of 200 mg kg−1), 18.93 mg kg−1 of Ni, 20.94 mg kg−1 of Cu, and 72.70 mg kg−1 of Zn. For the Beijing fluvo-aquic soil, the metal concentrations were 0.25 mg kg−1 of Cd (within the national threshold), 28.00 mg kg−1 of Pb, 32.30 mg kg−1 of Ni, 24.40 mg kg−1 of Cu, and 77.30 mg kg−1 of Zn. All Pb and Zn values in both soils were below the recommended WHO limits (Pb: 100 mg kg−1; Zn: 300 mg kg−1) for agricultural soils.
The biochar was prepared by pyrolyzing Pennisetum hydridum straws at 500 °C for 2 h [11,12]. The biochar had the following properties: total nitrogen, 2.25 g kg−1; total carbon, 216.76 g kg−1; C/N ratio, 96.34; pH, 6.85; Cd, 1.19 mg kg−1; Pb, 12.66 mg kg−1; Ni, 7.86 mg kg−1; Cu, 17.86 mg kg−1; and Zn, 109.75 mg kg−1. The earthworms (Eisenia fetida) were purchased from Beijing Deyi Earthworm Farm, China, and their heavy metal concentrations were Cd 0.51 mg kg−1, Pb 3.78 mg kg−1, Ni 8.06 mg kg−1, Cu 16.15 mg kg−1, and Zn 65.25 mg kg−1, which are expressed on a dry weight basis. Wheat (Triticum aestivum L. var. Jimai22) seeds were purchased from Beijing Seed Co., Ltd., Beijing, China.
2.2. Experimental Design
The MS·3 soil column experiment was performed at the Chinese Research Academy of Environmental Sciences, Beijing, in September 2019. PVC cylinders (diameter = 12 cm; height = 54 cm) were used in the experiment. The bottoms of the columns were covered with nylon mesh to prevent earthworms from escaping. Columns were packed with the soils at a bulk density of 1.4 g/cm3. To simulate the stratified structure of alkaline fluvo-aquic soils in northern China—where surface layers are typically more contaminated due to anthropogenic activities (e.g., sewage irrigation and industrial discharge)—the upper 0–20 cm layer was filled with Henan fluvo-aquic soil (Cd-contaminated), while the lower 20–50 cm layer contained Beijing fluvo-aquic soil (relatively uncontaminated). The two layers were separated by a nylon mesh to prevent mixing while allowing vertical water flow. This design specifically aimed to study Cd migration from the contaminated upper layer to the deeper soil under biochar amendment. After being packed in the columns, the soils were saturated with deionized water for 24 h and left to stand for about 10 days to reach equilibrium.
The experiment adopted a completely randomized design. Five treatments in three replicates were set up: (1) CK, where wheat was not grown, and neither biochar nor earthworms were added; (2) W, where wheat was grown but neither biochar nor earthworms were added; (3) E, where wheat was not grown and biochar was not applied, but earthworms were added; (4) W + E, where wheat was grown and earthworms were added, but biochar was not applied; and (5) B + W + E, where wheat was grown and earthworms and biochar were added. Biochar was added and mixed well with the Henan fluvo-aquic soil in the 0–20 cm layer before earthworms were added and wheat was grown in B + W + E.
The experiment was conducted in a greenhouse with a temperature of 23 ± 1 °C, a humidity of 55–60%, a light/dark cycle of 16/8 h, and a photon flux density of 1200 lux. The duration of the experiment was 28 days. During the experiment, approximately 15 mL of water was added to each column at 4 PM every day. At the end of the experiment, after wheat shoot lengths were recorded and wheat plants, including roots, were harvested, the PVC tubes were sawed so that the soil layers of 0–5, 5–10, 10–15, 15–20, 20–25, 25–30, 30–40, and 40–50 cm were obtained for sampling and earthworm collecting.
2.3. Sample Pretreatment and Analyses
Soil samples from each layer were air-dried, ground, and sieved to <2 mm (for soil pH determination) or <0.149 mm (for soil Cd determination). Soil pH was measured at a water/soil ratio of 5:2 using a pH meter (PHSe3C, Leici, Shanghai, China). Soil moisture was measured gravimetrically. Soil samples were digested using nitric–hydrochloric–hydrofluoric acid (5–2–2, v:v:v) in a microwave unit (CEM MARS 5, Matthews, NC, USA), and total Cd was measured using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7500c, Santa Clara, CA, USA) [19]. The method described by Leveque et al. [39] was used to determine the CaCl2-extractable Cd. Briefly, 2.00 g of soil was extracted with 20 mL of 0.01 mol L−1 CaCl2 solution, and Cd concentration was determined by ICP-MS (Agilent 7500c, Santa Clara, CA, USA).
Wheat samples were washed and dried. After fresh weights were recorded, the samples were dried in an oven at 90 °C for 30 min and then at 60 °C to constant weight. The dried wheat samples were powdered and digested using nitric acid–perchloric acid in a microwave unit, and Cd concentration was measured using ICP-MS (Agilent 7500c, Santa Clara, CA, USA). The Cd translocation factor was calculated as follows: shoot Cd/root Cd concentration × 100%.
Earthworms collected from each soil layer were counted and dried in an oven at 105 °C for 30 min and then at 70 °C to constant weight. The survival rate and mean weight change rate were calculated. Survival rate was calculated as follows: earthworm number at the end of the experiment/earthworm number at the beginning of the experiment × 100%. Similarly, the mean weight change rate was calculated as follows: earthworm weight at the end of the experiment/earthworm weight at the beginning of the experiment × 100%. Earthworm samples were digested using nitric acid–perchloric acid in a microwave unit, and Cd concentration was measured using ICP-MS (Agilent 7500c, Santa Clara, CA, USA).
2.4. Statistical Analyses
For each measurement, three replications were conducted. Data analyses were carried out using a one-way analysis of variance (ANOVA), and differences between treatments were assessed by Duncan’s multiple range test using SPSS 23.0 software, with the significance level being set at p < 0.05. The figures were plotted using Origin 8.01. The structural equation modeling analyses were conducted using AMOS 21.0 (Amos Development Corporation, Chicago, IL, USA). Structural equation modeling was used to evaluate the direct and indirect relationships between earthworm Cd or wheat growth and soil properties and soil Cd content. This approach can separate the direct and indirect effects that one variable may have on another and is therefore useful for exploring complex relationships in natural ecosystems [40].
3. Results
3.1. Soil pH and Water Content
Biochar application increased soil pH (Figure 1a). In the 0–10 cm layer, soil pH showed a descending order of B + W + E (8.6–8.9) > CK (8.3–8.4) > W + E, W, and E (8.1–8.2). In the 10–20 cm layer, the descending order of soil pH was B + W + E (9.1–9.2) > W and CK (8.4–8.6) > W + E and E (8.3–8.4). In the 20–50 cm layer, the descending order of soil pH was B + W + E (9.2–9.6) > W + E, W, E, and CK (8.4–8.7). These results showed that soil pH increased with depth in the 0–20 cm layer. For the 0–10 cm layer, the lower soil pH levels in W, E, and W + E compared to CK were due to root and earthworm secretions, whereas the significantly higher soil pH in B + W + E was due to biochar application. The soil pH decrease in the 10–20 cm layer was negligible in W but was obvious in E and W + E, which may be related to the activities of earthworms in this soil layer.

Figure 1.
Soil pH (a) and water content (b) in different soil layers in different treatments. CK: no wheat, no earthworm, and no biochar. W: only wheat. E: only earthworm. W + E: wheat + earthworm. B + W + E: biochar + wheat + earthworm. The error bars are standard deviations; n = 3.
Biochar application increased soil water content (Figure 1b). The soil water content in B + W + E increased with increasing depth. The treatments were in the descending order of B + W + E (24.7–40.9%) > CK and W + E (27.2–39.9%) > W and E (22.2–31.5%) in terms of soil water content. These results showed that biochar application improved soil water retention.
3.2. Total and CaCl2-Extractable Cd in the Soil Columns
Biochar application (B + W + E) decreased soil total and CaCl2-extractable Cd in all layers compared to the other treatments (Figure 2). For the 0–20 cm layer, soil total Cd was in the order of B + W + E (3.31–4.96 mg kg−1) < W + E (4.04–5.24 mg kg−1), W (4.46–5.45 mg kg−1), E (4.33–5.65 mg kg−1), and CK (4.57–5.45 mg kg−1) (Figure 2a). It was much lower in the 20–50 cm layer in all treatments compared to the 0–20 cm layer, with 1.58–2.16, 2.18–2.65, 2.10–4.55, 1.92–2.79, and 2.18–2.81 mg kg−1 in B + W + E, W + E, W, E, and CK, respectively. At the end of the experiment, soil total Cd in the 0–20 cm layer was markedly reduced compared to at the beginning of the experiment (6.44 mg kg−1), but that in the 20–50 cm layer was increased significantly compared to at the beginning of the experiment (0.25 mg kg−1). Soil total Cd increased with depth in the 0–10 cm layer and decreased with depth in the 20–50 cm layer. The results showed that total Cd in the upper soil layer was decreased, whereas that in the lower layer was increased due to Cd leaching from the upper layer. However, most of the total Cd remained in the upper soil layer.
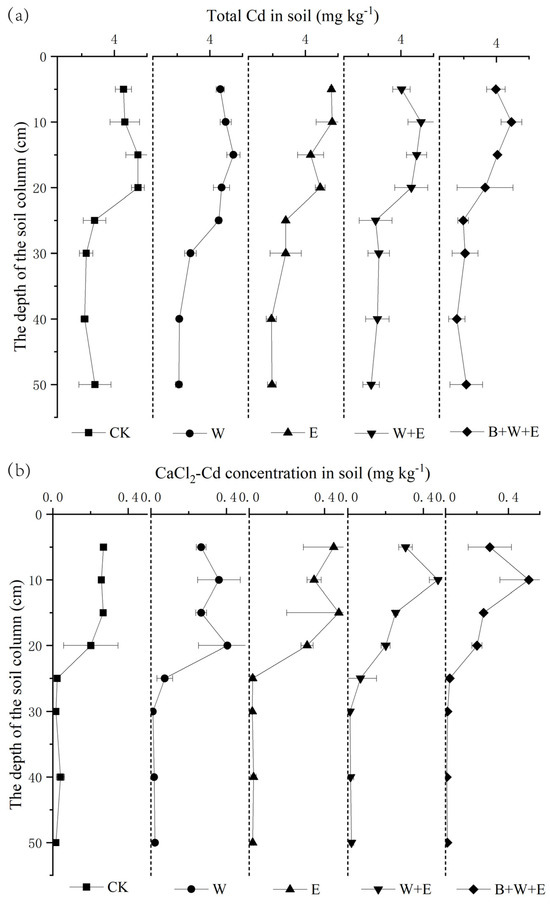
Figure 2.
Soil total Cd (a) and CaCl2-Cd in different soil layers in different treatments. CK: no wheat, no earthworm, and no biochar. W: only wheat. E: only earthworm. W + E: wheat + earthworm. B + W + E: biochar + wheat + earthworm. The error bars are standard deviations; n = 3. (b) soil CaCl2-extractable Cd was concentrated in the 0–20 cm layer in all treatments.
Similar to soil total Cd, soil CaCl2-extractable Cd was concentrated in the 0–20 cm layer in all treatments (Figure 2b). Soil CaCl2-extractable Cd in the 0–20 cm layer was higher in the W + E (0.20–0.48 mg kg−1), E (0.31–0.45 mg kg−1), and W (0.27–0.41 mg kg−1) treatments than in B + W + E (0.20–0.28 mg kg−1) and CK (0.20–0.27 mg kg−1), probably due to wheat root exudates and earthworm activities. In contrast, biochar application immobilized Cd and decreased Cd availability. In the 10–15 cm soil layer, CaCl2-extractable Cd was higher in the E treatment than in the other treatments, which may be because this layer was the main earthworm activity area in the E treatment. There was no significant difference in soil CaCl2-extractable Cd in the 20–50 cm layer (0.01–0.07 mg kg−1) between treatments.
3.3. Wheat Growth and Cd Uptake
Biochar application did not promote but inhibited wheat growth, and wheat grew better in W + E and W than in B + W + E, with larger biomass values (Figure 3). Biochar application (i.e., B + W + E) significantly decreased the Cd concentration in wheat shoots by 46.9% and 42.4% compared to W and W + E, respectively. In contrast, the Cd concentration in roots was reduced by 14.8% compared to W but increased by 12.3% compared to W + E. Compared to W, the Cd concentrations of wheat shoots and roots in W + E were reduced by 7.1% and 24.0%, respectively (Figure 4). The Cd translocation factor decreased significantly in B + W + E (0.14) compared to W (0.23) and W + E (0.28). The results showed that biochar application reduced Cd transportation from the roots to shoots, resulting in higher Cd accumulation in the roots compared to W + E.
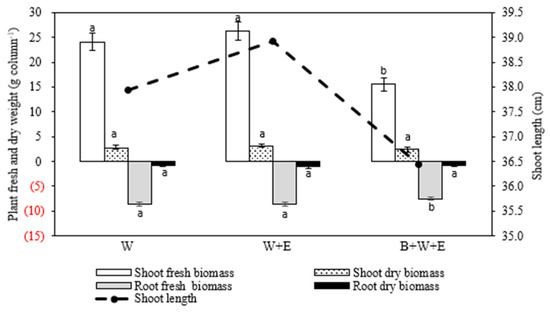
Figure 3.
Root and shoot biomass values and shoot lengths of the wheat plants in different treatments. W: only wheat. W + E: wheat + earthworm. B + W + E: biochar + wheat + earthworm. The same letters indicate no significant difference between treatments for the same parameter. Mean ± standard deviation (n = 3).
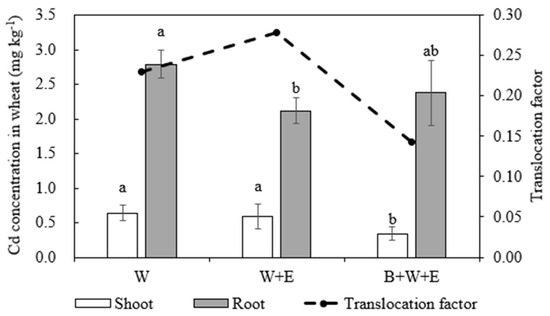
Figure 4.
Cd concentrations and translocation factors in wheat plants in different treatments. W: only wheat. W + E: wheat + earthworm. B + W + E: biochar + wheat + earthworm. The same letters indicate no significant difference between treatments for a same parameter. Mean ± standard deviation (n = 3).
3.4. Earthworm Distribution and Cd Concentration
At the end of the experiment, the earthworms were mainly distributed in the 0–5 cm layer in W + E and B + W + E but mainly in the 5–15 cm layer in E (Figure 5), indicating that the presence of wheat plants and biochar had influenced the activity zone of earthworms. One possible reason might be that the 0–5 cm layers in W + E and B + W + E were rich in organic matter, such as root exudates and debris, due to the planting of wheat, which, as food, might have attracted the earthworms. Biochar is a carbon-rich organic material. Therefore, more earthworms were found in the 0–5 cm layer of B + W + E (12) compared to W + E (10) (Figure 5).

Figure 5.
Number of earthworms collected from different soil layers in different treatments. E: only earthworm. W + E: wheat + earthworm. B + W + E: biochar + wheat + earthworm. The same capital letters indicate no significant difference between treatments for the same soil layer, and the same small letters indicate no significant difference between soil layers for the same treatment. Mean ± standard deviation (n = 3).
As shown in Figure 6a, the earthworm survival rate (75%) and mean weight change rate (94%) in B + W + E were lower than those in E (87% and 97%, respectively) and W + E (85% and 99%, respectively). The earthworm Cd concentration in B + W + E was 30% and 45%, which was significantly higher than those in E and W + E, respectively (Figure 6b). These results showed that wheat cultivation on the Cd-contaminated soil decreased the Cd concentration in earthworms, whereas biochar application increased the Cd concentration in earthworms, which posed a threat to earthworm survival.
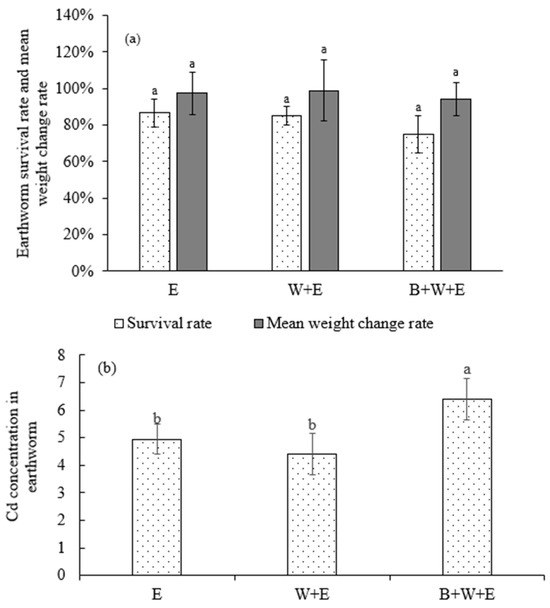
Figure 6.
Earthworm survival rate and weight change rate (a) and Cd concentration in earthworm (b) in different treatments. E: only earthworm. W + E: wheat + earthworm. B + W + E: biochar + wheat + earthworm. The earthworm survival rate was calculated as follows: earthworm number at the end of the experiment/earthworm number at the beginning of the experiment × 100%. The earthworm mean weight change rate was calculated as follows: earthworm weight at the end of the experiment/earthworm weight at the beginning of the experiment × 100%. The same letters indicate no significant difference between treatments. Mean ± standard deviation (n = 3).
3.5. Structural Equation Modeling Analysis
The structural equation modeling (SEM) was employed to quantify the responses of Cd accumulation in earthworms and wheat growth to soil properties and Cd availability (Figure 7). The SEM explained 77.0% (R2 = 0.770) of the variance in earthworm Cd content and 92.2% (R2 = 0.922) of the variance in wheat growth (Figure 7a,c), indicating high predictive accuracy.
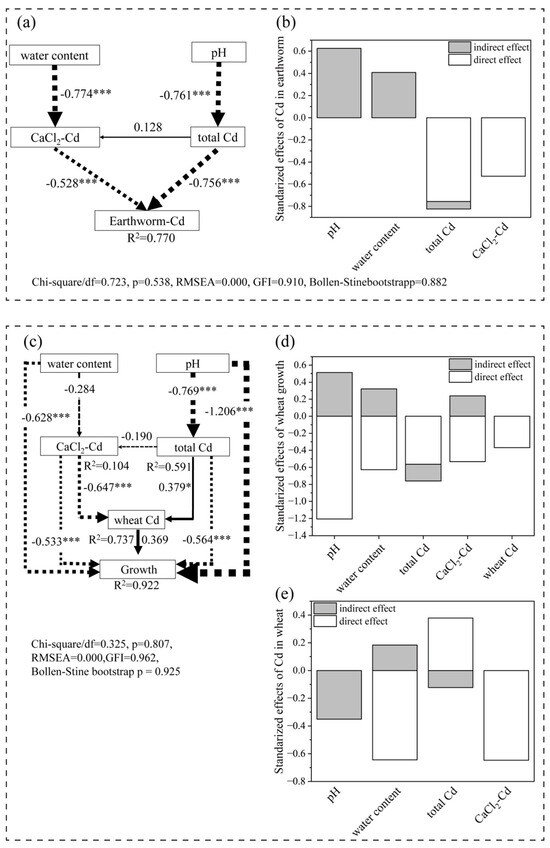
Figure 7.
Structural equation modeling (SEM) shows the direct and indirect effects of observed variables or latent variables on the cadmium (Cd) content in earthworms (a) and wheat growth (including Cd in wheat) (c). Each box represents a set of observed or latent variables. Solid line arrows and dashed lines arrows indicate positive and negative flows of causality, respectively. The higher path coefficients are shown as bold arrows. Line thickness corresponds to the magnitude of standardized coefficients (* p < 0.05 and *** p < 0.001). R2 value on the parameter signifies the variance explained by other parameters. Additionally, (b,d) display the decomposition of direct and indirect standardized effects for the key variables of earthworm Cd (b), wheat growth (d) and Cd in wheat (e), respectively. Wheat Cd accumulation was quantified through principal component analysis (PCA), integrating both aerial and root tissue cadmium concentrations, with the composite index expressed as factor analysis scores (FAC).
For earthworm Cd content (Figure 7a,b), pH and soil water content exhibited significant indirect negative effects, with path coefficients of −0.761 (p < 0.001) and −0.774 (p < 0.001), respectively. In contrast, total Cd and CaCl2-Cd showed strong direct negative effects on earthworm Cd accumulation, which was mediated through soil physicochemical interactions (path coefficients: −0.756 and −0.528, respectively; p < 0.001). These results suggest that while biochar-induced increases in pH and water retention indirectly reduced Cd bioavailability, elevated total Cd and CaCl2-Cd levels directly exacerbated Cd exposure to earthworms through complex soil–metal interactions.
For wheat growth (Figure 7c), pH remained the dominant driver, with a direct negative effect (path coefficient: −1.206 ***), which is consistent with the observed growth inhibition under alkaline stress. Soil water content and wheat Cd also contributed negatively to wheat growth (path coefficients: −0.628 *** and −0.369, respectively). Total Cd and CaCl2-Cd further suppressed wheat growth through both direct and indirect pathways (e.g., via pH modulation). The SEM for wheat Cd content (Figure 7e) highlighted CaCl2-Cd as the primary inhibitory factor (direct effect: −0.647), while pH indirectly mitigated Cd uptake through soil immobilization (indirect effect: −0.351).
3.6. The Fate of Cd in the MS·3 Soil Systems
The fates of Cd in the different treatments are shown in Table 1. Approximately 0.02%, 0.05–0.08%, 60.29–67.15%, and 32.84–39.69% of the total Cd were distributed in the wheat plants, earthworms, 0–20 cm soil layer, and 20–50 cm soil layer, respectively, in the treatments, demonstrating that the majority of Cd stayed in the upper soil layer. For the two MS·3 systems (B + W + E and W + E), the proportions of Cd in earthworms and the 0–20 cm soil layer were 84.44% and 6.79%, respectively, which were higher in B + W + E than in W + E. However, the proportion of Cd in the 20–50 cm soil layer was approximately 10.52% lower in B + W + E than in W + E. These results indicated that biochar application contributed to the Cd absorption by earthworms and Cd immobilization in the 0–20 cm soil layer.

Table 1.
Cd proportions (%) in the wheat plants, earthworms, and soil layers in the different treatments.
4. Discussion
The influences of biochar on soil pH and Cd bioavailability differ with different soils. For acidic soils, biochar application can mitigate soil acidity due to the presence of oxides, carbonates, and hydroxides of basic cations (Ca, Na, K, and Mg) [14] and the basic groups on the biochar surface [10,11,12]. In alkaline soils, biochar application reduces Cd bioavailability and promotes the transformation of available Cd to unavailable residual Cd [41,42]. In the alkaline soil column systems of this study, biochar application increased soil pH, which became more prominent with soil depth (Figure 1a). One possible reason is that biochar was oxidized to release surface basic ions (e.g., Ca2+ and K+) into the soil. These basic ions replaced the Al3+ and H+ ions adsorbed on the soil surface, which were then leached down the soil column, resulting in an increased soil pH. Soil pH has a negative influence on Cd immobilization by biochar [17]. In this research, there was no significant correlation between soil available Cd and pH in any soil layer, which may be related to the soil properties.
The SEM-driven insights emphasize the trade-offs between Cd mitigation and crop productivity (Figure 7c). While biochar effectively immobilized Cd (Figure 2a), its alkaline nature elevated soil pH beyond the optimal range for wheat (pH > 8.5), inducing nutrient imbalances and osmotic stress [17,43]. The pH range suitable for wheat growth is 6–8, and wheat growth is inhibited at pH > 8. Lu and Qiao [43] documented that the wheat emergence rate was 94% at pH 8.57 and decreased to 45% at pH 9.17, and wheat biomass correspondingly reduced by 48%. In B + W + E with a soil pH of 8.57–9.13 in the 0–20 cm layer, wheat fresh weight was reduced by 42% as compared to W + E. The dominant negative direct effect of pH on wheat growth (path coefficient: −1.206; p < 0.001) aligns with the significant biomass reduction in B + W + E (lower than W + E; Figure 3 and Figure 7). These findings echo studies emphasizing the need to balance biochar’s remediation efficacy with crop-specific pH tolerance thresholds [18,44]. Beyond alkaline stress, biochar’s high pH and ash content may induce nutrient imbalances (e.g., reduced availability of Fe, Mn, and P), as alkaline soils often limit micronutrient solubility. For instance, when the pH > 7, calcium phosphate precipitation is prone to occur, reducing the availability of phosphorus [45].
For wheat, the strong negative effect of CaCl2-Cd (coefficients = −0.647 ***) underscores biochar’s success in limiting Cd translocation to shoots (Figure 4 and Figure 7b). This aligns with findings that biochar reduces Cd bioavailability by increasing soil pH and promoting Cd adsorption onto stable organic–mineral complexes [18,41]. Following biochar amendment, a competition emerges between solution-phase CaCl2-Cd and biochar-adsorbed Cd. In soils with high biochar application, extractable Cd is preferentially adsorbed onto biochar surfaces rather than remaining freely dissolved in soil solution. Plant roots exhibit significantly higher uptake efficiency for free Cd2+ ions in solution compared to Cd adsorbed on biochar particles. Consequently, even if the total CaCl2-extractable Cd increases, the concentration of free Cd2+ ions in solution may decrease, leading to reduced Cd uptake by wheat. Soil pH indirectly reduces wheat Cd accumulation (indirect effect = −0.351) by promoting cadmium immobilization on biochar or soil particles, which further decreases free Cd2+ in solution. In this context, the statistically negative correlation between CaCl2-Cd and shoot Cd uptake represents a combined effect of pH-driven Cd speciation transformation and biochar adsorption. At elevated pH, a portion of CaCl2-extractable Cd exists in a “quasi-immobilized state”—chemically extractable but poorly bioavailable to plants due to strong binding with biochar functional groups or mineral surfaces.
Biochar has a well-developed porous structure. Its application can improve soil porosity, structure, aeration, and compressive strength as well as improve soil water and nutrient retention [41,46]. In this research, biochar application increased soil water content (Figure 1b). Yang et al. [41] showed that biochar application significantly increased soil water retention capacity at the matric potentials of −0.033 and −1.5 MPa, thereby increasing soil effective water content. While improved water retention can benefit crop growth under drought conditions, the SEM revealed a negative effect of soil water content on wheat growth (path coefficient: −0.628; p < 0.001). This may be attributed to root hypoxia exacerbated by prolonged waterlogging in biochar-amended alkaline soils.
This study was conducted in an alkaline fluvo-aquic soil system with sandy clay loam texture (clay content = 18–23%) and low organic matter (SOM = 1.35–1.55%). The high permeability of such soils may accelerate leaching of biochar aging products (e.g., soluble ash bases), while the low SOM content limits competitive adsorption of Cd by natural organic–mineral complexes, thus highlighting biochar’s Cd-immobilizing effect (Figure 2a). However, in alkaline soils with high clay content (>40%) or rich in carbonates, clay minerals (e.g., montmorillonite) or CaCO3 may dominate Cd fixation through ion exchange or coprecipitation [17,47], and the synergistic potential of biochar may vary depending on the soil’s inherent adsorption capacity.
Many studies have shown that biochar application has adverse effects on earthworms. It inhibits earthworm growth, reduces reproductive rates, and causes genotoxicity and weight loss [48,49]. In this research, biochar’s particulate and dissolved fractions likely intensified Cd toxicity to earthworms (Figure 6a,b), as evidenced by reduced survival rates (75% vs. 85–87%) and elevated Cd concentrations in B + W + E (30–45% higher than E and W + E). This was mainly due to four reasons. First, compared to E and W + E, the earthworms in B + W + E had a much higher Cd concentration (Figure 6b), which was due to earthworm ingestion of the Cd-binding biochar particles. The ingested Cd was toxic to the earthworms and led to earthworm mortality. Second, biochar application caused soil pH changes (Figure 1a). Soil pH in the 0–20 cm layer was increased in B + W + E by 0.49, 0.60, 0.71, and 0.68 units compared to CK, W, E, and W + E, respectively. Gruss et al. [50] pointed out that the soil pH increase caused by biochar application is unfavorable for earthworm survival. Third, the dissolved and particulate fractions of biochar are toxic to earthworms [51]. The particulate fraction of biochar adheres to earthworm skin and causes serious physical damage, eventually leading to earthworm death [46]. Compared to the particulate fraction, the dissolved fraction of biochar is more toxic to earthworms [51]. Fourth, persistent free radicals (PFRs) are produced during biochar preparation via pyrolysis, and such PFRs can cause oxidative damage to plants and earthworms by producing reactive oxygen species [52,53,54].
In addition, the SEM results reveal antagonistic interactions between earthworm activities and biochar in Cd dynamics. While earthworms typically enhance metal mobility through bioturbation and organic matter decomposition [55], biochar application attenuated this effect by stabilizing Cd in the upper soil layer (Table 1). In this study, this antagonistic effect is mainly manifested in the differences in Cd distribution: in the B + W + E treatment, 64.39% of the Cd remained in the upper 0–20 cm soil layer, while in the W + E treatment, the leaching of Cd to the 20–50 cm soil layer increased by 10.52% (Table 1). This indicates that biochar weakens the earthworm-driven remobilization of Cd by altering the soil metal speciation, which is consistent with the research results of Ibrahim et al. [47] and Xu et al. [56]. The direct inhibitory effects of total Cd and CaCl2-Cd on earthworm uptake (path coefficients: −0.756 and −0.528) suggest that biochar’s porous structure and functional groups (e.g., -C=O, -C-OH) effectively immobilize Cd via chemisorption or precipitation, reducing its bioavailability to soil fauna [11,17]. Thus, as shown by the SEM results, biochar plays a dual role in alkaline soils: it reduces the bioavailability of Cd for plants but inadvertently increases the Cd exposure of soil fauna.
5. Conclusions
Biochar application effectively reduces cadmium (Cd) mobility and bioavailability in alkaline fluvo-aquic soils within a multi-species soil system (MS·3). Biochar significantly increased soil pH and water retention while decreasing CaCl2-extractable Cd concentrations in the upper soil layers (0–20 cm), thereby reducing Cd translocation to wheat shoots by up to 46.9%. However, biochar amendment inhibited wheat growth due to elevated soil alkalinity and induced osmotic stress, highlighting a trade-off between Cd immobilization and crop productivity. Additionally, biochar application increased Cd accumulation in earthworms by 30–45%, likely due to biochar particle ingestion and persistent free radicals, which reduced earthworm survival rates. Structural equation modeling revealed that pH and total Cd directly influenced Cd bioaccumulation pathways, emphasizing the dual role of biochar in stabilizing soil Cd while posing risks to soil fauna. These findings underscore the need for optimizing biochar application rates and types to balance remediation efficacy and ecological safety in alkaline soils.
6. Environmental Significance
The research provides critical insights into biochar’s role in mitigating Cd pollution in alkaline agricultural soils, a less-studied context compared to acidic soils. By reducing Cd mobility and bioavailability, biochar can minimize Cd entry into the food chain, enhancing food safety in regions like Henan Province, China, where wheat production is threatened by Cd contamination. However, the study also highlights unintended ecological consequences, such as increased Cd toxicity to earthworms and inhibited crop growth under elevated pH conditions. These results emphasize the necessity of site-specific biochar strategies that consider soil pH thresholds, crop tolerance, and soil biodiversity conservation. The findings advocate for integrated soil management practices that combine biochar with pH-neutral amendments or organic matter to enhance both remediation efficiency and agricultural sustainability in Cd-contaminated alkaline environments.
Author Contributions
Conceptualization, D.L. and G.C.; methodology, D.L. and G.C.; writing—original draft, D.L.; writing—review and editing, D.L., C.L. and X.W.; data curation, C.L.; validation, H.H., D.W., Y.C., Z.W., F.L. and H.S., visualization, D.L. and C.L.; software, D.L. and C.L.; formal analysis, G.C.; supervision, X.W. and G.C.; funding acquisition, D.L.; resources, X.W. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Guangzhou basic research planning for basic and applied basic research project (2024A04J3256), the Special Fund for Scientific Innovation Strategy-construction of high level Academy of Agriculture Science (R2023YJ-YB3005), and the Guangdong Provincial Modern Agricultural Industry Common Key Technologies Research and Development Innovation Team Construction Project (Common Key Technologies for Agricultural Product Quality and Safety) under the Agricultural Sector Framework (2024CXTD18), Natural Science Foundation of Guangdong Province of China (2024A1515010715).
Data Availability Statement
The data that support this study are available upon reasonable request from the corresponding author.
Acknowledgments
Thank you to all those who helped with this study and to the research projects that sponsored it.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, F.; Zhang, Y.; Wu, T.; Wu, L.; Shi, G.; An, Y. The high-dimensional geographic dataset revealed significant differences in the migration ability of cadmium from various sources in paddy fields. Sci. Rep. 2023, 13, 1589. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.-Y.; Yu, X.-F.; Liu, Y.-J.; Zeng, X.-X.; Luo, F.-W.; Wang, X.-T.; Yang, X.; Wang, X.-Y.; Xue, X.; Yang, L.-J.; et al. Methyl jasmonate regulation of pectin polysaccharides in Cosmos bipinnatus roots: A mechanistic insight into alleviating cadmium toxicity. Environ. Pollut. 2024, 345, 123503. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Wang, W.; Lu, T.; Liu, L.; Yang, X.; Sun, X.; Qiu, G.; Hua, D.; Zhou, D. Zeolite-supported manganese oxides decrease the Cd uptake of wheat plants in Cd-contaminated weakly alkaline arable soils. J. Hazard. Mater. 2021, 419, 126464. [Google Scholar] [CrossRef]
- Wu, X.; Cai, Q.; Xu, Q.; Zhou, Z.; Shi, J. Wheat (Triticum aestivum L.) grains uptake of lead (Pb), transfer factors and prediction models for various types of soils from China. Ecotoxicol. Environ. Saf. 2020, 206, 111387. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, Y.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil contamination in China: Current status and mitigation strategies. Environ. Sci. Technol. 2015, 49, 750–759. [Google Scholar] [CrossRef]
- GB 2762-2017; National Food Safety Standard—Limits of Contaminants in Foods. National Health and Family Planning Commission of the People’s Republic of China and the China Food and Drug Administration: Beijing, China, 2017.
- Wang, X.; Lv, P.; Zhang, F.; Wang, W.; Liu, X.; Zhang, Q.; Mu, J.; Huang, X.; Bai, L.; Dai, J. Heavy Metal Accumulation in Maize and Wheat in Acidic Soil: A Comparative Study. Sustainability 2025, 17, 2084. [Google Scholar] [CrossRef]
- Han, S.; Li, H.; Rengel, Z.; Du, Z.; Hu, N.; Wang, Y.; Zhang, A. Biochar application promotes crops yield through regulating root development and the community structure of root endophytic fungi in wheat-maize rotation. Soil Tillage Res. 2023, 234, 105827. [Google Scholar] [CrossRef]
- Li, A.; Ye, C.; Jiang, Y.; Deng, D. Enhanced removal performance of magnesium-modified biochar for cadmium in wastewaters: Role of active functional groups, processes, and mechanisms. Bioresour. Technol. 2023, 386, 129515. [Google Scholar] [CrossRef]
- Yu, B.Z.; Li, D.Q.; Zhang, R.; He, H.Z.; Li, H.S.; Chen, G.K. Effects of heavy metals (Cd, Pb, Cu, Zn, and Ni) on Ipomoea aquatica Forsk. growth in a soil with containing metal-biochar application. Pol. J. Environ. Stud. 2020, 29, 2513–2524. [Google Scholar] [CrossRef]
- Li, D.Q.; Lai, C.H.; Li, Y.T.; Li, H.S.; Chen, G.K.; Lu, Q. Biochar improves Cd-contaminated soil and lowers Cd accumulation in Chinese flowering cabbage (Brassica parachinensis L.). Soil Tillage Res. 2021, 213, 105085. [Google Scholar] [CrossRef]
- He, M.J.; Xu, Z.B.; Hou, D.Y.; Gao, B.; Cao, X.D.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C.W. Waste-derived biochar for water pollution control and sustainable development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- Arwenyo, B.; Varco, J.J.; Dygert, A.; Brown, S.; Pittman, C.U.; Mlsna, T.M. Contribution of modified P-enriched biochar on pH buffering capacity of acidic soil. J. Environ. Manag. 2023, 339, 117863. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Wei, X.; Liu, Y.; Liang, J.; Shao, Y.; Huang, W.; Cheng, X. Different adsorption behaviors and mechanisms of a novel amino-functionalized hydrothermal biochar for hexavalent chromium and pentavalent antimony. Bioresour. Technol. 2020, 310, 123438. [Google Scholar] [CrossRef]
- Tang, J.; Ma, Y.; Deng, Z.; Li, P.; Qi, X.; Zhang, Z. One-pot preparation of layered double oxides-engineered biochar for the sustained removal of tetracycline in water. Bioresour. Technol. 2023, 381, 129119. [Google Scholar] [CrossRef]
- Wei, B.; Peng, Y.; Jeyakumar, P.; Lin, L.; Zhang, D.; Yang, M.; Zhu, J.; Lin, C.S.K.; Wang, H.; Wang, Z.; et al. Soil pH restricts the ability of biochar to passivate cadmium: A meta-analysis. Environ. Res. 2023, 219, 115110. [Google Scholar] [CrossRef]
- Liu, M.; Almatrafi, E.; Zhang, Y.; Xu, P.; Song, B.; Zhou, C.; Zeng, G.; Zhu, Y. A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: Applications and practical evaluations. Sci. Total Environ. 2022, 806, 150531. [Google Scholar] [CrossRef]
- Lai, C.H.; Li, D.Q.; Qin, J.H.; Li, J.; Yan, Z.G.; Chen, G.K. The migration of cadmium and lead in soil columns and their bioaccumulation in a multi-species soil system. Chemosphere 2021, 262, 127718. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, K.H.; Jiang, J.X.; Gao, X.; Xu, Y.M.; Wang, Y.L.; Xu, C.H.; Li, L.P.; Liang, X.F.; Shi, G.L. Enhancing the immobilization efficiency of mercapto-palygorskite on soil Cd through earthworm addition: Cd fractions, soil aggregates, and bacterial community. Appl. Soil Ecol. 2025, 209, 106024. [Google Scholar] [CrossRef]
- Zhang, C.M.; Liu, H.L.; Zhou, F.W.; Long, X.Z.; Liu, S.Q.; Wu, Y. Enhancing remediation efficiency of hyperaccumulators through earthworm addition: Evidence from a pot study on cadmium-contaminated soil. Sci. Total Environ. 2024, 934, 173169. [Google Scholar] [CrossRef]
- Wang, K.; Qiao, Y.; Zhang, H.; Yue, S.; Li, H.; Ji, X.; Liu, L. Bioaccumulation of heavy metals in earthworms from field contaminated soil in a subtropical area of China. Ecotoxicol. Environ. Saf. 2018, 148, 876–883. [Google Scholar] [CrossRef]
- Tong, F.; Xu, L.; Zhang, Y.; Wu, D.; Hu, F. Earthworm mucus contributes significantly to the accumulation of soil cadmium in tomato seedlings. Sci. Total Environ. 2024, 953, 176169. [Google Scholar] [CrossRef] [PubMed]
- Yuvaraj, A.; Thangaraj, R.; Ravindran, B.; Ravindran, B.; Chang, S.W.; Karmegam, N. Centrality of cattle solid wastes in vermicomposting technology: A cleaner resource recovery and biowaste recycling option for agricultural and environmental sustainability. Environ. Pollut. 2021, 268, 115688. [Google Scholar] [CrossRef]
- Hussain, N.; Chatterjee, S.K.; Maiti, T.K.; Goswami, L.; Das, S.; Deb, U.; Bhattacharya, S.S. Metal induced non-metallothionein protein in earthworm: A new pathway for cadmium detoxification in chloragogenous tissue. J. Hazard. Mater. 2021, 401, 123357. [Google Scholar] [CrossRef]
- Dedeke, G.A.; Owagboriaye, F.O.; Adebambo, A.O.; Ademolu, K.O. Earthworm metallothionein production as biomarker of heavy metal pollution in abattoir soil. Appl. Soil Ecol. 2016, 104, 42–47. [Google Scholar] [CrossRef]
- Schnug, L.; Jensen, J.; Scott-Fordsmand, J.J.; Leinaas, H.P. Toxicity of three biocides to springtails and earthworms in a soil multi-species (SMS) test system. Soil Biol. Biochem. 2014, 74, 115–126. [Google Scholar] [CrossRef]
- Fernández, C.; Alonso, C.; Babín, M.M.; Pro, J.; Carbonell, G.; Tarazona, J.V. Ecotoxicological assessment of doxycycline in aged pig manure using multispecies soil systems (MS-3). Sci. Total Environ. 2004, 323, 63–69. [Google Scholar] [CrossRef]
- Boleas, S.; Alonso, C.; Pro, J.; Babín, M.M.; Fernández, C.; Carbonell, G.; Tarazona, J.V. Effects of sulfachlorpyridazine in MS-3-arable land: A multispecies soil system for assessing the environmental fate and effects of veterinary medicines. Environ. Toxicol. Chem. 2005, 24, 811–819. [Google Scholar] [CrossRef]
- Boleas, S.; Alonso, C.; Pro, J.; Fernández, C.; Carbonell, G.; Tarazona, J.V. Toxicity of the antimicrobial oxytetracycline to soil organisms in a multispecies-soil system (MS-3) and influence of manure co-addition. J. Hazard. Mater. 2005, 122, 233–241. [Google Scholar] [CrossRef]
- Tarazona, J.V. Terrestrial Microcosms and Multispecies Soil Systems. Encycl. Toxicol. 2024, 8, 965–969. [Google Scholar]
- Fernández, M.D.; Cagigal, E.; Vega, M.M.; Urzelai, A.; Babín, M.; Pro, J.; Tarazona, J.V. Ecological risk assessment of contaminated soils through direct toxicity assessment. Ecotoxicol. Environ. Saf. 2005, 62, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.D.; Vega, M.M.; Tarazona, J.V. Risk-based ecological soil quality criteria for the characterization of contaminated soils. Combination of chemical and biological tools. Sci. Total Environ. 2006, 366, 466–484. [Google Scholar] [CrossRef]
- Carraturo, F.; Siciliano, A.; Giordano, A.; Di Capua, F.; Barone, F.; Casaletta, E.; Cicotti, F.; Guida, M.; Adani, F. Ecotoxicological assessment of waste-derived organic fertilizers and long-term monitoring of fertilized soils using a multi-matrix and multi-species approach. Sci. Total Environ. 2024, 912, 169341. [Google Scholar] [CrossRef]
- Fernández, M.D.; Alonso-Blázquez, M.N.; García-Gómez, C.; Babin, M. Evaluation of zinc oxide nanoparticle toxicity in sludge products applied to agricultural soil using multispecies soil systems. Sci. Total Environ. 2014, 497–498, 688–696. [Google Scholar] [CrossRef]
- Alonso, E.; Fernández, C.; Najera, I.; Pro, J.; Tarazona, J.V.; Carbonell, G. Assessing the influence of biota on metal mobility in a multi-species soil system (MS-3). Soil Sediment Contam. 2006, 15, 327–337. [Google Scholar] [CrossRef]
- Alonso, E.; González-Núñez, M.; Carbonell, G.; Fernández, C.; Tarazona, J.V. Bio-accumulation assessment via an adapted multi-species soil system (MS-3) and its application using cadmium. Ecotoxicol. Environ. Saf. 2009, 72, 1038–1044. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment and the State Administration for Market Regulation: Beijing, China, 2018.
- Leveque, T.; Capowiez, Y.; Schreck, E.; Xiong, T.; Foucault, Y.; Dumat, C. Earthworm bioturbation influences the phytoavailability of metals released by particles in cultivated soils. Environ. Pollut. 2014, 191, 199–206. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Qin, S.; Yang, G.; Fang, K.; Zhu, B.; Kuzyakov, Y.; Chen, P.; Xu, Y.; Yang, Y. Regulation of priming effect by soil organic matter stability over a broad geographic scale. Nat. Commun. 2019, 10, 5112. [Google Scholar] [CrossRef]
- Yang, K.; Wang, X.; Cheng, H.; Tao, S. Effect of aging on stabilization of Cd and Ni by biochars and enzyme activities in a historically contaminated alkaline agricultural soil simulated with wet–dry and freeze–thaw cycling. Environ. Pollut. 2021, 268 Pt A, 115846. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, G.; Li, X.; Zhang, X.; Hang, W.; Tang, M.; Gao, Y. Effects of biochar on the transformation of cadmium fractions in alkaline soil. Heliyon 2023, 9, e12949. [Google Scholar] [CrossRef]
- Lu, Y.X.; Qiao, Z.Y. Physical properties of alkaline soil and their effects on wheat seedling growth. Inn. Mong. Agric. Sci. Technol. 2006, 5, 38–39. (In Chinese) [Google Scholar]
- Martín-Franco, C.; Sánchez, J.T.; Alvarenga, P.; Peña, D.; Fernández-Rodríguez, F.; Vicente, L.A.; Albarrán, Á.; López-Piñeiro, A. Effects of fresh and field-aged holm-oak biochar on As, Cd and Pb bioaccumulation in different rice growing environments. Sci. Total Environ. 2023, 887, 164012. [Google Scholar] [CrossRef]
- Huang, C.Y.; Xu, J.M. Soil Science, 3rd ed.; China Agriculture Press: Beijing, China, 2010; pp. 170–188. [Google Scholar]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, L.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; El-Sherbini, M.A.A.; Selim, E.M.M. Effects of biochar on soil properties, heavy metal availability and uptake, and growth of summer squash grown in metal-contaminated soil. Sci. Hortic. 2022, 301, 111097. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Alo, M.N.; Onyekwere, A.M.; Crosse, J.D.; Nworie, O.; Chamba, E.B. Influence of biochar aaged in acidic soil on ecosystem engineers and two tropical agricultural plants. Ecotoxicol. Environ. Saf. 2018, 153, 116–126. [Google Scholar] [CrossRef]
- Elliston, T.; Oliver, I.W. Ecotoxicological assessments of biochar additions to soil employing earthworm species Eisenia fetida and lumbricus terrestris. Environ. Sci. Pollut. Res. 2020, 27, 33410–33418. [Google Scholar] [CrossRef]
- Gruss, I.; Twardowski, J.P.; Latawiec, A.; Medyńska-Juraszek, A.; Królczyk, J. Risk assessment of low-temperature biochar used as soil amendment on soil mesofauna. Environ. Sci. Pollut. Res. 2019, 26, 18230–18239. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, Y.; Deng, H.; Yu, H.; Feng, D.; Zhang, Y.; Ge, C.; Li, J. Significant contributions of biochar-derived dissolved matters to ecotoxicity to earthworms (Eisenia fetida) in soil with biochar amendment. Environ. Technol. Innov. 2023, 29, 102988. [Google Scholar] [CrossRef]
- Zhang, R.; Zimmerman, A.R.; Zhang, R.; Li, P.; Zheng, Y.; Gao, B. Persistent free radicals generated from a range of biochars and their physiological effects on wheat seedlings. Sci. Total Environ. 2024, 908, 168260. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Xu, M.; Liang, S.; Feng, Z.; Zhao, J. Mechanism of persulfate activation by biochar for the catalytic degradation of antibiotics: Synergistic effects of environmentally persistent free radicals and the defective structure of biochar. Sci. Total Environ. 2021, 794, 148707. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, J.; Zhou, J.; Wu, X.; Yang, K.; Ni, Z.; Liu, Z.; Jia, H. The overlooked toxicity of environmentally persistent free radicals (EPFRs) induced by anthracene transformation to earthworms (Eisenia fetida). Sci. Total Environ. 2022, 853, 158571. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, L.; Liu, J.; Deng, J.; Zou, J.; Zhang, X.; Shen, Z.; Chen, Y. Earthworm-mediated nitrification and gut digestive processes facilitate the remobilization of biochar-immobilized heavy metals. Environ. Pollut. 2023, 322, 121219. [Google Scholar] [CrossRef]
- Xu, P.; Sun, C.X.; Ye, X.Z.; Xiao, W.D.; Zhang, Q.; Wang, Q. The effect of biochar and crop straws on heavy metal bioavailability and plant accumulation in a Cd and Pb polluted soil. Ecotoxicol. Environ. Saf. 2016, 132, 94–100. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).