Abstract
Early blight, caused by fungi of the genus Alternaria, is one of the most destructive diseases affecting tomato plants, leading to a decrease in yield and commercial value. Studies so far on Alternaria spp. affecting tomato in Kazakhstan have been limited to morphological identification or molecular analysis, without an in-depth phylogenetic study and pathogenicity assessment. In this study, between 2023 and 2024, 61 isolates were obtained from tomato leaves with early blight symptoms and identified, based on conidial morphology and DNA sequencing, as A. tenuissima (54%) and A. alternata (46%). The pathogenicity assessment showed that the disease index for A. tenuissima was 21.7–53.3, while it was 41.7–60.0 for A. alternata, indicating greater aggressiveness of the latter species. The disease index varied by region, with the highest average value recorded for A. alternata from Almaty (55.7%), while 38.2% and 36.2% for A. tenuissima were recorded from Pavlodar and Akmola, respectively. Both species showed notable intraspecific variation in pathogenicity. To our knowledge, this is the first reported case of A. tenuissima detection as the causative agent of early blight in tomato plants in Kazakhstan. The results of this study may help facilitate the development of effective disease management strategies.
1. Introduction
Tomato (Solanum lycopersicum L.) is the second most important vegetable crop worldwide, with an annual production of more than 192.3 million metric tons over an area of 5.4 million hectares. The leading tomato-producing countries are China, India, and Turkey, which play key roles in the global agricultural sector and account for a significant share of the global market [1]. Tomato fruits are a source of vitamins A and C, folic acid, and the antioxidant lycopene, which helps reduce the risk of cancer and cardiovascular diseases [2]. The concentration of lycopene in tomato fruits varies depending on the variety and ranges from 0.9 to 4.2 mg per 100 g. In processed products such as tomato sauce and ketchup, the lycopene content is significantly higher, reaching 33–68 mg per 100 g [3]. Thus, tomatoes can be consumed fresh, processed, or canned. In Kazakhstan, tomatoes are the second-largest vegetable crop, with an annual production volume of approximately 792 thousand tons on an area of 28.8 thousand hectares; however, the average tomato yield is only 27.4 tons/ha (2023), which is 50% lower than the global average [1].
Tomato production is limited by the effects of various diseases, including those caused by fungi. Significant crop losses are caused by diseases such as late blight, caused by Phytophthora infestans [4], fusarium wilt (Fusarium oxysporium) [5], early blight (Alternaria linariae and A. alternata) [6], and septoria leaf spot (Septoria lycopersici) [7]. Among the fungal diseases affecting tomato plants, the most dangerous in terms of reducing the yield and commercial value of products is early blight caused by the fungus Alternaria. In some cases, yield losses caused by early blight can reach 79% [6]. Early blight caused by Alternaria spp. is characterized by necrotic leaf spots with concentric rings, which can lead to defoliation and reduced yield [8,9]. Tomato fruits at all stages of ripeness remain susceptible to infection, which typically manifest as dark, sunken lesions. On the stems, necrotic lesions are often elongated or striated and may form concentric rings in some cases [10].
The genus Alternaria comprises endophytic, saprophytic, and pathogenic species commonly found in the air, soil, plant debris, and food products. Pathogenic species are capable of long-term survival as mycelium or conidia on plant residues and as latent infections in seeds. Colonization of host plant tissues is often facilitated by mechanical injury or physiological weakening [11]. Infection is initiated through the secretion of various enzymes and secondary metabolites by the fungal pathogen, which contribute to the degradation of host cell structures and facilitate tissue penetration prior to active colonization [6].
Alternaria species produce approximately 70 mycotoxins [12,13,14]. The most extensively studied secondary metabolites produced by Alternaria species include alternariol (AOH), alternariol monomethyl ether (AME), altertoxins I and II (AT-I, AT-II), and tenuazonic acid (TeA) [10]. Some metabolites are species-specific, whereas others are synthesized by multiple species [15]. For example, Alternaria porri produces tentoxin, whereas A. tomatophila and A. solani produce altersolanol A, AT-I, and macrosporin. Alternaria alternata is known to produce TeA, AOH, and AME, also species-specific mycotoxins, and ALT, all of which are considered major food contaminants [10,16].
These secondary metabolites have genotoxic, mutagenic, carcinogenic, and cytotoxic properties and pose risks to human and animal health [17,18]. Early blight causes the greatest damage in areas with high temperatures alternating with heavy rainfall and high humidity. However, they can also occur in semi-arid climates [19].
Currently, the taxonomy of the genus Alternaria is still under discussion and revision. According to Simmons, Alternaria tomatophila is the most common and widespread pathogen causing early blight of tomato [20]. In 2014, Woudenberg et al. grouped isolates from Solanaceae, Cucurbitaceae, and Scrophulariaceae, all belonging to large-spore Alternaria species, into a new species designated A. lineariae [21]. This revision established a distinction between large- and small-spore forms (A. alternata and A. arborescens) isolated from Solanaceae. Morphological, molecular, and chemotaxonomic approaches have been used to differentiate the species [6].
Among the large-spored species, A. linariae (syn. A. tomatophila), A. solani, A. alternariacida, A. blumeae, A. crassa, A. grandis, and A. protenta are the primary causal agents of tomato early blight [22]. Among the small-spored species, the most important are A. alternata and A. tenuissima [19,23,24,25].
Traditionally, the identification of Alternaria spp. has been based on morphological characteristics [20,26], making it difficult to distinguish species, as many share similar morphological characteristics [21,27]. For precise identification, a combination of morphological features and molecular methods is required. In the morphological description of Alternaria spp., the following features are taxonomically important: the size and shape of the conidia and, in some cases, the size and shape of the conidiophores. Also important is the sporulation habitus—the general type of sporulation—which includes the presence of spore chains, their length, the nature of branching, the size of the spore “bushes”, and their density [8]. Molecular methods for identifying Alternaria are based on the use of specific primers to amplify conserved genetic regions. One of the most common genetic markers is the internal transcribed spacer (ITS) region, which is used as a standard barcode to identify fungi [28]. However, to improve the accuracy of genetic analysis, additional genes, such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), RNA polymerase II (rpb2), elongation factor 1-alpha (tef1), histone (H3), and Alternaria allergen gene (Alt a 1), are often sequenced [29,30,31].
Information on the population structure of Alternaria species that cause early blight in tomato is critical to better understand the distribution and importance of the different species and to develop effective management strategies to control this disease [6,19]. Key strategies for managing early blight in tomato include culture practice, fungicide application, and the use of resistant cultivars. Fungicide treatments remain the most widely used approach to reduce crop losses [6]. However, the timing of application in relation to environmental conditions and disease development is critical for achieving effective control [32]. In addition, the visual assessment of symptoms does not always facilitate an accurate identification of the causal species, such as distinguishing between lesions caused by A. linariae, A. solani, or other Alternaria spp. Therefore, further information on pathogen biology and disease aetiology is necessary for the development of reliable management strategies [19].
To our knowledge, studies on Alternaria species, which cause early blight in tomatoes in Kazakhstan, have so far been limited to either identification by morphological characteristics or molecular analysis, without in-depth phylogenetic studies and pathogenicity assessments [33,34]. However, morphological identification alone can be inaccurate, and the lack of data on the pathogenicity of isolates prevents an objective assessment of the threat these isolates pose to agricultural production. Therefore, we conducted a comprehensive study of Alternaria spp., integrating morphological and molecular identification with phylogenetic analysis alongside an assessment of the pathogenicity of the isolates on tomato.
2. Materials and Methods
2.1. Sample Collection and Fungal Isolation
Tomato leaves with typical early blight symptoms, characterized by brown necrotic leaf spots with concentric rings, were collected at the end of the 2023–2024 growing season from three regions in Kazakhstan (Figure 1).

Figure 1.
Early blight symptoms on tomato plants: (a) typical early blight symptoms on tomato leaves; (b) lesions on senescing foliage during the late growth stage.
Samples were collected from five points in each field (Table 1). Symptomatic leaves were placed in individual bags and transported to the Laboratory of Biotechnology and Plant Breeding. Between 2 and 5 symptomatic leaves were collected per field, resulting in a total of 70 samples from the three regions.

Table 1.
Numbers of Alternaria isolates collected from diseased tomato leaves collected in three regions of Kazakhstan.
To isolate the fungus, 1 cm × 1 cm pieces of affected tissue were treated with 70% ethanol for 1 min, washed three times with sterile distilled water, and placed on potato–carrot agar (PCA) (20 g potato, 20 g carrot, 20 g agar, and 1000 mL sterile distilled water). Subsequently, 100 mg/L ampicillin (Sigma-Aldrich, St. Louis, MO, USA) was added, and the tissues were incubated at 25 °C under natural light until sporulation occurred. Mycelial fragments were extracted from growing colonies under a stereomicroscope and transferred to fresh PCA nutrient medium in Petri dishes and incubated at 23 °C for 5 days. A pure culture of the fungus was obtained by transferring individual spores to a nutrient medium potato dextrose agar (PDA, Difco, Detroit, MI, USA) with subsequent cultivation at 23 °C. After sufficient growth, the pure culture was stored at 4 °C.
2.2. Morphological Characterization
PDA nutrient medium was used to study culture characteristics such as the colour, size, and texture of colonies. Mycelial disks of 5 mm diameter were cut from the edges of growing colonies of a 5-day culture, placed on nutrient medium in Petri dishes, and incubated in the dark at 25 °C, and the characteristics were assessed after 7 days. To study the morphological characteristics, the mycelium was transferred to PCA and incubated at 22 °C under fluorescent lamps with a photoperiod of 8 h of light and 16 h of darkness. After 7 days, the morphology of conidia (colour, shape, length, and width of the body and the number of septa of 50 randomly selected conidia of each isolate) and the nature of sporulation were assessed using a Zeiss Axio Scope A1 microscope (Carl Zeiss Microscopy GmbH, Jena, Germany) with a magnification of 400×. Samples were identified based on morphological characteristics according to previously published recommendations [20,31].
2.3. DNA Extraction and PCR Amplification
A total of 61 isolates of Alternaria were selected for molecular identification. The total genomic DNA of the isolates was isolated from pure cultures grown on PDA in the dark for 7 days at 22–23 °C. Mycelia of each isolate were collected using a sterile scalpel by scraping from the medium and were transferred to microtubes. DNA was extracted using the CTAB method [35]. The DNA pellet was dissolved in 100 μL TE buffer (10 mM Tris-HCl pH 8.0, 0.1 mM EDTA). The quality and quantity of isolated DNA were assessed using a NanoDrop1000 spectrophotometer (Thermo Scientific, Wilmington, NC, USA). The ITS1 and ITS4 primers were used to amplify the ITS region of nuclear ribosomal DNA, including the 5.8S rDNA gene [36]. The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene region was amplified using primers gpd1/gpd2 [37]. The histone 3 (H3) gene region was amplified using primers H3-1a and H3-1b [38]. PCR was carried out in a volume of 25 μL containing 1 μL DNA (5 ng), 2.5 μL PCR buffer (10×), 2 μL MgCl2 (25 mM), 2 μL of each forward and reverse primer (10 pmol) (Table 1), 0.25 μL Dream Taq polymerase, 1.5 μL dNTPs (10 mM), and deionized water. The same reaction mixture without template DNA was used as a negative control. Amplification was conducted under the following cycling conditions: pre-denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55–58 °C for 30 sec, and extension at 72 °C for 1 min. Final elongation was performed at 72 °C for 7 min in a SimpliAmp Thermal Cycler (Thermo Fisher Scientific Inc., Waltham, MA, USA). The PCR products were visualized on a 1% agarose gel in 1× TBE buffer supplemented with ethidium bromide and irradiated with ultraviolet light. A 1000 bp DNA Ladder (Fermentas) was used as a molecular weight marker. The electrophoresis results were documented using a GelDoc XR system (Bio-Rad, Hercules, CA, USA). PCR products were purified using alkaline phosphatase Sap and exonuclease ExoI (Thermo Fisher Scientific). The reaction was carried out in a 20 μL volume containing 1× Sap buffer, 10 μL PCR product, 3 units ExoI, and 1 unit Sap at 37 °C for 30 min, followed by enzyme inactivation at 75 °C for 15 min. DNA sequencing was performed using an ABI PRISM® BigDye™ Terminator v.3.1 reagent kit. The sequencing products were analysed using an Applied Biosystems 3730 DNA Analyser (Thermo Fisher Scientific, Foster City, CA, USA).
2.4. Pathogenicity Tests
The pathogenicity test was performed using separated compound leaves of 45-day-old tomato plants of the “Surprise” variety. Pathogenicity tests were conducted by individually inoculating leaves with 27 isolates, including 11 A. alternata and 16 A. tenuissima isolates (Table 1, Supplementary Table S1). These isolates were selected to represent the species composition of populations from the three studied regions. To determine the pathogenicity of Alternaria isolates, identified isolates were incubated on PCA for 7–10 days at 25 °C. Colonies on the PCA plates were covered with sterile distilled water and 0.01% Tween-80 to disperse the spores. The surface of the colonies was carefully scraped off, and the suspension was collected. After filtration, the spore suspension was adjusted to a concentration of 106 conidia/mL using a haemocytometer. For inoculation, the apical leaves were pre-disinfected in a 1% sodium hypochlorite (NaOCl) solution for 1 min, washed with sterile distilled water (1 min), and dried on sterile filter paper in a laminar flow hood. Two small punctures were made in each leaf. One leaf was placed in a Petri dish and housed in a plastic box (18 × 13 × 4 cm; length × width × height). Sterile moist filter paper was placed at the bottom of the box to maintain high humidity. Twenty microlitres of the spore suspension was applied to the upper surface of each leaflet (two spots per leaflet). The control group was inoculated with sterile distilled water. Three replicates were performed for each isolate, with 30 leaves per replicate. The plastic boxes were sealed to maintain high humidity and incubated at 25 °C, 90% relative humidity, and a photoperiod of 12 h light/12 h dark. After 14 days, the diameters of the lesions on the inoculated leaves were measured. Disease severity (DS) was scored using a 4-point scale [39]: 1 = no lesions, 2 = lesions < 1 mm in diameter, 3 = lesions 1–5 mm in diameter, and 4 = lesions > 5 mm in diameter. The disease index (DI) was calculated using the formula DI = [100 × ∑ (n × corresponding DS)]/(N × 4), where n is the number of infected leaflets corresponding to each disease rating and N is the total number of leaflets [40]. To confirm Koch’s postulates, fungi were re-isolated from the inoculated leaflets and identified based on their morphological features.
2.5. Phylogenetic Analysis
Nucleotide sequences of the analysed samples were collected and edited using SeqMan [41]. A sequence similarity search was performed using the BLAST algorithm in the National Center for Biotechnology Information (NCBI) database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) (accessed on 7 April 2025). The nucleotide sequences of the ITS, GAPDH, and H3 loci were aligned using ClustalW, concatenated, and used to construct a combined phylogenetic tree. The ends of the alignment were trimmed to avoid regions with missing data. The Kimura 2-parameter model with gamma-distributed rate variation (K2 + G) was used for ITS and GAPDH phylogenetic inference. The Tamura–Nei model with gamma distributed rate variation (TN93 + G) was used for ITS and H3. Phylogenetic analysis was performed using the Maximum Likelihood (ML) method with 1000 bootstrap replicates in MEGA 6 (version 6.06; http://www.megasoftware.net/ (accessed on 7 April 2025)) [42].
2.6. Data Analyses
Data were analysed using Python 3.11. The Pandas library (v2.2.1) was used for data processing and calculation of descriptive statistics. Visualizations, including boxplots, were generated using Matplotlib (v3.8.4) and Seaborn (v0.13.2). Pathogenicity was assessed at three levels: individual isolates, fungal species, and regions. For isolate-level analysis, mean values were calculated from three biological replicates per isolate. No pooling was performed between isolates at this level. For species- and region-level comparisons, disease incidence and disease index (DI) values were pooled by fungal species and by region, respectively, and reported as mean ± standard deviation (SD). To assess the effect of fungal species (A. alternata and A. tenuissima) on disease incidence (%), a one-way ANOVA was performed using the StatsModels package. Levene’s test (scipy.stats v1.13.0) was used to evaluate the homogeneity of variances. Since variances between isolates were not homogeneous, Dunnett’s T3 test (via scikit_posthocs) was applied for multiple comparisons of disease incidence between isolates. For the comparison of DI (%) between species, the one-way ANOVA revealed statistically significant differences. To support this result, the Least Significant Difference (LSD) test was additionally performed. To evaluate differences in the DI and disease incidence among fungal isolates within each species, the Kruskal–Wallis H-test was applied separately for A. alternata and A. tenuissima. For visual grouping of isolates by aggressiveness, descriptive statistics were calculated, and isolates were classified into three groups (a: high, b: moderate, and c: low) based on the quartiles of the mean DI values. Boxplots were used to visualize the distribution of the DI by species and region. All statistical tests were two-sided, and results were considered statistically significant at p ≤ 0.05.
3. Results
3.1. Morphological Identification of Alternaria Species Associated with Early Blight on Tomato
Between 2023 and 2024, 61 isolates were obtained from tomato leaves with early blight symptoms. Based on the conidial morphology and sporulation patterns, the isolated fungi were tentatively classified as A. tenuissima (54%) and A. alternata (46%). Colonies of 33 isolates identified as A. tenuissima on PDA medium were grey to light brown, with dense aerial mycelia and a thin white border (Figure 2). The conidia formed simple long chains consisting of 7–14 conidia with one or two lateral branches on the PCA. The conidiophores were short, 14.7–64.9 μm long and 3.2–6.3 μm wide, and arose singly. The conidia were ovoid or inversely club-shaped and 17.7–47.8 × 8.2–9.6 μm in size. The conidia contained 3 to 7 transverse septa and 0–2 longitudinal septa. The colony colour of the 28 isolates identified as A. alternata ranged from dark grey to almost dark olive with 3–4 concentric rings. The colonies were dense with distinct white margins. The conidiophores on PCA were solitary, straight, or curved and 18.2–59.6 μm × 3.3–7.2 μm in size. The conidia formed branching chains, were ovoid to ellipsoidal, brown in colour, and 20.6–42.8 × 7.8–8.6 μm in size. The conidia had 3–7 transverse and 0–4 longitudinal septa.
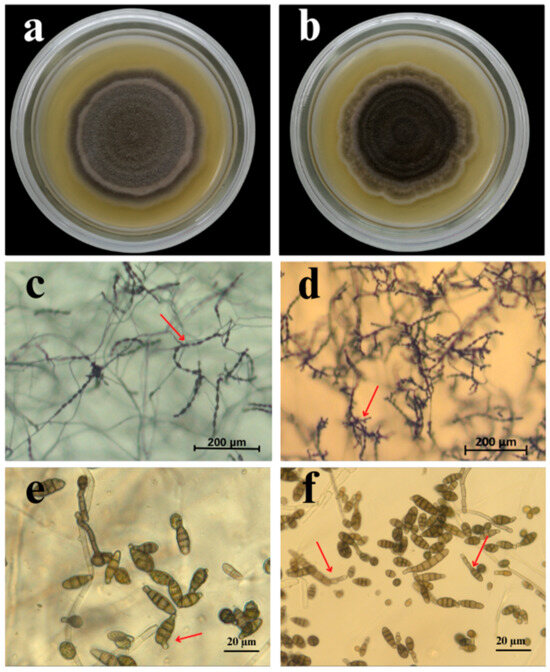
Figure 2.
Morphological features of Alternaria species isolated from tomato: (a,b) colony morphology of A. tennuissima and A. altrernata isolates on potato dextrose agar (PDA) plates, respectively; (c,d) sporulation patterns of A. tennuissima and A. altrernata isolates on potato carrot agar (PCA) plates, respectively; (e,f) conidia of A. tennuissima and A. altrernata isolates on PCA plates, respectively. Red arrows indicate characteristic differences in sporulation patterns (c,d) and conidial morphology between the two species (e,f).
3.2. DNA Sequencing and Phylogenetic Analysis
The PCR amplification of 61 Alternaria isolates with universal primers ITS1 and ITS4 yielded a 570 bp fragment that showed 100% homology to the sequences of A. alternata (GenBank Accession No. KP124298, KP124299) and A. tenuissima (AF347032). The amplification with primers gpd1 and gpd2 yielded 620 bp products that showed more than 99% homology with the sequences of A. alternata (GenBank Accession No. KP124155, KP124156) and A. tenuissima (AY278809). The PCR amplifications with primers H3-1a and H3-1b yielded about 546 bp fragment for 33 Alternaria isolates, showing over 99% identity to those of A. tenuissima (GenBank Accession No. MN505806, MN505803); 28 isolates (440 bp) were over 99% identity to those of A. alternata (GenBank Accession No. MN505801, MN506040). The resulting sequences of Alternaria isolates were deposited in GenBank (Supplementary Table S1).
The phylogenetic analysis based on concatenated ITS and GAPDH sequences indicated a clear separation of small- and large-spore Alternaria species. In turn, 27 of the studied isolates were grouped with the reference strains, A. alternata, A. arborescens, and A. tenuissima, with a high degree of support (bootstrap = 99%), confirming that they belonged to this phylogenetic lineage. A clear phylogenetic separation was also indicated between A. alternata and A. longipes, supported by a bootstrap value of 87%, indicating the evolutionary isolation of these species (Figure 3).
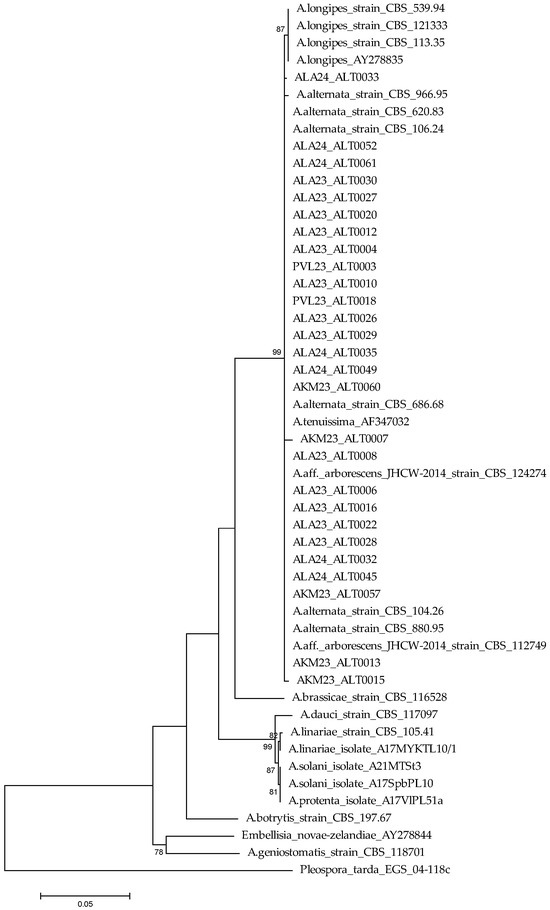
Figure 3.
Phylogenetic tree reconstructed by the Maximum Likelihood method based on combined rDNA internal transcribed spacer (ITS) and GAPDH sequence Alternaria isolates using MEGA 6 with 1000 bootstrap replications. Bootstrap values ≥ 70% were considered as significant and are indicated in the phylogenetic trees.
The phylogenetic analysis based on concatenated ITS and H3 sequences of 27 isolates of A. alternata and A. tenuissima, along with reference sequences from the NCBI database, allowed us to clearly distinguish between the two main clades. The upper clade included all isolates identified as A. tenuissima, forming a well-supported monophyletic lineage (bootstrap = 100%), which reflects their close genetic relationship. In contrast, the lower part of the tree contained A. alternata isolates, distributed across several subclades. This structure was strongly supported by a bootstrap value of 100%. In addition, the reference isolates A. brassicicola and A. solani formed a distinct clade, highlighting the evolutionary isolation of these large-spored species (Figure 4).
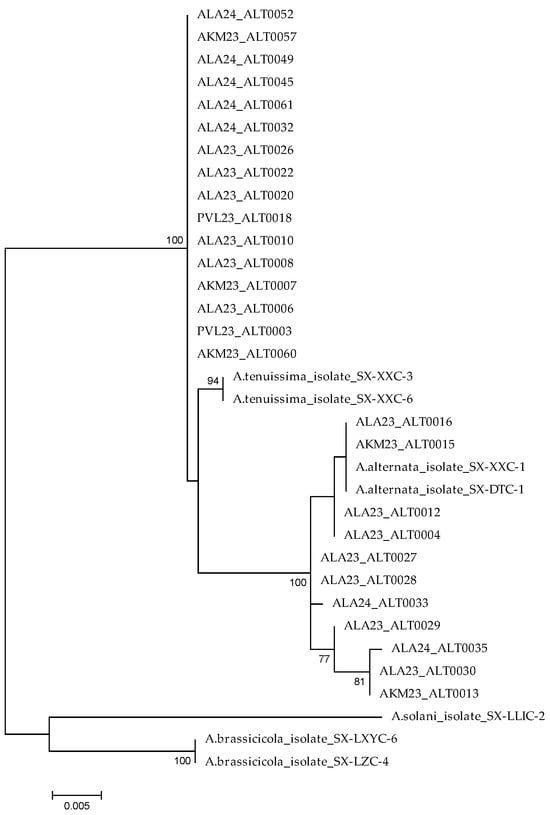
Figure 4.
Phylogenetic tree reconstructed by the Maximum Likelihood method based on combined rDNA internal transcribed spacer (ITS) and H3 sequences Alternaria isolates using MEGA 6 with 1000 bootstrap replications. Bootstrap values ≥ 70% were considered as significant and are indicated in the phylogenetic trees.
3.3. Pathogenicity Analysis
When tomato leaves were inoculated with a spore suspension of 27 Alternaria isolates, the first symptom of the disease appeared as brown necroses after 3 days. Isolates of A. alternata were characterized by the development of a typical yellow halo that enlarged as the lesions grew, eventually covering most of the inoculated leaf area, with the two inoculation points merging into a single lesion. In contrast, A. tenuissima isolates produced localized lesions confined to the inoculated areas. After 14 days, the spots became dark brown, oval, or round and ranged in size from 1 to 9 mm. Control samples inoculated with sterile distilled water did not show any symptoms of the disease (Figure 5).
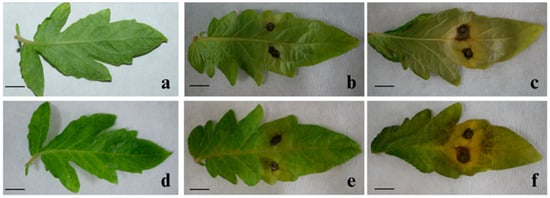
Figure 5.
Pathogenicity of the isolates of A. tenuissima and A. alternata on the detached leaves of tomato: (a,d) inoculated with sterile water (control); (b,e) inoculated with A. tenuissima; (c,f) inoculated with A. alternata, 14 days after inoculation. Scale bars: 1 cm.
The pathogens were re-isolated from symptomatic plant tissues, and their morphological features matched those of the original isolate, which was consistent with Koch’s postulates. To identify species-level differences in aggressiveness, a one-way ANOVA was performed to compare disease incidence (%) between the two fungal species (Alternaria alternata and A. tenuissima) and among individual isolates. Levene’s test indicated unequal variances between isolates, which necessitated the use of Dunnett’s T3 post hoc test for pairwise comparisons. However, this test did not reveal any significant differences between the individual isolates. Additionally, the comparison of disease incidence between the two species did not show a significant difference (p = 0.0879), which may suggest a similar infection frequency range under standardized inoculation conditions.
In contrast, a comparison of the DI (%) between species revealed a statistically significant difference (p < 0.001). A. alternata exhibited consistently higher DI values with lower variance, indicating stronger and more stable aggressiveness. A. tenuissima, on the other hand, demonstrated a wide range of DI values, which may reflect high intraspecific variability due to genetic differences among isolates, environmental sensitivity, or differences in virulence mechanisms.
To further assess intraspecific variability among isolates, Kruskal–Wallis H-tests were performed separately for A. alternata and A. tenuissima. For A. tenuissima, significant differences were observed among isolates in both disease incidence (H = 44.06, p = 1.08 × 10−4) and the DI (H = 43.43, p = 1.35 × 10−4). A. alternata also exhibited significant intraspecific variability regarding disease incidence (H = 26.38, p = 3.26 × 10−3) and the DI (H = 26.98, p = 2.62 × 10−3), although the variation was less pronounced compared to A. tenuissima. Thus, in addition to species-level differences, the data support a substantial influence of individual isolate characteristics on pathogenicity. To simplify the interpretation of isolate variability, descriptive statistics of the DI were calculated, and all isolates were grouped into three aggressiveness categories: “a” (top 25% by DI, high aggressiveness), “b” (middle 50%, moderate aggressiveness), and “c” (bottom 25%, low aggressiveness). Most A. alternata isolates fell into group “a”, confirming their consistently high pathogenicity. A. tenuissima isolates were distributed across all three groups, supporting the greater intraspecific variation revealed by the Kruskal–Wallis tests (Supplementary Table S2).
The mean DI values also support species-level differences. For A. tenuissima, disease incidence ranged from 50% to 100% (81.3 ± 13.5), and the DI ranged from 21.7 to 53.3 (39.2 ± 8.6). For A. alternata, disease incidence ranged from 73.3% to 100% (87.4 ± 7.2), and the DI from 41.7 to 60.0 (54.4 ± 4.4). These results highlight the higher and more stable aggressiveness of A. alternata isolates.
Additionally, geographic variation in pathogenicity was evaluated based on the origin of the isolates. Samples were originally collected from three regions: Akmola, Pavlodar, and Almaty. A. alternata was isolated only from Akmola and Almaty and was not detected in Pavlodar (ND). Isolates of A. alternata originating from Almaty exhibited the highest DI values (55.7 ± 3.9), suggesting the presence of more aggressive isolates in that region. A. tenuissima was isolated from all three regions. Among its isolates, the highest mean DI was observed for those from Almaty (40.1 ± 8.6), while lower values were recorded for isolates from Akmola (36.2 ± 9.2) and Pavlodar (38.2 ± 8.0). These findings may indicate a potential influence of regional environmental conditions on the pathogenic behaviour of the fungal isolates (Table 2).

Table 2.
Disease incidence and disease index of Alternaria isolates by region of origin.
To visually assess the variability of the data, a boxplot was constructed (Figure 6). The distribution of DI by species (Figure 6a) demonstrated that A. alternata isolates displayed higher and more consistent DI values. In the region-based boxplot (Figure 6b), the highest and most stable pathogenicity was observed in A. alternata isolates originating from Almaty, whereas A. tenuissima isolates from Pavlodar and Akmola exhibited considerable within-group variability.
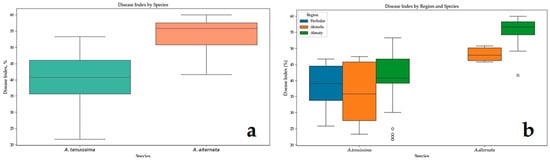
Figure 6.
Comparison of disease index for Alternaria species: (a) disease index for A. tenuissima and A. alternata; (b) disease index for A. tenuissima and A. alternata across different regions.
Overall, the results suggest that pathogenicity is influenced not only by species identity but also by isolate-specific traits and region of origin, highlighting the need for a multilayered assessment of the pathogen.
4. Discussion
This study presents the first data on Alternaria species associated with early blight of tomato in Kazakhstan based on morphological and molecular methods. The pathogenicity of the isolates was confirmed, and their taxonomic affiliations were clarified using phylogenetic analysis.
Existing data from Kazakhstan indicate that the main causative agent of early blight in tomatoes is A. alternata [33,34]. However, our study, based on morphological and molecular identification using three pairs of primers, showed that of 61 Alternaria isolates, 33 belonged to the species A. tenuissima and 28 belonged to A. alternata. These results are consistent with previous studies confirming that A. tenuissima and A. alternata are the pathogens that cause early blight of tomato in Iran [43]. To our knowledge, this is the first reported case of A. tenuissima causing early blight on tomato in Kazakhstan. Other species of the genus Alternaria (A. linariae, A. solani, A. arborescens, and A. grandis) that have been reported to cause early blight of tomato in other countries have not been detected in Kazakhstan.
This disease affects various crops worldwide, indicating a wide range of potential hosts for the pathogen. Early blight caused by species of Alternaria has been reported in tomato [43], potato [19,44], eggplant [45], wheat [46], pistachio [39], and onion [47]. Alternaria pathogens cause significant crop losses and contaminate produce with mycotoxins, posing a threat to food safety [11]. Understanding the species composition of the pathogen allows the development of effective plant protection strategies, as different species of Alternaria differ in their aggressiveness and ability to synthesize mycotoxins [48,49,50,51] (Supplementary Table S3).
The morphologically studied fungal species demonstrated distinct differences. A. tenuissima colonies were grey or light brown in colour, had dense aerial mycelium, and had a thin white border along the edge. A. alternata colonies varied in colour from dark grey to almost dark olive, with three to four distinct, concentric rings. A. tenuissima conidia formed predominantly simple, elongated chains, whereas A. alternata had branching conidia. Our results are consistent with those of previous studies [20,52].
The identification of Alternaria species based primarily on morphological characteristics is often difficult [53]. The wide range of hosts and significant morphological diversity of the representatives of this genus complicate species classification. Under such conditions, molecular methods provide reliable and objective criteria for species differentiation [30]. Molecular analysis allowed us to successfully identify the Alternaria species and confirm their identification based on morphological features. Furthermore, the amplification of the ITS and GAPDH genes yielded fragments of 570 and 620 bp, respectively. The amplification of the H3 gene yielded fragments of 546 (for 33 isolates) and 440 bp (for 28 isolates).
A comparison of the obtained sequences with those in the GenBank database allowed us to identify the isolates as A. alternata and A. tenuissima. Our results are in line with similar studies in which several Alternaria species and closely related fungi were identified based on the GAPDH sequences, H3 gene sequences, and ITS region sequences [54,55,56].
The taxonomy of A. alternata remains contentious. Based on a multigene phylogenetic analysis covering 35 morphological species described by Simmons (2007), this species was classified as a single species (A. alternata), as molecular data do not allow a reliable distinction between these taxa [20,31]. This approach, however, has caused confusion in subsequent taxonomic studies, especially when attempting to classify new isolates from different hosts [57]. The morphological boundaries of A. alternata remain poorly defined, and the question of the precise classification of representatives of this species remains open, requiring further research [58]. Phylogenetic analysis and accurate species identification present significant challenges for small-spore Alternaria species [46].
In the current study, the construction of a Maximum Likelihood tree based on ITS and GAPDH sequences did not allow for the effective differentiation of species within the small-spored Alternaria group, which is consistent with previous studies indicating the insufficient discriminatory power of this region in identifying closely related taxa [31,59]. In contrast, in the study by Rotondo et al. [60], representatives of A. arborescens formed a separate cluster. However, isolates belonging to A. alternata and A. tenuissima did not show a clear distinction, confirming their high degree of genetic similarity [50]. Similarly, Armitage et al. (2015) also reported that A. arborescens and A. gaisen formed distinct clades, whereas A. tenuissima, A. alternata, and A. mali isolates were grouped into a single phylogenetic lineage [53]. Despite the lack of clear phylogenetic divergence between A. alternata and A. tenuissima based on ITS and GAPDH gene sequences, the isolates show consistent morphological differences. Although the sporulation phenotype does not accurately reflect evolutionary relationships, it remains important for the classification of subgroups [58].
The phylogenetic analysis based on concatenated ITS and H3 gene sequences, supported by a bootstrap value of 100%, demonstrated a clear phylogenetic distinction between A. alternata and A. tenuissima. Similarly, in the study by Kang et al. [61], the use of ITS and H3 genes allowed for the separation of A. arborescens, A. infectoria, and A. tenuissima, which were also distinguishable morphologically.
These findings are further supported by the study of Shi et al. [54], who reported the detection of leaf spot caused by Alternaria species on Chinese cabbage. Our results are consistent with other studies that characterized Alternaria species [40,52,62].
The main objective of this study was to identify Alternaria species causing early blight of tomato in Kazakhstan and to evaluate the pathogenicity of the isolates for the subsequent development of effective disease control strategies, since these species exhibit different sensitivity to certain fungicides. The ANOVA results (p < 0.001) showed a significant difference between pathogen type and the DI. In general, A. alternata isolates were more aggressive than A. tenuissima isolates. However, in strawberries [39], A. tenuissima demonstrated a higher DI compared to A. alternata (20.2 ± 7.9 and 17.6 ± 11.6, respectively). These differences may be due to genetic variability within species, the influence of environmental conditions, and differences in resistance mechanisms among crops [59].
Our findings regarding intraspecific variation in pathogenicity are consistent with previous studies on other Alternaria species. For example, a study by Zhao et al. (2023) found that although all tested strains of A. melongenicola, A. solani, and A. yichangensis caused 100% disease incidence under controlled conditions, the disease index varied markedly among strains—from highly virulent ones such as A. solani YZU 151049 (62.5%) to weakly virulent or asymptomatic strains such as A. argyroxiphii (YZU 211300) and A. blumeae (YZU 171159) [22]. These findings underscore the importance of strain-level assessment when evaluating pathogenic potential, aligning with the significant intraspecific variation observed in both A. alternata and A. tenuissima isolates in our study.
The highest DI values observed for isolates originating from the Almaty region may be due to more favourable climatic conditions for pathogen development in that area. In particular, this region has a higher annual precipitation (400–600 mm) compared to the drier Akmola and Pavlodar regions (approximately 250–350 mm). The average air temperature during the growing season in the Almaty region is approximately 21 °C, whereas in Akmola and Pavlodar, it is 17 °C and 19 °C, respectively [63]. Humidity and temperature are key factors for conidial germination and infection of plants by Alternaria spp. [60,61]. The present findings suggest that both pathogen species and the region of isolate origin can substantially affect the aggressiveness of the isolates observed in this study. Isolates of A. alternata from Almaty showed the highest pathogenicity and may be of particular epidemiological relevance, requiring priority consideration in plant protection strategies.
In summary, our study provided novel insights into the species composition of Alternaria associated with early blight in tomato plants in Kazakhstan. By identifying the dominant species and clarifying their pathogenicity and phylogenetic relationships, our findings contribute to a better understanding of disease dynamics and support the development of effective management strategies.
5. Conclusions
This study established that A. alternata and A. tenuissima are the main causative agents of early blight on tomato in Kazakhstan, emphasizing their key role in tomato pathogenesis. Molecular identification showed that of the 61 isolates, 33 were A. tenuissima and 28 were A. alternata. However, A. tenuissima has not been registered as a tomato pathogen in Kazakhstan. The revealed higher DI of A. alternata (41.7–60.0%) compared to A. tenuissima (21.7–53.3%) indicates its higher aggressiveness. Considerable intraspecific variation in pathogenicity was observed in both species. Based on disease index values, isolates were grouped into three aggressiveness levels. A. alternata collected from the Almaty region showed the highest average DI (55.7%), whereas A. tenuissima from Pavlodar and Akmola had moderately lower DI values (38.2 and 36.2%, respectively). A. alternata was not isolated from any samples collected in the Pavlodar region. These results emphasize the need for further research aimed at screening tomato varieties in Kazakhstan for resistance to early blight caused by A. alternata and A. tenuissima, assessing their sensitivity to the fungicides used, and studying the spectrum of toxins produced by these pathogens. Further studies of these aspects will allow the development of more effective strategies for monitoring and combating early blight in tomato, which in turn will help reduce crop losses.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15051251/s1, Table S1: GenBank accession no. for sequences of rDNA ITS, GAPDH, and H3 gene of Alternaria isolates; Table S2. Pathogenicity assessment of representative Alternaria isolates on tomato leaves (Solanum lycopersicum); Table S3: Metabolic and pathogenic characteristics of Alternaria species.
Author Contributions
Conceptualization, A.K.; methodology, A.Y., A.A., A.M. and A.N.; validation, A.Y., A.A., Z.T. and B.A.; formal analysis, A.K. and A.Y.; investigation, A.Y., A.A., Z.T., A.M., B.A. and A.K.; data curation, A.N. and A.Y.; writing—original draft preparation, A.Y. and A.K.; writing—review and editing, A.Y. and A.K.; supervision, A.K.; project administration, A.K.; funding acquisition, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan. Grant No. AP19679502 “Search of new donors and sources of resistance-genes to early blight in tomato and development SCAR-markers to create resistant varieties” for 2023–2025.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ITS | Internal transcribed spacer |
| PCA | Potato–carrot agar |
| DI | Disease index |
| ANOVA | Analysis of variance |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| H3 | Histone H3 |
| DS | Disease severity |
References
- FAOSTAT Database. Available online: http://faostat.fao.org/site/339/default.aspx (accessed on 20 September 2024).
- Böhm, F.; Edge, R.; Truscott, G. Interactions of dietary carotenoids with activated (singlet) oxygen and free radicals: Potential effects for human health. Mol. Nutr. Food Res. 2012, 56, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Pajkovic, N. Multitargeted therapy of cancer by lycopene. Cancer Lett. 2008, 269, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, P.; Singh, P.; Kethiravan, D.; Ramathani, I.; Ramakrishnan, N. Late Blight in Tomato: Insights into the pathogenesis of the Aggressive Pathogen Phytophthora infestans and Future Research Priorities. Planta 2021, 253, 119. [Google Scholar] [CrossRef]
- Ali, M.; Hussain, S.; Nadeem, A.; Ullah, S.; Yasin, M. Studies on biological management of Fusarium Wilt of tomato. Gesunde Pflanz. 2023, 75, 1475–1483. [Google Scholar] [CrossRef]
- Adhikari, P.; Oh, Y.; Panthee, D.R. Current status of early blight resistance in tomato: An update. Int. J. Mol. Sci. 2017, 18, 2019. [Google Scholar] [CrossRef]
- Pandey, A.; Paudel, R.; Adhikari, T.B.; Panthee, D.R.; Louws, F.J. Septoria Leaf spot of tomatoes: Historical insights, present challenges, and future prospects. Horticulturae 2024, 10, 1299. [Google Scholar] [CrossRef]
- Gannibal, P.B. Monitoring of Alternaria Diseases in Agricultural Crops and Identification of Alternaria Fungi: A Methodological Guide; Levitin, M.M., Ed.; GNU VIZR of the Russian Academy of Agricultural Sciences: St. Petersburg, Russia, 2011; p. 70. [Google Scholar]
- Kokaeva, L.Y.; Belosokhov, A.F.; Doeva, L.Y.; Skolotneva, E.S.; Elansky, S.N. Distribution of Alternaria species on blighted potato and tomato leaves in Russia. J. Plant Dis. Prot. 2017, 125, 205–212. [Google Scholar] [CrossRef]
- Salotti, I.; Giorni, P.; Battilani, P. Biology, ecology, and epidemiology of Alternaria species affecting tomato: Ground information for the development of a predictive model. Front. Plant Sci. 2024, 15, 1430965. [Google Scholar] [CrossRef]
- Habib, W.; Masiello, M.; El Ghorayeb, R.; Gerges, E.; Susca, A.; Meca, G.; Moretti, A. Mycotoxin profile and phylogeny of pathogenic Alternaria species isolated from symptomatic tomato plants in Lebanon. Toxins 2021, 13, 513. [Google Scholar] [CrossRef]
- López, P.; Venema, D.; de Rijk, T.; de Kok, A.; Scholten, J.M.; Mol, H.G.J.; de Nijs, M. Occurrence of Alternaria toxins in Food Products in the Netherlands. Food Control 2016, 60, 196–204. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis, and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Pinto, V.E.F.; Patriarca, A. Alternaria species and Their Associated Mycotoxins. Mycotoxigenic Fungi. Methods Mol. Biol. 2017, 1542, 13–32. [Google Scholar] [CrossRef]
- Wenderoth, M.; Garganese, F.; Schmidt-Heydt, M.; Soukup, S.T.; Ippolito, A.; Sanzani, S.M.; Fischer, R. Alternariol as virulence and colonization factor of Alternaria alternata during plant infection. Mol. Microbiol. 2019, 112, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.; Dongo, A.; Pryor, B.M. Secondary metabolite profiling of Alternaria dauci, A. porri, A. solani, and A. tomatophila. Mycol. Res. 2008, 112, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Haro, M.L.; Cabrera, G.; Fernández Pinto, V.; Patriarca, A. Alternaria toxins in tomato products from the Argentinean market. Food Control 2023, 147, 109607. [Google Scholar] [CrossRef]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017, 1, 1569748. [Google Scholar]
- Bessadat, N.; Berruyer, R.; Hamon, B.; Bataille-Simoneau, N.; Benichou, S.; Kihal, M.; Henni, D.E.; Simoneau, P. Alternaria species associated with early blight epidemics on tomato and other Solanaceae crops in Northwestern Algeria. Eur. J. Plant Pathol. 2017, 148, 181–197. [Google Scholar] [CrossRef]
- Simmons, E.G. Alternaria: An Identification Manual; CBS Fungal Biodiversity Centre: Utrecht, The Netherlands, 2007. [Google Scholar]
- Woudenberg, J.H.; Truter, M.; Groenewald, J.Z.; Crous, P.W. Large-spored Alternaria pathogens in section Porri disentangled. Stud. Mycol. 2014, 79, 1–47. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, H.; Liu, H.-F.; Gao, G.-Y.; Zhang, Y.; Li, Z.-N.; Deng, J.-X. Pathogenicity and diversity of large-spored Alternaria associated with three solanaceous vegetables (Solanum tuberosum, S. lycopersicum and S. melongena) in China. Plant Pathol. 2023, 72, 376–391. [Google Scholar] [CrossRef]
- Rodrigues, T.T.M.S.; Berbee, M.L.; Simmons, E.G.; Cardoso, C.R.; Reis, A.; Maffia, L.A.; Mizubuti, E.S.G. First report of Alternaria tomatophila and A. grandis causing early blight on tomato and potato in Brazil. New Dis. Rep. 2010, 22, 28. [Google Scholar] [CrossRef]
- Bessadat, N.; Hamon, B.; Henni, D.E.; Simoneau, P. First report of tomato early blight caused by Alternaria grandis in Algeria. Plant Dis. 2016, 100, 533. [Google Scholar] [CrossRef]
- Alizadeh-Moghaddam, G.; Rezayatmand, Z.; Nasr-Esfahani, M.; Khozaei, M. Bio-genetic analysis of resistance in tomato to early blight disease, Alternaria alternata. Phytochemistry 2020, 179, 112486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y. Alternaria . In Flora Fungorum Sinicorum; Zhao, J.D., Zhang, X.Q., Eds.; Beijing Science Press: Beijing, China, 2003; Volume 16, pp. 1–283. [Google Scholar]
- Andersen, B.; Krøger, E.; Roberts, R.G. Chemical and Morphological Segregation of Alternaria arborescens, A. infectoria, and A. tenuissima Species-Groups. Mycol. Res. 2002, 106, 170–182. [Google Scholar] [CrossRef]
- Oviedo, M.S.; Sturm, M.E.; Reynoso, M.M.; Chulze, S.N.; Ramirez, M.L. Toxigenic profile and AFLP variability of Alternaria alternata and Alternaria infectoria occurring on wheat. Braz. J. Microbiol. 2013, 44, 447–455. [Google Scholar] [CrossRef]
- Kokaeva, L.Y.; Yarmeeva, M.M.; Kokaeva, Z.G.; Chudinova, E.M.; Balabko, P.N.; Elansky, S.N. Phylogenetic study of Alternaria potato and tomato pathogens in Russia. Diversity 2022, 14, 685. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Gannibal, P.B.; Dugan, F.M.; Pryor, B.M. Characterization of Alternaria isolates from the Infectoria species-group and a new taxon from Arrhenatherum, Pseudoalternaria arrhenatheria sp. nov. Mycol. Progress. 2014, 13, 257–276. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; De Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria Section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Wang, F.; Saito, S.; Michailides, T.J.; Xiao, C.L. Fungicide resistance in Alternaria alternata from blueberry in California and its impact on control of Alternaria rot. Plant Dis. 2022, 106, 1446–1453. [Google Scholar] [CrossRef]
- Abylaeva, U.A.; Sardar, A.A.; Tursunova, A.K.; Turbekova, S.M.; Abisheva, G.D. Isolation and identification of pathogenic fungi isolated from solanum lycopersicum (tomato) in the conditions of the Almaty region. Microbiol. Virol. 2023, 3, 243–260. [Google Scholar]
- Ismailova, E.T.; Cadanov, A.K.; Shemshura, O.N.; Seitbattalova, A.I.; Daugalieva, C.T.; Kaptagai, R.J. Morphological and molecular genetic characteristics of causative agents of the main fungal diseases of tomatoes growing in Almaty region. News of the National Academy of Sciences of the Republic of Kazakhstan, Series of Biological and Medical; Institute of Plant Biology and Biotechnology: Almaty, Kazakhstan, 2017; Volume 5, pp. 75–81. [Google Scholar]
- Huang, F.; Fu, Y.; Nie, D.; Stewart, J.E.; Peever, T.L.; Li, H. Identification of a novel phylogenetic lineage of Alternaria alternata causing citrus brown spot in China. Fungal Biol. 2015, 119, 320–330. [Google Scholar] [CrossRef] [PubMed]
- White, T.J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 1990. [Google Scholar]
- Berbee, M.L.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of Primer Sets Designed for Use with the PCR to Amplify Conserved Genes from Filamentous Ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Pryor, B.M.; Michailides, T.J. Morphological, pathogenic, and molecular characterization of Alternaria isolates associated with Alternaria Late Blight of pistachio. Phytopathology 2002, 92, 406–416. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, X.; Liu, S.; Hu, K.; Wu, X. Characterization of Alternaria species associated with black spot of strawberry in Beijing Municipality of China. Can. J. Plant Pathol. 2020, 42, 235–242. [Google Scholar] [CrossRef]
- Allex, C.F.; Shavlik, J.W.; Blattner, F.R. Neural network input representations that produce accurate consensus sequences from DNA fragment assemblies. Bioinformatics 1999, 15, 723–728. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Ramezani, Y.; Taheri, P.; Mamarabadi, M. Identification of Alternaria spp. Associated with tomato early blight in Iran and investigating some of their virulence factors. J. Plant Pathol. 2019, 101, 647–659. [Google Scholar] [CrossRef]
- Elansky, S.N.; Pobedinskaya, M.A.; Kokaeva, L.; Statsyuk, N.; Alexandrova, A. Molecular identification of the species composition of Russian isolates of pathogens causing early blight of potato and tomato. PPO-Spec. Rep. 2012, 15, 151–156. [Google Scholar]
- Shafique, M.S.; Amrao, L.; Saeed, S.; Ahmed, M.Z.; Ghuffar, S.; Anwaar, H.A.; Sheikh, U.A.A.; Khan, M.A.; Qadir, A.; Abdullah, A. Occurrence of leaf spot caused by Alternaria alternata on eggplant (Solanum melongena) in Pakistan. Plant Dis. 2021, 105, 1224. [Google Scholar] [CrossRef]
- Al-Nadabi, H.H.; Maharachchikumbura, S.S.N.; Agrama, H.; Al-Azri, M.; Nasehi, A.; Al-Sadi, A.M. Molecular characterization and pathogenicity of Alternaria species on wheat and date palms in Oman. Eur. J. Plant Pathol. 2018, 152, 577–588. [Google Scholar] [CrossRef]
- Ramjegathesh, R.; Ebenezar, E.G. Morphological and physiological characters of Alternaria alternata causing leaf blight disease of onion. J. Plant Pathol. 2012, 34, 34–44. [Google Scholar]
- Ismail, A.M.; Elshewy, E.S.; El-Ganainy, S.M.; Magistà, D.; Hamouda, A.F.; Alhudaib, K.A.; Ebrahim, W.; Almaghasla, M.I. Mycotoxins from Tomato Pathogenic Alternaria alternata and Their Combined Cytotoxic Effects on Human Cell Lines and Male Albino Rats. J. Fungi 2023, 9, 282. [Google Scholar] [CrossRef]
- Zwickel, T.; Kahl, S.M.; Klaffke, H.; Rychlik, M.; Müller, M.E.H. Spotlight on the Underdogs—An Analysis of Underrepresented Alternaria Mycotoxins Formed Depending on Varying Substrate, Time, and Temperature Conditions. Toxins 2016, 8, 344. [Google Scholar] [CrossRef]
- Nottensteiner, M.; Absmeier, C.; Zellner, M. QoI Fungicide Resistance Mutations in Alternaria solani and Alternaria alternata are Fully Established in Potato Growing Areas in Bavaria and Dual Resistance against SDHI Fungicides is Upcoming. Gesunde Pflanz. 2019, 71, 155–164. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Susca, A.; Ghionna, V.; Logrieco, A.F.; Franzoni, M.; Ravaglia, S.; Meca, G.; Moretti, A. Molecular identification and mycotoxin production by Alternaria species occurring on durum wheat, showing black point symptoms. Toxins 2020, 12, 275. [Google Scholar] [CrossRef]
- Sun, X.; Wang, C.; Gao, X.; Wu, X.; Fu, Y. Characterization of Alternaria species associated with black spot of strawberry in Dandong, China. Agronomy 2023, 13, 1014. [Google Scholar] [CrossRef]
- Armitage, A.D.; Barbara, D.J.; Harrison, R.J.; Lane, C.R.; Sreenivasaprasad, S.; Woodhall, J.W.; Clarkson, J.P. Discrete lineages within Alternaria alternata species Group: Identification using new highly variable loci and support from morphological characters. Fungal Biol. 2015, 119, 994–1006. [Google Scholar] [CrossRef]
- Shi, X.; Zeng, K.; Wang, X.; Liang, Z.; Wu, X. Characterization of Alternaria species causing leaf spot on Chinese cabbage in Shanxi province of China. J. Plant Pathol. 2021, 103, 283–293. [Google Scholar] [CrossRef]
- Ding, S.; Meinholz, K.; Cleveland, K.; Jordan, S.A.; Gevens, A.J. Diversity and virulence of Alternaria spp. causing potato early blight and brown spot in Wisconsin. Phytopathology 2019, 109, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Gou, Y.N.; Aung, S.L.L.; Htun, A.A.; Huang, C.X.; Deng, J.X. Alternaria species in section Alternaria associated with Iris plants in China. Front. Microbiol. 2022, 13, 1036950. [Google Scholar] [CrossRef] [PubMed]
- Aung, S.L.L.; Wu, L.; Yang, T.; Fu, Y.; Wu, Y.; Luo, C. Morphology and Molecular Characterization of a Fungus from the Alternaria alternata Species Complex Causing Black Spots on Pyrus sinkiangensis. Mycobiology 2020, 48, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Gupta, A.K.; Sharma, J.N.; Sharma, M. Characterization of Alternaria blotch disease of apple in Himachal Pradesh, India: Insights on morphology, pathogenicity, and molecular features. Mol. Biol. Rep. 2024, 51, 687. [Google Scholar] [CrossRef]
- Rotondo, F.; Collina, M.; Brunelli, A.; Pryor, B.M. Comparison of Alternaria spp. Collected in Italy from apple with A. mali and other AM-toxin producing strains. Phytopathology 2012, 102, 1130–1142. [Google Scholar] [CrossRef]
- Kang, J.C.; Crous, P.W.; Mchau, G.R.A.; Serdani, M.; Song, S.M. Phylogenetic Analysis of Alternaria spp. Associated with Apple Core Rot and Citrus Black Rot in South Africa. Mycol. Res. 2002, 106, 1151–1162. [Google Scholar] [CrossRef]
- Ma, G.P.; Bao, S.W.; Zhao, J.; Sui, Y.; Wu, X.H. Morphological and molecular characterization of Alternaria species causing leaf blight on watermelon in China. Plant Dis. 2021, 105, 60–70. [Google Scholar] [CrossRef]
- RSE “Kazhydromet”. Archive of Average Meteorological Indicators by Region. Available online: https://kazhydromet.kz (accessed on 5 April 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).