Abstract
The two-component signal transduction system EnvZ/OmpR is described to mediate response to osmotic stress, although it regulates genes involved in other processes such as virulence, fatty acid uptake, exopolysaccharide production, peptide transportation, and flagella production. Considering that some of these processes are known to be important for a successful symbiosis, the present study addresses the effects of extra envZ-like gene copies in the Mesorhizobium–chickpea symbiosis. Five Mesorhizobium-transformed strains, expressing the envZ-like gene from M. mediterraneum UPM-Ca36T, were evaluated in terms of symbiotic performance. Chickpea plants inoculated with envZ-transformed strains (PMI6envZ+ and EE7envZ+) showed a significantly higher symbiotic effectiveness as compared to the corresponding control. In plants inoculated with PMI6envZ+, a higher number of infection threads was observed, and nodules were visible 4 days earlier. Overall, our results showed that the overexpression of Env-like protein may influence the symbiotic process at different stages, leading to strain-dependent effects. This study contributes to elucidating the role of an EnvZ-like protein in the rhizobia–legume symbioses.
1. Introduction
The ability to sense changes in the external environment is fundamental to bacterial survival [1,2,3]. Changes in the surroundings can impose different types of stress, limiting bacterial growth and influencing the interactions with other prokaryotic or eukaryotic organisms. Bacteria respond to these fluctuations using a set of versatile systems that allow the detection of information about the environment and accordingly modulate gene expression [4].
Two-component regulatory systems that comprise a histidine kinase (HK) and a cognate response regulator (RR) can be used to respond to stress [5,6]. As a general mechanism, the sense of an environmental stress by the HK allows the autophosphorylation of a conserved histidine residue, and this phosphoryl group is subsequently transferred to a conserved aspartic acid residue of the RR. In its phosphorylated state, the RR usually binds to DNA target sequences promoting the transcription of specific target genes [6]. The well-characterised two-component regulatory system EnvZ/OmpR is activated in response to osmotic stress and regulates the expression of outer membrane porins OmpF and OmpC according to the osmolarity level [6,7]. At high-osmolarity conditions, EnvZ is autophosphorylated and further transfers the phosphoryl group to OmpR, which then binds to the promoter regions of ompF and ompC genes that encode outer membrane porins [8,9]. Both porins act in the nutrient exchange, but at high osmolarity, OmpC becomes the major porin in the outer membrane, while at low osmolarity conditions, OmpF is more abundant [6]. When the osmotic stress is relieved, EnvZ is able to act as a phosphatase and dephosphorylates the OmpR-P [10].
In Escherichia coli, envZ/ompR mutations affect directly or indirectly the expression of more than 100 genes, including genes involved in amino acid biosynthesis, such as isoleucine and cysteine, iron and maltose transport, and flagellar synthesis [11]. In Yersinia pestis, ompR mutation altered the expression of 224 genes, suggesting a global regulatory role for OmpR [12]. Besides its role on osmotolerance, the EnvZ/OmpR system has also been reported to influence other functions, such as virulence in pathogens, exopolysaccharide (EPS) production, fatty acid uptake, peptide transport, and flagella production [9,13,14,15,16,17,18,19], even in mammalian cells [20]. In addition, EnvZ/OmpR regulates the type III secretion system (T3SS) genes in pathogenic bacteria, such as Yersinia enterocolitica, Salmonella typhimurium, and Pseudomonas syringae [13,21,22].
Rhizobia are diazotrophic soil bacteria that can colonise the roots of many legume species and establish mutualistic symbioses (for example, [23]). Inside the root nodules, rhizobia reduce atmospheric nitrogen into ammonium, which is an N-compound that the host plant can use. In exchange, the host plant provides organic compounds resulting from photosynthesis, which are used as nutrient source by the microsymbiont. This biological nitrogen fixation process is particularly important in the context of agroecosystems, since it represents a natural way to generate an N-input into the crop and ultimately to the soil, thus allowing the reduction of synthetic N-fertiliser applications. These N-fertilisers have relevant environmental costs, since their synthesis requires high energetic input, which is largely dependent on fossil fuels [24]. In addition, N-fertilisers are easily leached to groundwater, leading to problems of nitrate contamination. For all these reasons, the optimisation of rhizobia–legume symbiosis is vital in the context of sustainable agriculture.
Rhizobia genomes are typically large and usually comprise several plasmids, which harbour mainly accessory genes. The genes directly involved in nodule development and nitrogen fixation, as for example nod, nif, or fix genes, are encoded in symbiotic plasmids or in chromosomal symbiosis islands (for a review, see [25]). Besides the well-characterised symbiosis genes, other genes associated with different molecular mechanisms, as for example stress response or secretion system genes, may influence the symbiotic plant–rhizobia interaction [26,27].
As mentioned, several studies have addressed the function of EnvZ/OmpR in numerous pathogenic bacteria, mostly Gammaproteobacteria. In rhizobia, which belong to Alpha- and Betaproteobacteria, there are few reports mentioning envZ and/or envZ-like genes [28,29,30]. To the best of our knowledge, there are no studies on the function of the EnvZ/OmpR two-component system in symbiotic bacteria. Nevertheless, most of the mechanisms that the EnvZ/OmpR system has been reported to influence in other bacteria are important in rhizobia–legume interaction. For example, the role of EPS has been studied using different rhizobia–legume symbioses, and its production is known to be important for root colonisation and host interaction (for a review, see [31]).
The aim of the present study was to evaluate the effects of overexpressing an envZ-like gene in the rhizobia–host interaction, namely, to evaluate if higher levels of EnvZ-like protein could lead to an improvement of the symbiotic performance in chickpea mesorhizobia. Our results show that the deregulation of the two-component regulatory system EnvZ/OmpR, by expression of extra envZ-like copies, may influence the symbiotic process by means of different mechanisms, in a strain-dependent way.
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
All bacterial strains and plasmids used in this study are mentioned in Table 1. A group of four chickpea Mesorhizobium strains previously isolated from Portuguese soils was used: V-15b-Viseu, ST-2-Setúbal, EE-7-ENMP, PMI-6-Portimão [32,33,34]. In addition, the type strain Mesorhizobium mediterraneum UPM-Ca36T was also used [35].
Tryptone–yeast (TY) or yeast–mannitol agar (YMA) medium [36] at 28 °C was used to grow mesorhizobia, and tetracycline (15 μg·mL−1) was added into the medium for pRK415-modified mesorhizobia strains. For bacteria modified with plasmids carrying the gfp and rfp genes, kanamycin (50 μg·mL−1) and gentamycin (15 μg·mL−1) were added to TY medium, respectively. E. coli strains were grown in Luria–Bertani (LB) medium [37] at 37 °C. For E. coli strains harbouring pRK415, LB was supplemented with tetracycline (15 μg·mL−1), and for the strain MT616 carrying pRK600, chloramphenicol (25 μg·mL−1) was added.

Table 1.
Bacterial strains and plasmids used in this work.
Table 1.
Bacterial strains and plasmids used in this work.
| Plasmids/Strains | Characteristics | Reference |
|---|---|---|
| pRK600 | pRK2013 npt::Tn9. Cmr | [38] |
| pRK415 | Broad host-range vector; Tcr | [39] |
| pRKenvZ | Plasmid pRK415 containing the envZ-like gene (locus tag CIT25_RS09080) from M. mediterraneum UPM-Ca36T; Tcr | This work |
| pMRGFP | Plasmid containing the gfp gene; Kmr | [40] |
| pMP4661 | Plasmid containing the rfp gene; Gmr | [41] |
| E. coli MT616 | Strain harbouring the helper plasmid pRK600 | [38] |
| E. coli DH5α | Competent cells | NZYTech |
| Mesorhizobium sp. V-15b | Isolated from chickpea root nodules (Portugal) | [32] |
| Mesorhizobium sp. ST-2 | Isolated from chickpea root nodules (Portugal) | [32] |
| Mesorhizobium sp. PMI-6 | Isolated from chickpea root nodules (Portugal) | [32] |
| Mesorhizobium sp. EE-7 | Isolated from chickpea root nodules (Portugal) | [32] |
| M. mediterraneum UPM-Ca36T | Isolated from chickpea root nodules (Spain) | [35] |
| V15benvZ+ | Mesorhizobium sp. V-15b harbouring pRKenvZ | This work |
| ST2envZ+ | Mesorhizobium sp. ST-2 harbouring pRKenvZ | This work |
| PMI6envZ+ | Mesorhizobium sp. PMI-6 harbouring pRKenvZ | This work |
| PMI6envZ+gfp | Mesorhizobium sp. PMI-6 harbouring pRKenvZ and pMRGFP | This work |
| EE7envZ+ | Mesorhizobium sp. EE-7 harbouring pRKenvZ | This work |
| EE7envZ+gfp | Mesorhizobium sp. EE-7 harbouring pRKenvZ and pMRGFP | This work |
| Ca36envZ+ | M. mediterraneum UPM-Ca36T harbouring pRKenvZ | This work |
| V15bpRK | Mesorhizobium sp. V-15b harbouring pRK415 | [42] |
| ST2pRK | Mesorhizobium sp. ST-2 harbouring pRK415 | [42] |
| PMI6pRK | Mesorhizobium sp. PMI-6 harbouring pRK415 | [42] |
| PMI6pRKgfp | Mesorhizobium sp. PMI-6 harbouring pRK415 and pMRGFP | [42] |
| PMI6pRKrfp | Mesorhizobium sp. PMI-6 harbouring pRK415 and pMP4661 | This work |
| EE7pRK | Mesorhizobium sp. EE-7 harbouring pRK415 | This work |
| EE7pRKgfp | Mesorhizobium sp. EE-7 harbouring pRK415 and pMRGFP | This work |
| EE7pRKrfp | Mesorhizobium sp. EE-7 harbouring pRK415 and pMP4661 | This work |
| Ca36pRK | M. mediterraneum UPM-Ca36T harbouring pRK415 | This work |
2.2. Phylogenetic Analysis
BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 13 April 2025) analyses of the envZ-like gene from M. mediterraneum UPM-Ca36T were performed to search for homologous genes in other rhizobia. The genomic context of envZ or envZ-like genes from Mesorhizobium species nodulating different hosts was compared to that of E. coli, using data available in the NCBI genome database. In addition, a protein domain analysis was performed with ScanProsite, an Interpro database member [43]. An envZ phylogenetic analysis was performed with a final set of 11 Mesorhizobium strains, together with other Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria (Table S1). For comparison purposes, a phylogenetic analysis of the 16S rRNA gene sequence was also performed, using the same set of strains. Sequences were aligned using the ClustalW algorithm, and MEGA X [44] was used to perform the phylogenetic analysis, including the determination of the best nucleotide substitution model using Modeltest [45]. The phylogenetic tree was generated using the neighbour-joining algorithm with 1000 bootstrap replications.
2.3. Modifying Mesorhizobia Strains with an Extra EnvZ-like Gene
The complete sequence of the envZ-like gene from M. mediterraneum UPM-Ca36T (=USDA 3392T), with the locus tag CIT25_RS09080, was amplified by PCR using 0.5 μL of DNA, 1× GC Buffer, 0.2 mM of each dNTP, 7.5 pmol of each primer, and 0.4 U of Phusion DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA) in a final volume of 25 μL. The primers designed for this PCR reaction were envZ-HindIII-F (5′-AAGCTTAATGAGACGTTTCCTGCCGCA-3′) and envZ-BamHI-R (5′-GGATCCCTACGTTGCCAGCGGCAAGC-3′). The amplification program was 30 s at 98 °C, 30 cycles of 10 s at 98 °C, 20 s at 56 °C, 28 s at 72 °C, and a final extension of 5 min at 72 °C. The obtained fragment of 1407 bp was cloned in pCR-BluntTM vector (Thermo Fisher Scientific, Waltham, MA, USA) and sequenced (Macrogen, Kellyville, Australia). Finally, the envZ-like gene from M. mediterraneum UPM-Ca36T was subcloned in the expression vector pRK415, using the restriction sites corresponding to HindIII and BamHI.
Five chickpea Mesorhizobium strains were modified with the plasmid pRKenvZ containing the envZ-like gene under the control of the lac promoter. Triparental conjugation with E. coli DH5α cells harbouring the plasmid pRKenvZ or pRK415 as a donor, as well as the helper strain E. coli MT616 containing pRK600, were carried out as previously described [46]. The pRKenvZ-modified mesorhizobia strains were named V15benvZ+, ST2envZ+, PMI6envZ+, EE7envZ+, and Ca36envZ+. The mesorhizobia strains EE-7-ENMP and UPM-Ca36T were modified with the plasmid pRK415 (control) and named EE7pRK and Ca36pRK, respectively. Mesorhizobium sp. strains V-15b-Viseu, ST-2-Setúbal, and PMI-6-Portimão modified with pRK415 were already available from a previous study [42].
To confirm the mesorhizobia acquisition of pRKenvZ and pRK415, total DNA was extracted according to [47] and used to amplify the multiple cloning site region. Using the M13F and M13R-pUC universal primers, a DNA fragment of 1529 bp will be amplified from pRKenvZ and 122 bp will be amplified from pRK415. The PCR reaction was performed using 5 μL of total DNA, 1× Green GoTaq® Flexi buffer, 0.2 mM of each dNTP, 1.5 mM MgCl2, 15 pmol of each universal primer, and 0.625U of GoTaq® G2 Flexi DNA Polymerase (Promega, Madison, WI, USA), in a final volume of 50 μL. The amplification program included an initial denaturation step of 2 min at 95 °C, followed by 30 cycles of 60 s at 95 °C, 45 s at 56 °C, 85 s at 72 °C, and a final extension of 5 min at 72 °C. Amplification fragment size was verified by agarose gel electrophoresis.
2.4. Evaluation of the Symbiotic Performance
To evaluate the symbiotic effectiveness of mesorhizobia strains, a pot trial was performed using chickpea pre-germinated seeds (variety ELIXIR, cultivar CHK3236) inoculated with the wild type, the strains harbouring pRKenvZ, and the strains harbouring pRK415, as described previously [32].
For plant inoculation purposes, rhizobia strains were grown in liquid TY medium, with the appropriate antibiotics, at 28 °C for 72 h. Cell cultures were centrifuged at 10.000× g and resuspended in fresh TY medium to an optical density (OD) at 540 nm of 1.0. Each chickpea seedling was transferred to a plastic pot with sterile vermiculite and inoculated with 1 mL of this bacterial suspension. Five replicates per treatment were performed. A nitrogen-free nutrient solution [48] was applied three times a week. Uninoculated chickpea plants were used as a negative control, and uninoculated plants receiving the nutrient solution supplemented with 0.1% of KNO3 were used as a positive control. Plants were kept in a growth chamber using a cycle of 16 h-light/8 h-dark and temperatures of 24 –day/18 –night, with a constant relative humidity of 65%.
A second pot trial was performed to evaluate the performance of plants inoculated with strains harbouring pRKenvZ under salinity stress. Plants inoculated the strains harbouring the empty expression vector (pRK415), as well as the abovementioned negative and positive control treatments, were also included in this second pot trial. All the procedures were the same as described above, with the only difference being the supplementation of the watering solution with 0.15% NaCl to impose the salinity stress [49]. The watering with the nutrient solution supplemented with NaCl started at 15 days after inoculation and was alternated with normal nutrient solution watering.
After 7 weeks, plants were harvested, and several parameters were measured, namely, number of nodules (NN) and average weight per nodule (AWN), as well as shoot dry weight (SDW) and root dry weight (RDW). In addition, the symbiotic effectiveness (SE) was estimated using the shoot dry weight values, namely, as a ratio of the difference between the positive and negative controls and the difference between the treatment and the negative control [50]. Statistical analysis was performed using the software SPSS statistics V.21 (SPSS Inc.; IBM, New York, NY, USA) and included one-way ANOVA and Tukey’s multiple range test (p < 0.05).
Considering the results obtained in this symbiotic performance trial, a subset of strains was selected for further analyses: PMI6envZ+ and EE7envZ+, as well as the corresponding control strains, PMI6pRK and EE7pRK.
2.5. Bacterial Growth in Salt Stress Conditions
To evaluate the effect of extra envZ-like copies in the tolerance to salinity stress, strains were grown in 5 mL of liquid TY medium supplemented with 0.75% NaCl (three replicates for each strain). The optical densities at 540 nm were measured every 24 h, for a total of 168 h. t-test (p < 0.05) was used to compare the growth of different strains at the same timepoint. Bacterial growth was also evaluated on YMA medium supplemented with 1.5% NaCl. Cell density was adjusted to an initial OD540 nm of 0.2, from which decimal dilutions up to 10−5 were performed. A volume of 10 μL from each dilution was inoculated in YMA plates with and without NaCl supplementation and growth was observed after 6 days of incubation at 28 °C.
2.6. Motility Assay and Mucoid Phenotype
The swimming ability was compared between the strains carrying the empty vector (pRK415) and those modified with extra envZ-like gene copies, using TY plates containing 0.25% agar [51]. A volume of 10 μL of cell suspension (OD540 nm of 0.1) was used to inoculate the centre of a TY plate, and the diameter of the growth was evaluated after 20 days of growth at 28 °C, as previously described [42].
In order to evaluate differences in the mucoid phenotype between the strains harbouring pRK415 or pRKenvZ, bacterial growth was observed in YMA plates supplemented with 25 mg Congo Red l-1 [52]. All tests were performed in triplicate.
2.7. Evaluation of Nodulation Kinetics
The time-course of nodulation was monitored using chickpea seeds (variety ELIXIR, cultivar CHK3236) inoculated with PMI6pRK, PMI6envZ+, EE7pRK, and EE7envZ+ strains (OD 540 nm of 0.6) and grown under hydroponic conditions, as previously described [53]. This trial was performed using eight plants per treatment and the same plant growth chamber settings described above for the evaluation of symbiotic performance. The number of nodules was evaluated every three days, for a total of 33 days. t-test (p < 0.05) was used to compare different treatments at the same timepoint.
2.8. Analysis of Rhizobia Infection Process
The infection process was investigated using confocal microscopy. Strains PMI6envZ+ and EE7envZ+ were modified with plasmid pMRGFP [40], which includes the gfp gene, while strains PMI6pRK and EE7pRK were modified with plasmid pMRGFP or pMP4661, which includes the rfp gene [41]. These plasmids were mobilised into mesorhizobia cells by triparental conjugation as described above, and their presence was confirmed by antibiotic resistance and fluorescence microscopy observations of cell cultures.
Pre-germinated chickpea seeds were inoculated with mesorhizobia strains tagged with GFP (single inoculation experiments) or RFP and GFP (co-inoculation experiments) [53]. A total of 6 plants per treatment were analysed, including mock inoculated treatment. Chickpea roots were stained using 10 μM propidium iodide (Sigma-Aldrich, Burlington, MA, USA) or 50 mg calcofluor white L−1 (Sigma-Aldrich, Burlington, MA, USA) and 10% potassium hydroxide [54]. At 4 days after inoculation, analyses of the infection process were performed using a Confocal Laser Scanning Microscope (Leica TCS SPE), which allowed the visualisation of fluorescence from GFP at 488 nm, RFP, and propidium iodide at 532 nm and calcofluor white at 405 nm. Using the Leica LASX software (https://www.leica-microsystems.com/products/microscope-software/p/leica-las-x-ls/downloads/, accessed on 13 April 2025), projections were made by stacks accumulation and adjusting individual channels.
3. Results
3.1. Phylogenetic Analysis of EnvZ/EnvZ-like Genes in the Genus Mesorhizobium
Analysis of the envZ-like gene from M. mediterraneum UPM-Ca36T using BLAST allowed the identification of highly similar sequences in several other Mesorhizobium genomes, comprising different species and isolated from distinct host legumes. Since most of these sequences are not annotated as envZ, the designation envZ-like genes was used in these cases. Analysis of the genomic context of envZ and envZ-like genes in Mesorhizobium strains showed that envZ and the corresponding response regulator, located immediately upstream, are usually positioned next to genes encoding ABC transporters (Figure 1 and Figure S1). In terms of the presence of conserved protein domains, all analysed EnvZ and EnvZ-like proteins presented a HAMP linker domain of 53 amino acids and a histidine kinase domain of approximately 200 amino acids at the C-terminal (Figure 1 and Figure S1).
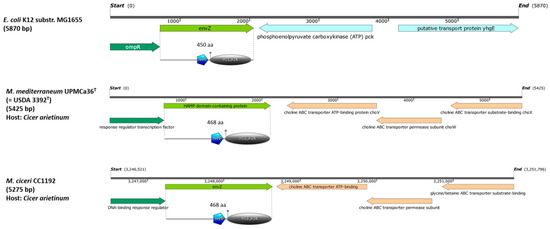
Figure 1.
Genomic context of envZ or envZ-like genes in E. coli and in two mesorhizobia species (M. mediterraneum UPM-Ca36T and M. ciceri CC1192) nodulating chickpea (Cicer arietinum). The annotation available on the NCBI database was used. envZ or envZ-like as well as the upstream response regulator are marked in green. For the envZ or envZ-like genes, the protein domain analysis is shown, with HAMP standing for HAMP domain and HIS_KIN standing for histidine kinase domain.
The phylogenetic analysis of the envZ and envZ-like genes (Figure 2) showed that all the Mesorhizobium strains form a monophyletic group, since the remaining Alphaproteobacteria, with an annotated envZ gene, form different lineages, including S. fredii NGR234 (a broad host range rhizobium). The Gammaproteobacteria included in the phylogeny grouped in a separate cluster, as expected, since the sequence of the envZ gene of these species showed low identity to the Mesorhizobium sequences. Using this same set of species, the 16S rRNA gene-based phylogeny (Figure S2) showed that the clusters generated reflected the different classes of phylum Pseudomonadota, with all the Alphaproteobacteria grouping in the same cluster. Overall, these analyses support the annotation of the gene from M. mediterraneum UPM-Ca36T (=USDA 3392T) with the locus tag CIT25_RS09080 as envZ-like, and for simplicity, this gene will be from now on referred as envZ. Despite the presence of genes encoding the EnvZ/OmpR two-component system in the genome of UPM-Ca36T, the two genes typically regulated by this sensor system (ompF and ompC) were not identified. Nevertheless, the presence of ompF and ompR cannot be completely ruled out, since its genome is still in the draft stage.
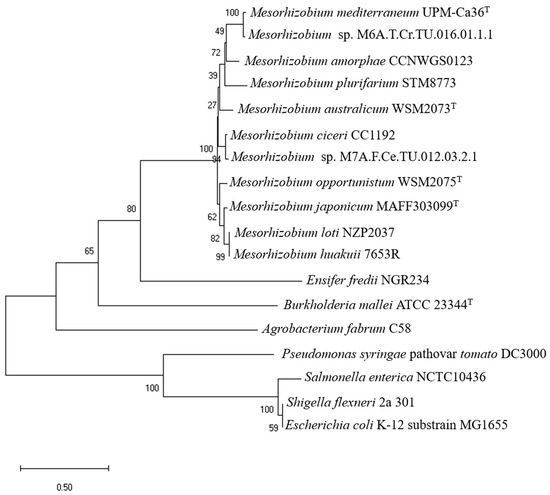
Figure 2.
Neighbour-joining phylogenetic tree based on envZ sequences. Tamura 3-parameter model with gamma distributed rate among sites was used. Percentage of bootstrap support (1000 replicates) is indicated on internal branches. Scale bar indicates 0.5 substitutions per site.
3.2. Symbiotic Performance
Five mesorhizobia strains overexpressing envZ were obtained by transformation with an expression vector. A previous study confirmed that this expression vector is suitable for ectopic and constitutive expression in chickpea mesorhizobia [42]. The mesorhizobia strains overexpressing envZ were tested under controlled conditions to assess if this higher expression levels could increase the symbiotic effectiveness. Despite the fact that envZ overexpression did not increase the number of nodules developed on chickpea plants for any of the five mesorhizobia tested, the symbiotic effectiveness (SE) was improved for PMI6envZ+ and EE7envZ+ with increases of 64% and 96%, respectively (Table 2). Shoot dry weight (SDW), which is used to calculate the SE, was significantly higher for plants inoculated with PMI6envZ+ and EE7envZ+ than for those inoculated with PMI6pRK and EE7pRK, respectively. From those two strains with extra envZ copies, only EE7envZ+ showed a significant increase in the average weight per nodule (AWN), which indicates that larger nodules were formed in plants inoculated with EE7envZ+, when compared to plants inoculated with the corresponding strain harbouring pRK415 (empty vector). Nodules from chickpea plants inoculated with ST2envZ+ also showed a significantly higher AWN, yet in this case, no differences on SE or SDW were detected. Regarding the root dry weight (RDW), significantly higher values were obtained in plant inoculated with EE7envZ+, PMI6envZ+, and ST2envZ+, when compared to the corresponding strains harbouring pRK415. These results showed that an increase on AWN or RDW is not directly reflected in an improvement of SE.

Table 2.
Plant parameters obtained from pot assays of chickpea plants inoculated with transformed mesorhizobia strains.
The homologous overexpression of envZ on UPM-Ca36T as well as the heterologous overexpression on V-15b did not improve any of the above-mentioned symbiotic parameters. For the five strains used in this study, the SE of the wild-type strains was similar to that of the corresponding strains containing pRK415 (no statistically significant differences were obtained).
The pot trial carried out under salinity conditions did not show a better performance of plant inoculated with envZ+ strains, as compared to the corresponding strains harbouring pRK415.
Taking these results into account, further analyses were performed using only the two strains that showed significant improvement in the SE, namely, PMI6envZ+ and EE7envZ+ strains, as well as their corresponding control (empty vector) strains.
3.3. Bacterial Growth Under Salt Stress Conditions
Since the envZ gene typically responds to an increase in osmolarity [6], the tolerance to salinity of the modified strains was evaluated on plates using YMA medium supplemented with 1.5% NaCl. This test showed that PMI6envZ+ was clearly more tolerant to salinity than the PMI6pRK strain, since the strain with extra envZ copies was able to grow even in the highest dilution (10−5), whereas PMI6pRK was not able to grow in any of the dilutions (Figure 3a). In addition, under control conditions (top panel of Figure 3a), it is evident there was an alteration of the mucoid phenotype of strain PMI6envZ+ (further discussed in the section “Exopolysaccharides production”). Under these same salinity conditions, both EE7pRK and EE7envZ+ strains were only able to grow until the 10−2 dilution, showing low tolerance to this salinity stress and no effect of the envZ extra copies (Figure 3b).
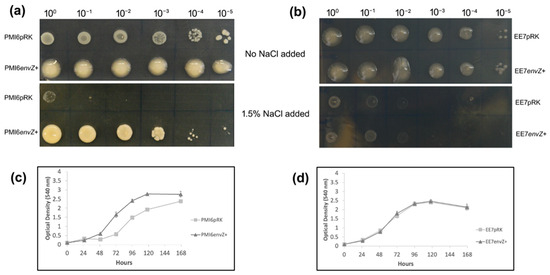
Figure 3.
Evaluation of tolerance to salinity stress. Plate test with YMA medium (no NaCl added) or YMA supplemented with 1.5% NaCl: (a) PMI6pRK and PMI6envZ+ strains; (b) EE7pRK and EE7envZ+ strains. Growth curves in liquid TY medium supplemented with NaCl 0.75%: (c) PMI6pRK and PMI6envZ+; (d) EE7pRK and EE7envZ+. Three replicates for each strain were analysed. Bars in (c,d) represent standard deviation.
The growth of the modified strains was also evaluated in liquid TY medium supplemented with 0.75% NaCl. Under this salinity condition, the PMI6envZ+ strain was able to grow faster in the exponential phase compared with the PMI6pRK strain (Figure 3c). Significant differences were found between PMI6pRK and PMI6envZ+ strains from 48 h of growth onwards (p < 0.05), using the t-test. Similarly to what was observed in the plate assay, no differences were observed between the EE7pRK and EE7envZ+ growth rate, and both strains reached the stationary phase at 120 h (Figure 3d).
3.4. Swimming
Since the motility of rhizobia strains may affect their infection ability [55,56], the effects of extra copies of envZ in the swimming ability was evaluated.
The strain PMI6envZ+ showed a migration zone larger than PMI6pRK (Figure S3a,b). This observation indicates a faster swimming ability of PMI6envZ+, which could contribute to improve the performance of this strain in the root hair infection process. The same test was performed for EE7envZ+ and EE7pRK, and no differences were observed between these strains (Figure S3c,d).
3.5. Exopolysaccharides Production
As the ability to form exopolysaccharides is a very important feature at the rhizobia-legume interaction [28], a plate assay was performed in order to verify whether envZ overexpression influences the mucoid phenotype, which is typical of most rhizobia. When growing in YMA, PMI6envZ+ showed a stronger mucoid phenotype than PMI6pRK (Figure S4a,b) or the corresponding wild-type strain. Although the EE-7 wild-type strain is naturally very mucoid, the strain EE7envZ+ showed a more accentuated mucoid phenotype than the control EE7pRK (Figure S4c,d), although this change is not as evident as in the case of PMI-6. In addition, and since the YMA plates were supplemented with Congo Red, a pH indicator, it was possible to track changes in the medium pH. In both PMI6envZ+ and EE7envZ+, the acidification of the culture medium was detected by the change from red to blue-violet coloration of the culture media. This pH change was more evident for PMI6envZ+, since the empty-vector strain did not acidify the medium, while in the case of EE7envZ+, the empty-vector strain already shows some degree of medium acidification.
3.6. Nodule Formation
To investigate the influence of extra-envZ copies in the rate of nodule formation, an assay with chickpea plants grown in hydroponic conditions was performed. Plants inoculated with PMI6envZ+ showed the first nodules at 10 days after inoculation (DAI), while plants inoculated with PMI6pRK showed the first nodules only at 14 DAI (Figure 4). Nevertheless, in an early phase of the assay, the number of nodules in plants inoculated with PMI6pRK was statistically higher (p < 0.05), namely, at 17 and 19 DAI. At 28 DAI, the strain overexpressing envZ began to show a significantly higher number of nodules (p < 0.05), and this number continued to be higher than in the plants inoculated with PMI6pRK until the end of the experiment (Figure 4a).
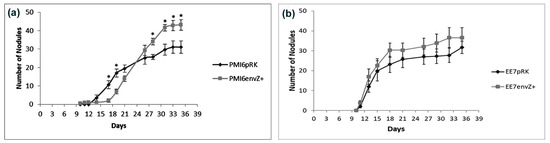
Figure 4.
Nodulation kinetics on chickpea plants. Average number of nodules for plants inoculated with (a) PMI6pRK or PMI6envZ+ strains; (b) EE7pRK or EE7envZ+ strains. Eight plants per treatment were analysed. Asterisks indicate statistically significant differences by independent sample t-test (p < 0.05). Bars represent standard error.
Similarly, plants that were inoculated with EE7envZ+ showed a higher number of nodules than plants inoculated with EE7pRK; however, in this case, the difference was not statistically significant (Figure 4b).
3.7. Colonisation and Infection Thread Formation
In order to investigate if the observed improvement of the symbiotic phenotype of PMI6envZ+ and EE7envZ+ relied on a higher competitiveness of these strains in the early stages of the interaction with the host roots, the infection process was evaluated using confocal microscopy. Considering the development and size of chickpea roots, it was not possible to accurately evaluate colonisation and infection thread formation in a quantitative manner. Instead, experiments of single and co-inoculation were performed and analysed qualitatively at different time points.
At 4 DAI, no differences were detected between chickpea seedlings inoculated with EE7pRKgfp and EE7envZ+gfp strains. Important infection parameters such as curling, formation of infection threads, and formation of caps on root hair tips were very similar between two strains (Figure 5d,e). Also at 4 DAI, chickpea seedlings inoculated with the PMI6envZ+gfp strain showed more infection threads than roots inoculated with PMI6pRKgfp (Figure 5a,b). Additionally, colonisation of the surface of the roots seemed more efficient for PMI6envZ+gfp than for PMI6pRKgfp. Despite the fact that no differences were detected in the infection process when EE7pRKgfp and EE7envZ+gfp strains were analysed separately (Figure 5d,e), upon co-inoculation, a higher amount of the green-tagged EE7envZ+gfp was observed in infection threads and in intracellular zones, suggesting a higher efficiency of the strain with extra envZ copies on these early symbiosis processes (Figure 5f). The same tendency was observed on the analysis of co-inoculation of PMI6envZ+gfp and PMI6pRKrfp strains (Figure 5c), which, in this case, agree with the observations made in the single inoculation analysis. Since strains were inoculated in a 1:1 mixture, the co-inoculation analysis suggests that competitiveness at the root hair infection stage is higher in the strains overexpressing envZ.
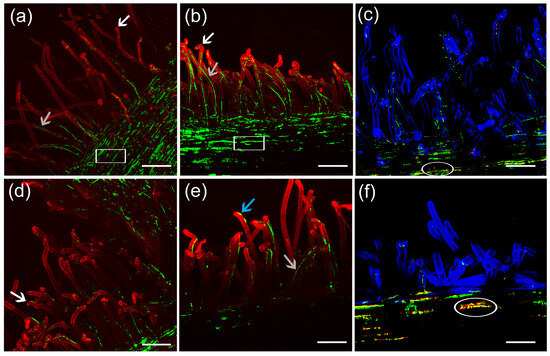
Figure 5.
Confocal laser scanning micrographs showing the initial infection process of chickpea roots inoculated with rhizobia tagged with green and red fluorescent protein (GFP and RFP, respectively). Single inoculation experiments: (a) PMI6pRKgfp, (b) PMI6envZ+gfp, (d) EE7pRKgfp, and (e) EE7envZ+gfp strains on roots stained with propidium iodide at 4 days after inoculation. Co-inoculation experiments: (c) PMI6pRKrfp and PMI6envZ+gfp; (f) EE7pRKrfp and EE7envZ+gfp strains on chickpea roots stained with calcofluor white at 6 days after inoculation. Root hair curling (white arrows); infection threads (gray arrows); caps on root hairs tips (blue arrows); rhizobia attached to the roots (square); and empty vector strains sharing the same intercellular space with those harbouring pRKenvZ (ellipses) are shown. Six plants per treatment were analysed. Scale bars: 75 μm (a,b,d,e); 50 μm (c,f).
4. Discussion
The EnvZ/OmpR two-component regulatory system regulates expression of outer membrane proteins (porins) in response to osmotic stress [6,57,58]. In addition, mutations in envZ or ompR affected the expression levels of several genes involved in different functions, as for example genes related to virulence in pathogens, fatty acid uptake, EPS production, peptide transportation, and flagella production [9,15,16]. To our knowledge, the potential role of this gene in rhizobia–legume symbiosis was not previously investigated; nonetheless, the existence of this system is mentioned in previous works [29,30]. Since most of the enumerated functions regulated by this system are relevant for the rhizobia interaction with their hosts, the aim of present study was to evaluate the effects of extra envZ copies on the symbiotic performance.
The envZ genes from the Mesorhizobium species analysed share over 80% of sequence similarity and form a monophyletic group, in a distinct lineage from the well-characterised envZ gene from E. coli. Nevertheless, for all these different species, the same conserved protein domains were found, namely, a HAMP linker domain, which is a domain common to histidine kinases and other chemoreceptors [28,59], and a histidine kinase domain, which includes several characteristic motifs, including a histidine residue that acts as the sensor part of the protein, since it can be phosphorylated [60]. Interestingly, in most Mesorhizobium, the envZ/ompR genes are encoded near choline and glycine betaine ABC transporter genes, which probably also play a role in osmoprotection, since the uptake of these important osmoprotectants is a common strategy to respond to salinity conditions in rhizobia [61].
As a first approach, to evaluate if extra envZ copies could improve the symbiotic plant parameters, a pot assay was performed using chickpea plants inoculated with the five mesorhizobia strains modified with the expression vectors pRKenvZ and pRK415. Only two strains, PMI6envZ+ and EE7envZ+, showed a significant improvement in the symbiotic effectiveness, compared to the respective control strain. Furthermore, EE7envZ+ induced the development of larger nodules compared to EE7pRK. Under salinity conditions, this outperformance of chickpea plants inoculated with the strains overexpressing envZ was no longer observed. The fact that closely related rhizobia respond differently to the overexpression of the same gene was previously reported in a study using highly conserved symbiosis genes [42]. This is probably due to differences in genomic background, since the expression levels using the pRK415 vector are likely to be consistence among different strains [42]. Further studies are required to understand if this strain-dependent response is due to the requirement of higher or lower levels of overexpression for the three strains not responding to the envZ overexpression.
As the main function of EnvZ is to sense the fluctuations of osmotic conditions in the environment [6], the modified strains were submitted to salinity tolerance assays. The PMI6envZ+ strain grew faster than the control in both liquid and solid medium supplemented with 1.5% NaCl, while EE7envZ did not show an enhanced tolerance to salinity. These findings support the same trend displayed by the symbiotic performance trial, i.e., the heterologous expression of envZ may affect different mechanisms in different strains.
To investigate which symbiotic traits were affected by the expression of extra envZ copies, further studies were performed with the modified EE-7 and PMI-6 strains. In general, a successful symbiosis involves rhizobia penetration through the infection thread, which requires the biosynthesis of functional Nod factors and EPS [62,63]. EPS may be important in lowering the legume immune response during this stage of rhizobia invasion [64,65]. Nevertheless, it is known that different rhizobia strains may produce different EPS, not only in terms of composition but also in terms of quantity [66]. Since the mucoid phenotype reflects the ability to produce EPS [67], the highest mucoid phenotype observed in the case of PMI6envZ+ may have improved the ability of these bacteria to infect the host root hairs. S. meliloti 1021 overexpressing exoR, which encodes an enzyme involved in succinoglycan biosynthesis, enhanced the symbiosis with Medicago truncatula [65]. In addition, the two-component regulatory system ExoS-ChvI, which positively regulates the synthesis of succinoglycan in S. meliloti, shares high similarity to the ChvG-ChvI, a member of the EnvZ-OmpR family [28]. EPS influences the symbiosis process in other rhizobia species as well; for example, R. leguminosarum bv trifolii strains overproducing EPS increased the shoot development and the number of nodules on clover roots, as well as nodule occupancy [68]. In addition, the observed influence of EnvZ in EPS production is in line with previous studies reporting that the EnvZ/OmpR two-component system regulates polysaccharide production in Erwinia amylovora and Salmonella typhi and outer membrane proteins in Xenorhabdus nematophilus [16,18,69].
Although the pot assay showed no significant difference in the number of nodules (NN) developed in chickpea plants inoculated with PMI6envZ+ or with PMI6pRK, the hydroponic conditions showed that the NN was significantly higher in plants inoculated with the strain overexpressing envZ in the later period of the experiment. This apparent divergence between the results was most likely due to the shorter duration of the hydroponic assay (5 weeks), compared with the pot experiment (8 weeks). These results suggest that the higher NN in plants inoculated with PMI6envZ+ might be temporary and yet benefit the plant in terms of N-fixation in this period and contribute to a better symbiotic performance.
The qualitative analysis of the early steps of the infection process showed that roots inoculated with PMI6envZ+gfp displayed a higher number of infection threads than the ones inoculated with the corresponding control strain. These results suggest that EnvZ may contribute to a more effective infection of plant root and this difference in the initial steps of the symbiotic process may account for the earlier nodulation and higher NN observed in hydroponic conditions for plants inoculated with PMI6envZ+. Upon single inoculation, no evident alteration in the number of infection threads was detected for EE7envZ+, when compared with the empty vector strain. However, upon co-inoculation, a higher density of EE7envZ+cells attached on the intracellular zone was observed, and this may contribute to the higher NN observed in plants inoculated with this strain in hydroponic conditions.
Several studies also showed that motile rhizobia are more efficient in nodulation [70,71] or more competitive on the plant root colonisation [55,72] than non-motile ones. Therefore, improvement of motility, as observed in the swimming assay for PMI6envZ+, could account to the more pronounced enhancement of the infection ability and competitiveness of PMI6envZ+ and is consistent with previous studies in other Gammaproteobacteria [9,11,16,73,74,75].
Previous studies reported that EnvZ/OmpR may regulate secretion system (SS) genes in pathogenic bacteria, such as S. typhimurium, P. syringae, and Y. enterocolitica [13,21,22]. Although there is no report showing the EnvZ/OmpR influence on SS in rhizobia, it is known that different types of SS are encoded in the genomes of these bacteria, and their importance for the symbiosis has been previously shown [76,77]. One possible explanation for the SE improvement of the strains overexpressing envZ could be the influence of EnvZ/OmpR in the regulation of SS in rhizobia. In fact, previous studies reported that T3SS in rhizobia are often co-regulated with nodulation genes. Nevertheless, the deletion of T3SS effector proteins that are delivered to the host cells may result in improvement, impairment, or no effect on the symbiotic performance [78].
The present study represents the first report on the role of envZ in the symbiotic performance of rhizobia. Nevertheless, another two-component system from the same family, ExoS/ChvI, has been previously reported to be essential to the establishment of an effective symbiosis in S. meliloti [79,80,81]. Further studies are required to better understand all the mechanisms regulated by the EnvZ/OmpR system in the rhizobia–legume interactions.
5. Conclusions
This study shows the involvement of the envZ-like genes in the rhizobia–legume symbiosis. Overexpression of envZ in PMI-6 strain improved some important traits, such as root colonisation and infection, number of nodules in an early phase of the symbiosis, and mucoid phenotype (EPS production). These same parameters evaluated in EE7envZ+ and EE7pRK did not reveal differences as evident as the ones revealed by PMI6 and its derivative. Overall, these results showed that the envZ extra-copies influenced the mesorhizobia–chickpea symbiosis at different stages.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15051235/s1. Figure S1: Genomic context of envZ or envZ-like genes in several Mesorhizobium species from different hosts, including other legume species from the Cicer genus. The annotation available on the NCBI database was used. envZ or envZ-like as well as the upstream response regulator are marked in green. For the envZ or envZ-like genes, the protein domain analysis is shown, with HAMP standing for HAMP domain and HIS_KIN standing for histidine kinase domain. Figure S2: Neighbour-joining phylogenetic tree based on 16S rRNA gene sequences. The Tamura–Nei model with gamma distributed rate among sites was used. Percentage of bootstrap support (1000 replicates) is indicated on internal branches. Scale bar indicates 0.02 substitutions per site. Figure S3: Swimming ability of (a) PMI6pRK, (b) PMI6envZ+, (c) EE7pRK, and (d) EE7envZ+ strains in TY medium containing 0.25% agar. Figure S4: Mucoid phenotype in YMA plates supplemented with Congo Red. (a) PMI6pRK, (b) PMI6envZ+, (c) EE7pRK, and (d) EE7envZ+. Table S1: List of bacterial strains used for phylogenetic analysis and the corresponding envZ or envZ-like gene and 16S rRNA gene locus tag. References [81,82,83,84,85,86,87,88,89,90,91,92,93] are cited in the Supplementary Materials.
Author Contributions
Conceptualisation, S.O.; methodology, J.R.d.-S., E.M. and A.A.; formal analysis, J.R.d.-S., E.M. and A.A.; writing—original draft preparation, J.R.d.-S.; writing—review and editing, E.M. and A.A.; funding acquisition, S.O. All authors have read and agreed to the published version of the manuscript, with the exception of S.O.
Funding
This research was funded by FEDER Funds through the Operational Program for Competitiveness Factors—COMPETE, and by National Funds through FCT—Foundation for Science and Technology under the Project UIDB/05183 and the Strategic Project UID/AGR/00115/2013, Project nº FCOMP-01-0124-FEDER-028316 (PTDC/BIA-EVF/4158/2012), project POCI-01-0145-FEDER-016810 (PTDC/AGR-PRO/2978/2014), and InAlentejo ALENT-07-0262-FEDER-001871. This publication includes experimental work carried out in the scope of the PhD fellowship granted to J.R. da-Silva (1254-13-8), and the corresponding funding from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) is acknowledged.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank MED (https://doi.org/10.54499/UIDB/05183/2020; https://doi.org/10.54499/UIDP/05183/2020, accessed on 13 April 2025) and CHANGE (https://doi.org/10.54499/LA/P/0121/2020, accessed on 13 April 2025). We thank Alvaro Peix from IRNASA-CSIC for providing pMP4661 and G. Mariano for technical assistance.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tschauner, K.; Hörnschemeyer, P.; Müller, V.S.; Hunke, S. Dynamic interaction between the CpxA sensor Kinase and the periplasmic accessory protein CpxP mediates signal recognition in E. coli. PLoS ONE 2014, 9, e107383. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Epstein, B.; Badgley, B.D.; Unno, T.; Xu, L.; Reese, J.; Gyaneshwar, P.; Denny, R.; Mudge, J.; Bharti, A.K.; et al. Comparative genomics of the core and accessory genomes of 48 Sinorhizobium strains comprising five genospecies. Genome Biol. 2013, 14, R17. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-T.; Tyler, B.M.; Setubal, J.C. Protein Secretion Systems in bacterial-host associations, and their description in the gene ontology. BMC Microbiol. 2009, 9, S2. [Google Scholar] [CrossRef] [PubMed]
- Boor, K.J. Bacterial Stress Responses: What doesn’t kill them can make them stronger. PLoS Biol. 2006, 4, e23. [Google Scholar] [CrossRef]
- Foo, Y.H.; Gao, Y.; Zhang, H.; Kenney, L.J. Cytoplasmic sensing by the inner membrane Histidine Kinase EnvZ. Prog. Biophys. Mol. Biol. 2015, 118, 119–129. [Google Scholar] [CrossRef]
- Wang, L.C.; Morgan, L.K.; Godakumbura, P.; Kenney, L.J.; Anand, G.S. The inner membrane histidine kinase EnvZ senses osmolality via helix-coil transitions in the cytoplasm: The mechanism of EnvZ osmosensing. EMBO J. 2012, 31, 2648–2659. [Google Scholar] [CrossRef]
- Pasqua, M.; Coluccia, M.; Eguchi, Y.; Okajima, T.; Grossi, M.; Prosseda, G.; Utsumi, R.; Colonna, B. Roles of Two-Component signal transduction systems in Shigella virulence. Biomolecules 2022, 12, 1321. [Google Scholar] [CrossRef]
- Yoshida, T.; Cai, S.J.; Inouye, M. Interaction of EnvZ, a sensory Histidine Kinase, with phosphorylated OmpR, the cognate Response Regulator. Mol. Microbiol. 2002, 46, 1283–1294. [Google Scholar] [CrossRef]
- Yuan, J.; Wei, B.; Shi, M.; Gao, H. Functional assessment of EnvZ/OmpR Two-Component System in Shewanella oneidensis. PLoS ONE 2011, 6, e23701. [Google Scholar] [CrossRef]
- Mattison, K.; Kenney, L.J. Phosphorylation alters the interaction of the response regulator OmpR with Its Sensor Kinase EnvZ. J. Biol. Chem. 2002, 277, 11143–11148. [Google Scholar] [CrossRef]
- Oshima, T.; Aiba, H.; Masuda, Y.; Kanaya, S.; Sugiura, M.; Wanner, B.L.; Mori, H.; Mizuno, T. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 2002, 46, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhang, Y.; Han, Y.; Yang, L.; Liu, X.; Guo, Z.; Tan, Y.; Huang, X.; Zhou, D.; Yang, R. Phenotypic and transcriptional analysis of the osmotic regulator OmpR in Yersinia pestis. BMC Microbiol. 2011, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Oropeza, R.; Kenney, L.J. Dual regulation by phospho-OmpR of ssrA/B Gene expression in Salmonella pathogenicity island 2. Mol. Microbiol. 2003, 48, 1131–1143. [Google Scholar] [CrossRef]
- Gerken, H.; Shetty, D.; Kern, B.; Kenney, L.J.; Misra, R. Effects of pleiotropic ompr and envz alleles of Escherichia coli on envelope stress and antibiotic sensitivity. J. Bacteriol. 2024, 206, e00172-24. [Google Scholar] [CrossRef]
- Ji, X.; Shi, A.; Wang, J.; Zhang, B.; Hu, Y.; Lv, H.; Wu, J.; Sun, Y.; Liu, J.-M.; Zhang, Y.; et al. EnvZ/OmpR controls protein expression and modifications in Cronobacter sakazakii for virulence and environmental resilience. J. Agric. Food Chem. 2024, 72, 18697–18707. [Google Scholar] [CrossRef]
- Li, W.; Ancona, V.; Zhao, Y. Co-regulation of polysaccharide production, motility, and expression of Type III Secretion genes by EnvZ/OmpR and GrrS/GrrA systems in Erwinia amylovora. Mol. Genet. Genom. 2014, 289, 63–75. [Google Scholar] [CrossRef]
- Mills, S.D.; Ruschkowski, S.R.; Stein, M.A.; Finlay, B.B. Trafficking of porin-deficient Salmonella typhimurium mutants inside HeLa cells: ompR and envZ mutants are defective for the formation of Salmonella -induced filaments. Infect. Immun. 1998, 66, 1806–1811. [Google Scholar] [CrossRef]
- Pickard, D.; Li, J.; Roberts, M.; Maskell, D.; Hone, D.; Levine, M.; Dougan, G.; Chatfield, S. Characterization of Defined ompR Mutants of Salmonella Typhi: ompR Is Involved in the regulation of vi polysaccharide expression. Infect. Immun. 1994, 62, 3984–3993. [Google Scholar] [CrossRef]
- Shin, S.; Park, C. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J. Bacteriol. 1995, 177, 4696–4702. [Google Scholar] [CrossRef]
- Leopold, A.V.; Verkhusha, V.V. Engineering signalling pathways in mammalian cells. Nat. Biomed. Eng. 2024, 8, 1523–1539. [Google Scholar] [CrossRef]
- Feng, X.; Walthers, D.; Oropeza, R.; Kenney, L.J. The response regulator SsrB activates transcription and binds to a region overlapping ompr binding sites at Salmonella pathogenicity island 2. Mol. Microbiol. 2004, 54, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Nieckarz, M.; Jaworska, K.; Raczkowska, A.; Brzostek, K. The regulatory circuit underlying downregulation of a Type III Secretion System in Yersinia enterocolitica by transcription factor OmpR. Int. J. Mol. Sci. 2022, 23, 4758. [Google Scholar] [CrossRef] [PubMed]
- Masson-Boivin, C.; Sachs, J.L. Symbiotic nitrogen fixation by rhizobia—the roots of a success story. Curr. Opin. Plant Biol. 2018, 44, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.K.; Rajput, V.D.; Kumari, A.; Espinosa-Saiz, D.; Menendez, E.; Minkina, T.; Dwivedi, P.; Mandzhieva, S. Plant growth-promoting rhizobacteria: A potential bio-asset for restoration of degraded soil and crop productivity with sustainable emerging techniques. Environ. Geochem. Health 2023, 45, 9321–9344. [Google Scholar] [CrossRef]
- Geddes, B.; Kearsley, J.; Morton, R.; DiCenzo, C.; Finan, T. The genomes of rhizobia. In Advances in Botanical Research; Frendo, P., Frugier, F., Masson-Boivin, C., Eds.; Elsevier: Toronto, ON, Canada, 2020; Volume 94, pp. 1–348. [Google Scholar]
- da-Silva, J.R.; Alexandre, A.; Brígido, C.; Oliveira, S. Can stress response genes be used to improve the symbiotic performance of rhizobia? AIMS Microbiol. 2017, 3, 365–382. [Google Scholar] [CrossRef]
- Paço, A.; da-Silva, J.R.; Eliziário, F.; Brígido, C.; Oliveira, S.; Alexandre, A. traG gene is conserved across Mesorhizobium spp. able to nodulate the same host plant and expressed in response to root exudates. BioMed Res. Int. 2019, 2019, 3715271. [Google Scholar] [CrossRef]
- Cheng, H.-P.; Walker, G.C. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI Two-Component regulatory system. J. Bacteriol. 1998, 180, 20–26. [Google Scholar] [CrossRef]
- David, M.; Daveran, M.-L.; Batut, J.; Dedieu, A.; Domergue, O.; Ghai, J.; Hertig, C.; Boistard, P.; Kahn, D. Cascade regulation of nif gene Expression in Rhizobium meliloti. Cell 1988, 54, 671–683. [Google Scholar] [CrossRef]
- Ronson, C.W.; Astwood, P.M.; Nixon, T.B.; Ausubel, F.M. Deduced products of c4-dicarboxylate transport regulatory genes of Rhizobium leguminosarum are homologous to nitrogen regulatory gene products. Nucl. Acids Res. 1987, 15, 7921–7934. [Google Scholar] [CrossRef]
- Acosta-Jurado, S.; Fuentes-Romero, F.; Ruiz-Sainz, J.-E.; Janczarek, M.; Vinardell, J.-M. Rhizobial Exopolysaccharides: Genetic regulation of their synthesis and relevance in symbiosis with legumes. Int. J. Mol. Sci. 2021, 22, 6233. [Google Scholar] [CrossRef]
- Alexandre, A.; Brígido, C.; Laranjo, M.; Rodrigues, S.; Oliveira, S. Survey of chickpea rhizobia diversity in portugal reveals the predominance of species distinct from Mesorhizobium ciceri and Mesorhizobium mediterraneum. Microb. Ecol. 2009, 58, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Brígido, C.; Alexandre, A.; Oliveira, S. Transcriptional analysis of major chaperone genes in salt-tolerant and salt-sensitive mesorhizobia. Microbiol. Res. 2012, 167, 623–629. [Google Scholar] [CrossRef]
- Laranjo, M.; Alexandre, A.; Rivas, R.; Velázquez, E.; Young, J.P.W.; Oliveira, S. Chickpea rhizobia symbiosis genes are highly conserved across multiple Mesorhizobium species: Mesorhizobia symbiosis genes are conserved. FEMS Microbiol. Ecol. 2008, 66, 391–400. [Google Scholar] [CrossRef]
- Nour, S.M.; Cleyet-Marel, J.-C.; Normand, P.; Fernandez, M.P. Genomic heterogeneity of strains nodulating chickpeas (Cicer arietinum l.) and description of Rhizobium mediterraneum sp. nov. Int. J. Syst. Bacteriol. 1995, 45, 640–648. [Google Scholar] [CrossRef]
- Somasegaran, P.; Hoben, H. Handbook for Rhizobia; Springer-Verlag: New York, NY, USA, 1994. [Google Scholar]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 2001. [Google Scholar]
- Finan, T.M.; Kunkel, B.; De Vos, G.F.; Signer, E.R. Second symbiotic megaplasmid in Rhizobium meliloti carrying exopolysaccharide and thiamine synthesis genes. J. Bacteriol. 1986, 167, 66–72. [Google Scholar] [CrossRef]
- Keen, N.T.; Tamaki, S.; Kobayashi, D.; Trollinger, D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 1988, 70, 191–197. [Google Scholar] [CrossRef]
- García-Fraile, P.; Carro, L.; Robledo, M.; Ramírez-Bahena, M.-H.; Flores-Félix, J.-D.; Fernández, M.T.; Mateos, P.F.; Rivas, R.; Igual, J.M.; Martínez-Molina, E.; et al. Rhizobium promotes non-legumes growth and quality in several Production Steps: Towards a biofertilization of edible raw vegetables healthy for humans. PLoS ONE 2012, 7, e38122. [Google Scholar] [CrossRef]
- Bloemberg, G.V.; Wijfjes, A.H.M.; Lamers, G.E.M.; Stuurman, N.; Lugtenberg, B.J.J. Simultaneous imaging of Pseudomonas fluorescens WCS365 populations expressing three different autofluorescent proteins in the rhizosphere: New perspectives for studying microbial communities. Mol. Plant-Microbe Interact. 2000, 13, 1170–1176. [Google Scholar] [CrossRef]
- da-Silva, J.R.; Menéndez, E.; Eliziário, F.; Mateos, P.F.; Alexandre, A.; Oliveira, S. Heterologous expression of nifA or nodD genes improves chickpea-Mesorhizobium symbiotic performance. Plant Soil 2019, 436, 607–621. [Google Scholar] [CrossRef]
- De Castro, E.; Sigrist, C.J.A.; Gattiker, A.; Bulliard, V.; Langendijk-Genevaux, P.S.; Gasteiger, E.; Bairoch, A.; Hulo, N. ScanProsite: Detection of PROSITE signature matches and prorule-associated functional and structural residues in proteins. Nucleic Acids Res. 2006, 34, W362–W365. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Brígido, C.; Glick, B.R.; Oliveira, S.; Alho, L. Mesorhizobium Ciceri LMS-1 Expressing an exogenous 1-Aminocyclopropane-1-Carboxylate (ACC) Deaminase Increases Its Nodulation Abilities and Chickpea plant resistance to soil constraints: M. ciceri (pRKACC) increases nodulation and plant growth. Lett. Appl. Microbiol. 2012, 55, 15–21. [Google Scholar] [CrossRef]
- Rivas, R.; Velázquez, E.; Valverde, A.; Mateos, P.F.; Martínez-Molina, E. A two primers random amplified polymorphic DNA procedure to obtain polymerase chain reaction fingerprints of bacterial species. Electrophoresis 2001, 22, 1086–1089. [Google Scholar] [CrossRef]
- Broughton, W.J.; Dilworth, M.J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef]
- Brígido, C.; Nascimento, F.X.; Duan, J.; Glick, B.R.; Oliveira, S. Expression of an Exogenous 1-Aminocyclopropane-1-Carboxylate Deaminase gene in Mesorhizobium spp. reduces the negative effects of salt stress in chickpea. FEMS Microbiol. Lett. 2013, 1, 46–53. [Google Scholar]
- Gibson, A. Evaluation of nitrogen fixation by legumes in the greenhouse and growth chamber. In Symbiotic Nitrogen Fixation Technology; Elkan, G., Ed.; Marcel Dekker: New York, NY, USA, 1987; pp. 321–363. [Google Scholar]
- Rouws, L.F.M.; Simões-Araújo, J.L.; Hemerly, A.S.; Baldani, J.I. Validation of a Tn5 transposon mutagenesis system for Gluconacetobacter diazotrophicus through characterization of a flagellar mutant. Arch. Microbiol. 2008, 189, 397–405. [Google Scholar] [CrossRef]
- Robledo, M.; Rivera, L.; Jiménez-Zurdo, J.I.; Rivas, R.; Dazzo, F.; Velázquez, E.; Martínez-Molina, E.; Hirsch, A.M.; Mateos, P.F. Role of Rhizobium endoglucanase CelC2 in cellulose biosynthesis and biofilm formation on plant roots and abiotic surfaces. Microb. Cell Fact. 2012, 11, 125. [Google Scholar] [CrossRef]
- Brígido, C.; Robledo, M.; Menéndez, E.; Mateos, P.F.; Oliveira, S. A ClpB chaperone knockout mutant of Mesorhizobium ciceri shows a delay in the root nodulation of chickpea plants. Mol. Plant-Microbe Interact. 2012, 25, 1594–1604. [Google Scholar] [CrossRef]
- Flores-Félix, J.; Menéndez, E.; Marcos-García, M.; Celador-Lera, L.; Rivas, R. Calcofluor white, an alternative to propidium iodide for plant tissues staining in studies of root colonization by fluorescent-tagged rhizobia. JABB 2015, 2, 65–70. [Google Scholar] [CrossRef]
- Caetano-Anolles, G.; Wall, L.; De Micheli, A.; Macchi, E.; Baur, W.; Favelukes, G. Role of Motility and Chemotaxis in Efficiency of Nodulation by Rhizobium meliloti. Plant Physiol. 1988, 86, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Gay-Fraret, J.; Ardissone, S.; Kambara, K.; Broughton, W.J.; Deakin, W.J.; Quéré, A. Cyclic-β-glucans of Rhizobium (Sinorhizobium) sp. strain NGR234 are required for hypo-osmotic adaptation, motility, and efficient symbiosis with host plants. FEMS Microbiol. Lett. 2012, 333, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Alphen, W.V.; Lugtenberg, B. Influence of osmolarity of the growth Medium on the outer membrane protein pattern of Escherichia coli. J. Bacteriol. 1977, 131, 623–630. [Google Scholar] [CrossRef]
- Bontemps-Gallo, S.; Madec, E.; Robbe-Masselot, C.; Souche, E.; Dondeyne, J.; Lacroix, J.-M. The opgc gene is required for opgs succinylation and is osmoregulated through RcsCDB and EnvZ/OmpR in the phytopathogen Dickeya dadantii. Sci. Rep. 2016, 6, 19619. [Google Scholar] [CrossRef]
- Aravind, L.; Ponting, C.P. The cytoplasmic helical linker domain of receptor histidine kinase and methyl-accepting proteins is common to many prokaryotic signalling proteins. FEMS Microbiol. Lett. 1999, 176, 111–116. [Google Scholar] [CrossRef]
- West, A.H.; Stock, A.M. Histidine Kinases and Response regulator proteins in Two-Component signaling systems. TIBS 2001, 26, 369–376. [Google Scholar] [CrossRef]
- Boncompagni, E.; Østerås, M.; Poggi, M.-C.; Le Rudulier, D. Occurrence of choline and glycine betaine uptake and metabolism in the family Rhizobiaceae and their roles in osmoprotection. Appl. Environ. Microbiol. 1999, 65, 2072–2077. [Google Scholar] [CrossRef]
- Jones, K.M.; Kobayashi, H.; Davies, B.W.; Taga, M.E.; Walker, G.C. How rhizobial symbionts invade plants: The Sinorhizobium–medicago model. Nat. Rev. Microbiol. 2007, 5, 619–633. [Google Scholar] [CrossRef]
- Klein, S. Interaction of nod and exo Rhizobium meliloti in alfalfa nodulation. Mol. Plant-Microbe Interact. 1988, 1, 94. [Google Scholar] [CrossRef]
- Jones, K.M.; Sharopova, N.; Lohar, D.P.; Zhang, J.Q.; VandenBosch, K.A.; Walker, G.C. Differential response of the plant Medicago truncatula to its symbiont Sinorhizobium meliloti or an exopolysaccharide-deficient mutant. Proc. Natl. Acad. Sci. USA 2008, 105, 704–709. [Google Scholar] [CrossRef]
- Jones, K.M. Increased production of the exopolysaccharide succinoglycan enhances Sinorhizobium meliloti 1021 symbiosis with the Host Plant Medicago truncatula. J. Bacteriol. 2012, 194, 4322–4331. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.K.; Maiti, T.K. Structure of extracellular polysaccharides (EPS) produced by rhizobia and their functions in legume–bacteria symbiosis:—A Review. Achiev. Life Sci. 2016, 10, 136–143. [Google Scholar] [CrossRef]
- Staehelin, C.; Forsberg, L.S.; D’Haeze, W.; Gao, M.-Y.; Carlson, R.W.; Xie, Z.-P.; Pellock, B.J.; Jones, K.M.; Walker, G.C.; Streit, W.R.; et al. Exo-oligosaccharides of Rhizobium sp. strain NGR234 are required for symbiosis with various legumes. J. Bacteriol. 2006, 188, 6168–6178. [Google Scholar] [CrossRef]
- Janczarek, M.; Jaroszuk-Ściseł, J.; Skorupska, A. Multiple copies of rosR and pssA genes enhance exopolysaccharide production, symbiotic competitiveness and clover nodulation in Rhizobium leguminosarum bv. trifolii. Antonie van Leeuwenhoek 2009, 96, 471–486. [Google Scholar] [CrossRef]
- Forst, S.; Tabatabai, N. Role of the histidine Kinase, EnvZ, in the production of outer membrane proteins in the symbiotic-pathogenic bacterium Xenorhabdus nematophilus. Appl. Environ. Microbiol. 1997, 63, 962–968. [Google Scholar] [CrossRef]
- Soby, S.; Bergman, K. Motility and chemotaxis of Rhizobium meliloti in soil. Appl. Environ. Microbiol. 1983, 46, 995–998. [Google Scholar] [CrossRef]
- Zheng, H.; Mao, Y.; Teng, J.; Zhu, Q.; Ling, J.; Zhong, Z. Flagellar-dependent motility in Mesorhizobium tianshanense is involved in the early stage of plant host interaction: Study of an flge mutant. Curr. Microbiol. 2015, 70, 219–227. [Google Scholar] [CrossRef]
- Mellor, H.Y.; Glenn, A.R.; Arwas, R.; Dilworth, M.J. Symbiotic and competitive properties of motility mutants of Rhizobium trifolii TA1. Arch. Microbiol. 1987, 148, 34–39. [Google Scholar] [CrossRef]
- Kim, D.; Boylan, B.; George, N.; Forst, S. Inactivation of ompR promotes precocious swarming and flhDC expression in Xenorhabdus nematophila. J. Bacteriol. 2003, 185, 5290–5294. [Google Scholar] [CrossRef]
- Prüß, B.M. Involvement of Two-Component Signaling on bacterial motility and biofilm development. J. Bacteriol. 2017, 199, e00259-17. [Google Scholar] [CrossRef]
- Tipton, K.A.; Rather, P.N. An ompR-envZ Two-Component System ortholog regulates phase variation, osmotic tolerance, motility, and virulence in Acinetobacter baumannii strain AB5075. J. Bacteriol. 2017, 199, e00705-16. [Google Scholar] [CrossRef] [PubMed]
- Hubber, A.M.; Sullivan, J.T.; Ronson, C.W. Symbiosis-Induced cascade regulation of the Mesorhizobium loti R7A VirB/D4 Type IV Secretion System. Mol. Plant-Microbe Interact. 2007, 20, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, S.; Kaneko, T.; Sato, S.; Saeki, K. Hijacking of leguminous nodulation signaling by the rhizobial Type III Secretion System. Proc. Natl. Acad. Sci. USA 2013, 110, 17131–17136. [Google Scholar] [CrossRef]
- Teulet, A.; Camuel, A.; Perret, X.; Giraud, E. The versatile roles of Type III Secretion Systems in Rhizobium-Legume symbioses. Annu. Rev. Microbiol. 2022, 76, 45–65. [Google Scholar] [CrossRef]
- Bélanger, L.; Dimmick, K.A.; Fleming, J.S.; Charles, T.C. Null mutations in Sinorhizobium meliloti exoS and chvI demonstrate the importance of this Two-Component Regulatory System for symbiosis. Mol. Microbiol. 2009, 74, 1223–1237. [Google Scholar] [CrossRef]
- Chen, E.J.; Fisher, R.F.; Perovich, V.M.; Sabio, E.A.; Long, S.R. Identification of direct transcriptional target genes of ExoS/ChvI Two-Component signaling in Sinorhizobium meliloti. J. Bacteriol. 2009, 191, 6833–6842. [Google Scholar] [CrossRef]
- Soto, M.J.; Sanjuán, J.; Olivares, J. Rhizobia and plant-pathogenic bacteria: Common infection weapons. Microbiology 2006, 152, 3167–3174. [Google Scholar] [CrossRef]
- Greenlon, A.; Chang, P.L.; Damtew, Z.M.; Muleta, A.; Carrasquilla-Garcia, N.; Kim, D.; Nguyen, H.P.; Suryawanshi, V.; Krieg, C.P.; Yadav, S.K.; et al. Global-level population Genomics reveals differential effects of geography and phylogeny on horizontal gene transfer in soil bacteria. Proc. Natl. Acad. Sci. USA 2019, 116, 15200–15209. [Google Scholar] [CrossRef]
- Diouf, F.; Diouf, D.; Klonowska, A.; Le Queré, A.; Bakhoum, N.; Fall, D.; Neyra, M.; Parrinello, H.; Diouf, M.; Ndoye, I.; et al. Genetic and genomic diversity studies of acacia symbionts in Senegal reveal new species of Mesorhizobium with a putative geographical pattern. PLoS ONE 2015, 10, e0117667. [Google Scholar] [CrossRef]
- Nandasena, K.; Yates, R.; Tiwari, R.; O’Hara, G.; Howieson, J.; Ninawi, M.; Chertkov, O.; Detter, C.; Tapia, R.; Han, S.; et al. Complete genome sequence of Mesorhizobium ciceri bv. biserrulae type strain (WSM1271T). Stand. Genom. Sci. 2013, 9, 462–472. [Google Scholar]
- Wang, S.; Hao, B.; Li, J.; Gu, H.; Peng, J.; Xie, F.; Zhao, X.; Frech, C.; Chen, N.; Ma, B.; et al. Whole-genome sequencing of Mesorhizobium huakuii 7653R provides molecular insights into host specificity and symbiosis island dynamics. BMC Genom. 2014, 15, 440. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Sullivan, J.; Ronson, C.; Tian, R.; Bräu, L.; Davenport, K.; Daligault, H.; Erkkila, T.; Goodwin, L.; Gu, W.; et al. Genome sequence of the Lotus spp. microsymbiont Mesorhizobium loti strain NZP2037. Stand. Genom. Sci. 2014, 9, 7. [Google Scholar] [CrossRef]
- Wang, X.; Luo, Y.; Liu, D.; Wang, J.; Wei, S.; Zhao, L. Complete genome sequence of the Robinia pseudoacacia L. symbiont Mesorhizobium amorphae CCNWGS0123. Stand. Genom. Sci 2018, 13, 18. [Google Scholar] [CrossRef]
- Haskett, T.; Wang, P.; Ramsay, J.; O’Hara, G.; Reeve, W.; Howieson, J.; Terpolilli, J. Complete genome sequence of Mesorhizobium ciceri strain CC1192, an efficient nitrogen-fixing microsymbiont of Cicer arietinum. Genome Announc. 2016, 4, e00516-16. [Google Scholar] [CrossRef]
- Schmeisser, C.; Liesegang, H.; Krysciak, D.; Bakkou, N.; Le Quéré, A.; Wollherr, A.; Heinemeyer, I.; Morgenstern, B.; Pommerening-Röser, A.; Flores, M.; et al. Rhizobium sp. strain NGR234 Possesses a remarkable number of secretion systems. Appl. Environ. Microbiol. 2009, 75, 4035–4045. [Google Scholar] [CrossRef]
- Wood, D.W.; Setubal, J.C.; Kaul, R.; Monks, D.E.; Kitajima, J.P.; Okura, V.K.; Zhou, Y.; Chen, L.; Wood, G.E.; Almeida, N.F.; et al. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 2001, 294, 2317–2323. [Google Scholar] [CrossRef]
- Buell, C.R.; Joardar, V.; Lindeberg, M.; Selengut, J.; Paulsen, I.T.; Gwinn, M.L.; Dodson, R.J.; Deboy, R.T.; Durkin, A.S.; Kolonay, J.F.; et al. The Complete genome sequence of the Arabidopsis and tomato pathogen Pseudomonas syringae Pv. Tomato DC3000. Proc. Natl. Acad. Sci. USA 2003, 100, 10181–10186. [Google Scholar] [CrossRef]
- Hayashi, K.; Morooka, N.; Yamamoto, Y.; Fujita, K.; Isono, K.; Choi, S.; Ohtsubo, E.; Baba, T.; Wanner, B.L.; Mori, H.; et al. Highly accurate genome sequences of Escherichia coli K-12 strains MG1655 and W3110. Mol. Syst. Biol. 2006, 2, 2006.0007. [Google Scholar] [CrossRef]
- Jin, Q. Genome sequence of Shigella flexneri 2a: Insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res. 2002, 30, 4432–4441. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).