Abstract
The growing prevalence of metabolic diseases underscores the necessity for enhancing the nutritional value of widely consumed foods. The present study investigated the impact of melatonin elicitation on the accumulation of flavanones and endogenous melatonin in lemons. Preharvest treatments of 0.1 and 1 mM were applied, followed by postharvest treatment of 1 mM, either individually or in combination, and then cold storage. The quantification of bioactive compounds was conducted in various plant components, namely juice, albedo, flavedo, and leaves, employing HPLC-DAD and HPLC-MS/MS methodologies. Preharvest application of 1 mM melatonin resulted in a 26% increase in flavanone concentration in juice at harvest, while postharvest treatment induced a 19% increase during storage. The combination of both treatments resulted in elevated levels of flavanone (a 27% increase). With regard to melatonin levels, the combined treatments resulted in a significant increase in all tissues; however, the postharvest application alone achieved the highest concentration (6.99 µg L−1), particularly in the juice. The results of this study demonstrate the efficacy of melatonin elicitation, particularly in postharvest treatments, as a practical strategy to enhance the functional quality of lemons. This approach has the potential to facilitate the development of health-promoting foods and the valorisation of citrus byproducts. Further research is required to elucidate the role of melatonin in modulating the bioavailability and health effects of lemon phytochemicals in humans.
1. Introduction
The contemporary global population is confronted with a mounting array of health challenges, with a particularly distressing escalation in metabolic diseases [1]. This surge is predominantly driven by substantial lifestyle modifications, encompassing inadequate dietary practices, diminished physical activity, and the pervasive presence of chronic stress [2]. Consequently, conditions such as obesity, type 2 diabetes, and cardiovascular diseases are becoming increasingly prevalent, affecting individuals across all age groups and socioeconomic strata. The consequences of these metabolic disorders are twofold. Firstly, it is evident that they have a detrimental effect on the quality of life of those affected. Secondly, they impose a considerable strain on healthcare systems worldwide. It is imperative that urgent measures are implemented and comprehensive strategies developed for the prevention and management of such disorders [3]. The multifaceted nature of the issue, which is characterised by the interplay of genetic predispositions, environmental factors, and behavioural choices, underscores the necessity for a multifaceted approach that addresses individual needs whilst also promoting population-wide health initiatives [4].
The maintenance of optimal health and the prevention of chronic diseases is contingent on the consumption of a diet that is rich in fruits and vegetables. These foods are abundant in essential nutrients, including vitamins, minerals, fibre, and bioactive compounds, such as phenolic compounds, which have been demonstrated to confer numerous health benefits [5]. Phenolic compounds, which are present in abundance in various fruits and vegetables, have been shown to possess potent antioxidant, anti-inflammatory, and anticancer properties [6]. These compounds have been demonstrated to possess the capacity to protect cells from damage caused by free radicals, thereby reducing the risk of cardiovascular diseases, type 2 diabetes, certain cancers, and neurodegenerative disorders [7]. A wide variety of colourful fruits and vegetables should be incorporated into one’s daily diet to ensure adequate intake of phenolic compounds and other essential nutrients, thereby promoting overall well-being and disease prevention.
The global significance of the lemon industry is substantial, with a recorded production output of approximately 10.1 million metric tons in 2023 [8]. The primary countries responsible for this production are Mexico, India, China, and Argentina, which collectively represent a significant proportion to the global supply. The versatility of and demand for lemons are demonstrated by their utilisation in various sectors, including food and beverage, cosmetics, and pharmaceuticals. Spain plays a pivotal role in the European market; in 2022, Spain’s lemon production reached approximately 700,000 metric tons [9], positioning it as one of the leading producers in Europe. The favourable climate and advanced agricultural practices in the country contribute to the high-quality nature of its lemon production, making it a key player in the international lemon trade.
The health benefits of lemons are well-documented, largely due to their high content of phenolic compounds, particularly flavanones [10]. These compounds have been demonstrated to possess significant antioxidant and anti-inflammatory properties, thereby conferring a range of health benefits [11]. Flavanones, such as hesperidin, which are found in abundance in lemons, have been shown to protect against cardiovascular diseases by improving blood vessel function and reducing blood pressure [12]. Moreover, the aforementioned compounds have been shown to possess antineoplastic properties, thereby impeding the growth of tumours and inducing apoptosis in cancerous cells [13]. Consequently, regular consumption of lemons can be considered a valuable dietary strategy for promoting overall health and reducing the risk of chronic diseases.
A range of strategies have been evaluated in order to enhance the quality and health-promoting benefits of fruits, with the objective of increasing the concentration of the aforementioned phenolic compounds. In this sense, chilling stress has been demonstrated to elicit phenolic compound synthesis due to increased phenylalanine ammonia-lyase (PAL) activity [14]. Furthermore, melatonin has garnered significant attention for its potential to enhance the accumulation of phenolic compounds, such as flavonoids, which contribute to antioxidant capacity and overall fruit quality [15,16,17]. These findings suggest that cold storage and melatonin have the potential to serve as effective tools for enhancing the quality of citrus fruits by modulating phenolic compounds and other beneficial characteristics.
However, as far as is known, there is a significant absence of knowledge regarding the phytochemical response of lemons to melatonin elicitation and cold-stress induction, particularly with regard to flavanone accumulation and endogenous melatonin levels across tissues. Therefore, the main aim of this study was to evaluate the efficacy of melatonin elicitation in lemons, applied in preharvest, postharvest, or a combination of both, in terms of enhancing the concentration of bioactive compounds, specifically flavanones. Additionally, the study aimed to quantify endogenous concentrations of bioactive compounds in different fruit tissues.
2. Materials and Methods
2.1. Plant Material, Experimental Design, and Storage Conditions
The experiment was conducted during the 2023–2024 growing season in a commercial plot in Orihuela (Alicante, Spain, 38°7′49.09″ N, 0°59′54.58″ W), under Mediterranean climate conditions (with a mean annual temperature of ≈19 °C and average rainfall of 283 mm) and standard growing practices for organic lemons. The organic lemon trees were 20 years old and had been grafted on Citrus macrophylla rootstock (Citrus limon (L.) Burm. f.), cv. Fino-95, which were planted at a spacing of 6 × 5 m, were selected at random for each treatment that was tested. Melatonin (Sigma-Aldrich, Madrid, Spain) treatments were carried out by applying 1.5 L per tree, applied with a foliar sprayer, of newly brewed melatonin solutions at 0.1 and 1 mM containing 1 mL L−1 Tween 20. In a similar manner, 5 L of distilled water with 1 mL L−1 Tween 20 was applied to the control trees. The selected melatonin concentrations were based on previous experiments which demonstrated that doses between 0.1 and 1 mM elicited the greatest increases in phenolic content in citrus fruits [18].
Each treatment utilised three replicates, with each replicate consisting of three trees (9 trees per treatment, n = 9). The application of each treatment was conducted on a monthly basis, commencing after the natural degreening stage and continuing until seven days prior to the harvest date (25 October and 21 November 2023, respectively), according to a previous study [19]. The fruits were harvested at the yellow commercial ripening stage and without physical damage or disorders. The fruits were then immediately transported to the laboratory. For the experiment, 30 lemons in optimal condition were utilised for each replication, and 10 fruits were harvested at random from each tree: a total of 90 fruits were harvested from each treatment, with the fruit being separated from the stalk and a small number of leaves, according to previous research [18]. Subsequently, three lots of 30 fruits of uniform size and colour were selected from each of the three field replicates (n = 3) to perform the different treatments tested. One lot of each replicate (30 fruits in total, n = 3) was subjected to tissue preparation to measure parameters at harvest (Harvest), while another lot composed of 30 fruits (n = 3) was stored for 21 days at 1 °C and a relative humidity (RH) of 85–90% (Cold Storage). Finally, the third lot of 30 fruits was dipped in a solution of melatonin at 1 mM for 15 min (in accordance with previous experiments), while the control fruits were immersed in distilled water [18,20]. After treatments, the fruits were placed on a bench for air-drying. Subsequently, they were stored at a temperature of 1 °C and 85–90% RH (Post + Cold Storage). The tissue preparation and analytical determinations of the second and third lots were performed after cold storage.
2.2. Preparation of Tissues and Juice from Lemon Fruit
2.2.1. Freeze-Dried Samples: Leaf, Flavedo and Albedo
Samples of leaf, flavedo, and albedo from lemon fruit were immediately cut into slices of 1 × 0.5 cm to obtain a homogeneous sample from each replicate. These samples were frozen in liquid N2 and freeze-dried in an Alpha 2–4 freeze drier (Christ Alpha 2–4; Braum Biotech, Osterode am Harz, Germany) for 1 d under reduced pressure, i.e., 2.2 MPa. The temperature in the drying chamber was recorded as being −25 °C, while the heating plate reached 15 °C, in accordance with a previously established protocol [21]. Subsequently, the samples were subjected to milling until a fine powder was obtained, after which they were vacuum-packed for use in the measurement of the endogenous melatonin content. After the preparation, the samples were stored in a freezer set at a temperature of at −20 °C, pending subsequent analysis. For the aforementioned determinations, the results were expressed on a fresh weight (FW) basis.
2.2.2. Fresh Juice
Samples were freshly squeezed from lemon fruits and subsequently stored in a freezer at −20 °C until further analysis to preserve the chemical composition. For the aforementioned determinations, the results were expressed on a FW basis.
2.3. Qualitative and Quantitative Analysis of Phenolic Compounds in the Juice
The identification and quantification of phenolic compounds were carried out through HPLC-DAD by applying a previously reported method [22]. Flavanones were quantified as hesperidin (a wavelength of 280 nm) and expressed as mg 100 mL−1 of sample (FW basis).
2.4. Extraction and Quantification of Endogenous Melatonin in the Leaf and Fruit Tissues
The sample processing method employed to determine the melatonin content was carried out in accordance with the protocol previously described by Fernández-Pachón et al. [23], with certain modifications. Briefly, 100 mg of tissue was mixed with 500 μL of DMSO, and vortexed for 5 min. Later, 1500 μL of methanol/water (1:1, v/v) was added to the previous mix and treated with mild ultrasonication for 10 min at room temperature. Lastly, the samples were centrifuged at 10,500 rpm for 5 min (Sigma 1–13, B. Braun Biotech International, Osterode, Germany), and supernatants were filtered through a 0.22 μm polyvinylidene fluoride (PVDF) filter (Millex HV13, Millipore, Bedford, MA, USA). Prior to analysis, samples were stored at −20 °C. In relation to the juices, the processing procedure entailed a series of steps, namely centrifugation, filtration, and storage, all of which were conducted under identical conditions.
The determination and quantification of melatonin was performed using a UHPLC-QqQ-MS/MS (UPCL-1290 Series and a 6460 QqQ-MS/MS; Agilent Technologies, Waldbronn, Germany) with an Acquity BEH C18 column (2.1 × 150 mm; 1.7 μm; Waters, Milford, MA, USA), following a previous method with slight modifications [23]. Briefly, chromatographic separation was conducted utilising a binary gradient composed of water (phase A) and acetonitrile (phase B), both of which were LC-grade solvents containing 0.1% formic acid (v/v). A linear gradient was applied at a flow rate of 0.30 mL min−1 with the following programme (time; %B): (0.00; 40), (1.50; 40), (1.51; 90), (3.50; 90), (3.51; 40). The injection volume was set at 5 μL, and the analysis was conducted in positive mode using multiple reaction monitoring (MRM). Nitrogen was utilised as collision gas, thereby enabling the fragmentation of compounds through collision-induced dissociation within the collision cell of the triple quadrupole mass spectrometer. The instrument parameters were configured in the following manner: a drying gas flow of 8 L min−1 was established, along with a sheath gas flow of 12 L min−1; the sheath gas temperature was set to 350 °C; the nebuliser pressure was set to at 30 psi; the capillary voltage was set to at 4000 V; and the nozzle voltage was set to at 1000 V. The MassHunter software version B 04.00 was utilised for the purpose of controlling the mass spectrometer and for the collection of data. Meanwhile, version B 03.01 was employed for data analysis, including peak integration and linear regression.
2.5. Statistical Analysis
The results are presented as the mean ± SD (n = 3). The statistical differences between the various experimental conditions were identified by means of one-way analyses of variance (ANOVA) and multiple range tests of Tukey in order to ascertain the differences between the various treatments and conditions. All statistical analyses were performed using SPSS 25.0 software (LEAD Technologies, Inc., Chicago, IL, USA). The level of statistical significance was set at p < 0.05.
3. Results and Discussion
3.1. Effect of Melatonin on Flavanones Concentration in the Juice of Lemon
The analysis of the flavanone profile of lemon juice revealed the presence of two major compounds, i.e., eriodictyol-7-O-rutinoside and hesperetin-7-O-rutinoside, which contributed equally to the total flavanone content. Figure 1 illustrates the total concentration of flavanones in juices for the different melatonin treatments (preharvest and preharvest plus postharvest) at the following times: harvest and after 21 days at cold storage.
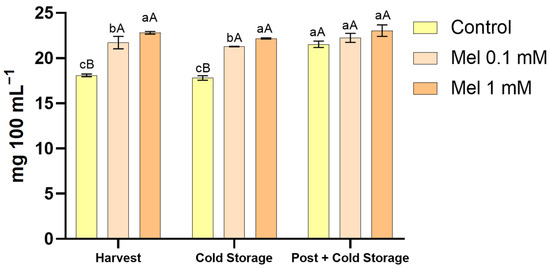
Figure 1.
Total concentration of flavanones in the lemon juice affected by the different melatonin preharvest treatments (0.1 and 1 mM concentrations) at harvest, after cold storage, and after postharvest application combined with cold storage. Lowercase letters denote statistically significant differences between treatments under same conditions, while uppercase letters indicate significant differences between conditions for the same treatment, with a significance level of p < 0.05. These distinctions were determined using one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test (n = 3) for each sampling date.
The results indicated that melatonin application significantly enhanced flavanone biosynthesis in a dose-dependent manner. The preharvest application of 1 mM melatonin led to the highest flavanone concentration at harvest time, with an increase of 26% over the control (18.09 ± 0.15 mg 100 mL−1), followed by 0.1 mM melatonin treatment, which resulted in a 20% increase (p < 0.05). These findings suggest that 1 mM melatonin is optimal for stimulating flavanone synthesis during fruit development. As demonstrated in previous studies, the application of this hormone in citrus cultivars has been shown to increase the concentration of phenolic compounds. However, literature on its effect in lemon crops is scarce. Jafari et al. demonstrated that the foliar application of melatonin resulted in an augmentation of the secondary metabolite production, predominantly hesperidin, in two lime cultivars [24].
Furthermore, this dose-dependent response is consistent with previous findings on melatonin’s role in secondary metabolite modulation in plant tissues, where it acts as a signal molecule to upregulate phenolic pathways. A recent study by Badiche et al. [19] demonstrated that the preharvest application of melatonin on ‘Verna’ lemon had a positive impact on bioactive compounds, with the most significant results being obtained at the highest tested dose (0.5 mM). In addition, the findings of Rastgoo et al. [25] corroborate our findings, evidencing a linear and positive correlation between melatonin dosage and the phenolic content in Lisbon lemons. This relationship was observed despite the implementation of melatonin postharvest treatment through the process of dipping. It is hypothesized that melatonin increases the activity of the phenylpropanoid pathway by promoting the expression of various genes involved in flavonoid synthesis. As demonstrated in the relevant literature, these include phenylalanine ammonia-lyase, cinnamate-4-hydroxylase, chalcone synthase, and flavanone 3-hydroxylase, among others [26,27].
Regarding cold storage after harvest, the levels of flavanone remained stable after 21 days under all conditions, indicating a high degree of stability of the compound when refrigerated. This stability may be attributable to the low temperatures, which minimise the activity of polyphenol oxidases and peroxidases, the enzymes responsible for phenolic degradation. This finding is consistent with those reported in the literature on the stability of citrus phenolics under refrigerated conditions [28,29].
Conversely, the postharvest application of melatonin, in conjunction with cold storage, exhibited an additive effect on flavanone concentration. The present study demonstrated that the application of this novel treatment to control fruits resulted in a significant increase in flavanone content, with an average increment of 19% being observed in comparison to control samples at harvest. In this sense, Ma et al. [15] investigated the impact of postharvest melatonin treatment on ‘Newhall’ navel orange, revealing that this treatment promoted the accumulation of antioxidants, including ascorbate and total phenols. In limes, Al-Qurashi et al. [30] showed an increase in total phenolic compounds and a concomitant reduction in the enzyme polyphenol oxidase following a melatonin dipping treatment. These findings are consistent with the results obtained in the present study. This booster effect suggests that melatonin enhances flavanone biosynthesis by sequentially activating secondary metabolite pathways, even under postharvest conditions [18]. However, the combination of preharvest and postharvest treatments did not result in a significant increase in flavanone content beyond the individual treatments. These results may be attributable to a potential saturation of the metabolic pathways responsible for flavanone synthesis when exposed to elevated melatonin concentrations.
In addition to the targeted analysis of eriodictyol-7-O-rutinoside and hesperetin-7-O-rutinoside, which are the predominant flavanones in lemon juice and are widely recognised for their health-promoting properties, previous literature suggests that melatonin may also modulate the levels of other lemon metabolites. Despite the preponderance of research conducted on lemon cultivars that has concentrated on the enhancement of total phenolic content, a study by Hosseini et al. demonstrated that melatonin application in lemon verbena increased the concentration of specific phenolic acids, including chicoric, caffeic, and chlorogenic acids [31]. Furthermore, melatonin has been observed to modulate the levels of other flavonoids, including quercetin, rutin, and catechin, in limes, though varietal distinctions may result in divergent responses [24]. Despite this broader modulatory role of melatonin in citrus metabolism, future research should expand the metabolic profiling to comprehensively assess its impact on lemon fruit quality.
Lastly, the enhanced flavanone content observed in response to melatonin treatment may further augment the health-promoting properties of lemon juice. Flavanones are recognised for their antioxidant, anti-diabetic, anti-inflammatory, antibacterial, antioxidant, antiviral, cytotoxic, and lipid-lowering properties [32]. In this study, the effects of melatonin-treated lemon juice (10 mM) on the health of K14HPV16 mice, a transgenic model of cancer induced by the human papillomavirus (HPV) [33], were evaluated. The findings indicated the presence of potential benefits, including enhancements in health indicators, immune responses, and tumour regressions. Furthermore, that study suggested the possibility of utilising the treatment as a complementary modality for conditions associated with HPV. Furthermore, it has been demonstrated that hesperidin consumption reduces systolic blood pressure in diabetic patients but has no effect on blood pressure in healthy individuals [34]. In addition, a recent study by Song et al. [35] demonstrated that hesperetin facilities the detoxification of aflatoxin B1 and potentially offers protection against neurotoxicity.
In addition to its impact on the accumulation of bioactive compounds, previous studies have demonstrated that melatonin application also improves key fruit quality parameters in lemon cultivars. Specifically, melatonin treatments have been shown to reduce weight loss and electrolyte leakage, maintain higher firmness, and lower the respiration rate during postharvest storage [18,19]. Furthermore, melatonin has been demonstrated to contribute to delayed fruit ripening, as evidenced by a reduced total soluble solids (TSS)/total acidity (TA) ratio in comparison to untreated fruits [36]. The present findings serve to reinforce the role of melatonin as an elicitor of bioactive metabolites, in addition to its function as a valuable tool for the extension of shelf-life and the preservation of the commercial quality of lemon fruits.
In view of the evident dose-dependent effect observed in this study, it is recommended that further research be conducted to assess the impact of additional melatonin concentrations, both within and beyond the 0.1–1.0 mM range. Exploration of intermediate or higher doses may provide a more precise understanding of the dose–response relationship, help to define the threshold for biosynthetic pathway saturation, and make it possible to optimise the concentration needed to maximise flavanone accumulation while ensuring fruit safety and quality. Such studies are essential to refine melatonin-based agronomic strategies for enhancing the functional value of lemon fruits.
3.2. Melatonin Distribution in the Leaf and Tissues of Lemon
The analysis of melatonin was conducted with the objective of evaluating its absorption and distribution across different tissues. HPLC-MS/MS facilitated the identification of the administered melatonin and two isomers (Table 1). It is important to note that the isomers were identified exclusively in lemon juice, accounting for 2% and 15% of the total amount, respectively.

Table 1.
Melatonin and its isomers identified and quantified in lemon samples.
The quantification of these compounds is represented in Figure 2, divided by the different samples analysed. An accumulation of leaves was observed in a dose-dependent manner, with significant variations among the treatments both at harvest and after cold storage. The 1 mM treatment exhibited the highest values. However, the postharvest treatment combined with cold storage led to the highest increase of melatonin in this tissue (77.42 ± 5.32 µg 100 g−1), followed by the combination of preharvest 0.1 mM and 1 mM melatonin treatments plus cold storage, which were 27% and 47% lower, respectively.
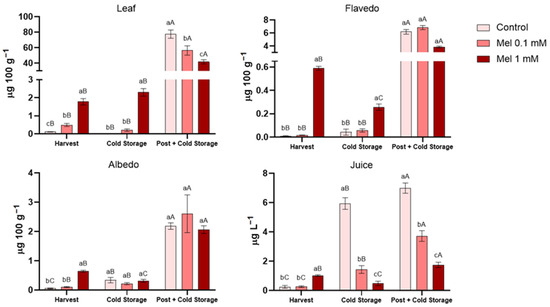
Figure 2.
Total concentration of endogenous melatonin in the samples analysed (leaf, flavedo, albedo and juice), affected by the different melatonin preharvest treatments (0.1 and 1 mM concentrations) at harvest, after cold storage, and after postharvest application combined with cold storage. Lowercase letters denote statistically significant differences between treatments under same conditions, while uppercase letters indicate significant differences between conditions for the same treatment, with a significance level of p < 0.05. These distinctions were determined using one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test (n = 3) for each sampling date.
In the flavedo, at harvest, the highest melatonin content was achieved with 1 mM preharvest treatment (0.59 ± 0.02 and µg 100 g−1), while the other treatments showed very low levels (0.01 µg 100 g−1, on average). The application of cold storage resulted in a modest increase in controls and 0.1 mM samples; however, a substantial decrease was observed in the 1 mM treatment. Conversely, after postharvest treatment, preharvest 0.1 mM samples reached the highest melatonin concentration (6.82 ± 0.32 µg 100 g−1), with the control group (which received only a postharvest treatment) and preharvest 1 mM samples exhibiting 10% and 44% lower concentrations, respectively.
The albedo exhibited a comparable trend to the flavedo at harvest. The application of a preharvest 1 mM treatment resulted in the attainment of the highest melatonin concentration (0.64 ± 0.03 µg 100 g−1). Furthermore, cold storage exhibited a similar effect to that observed for flavedo, with an increase for the control and preharvest 0.1 mM treatment and a decrease for the preharvest 1 mM treatment. The application of postharvest treatments resulted in a significant increase in melatonin levels, with an average increase of 2.28 µg 100 g−1. However, no significant differences were observed among the various treatments.
Finally, in juices, the highest recorded concentration of melatonin was observed in the preharvest 1 mM treatment (1.01 ± 0.06 µg L−1), which was four-fold greater than the other treatments at harvest. However, a 50% decrease in melatonin levels was observed after cold storage, while the control and 0.1 mM samples exhibited a significant increase in melatonin concentrations (5.93 and 1.43 µg L−1, respectively). Following the postharvest treatment, the control group, which received only a postharvest treatment, presented the highest concentration of melatonin (6.99 ± 0.35 µg L−1). This was followed by the combination of preharvest melatonin treatments at 0.1 mM and 1 mM, which resulted in concentrations 47% and 75% lower, respectively. Despite these increases, the melatonin concentrations in the juice remained low in comparison to those found in dietary supplements (e.g., 3 mg per tablet), i.e., 330 mL of juice provides approximately 2.5 µg, thereby supporting the safety of the consumption of lemons that have undergone this treatment.
Melatonin, a substance that has gained widespread recognition for its role in sleep regulation, has been demonstrated to offer a range of health benefits that extend beyond the improvement of sleep quality. Evidence suggests that the treatment has cardioprotective effects, including the reduction of hypertension, improvement in cardiovascular function, and the safeguarding of heart tissue from damage [37,38]. The aforementioned benefits are associated with its potent antioxidant and anti-inflammatory properties, which help to mitigate oxidative stress and inflammation, both of which are strongly linked with cardiovascular diseases [37,39]. Furthermore, melatonin has been demonstrated to enhance sleep quality, particularly in individuals experiencing sleep disturbances, such as postmenopausal women, and to alleviate anxiety and depression, as observed in diabetic haemodialysis patients [40,41]. The metabolic advantages of the intervention under investigation include glycaemic control through the reduction of fasting plasma glucose, insulin levels, and insulin resistance. In addition, improvements in lipid profiles are achieved through the reduction of triglycerides and the enhancement of HDL-cholesterol levels [39,40,41,42]. It is evident that the multifaceted effects of melatonin render it a promising compound for promoting overall health and addressing chronic conditions.
The results demonstrate the effective absorption and accumulation of exogenous melatonin across different lemon tissues, confirming the findings of Lin et al., who reported that exogenous melatonin treatments enhance endogenous melatonin levels through absorption mechanisms [43]. Also, Wang et al. suggested that endogenous melatonin levels increased in sweet cherries during the early storage period due to physical infiltration [44]. However, other studies have shown that exogenous melatonin may also stimulate its own biosynthesis via feedback regulation of the key genes involved in its metabolic pathway, including tryptophan decarboxylase, tryptamine 5-hydroxylase, serotonin N-acetyltransferase, N-acetylserotonin methyltransferase, and caffeic acid O-methyltransferase [45,46,47,48]. Moreover, a dose-dependent response was observed at harvest, with higher concentrations of melatonin associated with the preharvest 1 mM treatment; this aligns with prior research showing that higher doses of melatonin result in greater tissue accumulation in litchi and sweet cherries [49,50].
The effects of cold storage were tissue-specific, i.e., levels remained stable in leaf, flavedo, and albedo but changed markedly in juice. Consequently, in the control and preharvest 0.1 mM treatments, cold storage led to increased melatonin levels in juices, possibly due to stress-induced biosynthesis, as melatonin plays a pivotal role in countering oxidative stress by acting as an antioxidant [51,52]. In this sense, different abiotic stressors, including salinity, extreme temperatures (heat and cold), or drought, have been demonstrated to trigger an increase in melatonin levels by enhancing the expression of genes involved in its biosynthesis.
Conversely, in the preharvest 1 mM treatment, cold storage reduced melatonin content across tissues. This decline could be indicative of the utilisation of endogenous melatonin to mitigate oxidative stress, given its potent antioxidant properties. The balance between melatonin biosynthesis and consumption under stress is likely to determine its observed concentration in stored fruits. Recent studies by Yan et al. and Wang et al. conducted in cherry tomatoes and sweet cherries, respectively, showed that endogenous melatonin, previously augmented by postharvest melatonin treatment, was diminished after storage [44,46]. This finding is in alignment with the results obtained in this study. However, further research is required to elucidate the mechanism underlying the reduction of endogenous melatonin after cold storage.
The application of postharvest melatonin treatments in conjunction with cold storage has been demonstrated to result in a substantial enhancement of melatonin content across all tissues examined. This finding underscores the efficacy of this approach as a promising strategy for enriching fruits with this bioactive compound. The observed increase was attributable to the enhancement of the genes implicated in the synthesis of melatonin, in addition to the direct absorption of exogenous melatonin during the treatment process, as previously outlined in the literature. Postharvest treatments have been shown to facilitate a more direct and uniform absorption of melatonin into the fruit’s vascular system, which may, in turn, facilitate its accumulation in tissues. Furthermore, postharvest applications provide the flexibility to tailor treatments to specific fruit cultivars and storage conditions, thus ensuring the maximisation of shelf-life and quality preservation. A plethora of studies have repeatedly evidenced the efficacy of melatonin in decelerating the process of senescence, preserving firmness, and hindering decay when administered post-harvest [16,18,44,45].
It is interesting to note that the combination of preharvest and postharvest treatments demonstrated a lower effect compared to postharvest single treatment. The control group, on the other hand, showed the highest levels of melatonin in most tissues, or no significant differences were observed between treatments. This phenomenon could be associated with a potential saturation of the biosynthetic pathway responsible for melatonin synthesis.
From a pragmatic standpoint, postharvest treatments have been shown to be both effective and cost-effective, as well as suitable for commercial application. Furthermore, given the capacity of postharvest treatments to be applied uniformly to harvested fruit, they ensure consistent enrichment and facilitate the production of functional juices with enhanced melatonin content. These findings underscore the efficacy of postharvest treatments as a preferred strategy for developing health-promoting citrus products, particularly juices, which are extensively consumed and highly regarded for their nutritional benefits. Furthermore, the increased melatonin content in byproducts, such as flavedo and albedo, supports their revalorisation within the citrus industry, promoting their use as sources of bioactive compounds for nutraceuticals or functional food formulations.
4. Conclusions and Future Prospects
The present study demonstrated that melatonin application is an effective strategy to increase flavanone and endogenous melatonin levels in lemon fruits. Preharvest treatments were found to result in a dose-dependent increase in flavanone content, both at harvest and after cold storage. The most marked enhancements in flavanone and melatonin levels were observed after postharvest application (either individually or in combination). It is noteworthy that postharvest treatments alone were sufficient to significantly enrich the melatonin content, particularly in lemon juice. This highlights their practical applicability within current industrial workflows, as they represent the most feasible and efficient approach for commercial-scale implementation. The findings of this study provide valuable insights into the use of melatonin as a natural elicitor to improve the functional quality of lemons while also promoting the valorisation of citrus byproducts. In this regard, subsequent studies should investigate a more extensive range of melatonin concentrations in order to ascertain the most efficacious treatment across a range of lemon cultivars. Moreover, in view of the limited comprehension of the impact of melatonin on the bioavailability of phenolic compounds in human health, further studies are required to elucidate the mechanistic role of melatonin in modulating the absorption of health-promoting compounds present in different lemon tissues.
Author Contributions
Conceptualisation, D.V.; methodology, V.A. and M.E.G.-P.; validation, V.A., M.E.G.-P. and D.V.; formal analysis, V.A.; investigation, V.A. and M.E.G.-P.; resources, D.V.; data curation, V.A.; writing—original draft preparation, V.A. and M.E.G.-P.; writing—review and editing, all authors; visualisation, V.A.; supervision, D.V.; project administration, D.V.; funding acquisition, D.V. All authors have read and agreed to the published version of the manuscript.
Funding
Direcció General de Ciència i Investigació of the Generalitat Valenciana, Spain. Financial support through Prometeo Program (PROMETEO/2021/089).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank to Juan Miguel Valverde Veracruz for the permission to use his plots and the technical support received and to Conselleria d’Educació, Universitats i Ocupació of Generalitat Valenciana (GVA, Spain) for the Postdoc-scholarship of Vicente Agulló co-financed by the European Social Fund (grant number CIAPOS/2022/055). In addition, the authors thank BioRender (https://www.biorender.com/), (Toronto, ON, Canada) to provide pictures used in the Graphical Abstract.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kirwan, D. Global health: Current issues, future trends and foreign policy. Clin. Med. 2009, 9, 247–253. [Google Scholar] [CrossRef]
- Malik, V.S.; Hu, F.B.; Schulze, M.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud. Evaluación de la capacidad nacional para la prevención y el control de enfermedades no transmisibles. Inf. Encuesta Mund. 2021, 2023, 1–122. [Google Scholar]
- Clemente-Suárez, V.J.; Martín-Rodríguez, A.; Redondo-Flórez, L.; López-Mora, C.; Yáñez-Sepúlveda, R.; Tornero-Aguilera, J.F. New Insights and Potential Therapeutic Interventions in Metabolic Diseases. Int. J. Mol. Sci. 2023, 24, 10672. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/ (accessed on 16 April 2025).
- Ministerio de Agricultura, Pesca y Alimentación. Available online: https://www.mapa.gob.es/ (accessed on 16 April 2025).
- Home. Available online: https://www.usda.gov/ (accessed on 16 April 2025).
- Tripoli, E.; La Guardia, M.; Giammanco, S.; Di Majo, D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Taher, M.; Shukry, N.A.S.B.; Susanti, D.; Saleh, W.M.N.H.W.; Syukri, Y. Citrus flavonoids in preventing cardiovascular diseases. Plant-Deriv. Bioact. Chem. Mode Action 2020, 1, 495–508. [Google Scholar] [CrossRef]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lin, X.; Wei, Q.; Yang, X.; Zhang, Y.; Chen, J. Melatonin treatment delays postharvest senescence and maintains the organoleptic quality of ‘Newhall’ navel orange (Citrus sinensis (L.) Osbeck) by inhibiting respiration and enhancing antioxidant capacity. Sci. Hortic. 2021, 286, 110236. [Google Scholar] [CrossRef]
- Shah, H.M.S.; Singh, Z.; Hasan, M.U.; Kaur, J.; Afrifa-Yamoah, E.; Woodward, A. Melatonin application suppresses oxidative stress and maintains fruit quality of cold stored ‘Esperanza’ raspberries by regulating antioxidant system. Postharvest Biol. Technol. 2024, 207, 112597. [Google Scholar] [CrossRef]
- Aboryia, M.S.; Lo’Ay, A.A.; Omar, A.S.M. Reduction of chilling injury of “Washington” navel orange fruits by melatonin treatments during cold storage. Folia Hortic. 2021, 33, 343–353. [Google Scholar] [CrossRef]
- Badiche-El Hilali, F.; Valverde, J.M.; García-Pastor, M.E.; Serrano, M.; Castillo, S.; Valero, D. Melatonin Postharvest Treatment in Leafy ‘Fino’ Lemon Maintains Quality and Bioactive Compounds. Foods 2023, 12, 2979. [Google Scholar] [CrossRef]
- Badiche-El Hilali, F.; García-Pastor, M.E.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Melatonin as an Efficient and Eco-Friendly Tool to Increase Yield and to Maintain Quality Attributes during Lemon Storage. Int. J. Mol. Sci. 2024, 25, 10025. [Google Scholar] [CrossRef]
- Carrión-Antolí, A.; Lorente-Mento, J.M.; Valverde, J.M.; Castillo, S.; Valero, D.; Serrano, M. Effects of melatonin treatment on sweet cherry tree yield and fruit quality. Agronomy 2022, 12, 3. [Google Scholar] [CrossRef]
- García-Pastor, M.E.; Serrano, M.; Guillén, F.; Zapata, P.J.; Valero, D. Preharvest or a combination of preharvest and postharvest treatments with methyl jasmonate reduced chilling injury, by maintaining higher unsaturated fatty acids, and increased aril colour and phenolics content in pomegranate. Postharvest Biol. Technol. 2020, 167, 111226. [Google Scholar] [CrossRef]
- Agulló, V.; Domínguez-Perles, R.; Moreno, D.A.; Zafrilla, P.; García-Viguera, C. Alternative sweeteners modify the urinary excretion of flavanones metabolites ingested through a new maqui-berry beverage. Foods 2020, 9, 41. [Google Scholar] [CrossRef]
- Fernández-Pachõn, M.S.; Medina, S.; Herrero-Martín, G.; Cerrillo, I.; Berná, G.; Escudero-Lõpez, B.; Ferreres, F.; Martín, F.; García-Parrilla, M.C.; Gil-Izquierdo, A. Alcoholic fermentation induces melatonin synthesis in orange juice. J. Pineal Res. 2014, 56, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.; Shahsavar, A. The Effect of Foliar Application of Melatonin on Changes in Secondary Metabolite Contents in Two Citrus Species Under Drought Stress Conditions. Front. Plant Sci. 2021, 12, 692735. [Google Scholar] [CrossRef] [PubMed]
- Rastgoo, N.; Rastegar, S.; Rohani, A. Optimization of melatonin treatment using response surface methodology to enhance postharvest quality of lemon fruit during cold storage. J. Food Meas. Charact. 2024, 18, 2814–2833. [Google Scholar] [CrossRef]
- Liang, D.; Shen, Y.; Ni, Z.; Wang, Q.; Lei, Z.; Xu, N.; Deng, Q.; Lin, L.; Wang, J.; Lv, X.; et al. Exogenous melatonin application delays senescence of kiwifruit leaves by regulating the antioxidant capacity and biosynthesis of flavonoids. Front. Plant Sci. 2018, 9, 360644. [Google Scholar] [CrossRef]
- Khatam, A.S.; Rastegar, S.; Jahromi, A.A.; Khankahdani, H.H.; Bagherian, S.A.A. Biochemical and physiological mechanism induced by melatonin in Mexican lime (Citrus aurantifolia Swingle) plants: Cold and freezing stress. Acta Physiol. Plant. 2023, 45, 98. [Google Scholar] [CrossRef]
- Huynh, T.D.; Kha, C.T.; Nguyen, V.A. Changes in quality parameters and bioactive components of seedless lime fruit (Citrus latifolia) during cold storage. Food Res. 2024, 8, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Recent Advances of Polyphenol Oxidases in Plants. Molecules 2023, 28, 2158. [Google Scholar] [CrossRef]
- Al-Qurashi, A.D.; Awad, M.A. Effect of exogenous melatonin and chitosan treatments on quality and biochemical changes of ‘Balady Banzahir’ limed during shelf life. J. Anim. Plant Sci. 2023, 33, 310–319. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Samsampour, D.; Zahedi, S.M.; Zamanian, K.; Rahman, M.M.; Mostofa, M.G.; Tran, L.S.P. Melatonin alleviates drought impact on growth and essential oil yield of lemon verbena by enhancing antioxidant responses, mineral balance, and abscisic acid content. Physiol. Plant. 2021, 172, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, H.; Shompa, S.A.; Islam, M.M.; Alam, S.; Richi, F.T.; Emon, N.U.; Ashrafi, S.; Ahmed, N.U.; Chowdhury, M.N.R.; Fatema, N.; et al. Flavonoids: A treasure house of prospective pharmacological potentials. Heliyon 2024, 10, e27533. [Google Scholar] [CrossRef]
- Badiche-El Hilali, F.; Medeiros-Fonseca, B.; Silva, J.; Silvestre-Ferreira, A.C.; Pires, M.J.; Gil da Costa, R.M.; Peixoto, F.; Oliveira, P.A.; Valero, D. The Effect of Lemon Juice (Citrus limon L.) Treated with Melatonin on the Health Status and Treatment of K14HPV16 Mice. Antioxidants 2024, 13, 588. [Google Scholar] [CrossRef]
- Gao, H.; Chen, F.; Wang, S. Hesperidin reduces systolic blood pressure in diabetic patients and has no effect on blood pressure in healthy individuals: A systematic review and meta-analysis. Phytother. Res. 2024, 38, 3706–3719. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Hesperetin protects hippocampal neurons from the neurotoxicity of Aflatoxin B1 in mice. Ecotoxicol. Environ. Saf. 2024, 269, 115782. [Google Scholar] [CrossRef] [PubMed]
- Rastgoo, N.; Rastegar, S.; Rohani, A. Maintaining quality of Lisbon lemon (Citrus limon) in cold storage using natural elicitors. J. Hortic. Postharvest Res. 2024, 7, 99–114. [Google Scholar] [CrossRef]
- Jiki, Z.; Lecour, S.; Nduhirabandi, F. Cardiovascular Benefits of Dietary Melatonin: A Myth or a Reality? Front. Physiol. 2018, 9, 528. [Google Scholar] [CrossRef]
- Tobeiha, M.; Jafari, A.; Fadaei, S.; Mirazimi, S.M.A.; Dashti, F.; Amiri, A.; Khan, H.; Asemi, Z.; Reiter, R.J.; Hamblin, M.R.; et al. Evidence for the Benefits of Melatonin in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 888319. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Soleimani, A.; Bahmani, F.; Aghadavod, E.; Ramezani, R.; Reiter, R.J.; Mansournia, M.A.; Banikazemi, Z.; Soleimani, M.; Zaroudi, M.; et al. The Effects of Melatonin Supplementation on Parameters of Mental Health, Glycemic Control, Markers of Cardiometabolic Risk, and Oxidative Stress in Diabetic Hemodialysis Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Ren. Nutr. 2020, 30, 242–250. [Google Scholar] [CrossRef]
- Treister-Goltzman, Y.; Peleg, R. Melatonin and the health of menopausal women: A systematic review. J. Pineal Res. 2021, 71, e12743. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Nunes, B.P. Effects of melatonin supplementation on diabetes: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2021, 40, 4595–4605. [Google Scholar] [CrossRef]
- Lin, Y.; Fan, L.; Xia, X.; Wang, Z.; Yin, Y.; Cheng, Y.; Li, Z. Melatonin decreases resistance to postharvest green mold on citrus fruit by scavenging defense-related reactive oxygen species. Postharvest Biol. Technol. 2019, 153, 21–30. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, X.; Yang, Q.; Zhao, Q. Exogenous melatonin delays postharvest fruit senescence and maintains the quality of sweet cherries. Food Chem. 2019, 301, 125311. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Yan, R.; Li, S.; Cheng, Y.; Kebbeh, M.; Huan, C.; Zheng, X. Melatonin treatment maintains the quality of cherry tomato by regulating endogenous melatonin and ascorbate-glutathione cycle during room temperature. J. Food Biochem. 2022, 46, e14285. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Li, N.; Zhai, K.; Yin, Q.; Gu, Q.; Zhang, X.; Melencion, M.G.; Chen, Z. Crosstalk between melatonin and reactive oxygen species in fruits and vegetables post-harvest preservation: An update. Front. Nutr. 2023, 10, 1143511. [Google Scholar] [CrossRef]
- Xie, J.; Qin, Z.; Pan, J.; Li, J.; Li, X.; Khoo, H.E.; Dong, X. Melatonin treatment improves postharvest quality and regulates reactive oxygen species metabolism in “Feizixiao” litchi based on principal component analysis. Front. Plant Sci. 2022, 13, 965345. [Google Scholar] [CrossRef]
- Cortés-Montaña, D.; Bernalte-García, M.J.; Palomino-Vasco, M.; Serradilla, M.J.; Velardo-Micharet, B. Effect of preharvest melatonin applications at dusk on quality and bioactive compounds content of early sweet cherries. J. Sci. Food Agric. 2024, 104, 1583–1590. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Abbas, T.; Ahmad, I.; Nawaz, R.; Nazim, M.; Gatasheh, M.K.; Alamri, A.M.; Muneeb, A. Physiological responses and antioxidant properties of Citrus reticulata under different abiotic stresses mitigated by endogenous melatonin. Sci. Hortic. 2023, 322, 112442. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).