CdGLK1 Transcription Factor Confers Low-Light Tolerance in Bermudagrass via Coordinated Upregulation of Photosynthetic Genes and Enhanced Antioxidant Enzyme Activity
Abstract
1. Introduction
2. Materials and Methods
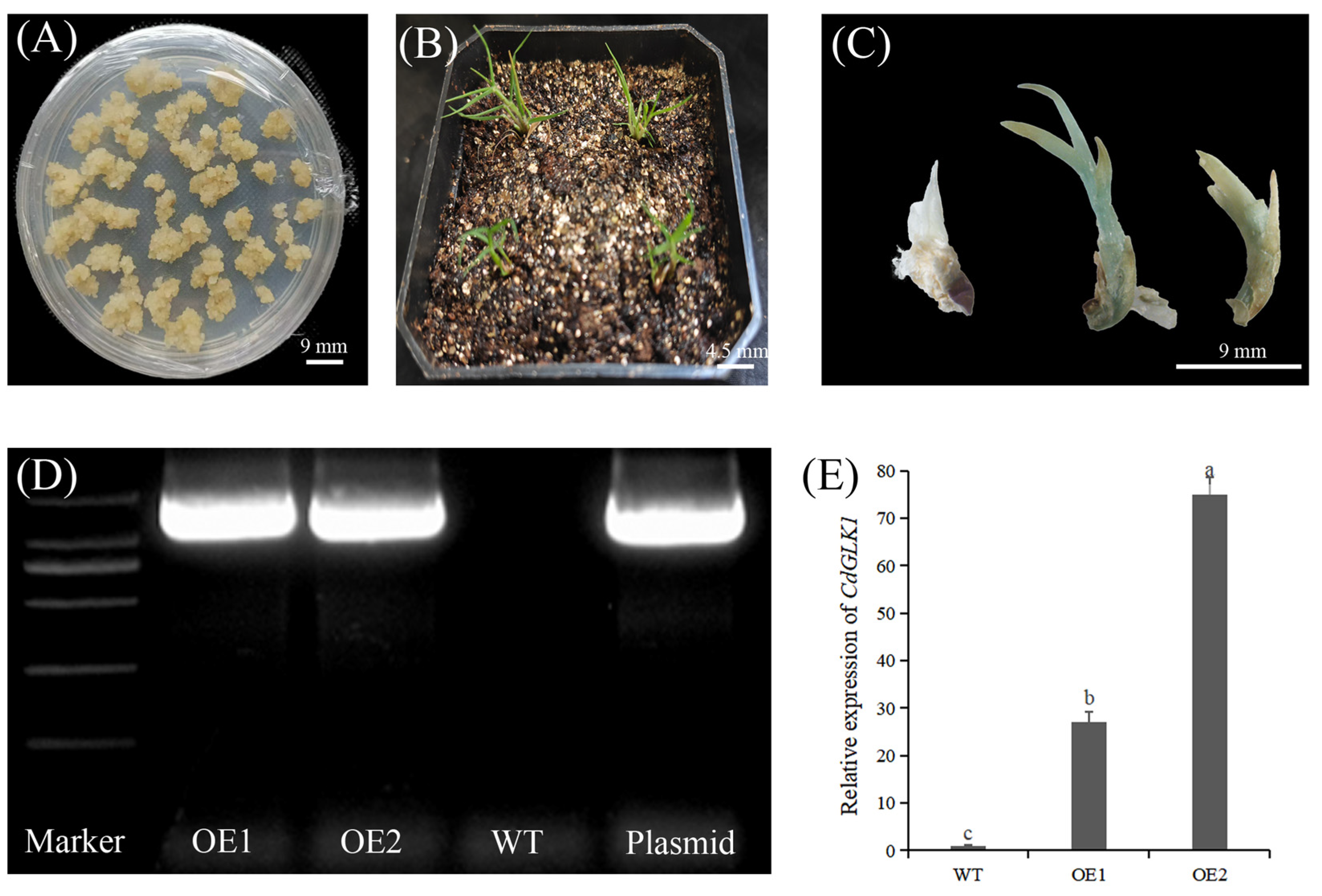
2.1. Plant Materials and Growth Conditions
2.2. Experimental Design and Treatments
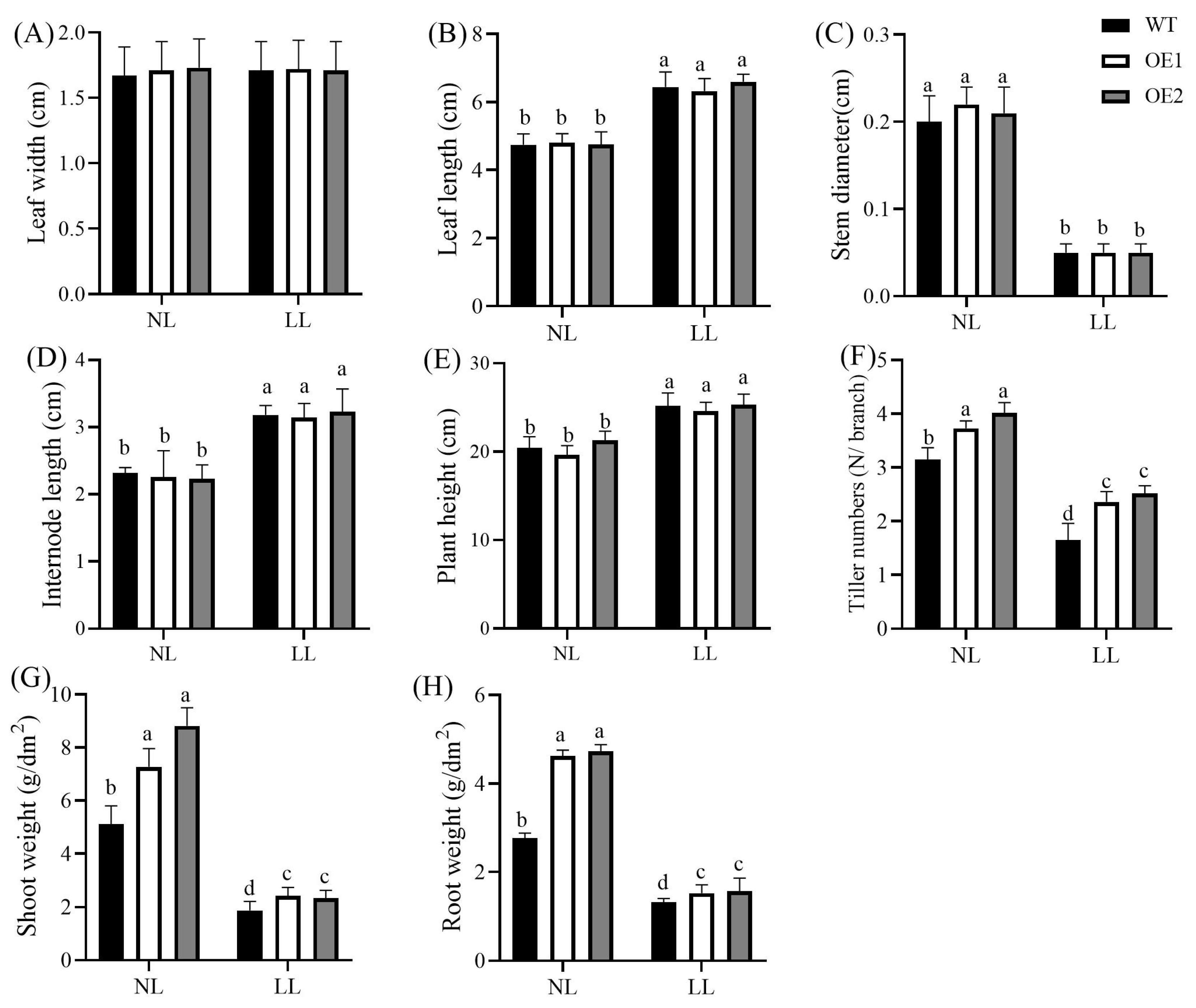
2.3. Assay of Morphological Parameters
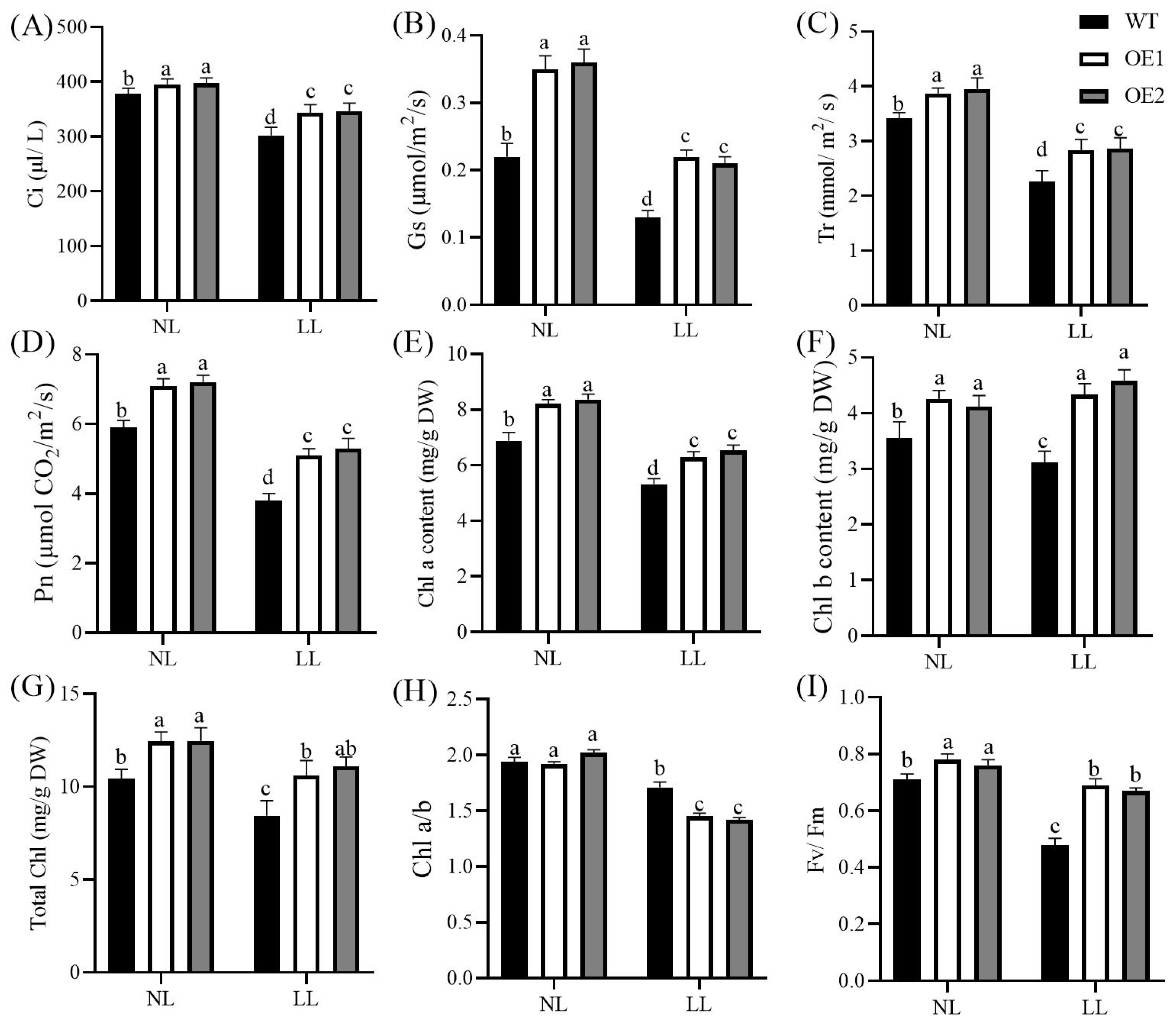
2.4. Measurements of Gas Exchange Parameters, Fv/Fm, and Chlorophyll Content
2.5. Quantification of Photosynthetic Enzyme Activities
2.6. Quantification of Antioxidant Enzyme Activities
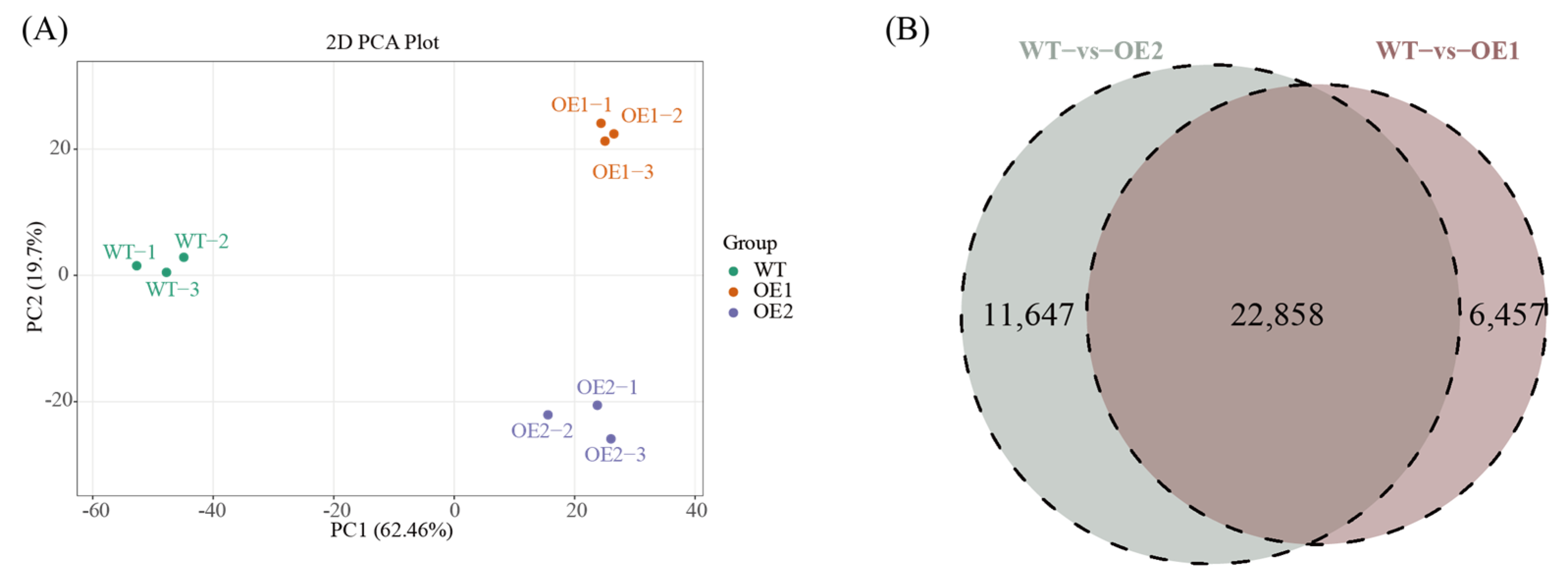
2.7. Transcriptome Profiling and Comparative Analysis
2.8. Quantitative Real-Time PCR Analysis
2.9. Statistical Analysis
3. Results
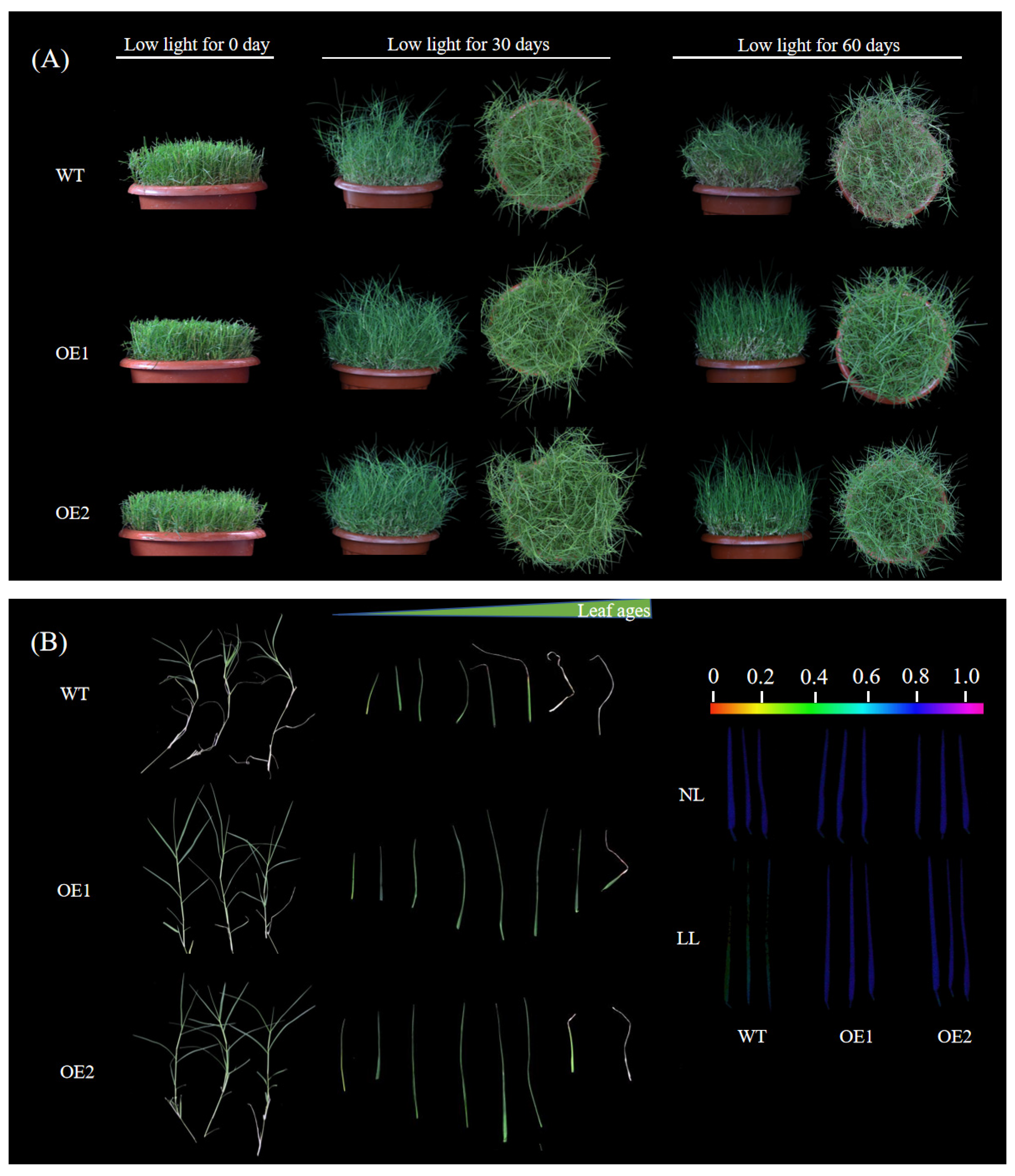
3.1. Overexpression of CdGLK1 Alters Bermudagrass Morphology Under Low Light
3.2. Overexpression of CdGLK1 Enhances Photosynthetic Capacity and Gas Exchange in Bermudagrass
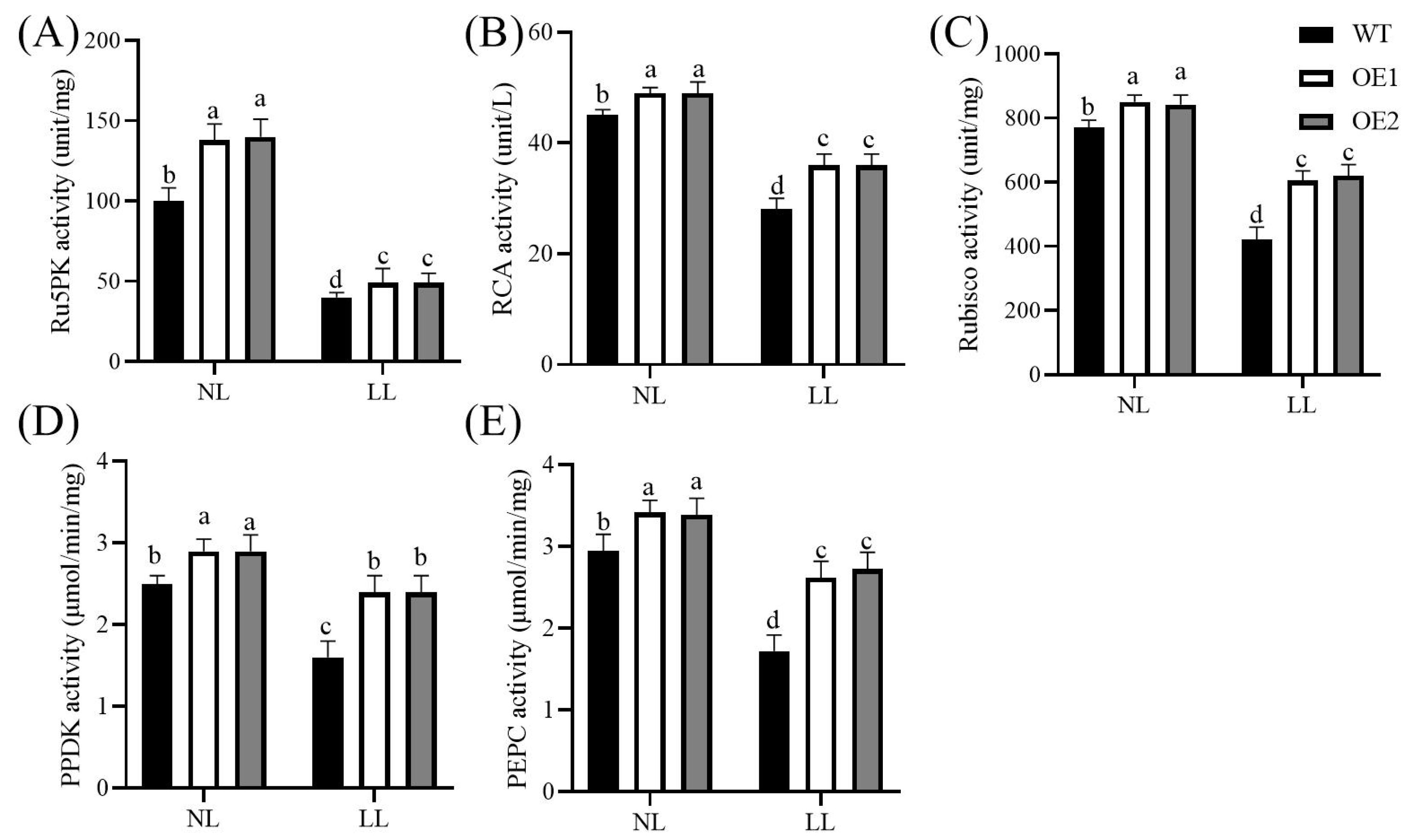
3.3. Overexpression of CdGLK1 Enhances the Activity of Photosynthetic Enzymes in Bermudagrass
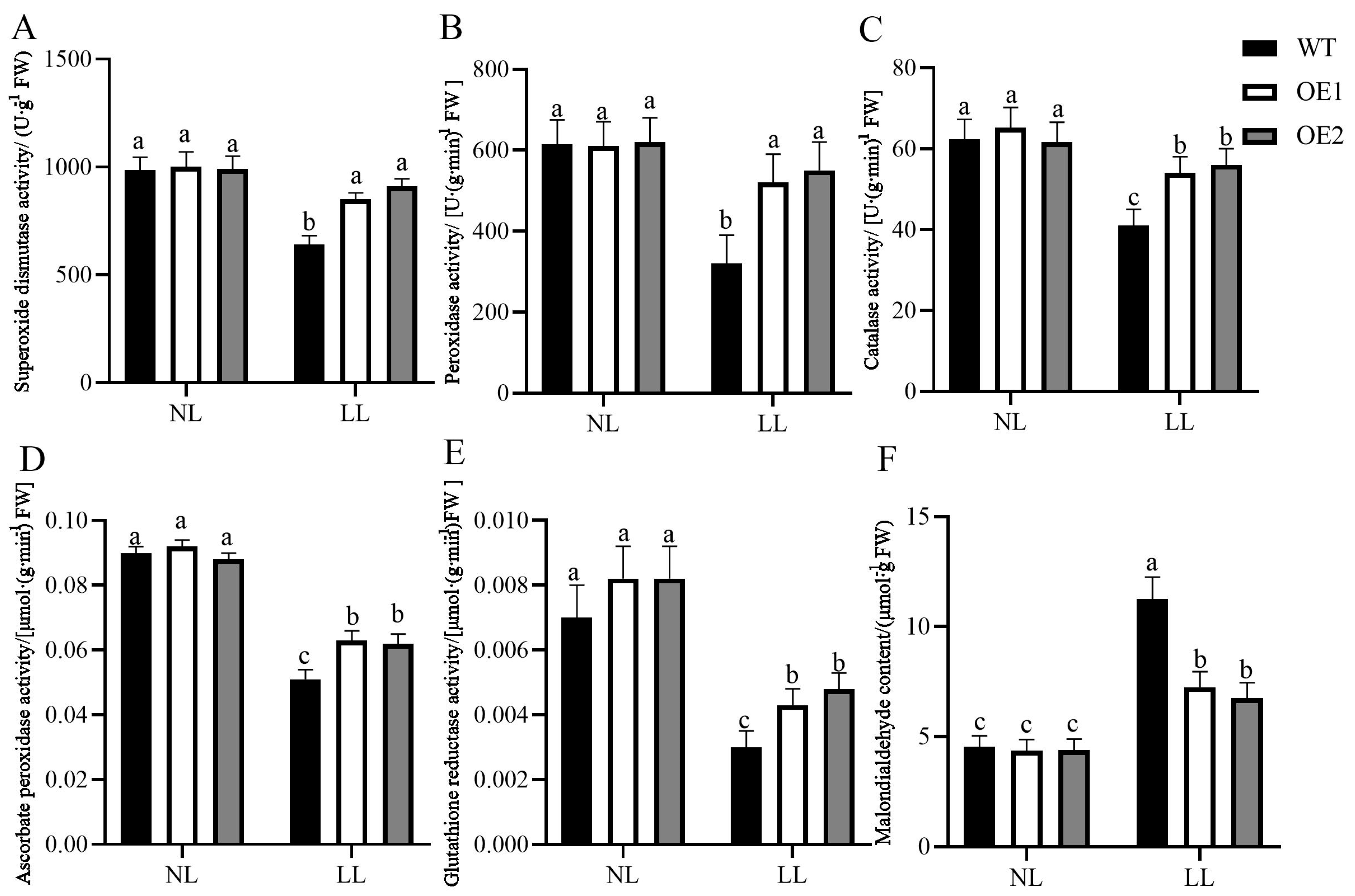
3.4. Overexpression of CdGLK1 Enhances Antioxidant Enzyme Activity in Bermudagrass Under Low Light
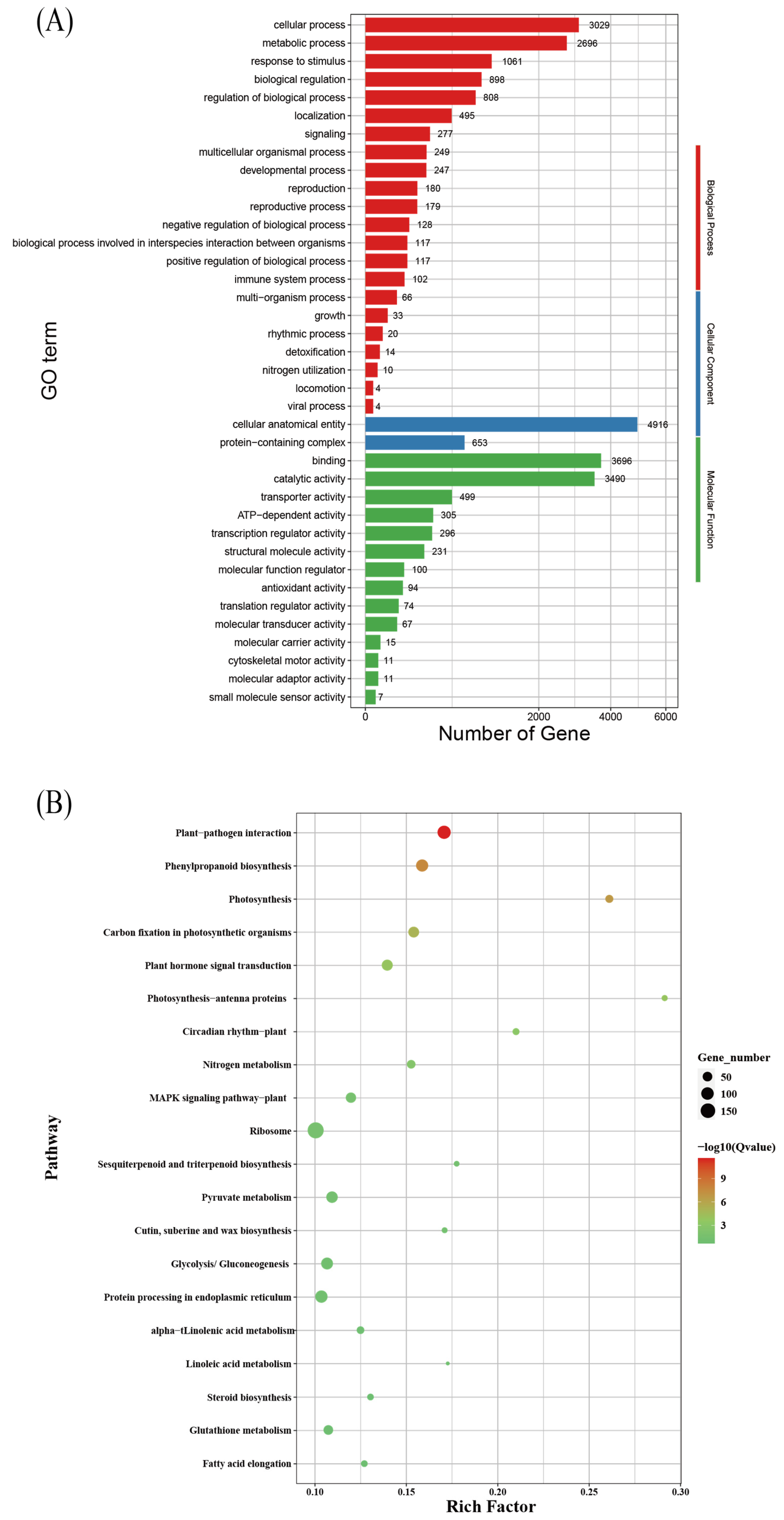
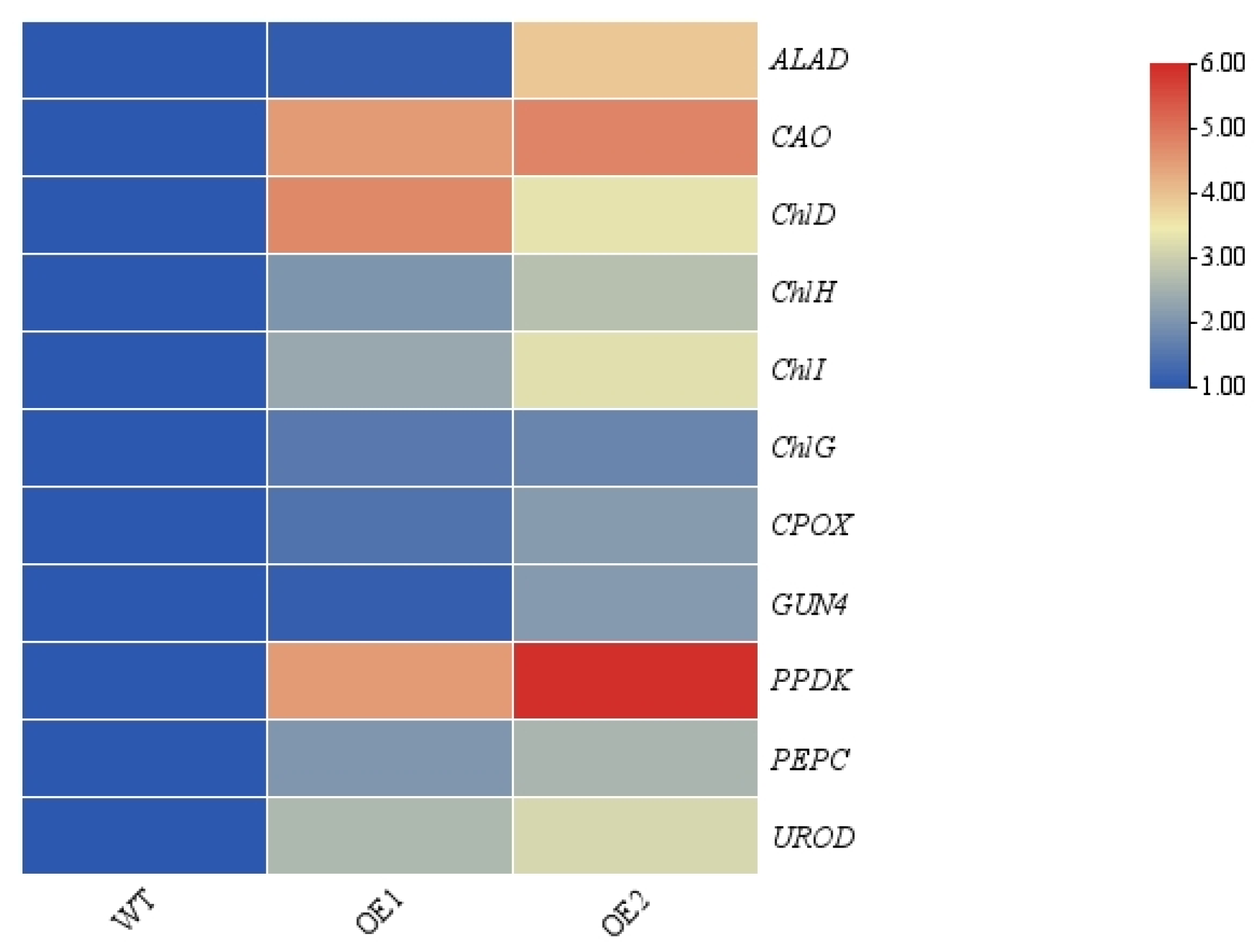
3.5. Enrichment and Analysis of Differentially Expressed Genes
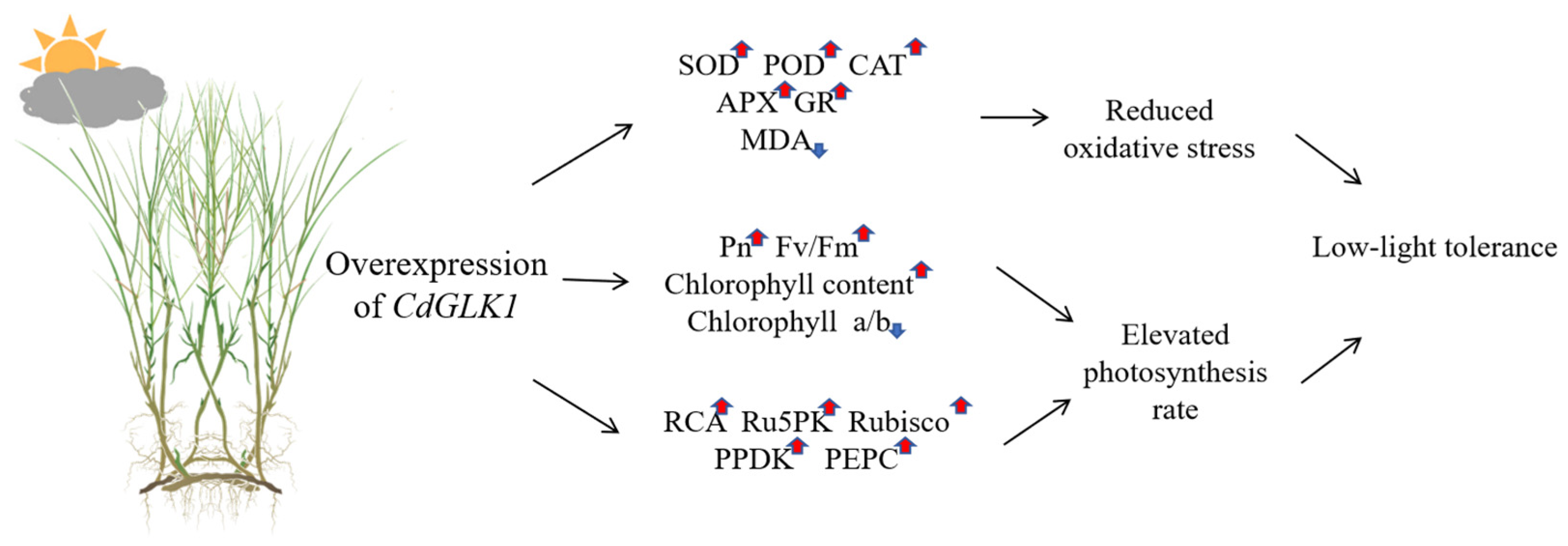
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Lee, G.; Carrow, R.N.; Duncan, R.R. Salinity tolerance of selected Seashore paspalums and bermudagrasses: Root and verdure responses and criteria. HortScience 2004, 39, 1143–1147. [Google Scholar] [CrossRef]
- Chen, J.; Thammina, C.; Li, W.; Yu, H.; Yer, H. Isolation of prostrate turfgrass mutants via screening of dwarf phenotype and characterization of a perennial ryegrass prostrate mutant. Hortic. Res. 2016, 3, 16031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fox, J.L.; Jespersen, D.; Baxter, L.L.; Snider, J.L.; Iersel, M.W.V.; Schwartz, B.M. Towards estimating shade response of bermudagrass (Cynodon spp.) using field-based photosynthetic properties. Grass Res. 2022, 2, 20–25. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, J.; Dong, L.; Sun, X.; Chen, J.; Xie, F.; Chen, Y. Shade responses of prostrate and upright turf-type bermudagrasses. Grass Res. 2022, 2, 62–70. [Google Scholar] [CrossRef]
- Frangedakis, E.; Yelina, N.E.; Billakurthi, K.; Hua, L.; Schreier, T.; Dickinson, P.J.; Tomaselli, M.; Haseloff, J.; Hibberd, J.M. MYB-related transcription factors control chloroplast biogenesis. Cell 2024, 187, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gommers, C.M.M.; Visser, E.J.W.; Onge, K.R.S.; Voesenek, L.; Pierik, R. Shade tolerance: When growing tall is not an option. Trends Plant Sci. 2013, 18, 65–71. [Google Scholar] [CrossRef]
- Wen, S.; Liu, B.; Long, S.; Gao, S.; Liu, Q.; Liu, T.; Xu, Y. Low nitrogen level improves low light tolerance in tall fescue by regulating carbon and nitrogen metabolism. Environ. Exp. Bot. 2021, 194, 104749. [Google Scholar] [CrossRef]
- Long, S.; Liu, Q.; Guo, H.; Li, X.; You, X.K.; Liu, B.W.; Gao, S.H.; Wen, S.Y.; Liu, T.Y.; Xu, Y.F. Integrated physiological and transcriptomic analyses reveal differential photosynthetic responses to low light stress in tall fescue cultivars. Sci. Hortic. 2022, 304, 111343. [Google Scholar] [CrossRef]
- Jiang, Y.; Ronny, R.; Robert, N. Assessment of low light tolerance of seashore paspalum and bermudagrass. Crop Sci. 2004, 44, 587–594. [Google Scholar] [CrossRef]
- Mcbee, G. Association of certain variations in light quality with the performance of selected turfgrasses. Crop Sci. 1969, 9, 14–17. [Google Scholar] [CrossRef]
- Waters, M.T.; Wang, P.; Korkaric, M.; Capper, R.G.; Saunders, N.J.; Langdale, J.A. GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 2009, 21, 1109–1128. [Google Scholar] [CrossRef] [PubMed]
- Cackett, L.; Luginbuehl, L.H.; Schreier, T.B.; Lopez, J.E.; Hibberd, J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2021, 229, 1355–1370. [Google Scholar] [CrossRef]
- Koichi, K.; Daichi, S.; Ko, N.; Daiki, F.; Hirohisa, K.; Masami, K.; Mayuko, S.; Kiminori, T.; Keiko, S.; Niyogi, K. Photosynthesis of root chloroplasts developed in Arabidopsis lines overexpressing GOLDEN2-LIKE transcription factors. Plant Cell Physiol. 2013, 54, 1365–1377. [Google Scholar]
- Carina, B.; Schwechheimer, C. B-GATA transcription factors—Insights into their structure, regulation, and role in plant development. Front. Plant Sci. 2015, 6, 90–95. [Google Scholar]
- Yan, Z.; Blakley, I.C.; Franco, J.M.; Zorrilla, F.; Yamburenko, M.V.; Roberto, S.; Kieber, J.J.; Loraine, A.E.; Eric, S.G. Coordination of chloroplast development through the action of the GNC and GLK transcription factor families. Plant Physiol. 2018, 178, 720–735. [Google Scholar]
- Wang, P.; Fouracre, J.; Kelly, S.; Karki, S.; Gowik, U.; Aubry, S.; Shaw, M.K.; Westhoff, P.; Inez, H.S.; Quick, W.P.; et al. Evolution of GOLDEN2-LIKE gene function in C3 and C4 plants. Planta 2012, 237, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Rossini, L.; Cribb, L.; Martin, D.J. The maize Golden2 gene defines a novel class of transcriptional regulators in plants. Plant Cell 2001, 13, 1231–1244. [Google Scholar] [CrossRef]
- Bravo, G.A.; Yasumura, Y.; Langdale, J.A. Specialization of the Golden2-like regulatory pathway during land plant evolution. New Phytol. 2009, 183, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Muramatsu, M.; Hakata, M.; Makoto, H.; Osamu, U.; Yoshiaki, N.; Hirohiko, H.; Makoto, T.; Hiroaki, I. Ectopic overexpression of the transcription factor OsGLK1 induces chloroplast development in non-green rice cells. Plant Cell Physiol. 2009, 50, 1933–1949. [Google Scholar] [CrossRef]
- Brand, A.; Borovsky, Y.; Hill, T.; Rahman, K.A.; Bellalou, A.; Deynze, A.V. CaGLK2 regulates natural variation of chlorophyll content and fruit color in pepper fruit. Theor. Appl. Genet. 2014, 127, 2139–2148. [Google Scholar] [CrossRef]
- Kim, N.; Jeong, J.; Kim, J.; Oh, J.; Choi, G. Shade represses photosynthetic genes by disrupting the DNA binding of GOLDEN2-LIKE1. Plant Physiol. 2023, 191, 2334–2352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, R.; Zeng, X.Y.; Lee, S.; Ye, L.H.; Tian, S.L.; Zhang, Y.J.; Busch, W.; Zhou, W.B.; Zhu, X.G.; et al. GLK transcription factors accompany ELONGATED HYPOCOTYL5 to orchestrate light-induced seedling development in Arabidopsis. Plant Physiol. 2024, 194, 2400–2421. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Liu, X.; Cao, Y.; Li, X. Genetic manipulation of bermudagrass photosynthetic biosynthesis using Agrobacterium-mediated transformation. Physiol. Plantarum 2022, 174, 13710. [Google Scholar] [CrossRef]
- Cole, L.F.; Mccallan, S.A.; Olga, P.; Rafay, A.; Virginie, S.; Lee, C. The role of the GOLDEN2-LIKE (GLK) transcription factor in regulating terpenoid indole alkaloid biosynthesis in Catharanthus roseus. Plant Cell Rep. 2024, 6, 43–49. [Google Scholar]
- Huixin, G.; Ranhong, L.; Yuming, Z.; Guifeng, L.; Su, C.; Jing, J. Loss of GLK1 transcription factor function reveals new insights in chlorophyll biosynthesis and chloroplast development. J. Exp. Bot. 2019, 12, 3125–3138. [Google Scholar]
- Tu, X.; Ren, S.; Shen, W.; Li, J.; Li, Y.; Li, C.; Li, Y.; Zong, Z.; Xie, W.; Grierson, D.; et al. Limited conservation in cross-species comparison of GLK transcription factor binding suggested wide-spread cistrome divergence. Nat. Commun. 2022, 13, 7632. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Gwak, D.; Kim, S.; Yi, T.; Ha, S. Molecular action of GOLDEN2-LIKE transcription factor family with diverse interacting promoters and proteins. Physiol. Plant. 2024, 1, 176–180. [Google Scholar] [CrossRef]
- Yang, Z.; Han, P.; Yu, J.; He, F.; Liu, J.; Chen, Y.; Zhuang, L. A Method of Particle Bombardment-Mediated Transformation System in Bermudagrass. Chinese Patent CN118006670A, 10 May 2024. [Google Scholar]
- Arnon, D. Copper enzymes isolated chloroplasts, polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Xu, L.; Yu, J.; Han, L.; Huang, B. Photosynthetic enzyme activities and gene expression associated with drought tolerance and post-drought recovery in kentucky bluegrass. Env. and Exp. Bot. 2013, 89, 28–35. [Google Scholar] [CrossRef]
- Marri, L.; Sparla, F.; Pupillo, P.; Trost, P. Co-ordinated gene expression of photosynthetic glyceraldehyde-3-phosphate dehydrogenase, phosphoribulokinase, and CP12 in Arabidopsis thaliana. J. Exp. Bot. 2005, 56, 73–80. [Google Scholar] [CrossRef]
- Upadhyaya, A.; Sankhla, D.; Davis, T.D.; Sankhla, N.; Smith, B.N. Effect of paclobutrazol on the activities of some enzymes of activated oxygen metabolism and lipid peroxidation in senescing soybean leaves. J. Plant Physiol. 1985, 121, 453–461. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, L. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Jaime, F.; Manuel, R. Molecular mechanisms of shade tolerance in plants. New Phytol. 2023, 239, 1190–1202. [Google Scholar]
- Qian, Y.; Engelke, M. Influence of trinexapac-ethyl on diamond zoysiagrass in a shade environment. Crop Sci. 1999, 39, 202–208. [Google Scholar] [CrossRef]
- Kephart, K.; Buxton, D. Forage quality responses of C3 and C4 perennial grasses to shade. Crop Sci. 1993, 33, 831–837. [Google Scholar] [CrossRef]
- Okeyo, D.; Fry, J.; Bremer, D.; Chandra, A.; Genovesi, A. Stolon growth and tillering of experimental zoysiagrasses in shade. Hortscience 2011, 46, 1418–1422. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, U. Shade tolerance: A key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Bastakis, E.; Hedtke, B.; Klermund, C.; Grimm, B.; Schwechheimer, C. LLM-Domain B-GATA transcription factors play multifaceted roles in controlling greening in Arabidopsis. Plant Cell 2018, 30, 582–599. [Google Scholar] [CrossRef] [PubMed]
- Philip, A.; Knapp, A.K. Response to short-term reductions in light in soybean leaves. Int. J. Plant Sci. 1998, 159, 805–810. [Google Scholar]
- Fitter, D.W.; Martin, D.J.; Copley, M.J.; Scotland, R.W.; Langdale, J.A. GLK gene pairs regulate chloroplast development in diverse plant species. Plant J. 2002, 31, 713–727. [Google Scholar] [CrossRef]
- Van Huylenbroeck, J.M.; Van Bockstaele, E. Effects of shading on photosynthetic capacity and growth of turfgrass species. Turfgrass 2001, 9, 353–359. [Google Scholar]
- Tanaka, R.; Koshino, Y.; Sawa, S.; Ishiguro, S.; Okada, K.; Tanaka, A. Overexpression of chlorophyllide a oxygenase (CAO) enlarges the antenna size of photosystem II in Arabidopsis thaliana. Plant J. 2010, 26, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Gan, P.; Liu, F.; Li, R.; Wang, S.; Luo, J. Chloroplasts—Beyond energy capture and carbon fixation: Tuning of photosynthesis in response to chilling stress. Int. J. Mol. Sci. 2019, 20, 5046. [Google Scholar] [CrossRef]
- Guo, M.; Yang, F.; Zhu, L.; Wang, L.; Qi, Z.; Fotopoulos, V.; Yu, J.; Zhou, J. Loss of cold tolerance is conferred by absence of the WRKY34 promoter fragment during tomato evolution. Nat. Commun. 2024, 15, 6667. [Google Scholar] [CrossRef]
- Tiwari, A.; Mamedov, F.; Fitzpatrick, D.; Gunell, S.; Tikkanen, M.; Aro, E.M. Differential FeS cluster photodamage plays a critical role in regulating excess electron flow through photosystem I. Nat. Plants 2024, 10, 1592–1603. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wei, S.; Gao, Y.; Pei, H.; Geng, R.; Lu, Z.; Wang, P.; Zhou, W. Maize GOLDEN2-LIKE proteins enhance drought tolerance in rice by promoting stomatal closure. Plant Physiol. 2024, 194, 774–786. [Google Scholar] [CrossRef]
- Chastain, C.J.; Chollet, R. Regulation of pyruvate, orthophosphate dikinase by ADP-/Pi-dependent reversible phosphorylation in C3 and C4 plants. Plant Physiol. Biochem. 2003, 41, 523–532. [Google Scholar] [CrossRef]
- Niu, K.J.; Ma, H.L. The positive effects of exogenous 5-aminolevulinic acid on the chlorophyll biosynthesis, photosystem, and Calvin cycle of kentucky bluegrass seedlings in response to osmotic stress. Environ. Exp. Bot. 2018, 155, 260–271. [Google Scholar] [CrossRef]
- Walker, R.P.; Paoletti, A.; Leegood, R.C.; Famiani, F. Phosphorylation of phosphoenolpyruvate carboxykinase (PEPCK) and phosphoenolpyruvate carboxylase (PEPC) in the flesh of fruits. Plant Physiol. Biochem. 2016, 108, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Doubnerova, V.; Rylova, H. What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci. 2011, 180, 575–583. [Google Scholar] [CrossRef]
- Pyngrope, S.; Bhoomika, K.; Dubey, R.S. Reactive oxygen species, ascorbate-glutathione pool, and enzymes of their metabolism in drought-sensitive and tolerant indica rice (Oryza sativa L.) seedlings subjected to progressing levels of water deficit. Protoplasma 2013, 250, 585–600. [Google Scholar] [CrossRef]
- Meloni, D.A.; Oliva, M.A.; Martinez, C.A.; Cambraia, J. Photosynthesis and activity of superoxide dismutase, peroxidase and glutathione reductase in cotton under salt stress. Environ. Exp. Bot. 2003, 49, 69–76. [Google Scholar] [CrossRef]
- Wang, J.; Dou, J.; Yue, Z.; Wang, J.; Chen, T.; Li, J.; Dai, H.; Dou, T.; Yu, J.; Liu, Z. Effect of hydrogen sulfide on cabbage photosynthesis under black rot stress. Plant Physiol. Biochem. 2024, 208, 08453. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Li, Y.; Wang, L. Knockout of stigmatic ascorbate peroxidase 1 (APX1) delays pollen rehydration and germination by mediating ROS homeostasis in Brassica napus L. Plant J. 2024, 119, 1258–1271. [Google Scholar] [CrossRef]
- Fu, J.J.; Luo, Y.L.; Sun, P.G.; Gao, J.Z.; Zhao, D.H.; Yang, P.Z.; Hu, T.M. Effects of shade stress on turfgrasses morphophysiology and rhizosphere soil bacterial communities. BMC Plant Biol. 2020, 20, 92. [Google Scholar] [CrossRef]
- Zhang, X.H.; Liu, Y.H.; Liu, Q.; Zong, B.; Yuan, X.P.; Sun, H.E.; Wang, J.; Zang, L.; Ma, Z.Z.; Liu, H.M.; et al. Nitric oxide is involved in abscisic acid-induced photosynthesis and antioxidant system of tall fescue seedlings response to low light stress. Environ. Exp. Bot. 2018, 155, 226–238. [Google Scholar] [CrossRef]
- Wherley, B.G.; Gardner, D.S.; Metzger, J.D. Tall fescue photomorphogenesis as influenced by changes in the spectral composition and light intensity. Crop Sci. 2005, 45, 562–568. [Google Scholar] [CrossRef]
| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| CdGLK1 | GCCCTGTTGTTTGGTGTTAC | CCGCAAGCTGGAGGCAC |
| PP2A | TACTACTTCCCTTGACGGC | AATACCCCAACCACATC |
| ALAD | TTATAAAATTATACCAGGA | TGATAATACAGTATCTCAT |
| CAO | ATCTCAGGCAAATGATGA | CCCCATCGATATGGAGTT |
| ChlD | ACGCGACCTTGGCTATCAA | ATATCTGGTACTTTCAACT |
| ChlH | ACTACCTTAACAATAGGAC | TGGTCTCTTAATCCAAGT |
| ChlI | GCCGACAGACGTGCCTCCG | TTGGCCCTGGCCTTGCAT |
| ChlG | GGCGCGGGCGTACATGATG | CCACCGCCTGGAGCTCAA |
| CPOX | AGAAGTCCTGAACATTGCA | TGAGAACCCTACTATCTG |
| GUN4 | TCGACTTCTCCCCACGCCG | CCCTTCCCCAACCTCCGC |
| PEPC | ATGGCGTCCGAGCGGCACC | ACAATTCCGGATCCTGGC |
| PPDK | GCAGAGGGTCTTCCACTTC | GATTAATGCTTCTGTACTT |
| UROD | CAGGCCCCACCTGGTGGAA | TGGGATCGAGGCTCAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, P.; Liu, J.; Yu, J.; Liu, Z.; He, F.; Yang, Z. CdGLK1 Transcription Factor Confers Low-Light Tolerance in Bermudagrass via Coordinated Upregulation of Photosynthetic Genes and Enhanced Antioxidant Enzyme Activity. Agronomy 2025, 15, 1225. https://doi.org/10.3390/agronomy15051225
Han P, Liu J, Yu J, Liu Z, He F, Yang Z. CdGLK1 Transcription Factor Confers Low-Light Tolerance in Bermudagrass via Coordinated Upregulation of Photosynthetic Genes and Enhanced Antioxidant Enzyme Activity. Agronomy. 2025; 15(5):1225. https://doi.org/10.3390/agronomy15051225
Chicago/Turabian StyleHan, Peng, Jun Liu, Jingjin Yu, Zhongpeng Liu, Fahui He, and Zhimin Yang. 2025. "CdGLK1 Transcription Factor Confers Low-Light Tolerance in Bermudagrass via Coordinated Upregulation of Photosynthetic Genes and Enhanced Antioxidant Enzyme Activity" Agronomy 15, no. 5: 1225. https://doi.org/10.3390/agronomy15051225
APA StyleHan, P., Liu, J., Yu, J., Liu, Z., He, F., & Yang, Z. (2025). CdGLK1 Transcription Factor Confers Low-Light Tolerance in Bermudagrass via Coordinated Upregulation of Photosynthetic Genes and Enhanced Antioxidant Enzyme Activity. Agronomy, 15(5), 1225. https://doi.org/10.3390/agronomy15051225





