Effects of Microbial Agents on Soil Improvement—A Review and Bibliometric Analysis
Abstract
1. Introduction
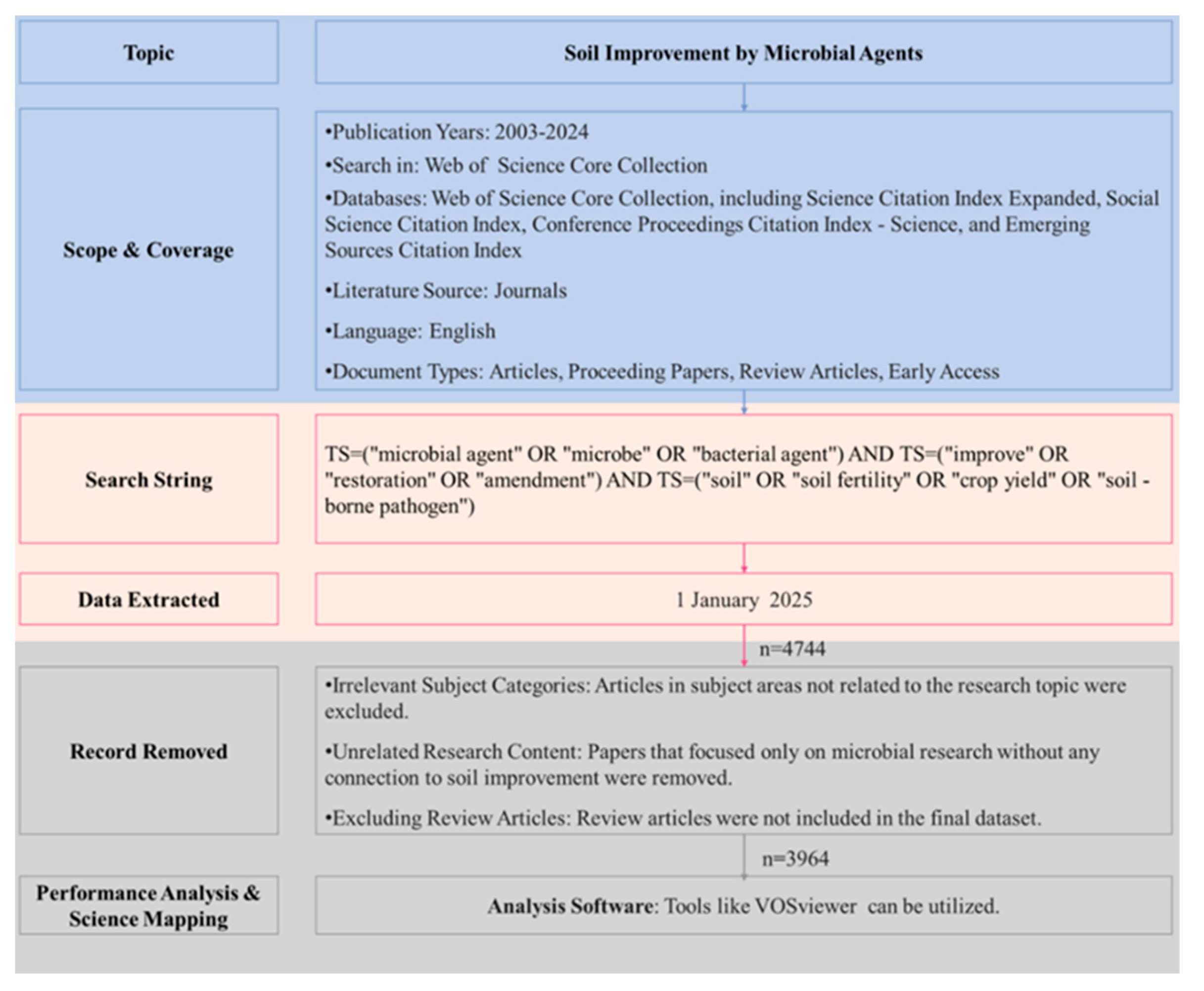
2. Materials and Methods
2.1. Data Sources
2.2. Analysis Method
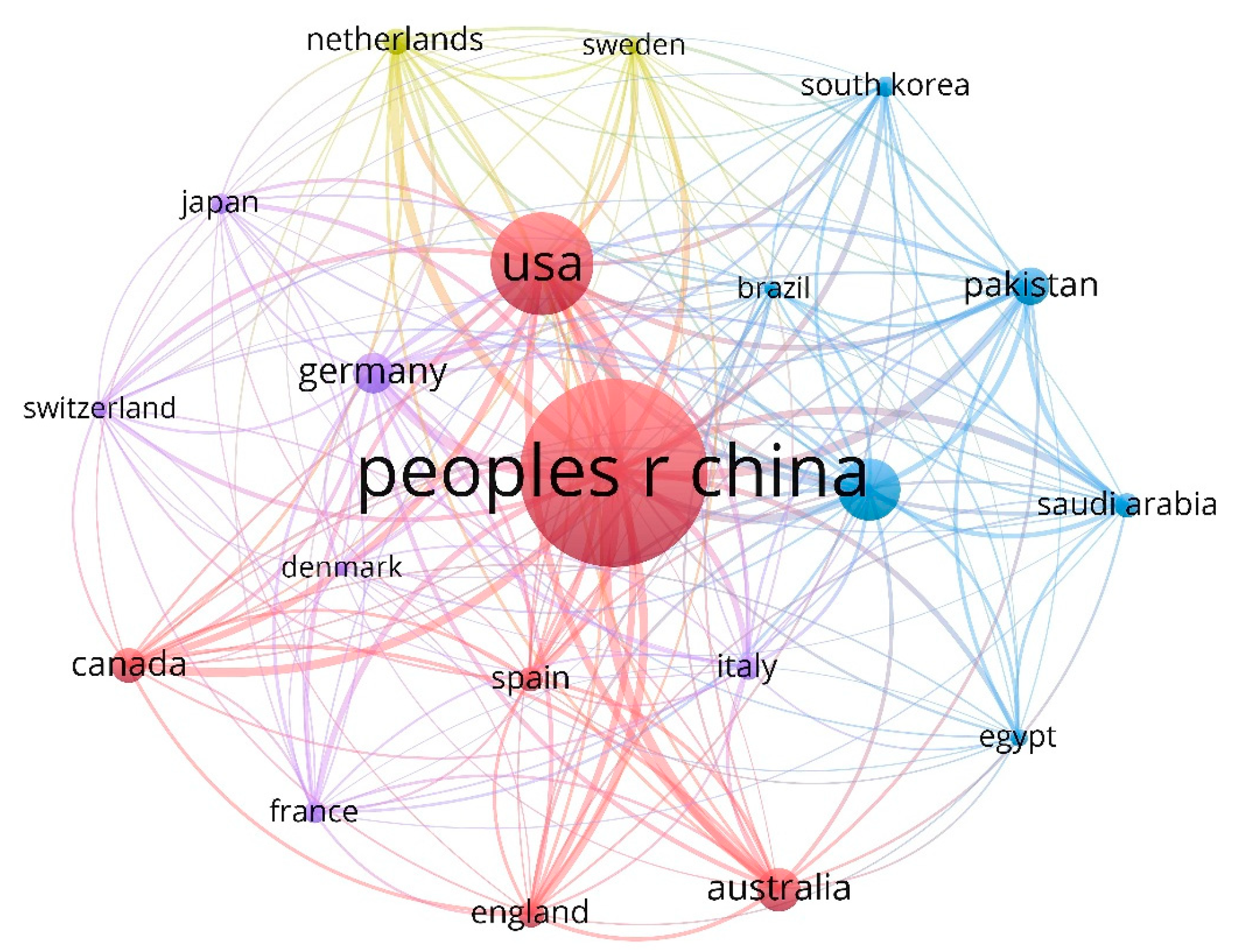
3. Bibliometric Analysis
3.1. Global Publication Trend Analysis
3.2. Visualization Analysis of High-Frequency Keyword Co-Occurrence
3.3. Analysis of Research Hotspots and Emerging Trends
4. Classification of Microbial Agents
4.1. Microbial Agent Formulation Types
4.2. Classification of Microbial Species and Functional Characteristics of Microbial Agents
4.3. Microbial Agents Can Be Classified on the Basis of Their Composition
5. Soil Improvement Effects of Microbial Agents and Influencing Factors
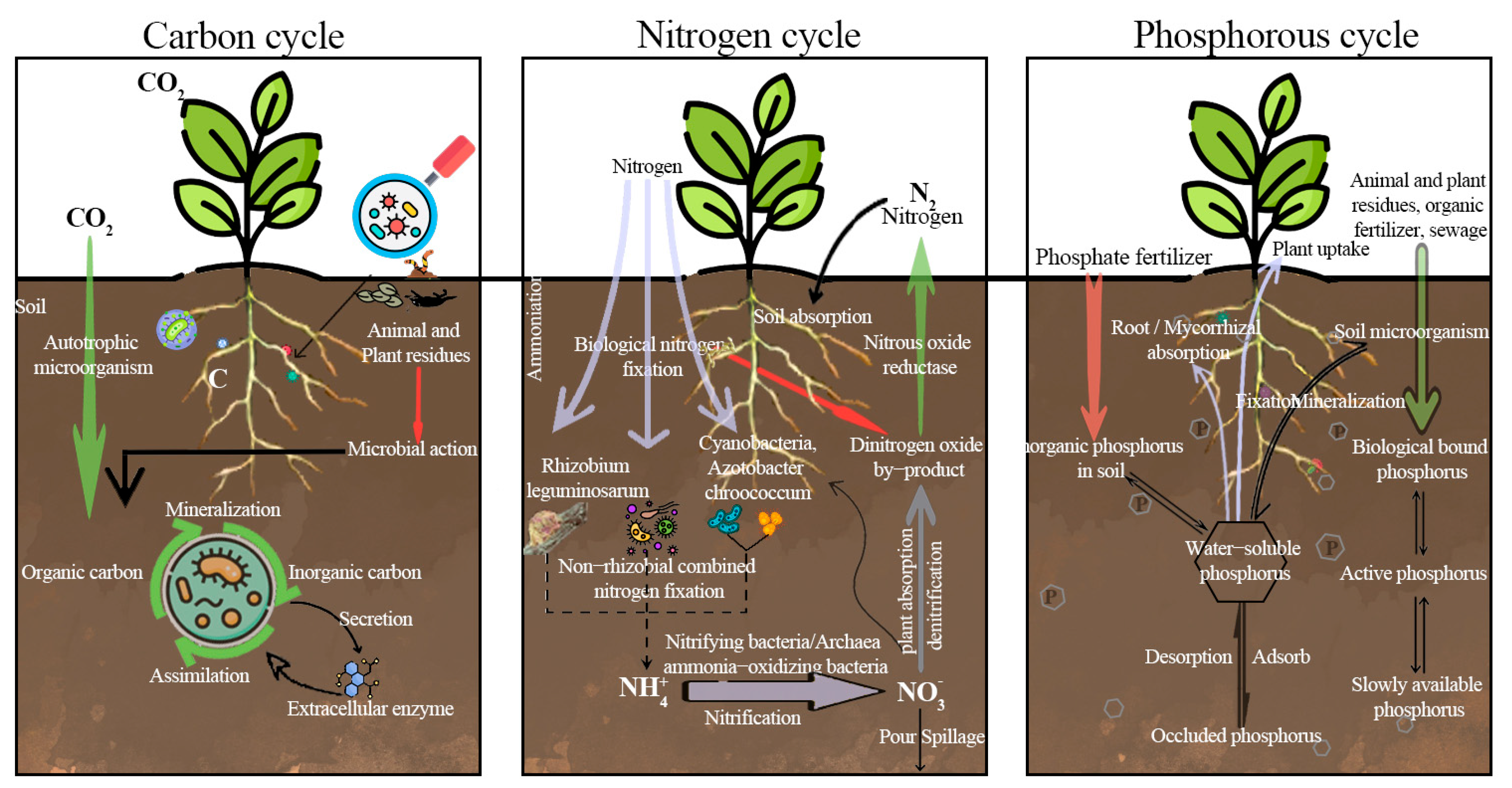
5.1. Role of Microorganisms in the Regulation of Soil Physicochemical Properties
5.1.1. Microorganisms Are Involved in the Assimilation and Mineralization of Organic Carbon
5.1.2. Regulatory Role of Microorganisms in Soil Biological Nitrogen Fixation
5.1.3. Microbial Regulation of Soil Phosphorus Availability
5.1.4. Microbial Improvement of Soil Physical Structure
5.2. Microbial Improvement of the Soil Biological Environment
5.2.1. Microbial Resistance to Stress and Control of Soil Pests and Diseases
5.2.2. Microbial Control of Pollution and Degradation of Harmful Substances
5.3. Microorganisms as Predictors of Soil Health
5.4. Limitations of Microbial Agent Application
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fierer, N.; Wood, S.A.; Bueno de Mesquita, C.P. How microbes can, and cannot, be used to assess soil health. Soil Biol. Biochem. 2021, 153, 108111. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Y.; Zhang, Y.; Shi, S. Legal System of Soil Pollution Remediation in China and Its Regulation and Guidance to Soil Pollution Remediation. Sustainability 2023, 15, 11504. [Google Scholar] [CrossRef]
- Sheer, A.; Sardar, M.F.; Younas, F.; Zhu, P.; Noreen, S.; Mehmood, T.; Farooqi, Z.U.R.; Fatima, S.; Guo, W.H. Trends and social aspects in the management and conversion of agricultural residues into valuable resources: A comprehensive approach to counter environmental degradation, food security, and climate change. Bioresour. Technol. 2024, 394, 130258. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Wu, J.; Lu, S.; Wang, Y.; Jiao, X.; Song, L. Soil and soil environmental quality monitoring in China: A review. Environ. Int. 2014, 69, 177–199. [Google Scholar] [CrossRef]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Wang, G.; Wang, X.; Wu, B.; Qi, L. Desertification and its mitigation strategy in China. J. Resour. Ecol. 2012, 3, 97–104. [Google Scholar] [CrossRef]
- Rahman, K.M.A.; Zhang, D. Effects of Fertilizer Broadcasting on the Excessive Use of Inorganic Fertilizers and Environmental Sustainability. Sustainability 2018, 10, 759. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Grandy, A.S.; Daly, A.B.; Bécu, T.; Cardinael, R.; Fontaine, S.; Jilling, A.; MacLaren, C.; Phillips, R.P. A microbial framework for nitrogen cycling solutions in agroecosystems. One Earth 2024, 7, 2103–2107. [Google Scholar] [CrossRef]
- Six, J.; Frey, S.D.; Thiet, R.K.; Batten, K.M. Bacterial contribution to carbon sequestration in soils. J. Plant Nutr. Soil Sci. 2006, 169, 350–353. [Google Scholar] [CrossRef]
- Sun, X.; Wang, W.; Yi, S.; Zheng, F.; Zhang, Z.; Alharbi, S.A.; Filimonenko, E.; Wang, Z.; Kuzyakov, Y. Microbial composition in saline and alkaline soils regulates plant growth with P-solubilizing bacteria. Appl. Soil Ecol. 2024, 203, 105653. [Google Scholar] [CrossRef]
- Liu, W.; He, C.; Han, S.; Lin, B.; Liu, W.; Dang, Y.P.; Zhao, X.; Zhang, H. Enhancing soil ecosystem multifunctionality through combined conservation tillage and legume-based crop rotation in the North China Plain. Agric. Ecosyst. Environ. 2025, 379, 109355. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Devi, M.K.; Kumar, P.S. Engineering microbes for enhancing the degradation of environmental pollutants: A detailed review on synthetic biology. Environ. Res. 2022, 214, 113868. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, S.; Houali, K.; Yadav, K.K.; Arabi, A.I.A.; Eltayeb, L.B.; AwjanAlreshidi, M.; Benguerba, Y.; Cabral-Pinto, M.M.; Nabti, E.H. Bioremediation techniques for soil organic pollution: Mechanisms, microorganisms, and technologies—A comprehensive review. Ecol. Eng. 2024, 207, 107338. [Google Scholar] [CrossRef]
- Semenov, M.; Blagodatskaya, E.; Stepanov, A.; Kuzyakov, Y. DNA-based determination of soil microbial biomass in alkaline and carbonaceous soils of semi-arid climate. J. Arid Environ. 2018, 150, 54–61. [Google Scholar] [CrossRef]
- Eren, A.M.; Banfield, J.F. Modern microbiology: Embracing complexity through integration across scales. Cell 2024, 187, 5151–5170. [Google Scholar] [CrossRef]
- He, Y.; Lan, Y.; Zhang, H.; Ye, S. Research characteristics and hotspots of the relationship between soil microorganisms and vegetation: A bibliometric analysis. Ecol. Indic. 2022, 141, 109145. [Google Scholar] [CrossRef]
- Zhu, J.; Hua, W. Visualizing the knowledge domain of sustainable development research between 1987 and 2015: A bibliometric analysis. Scientometrics 2017, 110, 893–914. [Google Scholar] [CrossRef]
- Nakagawa, S.; Samarasinghe, G.; Haddaway, N.R.; Westgate, M.J.; O’Dea, R.E.; Noble, D.W.; Lagisz, M. Research weaving: Visualizing the future of research synthesis. Trends Ecol. Evol. 2019, 34, 224–238. [Google Scholar] [CrossRef]
- Sun, S.; Xue, R.; Liu, M.; Wang, L.; Zhang, W. Research progress and hotspot analysis of rhizosphere microorganisms based on bibliometrics from 2012 to 2021. Front. Microbiol. 2023, 14, 1085387. [Google Scholar] [CrossRef]
- Nkongolo, K.K.; Narendrula-Kotha, R. Advances in monitoring soil microbial community dynamic and function. J. Appl. Genet. 2020, 61, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.S.; Pandey, V.C.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar] [CrossRef]
- Banerjee, S.; Van Der Heijden, M.G. Soil microbiomes and one health. Nat. Rev. Microbiol. 2023, 21, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Sivaram, A.K.; Abinandan, S.; Chen, C.; Venkateswartlu, K.; Megharaj, M. Microbial inoculant carriers: Soil health improvement and moisture retention in sustainable agriculture. Adv. Agron. 2023, 180, 35–91. [Google Scholar] [CrossRef]
- Kumar, A.; Das, A.; Singh, D.; Das, M.K.; Srivastava, G.P.; Singh, J.P.; Thapa, S.; Das, S.; Chakdar, H. Soil health restoration in degraded lands: A microbiological perspective. Land Degrad. Dev. 2023, 34, 5155–5170. [Google Scholar] [CrossRef]
- Deng, L.; Wang, T.; Luo, W.; He, L.; Liang, Z. Effects of a compound microbial agent and plants on soil properties, enzyme activities, and bacterial composition of Pisha sandstone. Environ. Sci. Pollut. Res. 2021, 28, 53353–53364. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, S.; Li, X.; Rong, K.; Li, J.; Jiang, L. Effects of microbial inoculant and additives on pile composting of cow manure. Front. Microbiol. 2023, 13, 1084171. [Google Scholar] [CrossRef]
- Pan, L.; Mao, L.; Zhang, H.; Wang, P.; Wu, C.; Xie, J.; Yu, B.; Sial, M.U.; Zhang, L.; Zhang, Y.; et al. Modified biochar as a more promising amendment agent for remediation of pesticide-contaminated soils: Modification methods, mechanisms, applications, and future perspectives. Appl. Sci. 2022, 12, 11544. [Google Scholar] [CrossRef]
- Veliz, E.A.; Martínez-Hidalgo, P.; Hirsch, A.M. Chitinase-producing bacteria and their role in biocontrol. AIMS Microbiol. 2017, 3, 689. [Google Scholar] [CrossRef]
- Funahashi, F.; Myrold, D.D.; Parke, J.L. The effects of soil solarization and application of a Trichoderma biocontrol agent on soil fungal and prokaryotic communities. Soil Sci. Soc. Am. J. 2022, 86, 369–383. [Google Scholar] [CrossRef]
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed]
- Morales-Cedeño, L.R.; del Carmen Orozco-Mosqueda, M.; Loeza-Lara, P.D.; Parra-Cota, F.I.; de Los Santos-Villalobos, S.; Santoyo, G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre-and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021, 242, 126612. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Jie, G.; She-Qi, Z.; Long-Xiang, S.; Wei, S.; Xun, Q.; Man-Li, D.; Ya-Nan, Y.; Xiao-Juan, W. Effects of adding compound microbial inoculum on microbial community diversity and enzymatic activity during co-composting. Environ. Eng. Sci. 2018, 35, 270–278. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field. Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Yuan, X.; Chen, S. Effect of three actinomycetes on cucumber root-knot nematode disease. J. Northwest A F Univ.-Nat. Sci. Ed. 2022, 50, 1–8. [Google Scholar] [CrossRef]
- Cui, N.; Wang, S.; Khorram, M.S.; Fang, H.; Yu, Y. Microbial degradation of fomesafen and detoxification of fomesafen-contaminated soil by the newly isolated strain Bacillus sp. FE-1 via a proposed biochemical degradation pathway. Sci. Total Environ. 2018, 616, 1612–1619. [Google Scholar] [CrossRef]
- Gu, J.; Qi, X.; Li, X.; Ren, Y.; Wang, X. Preparation of solid microbial inoculants and its application in aerobic composting. Chin. J. Environ. Eng. 2020, 14, 253–261. [Google Scholar] [CrossRef]
- Wen, D.; Wang, X.; Sun, K.N.; Wang, K.A.; Gao, J.W.; Zhang, W.; Yang, N. Effects of different forms of microbial agents on the growth and quality of Brassica rapa L. ssp. chinensis Makino (non-heading Chinese cabbage). Chin. J. Appl. Ecol. 2021, 32, 1777–1782. [Google Scholar] [CrossRef]
- Afanador-Barajas, L.N.; Navarro-Noya, Y.E.; Luna-Guido, M.L.; Dendooven, L. Impact of a bacterial consortium on the soil bacterial community structure and maize (Zea mays L.) cultivation. . Sci. Rep. 2021, 11, 13092. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Niu, B.; Wang, L.; Zhang, M.; Chen, Y.; Wang, J.; Gao, M. Functional characteristics of Bacillus siamensis 37402-1 and its growth-promoting effect on garlic. Soil Fertil. Sci. China 2024, 2, 185–192. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Li, Z.; Liu, L. Effects of arbuscular mycorrhizal fungi on nitrogen uptake of maize and soil N2O emissions in cinnamon soil. Acta Ecol. Sin. 2024, 44, 1972–1984. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Wu, W.; Jin, Q.; Wang, R.; Zhang, L.; Gao, F.; Zhao, Y.; Wang, W. Effects of bio-organic fertilizer and microbial agent on the growth of tea chrysanthemum and soil fertility under continuous cropping cultivation system in the mountainous area of Beijing. Soil Fertil. Sci. China 2023, 12, 107–113. [Google Scholar] [CrossRef]
- Dong, W.; Zhou, R.; Li, X.; Yan, H.; Zheng, J.; Peng, N.; Zhao, S. Effect of simplified inoculum agent on performance and microbiome during cow manure-composting at industrial-scale. Bioresour. Technol. 2024, 393, 130097. [Google Scholar] [CrossRef] [PubMed]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Song, J.; Brookes, P.C.; Shan, S.; Xu, J.; Liu, X. Effects of remediation agents on microbial community structure and function in soil aggregates contaminated with heavy metals. Geoderma 2022, 425, 116030. [Google Scholar] [CrossRef]
- Cheng, Z.; Lu, L.; Kennes, C.; Ye, J.; Yu, J.; Chen, D.; Chen, J. A composite microbial agent containing bacterial and fungal species: Optimization of the preparation process, analysis of characteristics, and use in the purification for volatile organic compounds. Bioresour. Technol. 2016, 218, 751–760. [Google Scholar] [CrossRef]
- Singh, V.; Kumar, B. A review of agricultural microbial inoculants and their carriers in bioformulation. Rhizosphere 2024, 29, 100843. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Furtak, K. Soil–Plant–Microbe interactions determine soil biological fertility by altering rhizospheric nutrient cycling and biocrust formation. Sustainability 2022, 15, 625. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The microbial engines that drive Earth’s biogeochemical cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef]
- Kuypers, M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Richardson, A.E.; Hocking, P.J.; Simpson, R.J.; George, T.S. Plant mechanisms to optimise access to soil phosphorus. Crop Pasture Sci. 2009, 60, 124–143. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Denaro, R.; Di Pippo, F.; Crisafi, F.; Rossetti, S. Biodegradation of Hydrocarbons in Marine Environment. In Water Pollution and Remediation: Organic Pollutants; Inamuddin, Ahamed, M.I., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer: Cham, Switzerland, 2021; Volume 54. [Google Scholar] [CrossRef]
- Singh, B. Organophosphorus-degrading bacteria: Ecology and industrial applications. Nat. Rev. Microbiol. 2009, 7, 156–164. [Google Scholar] [CrossRef]
- Nies, D. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef]
- Vázquez, M.M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Liang, C.; Zhu, X. The soil microbial carbon pump as a new concept for terrestrial carbon sequestration. Sci. China Earth Sci. 2021, 64, 545–558. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Ros, G.H.; Furtak, K.; Iqbal, H.M.; Parra-Saldívar, R. Soil carbon sequestration–An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022, 815, 152928. [Google Scholar] [CrossRef]
- Wang, M.; Guo, X.; Zhang, S.; Xiao, L.; Mishra, U.; Yang, Y.; Zhu, B.; Wang, G.; Mao, X.; Qian, T.; et al. Global soil profiles indicate depth-dependent soil carbon losses under a warmer climate. Nat. Commun. 2022, 13, 5514. [Google Scholar] [CrossRef]
- Jiang, S.; Jardinaud, M.F.; Gao, J.; Pecrix, Y.; Wen, J.; Mysore, K.; Xu, P.; Sanchez-Canizares, C.; Ruan, Y.; Li, Q.; et al. NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Science 2021, 374, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Sepp, S.K.; Vasar, M.; Davison, J.; Oja, J.; Anslan, S.; Al-Quraishy, S.; Al-Quraishy, S.; Bahram, M.; Bueno, C.G.; Cantero, J.J.; et al. Global diversity and distribution of nitrogen-fixing bacteria in the soil. Front. Plant Sci. 2023, 14, 1100235. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Ma, K.; Jiang, Y.; Chen, J.; Kou, Y.; Ge, Z.; Chen, Z.; Wei, X.; Yu, L. Endothelial miR-196b-5p regulates angiogenesis via the hypoxia/miR-196b-5p/HMGA2/HIF1α loop. Am. J. Physiol.-Cell Physiol. 2023, 324, C407–C419. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018, 612, 522–537. [Google Scholar] [CrossRef]
- Pang, F.; Li, Q.; Solanki, M.K.; Wang, Z.; Xing, Y.X.; Dong, D.F. Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 2024, 15, 1383813. [Google Scholar] [CrossRef]
- Guhra, T.; Stolze, K.; Totsche, K.U. Pathways of biogenically excreted organic matter into soil aggregates. Soil Biol. Biochem. 2022, 164, 108483. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, L.; Zhou, J.; Rengel, Z.; George, T.S.; Feng, G. Exploring the secrets of hyphosphere of arbuscular mycorrhizal fungi: Processes and ecological functions. Plant Soil 2022, 481, 1–22. [Google Scholar] [CrossRef]
- Wu, D.; Wang, W.; Yao, Y.; Li, H.; Wang, Q.; Niu, B. Microbial interactions within beneficial consortia promote soil health. Sci. Total Environ. 2023, 870, 165801. [Google Scholar] [CrossRef]
- Deveau, A.; Bonito, G.; Uehling, J.; Paoletti, M.; Becker, M.; Bindschedler, S.; Hacquard, S.; Hervé, V.; Labbé, J.; Lastovetsky, O.A.; et al. Bacterial–fungal interactions: Ecology, mechanisms and challenges. FEMS Microbiol. Rev. 2018, 42, 335–352. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, C.; Cao, N.; Ding, C.; Huang, Y.; Wang, J. Biodegradable and conventional microplastics exhibit distinct microbiome, functionality, and metabolome changes in soil. J. Hazard. Mater. 2022, 424, 127282. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, A.; Piotrowska-Seget, Z. Bioaugmentation as a strategy for cleaning up of soils contaminated with aromatic compounds. Microbiol. Res. 2010, 165, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, R.C.; van Es, H.M.; Buckley, D.H. Predicting measures of soil health using the microbiome and supervised machine learning. Soil Biol. Biochem. 2022, 164, 108472. [Google Scholar] [CrossRef]
- Muhonja, C.N.; Makonde, H.; Magoma, G.; Imbuga, M. Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PLoS ONE 2018, 13, e0198446. [Google Scholar] [CrossRef]
- Solanki, S.; Sinha, S.; Singh, R. Myco-degradation of microplastics: An account of identified pathways and analytical methods for their determination. Biodegradation 2022, 33, 529–556. [Google Scholar] [CrossRef]
- Yuan, J.; Ma, J.; Sun, Y.; Zhou, T.; Zhao, Y.; Yu, F. Microbial degradation and other environmental aspects of microplastics/plastics. Sci. Total Environ. 2020, 715, 136968. [Google Scholar] [CrossRef]
- Priya, A.K.; Gnanasekaran, L.; Dutta, K.; Rajendran, S.; Balakrishnan, D.; Soto-Moscoso, M. Biosorption of heavy metals by microorganisms: Evaluation of different underlying mechanisms. Chemosphere 2022, 307, 135957. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef]
- Mahto, K.U.; Priyadarshanee, M.; Samantaray, D.P.; Das, S. Bacterial biofilm and extracellular polymeric substances in the treatment of environmental pollutants: Beyond the protective role in survivability. J. Clean. Prod. 2022, 379, 134759. [Google Scholar] [CrossRef]
- Hu, M.; Li, Y.; Ge, C.; Zhang, Y.; Yao, H. Research status and application prospects of combined nitrogen fixation in gramineous plants. Chin. J. Eco-Agric. 2021, 29, 1815–1826. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]
- Hermans, S.M.; Buckley, H.L.; Case, B.S.; Curran-Cournane, F.; Taylor, M.; Lear, G. Using soil bacterial communities to predict physico-chemical variables and soil quality. Microbiome 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guan, Y. Microbial investigations of new hydrogel-biochar composites as soil amendments for simultaneous nitrogen-use improvement and heavy metal immobilization. J. Hazard. Mater. 2022, 424, 127154. [Google Scholar] [CrossRef]
- Toju, H.; Peay, K.G.; Yamamichi, M.; Narisawa, K.; Hiruma, K.; Naito, K.; Fukuda, S.; Ushio, M.; Nakaoka, S.; Onoda, Y.; et al. Core microbiomes for sustainable agroecosystems. Nat. Plants 2018, 4, 733. [Google Scholar] [CrossRef]
- Pirttilä, A.M.; Mohammad Parast Tabas, H.; Baruah, N.; Koskimäki, J.J. Biofertilizers and biocontrol agents for agriculture: How to identify and develop new potent microbial strains and traits. Microorganisms 2021, 9, 817. [Google Scholar] [CrossRef]
- Manfredini, A.; Malusà, E.; Costa, C.; Pallottino, F.; Mocali, S.; Pinzari, F.; Canfora, L. Current methods, common practices, and perspectives in tracking and monitoring bioinoculants in soil. Front. Microbiol. 2021, 12, 698491. [Google Scholar] [CrossRef]
- O’Callaghan, M.; Ballard, R.A.; Wright, D. Soil microbial inoculants for sustainable agriculture: Limitations and opportunities. Soil Use Manag. 2022, 38, 1340–1369. [Google Scholar] [CrossRef]
- Thompson, I.P.; Van Der Gast, C.J.; Ciric, L.; Singer, A.C. Bioaugmentation for bioremediation: The challenge of strain selection. Environ. Microbiol. 2005, 7, 909–915. [Google Scholar] [CrossRef]
- Kaminsky, L.M.; Trexler, R.V.; Malik, R.J.; Hockett, K.L.; Bell, T.H. The inherent conflicts in developing soil microbial inoculants. Trends Biotechnol. 2019, 37, 140–151. [Google Scholar] [CrossRef]

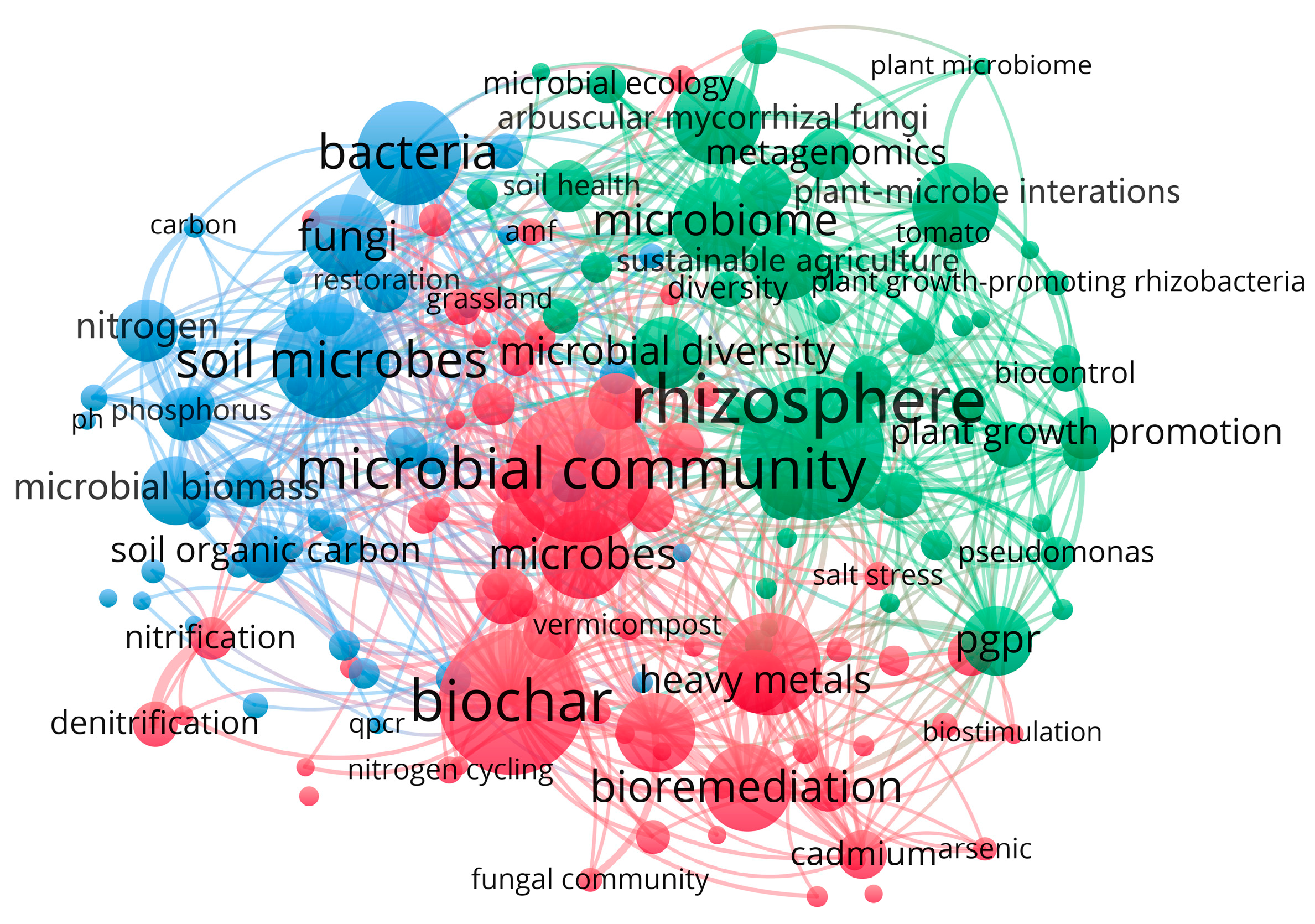


| Rank | Keyword | Frequency | Total Link Strength |
|---|---|---|---|
| 1 | rhizosphere | 156 | 254 |
| 2 | biochar | 159 | 202 |
| 3 | microbial community | 159 | 184 |
| 4 | soil microbes | 120 | 118 |
| 5 | phytoremediation | 103 | 133 |
| 6 | bacteria | 105 | 197 |
| 7 | sustainable agriculture | 68 | 101 |
| 8 | nitrogen | 55 | 102 |
| 9 | heavy metals | 57 | 82 |
| 10 | soil microbial community | 50 | 64 |
| Classification | Categorization | Functional Characteristic | Application | References |
|---|---|---|---|---|
| Dosage form | Liquid agents | (1) Advantages such as ease of preparation, ease of application, short activation time, and rapid effectiveness. (2) The ability to prevent soil-borne diseases, maintain the balance of rhizosphere microbial communities, and degrade toxic substances. | Applicable for seed/root treatment, drip irrigation/fertigation, and water purification/pollutant degradation. | [24] |
| Solid agents | (1) Promoting plant growth and increasing yield. (2) Enhancing the activity of urease and transaminase in soil, increasing the content of available nitrogen, and promoting nitrogen absorption. | Enhances long-term soil enhancement with latent-phase efficacy while functioning as basal fertilizer. | [24,25] | |
| Composite method | Single agents | (1) Single function, with limited adaptability. (2) Targeted antagonism against pathogens, pests, and soil-borne diseases. | Resolving single soil environment issues. | [25] |
| Composite agents | (1) Compared to single microbial agents, composite agents are more efficient and stable in soil, promoting crop growth. (2) They can significantly increase the nitrogen, phosphorus, and soluble leaf protein content in plants, thereby promoting plant growth. | Improve soil health and crop growth; inoculate leguminous crops to suppress wilt. | [26] | |
| Functional characteristic | Organic material composting agents | (1) Induces rapid thermophilic phase, enhancing organic matter conversion. (2) Eradicate pathogens, nematode eggs, and compost contaminants. (3) Effectively degrade odor-producing organic macromolecules and suppress putrefactive microorganisms. | Decompose diverse organic materials (e.g., straw, manure, distillers’ grains, plant litter, sewage sludge). | [27] |
| Soil remediation agents | (1) Promote the formation of soil aggregates, increase soil porosity, improve soil structure, and alleviate compaction. (2) Regulate soil pH, degrade pesticide residues, and immobilize heavy metals, mitigating salinization and alkalization. (3) Enhance soil water and nutrient retention capacity, promoting stable and high crop yields. | Remediate compacted/acidic soils and agrochemical/heavy metal co-contaminated sites. | [28] | |
| Disease-resistance agents | (1) Inhibit the proliferation of soil pathogens, effectively preventing root rot, damping-off, root-knot nematodes, and other replant diseases. (2) Enhance crop stress resistance, improving tolerance to early frost, cold, and lodging. | Applicable to field crops such as wheat, corn, and soybeans, as well as economic crops like vegetables, fruit trees, and medicinal plants. | [29,30] | |
| Growth-promoting agents | (1) Provide or activate nutrients, stimulate root growth, and increase yield. (2) Improve the quality of agricultural products by participating in the synthesis of plant cell materials, enhancing the content of beneficial components such as proteins, sugars, vitamins, and amino acids. | Applicable to field crops such as wheat, corn, and soybeans, as well as economic crops like vegetables, fruit trees, and medicinal plants. | [31,32] | |
| Microbial species | Bacterial agents | (1) Diverse in species, with strong reproductive ability and complex metabolism. (2) Enhance soil fertility, improve soil structure, increase soil bacterial population, and combat soil pests and diseases. | Enhances phosphorus/potassium solubilization and soil transformation while suppressing pathogenic microbes. | [33] |
| Fungal agents | (1) Form a network-like mycelial structure, increasing contact opportunities with contaminants on particulate surfaces. (2) Degrade diverse organic pollutants, including polycyclic aromatic hydrocarbons, petroleum hydrocarbons, and halogenated hydrocarbons. (3) Enrich indigenous microbiota, synergizing with native microbes-plant roots to boost remediation. | It is suitable for the remediation of sites with organic and composite contamination, as well as for land with poor soil structure. | [34] | |
| Actinomycete agents | (1) Assist plants in nutrient absorption. (2) Control the spread of plant pathogens, exhibiting strong antagonistic and parasitic effects on pathogenic organisms. (3) Actinomycetes secondary metabolites exhibit antimicrobial activity, enhance crop growth/yield, ameliorate soil ecology, and demonstrate eco-friendliness. | It can produce antibiotics and is used for wastewater treatment, as well as for the chemical control of root-knot nematodes. | [35] |
| Microbial Function | Mechanism | Genus/Species | References | First Author | Year |
|---|---|---|---|---|---|
| Carbon Cycle | Microbial decomposition of organic matter, CO2 fixation via photosynthesis. | Cyanobacteria, Streptomyces | [49] | Falkowski, P.G. | 2008 |
| Nitrogen Cycle | Nitrogen fixation, nitrification, denitrification, and ammonification. | Rhizobium, Nitrosomonas | [50] | Kuypers, M.M.M. | 2018 |
| Phosphorus Cycle | Solubilization, mineralization, and uptake via phosphatases. | Pseudomonas,Bacillus | [51] | Richardson, A.E. | 2009 |
| Soil Microbes Resist Pests/Diseases | Antibiotic production, induced systemic resistance (ISR). | Pseudomonas Fluorescens,Bacillus Subtilis | [52] | Pieterse, C.M.J. | 2014 |
| Microbial Degradation of Petroleum | Hydrocarbon degradation via oxygenases and biosurfactants. | Pseudomonas Putida,Alcanivorax Borkumensis | [53] | Denaro, R. | 2021 |
| Microbial Degradation of Pesticides | Enzymatic breakdown (e.g., hydrolases, oxidoreductases). | Arthrobacter,Pseudomonas | [54] | Singh, B.K. | 2009 |
| Microbial Heavy Metal Tolerance | Biosorption, bioaccumulation, and detoxification via metallothioneins. | Geobacter,Cupriavidus Metallidurans | [55] | Nies, D. | 1999 |
| Plastic Degradation | Enzymatic hydrolysis | Ideonella Sakaiensis,Aspergillus Niger | [56] | Yoshida, S. | 2016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, M.; Feng, T.; Wang, C.; Hao, X.; Yu, H. Effects of Microbial Agents on Soil Improvement—A Review and Bibliometric Analysis. Agronomy 2025, 15, 1223. https://doi.org/10.3390/agronomy15051223
Tan M, Feng T, Wang C, Hao X, Yu H. Effects of Microbial Agents on Soil Improvement—A Review and Bibliometric Analysis. Agronomy. 2025; 15(5):1223. https://doi.org/10.3390/agronomy15051223
Chicago/Turabian StyleTan, Mengdi, Tianjiao Feng, Cong Wang, Xiaozhen Hao, and Hang Yu. 2025. "Effects of Microbial Agents on Soil Improvement—A Review and Bibliometric Analysis" Agronomy 15, no. 5: 1223. https://doi.org/10.3390/agronomy15051223
APA StyleTan, M., Feng, T., Wang, C., Hao, X., & Yu, H. (2025). Effects of Microbial Agents on Soil Improvement—A Review and Bibliometric Analysis. Agronomy, 15(5), 1223. https://doi.org/10.3390/agronomy15051223






