Abstract
Salt stress can trigger endoplasmic reticulum (ER) stress and affect potato yield. The endomembrane system is tightly regulated in response to salt stress for maintaining cellular homeostasis. However, little is known about the genes involved in the ER-mediated cytoprotective pathways in potato plants. Previously characterized genes involved in the ER stress signaling pathway in Arabidopsis were used as prototypes. We identified 29 genes involved in ER stress response in the potato genome. Transcriptome data analysis showed that the expression levels of related genes were significantly different in different tissues. Most genes can response to β-aminobutyric acid, benzothiadiazole, salt, and mannitol. qRT-PCR assay revealed that they could respond to NaCl and tunicamycin, which was consistent with the fact that the promoter region of related genes contained ER-stress- and abiotic-stress-related cis-elements. Furthermore, we found that StbZIP60 has a splicing form, StbZIP60s, under salt and ER stress, which can be spliced at the CxGxxG site in the C terminus to create a frame shift through the excision of 23 base pairs. StbZIP60 is localized in the cytoplasm and nucleus, whereas most of the StbZIP60s translocated to the nucleus. This study provides a basis for further analyses of the functions of salt-stress- and ER-stress-related genes in potato plants.
1. Introduction
The endoplasmic reticulum (ER) is a particularly important organelle in eukaryotic organisms, which is responsible for the synthesis, assembly, transportation, and modification of secretory proteins and membrane proteins. About one-third of cellular proteins are synthetized in ER [1]. The process of protein processing and folding is finicky and only correctly folded and assembled proteins can be released. The ER quality control (ERQC) system guarantees the correct folding and transport of proteins in cells [2]. However, the folding process is also error-prone and can easily be disturbed by various abiotic and biotic stresses, such as drought, salinity, heat, and virus infection, resulting unfolded or misfolded proteins aggregating in the lumen, causing ER stress to the organism [1,2,3,4]. When plants are subjected to ER stress, their growth, development, and yield will be seriously affected.
To mitigate ER stress damage, plants take a series of measures: initiating the well-conserved unfolded protein response (UPR), a complex set of homeostatic mechanisms evolved to cope with ER stress in eukaryotes by promoting protein folding and maturation [5,6]; initiating endoplasmic-reticulum-associated degradation (ERAD) and autophagy processes to dispose of improperly folded proteins in the body, which can alleviate the ER burden [7,8,9]; decreasing the translational capacity of proteins to reduce proteins that need to be folded in the ER [10]; and initiating programmed cell death if severe or chronic stress conditions are present [11].
As an intracellular protective signal under ER stress, UPR has two signal transducers in plants. One is mediated by the ER membrane protein sensors ribonuclease and kinase inositol requiring 1 (IRE1), which can catalyze the unconventional splicing of bZIP60 mRNA at two “kissing” hairpin loops with conserved bases in each loop [12,13,14]. After splicing, bZIP60 lacks the transmembrane domain, and enters the nucleus from the endoplasmic membrane and binds to the promoters of related genes, such as bZIP60, HSFTF13, and BiP [15,16,17]. The other pathway is mediated by bZIP17/bZIP28, which are located in ER under normal conditions. While under abiotic stress, mainly salt stress, bZIP17 is cleaved by S1P and S2P, transported from the ER membrane into the Golgi apparatus and then into the nucleus [18,19], and drive genes expression. Upon ER stress, the ortholog bZIP28 is translocated from the ER to the Golgi, where it is subjected to intramembrane protein hydrolysis by Golgi-localized proteases [20,21,22]. Activated bZIP28 enters the nucleus, and upregulates the expression of ER-stress-induced genes [23].
Salt stress can disturb protein folding and ER homeostasis, and trigger ER stress [24]. Many genes involved in salt and ER stress pathways have been identified in plants. Mutations in Arabidopsis thaliana MNS5, a component of ERAD, result in hypersensitivity to salt and ER stresses [25]. Heat shock protein 70 (HSP70)-interacting protein is involved in NaCl and ER stress resistance in Arabidopsis by regulating HSP70 level [26]. SES1 plays a role of an ER-chaperone, which participates in ER stress and salt stress [27]. ER stress transducer AtbZIP17 mediates salt stress responses and can upregulate the expression of salt stress genes, such as ATHB-7 [19]. Nucleus- and cytoplasm-localized chaperone GmDNAJC7 is involved in the alkaline–salt, salt, and drought tolerance in Arabidopsis and soybean [28].
Potato (Solanum tuberosum L.) is the world’s fourth largest food crop [29], following maize, wheat, and rice, playing a key role in global food security and nutrient supply. Globally, more than 800 million hectares (about 6% of the world’s total land area) are affected by salinization, reducing crop yields by about 20% [30]. It is estimated that, by 2050, 50% of all arable land will impacted by high salinity [31]. Soil salinization has become a global concern. Potato is a shallow-rooted crop, and it is vulnerable to salt stress. High concentrations of salt inhibit potato growth by causing ionic toxicity, osmotic stress, and oxidative damage, ultimately hampering tuber yield. Therefore, the development of varieties that enhance salt stress resistance is important for improving potato’s yield. In recent years, screening for genes related to salt stress resistance and utilizing molecular breeding to improve potato salt stress resistance are research approaches that have received attention.
Transcriptome analysis was used to identify differentially expressed genes associated with ER stress and the UPR in potato by Venura [32]. Many genes involved in the salt stress pathway in potato were also identified by researchers [33,34]. However, whether genes involved in ER stress processes are involved in salt stress response is unknown. In this study, we took the genes involved in ER stress signal transduction, protein processing and folding, and degradation pathways in Arabidopsis thaliana as references; we identified their homologous genes in potato and studied their chromosomal localization, their expression profiles, their cis-element promoters, and their sub-localization. Meanwhile, we found that StbZIP60 has a spliced form, called StbZIP60s. This study will lay a foundation for studying the function of genes related to salt stress and ER stress in potato.
2. Materials and Methods
2.1. Plant Materials and Treatments
Three-week-old Xisen 6 potato seedlings, grown in an incubation chamber at 22 °C, were collected for expression pattern analysis. Roots, stems, and leaves were taken to create the tissue expression profiles of StbZIP60. The gene expression patterns under salt stress were analyzed by watering with 200 mM NaCl for 0, 1, 3, 6, 9, 12, 18, and 24 h, respectively. The seedlings were collected after spraying 5 μg/mL of tummycin for 0, 2, 4, 6, 8, 10, and 12 h for the analysis of gene expression patterns under ER stress. After sampling, all materials were placed in a −80 refrigerator and subsequently used for RNA extraction.
2.2. Identification and Property Analysis of Genes Related to ER Stress
The proteins sequence in Arabidopsis was downloaded from TAIR (www.arabidopsis.org/ (accessed on 14 October 2023)) (Table S1). The BLASTp tool in the Spud DB (spuddb.uga.edu/ (accessed on 14 October 2023)) was used to search for its homologous protein in potato, and the one with the smallest E value was selected as a candidate. The protein sequences, CDS sequences, and gene sequences of the candidate genes were determined based on the released genome-related files. Protein structural domains of gene in Arabidopsis and potato were analyzed using the NCBI Batch CD-search tool (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi?queries (accessed on 16 October 2023)). The molecular weight, theoretical pI, instability index, and grand average of hydropathicity (GRAVY) of the candidate proteins were determined by using ProtParam tool (https://web.expasy.org/protparam/ (accessed on 20 October 2023)). The subcellular localization of the candidate proteins was analyzed through the WoLF PSORT website (https://wolfpsort.hgc.jp/ (accessed on 20 October 2023)).
2.3. The Promoter Analysis of Candidate Genes
According to the gff file, the 2000 bp upstream sequence of the start codon (ATG) of the candidate gene was extracted using TBtools software (v2.130) [35], which was regarded as its promoter. The PlantCARE website (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 17 November 2024)) was used to analyze the promoters for abiotic-stress-related cis-elements, and Excel was used to determine whether the promoters contained the reported ERSE and UPRE cis-elements. The TBtools software was used for mapping.
2.4. Tissue Expression and Expression Pattern of Candidate Genes Under Stress Conditions
The expression levels of candidate genes in sepals, leaves, roots, shoots, callus, stolons, tubers, mature flowers, petioles, petals, stamens, carpels, and fruit were analyzed using the doubled monoploid potato DM 1-3 516 R44 gene expression matrix (TPM)-v6.1 transcriptome data downloaded from Spud DB. The gene’s expression levels were analyzed before and after treatment with P. infestans, BABA, BTH, salt, mannitol, heat, and phytohormone ABA, IAA, GA3, and BAP. Visualization was performed using TBtools.
Total RNA was extracted using RNAiso Plus (TaKaRa, Beijing, China). NanoDrop2000 was used to determine concentration and purity; then, they were reverse-transcribed into cDNA using the Evo M-MLV RT Kit with gDNA Clean (Accurate Biology, Hunan, China). To investigate the expression patterns of genes under salt stress and ER stress, twenty-two representative genes were randomly selected for qRT-PCR analysis. Specific primers shown in Table S2 were designed using Beacon Designer 8 (Premier Biosoft, Palo Alto, CA, USA). Real-time quantitative PCR was conducted using SYBR Green Pro Taq HS Premix (Accurate Biology, Changsha, China) on the CFX96 PCR instrument (Bio-Rad, Hercules, CA, USA). The qRT-PCR reaction system consisted of 7.5 μL of 2 ×SYBR Green Pro Taq HS Premix, 0.3 μL forward and reverse specific primers, 1.9 μL of ddH2O, and 5 μL 30-fold diluted cDNA. The reaction program was conducted at 95 °C for pre-denaturation for 30 s; followed by 95 °C denaturation for 5 s, 60 °C for 30 s, and 45 cycles. The melt curve was from 65 °C to 95 °C, with increments of 0.5 °C. Three replicates were performed for each sample. StActin was used for the reference gene. The relative expression levels were calculated by the 2–ΔΔCt method [36]. The data were visualized using GraphPad prism 6.
2.5. Splicing and Subcellular Localization Analysis of StbZIP60
Firstly, the Centroidfold (http://rtools.cbrc.jp/centroidfold/ (accessed on 3 October 2024)) website was used to analyze the mRNA secondary structure of StbZIP60. Specific primers (Table S2) were designed near the CxGxxG conserved site. qRT-PCR was used to detect the expression levels of StbZIP60up and StbZIP60s under salt stress or ER stress.
The CDS sequences of StbZIP60 and StbZIP60s were amplified using high-fidelity enzymes (Vazyme, Nanjing, China). The PCR program was set as follows: 95 °C for 5 min, 35 cycles of 95 °C for 15 s, 55 °C for 15 s, 72 °C for 30 s, 72 °C for 5 min, and 16 °C for 30 min. After recovering the DNA, it was ligated to binary expression vector pBWA(V)HS-eGFP to construct the pBWA(V)HS-eGFP-gene vector using the BioRun seamless cloning kit (BioRun, Wuhan, China). The recombinant vector was transformed into Agrobacterium tumefaciens GV3101, then was infiltrated into the epidermal cells of Nicotiana benthamiana leaves. Agrobacterium tumefaciens GV3101 containing the ER-localized marker mcherry-RFP was infiltrated as a control. After infiltration was conducted for 3–5 days, the position of GFP fluorescence in tobacco leaves was observed using laser confocal microscopy (Nikon, Japan).
2.6. Statistical Analysis
All the experimental determinations were repeated at least three times, and the final result was taken as the average value ± SD of multiple repetitions. Microsoft Excel 2021 was used for data processing. GraphPad Prism software was used for plotting and analyzing the variance and comparison of differences (Student’s t-test).
3. Results
3.1. Identification of ER-Stress-Related Genes in Potato
Taking the 29 genes reported to be involved in ER stress in Arabidopsis thaliana as the references, we found their homologous genes in potato by using the blast in SpuDB database (https://spuddb.uga.edu/ (accessed on 14 October 2023)). And their basic information, such as gene name, sequences (CDS, cDNA, protein, genomic), and protein properties (molecular weight, isoelectric point, etc.), is shown in the Supplementary Materials, Table S3. According to the gene nomenclature in Arabidopsis thaliana, the homologous genes in potato were renamed and classified according to activators, immediate downstream components, and downstream components (Table 1).

Table 1.
A list of known/predicted orthologous genes involved in ER stress pathways in potato and Arabidopsis.
There are three ER stress activators (bZIP17, bZIP28, and IRE1) in Arabidopsis; StbZIP17, StbZIP28, and StIRE1 also exist in potato. StbZIP17 and StbZIP28 are bZIP-type transmembrane proteins, and StIRE1A and StIRE1B are protein kinases, as categorized on the NCBI-CDD website (Table S4). This suggests that the role of ER stress activators in potato may be like that in Arabidopsis. We analyzed the conserved structural domains of related genes in downstream, including molecular chaperones/holdases, folding of glycoproteins, and molecules involved in ERAD pathways (Table S4). The structures of these proteins in potato are highly like those in Arabidopsis, suggesting that these genes may perform the same functions in potato as in Arabidopsis. To make the results more visible, we mapped the identified genes to chromosomes using the TBtools (Figure S1). The twenty-nine genes were distributed all chromosomes except chromosomes 5, 8, and 11. Most of the genes are located at the ends of the chromosomes. Noticeably, StIRE1B and StbZIP60 are physically remarkably close on chromosome 4, which are only 200 kb apart. These analyses lay the foundation for investigating the characteristic of genes involved in ER stress in potato.
3.2. Expression Profiles of Genes Related to ER Stress in Potato
We analyzed the expression patterns of identified genes in different tissues using transcriptome data on the SpuDB database (Table S5). The results showed that StbZIP28 and StIRE1A have a lower expression level (TPM < 1), while StSAR1, StDER, StUBC32, and StCDC48 have a higher expression level in all tissues detected (Figure 1A). StbZIP60 have a higher expression level in root and tuber (TPM > 150). The expression profiles of the identified genes under biotic and abiotic stresses were analyzed, including P. infestans infection, BABA, BTH, salt, mannitol, heat, ABA, IAA, GA3, and BAP treatment (Table S6). The results showed the expression levels of most genes changed within a 2-fold range under these treatments. Sixteen genes were found to have a response to BABA, BTH, salt, and mannitol. The expression of StIRE1A was downregulated after P. infestans, salt, mannitol, and heat treatments, while StIRE1B, StSES1, and StUFD1 were upregulated after salt and mannitol treatments (Figure 1B). This result indicated that these genes may be involved in salt and osmotic stress.
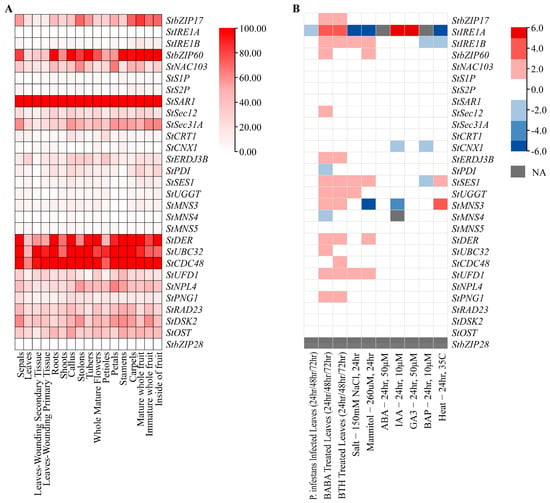
Figure 1.
Expression profiles of genes related to ER stress in potato. (A) The expression levels of genes in different tissues. The color bar represents the scale. (B) Expression level of genes under biotic, abiotic stress, and hormones conditions. The data are displayed in log2 fold change. The color bar represents the scale. NA indicates that data are unavailable.
3.3. Promoter Analysis of Genes Related to ER Stress in Potato
Promoter analysis of genes related to ER stress in potato is important in building an understanding of gene expression, allowing the analysis of the effect of cis-elements on their promoters. Using TBtools software, we extracted the promoter sequences of these genes (Table S7). After the analysis of their promoters, we found that their promoters contained a large number of cis-acting elements, such as those related to drought stress (Myb, Myb-binding site, MBS, MRE, CCAAT-box, DRE, etc.), high and low temperature responses (LTR, TCA, STRE), and ABA stress hormone responses (ABRE, AAGAA-motif). They were unevenly distributed on each gene promoter (Figure S2). Among the cis-elements responding to drought stress, the Myb cis-element was the most abundant, contained on the promoters of 27 genes (Figure 2A). Among the ABA-responsive cis-elements, ABRE was the most abundant and distributed on the promoters of 23 genes, followed by AAGAA-motif, which was distributed on the promoters of 21 genes (Figure 2A). Twenty-two genes contained STRE cis-elements on their promoters, amounting to a total of fifty-eight (Figure 2A). The presence of these abiotic stress response cis-elements on the promoters indicated that these genes might be involved in the abiotic stress response process. Because these genes are involved in ER stress signal transduction, protein processing, folding, and degradation, we also analyzed the cis-acting elements related to ER stress, including ERSE-I (CCAAT-N9-CCACG), ERSE-II (ATTGG-N-CCACG), XBP1-BS (GA-TGACGT-G(T/G)), UPRE-I (TGACTGR), UPRE-II (GATGACGCGTAC), and UPRE-III (TCATCG). Twelve genes contained cis-elements associated with ER stress on their promoters (Figure 2B). They contain more UPRE-1 and UPRE-III cis-elements compared to other ER-stress-response-associated cis-elements. These results indicated that some ER-stress-related genes may be involved in abiotic stress and ER stress.
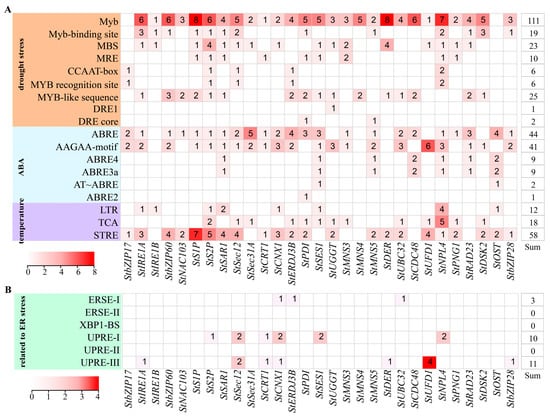
Figure 2.
Analysis of cis-elements on the promoters of ER-stress-related genes in potato. (A) The distribution and number of cis-elements related to drought, ABA hormone, and temperature on the gene’s promoter. (B) The cis-elements related to ER stress on the gene’s promoter.
3.4. Detection of the Expression Levels of Genes Under Salt Stress and ER Stress
Salt stress affects the processing and folding processes of proteins in the ER, leading to ER stress. We detected the relative expression level of the identified genes under salt stress or ER stress conditions. We found that most genes’ expression levels showed changes after 12 h of 200 mM NaCl treatment or 12 h of 5 μg/mL tummycin (TM) treatment compared with the control. Among them, 12/22 (StbZIP17, StIRE1A, StbZIP60, StS1P, StS2P, StSec12, StCRT, StMNS3, StUBC32, StCDC48, StPNG1, and StDER) genes can be significantly upregulated, while StNPL4 can be downregulated significantly after NaCl treatment. Eight genes (StbZIP17, StIRE1B, StbZIP60, StS2P, StSec12, StMNS3, StUBC32, and StPNG1) can be upregulated significantly after 12 h of TM treatment, while StNAC103, StPDI, StS1P, StUDF1, StNPL4, and StRAD23 can be downregulated significantly after TM treatment. The expression level of StbZIP17 was especially upregulated by nearly 2000-fold after salt stress, and was upregulated by more than 500-fold after TM treatment. StbZIP60 was upregulated by nearly 2-fold after salt stress, and upregulated by almost 4-fold after TM treatment (Figure 3). These results indicate that salt stress treatment influences the expression of genes involved in ER stress in potato. Genes involved in ER stress pathways in potato may be involved in salt stress processes.
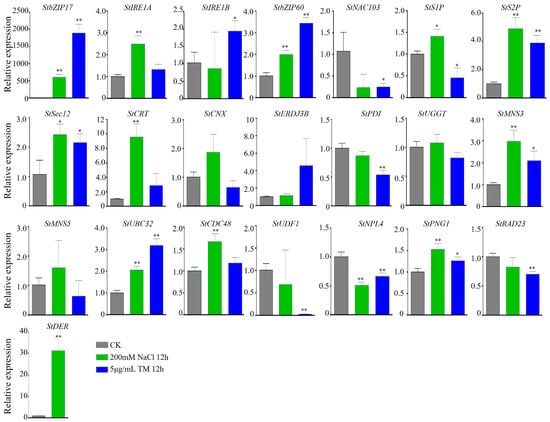
Figure 3.
The expression levels of genes under salt stress and ER stress. The three-week-old seedlings were treated with 200 mM NaCl or 5 μg/mL TM. And the gene expression levels were detected by RT-qPCR. StActin was used as an internal reference gene. Seedlings with no treatment were used as the control. The bars indicate the mean ± SD of three replicates. *, p < 0.05; **, p < 0.01 (Student’s t-test).
3.5. Analysis of Tissue Expression and Expression Pattern of StbZIP60 Under Salt Stress and ER Stress
bZIP60 plays a key role in salt stress and ER stress in plants [45]. We detected its expression in three tissue samples from the roots, stems, and leaves using qRT-PCR. The results showed that StbZIP60 is expressed in all tissues detected, and more in roots than in leaves and stems (Figure 4A). This result suggests that StbZIP60 is constitutively expressed in potato seedlings and may have a stronger role in roots than in stems and leaves.
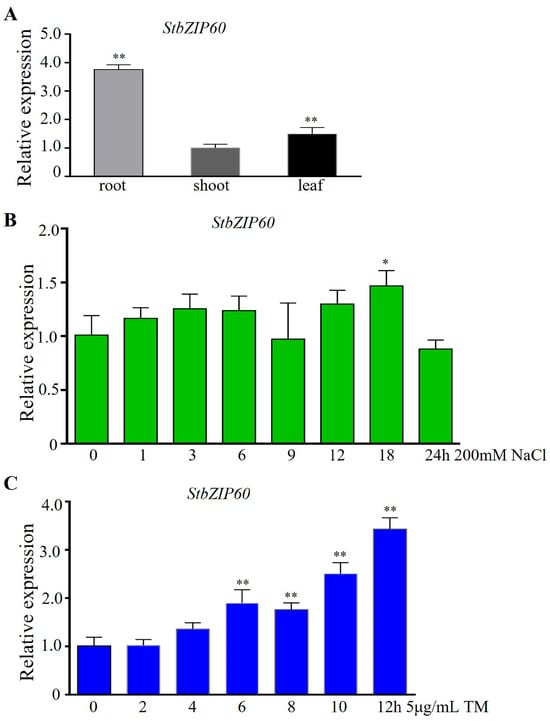
Figure 4.
The expression patterns of StbZIP60. (A) Expression levels of StbZIP60 in root, shoot, and leaf. StActin was used as an internal reference gene. (B) Expression level of StbZIP60 after 200 mM NaCl treatment for different periods of time. (C) Expression level of StbZIP60 after 5 μg/mL TM treatment for different periods of time. StActin was used as internal reference gene. Seedlings with no treatment as control. The bars indicate the mean ± SD of three replicates. *, p < 0.05; **, p < 0.01 (Student’s t-test).
We detected the expression levels of StbZIP60 after 200 mM NaCl treatment for various times (0, 1, 3, 6, 9, 12, 18, and 24 h). And the results showed that the expression of StbZIP60 showed a tendency of increasing and then decreasing; the expression level after 18 h of treatment was significantly different from that of the control (Figure 4B). This result indicates that StbZIP60 participated in the response to salt stress. We examined the expression levels of StbZIP60 after TM treatments for 0, 2, 4, 6, 8, 10, and 12 h. The results showed that the expression of StbZIP60 showed a rising trend, and the expression levels were significantly different from those of the control after 6, 8, 10, and 12 h treatments (Figure 4C). These results indicated that StbZIP60 was involved in ER stress. Taken together, these results suggest that StbZIP60 may be involved in ER stress triggered by salt stress.
3.6. Unconventional Splicing of StbZIP60 Under Salt Stress and ER Stress in Potato
bZIP60 mRNA splicing is activated by inositol-requiring enzyme-1 (IRE1) under ER stress and abiotic stress in plants [44,72,73]. To clarify whether StbZIP60 is spliced in potato under salt stress or ER stress, we firstly used the CentroidFold (http://rtools.cbrc.jp/centroidfold/ (accessed on 3 October 2024)) website to analyze the mRNA secondary structure of StbZIP60. The results showed that StbZIP60 forms a double stem–loop structure, and each stem–loop structure contains an IRE1-spliced CxGxxG conserved sequence (Figure 5A), indicating that StbZIP60 may be spliced (StbZIP60s) when subjected to stress. We used the InterPro website to predict that, under normal conditions, StbZIP60 belongs to transmembrane proteins with transmembrane structural domains located at amino acids 205–227 (Figure 5B). In contrast, after cutting off 23 bp, the transmembrane structural domain was removed, and its length was changed from the original 289 amino acids to 248 amino acids (Figure 5C), with the appearance of the nuclear localization signal NLS (PSNKGRKKD), which changed it into a nuclear localized protein.
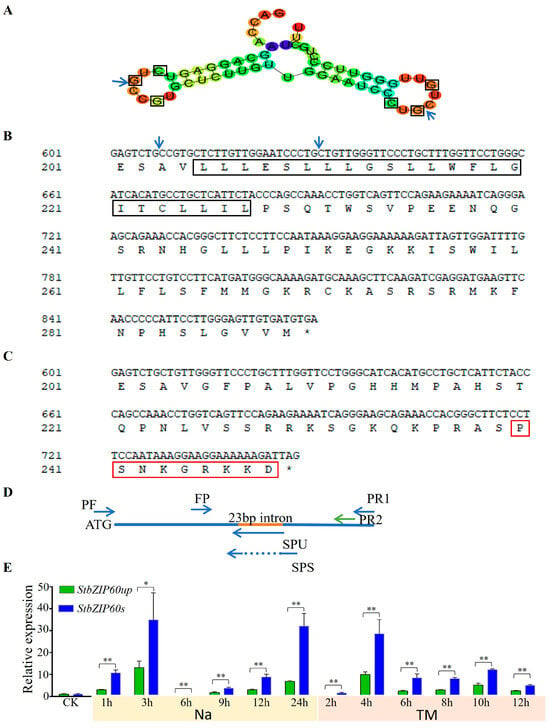
Figure 5.
Sequence analysis and expression levels of StbZIP60up and StbZIP60s under salt and TM conditions. (A) StbZIP60 forms two kissing loops predicted by Centroid fold website. Arrows indicate splicing sites, and the boxes indicate conserved bases CxGxxG in two loops. The color represents base pairing probabilities. (B) Amino acid sequence analysis of StbZIP60. Arrows indicate the splicing sites inferred from mRNA sequences. The amino acid sequences in the box are the transmembrane domain of StbZIP60 predicted by InterPro (https://www.ebi.ac.uk/interpro/ (accessed on 3 October 2024)). (C) Amino acid sequence analysis of StbZIP60s. A new C-terminus of StbZIP60s is forming, and the splicing results in the coding protein being shortened to 248 amino acids. The putative nuclear localization signal (NLS) sequence is highlighted in red. An asterisk denotes the stop codon. (D) The positions of primers used to detect StbZIP60up and StbZIP60s. The red line indicates the spliced 23 bp intron. Flanking primers (FPs) are conserved primer pairs for both StbZIP60up and StbZIP60s transcripts. SPU and SPS are specific reverse primers for StbZIP60up and StbZIP60s, respectively. The primers PF and PR1 used to clone the full CDS of StbZIP60, and PF and PR2 used to clone the full CDS of StbZIP60s. (E) The expression levels of StbZIP60up and StbZIP60s after different time periods of 200 mM NaCl or 5 μg/mL TM treatment. The bars indicate the mean ± SD of three replicates. *, p < 0.05; **, p < 0.01 (Student’s t-test).
We designed specific primers to detect StbZIP60up (unspliced) and StbZIP60s (Figure 5D). We found that StbZIP60 existed in two forms, StbZIP60up and StbZIP60s, under salt stress or TM stress conditions. These two forms can be induced by salt or TM treatments at different time intervals (Figure 5E), and the fold change of StbZIP60s was larger than that of StbZIP60up, leading to significantly different outcomes at all time intervals. We used specific primers PF and PR2 to clone the full length of StbZIP60s from Xisen 6 potato after salt stress treatment for 3 h. We found the presence of StbZIP60s was extremely low. We evaluated 20 positive clones of bacteria containing StbZIP60 fragments, and StbZIP60s was present in only 1 clone (Figure S3).
In order to determine the localization of StbZIP60 and StbZIP60s, we constructed the pBWA(V)HS-eGFP-StbZIP60 and pBWA(V)HS-eGFP-StbZIP60s vector (Figure 6A). After transforming to tobacco leaves, we found that eGFP-StbZIP60 was co-localized with ER marker mcherry-RFP. In addition, eGFP-StbZIP60 was also found in nucleus (Figure 6B). Meanwhile, most GFP-StbZIP60s were localized in the nucleus (Figure 6C). These results indicate that StbZIP60 is a membrane-bound transcription factor in potato. It can be transferred from the cytoplasm (ER) to the nucleus if the potato is subjected to salt stress or ER stress.
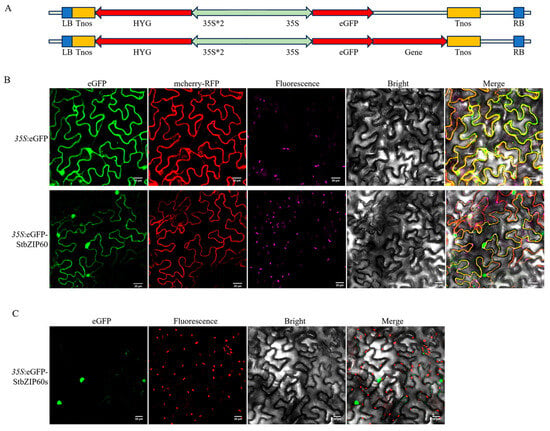
Figure 6.
Subcellular localization of StbZIP60 and StbZIP60s. (A) Vector diagram of 35S:eGFP, 35S:eGFP-StbZIP60, and 35S:eGFP-StbZIP60s. (B) Proteins were transiently transferred into epidermal cells of N. benthamiana leaves. 35S:eGFP vector was used as control, and mcherry-RFP is a marker for ER localization. Pink fluorescence represents chlorophyll; merged fluorescence is shown in yellow. Bars = 20 µm. (C) Subcellular localization of StbZIP60s. Red fluorescence belongs to chlorophyll. Bars = 20 µm.
4. Discussion
Salinity is a major global threat to agriculture, particularly in arid and semi-arid regions. ER is not only responsible for the processing and folding of proteins in organisms but also has an important role in responding to environmental stresses. Salt stresses disrupt protein folding and induce ER stress, which in turn activates the UPR and ERAD to aid in the refolding or degradation of misfolded proteins. At present, genes involved in the ER stress pathway have not been identified in potato, and it is unclear whether these genes are involved in salt stress. In this study, we used genes involved in ER stress processes in Arabidopsis thaliana as a reference to identify their homologs in potato. We analyzed the tissue and stress expression patterns, as well as the cis-elements in their promoter. Meanwhile, we found that the ER membrane-bound transcription factor StbZIP60 undergoes splicing in response to salt stress and ER stress, after which the protein is transferred from the ER to the nucleus. This study will lay the foundation for investigating the functions of these genes.
We identified 29 genes involved in ER pathways (including signaling ER stress sensing and signaling transduction pathways, molecular chaperones, glycoproteins, and the ERAD pathway) in potato (Table 1). The identification method is like the identification of genes involved in the ER pathway in soybean [74]. This suggests that ER stress signaling is conserved in potato, similar with Arabidopsis and soybean. Using published transcriptome data, we analyzed the expression of the identified genes in different tissues and under abiotic and biotic stresses (Figure 1). StSAR1 has a high expression in all tissues, which is a component of the COPII machinery and can interact with bZIP28 under ER stress [50]. All the gene expression levels were changed within a 2-fold range, with the exception of StIRE1A after P. infestans infection. And the expression levels of StIRE1A and StIRE1B increased after BABA and BTH treatments; this indicates that StIRE1 plays an important role in defense against pathogens. ER-localized StDMP2 acts as a positive regulator in the defense against Phytophthora infestans and was found to mediate ER homeostasis. StIRE1 has a higher expression in StDMP2 over-expression line; meanwhile, it has lower expression in stdmp2 after P. infestans infection [75]. More than 50% of the ER pathway genes were responsive to salt stress and osmotic stress (Figure 1), suggesting that they may be involved in salt stress and osmotic stress. Twenty-two genes were found to respond to NaCl or tunicamycin. Among them, the expression levels of seven genes (StbZIP17, StbZIP60, StS2P, StSec12, StMNS3, StUBC32, and StPNG1) were significantly upregulated, and StNPL4 was downregulated after salt or TM treatment (Figure 3). These findings confirm our hypothesis that genes involved in the ER stress pathway may be responsive to salt stress. This finding is consistent with the fact that the promoters of some genes contain multiple abiotic and ER stress response cis-elements (Figure 2).
The membrane-bound transcription factor bZIP60 plays an important role in ER stress and salt stress processes. The overexpression of AtbZIP60 was found to improve salt stress tolerance, while the mutant bzip60 was found to be sensitive to drought and salt stress in Arabidopsis thaliana [46,76]. We found that StbZIP60 was expressed in the roots, shoots, and leaves, with higher expression levels in the roots (Figure 4). SlbZIP60 is expressed in the roots, shoots, leaves, and flowers in tomato [72]. StbZIP60 can respond to NaCl and tunicamycin (Figure 3), and its promoters contain multiple abiotic stress response cis-elements (Figure 2). Therefore, we infer that StbZIP60 may be involved in ER stress and salt stress processes.
When plants are subjected to ER stress, IRE1-mediated bZIP60 mRNA splicing mechanisms are activated, and the splicing site is highly conserved in structure, usually containing the consensus CxGxxG motif [77,78]. We found that the CDS sequence of StbZIP60 contained this conserved splicing site (Figure 5A), indicating that StIRE1 may be spliced into StbZIP60 when potato is subjected to ER stress. Salt stress can induce ER stress, so we infer that StbZIP60 may have a spliced form under salt stress. Gene-specific primers were designed to detect the expression level of StbZIP60s and StbZIP60up during salt stress and ER stress using qRT-PCR. The results showed that the expression levels of StbZIP60s and StbZIP60up exhibited upregulation after stress treatments; this is with the exception of those treated for 6 h with NaCl. The upregulation fold change of StbZIP60s was higher than that of StbZIP60up, showing significant differences at all time intervals (Figure 5E). Using the cDNA treated with 200 mM NaCl for 3 h as a template, we screened the fragment containing the full length of the StbZIP60s ORF. The result showed that StbZIP60 underwent splicing after salt stress; the spliced site was consistent with that predicted by the Centroid fold website. But the splicing rate of StbZIP60 under salt stress was very low (1/20) (Figure S3). Researchers have reported, using an agarose gel assay, that AtbZIP60 and ZmbZIP60 do not undergo splicing under salt stress [45,73]. However, we cloned the StbZIP60s fragment using the pick single clone sequencing method. It is possible that the mechanism by which bZIP60 is involved in salt stress is different in different species, or it may be related to the detection method.
The localization of StbZIP60 was in the cytoplasm and the nucleus, while StbZIP60s were found in the nucleus (Figure 6). The different sub-localizations suggest that their functions may be different. Alternative splicing plays an important role in the adaptive responses of plants to various abiotic and biotic stresses [79]. Researchers have found that the spliced and unspliced forms of bZIP60 play different roles in the physiological responses to salt stress and ER stress [45]. Under drought stress conditions, the spliced form of BhbZIP60 (BhbZIP60s) trans-locates into the nucleus and binds to the cis-element of the UPR genes to mediate drought-induced ER stress responses in boea hygrometrica [44]. In maize, the spliced form of ZmbZIP60 was induced by heat stress [73,80]. In wheat, heat stress leads to the unconventional splicing of TabZIP60 and TabZIP60s activation, which can enhance heat tolerance by regulating the transcriptional reprogramming of downstream genes in Arabidopsis [81]. In the context of these findings, we ask the following questions for future researchers: What are the functions of StbZIP60 and StbZIP60s in response to salt stress? Does StbZIP60s exert transcriptional activation or transcriptional repression? Which cis-element does it bind to, and which genes are downstream targets? Does StbZIP60 undergo splicing under heat or drought stress in addition to salt stress? How does the organism maintain the dynamic stability of StbZIP60 and StbZIP60s? In addition, we need to further validate the functions of identified genes in maintaining ER homeostasis and salt stress adaptation, with the aim of providing a theoretical basis for the genetic improvement of potato stress tolerance.
5. Conclusions
In this study, 29 genes involved in the ER stress signaling pathway in the potato genome were systematically identified. We analyzed their chromosomal localizations, protein properties, and other basic information. Through transcriptome and qRT-PCR analyses, we found that the expression levels of these genes can respond to salt and TM treatments. Additionally, their promoter regions were enriched with cis-acting elements related to abiotic stress (e.g., Myb, LTR, ABRE, etc.) and ER stress (e.g., ERSE, UPRE, etc.), suggesting that they are involved in the salt-stress- and ER-stress-mediated pathways. We studied the characterization of StbZIP60 in detail. It was found to be expressed in the roots, stems, and leaves of potato, with relatively higher expression in roots; its expression level was induced by salt and TM. In addition, StbZIP60s, a spliced form of the StbZIP60 transcription factor, was identified for the first time in potato, which resulted in a code-shift by excising a 23 bp in the C-terminal conserved site (CxGxxG) and significantly altering subcellular localization. StbZIP60 was localized in the ER and the nucleus, whereas the StbZIP60s predominantly migrated to the nucleus. These findings not only expand our knowledge of the molecular mechanism of ER stress signaling pathways in potato, but also provide candidate genes for analyzing the regulatory network of salt tolerance in potato.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051224/s1, Figure S1: The distribution and location of the ER-stress-related genes in potato on chromosomes. Chromosome numbers are on the left and the scale is in mega bases (Mb); Figure S2: The distribution of cis-elements related to drought stress, high and low temperature response, and ABA stress hormone responses on gene promoters; Figure S3: Multiple sequence comparisons of 20 positive clones containing StbZIP60s fragments. The full length of StbZIP60s was cloned using PF and PR2 primers. Only clone #5 underwent splicing and the splicing site was consistent with those in the Centroidfold website’s prediction. The numbers on the left represent the names of the sequenced clone and the numbers on the right represent the number of bases; Table S1: The reference proteins sequence of Arabidopsis; Table S2: Primers used in the study; Table S3: The basic information of genes involved in ER stress pathways in potato; Table S4: The conserved structural domains of genes involved in ER stress pathways in potato and Arabidopsis; Table S5: Analysis of expression level of genes in different tissues; Table S6: Analysis of expression level of genes under abiotic, biotic, and hormone conditions; Table S7: The promoter sequence of the identified genes were extracted using the TBtools software. Table S8: The relative expression data of genes in this article.
Author Contributions
Conceptualization, P.G. and L.H.; Data curation, D.Z. and C.Z.; Formal analysis, Q.C.; Funding acquisition, P.G.; Investigation, I.P.S., R.Y., X.Z., and Y.L.; Methodology, P.G.; Project administration, P.G. and L.H.; Resources, P.G.; Software, D.Z.; Supervision, L.H.; Validation, D.Z., C.Z., Z.Q., P.S., and P.C.; Visualization, D.Z. and C.Z.; Writing—original draft, P.G.; Writing—review and editing, D.Z., C.Z., Z.Q., Q.C., I.P.S., P.S., P.C., R.Y., X.Z., Y.L., and L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (No. 32201714), Shandong Province Higher Educational Science and Technology Program (No.2024KJG070, No.2023KJ270), Innovation Team Program of Dezhou University (No. DZUQC202301), Key R&D Program of Shandong Province of China (No. 2022LZGC017), Doctoral Fund for Scientific Research of Dezhou University (No. 2020xjrc102).
Data Availability Statement
All data needed to evaluate the conclusions in this paper are present in the paper and/or the Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ER | endoplasmic reticulum |
| ERQC | ER quality control |
| UPR | unfolded protein response |
| ERAD | endoplasmic-reticulum-associated degradation |
| TM | tunicamycin |
| ERSE | ER stress responsive element |
| UPRE | UPR element |
References
- Reyes-Impellizzeri, S.; Moreno, A.A. The Endoplasmic Reticulum Role in the Plant Response to Abiotic Stress. Front. Plant Sci. 2021, 12, 755447. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R. Protein Quality Control in the Endoplasmic Reticulum of Plants. Annu. Rev. Plant Biol. 2018, 69, 147–172. [Google Scholar] [CrossRef] [PubMed]
- Angelos, E.; Ruberti, C.; Kim, S.J.; Brandizzi, F. Maintaining the factory: The roles of the unfolded protein response in cellular homeostasis in plants. Plant J. 2017, 90, 671–682. [Google Scholar] [CrossRef]
- Tintor, N.; Saijo, Y. ER-mediated control for abundance, quality, and signaling of transmembrane immune receptors in plants. Front. Plant Sci. 2014, 5, 65. [Google Scholar] [CrossRef]
- Ko, D.K.; Brandizzi, F. Dynamics of ER stress-induced gene regulation in plants. Nat. Rev. Genet. 2024, 25, 513–525. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, Y.; Wei, A.; Guo, M.; Li, Y.; Wang, J.; Wang, X.; Bao, Y. Unfolded protein response in balancing plant growth and stress tolerance. Front. Plant Sci. 2022, 13, 1019414. [Google Scholar] [CrossRef] [PubMed]
- Christianson, J.C.; Jarosch, E.; Sommer, T. Mechanisms of substrate processing during ER-associated protein degradation. Nat. Rev. Mol. Cell Biol. 2023, 24, 777–796. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, F.; Xie, Q. Insights into endoplasmic reticulum-associated degradation in plants. New Phytol. 2020, 226, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Nakatogawa, H. Autophagic degradation of the endoplasmic reticulum. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2020, 96, 1–9. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Simoni, E.B.; Oliveira, C.C.; Fraga, O.T.; Reis, P.A.B.; Fontes, E.P.B. Cell Death Signaling from Endoplasmic Reticulum Stress: Plant-Specific and Conserved Features. Front. Plant Sci. 2022, 13, 835738. [Google Scholar] [CrossRef]
- Nagashima, Y.; Mishiba, K.; Suzuki, E.; Shimada, Y.; Iwata, Y.; Koizumi, N. Arabidopsis IRE1 catalyses unconventional splicing of bZIP60 mRNA to produce the active transcription factor. Sci. Rep. 2011, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Humbert, S.; Liu, J.X.; Srivastava, R.; Rothstein, S.J.; Howell, S.H. Heat induces the splicing by IRE1 of a mRNA encoding a transcription factor involved in the unfolded protein response in Arabidopsis. Proc. Natl. Acad. Sci. USA 2011, 108, 7247–7252. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Dey, M.; Neculai, D.; Cao, C.; Dever, T.E.; Sicheri, F. Structure of the dual enzyme Ire1 reveals the basis for catalysis and regulation in nonconventional RNA splicing. Cell 2008, 132, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Iwata, Y.; Lee, M.H.; Koizumi, N. Analysis of a transcription factor using transient assay in Arabidopsis protoplasts. Methods Mol. Biol. 2011, 754, 107–117. [Google Scholar] [CrossRef]
- Li, Z.; Tang, J.; Srivastava, R.; Bassham, D.C.; Howell, S.H. The Transcription Factor bZIP60 Links the Unfolded Protein Response to the Heat Stress Response in Maize. Plant Cell 2020, 32, 3559–3575. [Google Scholar] [CrossRef]
- Iwata, Y.; Koizumi, N. An Arabidopsis transcription factor, AtbZIP60, regulates the endoplasmic reticulum stress response in a manner unique to plants. Proc. Natl. Acad. Sci. USA 2005, 102, 5280–5285. [Google Scholar] [CrossRef]
- Cho, Y. Arabidopsis AGB1 participates in salinity response through bZIP17-mediated unfolded protein response. BMC Plant Biol. 2024, 24, 586. [Google Scholar] [CrossRef]
- Liu, J.X.; Srivastava, R.; Che, P.; Howell, S.H. Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 2007, 51, 897–909. [Google Scholar] [CrossRef]
- Srivastava, R.; Deng, Y.; Howell, S.H. Stress sensing in plants by an ER stress sensor/transducer, bZIP28. Front. Plant Sci. 2014, 5, 59. [Google Scholar] [CrossRef]
- Che, P.; Bussell, J.D.; Zhou, W.; Estavillo, G.M.; Pogson, B.J.; Smith, S.M. Signaling from the endoplasmic reticulum activates brassinosteroid signaling and promotes acclimation to stress in Arabidopsis. Sci. Signal. 2010, 3, ra69. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, S.S.; Lu, S.J.; Liu, J.X. Site-1 protease cleavage site is important for the ER stress-induced activation of membrane-associated transcription factor bZIP28 in Arabidopsis. Sci. China Life Sci. 2015, 58, 270–275. [Google Scholar] [CrossRef]
- Liu, J.X.; Howell, S.H. bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 2010, 22, 782–796. [Google Scholar] [CrossRef]
- Manghwar, H.; Li, J. Endoplasmic Reticulum Stress and Unfolded Protein Response Signaling in Plants. Int. J. Mol. Sci. 2022, 23, 828. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Guo, C.; Ali, K.; Zheng, Q.; Wei, Q.; Zhu, Y.; Wang, L.; Li, G.; Li, W.; Zheng, B.; et al. A Non-redundant Function of MNS5: A Class I alpha-1, 2 Mannosidase, in the Regulation of Endoplasmic Reticulum-Associated Degradation of Misfolded Glycoproteins. Front. Plant Sci. 2022, 13, 873688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Duan, M.; Shan, L.; Zheng, L.; Liu, J. HIP is involved in NaCl and endoplasmic reticulum stress resistance in Arabidopsis. Plant Physiol. Biochem. 2024, 217, 109226. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Wang, J.; Li, H.; Xie, C.; Zhang, S.; Wu, C.; Yang, G.; Yan, K.; Huang, J.; Zheng, C. SENSITIVE TO SALT1, An Endoplasmic Reticulum-Localized Chaperone, Positively Regulates Salt Resistance. Plant Physiol. 2018, 178, 1390–1405. [Google Scholar] [CrossRef]
- Jin, T.; Shan, Z.; Zhou, S.; Yang, Q.; Gai, J.; Li, Y. GmDNAJC7 from Soybean Is Involved in Plant Tolerance to Alkaline-Salt, Salt, and Drought Stresses. Agronomy 2022, 12, 1419. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 January 2022).
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Butcher, K.; Wick, A.F.; DeSutter, T.; Chatterjee, A.; Harmon, J. Soil Salinity: A Threat to Global Food Security. Agron. J. 2016, 108, 2189–2200. [Google Scholar] [CrossRef]
- Herath, V.; Verchot, J. Comprehensive Transcriptome Analysis Reveals Genome-Wide Changes Associated with Endoplasmic Reticulum (ER) Stress in Potato (Solanum tuberosum L.). Int. J. Mol. Sci. 2022, 23, 13795. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, K.N.; Lal, M.K.; Tiwari, R.K.; Dev, D.; Kardile, H.B.; Patil, V.U.; Kumar, A.; Vanishree, G.; Kumar, D.; Bhardwaj, V.; et al. Salinity Stress in Potato: Understanding Physiological, Biochemical and Molecular Responses. Life 2021, 11, 545. [Google Scholar] [CrossRef]
- Han, X.; Yang, R.; Zhang, L.; Wei, Q.; Zhang, Y.; Wang, Y.; Shi, Y. A Review of Potato Salt Tolerance. Int. J. Mol. Sci. 2023, 24, 10726. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Demircan, N.; Ozgur, R.; Turkan, I.; Uzilday, B. Heavy metal toxicity leads to accumulation of insoluble proteins and induces endoplasmic reticulum stress-specific unfolded protein response in Arabidopsis thaliana. Environ. Sci. Pollut. Res. Int. 2024, 31, 53206–53218. [Google Scholar] [CrossRef]
- Gao, J.; Wang, M.J.; Wang, J.J.; Lu, H.P.; Liu, J.X. bZIP17 regulates heat stress tolerance at reproductive stage in Arabidopsis. aBIOTECH 2022, 3, 1–11. [Google Scholar] [CrossRef]
- Kim, J.S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. ER-Anchored Transcription Factors bZIP17 and bZIP28 Regulate Root Elongation. Plant Physiol. 2018, 176, 2221–2230. [Google Scholar] [CrossRef]
- Gao, H.; Brandizzi, F.; Benning, C.; Larkin, R.M. A membrane-tethered transcription factor defines a branch of the heat stress response in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 16398–16403. [Google Scholar] [CrossRef]
- Hong, Z.H.; Qing, T.; Schubert, D.; Kleinmanns, J.A.; Liu, J.X. BLISTER-regulated vegetative growth is dependent on the protein kinase domain of ER stress modulator IRE1A in Arabidopsis thaliana. PLoS Genet. 2019, 15, e1008563. [Google Scholar] [CrossRef]
- Gaguancela, O.A.; Zuniga, L.P.; Arias, A.V.; Halterman, D.; Flores, F.J.; Johansen, I.E.; Wang, A.; Yamaji, Y.; Verchot, J. The IRE1/bZIP60 Pathway and Bax Inhibitor 1 Suppress Systemic Accumulation of Potyviruses and Potexviruses in Arabidopsis and Nicotiana benthamiana Plants. Mol. Plant Microbe Interact. 2016, 29, 750–766. [Google Scholar] [CrossRef]
- Moreno, A.A.; Mukhtar, M.S.; Blanco, F.; Boatwright, J.L.; Moreno, I.; Jordan, M.R.; Chen, Y.; Brandizzi, F.; Dong, X.; Orellana, A.; et al. IRE1/bZIP60-mediated unfolded protein response plays distinct roles in plant immunity and abiotic stress responses. PLoS ONE 2012, 7, e31944. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Du, H.; Zhang, Z.; Xu, W.; Deng, X. BhbZIP60 from Resurrection Plant Boea hygrometrica is an mRNA Splicing-Activated Endoplasmic Reticulum Stress Regulator Involved in Drought Tolerance. Front. Plant Sci. 2017, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Valencia, C.; Moreno, A.A.; Sandoval-Ibanez, O.; Mitina, I.; Blanco-Herrera, F.; Cifuentes-Esquivel, N.; Orellana, A. bZIP17 and bZIP60 Regulate the Expression of BiP3 and Other Salt Stress Responsive Genes in an UPR-Independent Manner in Arabidopsis thaliana. J. Cell Biochem. 2015, 116, 1638–1645. [Google Scholar] [CrossRef]
- Fujita, M.; Mizukado, S.; Fujita, Y.; Ichikawa, T.; Nakazawa, M.; Seki, M.; Matsui, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Identification of stress-tolerance-related transcription-factor genes via mini-scale Full-length cDNA Over-eXpressor (FOX) gene hunting system. Biochem. Biophys. Res. Commun. 2007, 364, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liu, L.P.; Wang, Y.Z.; Yang, L.; Wang, M.J.; Liu, J.X. NAC103, a NAC family transcription factor, regulates ABA response during seed germination and seedling growth in Arabidopsis. Planta 2020, 252, 95. [Google Scholar] [CrossRef]
- Sun, L.; Yang, Z.T.; Song, Z.T.; Wang, M.J.; Sun, L.; Lu, S.J.; Liu, J.X. The plant-specific transcription factor gene NAC103 is induced by bZIP60 through a new cis-regulatory element to modulate the unfolded protein response in Arabidopsis. Plant J. 2013, 76, 274–286. [Google Scholar] [CrossRef]
- Iwata, Y.; Ashida, M.; Hasegawa, C.; Tabara, K.; Mishiba, K.I.; Koizumi, N. Activation of the Arabidopsis membrane-bound transcription factor bZIP28 is mediated by site-2 protease, but not site-1 protease. Plant J. 2017, 91, 408–415. [Google Scholar] [CrossRef]
- Srivastava, R.; Chen, Y.; Deng, Y.; Brandizzi, F.; Howell, S.H. Elements proximal to and within the transmembrane domain mediate the organelle-to-organelle movement of bZIP28 under ER stress conditions. Plant J. 2012, 70, 1033–1042. [Google Scholar] [CrossRef]
- Pastor-Cantizano, N.; Bernat-Silvestre, C.; Marcote, M.J.; Aniento, F. Loss of Arabidopsis p24 function affects ERD2 trafficking and Golgi structure, and activates the unfolded protein response. J. Cell Sci. 2018, 131, jcs203802. [Google Scholar] [CrossRef]
- Gimeno-Ferrer, F.; Pastor-Cantizano, N.; Bernat-Silvestre, C.; Selvi-Martinez, P.; Vera-Sirera, F.; Gao, C.; Perez-Amador, M.A.; Jiang, L.; Aniento, F.; Marcote, M.J. alpha2-COP is involved in early secretory traffic in Arabidopsis and is required for plant growth. J. Exp. Bot. 2017, 68, 391–401. [Google Scholar] [CrossRef]
- Joshi, R.; Paul, M.; Kumar, A.; Pandey, D. Role of calreticulin in biotic and abiotic stress signalling and tolerance mechanisms in plants. Gene 2019, 714, 144004. [Google Scholar] [CrossRef]
- Kim, J.H.; Nguyen, N.H.; Nguyen, N.T.; Hong, S.W.; Lee, H. Loss of all three calreticulins, CRT1, CRT2 and CRT3, causes enhanced sensitivity to water stress in Arabidopsis. Plant Cell Rep. 2013, 32, 1843–1853. [Google Scholar] [CrossRef] [PubMed]
- Montpetit, J.; Clua, J.; Hsieh, Y.F.; Vogiatzaki, E.; Muller, J.; Abel, S.; Strasser, R.; Poirier, Y. Endoplasmic reticulum calnexins participate in the primary root growth response to phosphate deficiency. Plant Physiol. 2023, 191, 1719–1733. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Qu, S.; Chowdhury, S.; Noxon, I.C.; Schonhoft, J.D.; Plate, L.; Powers, E.T.; Kelly, J.W.; Lander, G.C.; Wiseman, R.L. The endoplasmic reticulum HSP40 co-chaperone ERdj3/DNAJB11 assembles and functions as a tetramer. EMBO J. 2017, 36, 2296–2309. [Google Scholar] [CrossRef]
- Lu, D.P.; Christopher, D.A. Endoplasmic reticulum stress activates the expression of a sub-group of protein disulfide isomerase genes and AtbZIP60 modulates the response in Arabidopsis thaliana. Mol. Genet. Genom. 2008, 280, 199–210. [Google Scholar] [CrossRef]
- Guan, P.; Xie, C.; Zhao, D.; Wang, L.; Zheng, C. SES1 is vital for seedling establishment and post-germination growth under high-potassium stress conditions in Arabidopsis thaliana. PeerJ 2022, 10, e14282. [Google Scholar] [CrossRef]
- Guan, P.; Wang, J.; Xie, C.; Wu, C.; Yang, G.; Yan, K.; Zhang, S.; Zheng, C.; Huang, J. SES1 positively regulates heat stress resistance in Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 513, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Herrera, F.; Moreno, A.A.; Tapia, R.; Reyes, F.; Araya, M.; D’Alessio, C.; Parodi, A.; Orellana, A. The UDP-glucose: Glycoprotein glucosyltransferase (UGGT), a key enzyme in ER quality control, plays a significant role in plant growth as well as biotic and abiotic stress in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 127. [Google Scholar] [CrossRef]
- Farid, A.; Malinovsky, F.G.; Veit, C.; Schoberer, J.; Zipfel, C.; Strasser, R. Specialized roles of the conserved subunit OST3/6 of the oligosaccharyltransferase complex in innate immunity and tolerance to abiotic stresses. Plant Physiol. 2013, 162, 24–38. [Google Scholar] [CrossRef]
- Xia, T.; Zhan, Y.; Mu, Y.; Zhang, J.; Xu, W. MNSs-mediated N-glycan processing is essential for auxin homeostasis in Arabidopsis roots during alkaline response. iScience 2022, 25, 104298. [Google Scholar] [CrossRef]
- Huttner, S.; Veit, C.; Vavra, U.; Schoberer, J.; Liebminger, E.; Maresch, D.; Grass, J.; Altmann, F.; Mach, L.; Strasser, R. Arabidopsis Class I alpha-Mannosidases MNS4 and MNS5 Are Involved in Endoplasmic Reticulum-Associated Degradation of Misfolded Glycoproteins. Plant Cell 2014, 26, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Kamauchi, S.; Nakatani, H.; Nakano, C.; Urade, R. Gene expression in response to endoplasmic reticulum stress in Arabidopsis thaliana. FEBS J. 2005, 272, 3461–3476. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, R.; Wu, Y.; Wei, S.; Wang, Q.; Zheng, Y.; Xia, R.; Shang, X.; Yu, F.; Yang, X.; et al. ERAD-related E2 and E3 enzymes modulate the drought response by regulating the stability of PIP2 aquaporins. Plant Cell 2021, 33, 2883–2898. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Liu, L.; Zhao, Q.; Zhang, Z.; Li, Q.; Lin, B.; Wu, Y.; Tang, S.; Xie, Q. Arabidopsis ubiquitin conjugase UBC32 is an ERAD component that functions in brassinosteroid-mediated salt stress tolerance. Plant Cell 2012, 24, 233–244. [Google Scholar] [CrossRef]
- Ines, D.; Courty, P.E.; Wendehenne, D.; Rosnoblet, C. CDC48 in plants and its emerging function in plant immunity. Trends Plant Sci. 2024, 29, 786–798. [Google Scholar] [CrossRef]
- Wei, L.; Cheng, J.; Xiang, J.; Wu, J. Genome-wide identification and characterization of grapevine UFD1 genes during berry development and salt stress response. J. Plant Biochem. Biotechnol. 2022, 31, 592–601. [Google Scholar] [CrossRef]
- Bays, N.W.; Wilhovsky, S.K.; Goradia, A.; Hodgkiss-Harlow, K.; Hampton, R.Y. HRD4/NPL4 is required for the proteasomal processing of ubiquitinated ER proteins. Mol. Biol. Cell 2001, 12, 4114–4128. [Google Scholar] [CrossRef]
- Masahara-Negishi, Y.; Hosomi, A.; Della Mea, M.; Serafini-Fracassini, D.; Suzuki, T. A plant peptide: N-glycanase orthologue facilitates glycoprotein ER-associated degradation in yeast. Biochim. Biophys. Acta 2012, 1820, 1457–1462. [Google Scholar] [CrossRef]
- Raasi, S.; Wolf, D.H. Ubiquitin receptors and ERAD: A network of pathways to the proteasome. Semin. Cell Dev. Biol. 2007, 18, 780–791. [Google Scholar] [CrossRef]
- Kaur, N.; Kaitheri Kandoth, P. Tomato bZIP60 mRNA undergoes splicing in endoplasmic reticulum stress and in response to environmental stresses. Plant Physiol. Biochem. 2021, 160, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Humbert, S.; Howell, S.H. ZmbZIP60 mRNA is spliced in maize in response to ER stress. BMC Res. Notes 2012, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.; Silva, J.C.; Caetano, H.D.; Machado, J.P.; Mendes, G.C.; Reis, P.A.; Brustolini, O.J.; Dal-Bianco, M.; Fontes, E.P. Comprehensive analysis of the endoplasmic reticulum stress response in the soybean genome: Conserved and plant-specific features. BMC Genom. 2015, 16, 783. [Google Scholar] [CrossRef]
- Bi, W.; Chen, Y.; Song, Y.; Liu, J.; Zhao, T.; Sun, C.; Qin, J.; Tu, Z.; Li, Y.; Wang, X.; et al. Potato DMP2 positively regulates plant immunity by modulating endoplasmic reticulum homeostasis. J. Integr. Plant Biol. 2025. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Cai, H.; Guo, M.; Chai, M.; She, Z.; Ye, L.; Cheng, Y.; Wang, B.; Qin, Y. The bZIP Transcription Factor GmbZIP15 Negatively Regulates Salt- and Drought-Stress Responses in Soybean. Int. J. Mol. Sci. 2020, 21, 7778. [Google Scholar] [CrossRef]
- Hooks, K.B.; Griffiths-Jones, S. Conserved RNA structures in the non-canonical Hac1/Xbp1 intron. RNA Biol. 2011, 8, 552–556. [Google Scholar] [CrossRef]
- Howell, S.H. Evolution of the unfolded protein response in plants. Plant Cell Environ. 2021, 44, 2625–2635. [Google Scholar] [CrossRef]
- Alhabsi, A.; Ling, Y.; Crespi, M.; Reddy, A.S.N.; Mahfouz, M. Alternative Splicing Dynamics in Plant Adaptive Responses to Stress. Annu. Rev. Plant Biol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Li, Z.; Srivastava, R.; Tang, J.; Zheng, Z.; Howell, S.H. Cis-Effects Condition the Induction of a Major Unfolded Protein Response Factor, ZmbZIP60, in Response to Heat Stress in Maize. Front. Plant Sci. 2018, 9, 833. [Google Scholar] [CrossRef]
- Geng, X.; Zang, X.; Li, H.; Liu, Z.; Zhao, A.; Liu, J.; Peng, H.; Yao, Y.; Hu, Z.; Ni, Z.; et al. Unconventional splicing of wheat TabZIP60 confers heat tolerance in transgenic Arabidopsis. Plant Sci. 2018, 274, 252–260. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).