Optimizing the LED Light Spectrum for Enhanced Seed Germination of Lettuce cv. ‘Lollo Bionda’ in Controlled-Environment Agriculture
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Conditions, and Experimental Setup
2.2. LED Tubes and Light Treatments
2.3. Measurement of Germination Parameters
2.3.1. Germination Percentage (GP)
2.3.2. Mean Germination Time (MGT)
2.3.3. Germination Index (GI)
2.3.4. Seedling Vigor Assessment
2.3.5. Vigor Index (VI)
2.3.6. Seedling Emergence
2.3.7. Shoot and Root Length and Fresh Mass
2.4. Statistical Analysis
3. Results
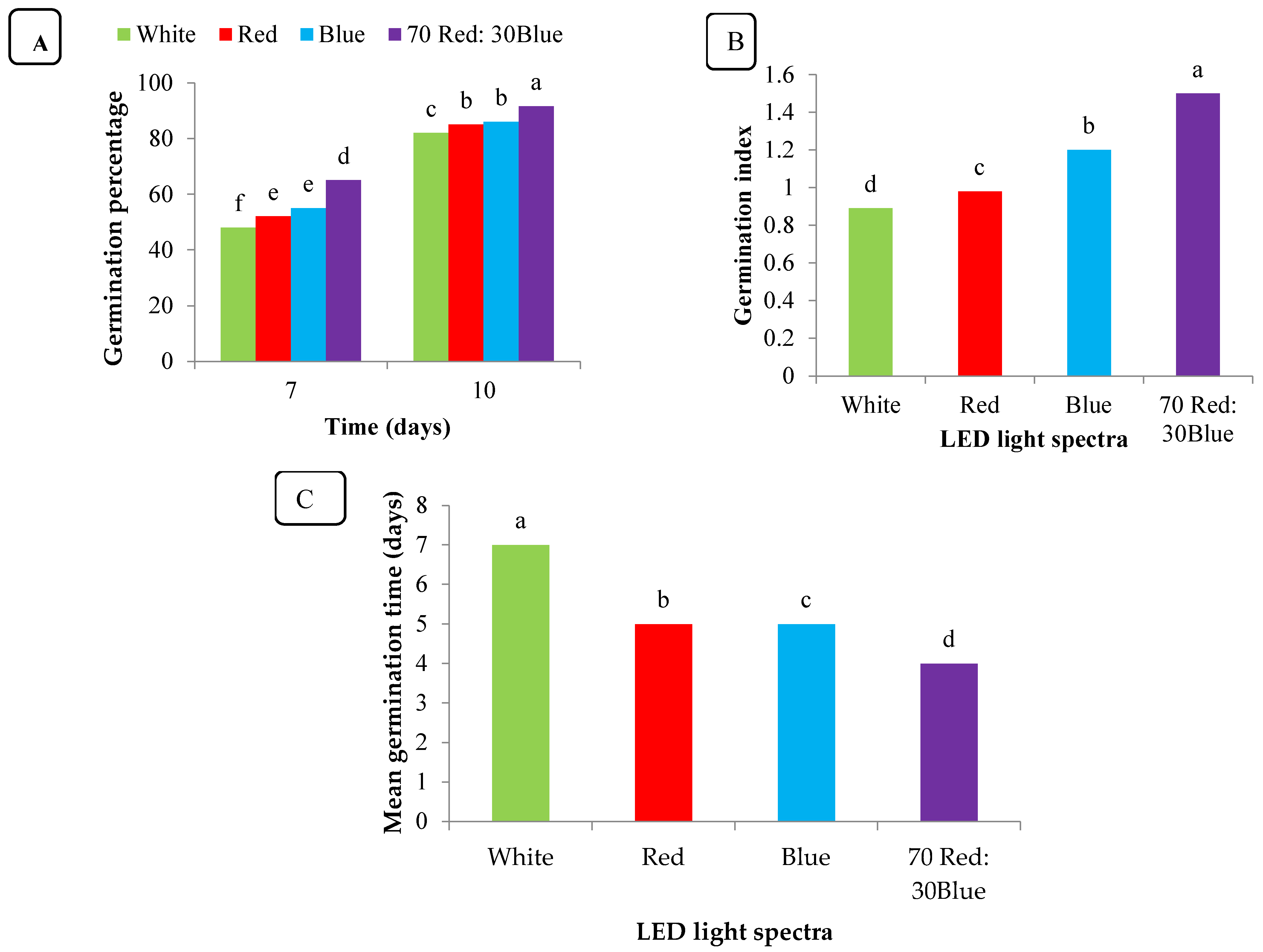
3.1. Effect of the LED Light Spectrum on Germination Traits
3.2. Vigor Index
3.3. Percentage of Emerged Seedlings
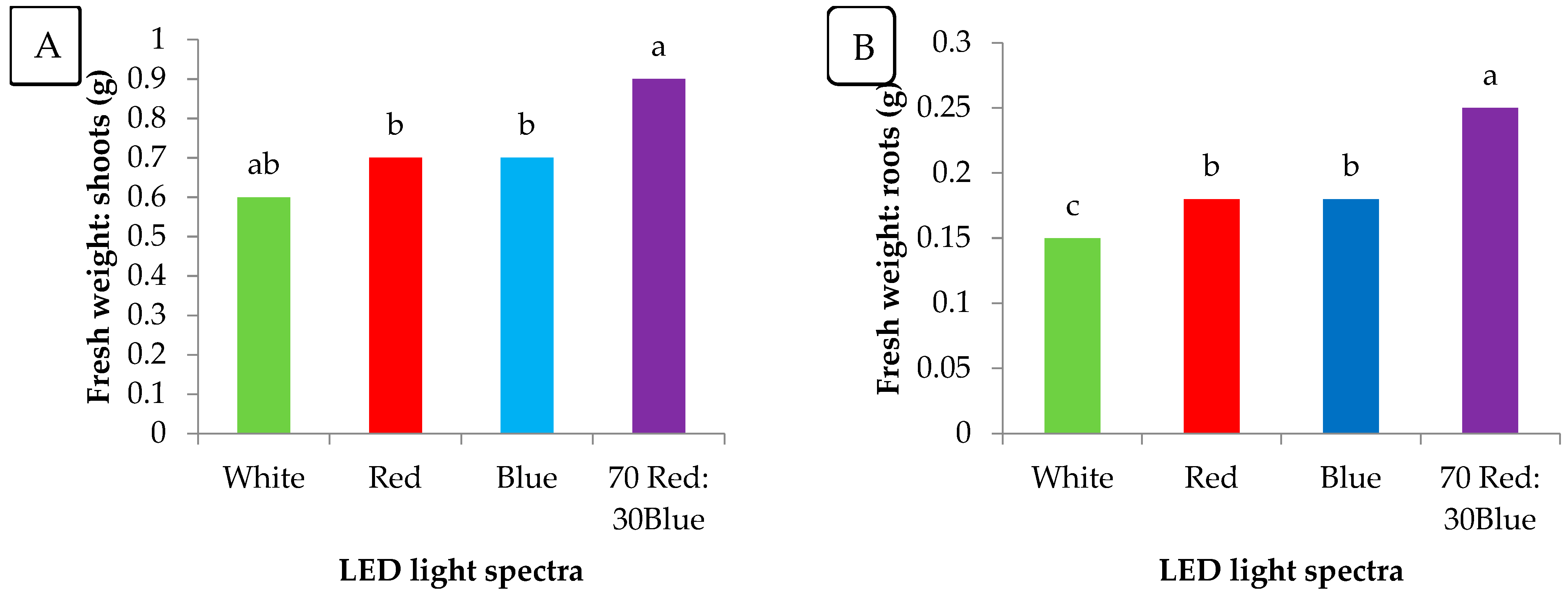
3.4. Shoot and Root Lengths
3.5. Fresh Weights of Shoots and Roots
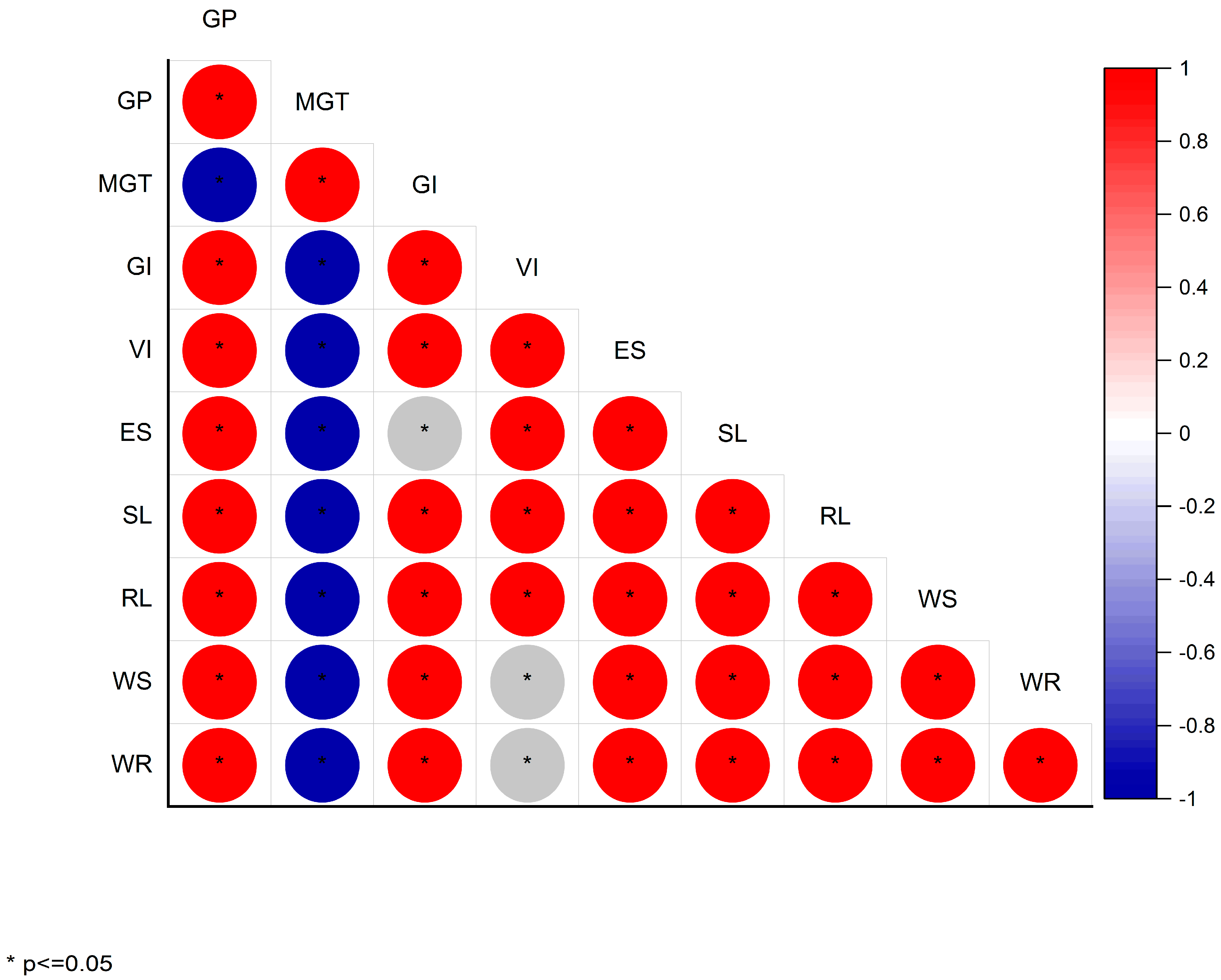
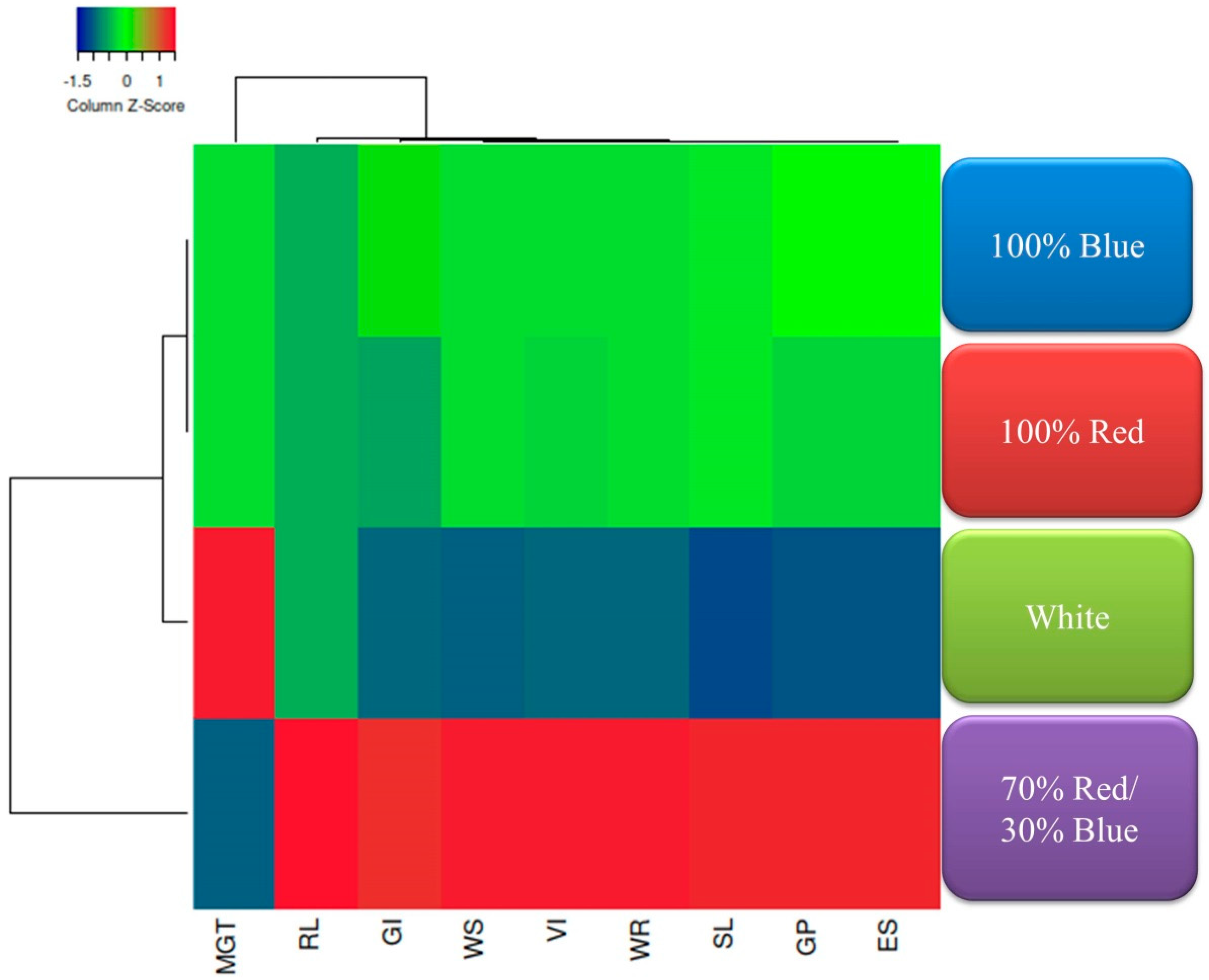
3.6. Correlation, Heatmap, and Multivariate Analyses
4. Discussion
4.1. Seed Germination Traits
4.2. Average Time for Germination
4.3. Seed Vigor
4.4. Early Growth
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gruda, N.; Bisbis, M.; Katsoulas, N.; Kittas, C. Smart greenhouse production practices to manage and mitigate the impact of climate change in protected cultivation. Acta Hortic. 2021, 1320, 189–196. [Google Scholar] [CrossRef]
- Gruda, N.S.; Samuolienė, G.; Dong, J.; Li, X. Environmental conditions and nutritional quality of vegetables in protected cultivation. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70139. [Google Scholar] [CrossRef]
- Eigenbrod, C.; Gruda, N. Urban vegetable for food security in cities. A review. Agron. Sustain. Dev. 2015, 35, 483–498. [Google Scholar] [CrossRef]
- Gruda, N.; Bisbis, M.; Tanny, J. Impacts of protected vegetable cultivation on climate change and adaptation strategies for cleaner production—A review. J. Clean. Prod. 2019, 225, 324–339. [Google Scholar] [CrossRef]
- Gruda, N. Assessing the impact of environmental factors on the quality of greenhouse produce. In Achieving Sustainable Greenhouse Cultivation; Burleigh Dodds Science Publishing: Sawston Cambridge, UK, 2019; pp. 413–444. [Google Scholar]
- Smith, B.J.; Rezazadeh, A.; Stafne, E.T.; Sakhanokho, H.F. Effect of light-emitting diodes, ultraviolet-B, and fluorescent supplemental greenhouse lights on strawberry plant growth and response to infection by the anthracnose pathogen Colletotrichum gloeosporioides. HortScience 2022, 57, 856–863. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.-H.; Park, H.S.; Min, C.-W.; Woo, J.H.; Choi, B.R.; Rahman, M.M.; Lee, K.-W. Light Quality Plays a Crucial Role in Regulating Germination, Photosynthetic Efficiency, Plant Development, Reactive Oxygen Species Production, Antioxidant Enzyme Activity, and Nutrient Acquisition in Alfalfa. Int. J. Mol. Sci. 2025, 26, 360. [Google Scholar] [CrossRef]
- Gargaro, M.; Murphy, R.J.; Harris, Z.M. Let-us investigate; A meta-analysis of influencing factors on lettuce crop yields within controlled-environment agriculture systems. Plants 2023, 12, 2623. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Yu-Xin, T.; Qi-Chang, Y. Optimal control of environmental conditions affecting lettuce plant growth in a controlled environment with artificial lighting: A review. S. Afr. J. Bot. 2020, 130, 75–89. [Google Scholar] [CrossRef]
- Bayley, D. Controlled Environment Production of Romaine Lettuce (Lactuca sativa). Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2020. [Google Scholar]
- Rahman, M.M.; Field, D.L.; Ahmed, S.M.; Hasan, M.T.; Basher, M.K.; Alameh, K. LED illumination for high-quality high-yield crop growth in protected cropping environments. Plants 2021, 10, 2470. [Google Scholar] [CrossRef]
- Rahman, M.B.; Chakma, J.D.; Momin, A.; Islam, S.; Uddin, M.A.; Islam, M.A.; Aryal, S. Smart Crop cultivation system using automated agriculture monitoring environment in the context of Bangladesh agriculture. Sensors 2023, 23, 8472. [Google Scholar] [CrossRef]
- Boros, I.F.; Székely, G.; Balázs, L.; Csambalik, L.; Sipos, L. Effects of LED lighting environments on lettuce (Lactuca sativa L.) in PFAL systems—A review. Sci. Hortic. 2023, 321, 112351. [Google Scholar] [CrossRef]
- Eichelsbacher, S.; Luksch, C.R.; Bienert, G.P.; Alcock, T.D.; Steppe, K.; Marcelis, L.F.; Orsini, F.; Rosenqvist, E.; Lambers, H.; Runkle, E. What Is the Limit of Vertical Farming Productivity? Food Energy Secur. 2025, 14, e70061. [Google Scholar] [CrossRef]
- Bączek-Kwinta, R.; Michałek, S. Smoke Compounds Compensate for Light Irrespective of Its Spectrum in Positively Photoblastic German Chamomile Seeds, Although Red Light Is Crucial. Agronomy 2025, 15, 700. [Google Scholar] [CrossRef]
- Lee, J.H.; Kwon, Y.B.; Roh, Y.H.; Choi, I.-L.; Kim, J.; Kim, Y.; Yoon, H.S.; Kang, H.-M. Effect of various LED light qualities, including wide red spectrum-LED, on the growth and quality of mini red romaine lettuce (cv. Breen). Plants 2023, 12, 2056. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, J.; Sun, Z.; Zhang, Z.; Hu, J. Multi-objective optimal regulation model and system based on whole plant photosynthesis and light use efficiency of lettuce. Comput. Electron. Agric. 2023, 206, 107617. [Google Scholar] [CrossRef]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Solano, C.J.; Hernández, J.A.; Suardíaz, J.; Barba-Espín, G. Impacts of LEDs in the red spectrum on the germination, early seedling growth and antioxidant metabolism of pea (Pisum sativum L.) and melon (Cucumis melo L.). Agriculture 2020, 10, 204. [Google Scholar] [CrossRef]
- Zaghdoud, C.; Ollio, I.; Solano, C.J.; Ochoa, J.; Suardiaz, J.; Fernández, J.A.; Martínez Ballesta, M.d.C. Red LED light improves pepper (Capsicum annuum L.) seed radicle emergence and growth through the modulation of aquaporins, hormone homeostasis, and metabolite remobilization. Int. J. Mol. Sci. 2023, 24, 4779. [Google Scholar] [CrossRef]
- Reale, A.L.; Santos, H.; Tirelli, G.; Moreno, L.; Pereira, W.; Bicalho, E. Colored led reduces energy use, affecting lettuce seed germination, growth, and antioxidant activity positively. Rev. Agronegócio Meio Ambiente 2024, 17, e12444. [Google Scholar] [CrossRef]
- Panțer, E.; Pele, M.; Drăghici, E.M. Influence of illumination with LEDs on growth and development of lettuce seedlings. Sci. Pap. Ser. B Hortic. 2015, LIX, 251–253. [Google Scholar]
- Basir, M.H.; Masri, I.N. Effect of Light Emitting Diode (LED) Spectrum at Seedlings Production for Optimal Growth on Different Type of Lettuce in MARDI Plant Factory. Adv. Agric. Food Res. J. 2021, 2, a0000229. [Google Scholar] [CrossRef]
- Joosen, R.V.L.; Arends, D.; Li, Y.; Willems, L.A.; Keurentjes, J.J.; Ligterink, W.; Jansen, R.C.; Hilhorst, H.W. Identifying genotype-by-environment interactions in the metabolism of germinating arabidopsis seeds using generalized genetical genomics. Plant Physiol. 2013, 162, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Rosental, L.; Nonogaki, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Belgaonkar, R.; Patil, S. Review on Impact of Led Lights on Seed Germination and Seedling Growth. Int. J. Sci. Eng. Technol. 2024, 12, 4. [Google Scholar] [CrossRef]
- Stutte, G.W. Light-emitting diodes for manipulating the phytochrome apparatus. HortScience 2009, 44, 231–234. [Google Scholar] [CrossRef]
- Hernández, R.; Eguchi, T.; Kubota, C. Growth and morphology of vegetable seedlings under different blue and red photon flux ratios using light-emitting diodes as sole-source lighting. In Proceedings of the VIII International Symposium on Light in Horticulture 1134, East Lansing, MI, USA, 22 May 2016; pp. 195–200. [Google Scholar]
- Nascimento, W.M.; Cantliffe, D.J.; Huber, D.J. 402 Lettuce Seed Germination and Endo-mannanase Activity are Stimulated by Ethylene at High Temperature. HortScience 1999, 34, 513B–513. [Google Scholar] [CrossRef]
- Al-Kinani, H.A.A.; Jerca, O.I.; Bobuțac, V.; Drăghici, E.M. Comparative study on lettuce growing in NFT and Ebb and Flow System. Sci. Pap. Ser. B Hortic. 2021, LXV, 361–368. [Google Scholar]
- Peñaloza, P.; Ramirez-Rosales, G.; McDonald, M.B.; Bennett, M.A. Lettuce (Lactuca sativa L.) seed quality evaluation using seed physical attributes, saturated salt accelerated aging and the seed vigour imaging system. Electron. J. Biotechnol. 2005, 8. [Google Scholar] [CrossRef]
- Mendoza-Paredes, J.E.; Castillo-González, A.M.; Avitia-García, E.; Valdéz-Aguilar, L.A.; García-Mateos, M.d.R. Effect of different blue: Red LED light ratios on habanero pepper (Capsicum chinense Jacq.) plants. Biotecnia 2021, 23, 110–119. [Google Scholar] [CrossRef]
- Ahsan, S.; Injamum-Ul-Hoque, M.; Shaffique, S.; Ayoobi, A.; Rahman, M.A.; Rahman, M.M.; Choi, H.W. Illuminating Cannabis sativa L.: The power of light in enhancing C. sativa growth and secondary metabolite production. Plants 2024, 13, 2774. [Google Scholar] [CrossRef] [PubMed]
- Trivellini, A.; Toscano, S.; Romano, D.; Ferrante, A. The role of blue and red light in the orchestration of secondary metabolites, nutrient transport and plant quality. Plants 2023, 12, 2026. [Google Scholar] [CrossRef]
- Tehrani, P.F.; Majd, A.; Mahmoodzadeh, H.; Satari, T.N. Effect of red and blue light-emitting diodes on germination, morphological and anatomical features of Brassica napus. Adv. Stud. Biol. 2016, 8, 173–180. [Google Scholar] [CrossRef]
- Choi, H.-S.; Cho, H.-T. Root hairs enhance Arabidopsis seedling survival upon soil disruption. Sci. Rep. 2019, 9, 11181. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef]
- Lymperopoulos, P.; Msanne, J.; Rabara, R. Phytochrome and phytohormones: Working in tandem for plant growth and development. Front. Plant Sci. 2018, 9, 1037. [Google Scholar] [CrossRef]
- Poppe, C.; Sweere, U.; Drumm-Herrel, H.; Schäfer, E. The blue light receptor cryptochrome 1 can act independently of phytochrome A and B in Arabidopsis thaliana. Plant J. 1998, 16, 465–471. [Google Scholar] [CrossRef]
- Sawada, Y.; Aoki, M.; Nakaminami, K.; Mitsuhashi, W.; Tatematsu, K.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Inoue, Y.; Nambara, E. Phytochrome-and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol. 2008, 146, 1386–1396. [Google Scholar] [CrossRef]
- Lee, W.-H.; Zebro, M.; Heo, J.-Y. Light-Emitting Diode Light Quality Influences Germination and Sprout Characteristics of Motherwort. J. Food Qual. 2023, 2023, 4644078. [Google Scholar] [CrossRef]
- Khajeh-Hosseini, M.; Lomholt, A.; Matthews, S. Mean germination time in the laboratory estimates the relative vigour and field performance of commercial seed lots of maize (Zea mays L.). Seed Sci. Technol. 2009, 37, 446–456. [Google Scholar] [CrossRef]
- Matthews, S.; Khajeh Hosseini, M. Mean germination time as an indicator of emergence performance in soil of seed lots of maize (Zea mays). Seed Sci. Technol. 2006, 34, 339–347. [Google Scholar] [CrossRef]
- McDonald, M.B. The history of seed vigor testing. J. Seed Technol. 1993, 17, 93–100. [Google Scholar]
- Qun, S.; Wang, J.-H.; Sun, B.-Q. Advances on seed vigor physiological and genetic mechanisms. Agric. Sci. China 2007, 6, 1060–1066. [Google Scholar]
- Hoque, M.; Islam, M.; Rahman, M.; Biswas, B.; Mahmud, N. Effect of Red, Blue, Green LEDs on the Germination and Seedling Growth of Rice. J. Environ. Sci. Nat. Resour. 2020, 13, 63–69. [Google Scholar] [CrossRef]
- Smith, O.; Welch, N.; McCoy, O. Studies on lettuce seed quality. II. Relationship of seed vigor to emergence, seedling weight, and yield. J. Am. Soc. Hortic. Sci. 1973, 98, 552–556. [Google Scholar] [CrossRef]
- Akbarian, B.; Matloobi, M.; Mahna, N. Effects of LED light on seed emergence and seedling quality of four bedding flowers. J. Ornam. Plants 2016, 6, 115–123. [Google Scholar]
- Hernández-Adasme, C.; Silva, H.; Escalona, V. In-door germination and seedling growth of green and red lettuce under LED-light spectrum and subsequent effect on baby leaf lettuce. Ital. J. Agron. 2022, 17, 1982. [Google Scholar] [CrossRef]
- Davarzani, M.; Aliniaeifard, S.; Mehrjerdi, M.Z.; Roozban, M.R.; Saeedi, S.A.; Gruda, N.S. Optimizing supplemental light spectrum improves growth and yield of cut roses. Sci. Rep. 2023, 13, 21381. [Google Scholar] [CrossRef]
- Fallah, S.; Aliniaeifard, S.; Zare Mehrjerdi, M.; Mirzaei, S.; Gruda, N.S. Night interruption with red and far-red light optimizes the phytochemical composition, enhances photosynthetic efficiency, and increases biomass partitioning in Italian basil. Plants 2024, 13, 3145. [Google Scholar] [CrossRef]
- Miao, Y.-X.; Wang, X.-Z.; Gao, L.-H.; Chen, Q.-Y.; Mei, Q. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED red and blue light component on growth and photosynthetic characteristics of coriander in plant factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Lee, H.; Han, G.; Cheong, E.J. Effect of different treatments and light quality on Ulmus pumila L. germination and seedling growth. For. Sci. Technol. 2021, 17, 162–168. [Google Scholar]
- Samuolienė, G.; Brazaitytė, A.; Urbonavičiūtė, A.; Šabajevienė, G.; Duchovskis, P. The effect of red and blue light component on the growth and development of frigo strawberries. Zemdirb. Agric. 2010, 97, 99–104. [Google Scholar]
- Zare, N.; Ghasemi, H.; Moosavi-Nezhad, M.; Aliniaeifard, S. Growth and Photosynthetic Performance of African Violet in Response to Light Quality and Phytohormones. J. Plant Growth Regul. 2024, 1–15. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H.; Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H. Dormancy and the control of germination. In Seeds: Physiology of Development and Germination, 3rd ed.; Springer: New York, NY, USA, 2013; pp. 247–297. [Google Scholar]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.; Roberts, E. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Yi, R.; Yan, J.; Xie, D. Light promotes jasmonate biosynthesis to regulate photomorphogenesis in Arabidopsis. Sci. China Life Sci. 2020, 63, 943–952. [Google Scholar] [CrossRef]
- Cheng, M.-C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome signaling networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef]
- Zhu, Y. Enhancing the Nutritional Quality of Red Leaf Lettuce by Optimizing End-of-Production Supplemental LED Lighting. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 2024. [Google Scholar]
- Yuan, X.; Hu, J.; Marcelis, L.F.; Heuvelink, E.; Peng, J.; Yang, X.; Yang, Q. Advanced Technologies in Plant Factories: Exploring Current and Future Economic and Environmental Benefits in Urban Horticulture. Hortic. Res. 2025, 12, uhaf024. [Google Scholar] [CrossRef] [PubMed]
- Baghalian, K.; Hajirezaei, M.-R.; Lawson, T. Current and future perspectives for controlled environment agriculture (CEA) in the 21st century. Front. Plant Sci. 2023, 14, 1334641. [Google Scholar] [CrossRef] [PubMed]
- Olle, M.; Viršile, A. The effects of light-emitting diode lighting on greenhouse plant growth and quality. Agric. Food Sci. 2013, 22, 223–234. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Dzakovich, M.P.; Gomez, C.; Lopez, R.; Burr, J.F.; Hernández, R.; Kubota, C.; Currey, C.J.; Meng, Q.; Runkle, E.S. Light-emitting diodes in horticulture. In Horticultural Reviews; Wiley: Hoboken, NJ, USA, 2015; Volume 43, pp. 1–88. [Google Scholar]
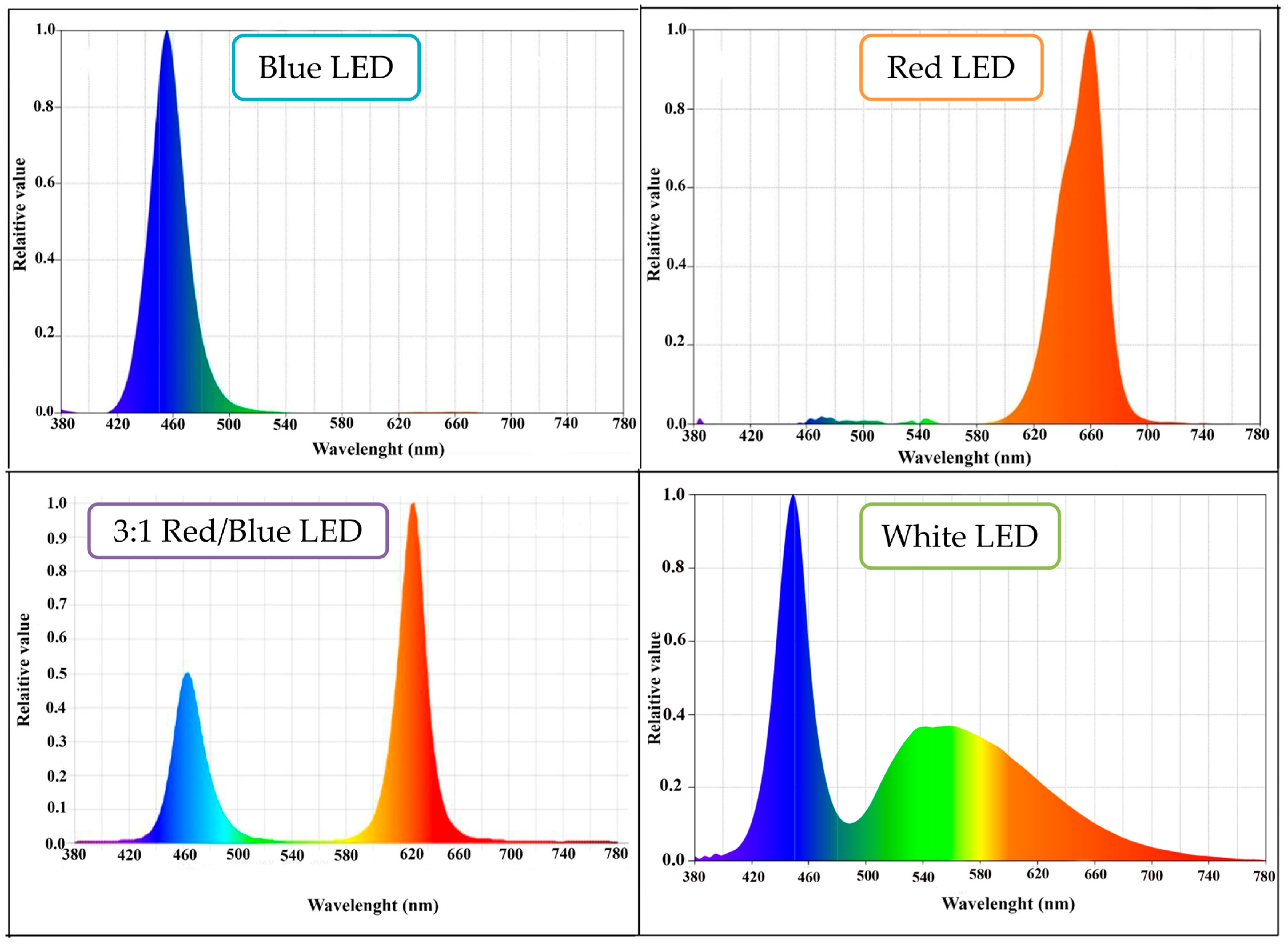




| Manufacturer | CRI (Color Rendering Index) | No. of LEDs | Light Coverage Area | Power Consumption | Lens Type | Input Voltage | DC Voltage | Output Current | Output Frequency |
|---|---|---|---|---|---|---|---|---|---|
| Iran Grow Light | 90% | 24 | 40 × 100 cm | 24 × 3 W | 90° | AC 100–260 V | 54–84 V | 600 mA ± 5% | 50/60 Hz |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soufi, H.R.; Roosta, H.R.; Gruda, N.S.; Khabisi, M.S. Optimizing the LED Light Spectrum for Enhanced Seed Germination of Lettuce cv. ‘Lollo Bionda’ in Controlled-Environment Agriculture. Agronomy 2025, 15, 1219. https://doi.org/10.3390/agronomy15051219
Soufi HR, Roosta HR, Gruda NS, Khabisi MS. Optimizing the LED Light Spectrum for Enhanced Seed Germination of Lettuce cv. ‘Lollo Bionda’ in Controlled-Environment Agriculture. Agronomy. 2025; 15(5):1219. https://doi.org/10.3390/agronomy15051219
Chicago/Turabian StyleSoufi, Hamid Reza, Hamid Reza Roosta, Nazim S. Gruda, and Mahdiyeh Shojaee Khabisi. 2025. "Optimizing the LED Light Spectrum for Enhanced Seed Germination of Lettuce cv. ‘Lollo Bionda’ in Controlled-Environment Agriculture" Agronomy 15, no. 5: 1219. https://doi.org/10.3390/agronomy15051219
APA StyleSoufi, H. R., Roosta, H. R., Gruda, N. S., & Khabisi, M. S. (2025). Optimizing the LED Light Spectrum for Enhanced Seed Germination of Lettuce cv. ‘Lollo Bionda’ in Controlled-Environment Agriculture. Agronomy, 15(5), 1219. https://doi.org/10.3390/agronomy15051219










