Abstract
The long-term arid climate in Xinjiang poses a major challenge to sustainable jujube production. In this study, we systematically evaluated the impacts of deficit irrigation (DI) by comparing a full irrigation control (CK) with six DI treatments—mild DI (75% CK) and severe DI (50% CK) water deficits applied during either flowering + fruit setting or fruit enlargement stages. The key findings demonstrate that flowering + fruit setting DI effectively balances water conservation with productivity. Mild DI (75% CK) during flowering + fruit setting reduced irrigation by 72 mm while maintaining near-optimal photosynthesis (95% recovery post-rewatering) and significantly improving fruit quality (5.49–10.28% higher sugar content, 3.40–5.06% larger fruit volume), despite a moderate 4.22–11.36% yield reduction. In contrast, severe DI caused irreversible physiological stress (only 75% photosynthetic recovery), and fruit-enlargement-stage DI uniformly compromised both yield and fruit size. An economic analysis confirmed flowering + fruit setting mild DI as optimal, generating 17,139–20,550 RMB·ha−1 profit through enhanced water use efficiency (WUE) and premium-quality fruit production. PLS-PM validation revealed that targeted flowering + fruit setting water deficit suppresses vegetative overgrowth while optimizing source–sink relationships, achieving a 23–31% WUE improvement without sacrificing marketable yield. Thus, mild DI during flowering + fruit setting is a climate-smart irrigation strategy for Xinjiang’s jujube industry, resolving water scarcity challenges with economic viability.
1. Introduction
Jujube (Zizyphus jujuba Mill.), a member of the Rhamnaceae family, is indigenous to China, Mongolia, and Central Asia. It is recognized as one of the characteristic forest fruits of Xinjiang. By the end of 2020, the total cultivation area of jujube in Xinjiang reached 413,495 hectares [1]. Jujube production in Xinjiang accounts for over 50% of the national total and contributes significantly to more than one-third of the increase in farmers’ income. The jujube planting industry has emerged as a key driver of rural economic growth. However, Xinjiang is situated in the interior of Eurasia, far from the ocean, and faces severe water scarcity with an average annual rainfall of only approximately 74.4 mm. The lack of water is a critical factor impacting crop growth in the region. To mitigate this issue, the promotion of deficit irrigation techniques, which offer advantages in water conservation, yield enhancement, and quality improvement, can partially alleviate water scarcity and support the rational and sustainable development of agriculture [2,3].
Deficit irrigation (DI) is an effective water-saving irrigation technique that artificially applies a water deficit during certain growth stages of crops to affect the distribution ratio of photosynthetic products to different tissues and organs, thereby improving crop yield, fruit quality, and water use efficiency [4]. This method specifies growth stages of crops, leading to physiological changes and differential water distribution, ultimately achieving water conservation and enhanced economic benefits [5,6]. Under water deficit stress, crops regulate the activity of key enzymes to cope with drought stress [7]. This is specifically manifested as a significant increase in levels of soluble sugars, proline, electrolyte leakage, malondialdehyde, and antioxidant enzyme activity in the leaves [8]. Upon alleviating drought stress, crops demonstrate remarkable recovery, with the degree of recovery negatively correlated with the level of water deficit, a phenomenon known as the water deficit compensation effect [9,10]. In summary, an appropriate water deficit can control plant vigor, consequently reduce pruning intensity, and increase the allocation ratio of photosynthetic products to reproductive organs [11]. This process ultimately aims to reduce pruning intensity, increase crop yield, improve fruit quality, and enhance water use efficiency [12,13]. However, the extent of this effect and the adaptability to water stress vary significantly among species such as peach trees [14], apple trees [15], and pear trees [16]. Notably, jujube trees exhibit increased water retention due to the waxy layer on their leaves, resulting in physiological responses to water regulation that differ from those of other fruit trees. Therefore, further research is necessary to explore the physiological regulation mechanisms of jujube trees under DI.
The flowering, early fruit, and fruit enlargement stages are critical for reproductive development in fruit trees. García-Tejero et al. [17] indicated that water deficits applied during the flowering stage can lead to a decrease in fruit quantity, thereby reducing crop yield. However, water deficits during the fruit enlargement stage are more likely to have a significant impact on yield. Some researchers have found that walnut (85% ETc) [18], pear trees (75% ETc) [19] and Mandarin trees (50% ETc) [20] may not experience a considerable reduction in yield when subjected to water deficits during the fruit enlargement stage. Furthermore, the water requirements of fruit trees can vary at different growth stages. For instance, Johnson et al. [21] noted that different crops exhibit varying responses to the compensatory effects of water deficits, highlighting species-specific and environmental variability. In addition to meteorological factors, the primary factors influencing yield response are crop type and soil texture. Increased irrigation frequency can lead to substantial yield differences among crops [22]. Additionally, some studies suggest that while water deficits during the flowering stage decrease fruit numbers, they significantly enhance crop quality, ultimately resulting in higher economic returns [23]. Therefore, the growth stage at which DI is applied has a significant impact on crop growth and yield. The timely and appropriate application of DI is crucial for regulating fruit growth and improving yield.
To address the critical need for optimizing deficit irrigation (DI) strategies in fruit tree cultivation, this study specifically investigates how jujube (Ziziphus jujuba Mill.) growth and yield respond to water deficits, with the ultimate goal of developing region-specific DI protocols that maximize water-use efficiency while maintaining productivity. The objectives of this study are (1) to quantitatively assess the effects of water deficits on physiological parameters and yield across different phenological stages using comparative analysis and partial least squares path modeling (PLS-PM); (2) to uncover the physiological and biochemical mechanisms that enable water conservation and sustained yield under DI conditions; and (3) to propose a suitable deficit drip irrigation model for jujube trees, thereby promoting the sustainable and high-quality development of the jujube industry in Xinjiang.
2. Materials and Methods
2.1. Study Location
The experiment was conducted at the fruit industry trial base of Xinjiang Agricultural University, located in Aksu Region, Xinjiang, from 2020 to 2021 (80°14′ E, 41°16′ N, altitude: 1133 m). The soil texture is mainly sandy loam (Table 1). This region has a typical temperate continental arid desert climate (Köppen climate classification: Bwk), characterized by significant temperature variations between day and night. In 2000–2020, the average annual precipitation is 74.41 mm, with an average annual temperature of 11.22 °C, minimum temperature of −15.32 °C, and maximum temperature of 41.58 °C. The annual total solar radiation ranges from 544.12 to 590.16 kJ·cm−2, while the annual sunshine duration varies between 2855 and 2967 h. The frost-free period lasts from 205 to 219 days, and the average annual effective accumulated temperature is 3950 °C. From 1 April to 31 October 2020 and 2021, the reference crop evapotranspiration (ET0) [24] in the experimental area ranged from 657.12 to 662.00 mm, with rainfall varying from 72.40 to 72.88 mm (Figure 1).

Table 1.
Physico-chemical parameters of the soil in the study area.
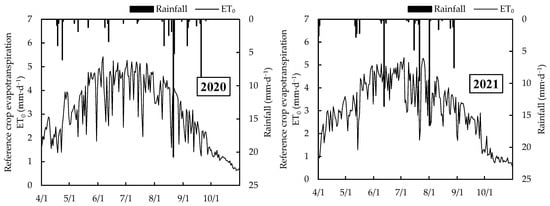
Figure 1.
The reference crop evapotranspiration and rainfall from 2020 to 2021.
2.2. Experimental Design
The tested crop was a 7-year-old gray jujube tree planted with a row spacing of 4 m × 1 m. Drip irrigation was employed, with the drip irrigation tape positioned 40 cm away from the jujube tree. The distance between drippers was 30 cm, with a flow rate of 2.3 L·h−1. Irrigation occurred every 7 days, totaling 17–18 sessions throughout the growth period, including 70 mm for spring irrigation and 30 mm for the fruit maturity stage. For the irrigation quota ETc = ET0 × Kc, we utilized the Penman–Monteith formula to calculate cumulative evapotranspiration for the preceding irrigation cycle ET0. The crop coefficient (Kc) was derived based on the research findings of Hong et al. [25]. In this study, we systematically evaluated the impacts of deficit irrigation (DI) by comparing a full irrigation control (CK) with six DI treatments, each with three replicates, following a randomized complete block design. The irrigation schedules for each treatment are detailed in Table 2. Each treatment area was 30 m long and 20 m wide, with a total of 150 trees. This experiment used drip irrigation for fertilization. The fertilization amount and agronomic measures were the same as those of other local agricultural growers.

Table 2.
Jujube irrigation system under different water deficit conditions.
2.3. Measurements
2.3.1. Crop Water Consumption and Evapotranspiration
Previous studies have shown that the water-absorbing roots of young jujube trees (diameter < 2 mm) are primarily concentrated in the 0 to 100 cm soil layer. Consequently, this study was focused on changes in soil moisture within this layer corresponding to the jujube root zone. The evapotranspiration of the jujube root zone was calculated using the water balance method [26]. A transparent plastic tube (TRIME tube) with a length of 150 cm was vertically positioned at distances of 30 cm and 50 cm between jujube plants, as well as at 30 cm, 50 cm, and 70 cm from the jujube row. The TRIME-IPH (TRIME-PICO-IPH, Channel Tech, Beijing, China) soil moisture measurement system was inserted into the TRIME tube to measure soil moisture at depths with ranges of 0–10, 10–20, 20–30, 30–40, 40–50, 50–60, 60–70, 70–80, 80–90, and 90–100 cm. An average soil moisture content of 0–100 cm from five pipes was taken as the soil moisture content for this experiment. The measurements were taken before and after each watering session. If there were changes in the heavy rainfall, additional testing was also required. The evapotranspiration (ET) of the jujube root zone was calculated using the water balance method, which dictates that
where
ET = I + P − ΔS − R − D
ET = crop evapotranspiration (mm);
I = the irrigation amount (mm);
P = the rainfall amount (mm);
ΔS = the change in soil moisture storage (mm);
R = the surface runoff (mm);
D = the deep percolation (mm).
2.3.2. Soil Evaporation
Soil evaporation (E) was determined using micro-lysimeters, which were prepared in-house from a PVC tube. The micro-lysimeters contained small, isolated volumes of bare soil mounted flush with or slightly above the soil surface, they and were weighed, using electronic balances with 0.001 kg precision, daily at 10:00 to determine water loss. The measurement duration is the entire reproductive period. Each micro-lysimeter was 11 cm in diameter and 15 cm in depth. The outer barrel was 12 cm in diameter and 20 cm in depth. Micro-lysimeter placement was targeted at 40 cm of the jujube row spacing and 50 cm of individual jujube plant spacing. The mean value measured by the micro-lysimeters was reported as the soil water evaporation.
2.3.3. Leaf Area Index
The leaf area index of the jujube was measured using a HemiView plant canopy analyzer (HMV1 v9, Harvesting Tech, Beijing, China). Each data acquisition period occurred before 9:00 a.m. (when there was no strong direct beam solar radiation). Beginning May 9, the leaf area index was determined every 7–10 days until the end of the growing season based on measurements from three jujube plants from each treatment.
2.3.4. Photosynthesis Parameters
The net photosynthetic rate, transpiration rate, and stomatal conductance of jujube leaves were measured using the CIRAS-3 photosynthesis measurement system (CIRAS-3, PP Systems, Mass., Amesbury, MA, USA). For each measurement, three south-facing leaves were selected. On the previous day at 12:00 p.m., the photosynthetic response curve was established to determine the optimal light intensity. On the measurement day, the built-in light source of the photosynthesis measurement system was adjusted to the optimal light intensity for the leaves, comprising 90% red light, 5% blue light, and 5% white light. Measurements were taken 1 to 2 times during the mid-stage.
3 + 3 of each growth phase, and the average was used as the corresponding photosynthetic parameter for that growth period.
2.3.5. Enzyme Activity Assay
The activities of two key enzymes, superoxide dismutase (SOD) and peroxidase (POD), were examined. Here, 1.0 g of fresh sample was obtained, and 9 mL of physiological saline was added. The sample was ground thoroughly and transferred to a centrifuge tube, where it was centrifuged (TGL-16M, JingxinTech, Beijing, China) at 1000× g at minus 4 °C for 20 min, and the supernatant was taken for testing. The absorbance (OD value) was measured at a wavelength of 450 nm using a light absorption full-wavelength microplate reader (ReadMax 1900, Shanpu Technology, Beijing, China). Three biological replicates were set for each treatment, and a standard curve was drawn to calculate the enzyme content. Measurements were taken once during the mid-term of each reproductive period.
2.3.6. Yield and Yield Components
For each replicate, three randomly selected jujube trees were used to measure individual yield, which was then extrapolated to represent the total yield of that replicate. Additionally, from each jujube tree, 20 fresh fruits were randomly selected using a quadrat sampling method. The longitudinal and transverse diameters of the fruits were measured, along with the fresh weight of each fruit.
After harvest, some fruits were stored in an insulated box at 3 °C for a specific duration prior to conducting quality assessments. The quality indicators measured included total sugar, reducing sugar, sucrose, total acidity, and vitamin C. Vitamin C content was determined using the 2,6-dichlorophenolindophenol (DCPIP) titration method. Total sugar, reducing sugar, and sucrose contents were measured using the phenol–sulfuric acid colorimetric method. Total acidity was assessed using acid–base titration methods.
The WUE and IWUE of jujube were calculated using the following equation [27]:
where
WUE = JY/ET
IWUE = JY/IW
WUE is the water-use efficiency for GY, (kg·ha−1·mm−1);
IWUE is the irrigation water-use efficiency for GY, (kg·ha−1·mm−1);
JY is jujube yield, (kg·ha−1);
ET is evapotranspiration of jujube during the growing season (mm);
IW irrigation water of jujube during the growing season (mm).
2.3.7. Meteorological
Meteorological data, including temperature, radiation, and rainfall, were measured every 30 min using a Watchdog small automatic weather station (Model 2700, Spectrum Technologies, Aurora, IL, USA).
2.4. Economic Calculation Formula
The calculation formula for farmland output value M and net income NI is as follows:
where Pi is the yield of jujube trees, kg·ha−1; and Yi represents the unit price of jujube fruit, RMB·kg−1.
M = Pi × Yi
NI = M − Iw − Ip − Io
This study established the unit prices of jujube fruits of varying volumes according to local simple grading standards, as illustrated in Table 3.

Table 3.
Simple classification of unit price of gray jujube in Aksu, China.
Iw represents the cost of irrigation water, RMB/ha. This study involved a survey of the surrounding irrigation areas and set the water price at 0.3 RMB·m−3.
Ip represents the fertilizer cost, RMB·ha−1. In this study, it was calculated based on local fertilizer prices, which were set at 6300 RMB·t−1.
Io represents other expenses, including spraying, weeding, and pruning (RMB/ha). In this study, local market prices are referenced, and the average for the period from 2020 to 2021 are calculated.
2.5. Data Analysis
The statistical analysis was conducted using DPS (PDS Inc., Rui Feng Information Technology Co., Ltd., Beijing, China). Two-way ANOVA was used to evaluate the variance of the statistical difference, and the means were separated using the least significant difference at a significance level of 5%.
3. Results
3.1. Evapotranspiration
Based on Figure 2, during the flowering stage, there was no significant difference in ET (evapotranspiration) between the mild DI and adequate irrigation treatments (p > 0.05). Severe DI led to a decrease within 2 years in ET of 11.18% to 18.62%. When the water deficit continued into the fruit setting stage, significant differences were observed among treatments C1, C2, and C3 (p < 0.05). This suggests that jujube trees possess a certain level of resistance to environmental stress; however, as the severity and duration of the deficit increase, the trees make adaptive adjustments to cope with external changes. Upon rehydration during the fruit enlargement stage, jujube trees exhibited a compensatory response to water deficit stress, resulting in a rapid recovery of ET in treatments C2 and C3. Although no significant difference was observed between treatments C1 and C2 (p > 0.05), treatment C3 still demonstrated a significant difference of 5.01% to 7.11% compared to C1 (p < 0.05).
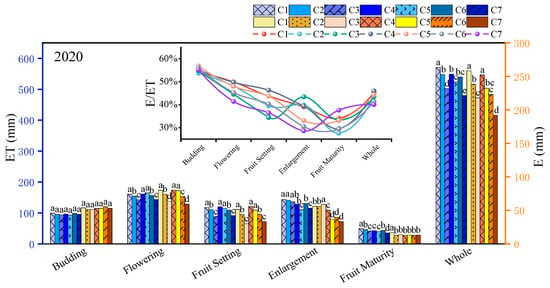
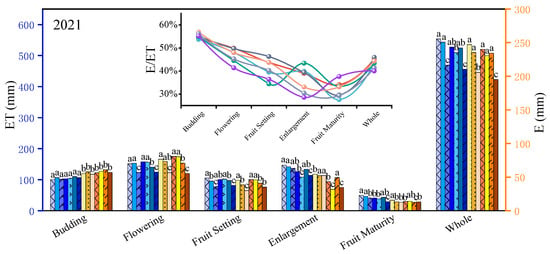
Figure 2.
Variation law of jujube evapotranspiration under deficit irrigation in different growth stages in 2020–2021. Note: ET, evapotranspiration, mm; E, soil evaporation, mm; E/ET, soil evaporation/evapotranspiration. Different lowercase letters in the same column indicate significant difference at the 5% level.
Following the application of DI during the fruit enlargement stage, the ET in treatments C4 and C5 decreased by 10.59% to 14.27% and by 22.92% to 25.98%, respectively. This decrease was significantly greater than that observed during the flowering + fruit setting stage, indicating that the fruit enlargement stage is more sensitive to water deficit. Additionally, comparisons of ET rates among treatments C4, C5, C6, and C7 during the fruit enlargement and maturity stages reveal that jujube trees can enhance drought resistance following prolonged exposure to mild DI. This improvement is attributed to increased root activity and a significantly enhanced capacity for water absorption following drought stress. However, prolonged severe deficit (C7) can inflict serious and irreparable damage to the trees, resulting in a significantly lower compensatory response in treatment C7 compared to treatment C5 (p < 0.05).
Furthermore, under water deficit conditions, the soil evaporation of jujube trees also showed a significant downward trend (p < 0.05). Overall, soil evaporation decreased as the irrigation quota decreased. The treatment with the highest soil evaporation was the control group (C1), accounting for 44.65% to 45.27% of the total evapotranspiration. The treatment with the lowest soil evaporation was C7, where soil evaporation accounted for 40.00% to 42.78% of the total evapotranspiration.
3.2. Growth Indices
Based on Table 4, DI during the flowering + fruit setting stage (C2, C3) significantly reduces the leaf area index, jujube fruit length, and annual increment of stem diameter by between 3.01% and 14.55% and decreases annual growth in plant height by between 10.95% and 26.17%. In contrast, DI during the fruit enlargement stage (C4, C5) results in a decrease of only 0.42% to 4.82% in the leaf area index, jujube fruit length, and annual increment of stem diameter, which is significantly lower than the effects observed during the flowering + fruit setting stage. The impact of DI during the flowering + fruit setting stage on various physiological indicators is notably greater than that during the fruit enlargement stage (p < 0.05). This analysis indicates that the flowering and fruit setting stages primarily facilitate vegetative growth. Introducing DI during these periods inhibits plant nutrient growth, reduces growth redundancy, and promotes nutrient storage in branches, ultimately enhancing fruit yield and quality later on. Conversely, the fruit enlargement stage focuses on reproductive growth, and the effect of DI on plant height and stem diameter is minimal during this stage.

Table 4.
Growth indices of jujube under different deficit irrigation (DI) treatments.
3.3. Photosynthesis
As shown in Figure 3, during the bud stage, all treatments received full irrigation, resulting in net photosynthetic rates that were generally consistent, ranging from 12.14 to 13.55 µmol/(m2·s). However, as the jujube trees progressed into the flowering + fruit setting stage, they began to experience the effects of water deficit. The crop possesses an adaptive regulatory mechanism that can mitigate or delay damage from water deficiency. No significant difference in net photosynthetic rates was observed between treatments C1 and C2 during the flowering stage (p > 0.05). In contrast, treatment C3, which experienced a greater degree of deficit, exhibited a significant decrease in net photosynthetic rate, declining by 10.71% relative to the other treatments (p < 0.05). As the duration of the water deficit increased, the adverse effects on leaves from mild deficit became increasingly evident. By the fruit setting stage, the reduction in net photosynthetic rate for treatment C2 had expanded to 9.85%, while for treatment C3, it increased to 17.55%. Following complete irrigation during the fruit enlargement stage, the net photosynthetic rates for treatments C2 and C3 showed a recovery, with treatment C2 recovering at a significantly higher rate than treatment C3. By the maturity stage, no significant difference was found between treatments C1 and C2 (p > 0.05), although both treatments differed significantly from treatment C3 (p < 0.05). This discrepancy may be attributed to the prolonged severe water stress, which caused substantial damage to the photosynthetic system of jujube leaves, limiting the effectiveness of the crop’s “self-repair” mechanisms.
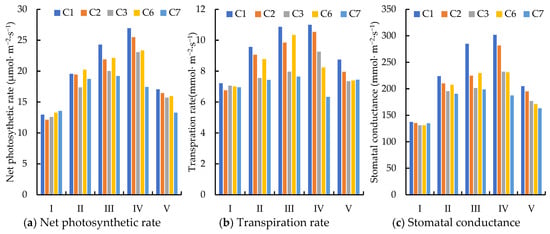
Figure 3.
Effect of deficit irrigation at flowering + fruit setting stage on photosynthetic characteristics of jujube. Note: I, budding; II, flowering; III, fruit setting; IV, fruit enlargement; V, fruit maturity.
Following DI, the transpiration rates of treatments C2 and C3 decreased compared to treatment C1, with the decline becoming more pronounced as the growth period progressed. The transpiration rate for treatment C2 decreased by 5.33% during flowering and by 9.39% at fruit set, while treatment C3 experienced a drop from 20.92% at flowering to 26.61% at fruit set, indicating a significant decrease (p < 0.05). After rehydration during the fruit enlargement stage, the transpiration rates of all treatments began to recover. However, by the maturity stage, the transpiration rate of treatment C2 remained 4.19% lower than that of treatment C1. The leaf transpiration rate for treatment C3 recovered more slowly, and despite rehydration, it was still 15.83% lower than that of treatment C1. Thus, the earlier water stress significantly affects the stomatal aperture of the leaves, resulting in a lower transpiration rate at maturity compared to the control group (p < 0.05).
According to Figure 4, following water deficit, the net photosynthetic rates under C4 and C5 treatments decreased by 7.98% and 21.75%, respectively. These reductions are significantly greater than the decreases observed in the photosynthetic index during the flowering + fruit setting stage, indicating that the fruit enlargement stage is more sensitive to water deficit. A comparison of the C6 and C7 treatments under long-term deficit conditions reveals no significant difference in net photosynthetic rate, transpiration rate and stomatal conductance between long-term mild water stress and short-term water stress (p > 0.05).
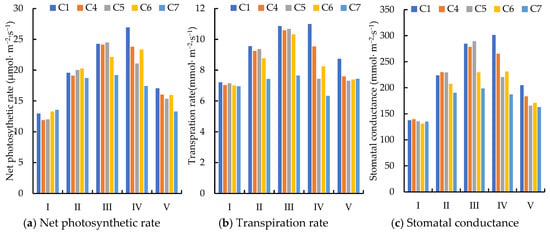
Figure 4.
Effect of deficit irrigation during fruit enlargement stage on photosynthetic characteristics of jujube. Note: I, budding; II, flowering; III, fruit setting; IV, fruit enlargement; V, fruit maturity.
3.4. Superoxide Dismutase and Peroxidase Content
Based on Table 5, under sufficient irrigation conditions, the SOD (superoxide dismutase) levels in jujube tree leaves exhibit a downward trend, whereas the POD (peroxidase) levels show an upward trend. When mild DI was imposed during the flowering period of jujube trees, no significant differences were observed in SOD levels. However, during the young fruit period, significant differences were noted. Water deficit results in an increase in SOD activity in the leaves of jujube trees, while rehydration leads to a rapid decline in SOD activity. Severe DI has a more pronounced impact on the leaves of jujube trees; specifically, the application of moderate water deficit during the flowering period results in significant differences in SOD levels (p < 0.05). After rehydration, SOD levels remain higher than those under sufficient irrigation conditions. Furthermore, comparisons between treatments C1 and C2 indicate that POD levels are more sensitive to water deficit than SOD levels, with any application of water deficit leading to significant differences in POD levels.

Table 5.
Effect of deficit irrigation treatments on superoxide dismutase (SOD) and peroxidase (POD) in leaves in 2020 and 2021.
3.5. Yield and Quality
According to Table 6, various levels of DI during different growth stages significantly influence the total sugar, total acid, and vitamin C content in fruits. A two-year experimental study indicated that implementing DI during the flowering + fruit setting stage markedly increased the total sugar and vitamin C content, with greater deficits corresponding to more pronounced increases. Under mild DI conditions during the fruit enlargement stage, the total sugar content of the fruits significantly rose, whereas severe DI resulted in a decrease in total sugar content. Overall, compared to the control group (C1 treatment), mild and severe DI during the flowering + fruit setting stage led to increases in total sugar content of 5.49% and 10.28%, respectively, while the total acid content decreased by 7.55% and 11.13%. Additionally, vitamin C content showed a slight increase in −1.02% and 7.16%. When mild DI was applied during the fruit enlargement stage, the total sugar content increased by 8.36%, total acid content decreased by 1.92%, and vitamin C content decreased by 1.41%. Conversely, under severe DI conditions, the total sugar and vitamin C content decreased by 6.12% and 12.69%, respectively, while the total acid content increased by 9.34%.

Table 6.
Effects of deficit irrigation on fruit quality in two years (2020–2021).
3.6. Economic Benefits
Based on Table 7, it seems that varying degrees of DI at different growth stages significantly impact the yield and economic benefits of jujube trees. A comparison of fruit volume and yield across different treatments reveals that DI during the flowering and early fruiting stages (C2 and C3 treatments) led to an increase in fruit volume by 3.40% to 5.06%, while simultaneously causing a decrease in jujube yield by 4.22% to 11.36%. Mild DI until the fruit enlargement stage (C6 and C7 treatments) under mild deficit conditions resulted in a 3.48% increase in fruit volume but a 6.96% decrease in fruit yield. In contrast, severe DI during this period resulted in a 26.44% decrease in fruit volume and a 21.50% decrease in jujube yield. When DI were applied exclusively during the fruit enlargement stage (C4 and C5 treatments), fruit volume decreased by 4.60% to 8.55%, and jujube yield decreased by 4.84% to 12.22%.

Table 7.
Jujube yield and economic benefits under deficit irrigation in two years (2020–2021).
The economic benefit analysis of jujube tree yield (Table 7) reveals that in 2020, the agricultural input–output ratio ranged from 1.46 to 2.52, with expected economic benefits of between RMB 4656 and 17,139. In 2021, driven by an increase in jujube tree yield, the agricultural input–output ratio rose to between 1.57 and 2.78, with expected economic benefits ranging from RMB 5787 to 20,803. Although DI may reduce the yield of jujube trees, the increase in fruit volume can enhance the price of dried jujubes, thereby improving economic benefits and supporting the application of DI. A comparison of the economic benefits across different treatments shows that in 2020, treatment C2 yielded the highest economic benefit at 17,139 RMB·ha−1, followed by treatments C1 and C3, which ranged from 14,408 to 14,783 RMB·ha−1. Treatment C7 recorded the lowest economic benefit at 7595 RMB·ha−1. In 2021, treatments C1 and C2 achieved the best economic benefits, ranging from 20,055 to 20,803 RMB·ha−1, while treatment C3 followed with an economic benefit of 18,259 RMB·ha−1. Treatment C7, again, had the lowest economic benefit at 5787 RMB·ha−1.
3.7. Correlation Analysis of Growth Index with Yield and Quality Based on Partial Least Squares Path Modeling (PLS-PM)
A partial least squares path modeling (PLS-PM) [28] approach was employed to analyze the relationships between growth indicators during the flowering + fruit setting stage (C1, C2, C3) and the fruit enlargement stage (C1, C4, C5), as well as their impacts on yield and quality. As depicted in Figure 5a, under water deficiency conditions, the direct effect coefficient of water consumption during the flowering + fruit setting stage on physiological indicators was 0.872 (p < 0.01), while the corresponding value during the fruit enlargement stage was only 0.245. Furthermore, the direct effect coefficient for both growth periods was negative (β = −0.345), indicating significant differences in the driving factors for the growth and development of jujube trees between the flowering + fruit setting stage and the fruit enlargement stage. Although growth parameters during the flowering + fruit setting stage contributed to a slight increase in jujube yield (direct effect coefficient 0.319), they exhibited a significant negative impact on quality (−0.613). This suggests that inhibiting growth during the flowering + fruit setting stage may positively affect fruit quality. Moreover, as illustrated in Figure 5b, the physiological indicators during the fruit enlargement stage were primarily driven by the irrigation quota for that growth period (direct effect coefficient 0.915). Additionally, growth performance during the fruit enlargement stage significantly influenced the yield and quality of jujube trees, with direct effects of 0.825 and 0.773, respectively, which were markedly higher than contributions from other growth stages. Thus, physiological indicators during the fruit enlargement stage are closely associated with fruit yield and quality, categorizing it as a water-sensitive period.
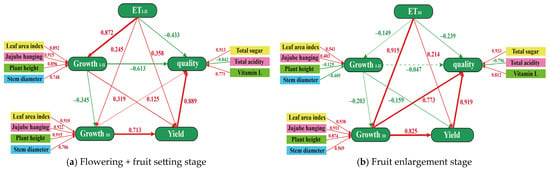
Figure 5.
Correlation analysis of growth index with yield and quality based on partial least squares path modeling (PLS-PM).
4. Discussion
Water scarcity is the most significant environmental factor limiting agricultural development in Xinjiang. Enhancing the efficiency of water resource utilization and achieving high economic returns are the primary objectives of modern agricultural production. Severe drought stress can inhibit the growth of aboveground nutrient organs and decrease the leaf area index [29]. Ballester et al. [30] noted that lower soil moisture content can modify a plant’s osmotic pressure, leading to greater accumulation of photosynthetic products in the trunk and a reduction in branches and leaves [31]. This study supports these findings: when jujube trees experience mild water shortages, the growth rates of pendant length, plant height, and LAI values are all significantly reduced. Concurrently, to enhance the growth and water absorption capacity of underground roots, some photosynthetic products are redirected to the trunk [32], which is least affected by water stress. However, severe water deficiency markedly impedes plant growth, resulting in a decline in various physiological indicators. Existing research indicates that water deficiency can trigger an increase in biochemical markers such as malondialdehyde, superoxide dismutase, and peroxidase, leading to a substantial accumulation of reactive oxygen species in the cell fluid, which damages the cell membrane [33]. This study found that a moderate water deficit level can rapidly increase the content of antioxidant enzymes (such as SOD and POD) in plant organs, helping them resist biotic stress. Furthermore, rehydrating crops after a period of water deficit significantly enhances their ability to cope with drought stress [34]. Antioxidant enzymes primarily protect plant cells by eliminating ROS [35] and, therefore, in some studies, the activity of SOD or POD is defined as an indicator of crop stress resistance. Additionally, in some studies, it has been found that while POD eliminates ROS, it also helps to increase the concentration of cell sap and to adjust the pressure difference inside and outside root tip cells [36] and enhance their passive water absorption capacity. However, the production of peroxidases generated by plants is limited to a certain level. Severe water deficits can lead to a sharp increase in ROS levels; simultaneously, insufficient water can hinder the production of peroxidases, resulting in significant damage to plant cells [37]. Additionally, the energy flow absorbed by PS II pigments in plant leaves directed towards the photochemical process decreases, lowering cellular energy consumption, increasing the efficiency of light energy utilization in non-photochemical quenching (NPQ), thereby protecting the photosynthetic system [38]. Following rehydration treatment, the activity of plant peroxidase increases significantly, enhancing the rate of cell metabolism and crop resistance and gradually restoring other biochemical indicators in the crops to their initial levels [39]. Nevertheless, the intensity of compensation for water deficiency varies among different crops, with jujube trees demonstrating greater resilience to water shortages compared to other fruit trees.
When plants are subjected to water stress, the roots produce a signaling substance (abscisic acid, ABA) that is transported to the aboveground parts, causing a decrease in the transpiration rate, photosynthetic rate, and chlorophyll content [40]. Candar et al. [41] found that crops exhibit a phenomenon known as “overcompensation” after rehydration, which is beneficial for the distribution and accumulation of photosynthetic products. Tognetti et al. [42] observed that, under physiological water stress, the stomatal conductance of leaves initially begins to decline, followed by decreases in the transpiration rate and photosynthetic rate. This study indicates that water deficit prompts a downward trend in the photosynthetic rate, stomatal conductance, and transpiration rate of jujube leaves, but the decrease is relatively small and slightly lower than that of other plants. For instance, the net photosynthetic rate in this study decreased by only 10% to 20% under deficit conditions, while studies by other researchers often report decreases of 20% to 30% [43]. This may be attributed to the presence of a waxy layer on both sides of jujube leaves that prevents water loss, resulting in a relatively lower decline. Water deficit has a significant impact on both the chlorophyll content of plant leaves and the growth of shoots. However, when comparing the dates at which chlorophyll content and shoot growth begin to be affected by water deficit, it was found that water stress first inhibits the growth of plant shoots, followed by adaptive adjustments to chlorophyll content to reduce photosynthetic intensity and water loss. Thus, the physiological adjustments made by plants in response to water deficit occur in a certain order, rather than simultaneously across all organs. Conversely, severe DI inflicts significant damage on the photosynthetic apparatus in leaves, resulting in the functional decline of both the stomata and leaf hydraulic systems before cell integrity is compromised. These detrimental effects are irreversible, even following short-term rehydration treatment [44]. Under mild water restriction, the plant’s adaptive mechanisms inhibit shoot growth to prioritize fruit development; however, excessive water deficit can adversely affect the plant, leading to cumulative damage that significantly impact fruit volume and quality, ultimately resulting in decreased yield.
The effects of DI applied at different growth stages on the growth of perennial fruit trees are complex. As noted by Poomkokrak et al. [45] and Mitra et al. [46], imposing water stress during the flowering stage can significantly reduce the number of fruits while increasing the weight of individual fruits. The results of this study align with these findings. When water stress occurs during the flowering + fruit setting stage, fruit trees demonstrate a decrease in fruit quantity, an increase in individual fruit weight, and an enhancement in fruit quality, thereby improving overall economic benefits [47]. Proper deficit treatment curtails the vegetative growth of crops, allowing for a greater allocation of photosynthetic products to flowers or fruits, thus enhancing yield and quality. However, under severe deficit conditions, there is a notable decrease in yield. This may result from the impact of severe deficit on photosynthesis, which outweighs its regulatory effects on vegetative and reproductive growth [48]. The fruit enlargement stage is critical for the water requirements of jujube trees; DI can severely inhibit the division and expansion of fruit flesh cells, as well as their absorption of water and nutrients, leading to reduced yield. This finding is consistent with Geerts et al. [49], who indicated that rewatering during the maturity stage of fruit trees has no significant impact on jujube yield. The fruit maturation stage is not a critical period for the water needs of jujube trees, as the growth of the flesh tissue is nearly complete, and DI has minimal effects on yield. Furthermore, as a perennial fruit tree, the age of jujube trees can influence their overall growth and development. Although the two-way ANOVA conducted in this experiment confirmed that the data involved in this study showed no significant interannual differences, some studies have indicated that as fruit tree age increases, the stress resistance gradually improves, thereby affecting the impact of water deficit on jujube trees.
5. Conclusions
This study involved the systematic evaluation of stage-specific deficit irrigation (DI) strategies for jujube trees, demonstrating that mild DI during flowering + fruit setting (70% field capacity) optimally balances water conservation with productivity, reducing irrigation by 72 mm while maintaining 97% of the full yield and improving fruit quality (soluble solids increased by 1.2–1.8°Brix). Physiologically, severe DI (≤50% field capacity) impaired stomatal conductance (28–41% reduction) and transpiration, limiting photosynthetic recovery to 75% post-rewatering versus 95% under mild DI, indicating irreversible hydraulic damage under prolonged stress. Stage-dependent responses emerged: flowering-stage DI minimized impacts on LAI (8% reduction) and photosynthesis (12% reduction), whereas fruit-enlargement-stage DI disproportionately affected structural growth (stem thickness: 18% reduction; jujube hanging: 22% reduction), aligning with vegetative–reproductive partitioning dynamics. An economic analysis identified flowering-stage DI as the most viable strategy, generating net profits of 17,139–20,550 RMB·ha−1, while fruit-enlargement-stage DI risked yield penalties (5–15% reduction) due to water sensitivity during rapid fruit expansion. These findings underscore the importance of precision irrigation scheduling by growth stage, with future research needed to integrate real-time soil–plant sensors for dynamic DI threshold adjustments and genotype-specific protocol optimization.
Author Contributions
Conceptualization, P.A.; methodology and software, W.Q.; supervision, Y.M. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Autonomous Region Youth Science Foundation (Grant No. 2023D01B28); the Central Guidance on Local Science and Technology Development Fund (Grant No. ZYYD2023A10); the National Natural Science Foundation of China (Grant No. 52069027); the Talent Development Fund of the Autonomous Region’s Tianchi Talents Introduction Program.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. Regretfully, the data are not publicly available due to the policies and confidentiality agreements adhered to in the laboratory.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- SBX. Xinjiang Statistical Yearbook in 2019; China Statistical Publishing House: Urumqi, China, 2020.
- Adet, L.; Rozendaal, D.; Tapi, A.; Zuidema, P.; Vaast, P.; Anten, N. Negative effects of water deficit on cocoa tree yield are partially mitigated by irrigation and potassium application. Agric. Water Manag. 2024, 296, 108789. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; Morote, E.; Navarro, J.M. Optimizing deficit irrigation in Monastrell vines grafted on rootstocks of different vigour under semi-arid conditions. Agric. Water Manag. 2024, 292, 108669. [Google Scholar] [CrossRef]
- Poma-Chamana, R.; Flores-Marquez, R.; Cordova-Tadeo, J.; Quello, A.; Arapa-Quispe, J.; Solórzano-Acosta, R. Transformation of Terraces with Irrigation Systems: Profitability and Water Savings in Potato Crop (Solanum tuberosum L.). Water 2025, 17, 668. [Google Scholar] [CrossRef]
- Gamboa, M.; Ortega-Farias, S.; de la Fuente, D.; Fuentes-Peñailillo, F.; Vargas, S.; Laurie, V. Grape ripening and phenolic content monitoring in Cabernet Sauvignon under regulated deficit irrigation using spectral reflectance indices. Sci. Hortic. 2024, 328, 112920. [Google Scholar] [CrossRef]
- Leng, F.; Zhou, J.; Wang, C.; Sun, L.; Zhang, Y.; Li, Y.; Wang, L.; Wang, S.; Zhang, X.; Xie, Z. Post-veraison different frequencies of water deficit strategies enhance Reliance grapes quality under root restriction. Food Chem. 2022, 390, 133181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, G.; Sun, Z.; Fan, G.; Xie, S.; Li, F.; Du, L. Physiological and growth responses of Lycium barbarum seedlings to water and salt stresses. Sci. Hortic. 2024, 337, 113506. [Google Scholar] [CrossRef]
- Delfani, K.; Asadi, M.; Golein, B.; Babakhani, B.; Jadid, R.R. Foliar Application of Glycine Betaine Affects Morpho-physiological, Biochemical and Fruit Quality Traits of Thomson Navel Orange Under Deficit Irrigation. J. Plant Growth Regul. 2022, 42, 2867–2883. [Google Scholar] [CrossRef]
- D’amico-Damião, V.; Dodd, I.C.; Oliveira, R.; Lúcio, J.C.; Rossatto, D.R.; Carvalho, R.F. Cryptochrome 1a of tomato mediates long-distance signaling of soil water deficit. Plant Sci. 2021, 303, 110763. [Google Scholar] [CrossRef]
- López-López, M.; Espadafor, M.; Testi, L.; Lorite, I.J.; Orgaz, F.; Fereres, E. Water use of irrigated almond trees when subjected to water deficits. Agric. Water Manag. 2018, 195, 84–93. [Google Scholar] [CrossRef]
- Campi, P.; Gaeta, L.; Mastrorilli, M.; Losciale, P. Innovative soil management and micro-climate modulation for saving water in peach orchards. Front. Plant Sci. 2020, 11, 1052. [Google Scholar] [CrossRef]
- Grilo, F.; Scalisi, A.; Pernice, F.; Morandi, B.; Bianco, R.L. Recurrent deficit irrigation and fruit harvest affect tree water relations and fruitlet growth in ‘Valencia’ orange. Eur. J. Hortic. Sci. 2019, 84, 177–187. [Google Scholar] [CrossRef]
- McKiernan, A.B.; Potts, B.M.; Brodribb, T.J.; Hovenden, M.J.; Davies, N.W.; McAdam, S.A.; Ross, J.J.; Rodemann, T.; O′Reilly-Wapstra, J.M. Responses to mild water deficit and rewatering differ among secondary metabolites but are similar among provenances within Eucalyptus species. Tree Physiol. 2015, 36, 133–147. [Google Scholar] [CrossRef]
- Rahmati, M.; Davarynejad, G.H.; Génard, M.; Bannayan, M.; Azizi, M.; Vercambre, G. Peach Water Relations, Gas Exchange, Growth and Shoot Mortality under Water Deficit in Semi-Arid Weather Conditions. PLoS ONE 2015, 10, e0120246. [Google Scholar] [CrossRef]
- Zheng, W.; Wen, M.; Zhao, Z.; Liu, J.; Wang, Z.; Zhai, B.; Li, Z. Black plastic mulch combined with summer cover crop increases the yield and water use efficiency of apple tree on the rainfed Loess Plateau. PLoS ONE 2017, 12, e0185705. [Google Scholar] [CrossRef] [PubMed]
- Blanco, V.; Willsea, N.; Campbell, T.; Howe, O.; Kalcsits, L. Combining thermal imaging and soil water content sensors to assess tree water status in pear trees. Front. Plant Sci. 2023, 14, 1197437. [Google Scholar] [CrossRef] [PubMed]
- García-Tejero, I.; Romero-Vicente, R.; Jiménez-Bocanegra, J.A.; Martínez-García, G.; Durán-Zuazo, V.H.; Muriel-Fernández, J.L. Response of citrus trees to deficit irrigation during different phenological periods in relation to yield, fruit quality, and water productivity. Agric. Water Manag. 2010, 97, 689–699. [Google Scholar] [CrossRef]
- Chen, F.; Cui, N.; Jiang, S.; Zhang, W.; Li, H.; Li, X.; Lv, M.; Liu, C.; Qiu, R.; Wang, Z. Effects of deficit drip irrigation at different growth stages on citrus leaf physiology, fruit growth, yield, and water productivity in South China. Agric. Water Manag. 2025, 307, 109206. [Google Scholar] [CrossRef]
- Guiqing, X.; Jinyao, L.; Haifang, H.; Tuqiang, C. Effect of deficit irrigation on physiological, morphological and fruit quality traits of six walnut tree cultivars in the inland area of Central Asia. Sci. Hortic. 2024, 329, 112951. [Google Scholar] [CrossRef]
- Mounzer, O.; Pedrero-Salcedo, F.; Nortes, P.A.; Bayona, J.-M.; Nicolás-Nicolás, E.; Alarcón, J.J. Transient soil salinity under the combined effect of reclaimed water and regulated deficit drip irrigation of Mandarin trees. Agric. Water Manag. 2013, 120, 23–29. [Google Scholar] [CrossRef]
- Johnson, R.S.; Handley, D.F. Using Water Stress to Control Vegetative Growth and Productivity of Temperate Fruit Trees. Hortscience Publ. Am. Soc. Hortic. Sci. 2000, 35, 1048–1049. [Google Scholar] [CrossRef]
- Fernández, J.E.; Moreno, F.; Cabrera, F.; Arrue, J.L.; Martín-Aranda, J. Drip irrigation, soil characteristics and the root distribution and root activity of olive trees. Plant Soil 1991, 133, 239–251. [Google Scholar] [CrossRef]
- Adu, M.O.; Yawson, D.O.; Armah, F.A.; Asare, P.A.; Frimpong, K.A. Meta-analysis of crop yields of full, deficit, and partial root-zone drying irrigation. Agric. Water Manag. 2018, 197, 79–90. [Google Scholar] [CrossRef]
- Allan, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. J. Hydrol. 1998, 285, 19–40. [Google Scholar]
- Hong, M.; Zhu, H.; Mo, H.; Zhao, J.; Ma, Y. The water consumption rule of jujube under different emitter flow rate and irrigation quota. Agric. Res. Arid. Areas 2014, 32, 72–77. [Google Scholar]
- Ai, P.; Ma, Y.; Hai, Y. Influence of jujube/cotton intercropping on soil temperature and crop evapotranspiration in an arid area. Agric. Water Manag. 2021, 256, 107118. [Google Scholar] [CrossRef]
- Anyia, A.O.; Herzog, H. Water-use efficiency, leaf area and leaf gas exchange of cowpeas under mid-season drought. Eur. J. Agron. 2004, 20, 327–339. [Google Scholar] [CrossRef]
- Rong, Q.; Chen, J.; Zhang, Y.; Tan, Z.; Wang, W.; Sun, C.; Guo, X.; Zhou, C.; Cai, H.; Zhao, X. The interaction between selenium and other elements in soil and rice roots shaped by straw and straw biochar regulated the enrichment of selenium in rice grain. Front. Plant Sci. 2024, 15, 1387460. [Google Scholar] [CrossRef]
- Lu, C.; Shi, X.; Yang, G.; Liu, K.; Yang, X.; Liu, B. Influences of water stress on SOD activity MDA and VC contents in leaves of strawberry. J. Hunan Agric. Univ. 1996, 05, 39–43. [Google Scholar]
- Ballester, C.; Castel, J.; Intrigliolo, D.S.; Castel, J.R. Response of Clementina de Nules citrus trees to regulated deficit irrigation. Yield components and fruit composition. Agric. Water Manag. 2011, 98, 1027–1032. [Google Scholar] [CrossRef]
- Zheng, R.; Kang, S.; Hu, X.; Li, S. Effects of water and nitrogen conditions on the diurnal variation of photosynthesis characteristic and yield of grapevine in arid oasis region. Trans. Chin. Soc. Agric. Eng. 2013, 29, 133–141. [Google Scholar]
- Peng, Y.; Liu, X.; Zhang, Y.; Leng, X.; Sun, G.; Huang, Y.; Yang, Q.; Yang, X. Effects of moistube fertigation on photosynthesis, yield, and use of water and fertilizer of mango (Mangifera indica L.) in dry and hot region. J. Soil Water Conserv. 2020, 34, 350–357. [Google Scholar]
- Caliskan, S.; Ozkaya, I.; Caliskan, M.E.; Arslan, M. The effects of nitrogen and iron fertilization on growth, yield and fertilizer use efficiency of soybean in a Mediterranean-type soil. Field Crops Res. 2008, 108, 126–132. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Cramer, G.R.; Ergül, A.; Grimplet, J.; Tillett, R.L.; Tattersall, E.A.R.; Bohlman, M.C.; Vincent, D.; Sonderegger, J.; Evans, J.; Osborne, C.; et al. Water and salinity stress in grapevines: Early and late changes in transcript and metabolite profiles. Funct. Integr. Genom. 2006, 7, 111–134. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Li, J.; Lian, S.; Zhang, J.; Shi, S. Effects of Deficit-Regulated Irrigation on Root-Growth Dynamics and Water-Use Efficiency of Winter Wheat in a Semi-Arid Area. Water 2024, 16, 2678. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, R.H.; Siddika, A.; Bardhan, K.; Hosen, S.; Prasad, P.V.V. Selenium and its nanoparticles modulate the metabolism of reactive oxygen species and morpho-physiology of wheat (Triticum aestivum L.) to combat oxidative stress under water deficit conditions. BMC Plant Biol. 2024, 24, 1–16. [Google Scholar] [CrossRef]
- Ma, F.; Kang, S.; Wang, M.; Pang, X.; Wang, J.; Li, Z. Effect of regulated deficit irrigation on water use efficiency and fruit quality of pear-jujube tree in greenhouse. Trans. Chin. Soc. Agric. Eng. 2006, 22, 37–43. [Google Scholar]
- Zhang, J.; Jiao, X.; Du, Q.; Song, X.; Ding, J.; Li, J. Effects of Vapor Pressure Deficit and Potassium Supply on Root Morphology, Potassium Uptake, and Biomass Allocation of Tomato Seedlings. J. Plant Growth Regul. 2020, 40, 509–518. [Google Scholar] [CrossRef]
- Vashisth, V.; Sharma, G.; Giri, J.; Sharma, A.K.; Tyagi, A.K. Rice A20/AN1 protein, OsSAP10, confers water-deficit stress tolerance via proteasome pathway and positive regulation of ABA signaling in Arabidopsis. Plant Cell Rep. 2024, 43, 1–21. [Google Scholar] [CrossRef]
- Candar, S.; Seçkin, G.U.; Kizildeniz, T.; Korkutal, I.; Bahar, E. Variations of Chlorophyll, Proline, and Abscisic Acid (ABA) Contents in Grapevines (Vitis vinifera L.) Under Water Deficit Conditions. Erwerbs-Obstbau 2023, 65, 1965–1977. [Google Scholar] [CrossRef]
- Tognetti, R.; Longobucco, A.; Miglietta, F.; Raschi, A. Water relations, stomatal response and transpiration of Quercus pubescens trees during summer in a Mediterranean carbon dioxide spring. Tree Physiol. 1999, 19, 261–270. [Google Scholar] [CrossRef]
- dos Santos, M.R.; Neves, B.R.; da Silva, B.L.; Donato, S.L.R. Yield, Water Use Efficiency and Physiological Characteristic of “Tommy Atkins” Mango under Partial Rootzone Drying Irrigation System. J. Water Resour. Prot. 2015, 07, 1029–1037. [Google Scholar] [CrossRef]
- Sonntag, F.; Naumann, M.; Pawelzik, E.; Smit, I. Improvement of cocktail tomato yield and consumer-oriented quality traits by potassium fertilization is driven by the cultivar. J. Sci. Food Agric. 2019, 99, 3350–3358. [Google Scholar] [CrossRef] [PubMed]
- Poomkokrak, J.; Sanevas, N.; Rungwattana, K. Fruit Quality and Plant Productivity of A Cherry Tomato (Solanum lycopersicum var. cerasiforme) Grown under Different Irrigation Regimes during the Reproductive Phase. Trends Sci. 2024, 21, 7589. [Google Scholar] [CrossRef]
- Rahmati, M.; Mirás-Avalos, J.M.; Valsesia, P.; Lescourret, F.; Génard, M.; Davarynejad, G.H.; Bannayan, M.; Azizi, M.; Vercambre, G. Disentangling the Effects of Water Stress on Carbon Acquisition, Vegetative Growth, and Fruit Quality of Peach Trees by Means of the QualiTree Model. Front. Plant Sci. 2018, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Gartung, J. Long-term productivity of early season peach trees under different irrigation methods and postharvest deficit irrigation. Agric. Water Manag. 2020, 230, 105940. [Google Scholar] [CrossRef]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef]
- Geerts, S.; Raes, D. Deficit irrigation as an on-farm strategy to maximize crop water productivity in dry areas. Agric. Water Manag. 2009, 96, 1275–1284. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).