Abstract
The increasing demand for medicinal and aromatic plants has expanded their cultivation. Concurrently, the utilization of Sideritis species has also increased, including under-evaluated species such as S. cypria Post. This study evaluated the impact of phosphorus (P: 50, 75, and 100 mg L−1) concentrations in hydroponic nutrient solution (NS), and foliar applications with iron (Fe) and zinc (Zn), to assess S. cypria yield and quality. Although fresh biomass was unaffected, reduced (50 mg L−1) and increased (100 mg L−1) P levels decreased dry matter content (DM). Furthermore, Zn spraying at 50 mg P L−1 increased DM by 10.2% compared to H2O sprayed plants. Increased P with foliar control reduced chlorophylls content by 45.6%, while foliar Zn negated this effect, increasing it by 71.9%. Leaf P accumulation was also reduced (up to 49.8%) under increased P levels, while foliar Fe modulated this response. Foliar applications enriched leaves with Fe and Zn. However, P levels determined the success of Zn biofortification. Intermediate (75 mg L−1) P levels decreased the phenolics content (up to 240%) and flavonoids (up to 190%), exhibiting reduced antioxidant activity and increased lipid peroxidation. In contrast, foliar applications regulated this effect, leading to reduced malondialdehyde (MDA) levels. Increased P levels enhanced the antioxidant capacity of plants, causing a 184% decrease in H2O2 contents under foliar control. Finally, a significant antibacterial activity was presented by the application of intermediate and increased P levels, regardless of foliar applications. Ultimately, the tailored NS provide sufficient S. cypria yield and quality, while foliar Fe and Zn can be successfully implemented to improve the nutritional status of S. cypria, through appropriate P management.
1. Introduction
The Sideritis L. genus (Lamiaceae), which includes around 150 species, is typically found in the Mediterranean area [1]. Several species of this genus are utilized in traditional medicine, due to their therapeutic properties against various ailments; gastritis, gastric ulcer, inflammations, wounds, burns and other conditions [2]. One of these species is Sideritis cypria Post., a herb that is native to Cyprus [3]. S. cypria is characterized by a chemical profile rich in secondary metabolites with strong antioxidant and cytotoxic activity attributed to apigenin and apigenin derivatives [4], and antimicrobial activity against Candida albicans and Gram-positive bacteria [5]. Despite its traditional use, research on optimal cultivation conditions remains limited. In particular, the optimal fertilization remains relatively undervalued, despite the significant influence of mineral concentrations and ratios for plant growth and metabolism [6].
The study of medicinal aromatic plants (MAPs), including S. cypria, is necessary for establishing their commercial cultivation and exploitation. As the demand for natural remedies and plant-based pharmaceutical products expands, so is the need to maintain sustained production of MAPs. This is due to the risks associated with the collection from wild populations, including overharvesting and species misidentification [7]. It is also important to standardize cultivation practices that ensure reproducible quality for optimal efficacy and safety [8]. To achieve this, several variables in the cultivation of MAPs need to be examined, as they influence the presence of bioactive secondary metabolites [9]. Optimal fertilization strategies are vital in enhancing growth and quality-related attributes of MAPs [10]. Towards this end, soilless cultivation is a suitable method of cultivating MAPs, as the control of mineral nutrition through the management of the nutrient solution (NS) can effectively modulate the synthesis and accumulation of plant bioactive compounds. In addition, soilless cultivation can be utilized to evaluate the nutritional requirements of underutilized plant species for large-scale commercialization [11].
Phosphorus (P), a vital plant macronutrient, has crucial roles in various cellular processes, enzyme activity and carbohydrates metabolism [12,13]. It participates in various physiological processes in plants, while its uptake influences the growth of plants and their secondary metabolism [10]. However, P availability in soils is often limited due to its strong fixation with soil minerals, rendering it largely inaccessible and often leading to P deficiencies [13]. This requires the use of mineral P fertilizers. Therefore, enhancing the efficiency of P is critical for improving agricultural productivity. This requires improved P acquisition and the use of P in processes related to growth, yield, and quality [6]. Excess P fertilization is a common issue in agriculture, contributing to eutrophication of aquatic ecosystems, while insufficient P availability constraints plant growth and development [14]. Phosphorus availability is interconnected with the presence of Fe and Zn in soil, having significant influence on their uptake and translocation in plants [15,16]. Cross-talks between the homeostasis of these nutrients have been investigated to an extent. Phosphorus deficiency has shown to disrupt Zn homeostasis, resulting in excessive Zn accumulation. Conversely, Zn-deficiency may cause P to considerably accumulate in plant tissues, suggesting a regulatory interplay between these nutrients [17]. Excessive P availability may also inhibit Zn uptake and translocation, referred as P-induced Zn deficiency [12]. Within plants, P and Fe exhibit antagonistic interactions; P deficiency can increase Fe accumulation in shoots due to the upregulation of Fe-responsive genes, while adequate P levels regulate Fe uptake and translocation [18]. The interplay between these elements may cause disruptions in energy transfer and storage, membrane processes, the biosynthesis and activation of various enzymes, photosynthesis and nitrogen (N) fixation [19], while their interaction contributes to the regulation of stress responses under nutrient deficiencies [15].
Low Fe and Zn contents in the human diet pose significant public health challenges. The inadequate uptake of Fe is a cause of Fe deficiency-induced anaemia, while Zn deficiency in humans may impair growth, delay wound healing, contribute to skeletal deformities, and increase susceptibility to diarrheal diseases [16,20]. Dietary Fe and Zn intake can be enhanced through biofortification of edible crops. Among available strategies, agronomic biofortification by foliar applications can be implemented to enhance the qualitative traits of non-woody shoot tissues [21]. Additionally, foliar applications of Fe and Zn have been connected to the enhancement of the phytochemical composition of plants, by modulating their secondary metabolism. For instance, foliar Fe effectively promoted salt tolerance and the antioxidant capacity of Trachyspermum ammi L. under saline stress conditions [22], by acting as a component of enzyme systems such as catalases and peroxidases [23]. Additionally, foliar Zn improved the total phenolic, antioxidant activity and essential oil yield of Satureja khuzistanica Jamzad [24]. Similarly, in Dracocephalum moldavica L., Zn supplementation promoted growth and secondary metabolite biosynthesis [25].
Given the complex interactions among P, Fe, and Zn, and their roles in plant secondary metabolism, antioxidant defense and stress tolerance, their assessment is pivotal in optimizing plant nutritional status. This is especially significant for under-evaluated MAPs such as S. cypria, as the exploration of these interactions is necessary for optimizing fertilization strategies. While a small number studies have examined the impact fertilization on S. cypria cultivation [6,26], few have investigated the impact of foliar applications. This gap in research limits the understanding of how these nutrients interact, especially in terms of their potential synergy or antagonism. Their investigation could also give greater detail regarding improvements in plant growth, yield, quality, and stress response. Thus, the objective of this research was to evaluate the effect of varying P levels in the hydroponic NS, on the growth and quality of S. cypria, with a focus on their effects on the absorption and homeostasis of Zn and Fe. The study also sought to determine the influence of foliar applications on mitigate human health concerns related to Zn and Fe deficiency through biofortification, while also investigating their influence on the secondary metabolism of S. cypria, particularly in relation to the synthesis of compounds with medicinal interest.
2. Materials and Methods
2.1. Plant Materials and Experimental Site
The study was carried out in a multi-span plastic greenhouse situated in Limassol, Cyprus, at 34.700120° N, 32.984276° E. Climate control included the automated use of fan and pad, shade screens, and vents. The experiment was conducted during the autumn—winter season, starting in November 2022. S. cypria seedlings, provided by the Ministry of Agriculture, Cyprus, were transplanted in 2.3 L pots filled with substrate mixture containing cocosoil and perlite (4:1, v/v), at the 7–10 leaf stage. Both cocosoil and perlite had negligible minerals available to plants [27]. Before transplanting, growing media was watered for 48 h to remove any salts accumulation and to maintain the physicochemical properties of the material. Each pot was placed on a plate for the collection of runoff. The experiment was conducted using a completely randomized design.
During the first 28 days after transplanting, all plants received the same starter NS (sNS) to ensure consistent early growth conditions. Afterwards, the three different NS compositions of the experiment were applied; (i) P50 (50 mg P L−1), (ii) P75 (75 mg P L−1), and (iii) P100 (100 mg P L−1), with the rest of the minerals remaining constant. The composition of the sNS, P50, P75, and P100, are presented in Table S1. Applications of the NS were conducted once or twice per week, depending on plant development and substrate moisture, using 50–100 mL NS pot−1. The electrical conductivity (EC) and pH were kept at 2.57 dS m−1 and 5.80–5.90, respectively. The various NS used in the current study were prepared using predetermined nutrient compositions, for the creation of stock A and B solutions. These were then diluted in irrigation water to achieve the target EC, while the formulation accounted for the use of acids (H3PO4 and HNO3) for maintaining optimal pH for soilless cultivation.
The experimental design was expanded to facilitate the implementation of foliar spraying with (i) Foliar control (pure dH2O), (ii) Foliar Fe (dH2O with Fe [0.5 mg L−1 FeSO4·7H2O or 1.8 mM Fe]), and (iii) Foliar Zn (dH2O with Zn [0.5 mg L−1 ZnSO4·7H2O or 1.74 mM Zn]). Foliar applications commenced at 58 days after transplanting (DAT) and were administered three times during the experiment. Foliar Zn and Fe concentrations and spraying intervals were supported by previous findings. No pesticides or other phytochemicals were used during the experiment. Mean temperatures were 21.3 °C (day) and 14.2 °C (night) (Figure S1). Mean day and night relative humidity were 60.4% (day) and 70.5% (night) (Figure S2).
The S. cypria plants used in the current experiment were divided to accommodate the three different P levels and three foliar applications. Thus, 9 treatments were used (3 P levels × 3 foliar applications) with each one holding 6 plants-replications. After the experiment was concluded, all plants were utilized for the agronomic evaluation of each treatment, and plant growth was evaluated on the basis of plant fresh weight (FW; g) and dry matter content (DM; %).
2.2. Leaf Photochemistry Features
Leaf photosynthetic attributes included the relative chlorophylls content measured by SPAD and the leaf chlorophyll fluorescence (Fv/Fm) with six replicates (individual plants) per treatment). Both were assessed before plant tissue sampling, using the SPAD-502 Plus (Konica Minolta, Tokyo, Japan) and the OS-30p fluorometer (Opti-sciences, Hertfordshire, UK), respectively.
Chlorophylls and carotenoids contents were measured by the methanol extraction of fresh plant tissue, and absorbance was measured at 470, 653, and 666 nm (Multiskan GO, Thermo Fischer Scientific, Waltham, MA, USA), as previously reported [9]. Results were presented in mg g−1 FW.
2.3. Plant Tissue Nutrient Content
After the experimental period, four leaf and root samples-replicates were obtained from pooled tissue of each treatment. Following drying at 42 °C and ash-burning at 550 °C for 4.5 h, samples were acid digested using 2 M HCl. The final extracts were used for macro- and micronutrient analyses. Elemental analysis was conducted using standardized procedures: (i) Flame photometry (Lasany Model 1832, Lasany International, Panchkula, India) for potassium (K) and sodium (Na) content, (ii) Molybdate-canadate colorimetric method with measurements at 470 nm (Multiskan GO, Thermo Fischer Scientific, Waltham, MA, USA) for P content, (iii) Kjeldahl method (BUCHI, Digest automat K-439 and Distillation Kjelflex K-360, Flawil, Switzerland) for N content, and iv) Atomic absorption spectrophotometry (PG Instruments AA500FG, Leicestershire, UK) for magnesium (Mg), calcium (Ca) and micronutrients (Fe, Zn, and copper-Cu) content, with the respective use of flame and graphite [9]. Results were presented in g kg−1 and mg kg−1 DW for macro- and micronutrients, respectively.
2.4. Total Phenols, Total Flavonoids, Antioxidant Capacity
Methanolic extracts of the samples leaf tissues (4 replicates/treatment) were prepared according to Chrysargyris et al. [9]. Total phenols were determined by employing the Folin–Ciocalteu method described previously [6], with measurements at 755 nm. Results were presented in gallic acid equivalents (mg GA g−1 FW).
Flavonoids determination utilized a modified aluminum chloride colorimetric assay, with measurements at 510 nm. Results were presented in rutin equivalents (mg rutin g−1 FW) [9].
Free radical-scavenging activity was determined via DPPH at 517 nm, and FRAP at 593 nm, while ABTS assay was employed with measurements at 734 nm (Multiskan GO, Thermo Fischer Scientific, Waltham, MA, USA). In all protocols, a Trolox ((±)-6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid) positive control was utilized, and the results were presented in Trolox equivalents (mg Trolox g−1 FW) [6].
2.5. Stress Indicators
Hydrogen peroxide (H2O2) was determined according to Loreto and Velikova [28], by measuring the absorbance at 390 nm. Results were presented as μmol H2O2 g−1 FW [9]. Malondialdehyde (MDA) content was determined as described previously [29] by measuring the absorbance of reaction mixtures at 532 nm, and correcting for non-specific absorbance at 600 nm. Results were presented as nmol MDA g−1 FW.
2.6. In Vitro Antibacterial Activity of Ethanolic Extract
2.6.1. Preparation of Ethanolic Extracts and Bacterial Cultures
Fresh plant material used for the extractions was first air-dried at 42 °C. The ethanolic extracts were prepared based on Lall et al. [30] using the maceration method in pure ethanol (1:2.5 v/v) (Merck, Darmstadt, Germany). The extracts remained in shaking for 72 h at 160 rpm and afterwards were filtered through a filter paper (Whatman No. 1, Merck, Darmstadt, Germany). A rotary evaporator (Laborota 4011 digital, Heidolph Instruments, Schwabach, Germany) was used for the removal of ethanol and the drying of the obtained extracts. The stock solutions for the S. cypria ethanolic extracts used in this study (8 mg mL−1) were prepared in 30% ethanol (concentration that did not impact the bacterial growth), while the following dilutions used were prepared in sterile dH2O.
Two Gram-negative (Escherichia coli ATCC 25922 and Salmonella enterica subsp. enterica ATCC 51741) and two Gram-positive (Staphylococcus aureus ATCC 11632 and Listeria monocytogenes ATCC 19111) bacterial cultures were freshly prepared in Brain heart infusion (BHI; Biokar, Beauvais Cedex, France) broth after incubation at 37 °C for 16–18 h reaching 108 colony forming units per mL (108 cfu mL−1) [31].
2.6.2. Disc Diffusion and Microdilution Method
The antibacterial activity of the prepared extracts was evaluated by two methods: (i) the disc diffusion and (ii) the microdilution method [31]. In brief, sterile filter paper discs (diameter of 6 mm) carrying 10 μL of ethanolic extract (4 mg mL−1) each were placed on the surface of solidified BHI agar medium inoculated with freshly prepared bacterial culture (100 μL). Incubation took place at 37 °C for 24 h and diameter of the inhibition zone (in mm) were measured. A standard antibiotic, ampicillin (Sigma-Aldrich, Taufkirchen, Germany) was used (100 μg mL−1) for reference.
For the microdilution method [31], a sterile 96-well plate was prepared with 50 μL of BHI broth, 5 μL of bacterial culture (106 cfu mL−1), and 45 μL of S. cypria ethanolic extracts serially diluted (two-fold dilutions) to concentrations ranging from 0.03 to 4 mg mL−1. The plate was sealed and incubated at 37 °C for 24 h measuring the absorbance at 600 nm every 30 min (Multiskan GO, Thermo Fischer Scientific, Waltham, MA, USA). At the end of incubation, the minimum inhibitory concentration (MIC) and the inhibitory concentration reducing the bacterial population by 50% (IC50) were determined and expressed as mg mL−1. Ampicillin was used as a reference antibiotic at concentrations ranging from 0.78 to 100 μg mL−1. In addition, positive (BHI broth and bacterial culture) and negative controls (plain BHI broth and ethanolic extract plus BHI broth) were prepared, ensuring the sterile conditions followed.
2.7. Statistical Analysis
Analysis of variance (ANOVA) on data was executed by the IBM SPSS version 26 (IBM Corp., New York, NY, USA) software. Results are expressed as mean ± standard error (SE). Post hoc comparisons were conducted using Duncan’s multiple range following ANOVA where the treatment impact reached significance at p < 0.05. Correlation analyses between metabolite concentrations were made using Pearson’s correlation coefficients using the R program (version 3.6.2).
3. Results
3.1. Plant Growth Parameters
Plant growth, as assayed by FW and DM, is presented in Table 1. DM was influenced by the experimental parameters, with the greatest values being observed for plants treated with P75. Under foliar control (H2O), P50 and P100 resulted in decreased DM compared to P75, with foliar Zn negating these reductions. However, foliar Fe improved DM only in the P100 treatment, as foliar Fe under P50 resulted in the lowest DM. Leaf FW remained unaffected by the treatments under examination.

Table 1.
The effect of foliar spraying (H2O, Fe, Zn), and P levels (P50, P75, P100; 50, 75, 100 mg L−1, respectively) on the fresh weight (FW; g) and dry matter (DM; %) of hydroponic Sideritis cypria.
3.2. Leaf Photochemistry Features
The highest SPAD values were obtained by applying P75 with foliar Fe, and P100 with foliar H2O, compared to the application of P100 under foliar Fe (Table 2). The lowest chlorophylls were found with the application of P100 with foliar H2O, whereas foliar Zn statistically increased these values (up to 71.9%). In contrast, the application of P75 with foliar Zn resulted in the highest chlorophyll content. The same treatment, however, had the lowest chlorophyll fluorescence, which was preceded by the application of P100 with foliar Zn. Finally, carotenoids were reduced when P100 was applied with foliar H2O, although this result did not persist with the application of foliar Fe and Zn.

Table 2.
The effect of foliar spraying (H2O, Fe, Zn), and P levels (P50, P75, P100; 50, 75, 100 mg L−1, respectively) on leaf SPAD, chlorophyll fluorescence (Fv/Fm), chlorophyll a (Chl a; mg g−1), chlorophyll b (Chl b; mg g−1), total chlorophylls (Total Chl; mg g−1), total carotenoids (Total Car; mg g−1) of hydroponic Sideritis cypria.
3.3. Plant Nutrient Content
The leaf and root macronutrient content of S. cypria is examined in Figure 1. Using P50, applications of foliar Fe and Zn decreased the leaves’ N content, compared to the control foliar application (Figure 1A1). A linear increase in N was observed with the rising P levels under the foliar Fe application. Meanwhile, root N content was consistently reduced with the application of P75, relative to P50 and P100 levels, regardless of foliar application (Figure 1A2). At P50 and P75, foliar Fe increased root N compared to foliar H2O and Zn, whereas at P100, root N was reduced with foliar Zn. Leaf P accumulation was directly influenced by the foliar applications; with foliar control, P was decreased with the raise of P in the NS, while the opposite occurred under the foliar Fe application (Figure 1B1). Interestingly, with foliar Zn, the application of P75 increased P levels in leaves and decreased them in roots (Figure 1B2). Leaf K was comparable among treatments, with the lowest being evident under the P100 treatment with foliar Zn (Figure 1C1). Reductions in the leaf Ca were evident when foliar Zn was applied with P ≤ 75 mg L−1 (Figure 1D1). Additionally, Ca in roots was significantly increased when P75 was applied (Figure 1D2). However, this application led to the reduction in Mg in leaves (Figure 1E1), while root Mg was enhanced with the use of the P100 NS under foliar Fe and Zn applications (Figure 1E2). Lastly, the Na of leaves was reduced by the application of P75, while reductions were also evident under foliar Zn (Figure 1F1), while increased Na content in roots were found in P100 treatments (Figure 1F2).
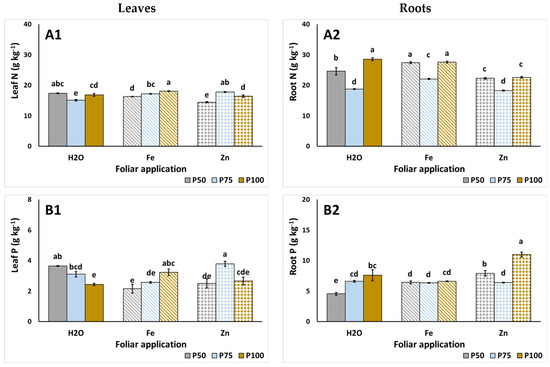
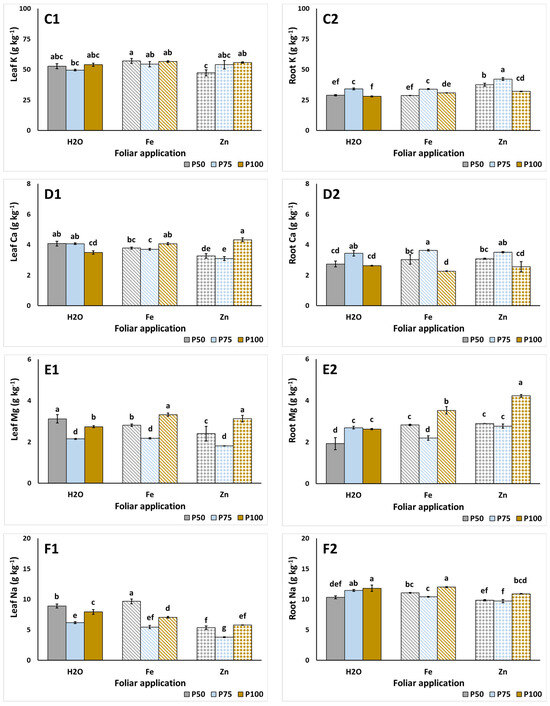
Figure 1.
The effect of foliar spraying (H2O, Fe, Zn), and P levels (P50, P75, P100; 50, 75, 100 mg L−1, respectively) on leaf and root macronutrients such as N (A1,A2), P (B1,B2), K (C1,C2), Ca (D1,D2), Mg (E1,E2), and Na (F1,F2), respectively, of hydroponic Sideritis cypria. Significant differences (p < 0.05) among the different applications are presented by different letters.
Leaf and root micronutrient content of S. cypria is examined in Figure 2. Leaf Fe accumulation was impacted by the application of foliar Fe, resulting in increased Fe levels, when compared to the rest of the foliar treatments (Figure 2A1). In addition, the application of P100 also increased Fe accumulation, with no significant changes found among foliar treatments. Root Fe accumulation was enhanced with the application of P75, compared to P50 and P100 (Figure 2A2). Foliar Fe enhanced leaf Cu and Zn were with the application of P75, compared to P50 and P100. However, decreases occurred with ≥ 75 mg P L−1 under the Zn foliar application (Figure 2B1,C1).

Figure 2.
The effect of foliar spraying (H2O, Fe, Zn), and P levels (P50, P75, P100; 50, 75, 100 mg L−1, respectively) on leaf and root micronutrients such as Fe (A1,A2), Cu (B1,B2), and Zn (C1,C2), respectively, of hydroponic Sideritis cypria. Significant differences (p < 0.05) among the different applications are presented by different letters.
3.4. Total Phenolics Content, Antioxidant Activity, and Flavonoids Content
The influence of the experimental parameters on total phenolics, antioxidant activity and flavonoids content is presented in Figure 3. In the control foliar application, total phenols, and the antioxidant capacity (as assayed by DPPH, FRAP and ABTS) and flavonoids of S. cypria plant leaves were increased with P50 and P100 applications in comparison to P75 (Figure 3A–E). In addition, Fe and Zn foliar applications increased phenols, DPPH and FRAP, at ≥75 mg P L−1, compared to 50 mg P L−1 in the NS (Figure 3B,C). ABTS was increased with the NS of ≥75 mg P L−1 under foliar Zn (Figure 3D), while flavonoids were increased with the NS of 50 mg P L−1 under foliar Fe and Zn applications (Figure 3E).

Figure 3.
The effect of foliar spraying (H2O, Fe, Zn) and P levels (P50, P75, P100; 50, 75, 100 mg L−1, respectively) on (A) phenols, antioxidant activity; (B) DPPH, (C) FRAP, (D) ABTS, and (E) flavonoids of hydroponic Sideritis cypria. Significant differences (p < 0.05) among the different applications are presented by different letters.
3.5. Stress Indicators
The effect of experimental factors on the stress response of plants is presented in Figure 4. In our study, the lowest H2O2 was observed under the P100 treatment with foliar control (Figure 4A). However, with the same P level, the foliar with Fe and Zn resulted in the increase of H2O2, with foliar Fe inducing the highest accumulation. A similar tendency was observed with the P75 treatment, while in the P50 treatment, only foliar Fe induced an increase in H2O2. Under the control foliar application, MDA was significantly stimulated with the P75 treatment, compared to P50 and P100 (Figure 4B). However, under Fe and Zn foliar applications, significant increases occurred with the P75 and P100, compared to the P50 treatment. Interestingly, with the P50 NS, no differences in MDA occurred among the foliar applications.

Figure 4.
The effect of foliar spraying (H2O, Fe, Zn), and P levels (P50, P75, P100; 50, 75, 100 mg L−1, respectively) on (A) H2O2 and (B) MDA of hydroponic Sideritis cypria. Significant differences (p < 0.05) among the different applications are presented by different letters.
3.6. Correlation Matrix
The correlation matrix of plant growth and physiology indices is shown in Figure 5. Among the factors that were changed, a positive correlation was found on the increased total phenols content with the increased flavonoids and antioxidant capacity (DPPH, FRAP) and to some extent to the increased leaf N content. Interestingly, leaf Mg content was negatively correlated with the relevant antagonistic cations in roots (K, Ca, and Fe) and the MDA content. Both leaf Fe and leaf Zn update were partially affected by the uptake of their antagonistic cations (K, Na, Ca, Mg, and Cu) in leaves or roots.
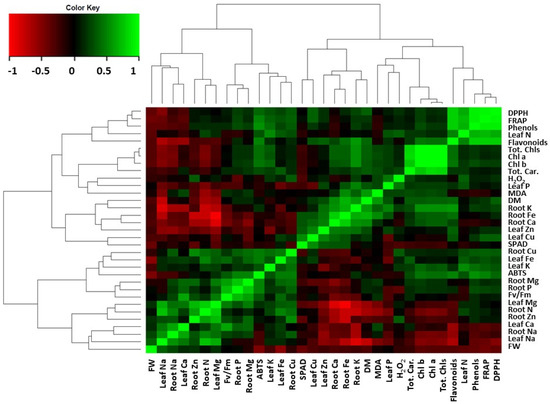
Figure 5.
Heat-map matrices of the correlation between growth and physiology indices of Sideritis cypria. Each square indicates r (Pearson’s correlation coefficient of a pair of metabolites).
Heat maps presented in Figure 6, according to the relative expression of plant growth, physiology, and mineral content of S. cypria plants grown under different P concentrations and foliar Fe or Zn revealed a variation in plant response among treatments. The increased P levels in the NS stimulated the total phenolics, flavonoids, and antioxidant capacity (assayed by FRAP and DPPH) without affecting the fresh biomass produced, while the content of chlorophylls and carotenoids, as well as H2O2 were decreased (Figure 6A). Iron foliar application stimulated the leaf N, Ca, Mg, Fe, and root Mg, Zn, Na, and Cu accumulation (Figure 6B). Zn foliar application stimulated minerals accumulation in leaves (Ca, Mg, and Fe) and roots (Mg and P) but slightly suppressed the accumulation of Cu in leaves and Cu and Zn in roots (Figure 6C).
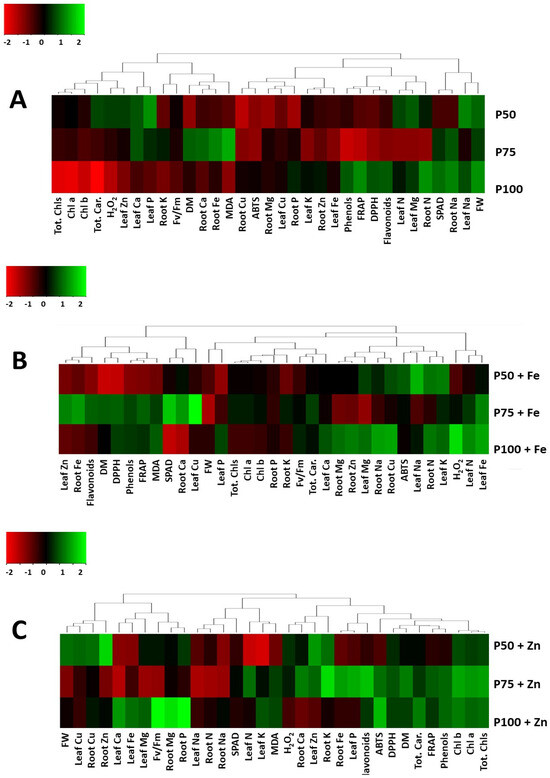
Figure 6.
Metabolite changes in Sideritis cypria. Heat map representing relative expression of growth and physiology elicited in plant tissue following foliar (A) with water (B) with Fe, and (C) with Zn applications under increased phosphorus (P) levels (75 and 100 mg L−1) as compared to the lower P-treated plants.
3.7. Antibacterial Activity of Ethanolic Extracts
Table 3 presents the effects of the investigated foliar spraying and P levels on the antibacterial activity of S. cypria ethanolic extracts. A low antibacterial activity towards E. coli and L. monocytogenes was observed, compared to ampicillin, while no significant changes were found between the plant extracts. Interestingly, the growth of S. enterica was not affected differently by the ethanolic extracts and/or antibiotics. On the other hand, plants subjected to P75 treatment resulted into an extract with significant antibacterial activity against S. aureus (similar DIZ to ampicillin 100 μg mL−1).

Table 3.
The effect of foliar spraying (H2O, Fe, Zn), and P levels (P50: 50 mg L−1, P75: 75 mg L−1, P100: 100 mg L−1) on the antibacterial activity of Sideritis cypria ethanolic extracts from plants grown hydroponically.
The plant extracts obtained from S. cypria plants treated with 100 mg P L−1 (with and without Fe and Zn foliar spraying), as well as zinc foliar spraying presented lower MIC (with P100 and foliar Zn showing the lowest MIC value among the P treatments) against E. coli compared to the other treatments (Table 3). Similarly, the growth of S. enterica was affected by extracts derived from plants grown with 100 mg P L−1 (with and without Fe and Zn foliar spraying) and foliar Zn (all P concentrations) as opposed to the other treatments. IC50 values for S. enterica were found lower with P75 (alone and with Zn foliar application) compared to P100. S. aureus was found to be susceptible (lower MIC and IC50 values) to the plant extracts obtained from plants treated with 75 mg P L−1, 100 mg P L−1 (with and without Fe and Zn foliar spraying) and 50 mg P L−1 with Zn foliar application (with P100 and foliar Zn showing the lowest MIC value among P treatments) compared to the other applied treatments. The growth of L. monocytogenes was influenced by plant extracts that derived from S. cypria plants treated with 100 mg P L−1 (with and without Fe and Zn foliar spraying) as well as P75 and its combination with Fe foliar spraying (lower MIC values). On the other hand, P75 presented lower IC50 values compared to P100 with foliar application. However, the overall antibacterial activity observed by the obtained ethanolic extracts was lower than the activity of the reference antibiotic (ampicillin).
4. Discussion
Efforts to improve the cultivation of MAPs for sustained production to support the needs of the industrial and culinary sectors are well underway [6]. Proper fertilizer management strategies are required to optimize crop nutrition and produce quality, while also enhancing nutrient use efficiency [32,33]. In addition, foliar spraying of micronutrients, aimed at improving plant growth, quality, and nutritional status, as well as to regulate nutrient deficiencies, present an efficient and sustainable agricultural practice for MAPs production. In the current research, different P levels in the hydroponic NS were examined, in conjunction with foliar Fe and Zn applications, to examine combined influence of these parameters on the growth, physiology, and quality of S. cypria, cultivated hydroponically.
In the current study, P fertilization and foliar applications did not influence plant fresh weight. However, the dry matter content of plants was negatively affected by the application of reduced (P50) or increased (P100) P levels, compared to the intermediate P level (P75), under the foliar control application. Decreased P availability may negatively influence the dry matter content of plants [34]. In addition, at high doses, luxury uptake of P may occur, potentially limiting Fe and Zn accumulation, due to the interference of P on their uptake, translocation, and utilization [35]. In this context, the supply of Fe and Zn via foliar spraying of the current study, mediated the plants’ response on dry matter content at reduced or increased P levels. Similarly, Fageria [36] reported higher dry matter yield with the improvement of Zn utilization in maize (Zea mays L.), while increases in leaf, stem, and root dry mass were found with the application of foliar FeSO4 in pepper (Capsicum annum L.) grown in aquaponics [37].
Phosphorus impacts various physiological processes, including photosynthesis and energy transfer via the formation of adenosine triphosphatase (ATP) [13,38]. In addition, the availability of P may influence the uptake of other nutrients (e.g., N, Fe, Zn), indirectly affecting vital processes such as chlorophyll production [39]. In the present study, SPAD was slightly reduced by the introduction of foliar Fe applications, under increased P levels in the NS. This, combined with elevated leaf P contents, shows a possible interaction among the elements on the plant photosynthetic performance, and further research is needed in that direction. In the current study, increased P levels under foliar control resulted in decreased chlorophylls (Chl a, Chl b, total Chl) contents, showing a correlation between increased P levels and the accumulation of chlorophylls in the leaves of S. cypria. Similarly, Siedliska et al. [40] reported that applying P over the recommended levels in the NS resulted in the decrease in leaf chlorophyll contents in sugar beet (Beta vulgaris L.), celery (Aqium graveolens L.), and strawberry (Fragaria × ananassa Duchesne). The decline of chlorophyll content under increased P addition may be associated with the interactions between P, Fe, and Zn. Iron is a prerequisite for the activity of aminolevulinic acid (ALA) synthase, involved in the first step of the tetrapyrrole biosynthetic pathway for chlorophyll production, while zinc is a component of the chlorophyllase enzyme, which is responsible for the last step in the biosynthesis of chlorophyll [41,42]. Thus, enhancement of the uptake of these micronutrients may mediate this response. In the present research, this was mostly related to the foliar application of Zn, which facilitated the increase in chlorophylls at mostly increased P in the NS. These findings are in accordance with those of Roosta and Mohsenian [37] and Derakhshani et al. [43], which reported an increase in chlorophyll production under Zn supply. In addition, foliar Fe was positively associated with increased Chl b contents, as previously reported by Roosta and Mohsenian [37].
The mineral composition of plants is largely affected by the availability and balance of macronutrients such as P, and micronutrients such as Zn and Fe [44,45]. For instance, P fertilization can influence the acquisition and utilization of N, while a complex tripartite interaction between P, Fe, and Zn has also been documented [46,47]. In the current study, leaf and root N was positively influenced by the application of reduced or increased P levels. In general, suitable P application promotes the accumulation of N, with P limitation negatively influencing N acquisition and use efficiency [48]. However, as reported previously, the reduced P levels of the present study are not considered as “P shortage” [6]. Phosphorus levels in leaves of plants treated with foliar control exhibited a decreasing trend in response to progressively higher P levels in the NS. Conversely, P in roots was increased in response to elevated P availability. This may be explained by the reduction in the delivery of P to the shoot, under P limitation [14]. This occurs due to the upregulation of PHT and PHO1 genes by P deficiency, to enhance P uptake. Under high external P, these genes are downregulated, favoring increased P storage in roots rather than transport to shoots, to limit excess accumulation [49]. In contrast, under foliar Fe, increased P levels in the NS caused a subsequent increase in leaf P content. Similarly, Moshtagh and Aminpanah [50] reported that foliar Fe application significantly raised leaf P contents of common bean (Phaseolus vulgaris L.). Under foliar Zn, P contents were enhanced under the intermediate P treatment. In line with this, Santos et al. [51] reported that the adequate P levels increased the shoot P accumulation in cotton (Gossypium hirsutum L.) under adequate Zn application. This is attributed to the specificity of Zn in the regulation of genes linked with P uptake [47]. However, in the current study, as neither P or Zn were in excess or deficiency, a narrower range of both nutrient levels could provide more information regarding their interactions. Excess P may also induce Ca deficiency [52]. This is mirrored with the results of the current study, as leaf Ca was reduced at P100 in the foliar control application. In addition, root Ca was the highest under intermediate P levels across all foliar applications. Leaf Fe and Zn were positively corelated with the applications of foliar Fe and Zn, respectively, affirming the efficacy of Fe biofortification procedures for improving the nutritive value of plants [53]. Under foliar Fe, Fe levels in leaves ranged from 346.2 to 465.5 mg kg−1, significantly exceeding previously reported levels of Chrysargyris and Tzortzakis [6], which ranged from roughly 60 to 120 mg kg−1. However, Lytra et al. [54] observed elevated Fe levels in cultivated S. cypria, at 398.9 mg kg−1, suggesting that this variation may be due to different agronomic and environmental factors, and the developmental stage of S. cypria plants. Improper management of foliar Fe applications may cause iron-induced phytotoxicity, and the effects depend on the Fe rate, source, and plant species [55]. However, the foliar sprays applied in the current studies did not induce such effect, and this is re-affirmed previously on S. cypria [56]. Moreover, although foliar Zn resulted in a general increase in leaf Zn accumulation, levels remained between 9.4 and 26.8 mg kg−1, below the levels reported by Lytra et al. [54]. These levels are well below the ones considered to cause Zn phytotoxicity (300 μg g−1 DW) [57]. Interestingly, the study of Chrysargyris et al. [56] highlighted that, even at foliar Zn levels higher than the ones used in the current study, accumulation in hydroponic S. cypria was minimal, mainly due to adequate nutrition through the NS. Additionally, in the current study, increased P levels reduced the accumulation of Zn. A similar result indicating that the high P levels reduced the Zn content of chia (Salvia hispanica L.) leaves has been reported by Korkmaz et al. [12], affirming the negative interaction between Zn and P. Finally, both Cu and Zn contents in leaves were positively influenced by intermediate P levels in the NS, under the foliar Fe applications. This may be related to the sufficiency of P and Fe, influencing the uptake of other elements including Cu and Zn [15], as well as the root growth of S. cypria plants [58]. Additionally, Zn foliar applications may alleviate the accumulation of Cu, especially under instances of Cu toxicity [26].
Plant nutrient efficiency is involved in the synthesis of various secondary metabolites such as phenolics, flavonoids, polyamines, and anthocyanins [13]. Antioxidants have a key role in the protection against the harmful effects of oxygen radicals. Phosphorus fertilization has been linked to increased accumulation of phenolic compounds [10]. In MAPs, several studies have highlighted the importance of adequate levels of P for improving total phenolic content and the overall quality of MAPs [59]. In the current study, under foliar control, the intermediate P levels in the NS produced plants with decreased phenolics content, flavonoids, and antioxidant activity, compared with the P50 and P100 treatments. This, coupled with increased lipid peroxidation, may be associated with the incapability of antioxidants to scavenge ROS. Nonetheless, the decrease in H2O2 content at intermediate P levels may be related to the enzymatic antioxidant activity, as observed by Elsafy et al. [60], who linked P availability to the activation of various enzymes, such as catalase (CAT), superoxide dismutase (SOD) and peroxidase (POD). Additionally, increased P in the NS enhanced the total phenolics content, flavonoids, and antioxidant activity of S. cypria plants, causing a decrease in H2O2 contents. This may be related to increases in enzymatic activities and transcription factors linked with the biosynthesis of bioactive compounds such as phenolics and flavonoids [60]. Interestingly, foliar applications of Fe and Zn modulated the response of S. cypria subjected to intermediate P levels, enhancing the scavenging mechanism, prompting increased phenolics, flavonoids and antioxidant activity. In addition, MDA levels were reduced under these conditions, while H2O2 was increased, exhibiting a potential alleviatory effect of enzymatic and non-enzymatic antioxidant activity. Foliar Fe has been associated with enhanced antioxidant enzyme activities, as well as total phenolic and antioxidants contents, in the cultivation of T. ammi L. and D. moldavica L. under saline conditions, improving plant resilience under stress [22,61]. Similarly, Zn has been reportedly associated with enhanced antioxidant enzyme activity on wheat (Triticum aestivum L.) cultivated under drought conditions [24], while increased concentrations of Zn were linked to increased total phenolics and flavonoids in habanero peppers (Capsicum chinense Jacq.) [62].
The cultivation conditions (among other parameters) have a significant impact (among other parameters) in the biological activities of MAP [63]. S. cypria ethanolic extracts previously showed promising effectiveness against various bacteria and fungi [64,65]. This could be associated with the fact that the genus of Sideritis L. is known to be rich in various secondary metabolites with the majority being terpenes and phenolic compounds [54]. The current study showed that plants grown under increased P levels (100 mg L−1) with or without foliar spraying along with Zn foliar application at lower P levels resulted into extracts with considering antibacterial activity (lower MIC values) compared to other treatments. Furthermore, the results of this study are in accordance with prior reports where S. cypria extracts presented a varying activity against both Gram-positive and negative bacteria [65,66]. This could be linked to the increase in antioxidant activity, phenols, and flavonoid content caused by the applied treatments. Increased P levels during cultivation have been associated with an increase in plant’s polyphenols [10]. In addition, micronutrients such as Fe, Zn, and selenium (Se) has shown to cause an increase in non-enzymatic antioxidant mechanisms such the production of glutathione, ascorbic acid, and proline, in an attempt to reduce the production of reactive oxygen species (ROS) due to oxidative stress (indicated mainly by high MDA levels) [67]. Antioxidant enzymatic activity (including SOD, CAT, POD) might also ignite as part of the plant’s defense mechanisms in order to eliminate the production and fatal consequences of ROS. Furthermore, the bacterial species’ susceptibility to the plants extracts is determined by their physiological and biochemical nature [68]. Thus, further investigation is necessary to fully address these pathways and mechanisms of action.
Even though the antibacterial activity of the assessed S. cypria ethanolic extracts was lower than the activity of ampicillin, a previous study showed that ethanolic extracts of S. cypria exhibited higher antibacterial and antifungal activity than commercially used preservatives such as sodium benzoate (E211) and potassium metabisulphite (E224) [65]. The antibacterial activity observed from the ethanolic extracts of the current study could be categorized as moderate according to previous mentions (ranges: 0.1 < MIC < 0.63 mg mL−1) [68]. Panayi et al. [64] also reported moderate antibacterial activity of S. cypria ethanolic extracts against E. coli. These findings could provide useful information for further investigation such as the possible combination of plant extracts with antibiotics already used in pharmaceuticals. By using lower antibiotic concentrations paired with plant extracts could be an option to contribute to the reduction in conventional antibiotic excessive use and therefore fight the alarmingly high levels of antibiotic resistance due to potential synergism [69]. Saci et al. [70] showed that oregano (Origanum vulgare) hydroethanolic extract alone presented moderate antibacterial activity against avian pathogenic strains of E. coli, whereas the extract’s combinations with ampicillin and tetracycline exhibited synergistic effects against resistant strains. However, caution should be taken during the combinations as antagonistic effects might also appear [71].
The increasing utilization of MAPs in the pharmaceutical industry has encouraged the scientific interest for underexplored species such as S. cypria, and for the production and quality enhancement strategies in agricultural practices, including tailored nutrition and biofortification [6,25]. Soilless cultivation is an effective means of evaluating and regulating the nutrition of such species, for achieving a balance between yield and quality. In addition, agronomic biofortification via foliar spraying is an effective means of enhancing the nutritive quality of crops, without requiring breeding or genetic engineering [21]. Ultimately, the foliar applications of Fe and Zn present a sustainable solution for addressing malnutrition and improving the quality of crops. This is especially significant for MAPs as industry standards dictate high and stable yield and quality [26]. In fact, as indicated previously, sub-optimal concentrations of Fe and Zn foliar applications may reduce plant yield and dry matter content. Thus, optimal dosing is critical to avoiding negative effects on commercial S. cypria cultivation [56]. For MAPs, the implementation of agronomic biofortification can present a viable and streamlined method of increasing the concentration of micronutrients and desirable secondary metabolites that attract the interest of producers and consumers. This, combined with the high economic cost of dealing with “hidden hunger” correlated with malnutrition of micronutrients, highlights the importance of mineral enrichment [72].
5. Conclusions
The current work was undertaken to investigate the effects of varying P levels (50, 75, 100 mg L−1) in the NS of S. cypria plants grown in substrate cultivation, under different micronutrient (Fe, Zn) foliar applications, to assess their impact on plant growth, nutritional status and qualitative characteristics, and their potential interrelation with P levels. While the varying P levels in the NS did not influence the fresh weight produced of S. cypria plants, reduced (50 mg L−1) and increased (100 mg L−1) P levels decreased dry matter content. However, foliar Zn and Fe modulated this response. Additionally, a decline in the content of chlorophylls was observed under increased P in the NS, while the foliar Zn enhanced it. Increasing the P levels of the NS without foliar applications reduced P in leaves but increased P in roots, highlighting the adaptive responses of plants to limiting excess accumulation. Foliar Zn regulated the uptake of P, as it accumulated more in roots, especially with increased P levels. This highlights its potential influence on genes associated with P uptake. Foliar Fe and Zn applications positively influenced the content of Fe and Zn in leaves, respectively, establishing the efficacy of agronomic biofortification on S. cypria. However, P had a significant influence on Zn biofortification. Intermediate P levels caused a decrease in phenolics content, flavonoids, and antioxidant activity and increased lipid peroxidation due to the decreased scavenging ability. However, Fe and Zn foliar spraying controlled this response, leading to enhancements in total phenolics content and antioxidants, and reductions in MDA levels, revealing a possible alleviation effect. At increased P levels in the NS, total phenolics content, flavonoids, and antioxidants were enhanced, causing a decrease in H2O2 contents. Finally, application of ≥75 mg P L−1 with or without foliar applications had significant antibacterial activity against the majority of the tested bacteria compared to reduced P levels. Nonetheless, this was lower than the activity of ampicillin. The core result of the present research is that exposure of S. cypria to foliar Fe and Zn could improve the qualitative and nutritional status of S. cypria plants, without influencing yield. These responses were mediated by the different P levels, highlighting the importance of appropriate nutrition strategies. Ultimately, P levels in the NS can be tailored to provide sufficient S. cypria yield and quality, while foliar Fe and Zn can be applied to achieve sustainable yields and final products with tailored bioactive compounds content and high nutritional quality.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051178/s1, Table S1: Electrical conductivity (EC) and nutrient concentrations in the nutrient solution (NS) supplied to S. cypria plants grown in soilless cultivation under three levels in phosphorus (P50: 50 mg L−1, P75: 75 mg L−1, P100: 100 mg L−1). Figure S1: Mean day and night temperatures during the cultivation period. Figure S2: Mean day and night relative humidity (RH) during the cultivation period.
Author Contributions
Conceptualization, A.C. and N.T.; methodology, G.N., A.C. and P.X.; software, A.C. and P.X.; validation, G.N., A.C. and G.B.; formal analysis, G.N., P.X. and A.C.; investigation, G.N., A.C. and P.X.; resources, G.B. and N.T.; data curation, G.N., A.C. and G.B.; writing—original draft preparation, G.N., A.C., P.X. and N.T.; writing—review and editing, A.C., G.B. and N.T.; visualization, G.B. and N.T.; supervision, A.C. and N.T.; project administration, N.T.; funding acquisition, N.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Project “Opti-AromaQ” EXCELLENCE/0421/0299, which is co-financed by the European Union and the Republic of Cyprus through the Research and Innovation Foundation.
Data Availability Statement
The authors declare data availability only upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aneva, I.; Zhelev, P.; Kozuharova, E.; Danova, K.; Nabavi, S.F.; Behzad, S. Genus Sideritis, section Empedoclia in southeastern Europe and Turkey—Studies in ethnopharmacology and recent progress of biological activities. DARU J. Pharm. Sci. 2019, 27, 407–421. [Google Scholar] [CrossRef]
- González-Burgos, E.; Carretero, M.E.; Gómez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities—A review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef]
- Christodoulou, C.S.; Griffiths, G.H.; Vogiatzakis, I.N. Systematic Conservation Planning in a Mediterranean island context: The example of Cyprus. Glob. Ecol. Conserv. 2021, 32, e01907. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Ekinci, M.; Ercisli, S.; Ozturk, H.I.; Aydin, M.; Ilhan, E.; Vicas, S.I.; Iancu, C.V.; Gitea, D.; et al. Composition of Anthocyanins, Specific Sugars, and Organic Acids in Wild Edible Aromatic and Medicinal Vegetables. Horticulturae 2025, 11, 145. [Google Scholar] [CrossRef]
- Hanoğlu, D.Y.; Hanoğlu, A.; Güvenir, M.; Süer, K.; Demirci, B.; Başer, K.H.C.; Yavuz, D.Ö. Chemical composition and antimicrobial activity of the essential oil of Sideritis cypria Post endemic in Northern Cyprus. J. Essent. Oil Res. 2017, 29, 228–232. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Nitrogen, phosphorus, and potassium requirements to improve Sideritis cypria growth, nutrient and water use efficiency in hydroponic cultivation. Heliyon 2025, 11, e40755. [Google Scholar] [CrossRef]
- Atherton, H.R.; Li, P. Hydroponic Cultivation of Medicinal Plants—Plant Organs and Hydroponic Systems: Techniques and Trends. Horticulturae 2023, 9, 349. [Google Scholar] [CrossRef]
- Nafiu, M.O.; Hamid, A.A.; Muritala, H.F.; Adeyemi, S.B. Preparation, Standardization, and Quality Control of Medicinal Plants in Africa. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 171–204. [Google Scholar]
- Chrysargyris, A.; Panayiotou, C.; Tzortzakis, N. Nitrogen and phosphorus levels affected plant growth, essential oil composition and antioxidant status of lavender plant (Lavandula angustifolia Mill.). Ind. Crops Prod. 2016, 83, 577–586. [Google Scholar] [CrossRef]
- Peng, L.-C.; Ng, L.-T. Impacts of Nitrogen and Phosphorus Fertilization on Biomass, Polyphenol Contents, and Essential Oil Yield and Composition of Vitex negundo Linn. Agriculture 2022, 12, 859. [Google Scholar] [CrossRef]
- Ceccanti, C.; Brizzi, A.; Landi, M.; Incrocci, L.; Pardossi, A.; Guidi, L. Evaluation of Major Minerals and Trace Elements in Wild and Domesticated Edible Herbs Traditionally Used in the Mediterranean Area. Biol. Trace Elem. Res. 2021, 199, 3553–3561. [Google Scholar] [CrossRef]
- Korkmaz, K.; Akgün, M.; Özcan, M.M.; Özkutlu, F.; Kara, Ş.M. Interaction effects of phosphorus (P) and zinc (Zn) on dry matter, concentration and uptake of P and Zn in chia. J. Plant Nutr. 2021, 44, 755–764. [Google Scholar] [CrossRef]
- Malhotra, H.; Vandana; Sharma, S.; Pandey, R. Phosphorus Nutrition: Plant Growth in Response to Deficiency and Excess. In Plant Nutrients and Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Fujita, M., Oku, H., Nahar, K., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2018; pp. 171–190. [Google Scholar] [CrossRef]
- White, P.J.; Hammond, J.P. Phosphorus nutrition of terrestrial plants. In The Ecophysiology of Plant-Phosphorus Interactions; Springer: Dordrecht, The Netherlands, 2008; pp. 51–81. [Google Scholar]
- Yang, X.; Liu, C.; Liang, C.; Wang, T.; Tian, J. The Phosphorus-Iron Nexus: Decoding the Nutrients Interaction in Soil and Plant. Int. J. Mol. Sci. 2024, 25, 6992. [Google Scholar] [CrossRef]
- Bouain, N.; Shahzad, Z.; Rouached, A.; Khan, G.A.; Berthomieu, P.; Abdelly, C.; Poirier, Y.; Rouached, H. Phosphate and zinc transport and signalling in plants: Toward a better understanding of their homeostasis interaction. J. Exp. Bot. 2014, 65, 5725–5741. [Google Scholar] [CrossRef]
- Xie, X.; Hu, W.; Fan, X.; Chen, H.; Tang, M. Interactions Between Phosphorus, Zinc, and Iron Homeostasis in Nonmycorrhizal and Mycorrhizal Plants. Front. Plant Sci. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Fan, X.; Zhou, X.; Chen, H.; Tang, M.; Xie, X. Cross-Talks Between Macro- and Micronutrient Uptake and Signaling in Plants. Front. Plant Sci. 2021, 12, 663477. [Google Scholar] [CrossRef]
- Rotaru, V.; Sinclair, T.R. Influence of Plant Phosphorus and Iron Concentrations on Growth of Soybean. J. Plant Nutr. 2009, 32, 1513–1526. [Google Scholar] [CrossRef]
- Sharma, S.; Malhotra, H.; Borah, P.; Meena, M.K.; Bindraban, P.; Chandra, S.; Pande, V.; Pandey, R. Foliar application of organic and inorganic iron formulation induces differential detoxification response to improve growth and biofortification in soybean. Plant Physiol. Rep. 2019, 24, 119–128. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Physiological limits to zinc biofortification of edible crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef]
- Abdoli, S.; Ghassemi-Golezani, K.; Alizadeh-Salteh, S. Responses of ajowan (Trachyspermum ammi L.) to exogenous salicylic acid and iron oxide nanoparticles under salt stress. Environ. Sci. Pollut. Res. 2020, 27, 36939–36953. [Google Scholar] [CrossRef]
- Rout, G.R.; Sahoo, S. Role of Iron in Plant Growth and Metabolism. Rev. Agric. Sci. 2015, 3, 1–24. [Google Scholar] [CrossRef]
- Sattar, A.; Wang, X.; Ul-Allah, S.; Sher, A.; Ijaz, M.; Irfan, M.; Abbas, T.; Hussain, S.; Nawaz, F.; Al-Hashimi, A.; et al. Foliar application of zinc improves morpho-physiological and antioxidant defense mechanisms, and agronomic grain biofortification of wheat (Triticum aestivum L.) under water stress. Saudi J. Biol. Sci. 2022, 29, 1699–1706. [Google Scholar] [CrossRef]
- Nekoukhou, M.; Fallah, S.; Abbasi-Surki, A.; Pokhrel, L.R.; Rostamnejadi, A. Improved efficacy of foliar application of zinc oxide nanoparticles on zinc biofortification, primary productivity and secondary metabolite production in dragonhead. J. Clean. Prod. 2022, 379, 134803. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Neofytou, G.; Chrysargyris, A. Nitrogen Fertilization Coupled with Zinc Foliar Applications Modulate the Production, Quality, and Stress Response of Sideritis cypria Plants Grown Hydroponically Under Excess Copper Concentrations. Plants 2025, 14, 691. [Google Scholar] [CrossRef]
- Marinou, E.; Chrysargyris, A.; Tzortzakis, N. Use of sawdust, coco soil and pumice in hydroponically grown strawberry. Plant Soil Environ. 2013, 59, 452–459. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Lall, N.; Chrysargyris, A.; Lambrechts, I.; Fibrich, B.; Blom Van Staden, A.; Twilley, D.; de Canha, M.N.; Oosthuizen, C.B.; Bodiba, D.; Tzortzakis, N. Sideritis perfoliata (Subsp. Perfoliata) Nutritive Value and Its Potential Medicinal Properties. Antioxidants 2019, 8, 521. [Google Scholar]
- Chrysargyris, A.; Xylia, P.; Botsaris, G.; Tzortzakis, N. Antioxidant and antibacterial activities, mineral and essential oil composition of spearmint (Mentha spicata L.) affected by the potassium levels. Ind. Crops Prod. 2017, 103, 202–212. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Change 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Yang, M.; He, W.; Li, Y.; Qiu, J.; Liu, F.; Li, R.; Qiu, Y. Effects of low levels of nitrogen or phosphorus provided in hydroponic culture on brown planthopper feeding and survival. Int. J. Pest Manag. 2021, 67, 89–98. [Google Scholar] [CrossRef]
- Dordas, C. Dry matter, nitrogen and phosphorus accumulation, partitioning and remobilization as affected by N and P fertilization and source–sink relations. Eur. J. Agron. 2009, 30, 129–139. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
- Fageria, N.K. Influence of micronutrients on dry matter yield and interaction with other nutrients in annual crops. Pesqui. Agropecuária Bras. 2002, 37, 1765–1772. [Google Scholar] [CrossRef]
- Roosta, H.R.; Mohsenian, Y. Effects of foliar spray of different Fe sources on pepper (Capsicum annum L.) plants in aquaponic system. Sci. Hortic. 2012, 146, 182–191. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus Acquisition and Utilization in Plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Lefever, K.; Laubscher, C.P.; Ndakidemi, P.A.; Nchu, F. Effects of pH and Phosphorus Concentrations on the Chlorophyll Responses of Salvia chamelaeagnea (Lamiaceae) Grown in Hydroponics. In Chlorophyll; InTech: Houston, TX, USA, 2017. [Google Scholar]
- Siedliska, A.; Baranowski, P.; Pastuszka-Woźniak, J.; Zubik, M.; Krzyszczak, J. Identification of plant leaf phosphorus content at different growth stages based on hyperspectral reflectance. BMC Plant Biol. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- El Naqma, K.; Elawady, R.; Ramadan, M.; Elsherpiny, M. Improving Soil Phosphorus Availability and Its Influence on Faba Bean performance: Exploring Mineral, Bio and Organic Fertilization with Foliar application of Iron and Zinc. Egypt. J. Soil Sci. 2024, 64, 619–630. [Google Scholar] [CrossRef]
- Mousavi, S.R. Zinc in Crop Production and Interaction with Phosphorus. Aust. J. Basic Appl. Sci. 2011, 5, 1503–1509. [Google Scholar]
- Derakhshani, Z.; Hassani, A.; Sadaghiani, M.H.R.; Hassanpouraghdam, M.B.; Khalifani, B.H.; Dalkani, M. Effect of Zinc Application on Growth and Some Biochemical Characteristics of Costmary (Chrysanthemum balsamita L.). Commun. Soil Sci. Plant Anal. 2011, 42, 2493–2503. [Google Scholar] [CrossRef]
- Asle-Mohammadi, Z.; Kharazmi, M.; Sheikhi, H.; Mohammadkhani, N.; Nicola, S. Foliar Application of Fe, Zn, and Mn as a Practical Strategy to Alleviate the Soil Cu Toxicity and Stimulate the Physiological and Biochemical Properties of Peppermint (Mentha piperita L.). J. Soil Sci. Plant Nutr. 2024, 24, 371–388. [Google Scholar] [CrossRef]
- Ranade-Malvi, U. Interaction of micronutrients with major nutrients with special reference to potassium. Karnataka J. Agric. Sci 2011, 24, 106–109. [Google Scholar]
- Du, M.; Zhang, W.; Gao, J.; Liu, M.; Zhou, Y.; He, D.; Zhao, Y.; Liu, S. Improvement of Root Characteristics Due to Nitrogen, Phosphorus, and Potassium Interactions Increases Rice (Oryza sativa L.) Yield and Nitrogen Use Efficiency. Agronomy 2021, 12, 23. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, S.S.; Mohapatra, T. Interaction Between Macro- and Micro-Nutrients in Plants. Front. Plant Sci. 2021, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Qin, L.; Bao, L.; Li, Y.; Li, X. Critical nutrient thresholds needed to control eutrophication and synergistic interactions between phosphorus and different nitrogen sources. Environ. Sci. Pollut. Res. 2016, 23, 21008–21019. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wu, W. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef]
- Moshtagh, S.; Aminpanah, H. Effects of Phosphorus Rate and Iron Foliar Application on Green Bean (Phaseolus vulgaris L.) Growth and Yield. Agric. Conspec. Sci. 2016, 80, 139–146. [Google Scholar]
- Santos, E.F.; Pongrac, P.; Reis, A.R.; Rabêlo, F.H.S.; Azevedo, R.A.; White, P.J.; Lavres, J. Unravelling homeostasis effects of phosphorus and zinc nutrition by leaf photochemistry and metabolic adjustment in cotton plants. Sci. Rep. 2021, 11, 13746. [Google Scholar] [CrossRef]
- Mardamootoo, T.; Du Preez, C.C.; Barnard, J.H. Phosphorus management issues for crop production: A review. Afr. J. Agric. Res. 2021, 17, 939–952. [Google Scholar]
- Guardiola-Márquez, C.E.; del Martínez-Ballesta, M.C.; García-Sánchez, C.V.; Bojorquez-Rodríguez, E.M.; Jacobo-Velázquez, D.A.; Martínez-Hernández, G.B. Effects of foliar and root application of Zn and Fe bio-nanofertilizers on the glucosinolate, Zn and Fe contents of Pak choi (Brassica rapa Subsp. Chinensis) grown under hydroponic and pot cultivation. Sci. Hortic. 2024, 338, 113654. [Google Scholar] [CrossRef]
- Lytra, K.; Tomou, E.; Chrysargyris, A.; Drouza, C.C.; Skaltsa, H.; Tzortzakis, N. Traditionally Used Sideritis cypria Post: Phytochemistry, Nutritional Content, Bioactive Compounds of Cultivated Populations. Front. Pharmacol. 2020, 11, 650. [Google Scholar] [CrossRef]
- Broschat, T.K.; Moore, K.K. Phytotoxicity of several iron fertilizers and their effects on Fe, Mn, Zn, Cu, and P content of African marigolds and zonal geraniums. HortScience 2004, 39, 595–598. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Tzortzakis, N. Iron and Zinc Foliar Spraying Affected Sideritis cypria Post. Growth, Mineral Content and Antioxidant Properties. Plants 2025, 14, 840. [Google Scholar] [CrossRef]
- Sahin, S.; Yucel, H.; Saglam, N.; Aydın, M.; Cakmak, P.; Gebologlu, N. Foliar Applications Of Ca, Zn, And Urea On Crispy Lettuce In Soilless Culture. Soil-Water J. 2013, 2, 23–30. [Google Scholar]
- Mahmoud, A.W.M.; Ayad, A.A.; Abdel-Aziz, H.S.M.; Williams, L.L.; El-Shazoly, R.M.; Abdel-Wahab, A.; Abdeldaym, E.A. Foliar Application of Different Iron Sources Improves Morpho-Physiological Traits and Nutritional Quality of Broad Bean Grown in Sandy Soil. Plants 2022, 11, 2599. [Google Scholar] [CrossRef] [PubMed]
- Nell, M.; Vötsch, M.; Vierheilig, H.; Steinkellner, S.; Zitterl-Eglseer, K.; Franz, C.; Novak, J. Effect of phosphorus uptake on growth and secondary metabolites of garden sage (Salvia officinalis L.). J. Sci. Food Agric. 2009, 89, 1090–1096. [Google Scholar] [CrossRef]
- Elsafy, M.; Tia, N.A.J.; Sir Elkhatim, K.A.; Othman, M.H.; Hassan, A.B.; Rahmatov, M.; Abdelhalim, T.S. Unveiling the influences of P fertilization on bioactive compounds and antioxidant activity in grains of four sorghum cultivars. PLoS ONE 2024, 19, e0311756. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef]
- Lytra, K.; Tomou, E.; Chrysargyris, A.; Christofi, M.; Miltiadous, P.; Tzortzakis, N.; Skaltsa, H. Bio-Guided Investigation of Sideritis cypria Methanol Extract Driven by in Vitro Antioxidant and Cytotoxic Assays. Chem. Biodivers. 2021, 18, e2000966. [Google Scholar] [CrossRef]
- Panayi, T.; Sarigiannis, Y.; Mourelatou, E.; Hapeshis, E.; Papaneophytou, C. Anti-quorum-sensing potential of ethanolic extracts of aromatic plants from the flora of Cyprus. Plants 2022, 11, 2632. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Petrovic, J.D.; Tomou, E.; Kyriakou, K.; Xylia, P.; Kotsoni, A.; Gkretsi, V.; Miltiadous, P.; Skaltsa, H.; Sokovi, M.D.; et al. Phytochemical Profiles and Biological Activities of Plant Extracts from Aromatic Plants Cultivated in Cyprus. Biology 2024, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Çarıkçı, S.; Kılıç, T.; Gören, A.C.; Dirmenci, T.; Alim Toraman, G.Ö.; Topçu, G. Chemical profile of the Anatolian Sideritis species with bioactivity studies. Pharm. Biol. 2023, 61, 1484–1511. [Google Scholar] [CrossRef] [PubMed]
- Szerement, J.; Szatanik-Kloc, A.; Mokrzycki, J.; Mierzwa-Hersztek, M. Agronomic Biofortification with Se, Zn, and Fe: An Effective Strategy to Enhance Crop Nutritional Quality and Stress Defense—A Review; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; Volume 22, ISBN 0123456789. [Google Scholar]
- Akinboye, A.O.; Adeyemo, R.O.; Karzis, J.; Petzer, I.M.; McGaw, L.J. Susceptibility patterns of Escherichia coli and streptococcal isolates from bovine mastitis cases to antibiotics and selected South African plant extracts with known antibacterial activities. S. Afr. J. Bot. 2024, 166, 14–26. [Google Scholar] [CrossRef]
- Lahmar, A.; Bedoui, A.; Mokdad-Bzeouich, I.; Dhaouifi, Z.; Kalboussi, Z.; Cheraif, I.; Ghedira, K.; Chekir-Ghedira, L. Reversal of resistance in bacteria underlies synergistic effect of essential oils with conventional antibiotics. Microb. Pathog. 2017, 106, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Saci, S.; Msela, A.; Saoudi, B.; Sebbane, H.; Trabelsi, L.; Alam, M.; Ernst, B.; Benguerba, Y.; Houali, K. Assessment of antibacterial activity, modes of action, and synergistic effects of Origanum vulgare hydroethanolic extract with antibiotics against avian pathogenic Escherichia coli. Fitoterapia 2024, 177, 106055. [Google Scholar] [CrossRef]
- Farhat, G.; Cheng, L.; Al-Dujaili, E.A.S.; Zubko, M. Antimicrobial Potential of Pomegranate and Lemon Extracts Alone or in Combination with Antibiotics against Pathogens. Int. J. Mol. Sci. 2024, 25, 6943. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Kyriacou, M.; Soteriou, G.A.; Graziani, G.; De Pascale, S.; Rouphael, Y. Zinc biofortification of hydroponically grown basil: Stress physiological responses and impact on antioxidant secondary metabolites of genotypic variants. Front. Plant Sci. 2022, 13, 1049004. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).