The Effect of Pesticide Solutions on the Deposition of Bubble-Containing Droplets
Abstract
1. Introduction
2. Materials and Methods
2.1. Generation Method of Bubble-Containing Droplets
2.2. Pesticide Solutions and Deposition Targets
2.3. Dynamic Viscosity Measurement Method for the Liquid
3. Results and Discussion
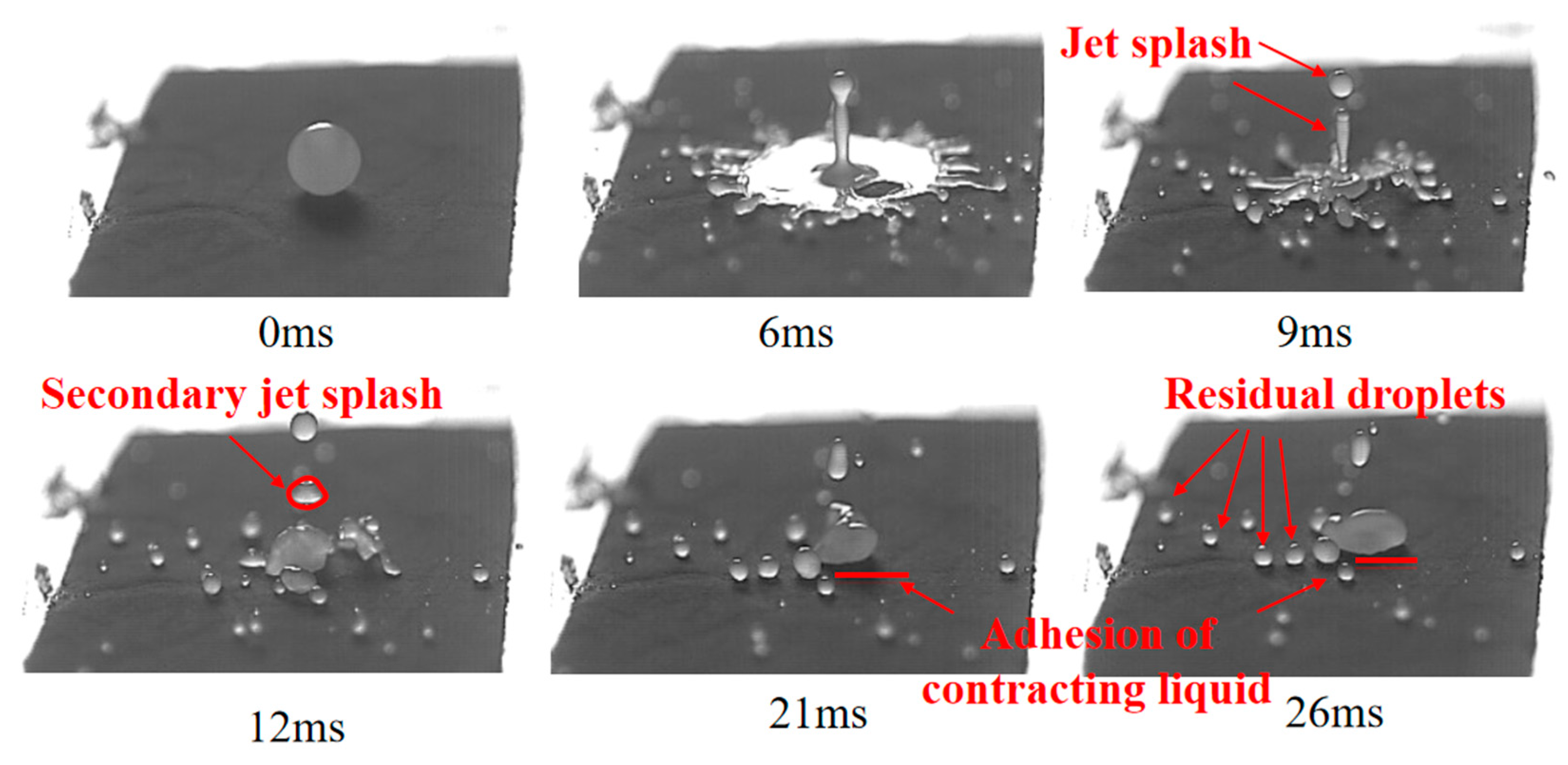
3.1. Deposition of Bubble-Containing Droplets on Hydrophilic Surfaces
3.1.1. Deposition of the Oil-Based Emulsion Bubble-Containing Droplets on Hydrophilic Surfaces
3.1.2. Deposition of Suspension Solution Bubble-Containing Droplets on Hydrophilic Surfaces
3.1.3. Deposition of Aqueous Solution Bubble-Containing Droplets on Hydrophilic Surfaces
3.2. Deposition of Bubble-Containing Droplets on Hydrophobic Plant Leaves
3.2.1. Deposition of Oil-Based Emulsion Bubble-Containing Droplets on Hydrophobic Leaf Surfaces
3.2.2. Deposition of Suspension Bubble-Containing Droplets on Hydrophobic Leaf Surfaces
3.2.3. Deposition of Aqueous Solutions Bubble-Containing Droplets on Hydrophobic Leaf Surfaces
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, W.-C.; Qiu, B.-J.; Xue, X.-Y.; Chen, C.; Xu, Z.-F.; Zhou, Q.-Q. Droplet deposition and control effect of insecticides sprayed with an unmanned aerial vehicle against plant hoppers. Crop Prot. 2016, 85, 79–88. [Google Scholar] [CrossRef]
- Wu, S.; Liu, J.Z.; Wang, J.S.; Hao, D.H.; Wang, R.K. The motion of strawberry leaves in an air-assisted spray field and its influence on droplet deposition. Trans. Asabe 2021, 64, 83–93. [Google Scholar] [CrossRef]
- Zhou, Q.; Xue, X.; Chen, C.; Cai, C.; Jiao, Y. Canopy deposition characteristics of different orchard pesticide dose models. Int. J. Agric. Biol. Eng. 2023, 16, 1–6. [Google Scholar] [CrossRef]
- Makhnenko, I.; Alonzi, E.R.; Fredericks, S.A.; Colby, C.M.; Dutcher, C.S. A review of liquid sheet breakup: Perspectives from agricultural sprays. J. Aerosol Sci. 2021, 157, 105805. [Google Scholar] [CrossRef]
- Li, J.; Nie, Z.; Chen, Y.; Ge, D.; Li, M. Development of boom posture adjustment and control system for wide spray boom. Agriculture 2023, 13, 2162. [Google Scholar] [CrossRef]
- Szarka, A.Z.; Kruger, G.R.; Golus, J.; Rodgers, C.; Perkins, D.; Brain, R.A. Spray drift deposition comparison of fluorimetry and analytical confirmation techniques. Pest Manag. Sci. 2021, 77, 4192–4199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhai, C.; Zhang, M.; Zhang, C.; Zou, W.; Zhao, C. Staggered-Phase spray control: A method for eliminating the inhomogeneity of deposition in low-frequency pulse-width modulation (PWM) variable spray. Agriculture 2024, 14, 465. [Google Scholar] [CrossRef]
- Bueno, M.R.; da Cunha, J.P.A.R.; de Santana, D.G. Assessment of spray drift from pesticide applications in soybean crops. Biosyst. Eng. 2017, 154, 35–45. [Google Scholar] [CrossRef]
- Liao, J.; Hewitt, A.J.; Wang, P.; Luo, X.; Zang, Y.; Zhou, Z.; Lan, Y.; O’Donnell, C. Development of droplet characteristics prediction models for air induction nozzles based on wind tunnel tests. Int. J. Agric. Biol. Eng. 2019, 12, 1–6. [Google Scholar] [CrossRef]
- Liao, J.; Luo, X.; Wang, P.; Zhou, Z.; O’Donnell, C.C.; Zang, Y.; Hewitt, A.J. Analysis of the influence of different parameters on droplet characteristics and droplet size classification categories for air induction nozzle. Agronomy 2020, 10, 256. [Google Scholar] [CrossRef]
- Butts, T.R.; Luck, J.D.; Fritz, B.K.; Hoffmann, W.C.; Kruger, G.R. Evaluation of spray pattern uniformity using three unique analyses as impacted by nozzle, pressure, and pulse-width modulation duty cycle. Pest Manag. Sci. 2019, 75, 1875–1886. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.C.; Chechetto, R.G.; O’Donnell, C.C.; Dorr, G.J.; Moore, J.H.; Baker, G.J.; Powis, K.J.; Hewitt, A.J. Determining the drift potential of Venturi nozzles compared with standard nozzles across three insecticide spray solutions in a wind tunnel. Pest Manag. Sci. 2016, 72, 1460–1466. [Google Scholar] [CrossRef]
- Dafsari, R.A.; Yu, S.; Choi, Y.; Lee, J. Effect of geometrical parameters of air-induction nozzles on droplet characteristics and behaviour. Biosyst. Eng. 2021, 209, 14–29. [Google Scholar] [CrossRef]
- McArtney, S.J.; Obermiller, J.D. Comparative performance of air-induction and conventional nozzles on an axial fan sprayer in medium density apple orchards. Horttechnology 2008, 18, 365–371. [Google Scholar] [CrossRef]
- De Cock, N.; Massinon, M.; Salah, S.O.T.; Lebeau, F. Investigation on optimal spray properties for ground based agricultural applications using deposition and retention models. Biosyst. Eng. 2017, 162, 99–111. [Google Scholar] [CrossRef]
- Shi, Q.; Mao, H.; Guan, X. Numerical simulation and experimental verification of the deposition concentration of an unmanned aerial vehicle. Appl. Eng. Agric. 2019, 35, 367–376. [Google Scholar] [CrossRef]
- Song, M.; Hu, D.; Zheng, X.; Wang, L.; Yu, Z.; An, W.; Na, R.; Li, C.; Li, N.; Lu, Z.; et al. Enhancing droplet deposition on wired and curved superhydrophobic leaves. ACS Nano 2019, 13, 7966–7974. [Google Scholar] [CrossRef]
- Xi, T.; Li, C.; Qiu, W.; Wang, H.; Lv, X.; Han, C.; Ahmad, F. Droplet deposition behavior on a pear leaf surface under wind-induced vibration. Appl. Eng. Agric. 2020, 36, 913–926. [Google Scholar] [CrossRef]
- Cheng, X.; Sun, T.-P.; Gordillo, L. Drop impact dynamics: Impact force and stress distributions. Annu. Rev. Fluid Mech. 2022, 54, 57–81. [Google Scholar] [CrossRef]
- Philippi, J.; Lagree, P.-Y.; Antkowiak, A. Drop impact on a solid surface: Short-time self-similarity. J. Fluid Mech. 2016, 795, 96–135. [Google Scholar] [CrossRef]
- Yarin, A.L. Drop impact dynamics: Splashing, spreading, receding, bouncing. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, Y.; Xu, E.; Meng, X.; Wu, G.; Li, H.Z. Dynamical behaviors of water drop impact on superhydrophobic surfaces with the macro-textured cavity. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 657, 130583. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, M.; Sun, Z.; Li, L.-J.; Guo, X.-Y.; Xu, Y.; Wu, X.-M. Regulating droplet impact and wetting behaviors on hydrophobic leaves using a nonionic surfactant. J. Colloid Interface Sci. 2023, 629, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yu, M.; Sun, Z.; Pan, S.-H.; Wang, Y.-M.; Ma, Y.-J.; Guo, X.-Y.; Xu, Y.; Wu, X.-M. Effect of hydrophilic chain length on the impact and wetting behavior of nonionic surfactants regulating droplets on hydrophobic surfaces. Surf. Interfaces 2023, 41, 103260. [Google Scholar] [CrossRef]
- Sanjay, V.; Chantelot, P.; Lohse, D. When does an impacting drop stop bouncing? J. Fluid Mech. 2023, 958, A26. [Google Scholar] [CrossRef]
- Hu, J.; Liu, C.; Wang, Z.; Li, Y.; Song, J.; Liu, Y.; Chu, X. Motion model for describing the quantity of air in droplets through changing the structure of air induction nozzle. Int. J. Agric. Biol. Eng. 2021, 14, 35–40. [Google Scholar] [CrossRef]
- Post, S.L. Drift of droplets from air-induction nozzles. Trans. Asabe 2019, 62, 1683–1687. [Google Scholar] [CrossRef]
- Gong, C.; Li, D.; Kang, C. Effect of oil-based emulsion on air bubbles in the spray sheet produced through the air-induction nozzle. Pest Manag. Sci. 2022, 78, 5347–5357. [Google Scholar] [CrossRef]
- Gulyaev, I.P.; Solonenko, O.P. Hollow droplets impacting onto a solid surface. Exp. Fluids 2013, 54, 1432. [Google Scholar] [CrossRef]
- Li, D.; Zhang, D.; Zheng, Z. Numerical analysis of hollow droplet impacts on a dry flat surface. Int. J. Heat Mass Transf. 2019, 129, 753–763. [Google Scholar] [CrossRef]
- Nasiri, M.; Amini, G.; Moreau, C.; Dolatabadi, A. Hollow droplet impact on a solid surface. Int. J. Multiph. Flow 2021, 143, 103740. [Google Scholar] [CrossRef]
- Yang, W.; Jia, W.; Ou, M.; Zhong, W.; Jiang, L.; Wang, X. Effect of Physical Properties of an Emulsion Pesticide on the Atomisation Process and the Spatial Distribution of Droplet Size. Agriculture 2022, 12, 949. [Google Scholar] [CrossRef]
- Yang, W.; Zhong, W.; Jia, W.; Ou, M.; Dong, X.; Zhang, T.; Ding, S.; Yu, P. The effect of oil-in-water emulsion pesticide on the evolution of liquid sheet rim disintegration and the spraying distribution. Crop Prot. 2024, 177, 106547. [Google Scholar] [CrossRef]
- Cao, C.; Liu, M.; Ma, X.; Chen, Y.; Huang, Q. Enhancing droplets deposition on superhydrophobic plant leaves by bio-based surfactant: Experimental characterization and molecular dynamics simulations. J. Mol. Liq. 2023, 387, 122696. [Google Scholar] [CrossRef]




| Concentration | Surface Tension (m N/m) | Dynamic Viscosity (mPa·s) |
|---|---|---|
| 0% | 72.14 | 0.83 |
| 0.1% | 43.40 | 0.81 |
| 0.2% | 38.76 | 0.78 |
| 0.4% | 34.73 | 0.80 |
| 0.8% | 33.64 | 0.86 |
| Concentration | Surface Tension (m N/m) | Dynamic Viscosity (mPa·s) |
|---|---|---|
| 0% | 72.14 | 0.83 |
| 0.1% | 64.42 | 1.04 |
| 0.2% | 53.60 | 1.13 |
| 0.4% | 45.93 | 1.20 |
| 0.8% | 37.33 | 1.21 |
| Concentration | Surface Tension (m N/m) | Dynamic Viscosity (mPa·s) |
|---|---|---|
| 0% | 72.14 | 0.83 |
| 0.0025% | 70.29 | 0.87 |
| 0.0050% | 64.91 | 0.86 |
| 0.01% | 52.68 | 0.83 |
| 0.02% | 31.10 | 0.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Jia, F.; Gong, C.; Kang, C. The Effect of Pesticide Solutions on the Deposition of Bubble-Containing Droplets. Agronomy 2025, 15, 1172. https://doi.org/10.3390/agronomy15051172
Yan M, Jia F, Gong C, Kang C. The Effect of Pesticide Solutions on the Deposition of Bubble-Containing Droplets. Agronomy. 2025; 15(5):1172. https://doi.org/10.3390/agronomy15051172
Chicago/Turabian StyleYan, Mingzhi, Feng Jia, Chen Gong, and Can Kang. 2025. "The Effect of Pesticide Solutions on the Deposition of Bubble-Containing Droplets" Agronomy 15, no. 5: 1172. https://doi.org/10.3390/agronomy15051172
APA StyleYan, M., Jia, F., Gong, C., & Kang, C. (2025). The Effect of Pesticide Solutions on the Deposition of Bubble-Containing Droplets. Agronomy, 15(5), 1172. https://doi.org/10.3390/agronomy15051172






