Abstract
The Colorado Potato Beetle (CPB, Leptinotarsa decemlineata Say, Coleoptera: Chrysomelidae) remains a destructive agricultural pest worldwide that continually overcomes conventional control methods. In recent years, RNA interference (RNAi) has emerged as an alternative for its management; however, although promising results have been reported, its effectiveness has been influenced by several factors, including the length of double-stranded RNA (dsRNA), the delivery method, stability, and especially the selection of the target gene. In this study, we designed and synthesized 290 bp dsRNAs targeting the SERCA and CPR genes from L. decemlineata, which encode the Sarco/Endoplasmic Reticulum Ca2⁺-ATPase and NADPH–Cytochrome P450 Reductase, respectively. Both dsRNAs successfully reduced transcript levels in larvae, with dsSERCA achieving ~60% knockdown by day 3 and dsCPR achieving ~50% knockdown by day 7. Furthermore, both treatments affected the larval growth and survival rate. However, while the dsCPR-treated larvae showed a 59% reduction in weight gain, the administration of dsSERCA had a strong phenotypic effect on the larvae, leading to decreased feeding, a 50.4% reduction in weight gain, and ultimately, 100% mortality. These results suggest that the SERCA and CPR genes could be promising targets for L. decemlineata control and emphasize the importance of appropriate target gene selection for RNAi silencing, as well as the need to explore and validate new genes for RNAi-mediated pest management.
1. Introduction
Potato plants (Solanum tuberosum L.) are considered the world’s most important non-cereal crop. In 2023 alone, more than 17 million hectares of potato plants were cultivated in over 150 countries, yielding approximately 383 million metric tons of potato with an estimated market value of USD 123 billion [1], highlighting its fundamental role in global food security.
Despite continuous advancements in productivity, evidenced by an increase of 9.6 million metric tons from 2022 to 2023, potato production remains highly vulnerable to pest infestations. Collectively, pests are responsible for annual yield losses of up to 40%, representing a major threat to food security [2,3,4]. Among these, L. decemlineata is one of the most destructive pests; each of its larvae can defoliate up to 40 cm2 of foliage [5]; however, under severe infestation, total crop losses are frequent, resulting in substantial reductions in tuber yield [6,7].
To reduce the economic impact caused by L. decemlineata, some management strategies have been used, including cultural practices and biological control [8,9,10,11]. However, the long-term effectiveness of these approaches has been severely compromised; therefore, chemical control remains the main control strategy, but the repeated use of insecticides has led to reduced efficacy. Although the precise mechanism of the rapid adaptation of L. decemlineata to new toxins is not yet fully understood [12,13,14], factors such as poor pest management practices, over-reliance on chemical control, and the beetle’s exceptional genetic adaptability—facilitated by an extensive network of xenobiotic detoxification pathways—have contributed to the evolution of resistance [12,15,16]. To date, L. decemlineata has developed resistance against 57 active ingredients [17], earning it the designation of a “super pest”.
The growing resistance of agricultural pests to pesticides has driven a continuous increase in global insecticide application rates worldwide, rising from 1.23 kg ha⁻1 in 1990 to 2.23 kg ha⁻1 in 2022 [18]. However, this intensive use has not only caused the development of insect resistance but also led to environmental and public health problems [19,20], underscoring the need for alternative control methods with greater long-term efficacy.
In recent years, RNAi has emerged as a next-generation approach for pest control [21]. It is based on a conserved post-transcriptional gene silencing mechanism triggered by dsRNA. This dsRNA is processed by the RNase III enzyme Dicer (~200 kDa), generating small interfering RNAs (siRNAs) of a length of 21–24 nucleotides [22]. These siRNAs are subsequently incorporated into the RNA-induced silencing complex (RISC), which guides the degradation of complementary messenger RNA (mRNA), ultimately preventing protein translation [23].
Taking advantage of this mechanism, RNAi has been implemented to silence genes in a wide range of species, including Bemisia tabaci [24,25], Tetranychus urticae [26], Helicoverpa armigera [27,28], Spodoptera litura [29], Chilo partellus [29], Plutella xylostella [29,30], Maruca vitrata [29], Tuta absoluta [31], Myzus persicae [32,33], Diabrotica virgifera virgifera [34,35], Apolygus lucorum [36,37], Sitobion avenae [38,39], Chilo suppressalis [40,41], and Diaphorina citri [42,43].
L. decemlineata has also been targeted by RNAi. To date, more than 26 genes have been silenced, including β-Actin, Sec23, ATPase, COPβ, Mesh, Ran, Tai, EcR, HR3, HR4, CncC, Prohibitin-1, FTZ-F1, Shd, SAHase, NAT1, alt, UAP, TPS, TRE, JHAMT, ILP, JHEH, CHS, p5cdh, E75, and ACE [14,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. These studies have demonstrated significant phenotypic effects, such as developmental defects, reduced feeding, and increased mortality; however, the magnitude and timing of these effects have varied depending on the gene targeted.
Despite the advances in RNAi technology for pest management, its large-scale implementation remains limited. Currently, only two RNAi-based products—SmartStax PRO® [65,66,67] and CalanthaTM (Ledprona) [68,69]—are commercially available. The limited number of available RNAi-based products may be due to their high production costs and strict regulatory requirements [70,71,72]. However, one of the greatest challenges lies in the technical limitations of RNAi. While gene silencing has been successfully achieved in several organisms, factors such as species-specific responses, dsRNA delivery methods, dsRNA processing efficiency, and the identification of optimal target genes are still under investigation [44,73,74,75,76].
In this context, two key genes in L. decemlineata, CPR and SERCA, stand out due to their essential roles in several biochemical pathways critical for insect survival. The CPR gene encodes NADPH–Cytochrome P450 Reductase (CPR), an enzyme responsible for transferring electrons from Nicotinamide Adenine Dinucleotide Phosphate (NADPH) to Cytochrome P450s [77,78], enabling vital functions such as detoxification, hormone metabolism, pheromone processing, and cuticular hydrocarbon synthesis [78,79,80,81,82,83]. Therefore, silencing CPR disrupts these metabolic processes, increasing the insect’s sensitivity to xenobiotics and reducing its survival in adverse environments [80,84,85].
Similarly, the SERCA gene plays a fundamental role in cellular homeostasis by regulating intracellular calcium levels. It encodes the Sarco/Endoplasmic Reticulum Ca2⁺-ATPase (SERCA), a P-type ATPase responsible for transporting Ca2⁺ from the cytosol into the Sarcoplasmic and Endoplasmic Reticulum (SR/ER) [86]. By maintaining low cytosolic calcium concentrations and higher levels within organelles [87,88,89,90], SERCA supports essential biological processes such as muscle contraction, neuronal signaling, hormonal secretion, and stress responses [91,92,93,94]. Disruptions in SERCA function result in cytotoxic calcium accumulation, triggering developmental defects, impaired mobility, and increased mortality [95,96,97]. Due to its critical role in insect physiology, SERCA has been proposed as a potential target for RNAi-based pest control; however, it has not yet been silenced in L. decemlineata.
In this study, we investigated the RNAi-mediated silencing of the SERCA and CPR genes in L. decemlineata to identify which gene silencing induces the most significant effects, including transcript reduction, developmental impairments, and mortality, and analyze how these effects evolve over time. Identifying the gene with the greatest impact will provide valuable insights into RNAi-based pest control strategies, paving the way for more effective and targeted approaches.
2. Materials and Methods
2.1. Selection of Target Genes
The SERCA and CPR genes were selected for silencing because their products are involved in key metabolic processes of L. decemlineata: SERCA encodes the Sarcoplasmic/Endoplasmic Reticulum-type calcium-transporting ATPase, whereas CPR encodes the NADPH–Cytochrome P450 Reductase. Their gene sequences were retrieved from the National Center for Biotechnology Information (NCBI) database (accession numbers MW194237 and MN275229, respectively).
2.2. In Silico Analysis of RNAi Effectiveness and Off-Target Effects
Fragments of the SERCA and CPR genes were analyzed for their theoretical RNA interference effectiveness and potential off-target effects using the QUT Nicotiana benthamiana RNA Target Tester (https://benthgenome.qut.edu.au/tools/TKsoft3x.php (accessed on 23 June 2024)). The analysis was performed against gene sequences from Homo sapiens, Bos taurus, Mus musculus, Oryctolagus cuniculus, Drosophila melanogaster, T. urticae, Tribolium castaneum, Apis mellifera, Copidosoma floridanum, Coccinella septempunctata, H. armigera, B. tabaci, Trichogramma pretiosum, M. persicae, D. virgifera virgifera, Chrysoperla carnea, Solenopsis invicta, and Frankliniella occidentalis. The accession numbers for these sequences can be found in Supplementary Material Table S1.
2.3. Synthesis and Cloning of SERCA and CPR Fragments
SERCA and CPR gene fragments were processed using the software SnapGene 5.4.3 (www.snapgene.com (accessed on 2 February 2018)), adding KpnI and XbaI restriction sites to the 5′ and 3′ ends of the SERCA sequence, and SwaI and AvrII restriction sites to the 5′ and 3′ ends of the CPR sequence for cloning. To ensure that the observed effects of dsRNA were due to the target gene, both sequences had the same length, with a 40% GC content. The final sequences were synthesized by GeneScript Inc. (Piscataway, NJ, USA) and cloned into the pBJC9 vector (GenBank: PV425440). Recombinant vectors were introduced into the Escherichia coli strain DH5α (Invitrogen®, Carlsbad, CA, USA) for plasmid maintenance and propagation.
2.4. In Vitro dsRNA Synthesis
The in vitro synthesis of dsRNA was conducted using the T7 RiboMAX™ Express RNAi System Kit (Promega, cat. P1700, Madison, WI, USA), in accordance with the manufacturer’s protocol. Plasmid DNAs containing both sequences were extracted from E. coli DH5α using the QIAGEN Plasmid Midiprep Kit (QIAGEN®, Valencia, CA, USA). The plasmid DNA was subsequently used to generate templates for in vitro transcription. For each dsRNA, both sense and antisense strands were amplified using custom primers designed using Primer3 (https://primer3.ut.ee (accessed on 18 July 2024)) and synthesized by Integrated DNA Technologies (IDT, Coralville, IA, USA). The minimal T7 promoter sequence (5′TAATACGACTCACTATAGG3′) was added to the 5′ end of both primers (Table 1). The amplifications were performed under the following conditions: 3 min of denaturation at 94 °C, followed by 40 cycles of amplification (45 s at 94 °C, 45 s at 61 °C, 1 min at 72 °C), and a final extension step of 5 min at 72 °C.

Table 1.
Primers used for in vitro dsRNA synthesis.
The transcription reaction was prepared using 10 µL of RiboMAX™ Express T7 buffer, 2 µL of T7 Express Enzyme Mix, and 200 ng of both sense and antisense DNA that were previously amplified, to reach a final volume of 20 µL. The reaction mixture was incubated at 37 °C for 1 h, followed by a heat treatment at 70 °C for 10 min. After incubation, the mixture was cooled gradually to room temperature over 20 min to promote proper RNA strand alignment. To remove any single-stranded RNA (ssRNA) and template DNA, the reaction was treated with RNase A (Thermo Fisher Scientific, Waltham, MA, USA) and DNase I (Thermo Fisher Scientific) at 37 °C for 30 min. Subsequently, dsRNA was precipitated using 3 M sodium acetate (pH 5.2) and isopropanol. The obtained pellet was washed with cold 70% ethanol and resuspended in nuclease-free water. The integrity of the dsRNA was resolved using electrophoresis on a 1.8% agarose gel, and its concentration was determined by measuring the absorbance at 260 nm using a UV/VIS NanoDrop One Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The dsRNA was stored at −70 °C until use.
2.5. Insect Growth Conditions
Adult beetles of L. decemlineata were collected from the field on silverleaf nightshade (Solanum elaeagnifolium Cav.) and buffalobur (Solanum rostratum Dunal) plants in Chihuahua, Chihuahua, Mexico (28°43′47″ N, 105°58′11″ W), during the period of August and September 2024. The beetles were maintained in a controlled environment within a terrarium, set to a temperature of 26 ± 2 °C, with relative humidity between 50% and 60%, and a 14 h light–10 h dark photoperiod (1200 lux). Silverleaf nightshade plants were provided as a food source.
To ensure a uniform larval size for growth and survival assays, adult beetles were allowed to lay eggs, which were subsequently collected and placed on fresh silverleaf nightshade leaves to hatch. The newly hatched first-instar larvae were given young leaves from one-month-old silverleaf nightshade plants to feed on.
For RNAi feeding assays, synchronized second-instar larvae were selected, individually weighed, and grouped into three biological replicates, each containing 30 larvae. Prior to the experiment, the larvae were starved for 6 h. Each group was then placed into Petri dishes and fed with 23 cm2 leaf discs of silverleaf nightshade, which were treated with dsRNA diluted in nuclease-free water to a final concentration of 8 ng cm⁻2 [98]. Silverleaf nightshade leaves painted with nuclease-free water were used as controls [98]. The leaves were replaced with fresh dsRNA-painted leaves every 24 h for 7 d. The larvae were weighed on days 3, 5, and 7 of the feeding assays, and mortality was recorded daily. At the end of the experiment, the larvae were stored at −70 °C until processing.
2.6. RNA Extraction
Frozen larvae were homogenized in liquid nitrogen, and 50–100 mg of powder was used to extract RNAi using the Total RNA Purification Kit (Jena Bioscience, Jena, Germany) following the manufacturer’s instructions. The RNA was treated with DNase I (Thermo Fisher Scientific) to eliminate residual DNA contamination. To verify RNA integrity, electrophoresis was performed with a 1% agarose gel. The RNA concentration was determined by measuring the absorbance at 260 nm using a UV/VIS NanoDrop One Spectrophotometer (Thermo Fisher Scientific).
2.7. Quantitative Real-Time PCR (qPCR)
qPCR was used to determine the expression levels of the SERCA and CPR genes in larval tissues. The primers for qPCR were designed using Primer3 (https://primer3.ut.ee (accessed on 18 July 2024)) and the RealTime qPCR tool from IDT (https://www.idtdna.com/scitools/ (accessed on 18 July 2024)), followed by further analysis using the OligoAnalyzer™ Tool (https://www.idtdna.com/calc/analyzer (accessed on 18 July 2024)). The specific primers used to amplify the SERCA gene were Fw—5′TTCCAGCGGAAGAAGGAAAG3′ and Rv—5′TTCTACGAAGGCCGTGAATG3′, whereas the primers used to amplify the CPR gene were Fw—5′CGCAGTCGTCAGCTTCTATT3′ and Rv—5′CGAGGTATCCGATGTACTTTGT3′. Additionally, the primers Fw—5′GCGGGAGAATGTACAGAGGA3′ and Rv—5′AAGTCTTCACGGAGCTTGGA3′ were used to amplify the Rps18 gene [99]. All primers were synthesized by IDT.
For cDNA synthesis, SuperScript® IV Reverse Transcriptase (Thermo Fisher Scientific) was used according to the manufacturer’s guidelines. The reverse primers were aligned with the template RNA, and the reaction mixture contained the following: 2 µM of gene-specific reverse primer, 10 mM dNTP mix (Promega), and 500 ng of RNA template in a final volume of 13 µL. The reaction mixture was incubated at 65 °C for 5 min, followed by cooling on ice for 1 min. The RT reaction was prepared by mixing 5X SSIV Buffer, 100 mM DTT, and 200 units of SuperScript IV® Reverse Transcriptase in a final volume of 7 µL. RT mixture was then added to the aligned RNA and incubated at 55 °C for 10 min, followed by 80 °C for 10 min to inactivate the reaction.
qPCR was performed using 5 µL of iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA), 5 µM of forward primer, 5 µM of reverse primer, and 2 µL of cDNA, resulting in a final reaction volume of 10 µL. The mixture was placed in the CFX96 Real-Time System (Bio-Rad) and subjected to the following thermal cycling conditions: an initial denaturation step at 95 °C for 1 min, followed by 39 amplification cycles (denaturation at 95 °C for 10 s, annealing at 52 °C for 30 s, and extension at 65 °C for 5 s). The data were normalized to the mRNA levels of the Rps18 gene, which encodes ribosomal protein S18 in L. decemlineata [99]. The relative expression levels of the SERCA and CPR genes were calculated using the ΔΔCt method [100]. Each reaction was repeated three times to minimize the intra-experimental variation [99], and the results were analyzed with the software Bio-Rad CFX Manager 3.1.
2.8. Statistical Analysis
The data analysis was performed using the statistical software Minitab® 20.3. A one-way ANOVA was conducted with a significance level set at p ≤ 0.05. Post hoc comparisons were made using Tukey’s mean separation test.
3. Results
Sarco/Endoplasmic Reticulum Ca2⁺-ATPase and NADPH–Cytochrome P450 Reductase are two crucial enzymes involved in distinct metabolic pathways of L. decemlineata and are therefore considered potential targets for pest control. To investigate the effects of gene silencing on SERCA and CPR, the coding sequences of both genes were retrieved from the NCBI (SERCA: 3060 bp, MW194237, and CPR: 2040 bp, MN275229). From each sequence, a 290 bp fragment was extracted, from nucleotide +1 to nucleotide 290 (Figure 1). These sequences were deposited in GenBank under the accession numbers PV233719 and PV233720, respectively. A bioinformatics analysis was conducted to evaluate the silencing potential of the fragments using the RNA Target Tester, which generated all possible 21 bp oligomers. The results indicated a strong RNA interference potential for both fragments: the 290 bp fragments from the SERCA and CPR genes produced 270 unique siRNAs, each with a potency score of 100% (Figure 2). We performed an analysis with RNAcofold to determine the thermodynamic stability of the sequences; the results showed that the dsRNA targeting the SERCA gene (dsSERCA) had a ΔG (minimum free energy, MFE) of −546.20 kcal/mol, with a frequency of 13.73% in the thermodynamic ensemble, suggesting a higher tendency to adopt a single, stable conformation. In contrast, the dsRNA targeting the CPR gene (dsCPR) showed a ΔG of −555.80 kcal/mol with a frequency of 17.95%, indicating greater stability compared to dsSERCA, along with a lower propensity to form multiple conformations.
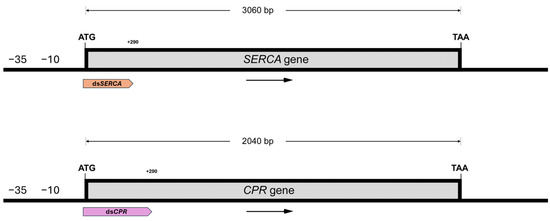
Figure 1.
RNA sequences designed for the silencing of the SERCA gene (GenBank: MW194237) and the CPR gene (GenBank: MN275229) in L. decemlineata. Both sequences target the 5′ region of the genes starting from position +1 and exhibit a GC content of 40%.
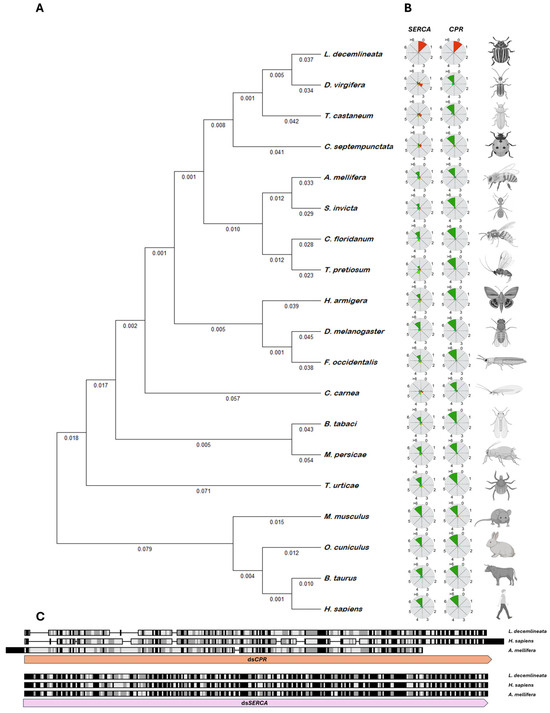
Figure 2.
(A) Phylogenetic tree based on concatenated nucleic acid sequences from the SERCA and CPR genes of L. decemlineata, B. taurus, M. musculus, O. cuniculus, D. melanogaster, T. urticae, T. castaneum, A. mellifera, C. floridanum, C. septempunctata, H. armigera, B. tabaci, T. pretiosum, M. persicae, D. virgifera virgifera, C. carnea, S. invicta, F. occidentalis, and H. sapiens, constructed using the Neighbor-Joining Method with the software Molecular Evolutionary Genetics Analysis (MEGA) 11.0.10. (B) Theoretical potency analysis of each species was calculated using the QUT Nicotiana benthamiana RNA Target Tester Platform. Circles indicate the theoretical effectiveness of siRNAs produced by each dsRNA sequence designed for L. decemlineata. Twenty-one nucleotide siRNA sequences with 0, 1, or 2 mismatches are expected to target gene cleavage (red), while those with >3 mismatches are expected to have no significant RNAi effect (green). Sequences shown in orange are classified as potentially having an RNAi effect. (C) Alignment of CPR and SERCA coding sequences (+1 to +290 bp) from H. sapiens, A. mellifera, and L. decemlineata. Conserved residues are marked in gray and black boxes. The lower boxes indicate the target region of the dsSERCA and dsCPR sequences.
Concurrently, an off-target analysis was performed to assess the potential interference of the designed fragments with the SERCA and CPR genes from a range of organisms, including B. taurus, M. musculus, O. cuniculus, D. melanogaster, T. urticae, T. castaneum, A. mellifera, C. floridanum, C. septempunctata, H. armigera, B. tabaci, T. pretiosum, M. persicae, D. virgifera virgifera, C. carnea, S. invicta, F. occidentalis, and H. sapiens. The analysis confirmed that both designed sequences exhibited more than three mismatches per 21-mer, suggesting minimal off-target effects. This indicates a high level of specificity and a reduced risk of unintended silencing (Figure 2B,C). Consequently, both sequences were designed with appropriate restriction sites at the 5′ and 3′ ends, respectively. The final sequences were synthesized by GenScript Inc. and cloned into the pBJC9 vector, resulting in the constructs pMAM1 (SERCA—290 bp) and pMAM2 (CPR—290 bp). The vector sequences were deposited in GenBank under the accession numbers PV261951 and PV261952, respectively.
3.1. In Vitro Synthesis of dsRNA Targeting SERCA and CPR Sequences
Larval feeding assays were performed using the dsRNA targeting the SERCA and CPR genes. The pMAM1 and pMAM2 vectors, which harbor the SERCA and CPR gene fragments from L. decemlineata, respectively, were used as templates for the in vitro synthesis of dsRNA, yielding fragments of approximately 290 bp, corresponding to the expected sizes of the designed sequences (Figure 3A).
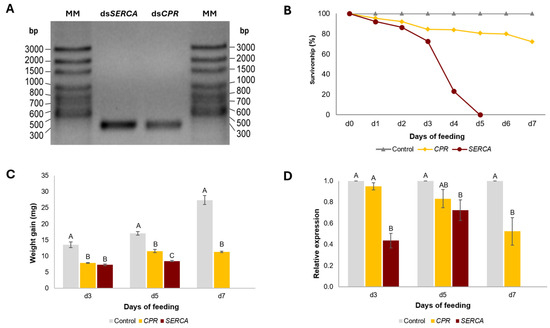
Figure 3.
Synthesis and feeding assays of dsSERCA and dsCPR in Leptinotarsa decemlineata. (A) In vitro synthesis of dsSERCA and dsCPR (290 bp). (B) Survivorship percentage. (C) Average larval weight after days 3, 5, and 7 of treatment with dsSERCA and dsCPR. (D) Relative expression levels of SERCA and CPR genes in L. decemlineata larvae. Data are presented as means ± standard errors. Letters above each bar indicate significant differences between groups, determined by one-way ANOVA followed by Tukey’s test in Minitab® 20.3.
3.2. Effects of Larval Feeding with dsRNA Targeting SERCA and CPR Genes
Second-instar larvae of L. decemlineata were selected for the insecticidal bioassays, given their higher susceptibility to dsRNA compared to third-instar larvae [101]. In the bioassays, larvae that consumed dsSERCA (8 ng cm⁻2) with a length of 290 bp exhibited high mortality levels throughout the experiment compared to the control. On day 1, mortality was 8%, increasing to 14% on day 2. By day 3, cumulative mortality reached 27%, and by day 4, it rose dramatically to 77%. Remarkably, 100% mortality was observed by day 5. In comparison, larvae fed with dsRNA targeting the CPR gene (dsCPR) exhibited a more gradual mortality progression. On day 1, mortality was 4%, increasing to 8% on day 2, and reaching 28% on day 7 (Figure 3B, Table A1).
Regarding weight gain, both treatments resulted in a significant weight reduction. Larvae fed with dsSERCA showed a 46.3% decrease in weight gain on day 3; these larvae stopped feeding, leading to a halt in their growth. As a result, weight gain decreased by 50.4% by day 5. In contrast, larvae fed with dsCPR exhibited a 42% reduction in weight gain on day 3, followed by a slight recovery on day 5 (9.8%). However, by day 7, weight gain had significantly decreased, with a 59% reduction compared to the control (Figure 3C, Table A2). Thus, both dsSERCA- and dsCPR-treated larvae exhibited smaller sizes; however, only the dsCPR-treated larvae showed mottling on the dorsum (Figure 4).
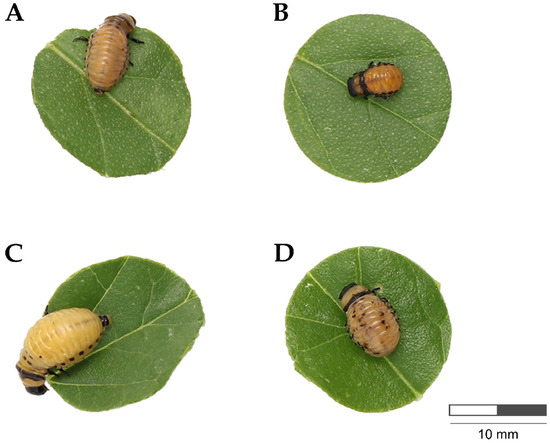
Figure 4.
Phenotype of L. decemlineata larvae fed with silverleaf nightshade leaves painted with dsSERCA and dsCPR (8 ng cm⁻2). (A) Larvae fed with leaves without dsRNA (control), showing the typical phenotype at day 5. (B) Larvae fed with leaves painted with dsSERCA at day 5. (C) Larvae fed with leaves without dsRNA (control), showing the typical phenotype at day 7. (D) Larvae fed with leaves painted with dsCPR at day 7.
In the bioassays, larvae fed leaves treated with dsSERCA showed a significant decrease in transcript levels. On day 3, transcript levels in dsSERCA-treated larvae were markedly reduced compared to those in the control (~60%). In contrast, larvae treated with dsCPR did not show a significant difference from the control at this time point. By day 5, transcript levels in dsSERCA-treated larvae continued to decline significantly (~24%). While larvae fed with dsCPR also showed a decrease in transcript levels, no significant difference was observed compared to day 3. However, by day 7, a substantial reduction in transcript levels was evident in the dsCPR-treated larvae (~50%) (Figure 3D, Table A3).
4. Discussion
Ensuring food security has always been a challenge, but with the global population projected to surpass 9.8 billion people in the next three decades [102], the certainty of food availability is becoming an urgent global issue. Given that approximately 80% of the food consumed originates from plants [103,104], enhancing agricultural production is critical to meet the growing global demand [105]. A decade ago, it was estimated that food production would need to increase by 70% to guarantee long-term food security [106,107]. However, later studies estimated an even more daunting level of 110% [108,109]. Achieving such levels of sustained production is complicated by the limited availability of arable land, the changing environmental conditions, market restrictions, and, most critically, the escalating threat of increasingly destructive pests [108,110,111,112].
Pests have long been a persistent challenge in agriculture, although only 10% of the 70,000 known pest species worldwide are considered serious threats [113]; they are responsible for approximately 40% of annual crop production losses [114]. Among these, L. decemlineata stands out due to its ability to rapidly defoliate infested crops, resulting in substantial losses and complicating control efforts [6]. While strategies such as biological control using Beauveria bassiana, C. carnea, and Pterostichus melanarius [8,9,11], as well as cultural control methods like crop rotation [10], have been implemented, the beetle remains a persistent problem. Chemical insecticides, although widely used, have also been losing effectiveness over time [17,69,115,116].
In the search for alternative pest control strategies that avoid the problems commonly associated with pesticides, RNAi has emerged as a revolutionary third-generation pest control strategy, offering high target specificity and significantly reduced environmental impact. Nevertheless, insects have shown they can also develop resistance to this technology, as observed in D. virgifera virgifera [117].
L. decemlineata has also been shown to be capable of developing resistance to RNAi [118], and due to its high genetic variability [7], different geographic populations may respond differently to RNAi-based treatments. This variability has already been observed with the commercial dsRNA product Calantha™, which targets the gene PSMB5 (proteasome subunit beta 5), showing inconsistent efficacy across L. decemlineata populations [119]. Therefore, the identification and validation of novel target genes whose silencing can effectively control pests of agricultural importance remain an important task. In this context, this study represents the first attempt to explore the silencing of SERCA and CPR genes, both involved in the essential biological processes of L. decemlineata.
The SERCA gene encodes the Sarco/Endoplasmic Reticulum Ca2⁺-ATPase, a crucial enzyme for muscle function. It is particularly important in the flight muscles, where rapid calcium cycling is required for sustained contractions. Additionally, SERCA is involved in neuronal activity, hormonal secretion, development, and stress responses [91,92,93,94]. Similarly, the CPR gene encodes NADPH–Cytochrome P450 Reductase, responsible for transferring electrons from NADPH to the heme center of Cytochrome P450s, thereby regulating their enzymatic activity [77,78]. As a result, CPR enables Cytochrome P450s to perform critical functions such as detoxification, hormone synthesis, and metabolism [78,79,80,81,82,83].
In this study, silencing the SERCA gene resulted in a rapid decline in larval survival, culminating in 100% mortality by day 5 of the experiment. This highlights the critical role of SERCA in larval physiology and suggests that the interference of genes implicated in calcium regulation exerts profound physiological effects on L. decemlineata. This result is consistent with reports indicating that impaired SERCA function leads to muscle dysfunction and cardiac disorders, in addition to compromising critical processes such as the Notch signaling pathway, intestinal actin cytoskeleton assembly, and chitin synthesis [97,120,121].
Other genes, when silenced, have also resulted in high mortality. For example, silencing β-Actin produced 100% mortality by day 5 using transgenic plants expressing dsRNA [99]. Similarly, silencing the Ran gene caused the complete inhibition of larval growth, leading to 100% mortality in just three days [47], while silencing the EcR gene resulted in up to 80% mortality by day 3 [50].
On the other hand, silencing CPR resulted in a more gradual mortality pattern, indicating a less immediate but still significant impact on larval viability. The 28% mortality observed on day 7 after CPR silencing is comparable to the 14.5–32.2% mortality reported for JHAMT silencing in L. decemlineata by day 6 [60], and slightly comparable to the 47% mortality observed on day 9 after silencing the ACE1 gene [44].
Although the mortality induced by CPR silencing in this study was lower than that observed with SERCA silencing, previous research has shown that CPR knockdown disrupts electron transfer, affecting the metabolic activity of Cytochrome P450 enzymes and compromising xenobiotic detoxification [122,123,124]. Consequently, several studies have highlighted an increased susceptibility of insects to various insecticides following CPR disruption. For instance, CPR gene silencing has been shown to enhance the sensitivity of Sitophilus zeamais, Aphis gossypii, T. urticae, Tetranychus cinnabarinus, Laodelphax striatellus, Locusta migratoria, Nilaparvata lugens, and Bactrocera dorsalis to malathion, cypermethrin, imidacloprid, fenpropathrin, sulfoxaflor, buprofezin, carbaryl, terpinen-4-ol, abamectin, bifenthrin, and fenpyroximate [85,123,125,126,127,128,129,130].
While CPR silencing alone did not cause high mortality, its potential role in increasing L. decemlineata susceptibility to insecticides remains an unexplored avenue. Furthermore, given that most insects possess a single CPR gene [80], novel research evaluating the synergistic effects of CPR knockdown combined with insecticide exposure could provide valuable insights into novel CPB management strategies.
The effects of RNAi in L. decemlineata have shown considerable variability, with different genes eliciting distinct levels of mortality and growth inhibition. For instance, Tai silencing led to approximately 20% larval mortality and an 80% pupation failure rate [48], and Mesh gene silencing caused 77% mortality by day 7 [46], whereas targeting Sec23, vATPase E, vATPase B, COPβ, β-Actin, and Shd led to mortality rates of up to 80% by day 12 [45,53], underscoring that mortality outcomes depend on the specific gene being targeted.
Despite the advances in RNAi-based control strategies for L. decemlineata, further optimization is required, particularly in identifying genes whose silencing induces high mortality during the early larval instars, which represent the stages of development where the greatest defoliation damage occurs [5].
In this study, both dsSERCA and dsCPR treatments significantly reduced larval weight gain compared to the control group. While no significant differences were observed between treatments on day 3, SERCA silencing had a more severe impact in the following days, leading to a complete stagnation in weight gain until larval death on day 5. In contrast, CPR silencing also reduced weight gain but to a lesser extent, allowing larvae to survive longer.
Interestingly, despite the similarity in phenotypic outcomes between dsSERCA and dsCPR, transcript analysis showed distinct expression patterns. CPR transcripts showed a more gradual decline throughout the experiment, corresponding to a less pronounced impact on weight gain and a progressive reduction in survival. However, SERCA transcript levels showed a marked decrease on day 3, followed by an unexpected increase in surviving larvae on day 5.
Since RNAi is a knockdown, not a knockout, method, complete gene silencing does not occur, and the effect is typically transient. Thus, the continuous and large-scale delivery of dsRNA targeting the gene is necessary to sustain insect mortality [45]. Based on this, we hypothesize that the increase in transcript levels observed on day 5 in larvae treated with dsSERCA could be due to the cessation of larval feeding, which would likely reduce dsRNA uptake and consequently diminish the effectiveness of gene silencing. Additionally, an alternative explanation involves regulatory feedback mechanisms, where gene expression is modulated beyond the direct effect of RNAi. Compensatory mechanisms, such as the upregulation of paralogous genes or the re-expression of the target gene itself, have been reported following RNAi treatment [131]. Notably, compensatory upregulation at the nuclear level can lead to increased RNA detection with qPCR, even if post-transcriptional repression in the cytoplasm remains sufficient to elicit a strong phenotype. Moreover, certain genes may remain functional even with residual transcript levels above 30–40%, which can still induce significant phenotypic effects, further complicating the interpretation of RNAi efficacy [132].
This phenomenon has been observed in other studies where the silencing of genes such as vacuolar-ATPase, acetylcholinesterase, arginine kinase, and chymotrypsin in species like C. partellus, Liriomyza trifolii, and Euschistus heros showed similar outcomes [133,134,135]. Similarly, the silencing of the JHAMT1, SAHase, ACE1, and Shd genes in L. decemlineata has demonstrated that transcript levels do not always correlate directly with phenotypic outcomes such as weight gain or mortality rates [44,53,54,60].
Given these complexities, qPCR results should be interpreted with caution, as they may not fully capture the functional consequences of gene silencing. In contrast, phenotypic quantification remains the most reliable method for assessing the efficacy of gene-targeted RNAi in a biological process [132].
A critical aspect influencing RNAi efficacy is dsRNA stability. In this study, both dsSERCA and dsCPR exhibited the same length (290 bp) and GC content (40%), yet their predicted stability differed significantly. dsSERCA exhibited lower thermodynamic stability compared to dsCPR. Interestingly, studies using hairpin RNAs have shown this trend, where lower thermodynamic stability is correlated with enhanced gene silencing, likely due to improved accessibility for Dicer processing [136]. Therefore, although structural stability is desirable to prevent premature degradation in the environment until ingestion by insects [137], overly stable structures may hinder Dicer recognition or cleavage efficiency, ultimately reducing RNAi efficiency.
In addition to dsRNA stability, the choice of the mRNA target region is another crucial factor. There have been contradictory results reported regarding which mRNA region (5′ or 3′) results in more effective silencing [138,139,140]. However, beyond the position within the transcript, the structural properties of the target sites—such as GC content, secondary structure, accessibility, and the number of hydrogen bonds that stabilize the mRNA—may play a more decisive role in determining RNAi efficiency [141,142,143,144]. While more research is needed to elucidate the factors that govern RNAi efficacy, evidence suggests that optimizing the length and stability of dsRNA, delivery methods, and the target genes are the most crucial factors for improving gene silencing efficiency. Given its specificity and potential for sustainable pest control, RNAi remains a valuable tool in modern agriculture.
5. Conclusions
Our study demonstrates that silencing the SERCA gene is a promising target for controlling L. decemlineata, and additional research should further explore the suitability of this gene in pest control. However, while silencing the CPR gene was less effective, it remains a gene of interest because it plays a crucial role in the function of Cytochrome P450s, which are involved in detoxification processes. Therefore, the silencing of the CPR gene could be combined with insecticides to enhance the control of CPB.
As pests continue to develop resistance to conventional insecticides, RNAi is an alternative pest management technology with high specificity and minimal environmental risks. However, for its successful integration into pest management strategies, further research is needed to refine delivery methods, optimize dsRNA design, and explore genetic targets that maximize RNAi efficacy. A holistic approach combining RNAi with other pest control measures will ensure its effectiveness and long-term sustainability in agriculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051151/s1, Table S1: Sequences from the National Center for Biotechnology Information (NCBI) database used in off-target analysis.
Author Contributions
Conceptualization, M.M.-A. and E.A.E.-S.; methodology, M.M.-A., T.S.S.-C., J.A.T.-C., S.R.S.-G., B.F.I.-F., and M.J.A.-J.; software, S.R.S.-G. and L.I.S.-E.; validation, M.J.A.-J.; formal analysis, M.M.-A., B.F.I.-F., Q.R.-C., and E.A.E.-S.; investigation, M.M.-A., J.A.T.-C., M.J.A.-J., and C.D.G.-B.; resources, M.M.-A., J.A.T.-C., and C.D.G.-B.; data curation, S.R.S.-G.; writing—original draft preparation, M.M.-A., J.A.T.-C., and E.A.E.-S.; writing—review and editing, M.M.-A., T.S.S.-C., S.R.S.-G., B.F.I.-F., M.J.A.-J., C.D.G.-B., Q.R.-C., L.I.S.-E., and E.A.E.-S.; visualization, T.S.S.-C., S.R.S.-G., B.F.I.-F., and Q.R.-C.; supervision, L.I.S.-E.; project administration, T.S.S.-C., C.D.G.-B., Q.R.-C., and E.A.E.-S.; funding acquisition, L.I.S.-E. and E.A.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT), Mexico, through the project CF-2023-I-1539.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
We want to thank Donald C. Weber, USDA, for his guidance in the care of the Colorado Potato Beetle, as well as Anahi Fierro Neri, for her support in insect management. We also wish to express our gratitude to the Facultad de Ciencias Químicas, Universidad Autónoma de Chihuahua, Mexico, for their collaboration in the development of this research. Finally, we extend our thanks to the Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONAHCYT), Mexico, for funding this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Survivorship percentage of L. decemlineata larvae treated with dsRNA targeting the SERCA and CPR genes.
Table A1.
Survivorship percentage of L. decemlineata larvae treated with dsRNA targeting the SERCA and CPR genes.
| Treatment | d0 | d1 | d2 | d3 | d4 | d5 | d6 | d7 |
| Control | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| dsSERCA | 100 | 92 | 86 | 73 | 23 | — | — | — |
| dsCPR | 100 | 96 | 92 | 85 | 84 | 81 | 80 | 72 |

Table A2.
Mean weight gain of L. decemlineata larvae treated with dsRNA targeting the SERCA and CPR genes.
Table A2.
Mean weight gain of L. decemlineata larvae treated with dsRNA targeting the SERCA and CPR genes.
| Treatment | d3 | d5 | d7 |
| Control | 13.57 | 17.09 | 27.39 |
| dsCPR | 7.87 | 11.58 | 11.28 |
| dsSERCA | 7.29 | 8.47 | — |
| Treatment | Standard error | ||
| d3 | d5 | d7 | |
| Control | 0.89 | 0.55 | 1.38 |
| dsCPR | 0.08 | 0.46 | 0.24 |
| dsSERCA | 0.26 | 0.22 | — |
| Treatment | Post-hoc Tukey | ||
| d3 | d5 | d7 | |
| Control | A | A | A |
| dsCPR | B | B | B |
| dsSERCA | B | C | — |

Table A3.
Mean relative expression of the SERCA and CPR genes in L. decemlineata larvae treated with dsRNA.
Table A3.
Mean relative expression of the SERCA and CPR genes in L. decemlineata larvae treated with dsRNA.
| Treatment | d3 | d5 | d7 |
| Control | 1.00 | 1.00 | 1.00 |
| dsCPR | 0.95 | 0.83 | 0.52 |
| dsSERCA | 0.44 | 0.72 | — |
| Treatment | Standard error | ||
| d3 | d5 | d7 | |
| Control | 0.00 | 0.00 | 0.00 |
| dsCPR | 0.03 | 0.09 | 0.13 |
| dsSERCA | 0.07 | 0.10 | — |
| Treatment | Post-hoc Tukey | ||
| d3 | d5 | d7 | |
| Control | A | A | A |
| dsCPR | A | AB | B |
| dsSERCA | B | B | — |
References
- FAOSTAT. Food and Agriculture Organization of the United Nations: Crops and Livestock Products. Available online: https://www.fao.org/faostat/es/#data/QCL (accessed on 25 February 2025).
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, K. Do Russia and Eastern Europe need GM plants? New Biotechnol. 2010, 27, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Ferro, D.N.; Logan, J.A.; Voss, R.H.; Elkinton, J.S. Colorado Potato Beetle (Coleoptera: Chrysomelidae) temperature-dependent growth and feeding rates. Environ. Entomol. 1985, 14, 343–348. [Google Scholar] [CrossRef]
- Weber, D. Colorado Beetle: Pest on the move. Pestic. Outlook 2003, 14, 256–259. [Google Scholar] [CrossRef]
- Alyokhin, A.; Benkovskayab, G.; Udalovc, M. Colorado Potato Beetle. In Insect Pests of Potato, Global Perspectives on Biology and Management, 2nd ed.; Alyokhin, A., Rondon, S.I., Gao, Y., Eds.; Elsevier: London, UK, 2022; Volume 1, p. 503. [Google Scholar]
- Alvarez, J.M.; Srinivasan, R.; Cervantes, F.A. Occurrence of the Carabid Beetle, Pterostichus melanarius (Illiger), in potato ecosystems of Idaho and its predatory potential on the Colorado Potato Beetle and aphids. Am. J. Potato Res. 2013, 90, 83–92. [Google Scholar] [CrossRef]
- Sablon, L.; Haubruge, E.; Verheggen, F.J. Consumption of immature stages of Colorado Potato Beetle by Chrysoperla carnea (Neuroptera: Chrysopidae) larvae in the laboratory. Am. J. Potato Res. 2013, 90, 51–57. [Google Scholar] [CrossRef]
- Speese, J.; Sterrett, S.B. Crop rotation reduces the cost of Colorado Potato Beetle control in potatoes. HortTechnology 1998, 8, 229–234. [Google Scholar] [CrossRef]
- Wraight, S.P.; Ramos, M.E. Delayed efficacy of Beauveria bassiana foliar spray applications against Colorado Potato Beetle: Impacts of number and timing of applications on larval and next-generation adult populations. Biol. Control 2015, 83, 51–67. [Google Scholar] [CrossRef]
- Chen, Y.H.; Cohen, Z.P.; Bueno, E.M.; Christensen, B.M.; Schoville, S.D. Rapid evolution of insecticide resistance in the Colorado Potato Beetle, Leptinotarsa decemlineata. Curr. Opin. Insect Sci. 2023, 55, 101000. [Google Scholar] [CrossRef]
- Pélissié, B.; Chen, Y.H.; Cohen, Z.P.; Crossley, M.S.; Hawthorne, D.J.; Izzo, V.; Schoville, S.D. Genome resequencing reveals rapid, repeated evolution in the Colorado Potato Beetle. Mol. Biol. Evol. 2022, 39, msac016. [Google Scholar] [CrossRef] [PubMed]
- Gaddelapati, S.C.; Kalsi, M.; Roy, A.; Palli, S.R. Cap ‘n’ collar C regulates genes responsible for imidacloprid resistance in the Colorado Potato Beetle, Leptinotarsa decemlineata. Insect Biochem. Mol. Biol. 2018, 99, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Schoville, S.D.; Chen, Y.H.; Andersson, M.N.; Benoit, J.B.; Bhandari, A.; Bowsher, J.H.; Brevik, K.; Cappelle, K.; Chen, M.-J.M.; Childers, A.K.; et al. A model species for agricultural pest genomics: The genome of the Colorado Potato Beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae). Sci. Rep. 2018, 8, 1931. [Google Scholar] [CrossRef]
- Zhu, F.; Moural, T.W.; Nelson, D.R.; Palli, S.R. A specialist herbivore pest adaptation to xenobiotics through up-regulation of multiple Cytochrome P450s. Sci. Rep. 2016, 6, 20421. [Google Scholar] [CrossRef]
- APRD. Arthropod Pesticide Resistance Database: Leptinotarsa decemlineata. Available online: https://www.pesticideresistance.org (accessed on 25 February 2025).
- FAO. Food and Agriculture Organization of the United Nations Statistical Databases: Pesticides Indicators. Available online: https://www.fao.org/faostat/en/#data/RP (accessed on 4 February 2025).
- Barathi, S.; Sabapathi, N.; Kandasamy, S.; Lee, J. Present status of insecticide impacts and eco-friendly approaches for remediation-a review. Environ. Res. 2024, 240, 117432. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M. Poisoning by pesticides. Medicine 2020, 48, 214–217. [Google Scholar] [CrossRef]
- Christiaens, O.; Whyard, S.; Vélez, A.M.; Smagghe, G. Double-stranded RNA technology to control insect pests: Current status and challenges. Front. Plant Sci. 2020, 11, 451. [Google Scholar] [CrossRef]
- Svoboda, P. Key mechanistic principles and considerations concerning RNA interference. Front. Plant Sci. 2020, 11, 1237. [Google Scholar] [CrossRef]
- Gordon, K.H.J.; Waterhouse, P.M. RNAi for insect-proof plants. Nat. Biotechnol. 2007, 25, 1231–1232. [Google Scholar] [CrossRef]
- Eakteiman, G.; Moses-Koch, R.; Moshitzky, P.; Mestre-Rincon, N.; Vassão, D.G.; Luck, K.; Sertchook, R.; Malka, O.; Morin, S. Targeting detoxification genes by phloem-mediated RNAi: A new approach for controlling phloem-feeding insect pests. Insect Biochem. Mol. Biol. 2018, 100, 10–21. [Google Scholar] [CrossRef]
- Xu, H.-X.; Qian, L.-X.; Wang, X.-W.; Shao, R.-X.; Hong, Y.; Liu, S.-S.; Wang, X.-W. A salivary effector enables whitefly to feed on host plants by eliciting salicylic acid-signaling pathway. Proc. Natl. Acad. Sci. USA 2019, 116, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.H.; Lee, S.H.; Park, Y.-D. Development of mite (Tetranychus urticae)-resistant transgenic Chinese cabbage using plant-mediated RNA interference. Hortic. Environ. Biotechnol. 2020, 61, 305–315. [Google Scholar] [CrossRef]
- Saini, R.P.; Raman, V.; Dhandapani, G.; Malhotra, E.V.; Sreevathsa, R.; Kumar, P.A.; Sharma, T.R.; Pattanayak, D. Silencing of HaAce1 gene by host-delivered artificial microRNA disrupts growth and development of Helicoverpa armigera. PLoS ONE 2018, 13, e0194150. [Google Scholar] [CrossRef] [PubMed]
- Bally, J.; Fishilevich, E.; Doran, R.L.; Lee, K.; de Campos, S.B.; German, M.A.; Narva, K.E.; Waterhouse, P.M. Plin-amiR, a pre-microRNA-based technology for controlling herbivorous insect pests. Plant Biotechnol. J. 2020, 18, 1925–1932. [Google Scholar] [CrossRef]
- Rana, S.; Rajurkar, A.B.; Kumar, K.; Mohankumar, S. Comparative analysis of Chitin Synthase A dsRNA mediated RNA interference for management of crop pests of different families of Lepidoptera. Front. Plant Sci. 2020, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Liu, Z.; Chen, J.; Sun, G.; Jiang, Y.; Li, M.; Xiong, L.; Chen, S.; Zhou, Y.; Asad, M.; et al. Silencing arginine kinase/integrin β1 subunit by transgenic plant expressing dsRNA inhibits the development and survival of Plutella xylostella. Pest Manag. Sci. 2020, 76, 1761–1771. [Google Scholar] [CrossRef]
- Camargo, R.A.; Barbosa, G.O.; Possignolo, I.P.; Peres, L.E.; Lam, E.; Lima, J.E.; Figueira, A.; Marques-Souza, H. RNA interference as a gene silencing tool to control Tuta absoluta in tomato (Solanum lycopersicum). PeerJ 2016, 4, e2673. [Google Scholar] [CrossRef]
- Murtaza, S.; Tabassum, B.; Tariq, M.; Riaz, S.; Yousaf, I.; Jabbar, B.; Khan, A.; Samuel, A.O.; Zameer, M.; Nasir, I.A. Silencing a Myzus persicae macrophage inhibitory factor by plant-mediated RNAi induces enhanced aphid mortality coupled with boosted RNAi efficacy in transgenic potato lines. Mol. Biotechnol. 2022, 64, 1152–1163. [Google Scholar] [CrossRef]
- Rauf, I.; Asif, M.; Amin, I.; Naqvi, R.Z.; Umer, N.; Mansoor, S.; Jander, G. Silencing cathepsin L expression reduces Myzus persicae protein content and the nutritional value as prey for Coccinella septempunctata. Insect Mol. Biol. 2019, 28, 785–797. [Google Scholar] [CrossRef]
- Niu, X.; Kassa, A.; Hu, X.; Robeson, J.; McMahon, M.; Richtman, N.M.; Steimel, J.P.; Kernodle, B.M.; Crane, V.C.; Sandahl, G. Control of western corn rootworm (Diabrotica virgifera virgifera) reproduction through plant-mediated RNA interference. Sci. Rep. 2017, 7, 12591. [Google Scholar] [CrossRef]
- Bolognesi, R.; Ramaseshadri, P.; Anderson, J.; Bachman, P.; Clinton, W.; Flannagan, R.; Ilagan, O.; Lawrence, C.; Levine, S.; Moar, W.; et al. Characterizing the mechanism of action of double-stranded RNA activity against Western Corn Rootworm (Diabrotica virgifera virgifera LeConte). PLoS ONE 2012, 7, e47534. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yang, B.; Zhang, A.; Ding, D.; Wang, G. Plant-mediated RNAi for controlling Apolygus lucorum. Front. Plant Sci. 2019, 10, 64. [Google Scholar] [CrossRef]
- Liang, S.; Luo, J.; Alariqi, M.; Xu, Z.; Wang, A.; Zafar, M.N.; Ren, J.; Wang, F.; Liu, X.; Xin, Y. Silencing of a LIM gene in cotton exhibits enhanced resistance against Apolygus lucorum. J. Cell. Physiol. 2021, 236, 5921–5936. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, J.; Francis, F.; Chen, J. Molecular characterization and gene silencing of Laccase 1 in the grain aphid, Sitobion avenae. Arch. Insect Biochem. Physiol. 2018, 97, e21446. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Q.; Li, X.; Sun, Y.; Wang, H.; Xia, L. Double-stranded RNA in the biological control of grain aphid (Sitobion avenae F.). Funct. Integr. Genom. 2015, 15, 211–223. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, Y.-C.; Li, P.; Niu, C.-Y. The effect of silencing 20E biosynthesis relative genes by feeding bacterially expressed dsRNA on the larval development of Chilo suppressalis. Sci. Rep. 2016, 6, 28697. [Google Scholar] [CrossRef]
- Mao, C.; Zhu, X.; Wang, P.; Sun, Y.; Huang, R.; Zhao, M.; Hull, J.J.; Lin, Y.; Zhou, F.; Chen, H. Transgenic double-stranded RNA rice, a potential strategy for controlling striped stem borer (Chilo suppressalis). Pest Manag. Sci. 2022, 78, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Saberi, E.; Mondal, M.; Paredes-Montero, J.R.; Nawaz, K.; Brown, J.K.; Qureshi, J.A. Optimal dsRNA concentration for RNA interference in Asian Citrus Psyllid. Insects 2024, 15, 58. [Google Scholar] [CrossRef]
- Killiny, N.; Hajeri, S.; Tiwari, S.; Gowda, S.; Stelinski, L.L. Double-stranded RNA uptake through topical application, mediates silencing of five CYP4 Genes and suppresses insecticide resistance in Diaphorina citri. PLoS ONE 2014, 9, e110536. [Google Scholar] [CrossRef]
- Julian-Chávez, B.; Siqueiros-Cendón, T.S.; Torres-Castillo, J.A.; Sinagawa-García, S.R.; Abraham-Juárez, M.J.; González-Barriga, C.D.; Rascón-Cruz, Q.; Siañez-Estrada, L.I.; Arévalo-Gallegos, S.; Espinoza-Sánchez, E.A. Silencing ACE1 gene with dsRNA of different lengths impairs larval development in Leptinotarsa decemlineata. Insects 2024, 15, 1000. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, J.; Palli, R.; Ferguson, J.; Palli, S.R. Ingested RNA interference for managing the populations of the Colorado Potato Beetle, Leptinotarsa decemlineata. Pest Manag. Sci. 2011, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Petek, M.; Coll, A.; Ferenc, R.; Razinger, J.; Gruden, K. Validating the potential of double-stranded RNA targeting Colorado Potato Beetle mesh gene in laboratory and field trials. Front. Plant Sci. 2020, 11, 1250. [Google Scholar] [CrossRef]
- Shen, C.-H.; Jin, L.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. RNA interference targeting Ras GTPase gene Ran causes larval and adult lethality in Leptinotarsa decemlineata. Pest Manag. Sci. 2022, 78, 3849–3858. [Google Scholar] [CrossRef]
- Xu, Q.-Y.; Deng, P.; Mu, L.-L.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. Silencing Taiman impairs larval development in Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2019, 160, 30–39. [Google Scholar] [CrossRef]
- Xu, Q.-Y.; Meng, Q.-W.; Deng, P.; Guo, W.-C.; Li, G.-Q. Leptinotarsa hormone receptor 4 (HR4) tunes ecdysteroidogenesis and mediates 20-hydroxyecdysone signaling during larval-pupal metamorphosis. Insect Biochem. Mol. Biol. 2018, 94, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Aksoy, E.; Çalışkan, M.E.; Bakhsh, A. Transgenic potato lines expressing hairpin RNAi construct of molting-associated EcR gene exhibit enhanced resistance against Colorado Potato Beetle (Leptinotarsa decemlineata, Say). Transgenic Res. 2019, 28, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Campuzano, C.; Martínez-Ramírez, A.C.; Contreras, E.; Rausell, C.; Real, M.D. Prohibitin, an essential protein for Colorado Potato Beetle larval viability, is relevant to Bacillus thuringiensis Cry3Aa toxicity. Pestic. Biochem. Physiol. 2013, 107, 299–308. [Google Scholar] [CrossRef]
- Liu, X.-P.; Fu, K.-Y.; Lü, F.-G.; Meng, Q.-W.; Guo, W.-C.; Li, G.-Q. Involvement of FTZ-F1 in the regulation of pupation in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2014, 55, 51–60. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, X.-P.; Wan, P.-J.; Shi, X.-Q.; Guo, W.-C.; Li, G.-Q. The P450 enzyme Shade mediates the hydroxylation of ecdysone to 20-hydroxyecdysone in the Colorado Potato Beetle, Leptinotarsa decemlineata. Insect Mol. Biol. 2014, 23, 632–643. [Google Scholar] [CrossRef]
- Zhou, L.-T.; Jia, S.; Wan, P.-J.; Kong, Y.; Guo, W.-C.; Ahmat, T.; Li, G.-Q. RNA interference of a putative S-adenosyl-L-homocysteine hydrolase gene affects larval performance in Leptinotarsa decemlineata (Say). J. Insect Physiol. 2013, 59, 1049–1056. [Google Scholar] [CrossRef]
- Fu, K.-Y.; Guo, W.-C.; Ahmat, T.; Li, G.-Q. Knockdown of a nutrient amino acid transporter gene LdNAT1 reduces free neutral amino acid contents and impairs Leptinotarsa decemlineata pupation. Sci. Rep. 2015, 5, 18124. [Google Scholar] [CrossRef]
- Guo, W.-C.; Liu, X.-P.; Fu, K.-Y.; Shi, J.-F.; Lü, F.-G.; Li, G.-Q. Functions of nuclear receptor HR3 during larval-pupal molting in Leptinotarsa decemlineata (Say) revealed by in vivo RNA interference. Insect Biochem. Mol. Biol. 2015, 63, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.-J.; Fu, K.-Y.; Lü, F.-G.; Guo, W.-C.; Li, G.-Q. Knockdown of a putative alanine aminotransferase gene affects amino acid content and flight capacity in the Colorado Potato Beetle Leptinotarsa decemlineata. Amino Acids 2015, 47, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-F.; Fu, J.; Mu, L.-L.; Guo, W.-C.; Li, G.-Q. Two Leptinotarsa uridine diphosphate N-acetylglucosamine pyrophosphorylases are specialized for chitin synthesis in larval epidermal cuticle and midgut peritrophic matrix. Insect Biochem. Mol. Biol. 2016, 68, 1–12. [Google Scholar] [CrossRef]
- Shi, J.-F.; Xu, Q.-Y.; Sun, Q.-K.; Meng, Q.-W.; Mu, L.-L.; Guo, W.-C.; Li, G.-Q. Physiological roles of trehalose in Leptinotarsa larvae revealed by RNA interference of trehalose-6-phosphate synthase and trehalase genes. Insect Biochem. Mol. Biol. 2016, 77, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.-Y.; Li, Q.; Zhou, L.-T.; Meng, Q.-W.; Lü, F.-G.; Guo, W.-C.; Li, G.-Q. Knockdown of juvenile hormone acid methyl transferase severely affects the performance of Leptinotarsa decemlineata (Say) larvae and adults. Pest Manag. Sci. 2016, 72, 1231–1241. [Google Scholar] [CrossRef]
- Lü, F.-G.; Fu, K.-Y.; Guo, W.-C.; Li, G.-Q. Characterization of two juvenile hormone epoxide hydrolases by RNA interference in the Colorado potato beetle. Gene 2015, 570, 264–271. [Google Scholar] [CrossRef]
- Shi, J.F.; Mu, L.L.; Chen, X.; Guo, W.C.; Li, G.Q. RNA interference of chitin synthase genes inhibits chitin biosynthesis and affects larval performance in Leptinotarsa decemlineata (Say). Int. J. Biol. Sci. 2016, 12, 1319–1331. [Google Scholar] [CrossRef]
- Wan, P.-J.; Fu, K.-Y.; Lü, F.-G.; Wang, X.-X.; Guo, W.-C.; Li, G.-Q. Knocking down a putative -pyrroline-5-carboxylate dehydrogenase gene by RNA interference inhibits flight and causes adult lethality in the Colorado Potato Beetle Leptinotarsa decemlineata (Say). Pest Manag. Sci. 2015, 71, 1387–1396. [Google Scholar] [CrossRef]
- Guo, W.-C.; Liu, X.-P.; Fu, K.-Y.; Shi, J.-F.; Lü, F.-G.; Li, G.-Q. Nuclear receptor ecdysone-induced protein 75 is required for larval–pupal metamorphosis in the Colorado Potato Beetle Leptinotarsa decemlineata (Say). Insect Mol. Biol. 2016, 25, 44–57. [Google Scholar] [CrossRef]
- EPA. U.S. Environmental Protection Agency, Registers Innovative Tool to Control Corn Rootworm. Available online: https://www.epa.gov/ (accessed on 13 November 2024).
- Head, G.P.; Carroll, M.W.; Evans, S.P.; Rule, D.M.; Willse, A.R.; Clark, T.L.; Storer, N.P.; Flannagan, R.D.; Samuel, L.W.; Meinke, L.J. Evaluation of SmartStax and SmartStax PRO maize against western corn rootworm and northern corn rootworm: Efficacy and resistance management. Pest Manag. Sci. 2017, 73, 1883–1899. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. GM Approval Database. Available online: https://www.isaaa.org/gmapprovaldatabase/ (accessed on 25 March 2025).
- EPA. U.S. Environmental Protection Agency: EPA Registers Novel Pesticide Technology for Potato Crops. Available online: https://www.epa.gov/pesticides/epa-registers-novel-pesticide-technology-potato-crops (accessed on 26 February 2025).
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.-W.; et al. First sprayable double-stranded RNA-based biopesticide product targets proteasome subunit beta type-5 in Colorado Potato Beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 141530. [Google Scholar] [CrossRef]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and regulatory approaches in the USA and in the EU for dsRNA-based externally applied pesticides for plant protection. Front. Plant Sci. 2021, 12, 682387. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef] [PubMed]
- Verdonckt, T.W.; Vanden, B.J. Methods for the cost-effective production of bacteria-derived double-stranded RNA for in vitro knockdown studies. Front. Physiol. 2022, 13, 836106. [Google Scholar] [CrossRef]
- Yu, N.; Christiaens, O.; Liu, J.; Niu, J.; Cappelle, K.; Caccia, S.; Huvenne, H.; Smagghe, G. Delivery of dsRNA for RNAi in insects: An overview and future directions. Insect Sci. 2013, 20, 4–14. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef]
- Christiaens, O.; Swevers, L.; Smagghe, G. DsRNA degradation in the pea aphid (Acyrthosiphon pisum) associated with lack of response in RNAi feeding and injection assay. Peptides 2014, 53, 307–314. [Google Scholar] [CrossRef]
- Shukla, J.N.; Megha, K.; Amit, S.; E. Narva, K.; Elane, F.; Satnam, S.; Kanakachari, M.; Palli, S.R. Reduced stability and intracellular transport of dsRNA contribute to poor RNAi response in lepidopteran insects. RNA Biol. 2016, 13, 656–669. [Google Scholar] [CrossRef]
- Lu, A.Y.H.; Junk, K.W.; Coon, M.J. Resolution of the Cytochrome P-450-containing ω-hydroxylation system of liver microsomes into three components. J. Biol. Chem. 1969, 244, 3714–3721. [Google Scholar] [CrossRef]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef]
- Feyereisen, R. Origin and evolution of the CYP4G subfamily in insects, cytochrome P450 enzymes involved in cuticular hydrocarbon synthesis. Mol. Phylogenet. Evol. 2020, 143, 106695. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Sams, S.; Moural, T.; Haynes, K.F.; Potter, M.F.; Palli, S.R. RNA Interference of NADPH-Cytochrome P450 Reductase results in reduced insecticide resistance in the bed bug, Cimex lectularius. PLoS ONE 2012, 7, e31037. [Google Scholar] [CrossRef]
- Rewitz, K.F.; O’Connor, M.B.; Gilbert, L.I. Molecular evolution of the insect Halloween family of cytochrome P450s: Phylogeny, gene organization and functional conservation. Insect Biochem. Mol. Biol. 2007, 37, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Maïbèche-Coisne, M.; Nikonov, A.A.; Ishida, Y.; Jacquin-Joly, E.; Leal, W.S. Pheromone anosmia in a scarab beetle induced by in vivo inhibition of a pheromone-degrading enzyme. Proc. Natl. Acad. Sci. USA 2004, 101, 11459–11464. [Google Scholar] [CrossRef]
- Sandstrom, P.; Welch, W.H.; Blomquist, G.J.; Tittiger, C. Functional expression of a bark beetle cytochrome P450 that hydroxylates myrcene to ipsdienol. Insect Biochem. Mol. Biol. 2006, 36, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Lycett, G.J.; McLaughlin, L.A.; Ranson, H.; Hemingway, J.; Kafatos, F.C.; Loukeris, T.G.; Paine, M.J.I. Anopheles gambiae P450 reductase is highly expressed in oenocytes and in vivo knockdown increases permethrin susceptibility. Insect Mol. Biol. 2006, 15, 321–327. [Google Scholar] [CrossRef]
- Huang, Y.; Lu, X.P.; Wang, L.L.; Wei, D.; Feng, Z.J.; Zhang, Q.; Xiao, L.F.; Dou, W.; Wang, J.J. Functional characterization of NADPH-cytochrome P450 reductase from Bactrocera dorsalis: Possible involvement in susceptibility to malathion. Sci. Rep. 2015, 5, 18394. [Google Scholar] [CrossRef]
- Palmgren, M.G.; Nissen, P. P-Type ATPases. Annu. Rev. 2011, 40, 243–266. [Google Scholar] [CrossRef]
- Berchtold, M.W.; Brinkmeier, H.; Müntener, M. Calcium Ion in skeletal muscle: Its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000, 80, 1215–1265. [Google Scholar] [CrossRef]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.L.E.; Young, H.S. The SarcoEndoplasmic Reticulum Calcium ATPase. In Membrane Protein Complexes: Structure and Function; Harris, J.R., Boekema, E.J., Eds.; Springer: Singapore, 2018; pp. 229–258. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Endo, M. Calcium-induced calcium release in skeletal muscle. Physiol. Rev. 2009, 89, 1153–1176. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Jassar, O.; Ghanim, M. The plant pathogenic bacterium Candidatus Liberibacter solanacearum induces calcium-regulated autophagy in midgut cells of its insect vector Bactericera trigonica. Microbiol. Spectr. 2023, 11, e0130123. [Google Scholar] [CrossRef]
- Sanyal, S.; Consoulas, C.; Kuromi, H.; Basole, A.; Mukai, L.; Kidokoro, Y.; Krishnan, K.S.; Ramaswami, M. Analysis of conditional paralytic mutants in Drosophila sarco-endoplasmic reticulum calcium ATPase reveals novel mechanisms for regulating membrane excitability. Genetics 2005, 169, 737–750. [Google Scholar] [CrossRef]
- Jayakumar, S.; Hasan, G. Neuronal calcium signaling in metabolic regulation and adaptation to nutrient stress. Front. Neural Circuits 2018, 12, 25. [Google Scholar] [CrossRef]
- Denmeade, S.R.; Isaacs, J.T. The SERCA pump as a therapeutic target: Making a “smart bomb” for prostate cancer. Cancer Biol. Ther. 2005, 4, 14–22. [Google Scholar] [CrossRef]
- Xu, H.; Van Remmen, H. The SarcoEndoplasmic Reticulum Calcium ATPase (SERCA) pump: A potential target for intervention in aging and skeletal muscle pathologies. Skelet. Muscle 2021, 11, 25. [Google Scholar] [CrossRef]
- Zhao, B.; Lucas, K.J.; Saha, T.T.; Ha, J.; Ling, L.; Kokoza, V.A.; Roy, S.; Raikhel, A.S. MicroRNA-275 targets sarco/endoplasmic reticulum Ca2+ adenosine triphosphatase (SERCA) to control key functions in the mosquito gut. PLoS Genet. Genet. 2017, 13, e1006943. [Google Scholar] [CrossRef]
- He, W.; Xu, W.; Xu, L.; Fu, K.; Guo, W.; Bock, R.; Zhang, J. Length-dependent accumulation of double-stranded RNAs in plastids affects RNA interference efficiency in the Colorado Potato Beetle. J. Exp. Bot. 2020, 71, 2670–2677. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, S.A.; Hasse, C.; Ruf, S.; Heckel, D.G.; Bock, R. Full crop protection from an insect pest by expression of long double-stranded RNAs in plastids. Science 2015, 347, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Guo, W.-C.; Fu, K.-Y.; Yang, S.; Li, X.-X.; Li, G.-Q. Instar-dependent systemic RNA interference response in Leptinotarsa decemlineata larvae. Pestic. Biochem. Physiol. 2015, 123, 64–73. [Google Scholar] [CrossRef] [PubMed]
- UN. United Nations. Department of Economic and Social Affairs. Population Division. 2022 Revision of World Population Prospects. Available online: https://population.un.org/wpp/ (accessed on 20 March 2025).
- FAO. Food and Agriculture Organization of the United Nations. Plant Health 2020. Available online: https://www.fao.org/plant-health-2020/en/ (accessed on 25 February 2025).
- Rizzo, D.M.; Lichtveld, M.; Mazet, J.A.K.; Togami, E.; Miller, S.A. Plant health and its effects on food safety and security in a One Health framework: Four case studies. One Health Outlook 2021, 3, 6. [Google Scholar] [CrossRef]
- Hemathilake, D.M.K.S.; Gunathilake, D.M.C.C. Chapter 31—Agricultural productivity and food supply to meet increased demands. In Future Foods; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 539–553. [Google Scholar] [CrossRef]
- Tester, M.; Langridge, P. Breeding technologies to increase crop production in a changing world. Science 2010, 327, 818–822. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations. Feeding the World, Eradicating Hunger. Available online: https://www.fao.org/fileadmin/templates/wsfs/Summit/WSFS_Issues_papers/WSFS_Background_paper_Feeding_the_world.pdf (accessed on 25 February 2025).
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The Impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Hens, L.; Quynh, L.X. Environmental Space. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef]
- Pimentel, D. Techniques for Reducing Pesticide Use: Economic and Environmental Benefits; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Paoletti, M.G.; Pimentel, D. Environmental risks of pesticides versus genetic engineering for agricultural pest control. J. Agric. Environ. Ethics 2000, 12, 279–303. [Google Scholar] [CrossRef]
- Grapputo, A.; Boman, S.; LindstrÖM, L.; Lyytinen, A.; Mappes, J. The voyage of an invasive species across continents: Genetic diversity of North American and European Colorado Potato Beetle populations. Mol. Ecol. 2005, 14, 4207–4219. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, R.A. The Colorado Potato Beetle: 125 years of mismanagement. Bull. Entomol. Soc. Am. 1987, 33, 142–150. [Google Scholar] [CrossRef]
- Khajuria, C.; Ivashuta, S.; Wiggins, E.; Flagel, L.; Moar, W.; Pleau, M.; Miller, K.; Zhang, Y.; Ramaseshadri, P.; Jiang, C.; et al. Development and characterization of the first dsRNA-resistant insect population from western corn rootworm, Diabrotica virgifera virgifera LeConte. PLoS ONE 2018, 13, e0197059. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Dee, J.; Moar, W.; Dufner-Beattie, J.; Baum, J.; Dias, N.P.; Alyokhin, A.; Buzza, A.; Rondon, S.I.; Clough, M.; et al. Selection for high levels of resistance to double-stranded RNA (dsRNA) in Colorado potato beetle (Leptinotarsa decemlineata Say) using non-transgenic foliar delivery. Sci. Rep. 2021, 11, 6523. [Google Scholar] [CrossRef]
- Pallis, S.; Alyokhin, A.; Manley, B.; Rodrigues, T.B.; Barnes, E.; Narva, K. Baseline susceptibility to a novel dsRNA-based insecticide across US populations of Colorado Potato Beetle. Agriculture 2023, 13, 2283. [Google Scholar] [CrossRef]
- Sanyal, S.; Jennings, T.; Dowse, H.; Ramaswami, M. Conditional mutations in SERCA, the Sarco-endoplasmic reticulum Ca2+-ATPase, alter heart rate and rhythmicity in Drosophila. J. Comp. Physiol. B 2006, 176, 253–263. [Google Scholar] [CrossRef]
- Zhu, W.; Duan, Y.; Chen, J.; Merzendorfer, H.; Zou, X.; Yang, Q. SERCA interacts with chitin synthase and participates in cuticular chitin biogenesis in Drosophila. Insect Biochem. Mol. Biol. 2022, 145, 103783. [Google Scholar] [CrossRef]
- Chen, X.E.; Zhang, Y. Identification and characterization of NADPH-dependent cytochrome P450 reductase gene and cytochrome b5 gene from Plutella xylostella: Possible involvement in resistance to beta-cypermethrin. Gene 2015, 558, 208–214. [Google Scholar] [CrossRef]
- Huang, Y.; Liao, M.; Yang, Q.; Shi, S.; Xiao, J.; Cao, H. Knockdown of NADPH-cytochrome P450 reductase and CYP6MS1 increases the susceptibility of Sitophilus zeamais to terpinen-4-ol. Pestic. Biochem. Physiol. 2020, 162, 15–22. [Google Scholar] [CrossRef]
- Liu, N.; Li, M.; Gong, Y.; Liu, F.; Li, T. Cytochrome P450s—Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; He, Y.; Ma, K. RNA interference of NADPH-cytochrome P450 reductase increases the susceptibility of Aphis gossypii Glover to sulfoxaflor. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 274, 109745. [Google Scholar] [CrossRef] [PubMed]
- Adesanya, A.W.; Cardenas, A.; Lavine, M.D.; Walsh, D.B.; Lavine, L.C.; Zhu, F. RNA interference of NADPH-cytochrome P450 reductase increases susceptibilities to multiple acaricides in Tetranychus urticae. Pestic. Biochem. Physiol. 2020, 165, 104550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Wang, L.; Yao, J.; Guo, H.; Fang, J. Knockdown of NADPH-cytochrome P450 reductase results in reduced resistance to buprofezin in the small brown planthopper, Laodelphax striatellus (fallén). Pestic. Biochem. Physiol. 2016, 127, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Liu, J.; Li, Y.; Liu, X.; Wu, H.; Ma, E.; Zhang, J. Knockdown of NADPH-cytochrome P450 reductase increases the susceptibility to carbaryl in the migratory locust, Locusta migratoria. Chemosphere 2017, 188, 517–524. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Shen, G.; Xu, Z.; Wei, P.; Zhang, Y.; Xu, Q.; He, L. Silencing NADPH-cytochrome P450 reductase results in reduced acaricide resistance in Tetranychus cinnabarinus (Boisduval). Sci. Rep. 2015, 5, 15581. [Google Scholar] [CrossRef]
- Liu, S.; Liang, Q.-M.; Zhou, W.-W.; Jiang, Y.-D.; Zhu, Q.-Z.; Yu, H.; Zhang, C.-X.; Gurr, G.M.; Zhu, Z.-R. RNA interference of NADPH–cytochrome P450 reductase of the rice brown planthopper, Nilaparvata lugens, increases susceptibility to insecticides. Pest Manag. Sci. 2015, 71, 32–39. [Google Scholar] [CrossRef]
- Kitzmann, P.; Schwirz, J.; Schmitt-Engel, C.; Bucher, G. RNAi phenotypes are influenced by the genetic background of the injected strain. BMC Genom. 2013, 14, 5. [Google Scholar] [CrossRef]
- Mehlhorn, S.; Hunnekuhl, V.S.; Geibel, S.; Nauen, R.; Bucher, G. Establishing RNAi for basic research and pest control and identification of the most efficient target genes for pest control: A brief guide. Front. Zool. 2021, 18, 60. [Google Scholar] [CrossRef]
- Cagliari, D.; Dias, N.P.; dos Santos, E.Á.; Rickes, L.N.; Kremer, F.S.; Farias, J.R.; Lenz, G.; Galdeano, D.M.; Garcia, F.R.M.; Smagghe, G.; et al. First transcriptome of the Neotropical pest Euschistus heros (Hemiptera: Pentatomidae) with dissection of its siRNA machinery. Sci. Rep. 2020, 10, 4856. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Wang, Y.-C.; Zhang, X.-X.; Iqbal, J.; Du, Y.-Z. RNA interference of genes encoding the Vacuolar-ATPase in Liriomyza trifoliis. Insects 2021, 12, 41. [Google Scholar] [CrossRef]
- Adeyinka, O.S.; Nasir, I.A.; Riaz, S.; Yousaf, I.; Toufiq, N.; Okiki, A.P.; Tabassum, B. A Protective dsRNA is crucial for optimum RNAi gene silencing in Chilo partellus. Int. J. Agric. Biol. 2021, 25, 1238–1248. [Google Scholar] [CrossRef]
- Westerhout, E.M.; Berkhout, B. A systematic analysis of the effect of target RNA structure on RNA interference. Nucleic Acids Res. 2007, 35, 4322–4330. [Google Scholar] [CrossRef]
- Cao, M.; Gatehouse, J.A.; Fitches, E.C. A Systematic study of RNAi effects and dsRNA stability in Tribolium castaneum and Acyrthosiphon pisum, following injection and ingestion of analogous dsRNAs. Int. J. Mol. Sci. 2018, 19, 1079. [Google Scholar] [CrossRef]
- Elbashir, S.M.; Harborth, J.; Weber, K.; Tuschl, T. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 2002, 26, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Teimoori-Toolabi, L.; Hashemi, S.; Azadmanesh, K.; Eghbalpour, F.; Safavifar, F.; Khorramizadeh, M.R. Silencing the wild-type and mutant K-ras increases the resistance to 5-flurouracil in HCT-116 as a colorectal cancer cell line. Anti-Cancer Drugs 2015, 26, 187–196. [Google Scholar] [CrossRef]
- Fakhr, E.; Zare, F.; Teimoori-Toolabi, L. Precise and efficient siRNA design: A key point in competent gene silencing. Cancer Gene Ther. 2016, 23, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, J.; Lenartowicz, E.; Soszynska-Jozwiak, M.; Szutkowska, B.; Kierzek, R.; Kierzek, E. RNA secondary structure motifs of the influenza A virus as targets for siRNA-mediated RNA interference. Mol. Ther. Nucleic Acids 2020, 19, 627–642. [Google Scholar] [CrossRef]
- Luo, K.Q.; Chang, D.C. The gene-silencing efficiency of siRNA is strongly dependent on the local structure of mRNA at the targeted region. Biochem. Biophys. Res. Commun. 2004, 318, 303–310. [Google Scholar] [CrossRef]
- Shao, Y.; Chan, C.Y.; Maliyekkel, A.; Lawrence, C.E.; Roninson, I.B.; Ding, Y. Effect of target secondary structure on RNAi efficiency. RNA 2007, 13, 1631–1640. [Google Scholar] [CrossRef]
- Lu, Z.J.; Mathews, D.H. Efficient siRNA selection using hybridization thermodynamics. Nucleic Acids Res. 2007, 36, 640–647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).