Suitability Analysis of Crops for Sloping Farmland in Arid Sandy Regions with Traditional Farming Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
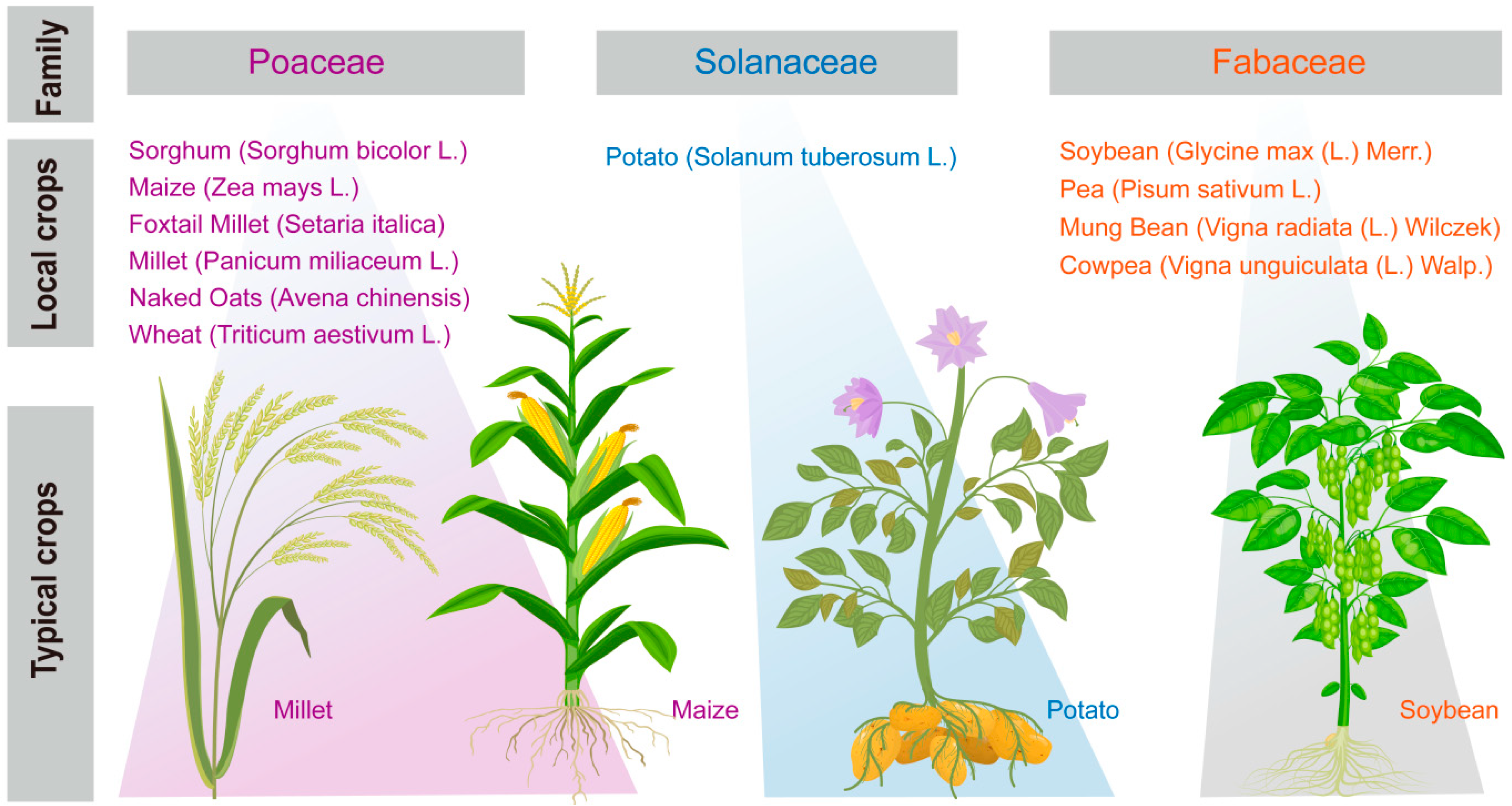
2.2. Selection of Crops
2.3. Evaluation Indicators
2.4. Experimental Design
- (1)
- Root system characterization: Root traits were assessed through systematic field sampling. Sampling strategy: Nine randomized sampling points were selected across varying slope positions and elevations, with six plants per crop species randomly selected from each. Crop height and root depth were recorded using calibrated tools. Root-to-shoot ratio (R/S) was calculated by separating, oven-drying (65 °C, 48 h), and weighing aboveground biomass and roots. Root length density and root density were quantified via high-resolution root imaging. Topsoil bulk density (0–20 cm depth) was measured using undisturbed soil cores extracted via the ring knife method.
- (2)
- Nitrogen fixation: Data compiled from peer-reviewed studies on nitrogen fixation rates in arid-region crops.
- (3)
- Anti-wind erosion assessment: Wind erosion resistance was evaluated through field measurements of four key parameters: stubble height (measured using graduated poles at 20 random points per plot), land cover percentage (quantified via drone-based multispectral imaging (SZ DJI, Shenzhen, China)), soil bulk density (determined by the core method at 0–15 cm depth), and surface roughness (assessed using a 1 m profile laser scanner with 2 mm resolution (Creality 3D, Shenzhen, China)). Wind speed attenuation (%) was derived from empirical relationships between stubble height and aerodynamic drag coefficients. Wind erosion intensity (t ha−1 yr−1) was calculated using the Revised Wind Erosion Equation (RWEQ) [32], calibrated with site-specific soil texture, stubble height, and climatic data.
- (4)
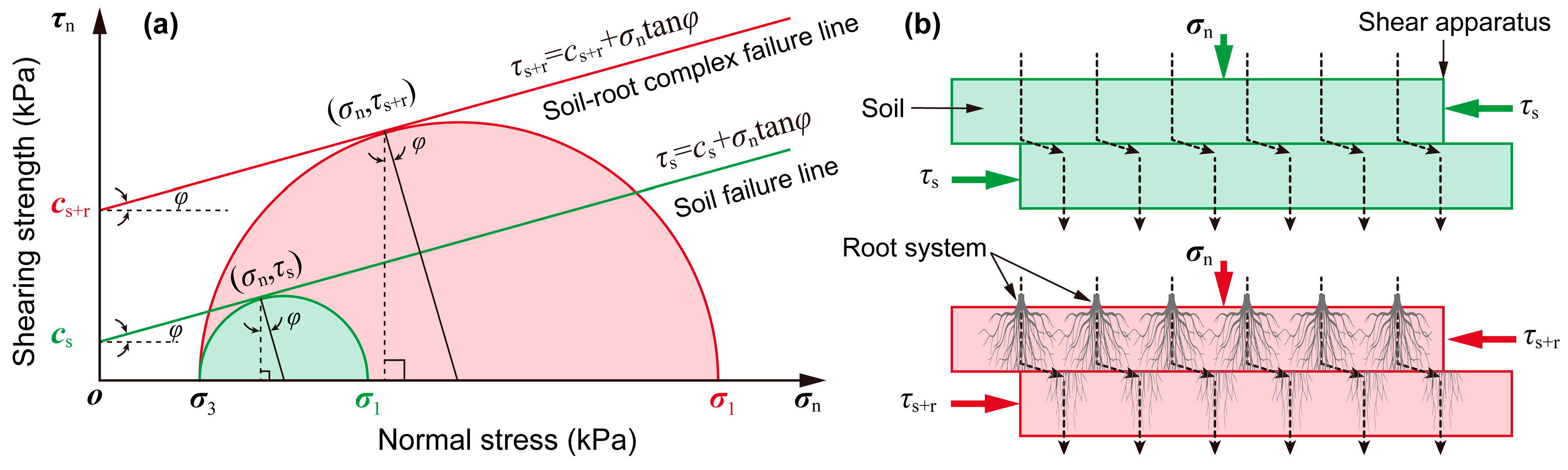
- Roots reinforcement: The shear strength of root–soil composites was measured using a 2012-HPF large-scale direct shear apparatus (Geocomp, Boston, USA). Undisturbed soil samples containing intact root systems were subjected to incremental normal stresses (50–200 kPa) to determine cohesion (c) and internal friction angle (φ).
- (5)
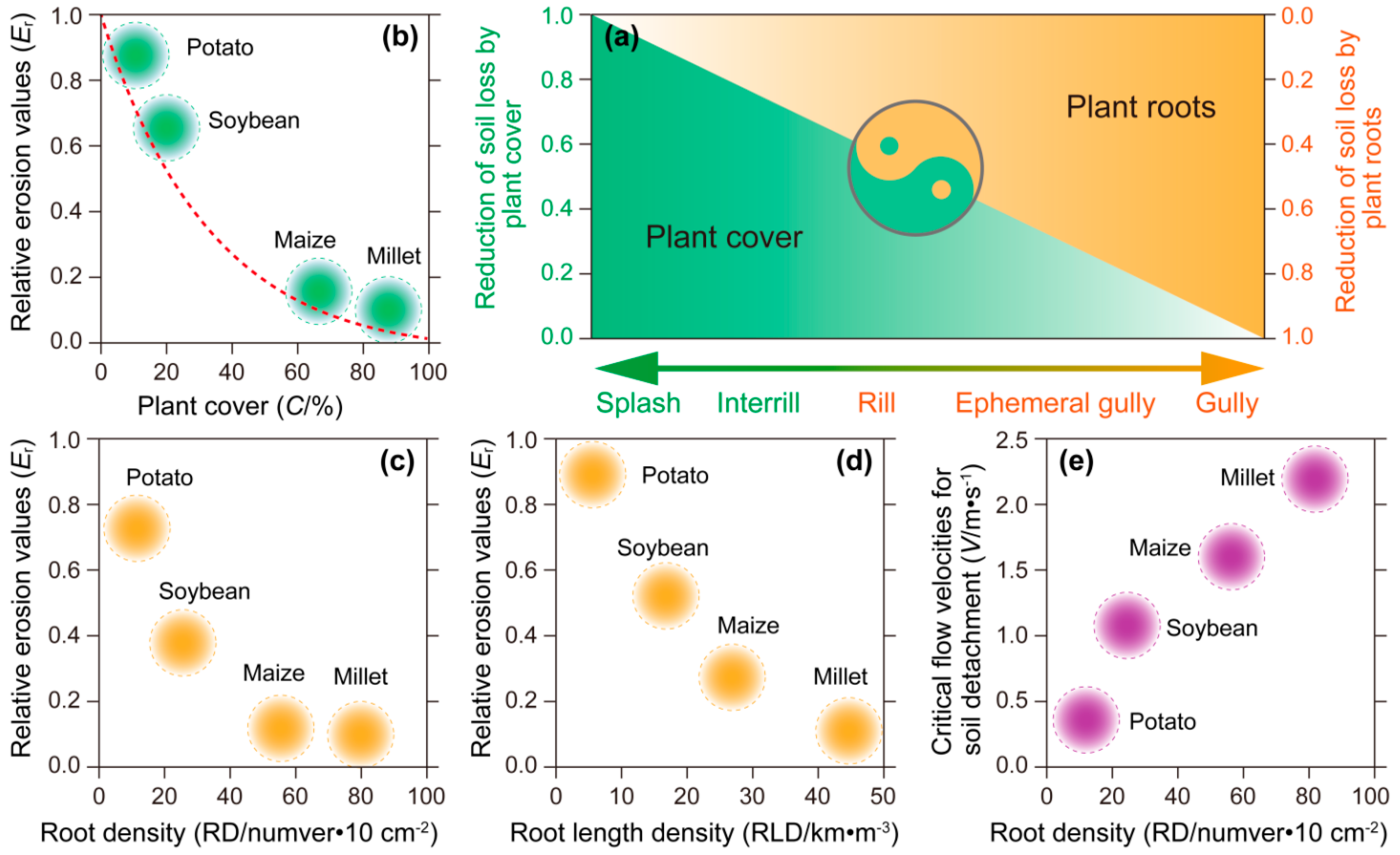
- Anti-water erosion capacity was assessed through the following: plant coverage rates (%) were quantified using ground-truth quadrat surveys; soil erosion rates were measured via sediment traps (Hohhot, China) installed in the Study Area. Data normalization: Erosion values were converted to relative erosion rates (%) by comparing them with bare soil control plots.
- (6)
- Water conservation efficiency was evaluated based on the following: the evaluation of this indicator can be based on the plant’s root system’s ability to absorb and store water, as well as the plant’s canopy’s ability to reduce evaporation, based on field observations and measurements. All field data were collected in situ.
3. Results
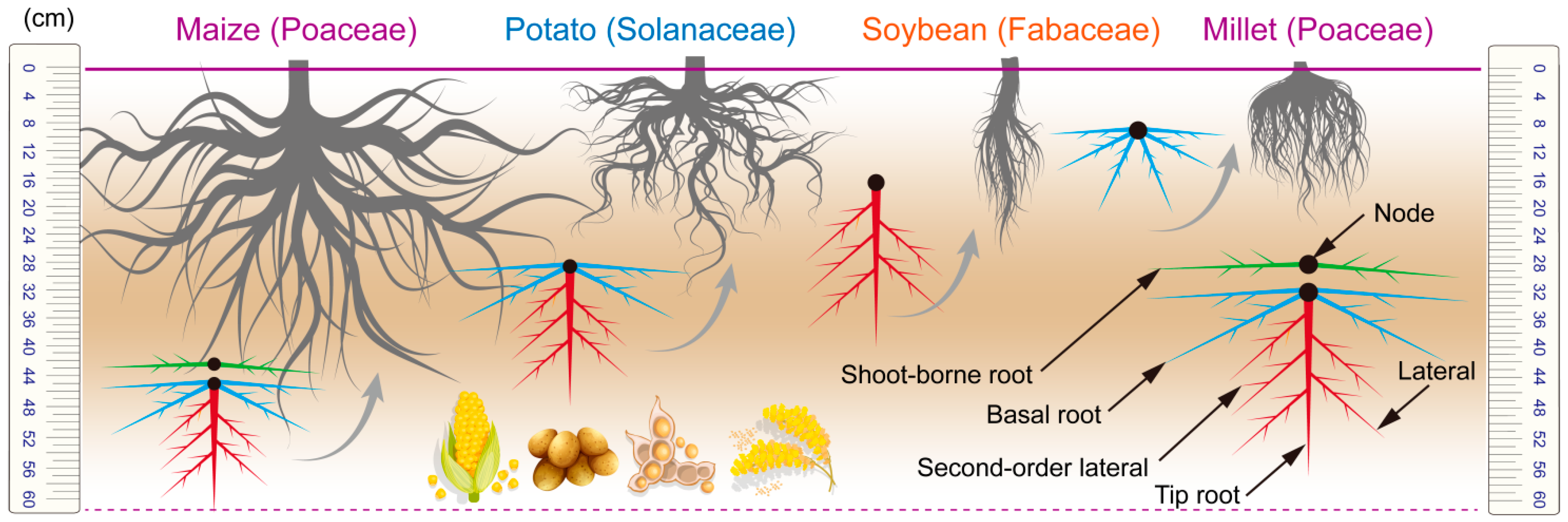
3.1. Root Characteristics
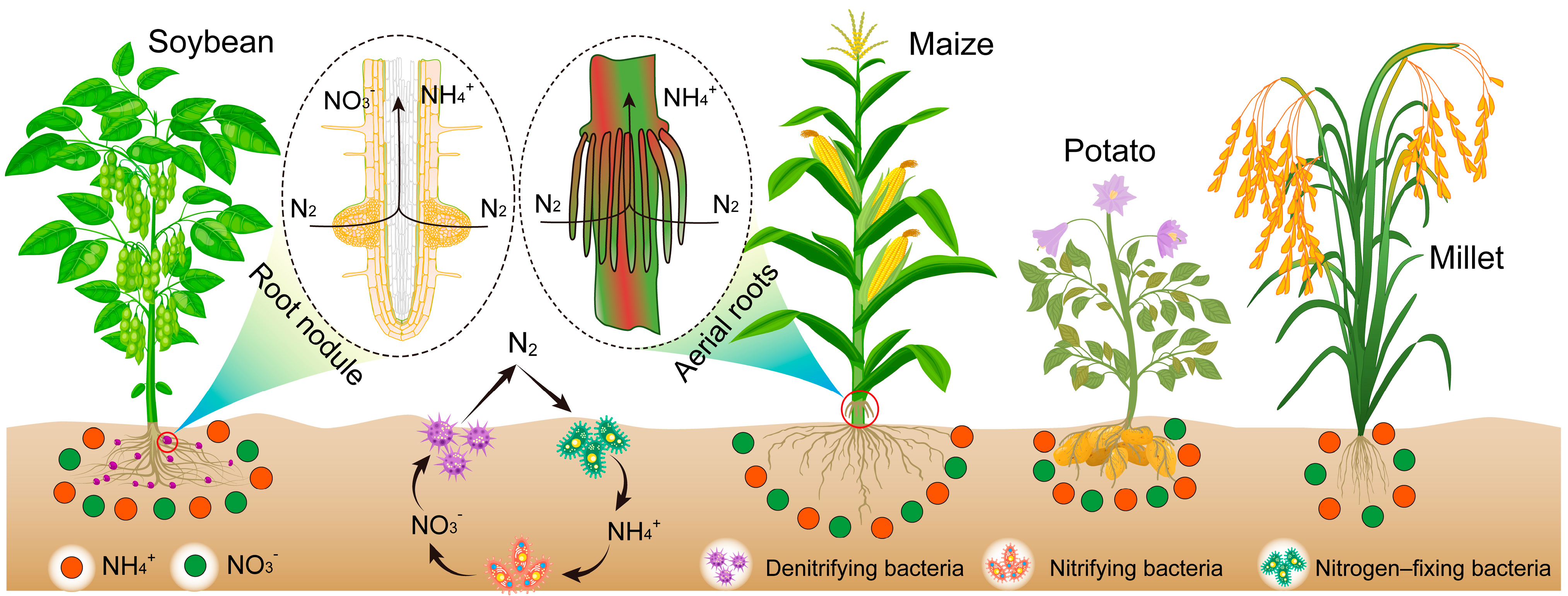
3.2. Nitrogen Fixation Effect
3.3. Anti-Wind Erosion Effect
3.4. Roots Reinforcement Effect
3.5. Anti-Water Erosion Effect
3.6. Water Conservation Effect
4. Discussion
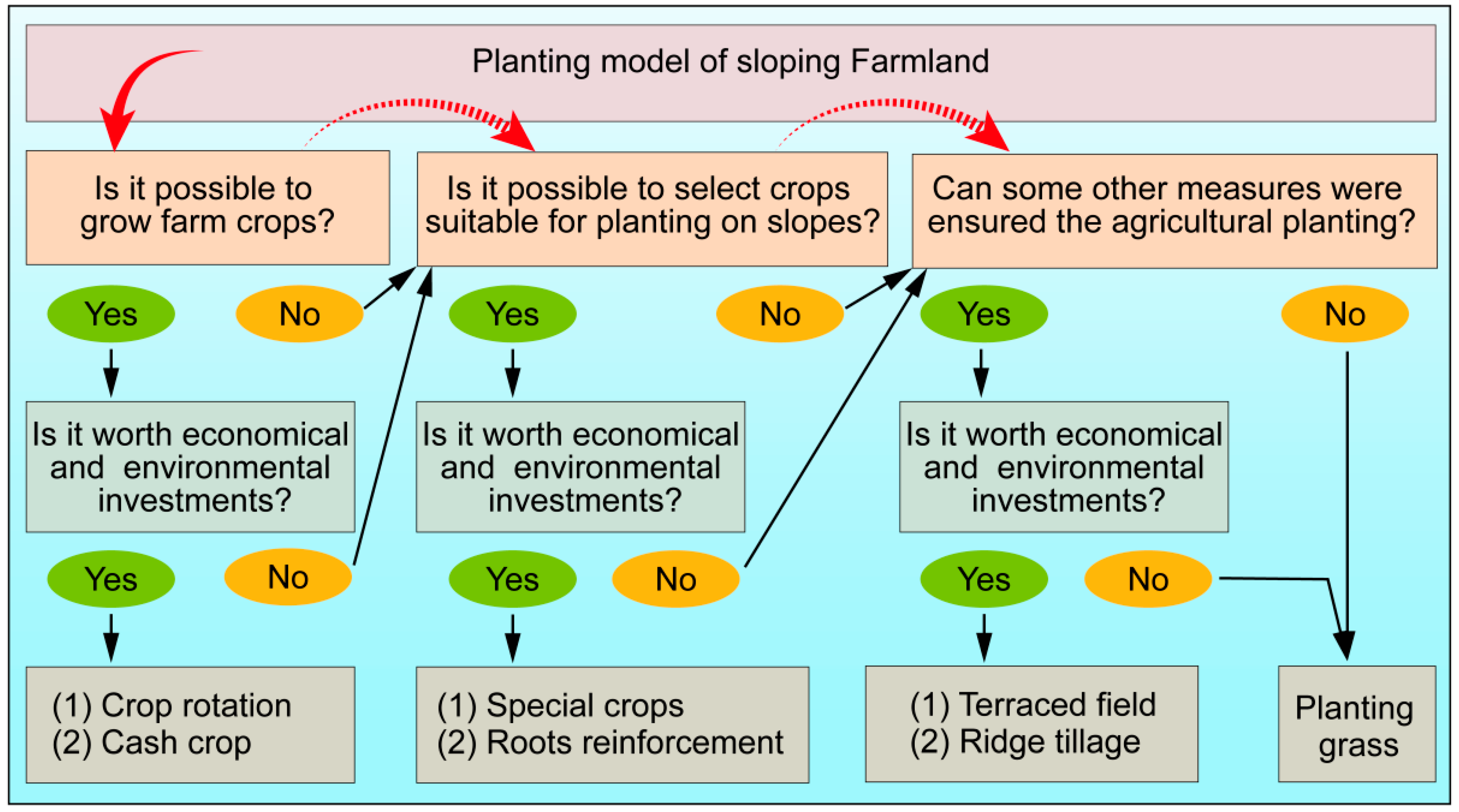
4.1. Crops Suitable for Sloping Farmland
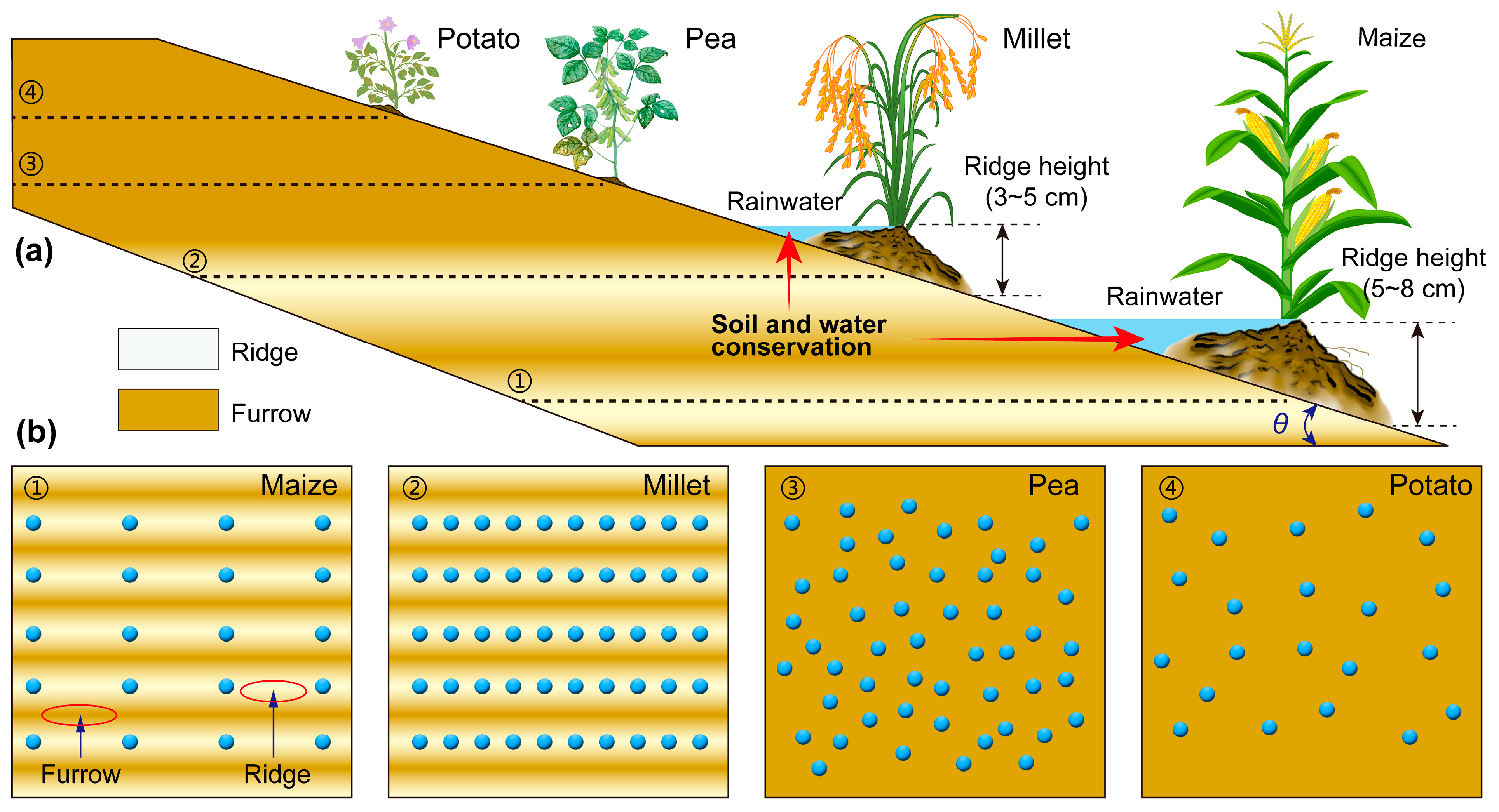
4.2. Cropping Plan for Sloping Farmland
5. Conclusions
- (1)
- The four indigenous crops, namely maize, millet, soybean, and potato, exhibit significant differences in their root systems, which determine their suitability for cultivation on sloping farmland. Maize and millet, with their extensive and deep root systems, are better equipped to anchor the soil and resist erosion. Specifically, maize exhibits peak root density (2.8 ± 0.4 cm/cm3) at 20–40 cm soil depth, while millet shows concentrated topsoil root density (4.2 ± 0.6 cm/cm3) within the upper 15 cm. In contrast, soybean and potato have shallower and less extensive root systems. Soybean displays a root density of 1.5 ± 0.3 cm/cm3 limited to the top 10 cm, whereas potato exhibits minimal root development (0.4 ± 0.5 cm/cm3) beyond 25 cm depth, making them less suitable for sloping farmland.
- (2)
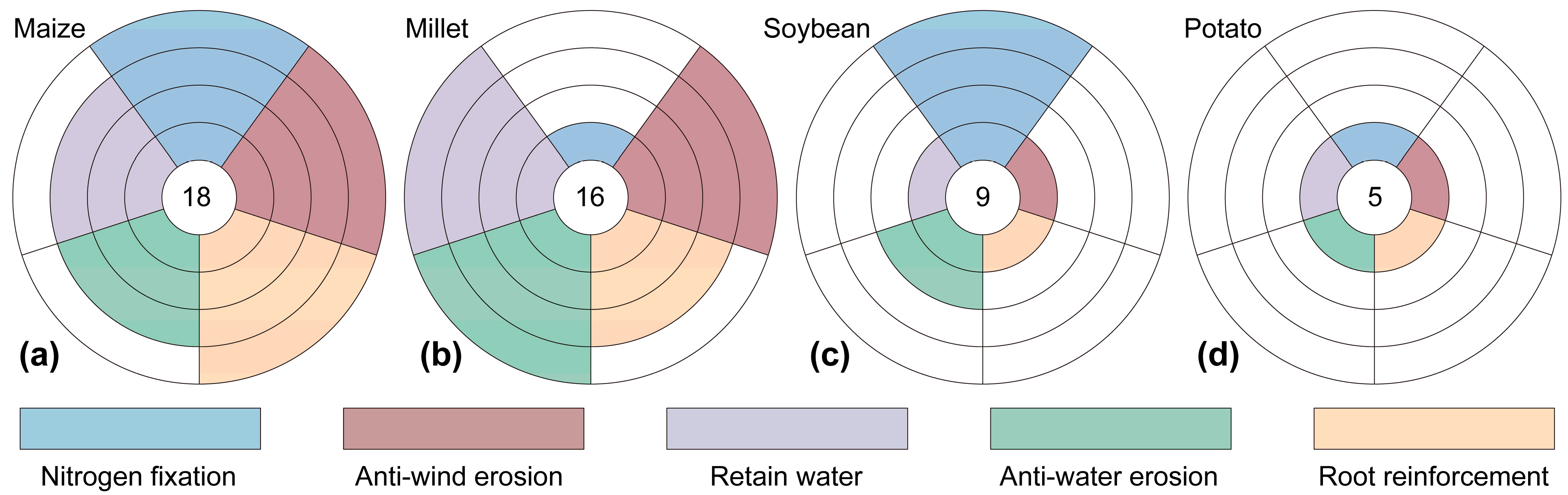
- The evaluation of the five indicators (nitrogen fixation, anti-wind erosion, root reinforcement, anti-water erosion, and water conservation) revealed that maize demonstrated superior adaptability with the highest composite score of 18, exhibiting robust performance across all indices. Millet ranked second with a score of 16, showing particular strength in soil and water conservation. In contrast, soybean and potato displayed markedly lower suitability, scoring 9 and 5, respectively, with potatoes showing negligible soil and water conservation capacity. This indicates that maize and millet are the most suitable crops for sloping farmland in sandy and arid regions.
- (3)
- Sloping farmland in sandy and arid regions face severe challenges of soil erosion and nutrient loss. The cultivation of maize and millet can effectively mitigate these problems. Their deep and extensive root systems help to stabilize the soil, reduce water and wind erosion, and enhance water retention, thereby contributing to the conservation of soil and nutrients on sloping farmland.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menegat, S.; Ledo, A.; Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 2022, 12, 14490. [Google Scholar] [CrossRef]
- Barros, B.G.F.; Freitas, A.D.S.; Tabosa, J.N.; Lyra, M.C.C.P.; Santo Mergulhao, A.C.E.; Silva, A.F.; Oliveira, W.S.; Fernandes-Júnior, P.I.; Sampaio, E.V.S.B. Biological nitrogen fixation in field-grown sorghum under different edaphoclimatic conditions is confirmed by N isotopic signatures. Nutr. Cycl. Agroecosyst. 2020, 117, 93–101. [Google Scholar] [CrossRef]
- Wolf, E.S.A.; Vela, S.; Wilker, J.; Davis, A.; Robert, M.; Infante, V.; Venado, R.E.; Voiniciuc, C.; Ané, J.M.; Vermerris, W. Identification of genetic and environmental factors influencing aerial root traits that support biological nitrogen fixation in sorghum. G3 Genes Genomes Genet. 2024, 14, jkad285. [Google Scholar] [CrossRef]
- Yun, J.X.; Wang, C.; Zhang, F.R.; Chen, L.; Sun, Z.X.; Cai, Y.P.; Luo, Y.Q.; Liao, J.W.; Wang, Y.L.; Cha, Y.Y.; et al. A nitrogen fixing symbiosis-specific pathway required for legume flowering. Sci. Adv. 2023, 9, eade1150. [Google Scholar] [CrossRef]
- Van Deynze, A.; Zamora, P.; Delaux, P.M.; Bennett, A.B.; Jayaraman, D.; Rajasekar, S.; Graham, D.; Maeda, J.; Gibson, D.; Schwartz, K.D.; et al. Nitrogen fixation in a landrace of maize is supported by a mucilage-associated diazotrophic microbiota. PLoS Biol. 2018, 16, e2006352. [Google Scholar] [CrossRef]
- Connolly, L.N.; Lorenz, N.; Maleki, K.; Kayafas, N.; Dick, R.P.; Mercer, K.L. Nitrogen fixation rates and aerial root production among maize landraces. Front. Plant Sci. 2025, 16, 1502884. [Google Scholar] [CrossRef]
- Pankievicz, V.C.S.; Delaux, P.M.; Infante, V.; Hirsch, H.H.; Rajasekar, S.; Zamora, P.; Jayaraman, D.; Calderon, C.I.; Bennett, A.; Ané, J.M. Nitrogen fixation and mucilage production on maize aerial roots is controlled by aerial root development and border cell functions. Front. Plant Sci. 2022, 13, 977056. [Google Scholar] [CrossRef]
- Mazumder, S.; Bhattacharya, D.; Lahiri, D.; Nag, M. Rhizobacteria and arbuscular mycorrhizal fungi (AMF) community in growth management and mitigating stress in millets: A plant-soil microbe symbiotic relationship. Curr. Microbiol. 2025, 82, 242. [Google Scholar] [CrossRef]
- Darapuneni, M.K.; Idowu, O.J.; Sarihan, B.; DuBois, D.; Grover, K.; Sanogo, S.; Djaman, K.; Lauriault, L.; Omer, M.; Dodla, S. Growth characteristics of summer cover crop grasses and their relation to soil aggregate stability and wind erosion control in arid southwest. Appl. Eng. Agric. 2021, 37, 11–23. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.Y.; Yu, J.; Shi, X.H.; Jia, L.G.; Fan, M.S. Implementing a soil ammonium N fertilizer management for synchronizing potato N demands. Heliyon 2024, 10, e30456. [Google Scholar] [CrossRef]
- Meng, J.; Li, W.Y.; Qi, F.Y.; Yang, T.X.; Li, N.; Wan, J.; Li, X.Q.; Jiang, Y.J.; Wang, C.H.; Huang, M.L.; et al. Knockdown of microRNA390 enhances maize brace root growth. Int. J. Mol. Sci. 2024, 25, 6791. [Google Scholar] [CrossRef]
- Hostetler, A.N.; Khangura, R.S.; Dilkes, B.P.; Sparks, E.E. Bracing for sustainable agriculture: The development and function of brace roots in members of Poaceae. Curr. Opin. Plant Biol. 2021, 59, 101985. [Google Scholar] [CrossRef]
- Hostetler, A.N.; Erndwein, L.; Ganji, E.; Reneau, J.W.; Killian, M.L.; Sparks, E.E. Maize brace root mechanics vary by whorl, genotype and reproductive stage. Ann. Bot. 2022, 129, 657–668. [Google Scholar] [CrossRef]
- Hostetler, A.N.; Erndwein, L.; Reneau, J.W.; Stager, A.; Tanner, H.G.; Cook, D.; Sparks, E.E. Multiple brace root phenotypes promote anchorage and limit root lodging in maize. Plant Cell Environ. 2022, 45, 1573–1583. [Google Scholar] [CrossRef]
- Burak, E.; Dodd, I.C.; Quinton, J.N. Do root hairs of barley and maize roots reinforce soil under shear stress? Geoderma 2021, 383, 114740. [Google Scholar] [CrossRef]
- Stubbs, C.J.; Cook, D.D.; Niklas, K.J. A general review of the biomechanics of root anchorage. J. Exp. Bot. 2019, 70, 3439–3451. [Google Scholar] [CrossRef]
- Goodman, A.M.; Ennos, A.R. The effects of mechanical stimulation on the morphology and mechanics of maize roots grown in an aerated nutrient solution. Int. J. Plant Sci. 2001, 162, 691–696. [Google Scholar] [CrossRef]
- Reneau, J.W.; Khangura, R.S.; Stager, A.; Erndwein, L.; Weldekidan, T.; Cook, D.D.; Dilkes, B.P.; Sparks, E.E. Maize brace roots provide stalk anchorage. Plant Direct 2020, 4, e00284. [Google Scholar] [CrossRef]
- Hao, J.Q.; Song, J.J.; Gao, G.X.; Xu, W.; Bai, J.Z.; Feng, Y.Z.; Wang, X. Mitigation of the ratio of soil dissolved organic carbon to available phosphorus effectively improves crop productivity under mulching measures on the Loess Plateau. Agronomy 2023, 13, 1810. [Google Scholar] [CrossRef]
- Chiurazzi, M.; Frugis, G.; Navazio, L. Symbiotic nitrogen fixation: A launchpad for investigating old and new challenges. J. Exp. Bot. 2025, 76, 1473–1477. [Google Scholar] [CrossRef]
- Hao, G.L.; Wang, L.G.; Liu, X.F. Methods for studying the effect of plant roots on soil mechanical reinforcement: A review. J. Soil Sci. Plant Nutr. 2023, 23, 2893–2912. [Google Scholar] [CrossRef]
- Li, X.F.; Li, Z.Q. What determines symbiotic nitrogen fixation efficiency in Rhizobium: Recent insights into Rhizobium leguminosarum. Arch. Microbiol. 2023, 205, 300. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, W.W.; Zhou, A.; Pereira, P. Water and wind erosion response to ecological restoration measures in China’s drylands. Geoderma 2023, 435, 116514. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, W.J.; Li, Q.; Sun, H.; Feng, Z.D.; Zou, J.T. Erosion-reducing potential of Salix psammophila roots in the water-wind crisscrossed erosion region of the Chinese Loess Plateau: A simulated investigation. Front. Environ. Sci. 2023, 10, 1109174. [Google Scholar] [CrossRef]
- Dahanayake, A.C.; Webb, J.A.; Greet, J.; Brookes, J.D. How do plants reduce erosion? An eco evidence assessment. Plant Ecol. 2024, 225, 593–604. [Google Scholar] [CrossRef]
- Kumi, F.; Obour, P.B.; Arthur, E.; Moore, S.E.; Asare, P.A.; Asiedu, J.; Angnuureng, D.B.; Atiah, K.; Amoah, K.K.; Amponsah, S.K.; et al. Quantifying root-induced soil strength, measured as soil penetration resistance, from different crop plants and soil types. Soil Tillage Res. 2023, 233, 105811. [Google Scholar] [CrossRef]
- Oshunsanya, S.O.; Yu, H.Q.; Opara, C.C.; Odebode, A.M.; Oluwatuyi, T.S.; Babalola, O. Integration effect of vetiver grass strips with maize population density on soil erosion under two contrasting slopes of rainforest agroecology. Catena 2023, 221, 106768. [Google Scholar] [CrossRef]
- Desta, G.; Tamene, L.; Abera, W.; Amede, T.; Whitbread, A. Effects of land management practices and land cover types on soil loss and crop productivity in Ethiopia: A review. Int. Soil Water Conserv. Res. 2021, 9, 544–554. [Google Scholar] [CrossRef]
- Ying, C.Y.; Li, C.L.; Li, L.X.; Zhou, C. Combined effects of polymer SH and ryegrass on the water-holding characteristics of loess. J. Arid. Land 2024, 16, 1686–1700. [Google Scholar] [CrossRef]
- Sun, J.Q.; Lu, P.; Cao, Y.X.; Zhang, N.C.; Wu, F.Q.; Li, P. Effects of different crop root systems on soil detachment by concentrated flow on the Loess Plateau in China. Water 2022, 14, 772. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, Q.W.; Lu, C.; Li, H.K.; Wang, H.; Wu, F.Q. Variations in soil detachment by rill flow during crop growth stages in sloping farmlands on the Loess Plateau. Catena 2022, 216, 106375. [Google Scholar] [CrossRef]
- Yang, X.Y.; Yang, Q.K.; Zhu, H.A.; Wang, L.; Wang, C.M.; Pang, G.W.; Du, C.Z.; Mubeen, M.; Waleed, M.; Hussain, S. Quantitative evaluation of soil water and wind erosion rates in Pakistan. Remote Sens. 2023, 15, 2404. [Google Scholar] [CrossRef]
- Shi, Y.C.; Gahagan, A.C.; Morrison, M.J.; Gregorich, E.; Lapen, D.R.; Chen, W. Stratified effects of tillage and crop rotations on soil microbes in carbon and nitrogen cycles at different soil depths in long-term corn, soybean, and wheat cultivation. Microorganisms 2024, 12, 1635. [Google Scholar] [CrossRef]
- Liu, Z.J.; Kong, X.Y.; Long, Y.P.; Liu, S.R.; Zhang, H.; Jia, J.B.; Cui, W.H.; Zhang, Z.M.; Song, X.W.; Qiu, L.J.; et al. Integrated single-nucleus and spatial transcriptomics captures transitional states in soybean nodule maturation. Nat. Plants 2023, 9, 515–524. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Marriel, I.E.; de Sousa, S.M.; Lana, U.G.D.P.; Mattos, B.B.; Oliveira, C.A.D.; Gomes, E.A. Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz. J. Microbiol. 2018, 49, 40–46. [Google Scholar] [CrossRef]
- Flórez-Zapata, N.; García, J.C.; Del Portillo, P.; Restrepo, S.; Uribe-Velez, D. Composition and function of the microbial community related with the nitrogen cycling on the potato rhizosphere. Acta Biológica Colomb. 2013, 18, 449–464. [Google Scholar]
- Rahmat, Z.; Sohail, M.N.; Perrine-Walker, F.; Kaiser, B.N. Balancing nitrate acquisition strategies in symbiotic legumes. Planta 2023, 258, 12. [Google Scholar] [CrossRef]
- Chalk, P.M. The strategic role of 15N in quantifying the contribution of endophytic N2 fixation to the N nutrition of non-legumes. Symbiosis 2016, 69, 63–80. [Google Scholar] [CrossRef]
- Lumactud, R.A.; Dollete, D.; Liyanage, D.K.; Szczyglowski, K.; Hill, B.; Thilakarathna, M.S.S. The effect of drought stress on nodulation, plant growth, and nitrogen fixation in soybean during early plant growth. J. Agron. Crop Sci. 2023, 209, 345–354. [Google Scholar] [CrossRef]
- Nazari, M.; Bickel, S.; Kuzyakov, Y.; Bilyera, N.; Zarebanadkouki, M.; Wassermann, B.; Dippold, M.A. Root mucilage nitrogen for rhizosphere microorganisms under drought. Biol. Fertil. Soils 2024, 60, 639–647. [Google Scholar] [CrossRef]
- Jiang, X.L.; Liu, W.J.; Yang, H.; Wang, H.D.; Li, Z.Y. Study on mechanical characteristics of living stumps and reinforcement mechanisms of slopes. Sustainability 2024, 16, 4294. [Google Scholar] [CrossRef]
- Comino, E.; Druetta, A. The effect of Poaceae roots on the shear strength of soils in the Italian alpine environment. Soil Tillage Res. 2010, 106, 194–201. [Google Scholar] [CrossRef]
- Tan, H.M.; Chen, F.M.; Chen, J.; Gao, Y.F. Direct shear tests of shear strength of soils reinforced by geomats and plant roots. Geotext. Geomembr. 2019, 47, 780–791. [Google Scholar] [CrossRef]
- Lian, B.Q.; Peng, J.B.; Zhan, H.B.; Wang, X.G. Mechanical response of root-reinforced loess with various water contents. Soil Tillage Res. 2019, 193, 85–94. [Google Scholar] [CrossRef]
- Fan, C.C.; Su, C.F. Role of roots in the shear strength of root-reinforced soils with high moisture content. Ecol. Eng. 2008, 33, 157–166. [Google Scholar] [CrossRef]
- Badhon, F.F.; Islam, M.A.; Islam, M.S. Experimental evaluation of additional shear strength for vetiver root-reinforced soil. In Geo-Congress 2024: Soil Improvement, Sustainability, Geoenvironmental, and Cold Regions Engineering; Geotechnical Special Publication 351; Geo-Congress on Bridging Government, Industry, and Academia for Resilient Mega-Communities: Vancouver, BC, Canada, 2024; pp. 207–217. [Google Scholar]
- Katuwal, S.; Vermang, J.; Cornelis, W.M.; Gabriels, D.; Moldrup, P.; De Jonge, L.W. Effect of root density on erosion and erodibility of a loamy soil under simulated rain. Soil Sci. 2013, 178, 29–36. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, K.D.; Liu, C.L.; Cen, Y.D.; Xia, J.Q. Effects of different vegetation components on soil erosion and response to rainfall intensity under simulated rainfall. Catena 2024, 235, 107652. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, G.; Wang, G. Effects of canopy and roots of patchy distributed Artemisia capillaris on runoff, sediment, and the spatial variability of soil erosion at the plot scale. Soil Sci. 2012, 177, 409–415. [Google Scholar] [CrossRef]
- Yin, Q.Y.; Liu, J.B.; Zhang, B. Impact of vegetation canopy, litter, and roots on soil erosion under complex rainfall regimes: A case study with artemisia sacrorum in Loess Hilly Region of China. Land Degrad. Dev. 2025, 36, 1371–1383. [Google Scholar] [CrossRef]
- Gyssels, G.; Poesen, J.; Bochet, E.; Li, Y. Impact of plant roots on the resistance of soils to erosion by water: A review. Prog. Phys. Geog. 2005, 29, 189–217. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Rodríguez Pleguezuelo, C.R. Soil-erosion and runoff prevention by plant covers. A review. Agron. Sustain. Dev. 2008, 28, 65–86. [Google Scholar] [CrossRef]
- Vannoppen, W.; Vanmaercke, M.; De Baets, S.; Poesen, J. A review of the mechanical effects of plant roots on concentrated flow erosion rates. Earth-Sci. Rev. 2015, 150, 666–678. [Google Scholar] [CrossRef]
- Duan, G.H.; Leng, C.Q.; Zhang, Z.Y.; Zhang, C.; Wen, Z.M. Quantitative study on the effects of vegetation and soil on runoff and sediment in the Loess Plateau. Forests 2024, 15, 1341. [Google Scholar] [CrossRef]
- Dalzell, B.J.; Fissore, C.; Nater, E.A. Topography and land use impact erosion and soil organic carbon burial over decadal timescales. Catena 2022, 218, 106578. [Google Scholar] [CrossRef]
- Zhou, S.; Li, P.; Zhang, Y.; Zhang, N.C.; Cao, Y.X. Formation of new erosion-deposition patterns after farmland conversion: The major role of topography. Catena 2023, 231, 107349. [Google Scholar] [CrossRef]
- Morbidelli, R.; Saltalippi, C.; Flammini, A.; Cifrodelli, M.; Corradini, C.; Govindaraju, R.S. Infiltration on sloping surfaces: Laboratory experimental evidence and implications for infiltration modeling. J. Hydrol. 2015, 523, 79–85. [Google Scholar] [CrossRef]
- Luo, J.; Zheng, Z.C.; Li, T.X.; He, S.Q.; Zhang, X.Z.; Huang, H.G.; Wang, Y.D. Quantifying the contributions of soil surface microtopography and sediment concentration to rill erosion. Sci. Total Environ. 2021, 752, 141886. [Google Scholar] [CrossRef]

| Parameter | Maize | Millet | Soybean | Potato |
|---|---|---|---|---|
| Height (m) | 1.5–2.0 | 1.2–1.5 | 0.5–1.0 | 0.2–0.6 |
| Max root depth (m) | 0.2–0.5 | 0.1–0.3 | 0.15–0.3 | 0.3–0.6 |
| Root density (cm/cm3) | 2.8 ± 0.4 | 4.2 ± 0.6 | 1.5 ± 0.3 | 0.4 ± 0.5 |
| Root-to-shoot ratio (R/S) | 0.25–0.35 | 0.45–0.55 | 0.20–0.25 | / |
| Parameter | Soybean | Maize | Millet | Potato |
|---|---|---|---|---|
| Fixation type | Symbiotic | Associative | Endophytic | None |
| Key organisms | Bradyrhizobium | Azospirillum | Diazotrophs | - |
| N2 fixed (kg ha−1 yr−1) | 40–60 | 10–20 | No data | ≈0 |
| Energy cost (photosynthate) | High (15% C) | Moderate (5% C) | - | - |
| Crop Type | Stubble Height (cm) | Land Cover (%) | Soil Density (g/cm3) | Surface Roughness (cm) | Wind Speed Attenuation (%) | Wind Erosion Intensity (t ha−1 yr−1) |
|---|---|---|---|---|---|---|
| Millet | 6–12 | 38–50 | 1.3 | 4.2 | 51–76 | 1.3 |
| Maize | 10–18 | 21–36 | 1.1 | 3.3 | 49–62 | 1.4 |
| Soybean | 0 | 0 | 1.0 | / | / | 9.4 |
| Potato | 0 | 0 | 0.9 | / | / | 12.2 |
| Material | Soil | Soil + Maize | Soil + Soybean | Soil + Millet | Soil + Potato |
|---|---|---|---|---|---|
| Shearing strength (kPa) | 9.69 | 79.78 | 22.97 | 85.26 | 19.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Zhang, H.; Chi, Q.; Zhao, B.; Wang, P. Suitability Analysis of Crops for Sloping Farmland in Arid Sandy Regions with Traditional Farming Methods. Agronomy 2025, 15, 1150. https://doi.org/10.3390/agronomy15051150
Li S, Zhang H, Chi Q, Zhao B, Wang P. Suitability Analysis of Crops for Sloping Farmland in Arid Sandy Regions with Traditional Farming Methods. Agronomy. 2025; 15(5):1150. https://doi.org/10.3390/agronomy15051150
Chicago/Turabian StyleLi, Shuanhu, Haonan Zhang, Qingguo Chi, Bohan Zhao, and Ping Wang. 2025. "Suitability Analysis of Crops for Sloping Farmland in Arid Sandy Regions with Traditional Farming Methods" Agronomy 15, no. 5: 1150. https://doi.org/10.3390/agronomy15051150
APA StyleLi, S., Zhang, H., Chi, Q., Zhao, B., & Wang, P. (2025). Suitability Analysis of Crops for Sloping Farmland in Arid Sandy Regions with Traditional Farming Methods. Agronomy, 15(5), 1150. https://doi.org/10.3390/agronomy15051150





