Optimizing Protein-Rich Young Vegetative Quinoa (Chenopodium quinoa) Growth: Effects of Inter-Row Spacing and Genotype in Mediterranean Summer Cultivation
Abstract
1. Introduction
2. Materials and Methods
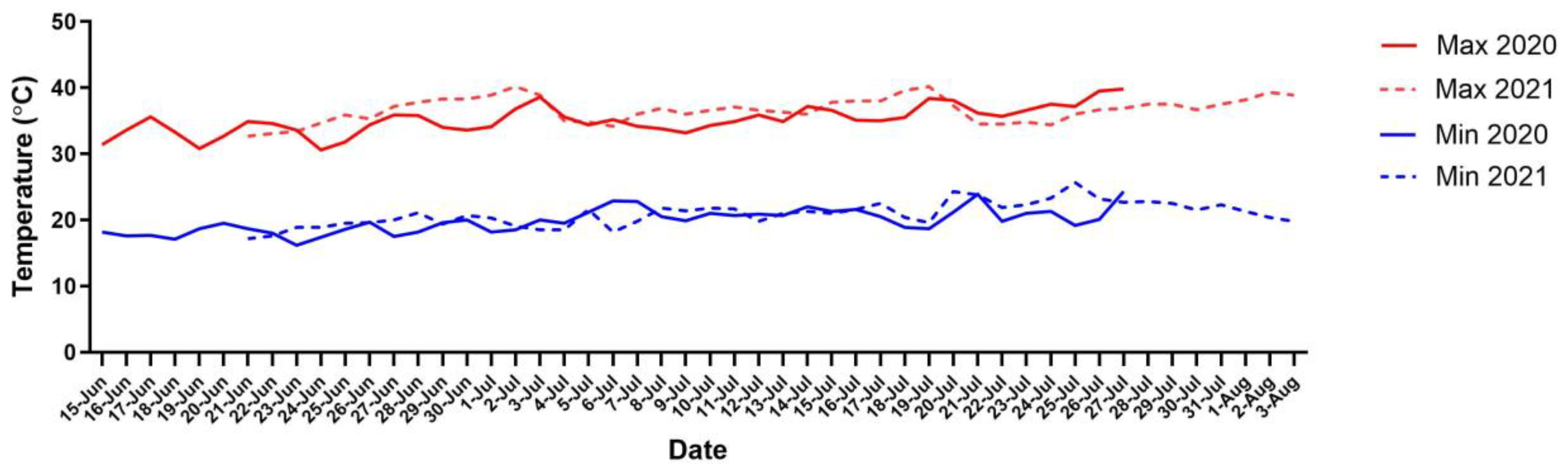
2.1. Experimental Site
2.2. Plant Material and Experimental Design
2.3. Plant Density at Harvest and Yield Measurements
2.4. Plant PC, Protein Yield, and Amino Acid Composition
2.5. Statistical Analysis
3. Results
3.1. Effect of Row Spacing on YVQ
3.1.1. Plant Density and DM
3.1.2. Fresh and DM Yield
3.1.3. PC and Protein Yield
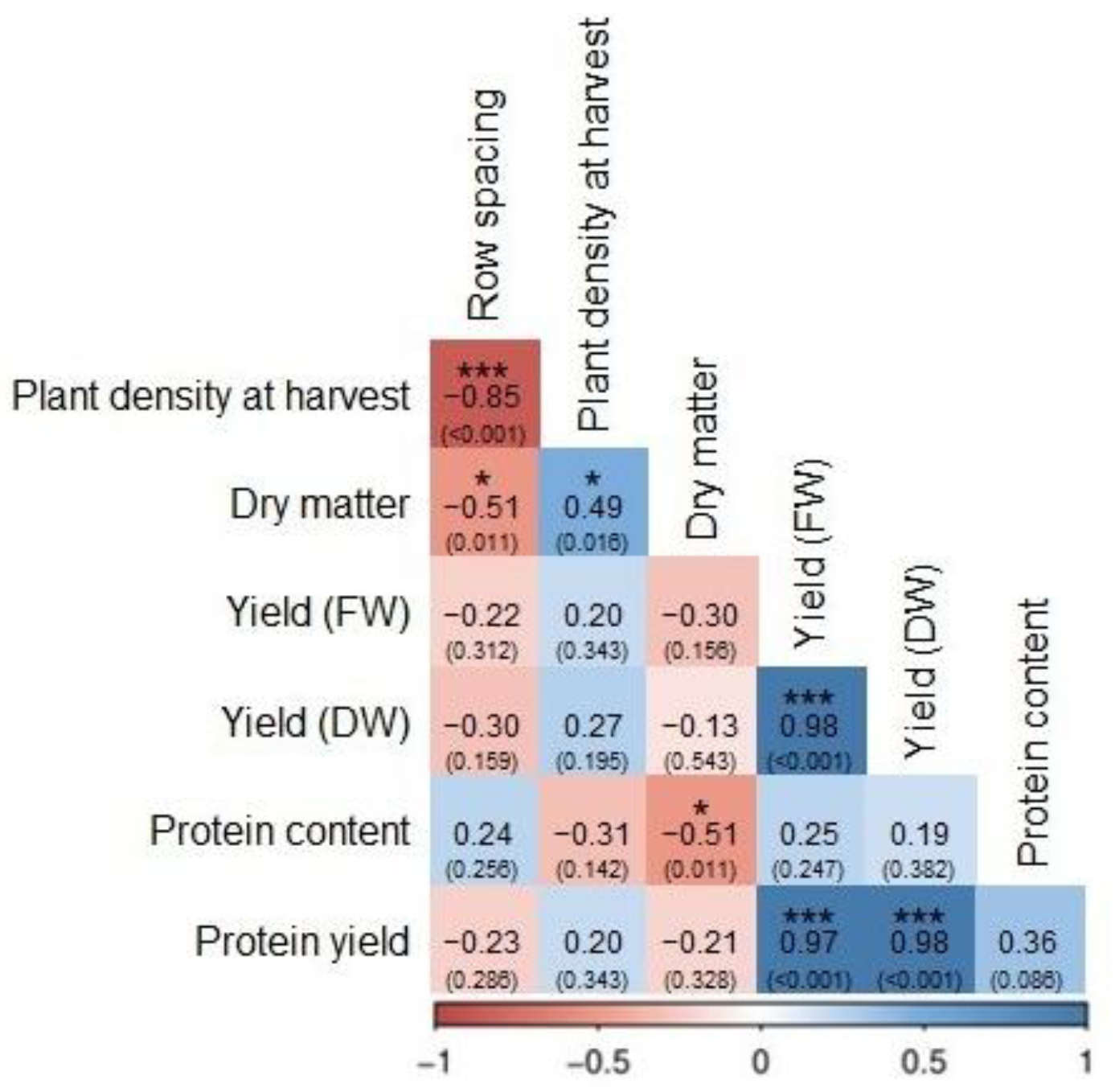
3.1.4. Correlations Between Traits
3.2. Effect of Genotypes on YVQ
3.2.1. Plant Density and DM
3.2.2. Fresh and DM Yield
3.2.3. PC and Protein Yield
3.3. Amino Acid Composition of YVQ
4. Discussion
4.1. Effect of Row Spacing on Yield Parameters
4.2. Effect of Genotype on Yield Parameters
4.3. Amino Acid Composition of YVQ
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAS | days after sowing |
| DM | dry matter |
| FW | fresh weight |
| EAAs | essential amino acids |
| PC | protein content |
| YVQ | young vegetative quinoa |
References
- Grandview Research Global Protein Ingredients Market Size Report, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/protein-ingredients-market (accessed on 29 January 2023).
- De Boer, J.; Aiking, H. On the Merits of Plant-Based Proteins for Global Food Security: Marrying Macro and Micro Perspectives. Ecol. Econ. 2011, 70, 1259–1265. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-Based Food and Protein Trend from a Business Perspective: Markets, Consumers, and the Challenges and Opportunities in the Future. Crit. Rev. Food Sci. Nutr. 2020, 61, 3119–3128. [Google Scholar] [CrossRef]
- Pam Ismail, B.; Senaratne-Lenagala, L.; Stube, A.; Brackenridge, A. Protein Demand: Review of Plant and Animal Proteins Used in Alternative Protein Product Development and Production. Anim. Front. 2020, 10, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, F.; Bazile, D.; Biaggi, C.; Callo-concha, D.; Jacquet, J.; Jemal, O.M.; King, O.I.; Mbosso, C.; Padulosi, S.; Speelman, E.N.; et al. When Neglected Species Gain Global Interest: Lessons Learned from Quinoa’s Boom and Bust for Teff and Minor Millet. Glob. Food Secur. 2022, 32, 100613. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7, 850. [Google Scholar] [CrossRef]
- Choukr-Allah, R.; Rao, N.K.; Hirich, A.; Shahid, M.; Alshankiti, A.; Toderich, K.; Gill, S.; Butt, K.U.R. Quinoa for Marginal Environments: Toward Future Food and Nutritional Security in MENA and Central Asia Regions. Front. Plant Sci. 2016, 7, 346. [Google Scholar] [CrossRef]
- Pulvento, C.; Bazile, D. Worldwide Evaluations of Quinoa—Biodiversity and Food Security under Climate Change Pressures: Advances and Perspectives. Plants 2023, 12, 868. [Google Scholar] [CrossRef]
- Scanlin, L.; Lewis, K.A. Quinoa as a Sustainable Protein Source: Production, Nutrition, and Processing. In Sustainable Protein Sources; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 223–238. ISBN 9780128027769. [Google Scholar]
- García-Parra, M.; Zurita-Silva, A.; Stechauner-Rohringer, R.; Roa-Acosta, D.; Jacobsen, S.E. Quinoa (Chenopodium quinoa Willd.) and Its Relationship with Agroclimatic Characteristics: A Colombian Perspective. Chil. J. Agric. Res. 2020, 80, 290–302. [Google Scholar] [CrossRef]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; CFR Ferreira, I. Chemical and Nutritional Characterization of Chenopodium quinoa Willd (Quinoa) Grains: A Good Alternative to Nutritious Food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef]
- Angeli, V.; Silva, P.M.; Massuela, D.C.; Khan, M.W.; Hamar, A.; Khajehei, F.; Graeff-Hönninger, S.; Piatti, C. Quinoa (Chenopodium quinoa Willd.): An Overview of the Potentials of the “Golden Grain” and Socio-Economic and Environmental Aspects of Its Cultivation and Marketization. Foods 2020, 9, 216. [Google Scholar] [CrossRef]
- Ceyhun Sezgin, A.; Sanlier, N. A New Generation Plant for the Conventional Cuisine: Quinoa (Chenopodium quinoa Willd.). Trends Food Sci. Technol. 2019, 86, 51–58. [Google Scholar] [CrossRef]
- Dakhili, S.; Abdolalizadeh, L.; Hosseini, S.M.; Shojaee-Aliabadi, S.; Mirmoghtadaie, L. Quinoa Protein: Composition, Structure and Functional Properties. Food Chem. 2019, 299, 125–161. [Google Scholar] [CrossRef]
- Noulas, C.; Tziouvalekas, M.; Vlachostergios, D.; Baxevanos, D.; Karyotis, T.; Iliadis, C. Adaptation, Agronomic Potential, and Current Perspectives of Quinoa Under Mediterranean Conditions: Case Studies from the Lowlands of Central Greece. Commun. Soil. Sci. Plant Anal. 2017, 48, 2612–2629. [Google Scholar]
- Jacobsen, S.E. The Scope for Adaptation of Quinoa in Northern Latitudes of Europe. J. Agron. Crop Sci. 2017, 203, 603–613. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.E.; Bazile, D.; Condori, B. Global Expansion of Quinoa and Challenges for the Andean Region. Glob. Food Secur. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Sellami, M.H.; Pulvento, C.; Lavini, A. Agronomic Practices and Performances of Quinoa under Field Conditions: A Systematic Review. Plants 2021, 10, 72. [Google Scholar] [CrossRef]
- Asher, A.; Dagan, R.; Galili, S.; Rubinovich, L. Effect of Row Spacing on Quinoa (Chenopodium quinoa) Growth, Yield, and Grain Quality under a Mediterranean Climate. Agriculture 2022, 12, 1298. [Google Scholar] [CrossRef]
- Wang, N.; Wang, F.; Shock, C.C.; Meng, C.; Qiao, L. Effects of Management Practices on Quinoa Growth, Seed Yield, and Quality. Agronomy 2020, 10, 445. [Google Scholar] [CrossRef]
- Walters, H.; Carpenter-Boggs, L.; Desta, K.; Yan, L.; Matanguihan, J.; Murphy, K. Effect of Irrigation, Intercrop, and Cultivar on Agronomic and Nutritional Characteristics of Quinoa. Agroecol. Sustain. Food Syst. 2016, 40, 783–803. [Google Scholar] [CrossRef]
- Asher, A.; Galili, S.; Whitney, T.; Rubinovich, L. The Potential of Quinoa (Chenopodium quinoa) Cultivation in Israel as a Dual-Purpose Crop for Grain Production and Livestock Feed. Sci. Hortic. 2020, 272, 109534. [Google Scholar] [CrossRef]
- Filik, G. Biodegradability of Quinoa Stalks: The Potential of Quinoa Stalks as a Forage Source or as Biomass for Energy Production. Fuel 2020, 266, 117064. [Google Scholar] [CrossRef]
- Matías, J.; Cruz, V.; Reguera, M. Heat Stress Impact on Yield and Composition of Quinoa Straw under Mediterranean Field Conditions. Plants 2021, 10, 955. [Google Scholar] [CrossRef]
- Ramos, N.; Cruz, A.M. Evaluation of Seven Seasonal Crops for Forage Production during the Dry Season in Cuba. Cuba. J. Agric. Sci. 2002, 36, 271–276. [Google Scholar]
- Ebeid, H.M.; Kholif, A.E.; El-Bordeny, N.; Chrenkova, M.; Mlynekova, Z.; Hansen, H.H. Nutritive Value of Quinoa (Chenopodium quinoa) as a Feed for Ruminants: In Sacco Degradability and in Vitro Gas Production. Environ. Sci. Pollut. Res. Int. 2022, 29, 35241–35252. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Tan, J.; Zeng, C.; Wang, J.; Huang, J.; Hu, Y.; Wu, Q.; Wu, X.; Liu, C.; et al. Quinoa Greens as a Novel Plant Food: A Review of Its Nutritional Composition, Functional Activities, and Food Applications. Crit. Rev. Food Sci. Nutr. 2024, 1–21. [Google Scholar] [CrossRef]
- Rubinovich, L.; Dagan, R.; Lugasi, Y.; Galili, S.; Asher, A. The Potential of Young Vegetative Quinoa (Chenopodium quinoa) as a New Sustainable Protein-Rich Winter Leafy Crop under Mediterranean Climate. PLoS ONE 2023, 18, e0290000. [Google Scholar] [CrossRef] [PubMed]
- Pathan, S.; Siddiqui, R.A. Nutritional Composition and Bioactive Components in Quinoa (Chenopodium quinoa Willd.) Greens: A Review. Nutrients 2022, 14, 558. [Google Scholar] [CrossRef]
- Adamczewska-Sowińska, K.; Sowiński, J.; Jama-Rodzeńska, A. The Effect of Sowing Date and Harvest Time on Leafy Greens of Quinoa (Chenopodium quinoa Willd.) Yield and Selected Nutritional Parameters. Agriculture 2021, 11, 405. [Google Scholar] [CrossRef]
- Pathan, S.; Ndunguru, G.; Islam, M.R.; Jhumur, S.T.; Ayele, A.G. Production of Quinoa Leafy Greens in High Tunnel for Season Extension in Missouri. Horticulturae 2023, 9, 209. [Google Scholar] [CrossRef]
- Vazquez-Luna, A.; Cortés, V.P.; Carmona, F.F.; Díaz-Sobac, R. Quinoa Leaf as a Nutritional Alternative. Cienc. Investig. Agrar. 2019, 46, 137–143. [Google Scholar] [CrossRef]
- Pathan, S.; Eivazi, F.; Valliyodan, B.; Paul, K.; Ndunguru, G.; Clark, K. Nutritional Composition of the Green Leaves of Quinoa (Chenopodium quinoa Willd.). J. Food Res. 2019, 8, 55. [Google Scholar] [CrossRef]
- Gómez, M.J.R.; Magro, P.C.; Blázquez, M.R.; Maestro-Gaitán, I.; Iñiguez, F.M.S.; Sobrado, V.C.; Prieto, J.M. Nutritional Composition of Quinoa Leafy Greens: An Underutilized Plant-Based Food with the Potential of Contributing to Current Dietary Trends. Food Res. Int. 2024, 178, 113862. [Google Scholar] [CrossRef] [PubMed]
- Gawlik-Dziki, U.; Świeca, M.; Sułkowski, M.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and Anticancer Activities of Chenopodium quinoa Leaves Extracts—In Vitro Study. Food Chem. Toxicol. 2013, 57, 154–160. [Google Scholar] [CrossRef]
- Stoleru, V.; Jacobsen, S.E.; Vitanescu, M.; Jitareanu, G.; Butnariu, M.; Munteanu, N.; Stan, T.; Teliban, G.C.; Cojocaru, A.; Mihalache, G. Nutritional and Antinutritional Compounds in Leaves of Quinoa. Food Biosci. 2022, 45, 101494. [Google Scholar] [CrossRef]
- Lim, J.G.; Park, H.M.; Yoon, K.S. Analysis of Saponin Composition and Comparison of the Antioxidant Activity of Various Parts of the Quinoa Plant (Chenopodium quinoa Willd.). Food Sci. Nutr. 2020, 8, 694–702. [Google Scholar] [CrossRef]
- Dick Mastebroek, H.; Limburg, H.; Gilles, T.; Marvin, H.J. Occurrence of Sapogenins in Leaves and Seeds of Quinoa (Chenopodium quinoa Willd). J. Sci. Food Agric. 2000, 80, 152–156. [Google Scholar] [CrossRef]
- Bernardo Solíz-Guerrero, J.; Jasso De Rodriguez, D.; Rodríguez-García, R.; Luis Angulo-Sánchez, J.; Méndez-Padilla, G. Quinoa Saponins: Concentration and Composition Analysis. In Trends in New Crops and New Uses; Janick, J., Whipkey, A., Eds.; ASHS Press: Alexandria, VA, USA, 2002. [Google Scholar]
- Abd El-Samad, E.H.; Hussin, S.A.; El-Naggar, A.M.; El-Bordeny, N.E.; Eisa, S.S. The Potential Use of Quinoa as a New Non-Traditional Leafy Vegetable Crop. Biosci. Res. 2018, 15, 3387–3403. [Google Scholar]
- Sief, A.S.; El-Deepah, H.R.A.; Kamel, A.S.M.; Ibrahim, J.F. Effect of Various Inter and Intra Spaces on the Yield and Quality of Quinoa (Chenopodium quinoa Willd.). J. Plant Prod. Mansoura Univ. 2015, 6, 371–383. [Google Scholar] [CrossRef]
- Bellalou, A.; Daklo-Keren, M.; Abu Aklin, W.; Sokolskaya, R.; Rubinovich, L.; Asher, A.; Galili, S. Germination of Chenopodium quinoa Cv. ‘Mint Vanilla’ Seeds under Different Abiotic Stress Conditions. Seed Sci. Technol. 2022, 50, 41–45. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- WHO. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint WHO/FAO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007; ISBN 9789241209359. [Google Scholar]
- Alvar-Beltrán, J.; Verdi, L.; Marta, A.D.; Dao, A.; Vivoli, R.; Sanou, J.; Orlandini, S. The Effect of Heat Stress on Quinoa (Cv. Titicaca) under Controlled Climatic Conditions. J. Agric. Sci. 2020, 158, 255–261. [Google Scholar] [CrossRef]
- Hinojosa, L.; Sanad, M.N.M.E.; Jarvis, D.E.; Steel, P.; Murphy, K.; Smertenko, A. Impact of Heat and Drought Stress on Peroxisome Proliferation in Quinoa. Plant J. 2019, 99, 1144–1158. [Google Scholar] [CrossRef]
- Abbas, G.; Areej, F.; Asad, S.A.; Saqib, M.; Anwar-ul-Haq, M.; Afzal, S.; Murtaza, B.; Amjad, M.; Naeem, M.A.; Akram, M.; et al. Differential Effect of Heat Stress on Drought and Salt Tolerance Potential of Quinoa Genotypes: A Physiological and Biochemical Investigation. Plants 2023, 12, 774. [Google Scholar] [CrossRef] [PubMed]
- Matías, J.; Rodríguez, M.J.; Cruz, V.; Calvo, P.; Reguera, M. Heat Stress Lowers Yields, Alters Nutrient Uptake and Changes Seed Quality in Quinoa Grown under Mediterranean Field Conditions. J. Agron. Crop Sci. 2021, 207, 481–491. [Google Scholar] [CrossRef]
- Alon, E.; Shapira, O.; Azoulay-Shemer, T.; Rubinovich, L. Shading Nets Reduce Canopy Temperature and Improve Photosynthetic Performance in ‘Pinkerton’ Avocado Trees during Extreme Heat Events. Agronomy 2022, 12, 1360. [Google Scholar] [CrossRef]
- Spehar, C.R.; da Rocha, J.E.S. Effect of Sowing Density on Plant Growth and Development of Quinoa, Genotype 4.5, in the Brazilian Savannah Highlands. Biosci. J. 2009, 25, 53–58. [Google Scholar]
- Risi, J.; Galwey, N.W. Effects of Sowing Date and Sowing Rate on Plant Development and Grain Yield of Quinoa (Chenopodium quinoa) in a Temperate Environment. J. Agric. Sci. 1991, 117, 325–332. [Google Scholar] [CrossRef]
- Bhargava, A.; Sudhir, S.; Deepak, O. Effect of Sowing Dates and Row Spacings on Yield and Quality Components of Quinoa (Chenopodium quinoa) Leaves. Indian. J. Agric. Sci. 2007, 77, 748–751. [Google Scholar]
- Prommarak, S. Response of Quinoa to Emergence Test and Row Spacing in Chiang Mai-Lumphun Valley Lowland Area. Khon Kaen Agric. J. 2014, 42, 8–14. [Google Scholar]
- Casini, P.; Biancofiore, G. Influence of Row Spacing on Canopy and Seed Production in Grain Amaranth (Amaranthus cruentus L.). Agron. Res. 2020, 18, 53–62. [Google Scholar] [CrossRef]
- Phillipus Du Plooy, C.; Beletse, Y.; Mulandana, N.; Mamadi, N.; Plooy, C. DU Effect of Spacing and Transplanting Time on Amaranths Yield. In African Crop Science Conference Proceedings; African Crop Science Society: Kampala, Uganda, 2009; Volume 9, pp. 243–246. [Google Scholar]
- Nascimento, C.S.; Filho, A.B.C.; Mendoza-Cortez, J.W.; Nascimento, C.S.; Neto, F.B.; Grangeiro, L.C. Effect of Population Density of Lettuce Intercropped with Rocket on Productivity and Land-Use Efficiency. PLoS ONE 2018, 13, e0194756. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.E.; Stølen, O.; Jørgensen, I. Cultivation of Quinoa (Chenopodium quinoa) under Temperate Climatic Conditions in Denmark. J. Agric. Sci. 1994, 122, 47–52. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2022: Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; FAO: Rome, Italy, 2022. [Google Scholar]
- Delgado-Iniesta, M.J.; Girona-Ruíz, A.; Sánchez-Navarro, A. Agro-Ecological Impact of Irrigation and Nutrient Management on Spinach (Spinacia oleracea L.) Grown in Semi-Arid Conditions. Land 2023, 12, 293. [Google Scholar] [CrossRef]
| Year | Row Spacing (cm) | Plant Density at Harvest (Plant m−2) | Dry Matter (%) | Fresh Yield (kg ha−1) | Dry Yield (kg DM ha−1) | Protein Content (%) | Protein Yield (kg ha−1) |
|---|---|---|---|---|---|---|---|
| 16 | 336 ± 6 a | 13.1 ± 0.6 | 28,469 ± 639 a | 3737 ± 243 a | 23.9 ± 1.4 | 884 ± 24 a | |
| 2020 | 26 | 193 ± 27 b | 13.4 ± 0.7 | 27,262 ± 251 a | 3662 ± 176 a | 23.5 ± 1.1 | 865 ± 76 a |
| 80 | 55 ± 4 c | 11.4 ± 0.2 | 20,986 ± 555 b | 2396 ± 54 b | 26.6 ± 2.2 | 633 ± 39 b | |
| 16 | 345 ± 37 a | 14.4 ± 0.1 a | 7423 ± 209 | 1072 ± 41 | 20.5 ± 0.4 | 220 ± 13 | |
| 2021 | 26 | 166 ± 157 b | 13.4 ± 0.1 b | 6747 ± 1609 | 902 ± 212 | 23.8 ± 1.3 | 209 ± 43 |
| 80 | 65 ± 3 c | 13.3 ± 0.4 b | 4957 ± 775 | 661 ± 105 | 22.3 ± 2 | 147 ± 28 |
| Year | Accession | Plant Density at Harvest (Plant m−2) | Dry Matter (%) | Fresh Yield (kg ha−1) | Dry Yield (kg DM ha−1) | Protein Content (%) | Protein Yield (kg ha−1) |
|---|---|---|---|---|---|---|---|
| Red Head | 176 ± 6 a | 12.2 ± 0.6 | 17,776 ± 794 | 2175 ± 196 | 24.4 ± 0.8 | 533 ± 56 | |
| Mint Vanilla | 183 ± 16 a | 13.1 ± 0.3 | 16,852 ± 389 | 2199 ± 54 | 26.3 ± 1.1 | 579 ± 25 | |
| 2020 | Ivory | 207 ± 14 a | 13.6 ± 0.6 | 15,969 ± 752 | 2182 ± 173 | 24.6 ± 2.1 | 539 ± 69 |
| Oro de Valle | 111 ± 4 b | 12.3 ± 0.4 | 14,923 ± 1056 | 1852 ± 182 | 26.5 ± 1.7 | 486 ± 40 | |
| Peppermint | 186 ± 6 a | 12.7 ± 0.7 | 16,531 ± 1609 | 2136 ± 298 | 25.9 ± 1.4 | 556 ± 85 | |
| Red Head | 279 ± 7 a | 14 ± 0.4 | 9342 ± 1620 | 1292 ± 209 | 22.7 ± 1 | 290 ± 44 | |
| Mint Vanilla | 168 ± 13 b | 14.5 ± 0.3 | 8337 ± 686 | 1209 ± 98 | 21.9 ± 0.5 | 265 ± 19 | |
| 2021 | Ivory | 181 ± 14 b | 14.5 ± 0.4 | 9821 ± 1241 | 1417 ± 177 | 21.5 ± 1 | 300 ± 29 |
| Oro de Valle | 163 ± 6 b | 15.1 ± 0.2 | 7477 ± 1387 | 1122 ± 198 | 23.4 ± 0.7 | 260 ± 42 | |
| Peppermint | 213 ± 20 b | 15.1 ± 0.5 | 9847 ± 793 | 1494 ± 153 | 21.2 ± 1.3 | 315 ± 33 |
| Amino Acids (g 100 g DM−1) | Recommended Daily Intake (g per 70 kg Body Weight) a | |
|---|---|---|
| Essential | ||
| Histidine | 0.23 ± 0.01 | 0.7 |
| Isoleucine | 0.55 ± 0.04 | 1.4 |
| Leucine | 1.01 ± 0.05 | 2.73 |
| Lysine | 0.51 ± 0.04 | 2.1 |
| Methionine | 0.22 ± 0.01 | 0.7 |
| Phenylalanine | 0.65 ± 0.04 | 1.75 b |
| Threonine | 0.63 ± 0.03 | 1.05 |
| Tryptophan | 0.0588 ± 0.0069 | 0.28 |
| Valine | 0.64 ± 0.05 | 1.82 |
| Non-essential | ||
| Alanine | 0.74 ± 0.05 | |
| Arginine | 0.64 ± 0.04 | |
| Aspartic acid | 1.24 ± 0.06 | |
| Cystine + Cysteine | 0.20 ± 0.01 | |
| Glutamic acid | 1.54 ± 0.5 | |
| Glycine | 0.75 ± 0.03 | |
| Proline | 0.63 ± 0.03 | |
| Serine | 0.65 ± 0.03 | |
| Tyrosine | 0.36 ± 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubinovich, L.; Dagan, R.; Galili, S.; Asher, A. Optimizing Protein-Rich Young Vegetative Quinoa (Chenopodium quinoa) Growth: Effects of Inter-Row Spacing and Genotype in Mediterranean Summer Cultivation. Agronomy 2025, 15, 1102. https://doi.org/10.3390/agronomy15051102
Rubinovich L, Dagan R, Galili S, Asher A. Optimizing Protein-Rich Young Vegetative Quinoa (Chenopodium quinoa) Growth: Effects of Inter-Row Spacing and Genotype in Mediterranean Summer Cultivation. Agronomy. 2025; 15(5):1102. https://doi.org/10.3390/agronomy15051102
Chicago/Turabian StyleRubinovich, Lior, Reut Dagan, Shmuel Galili, and Aviv Asher. 2025. "Optimizing Protein-Rich Young Vegetative Quinoa (Chenopodium quinoa) Growth: Effects of Inter-Row Spacing and Genotype in Mediterranean Summer Cultivation" Agronomy 15, no. 5: 1102. https://doi.org/10.3390/agronomy15051102
APA StyleRubinovich, L., Dagan, R., Galili, S., & Asher, A. (2025). Optimizing Protein-Rich Young Vegetative Quinoa (Chenopodium quinoa) Growth: Effects of Inter-Row Spacing and Genotype in Mediterranean Summer Cultivation. Agronomy, 15(5), 1102. https://doi.org/10.3390/agronomy15051102






