Effects of Wood Ash Fertilizer on Element Dynamics in Soil Solution and Crop Uptake
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Wood Ash
2.3. Physicochemical Properties of Wood Ash
2.4. Pot Experiment
2.5. Sampling and Analysis
2.5.1. Soil Solution
2.5.2. Crop Growth Evaluation
2.6. Data Analyses
3. Results
3.1. Physicochemical Properties of Wood Ash
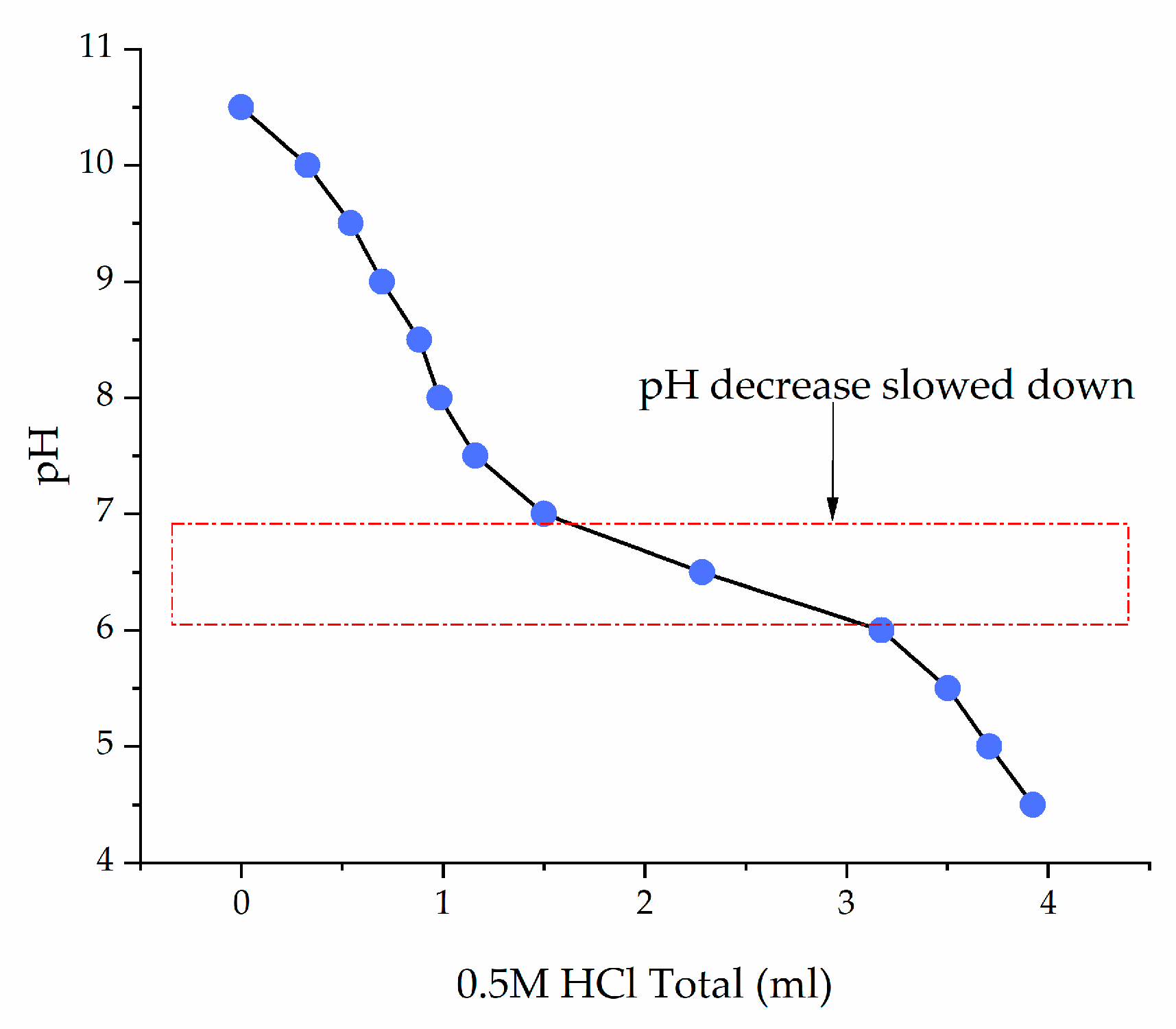
3.2. Soil Solution pH
3.3. Elemental Concentration in Soil Solution and Crops
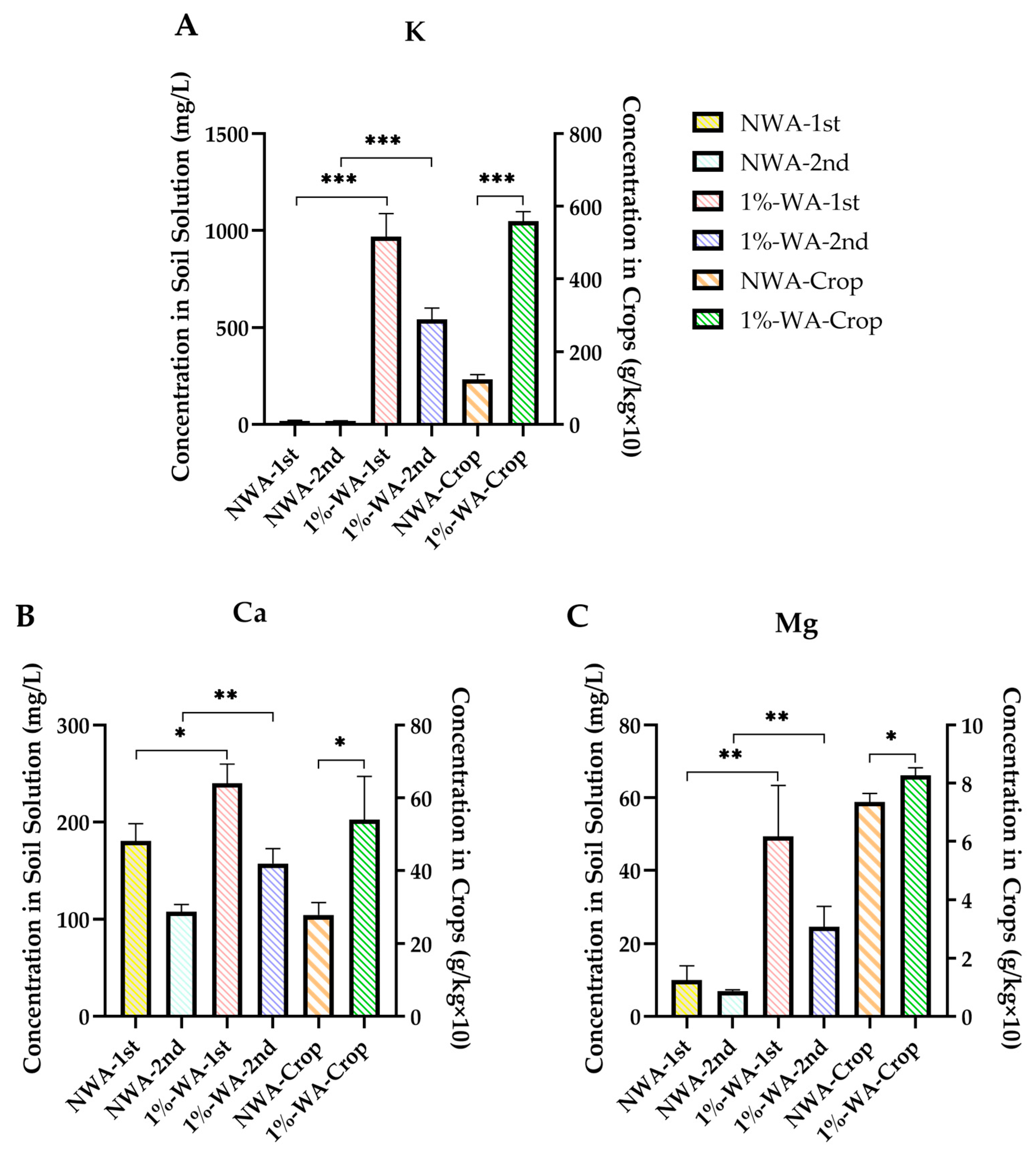
3.3.1. K, Ca, and Mg
3.3.2. P and Mo
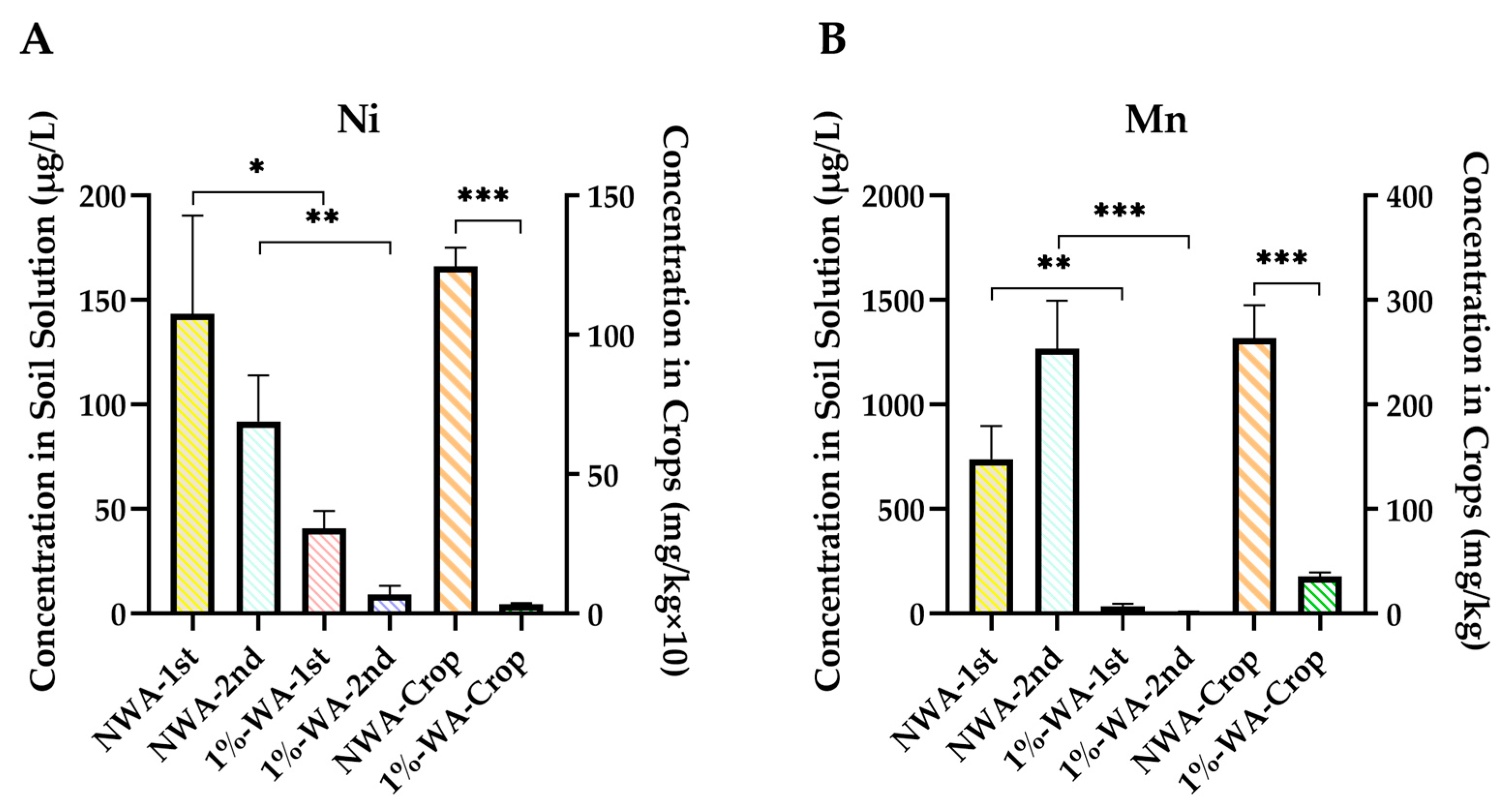
3.3.3. Ni, Mn, Zn, and Cd
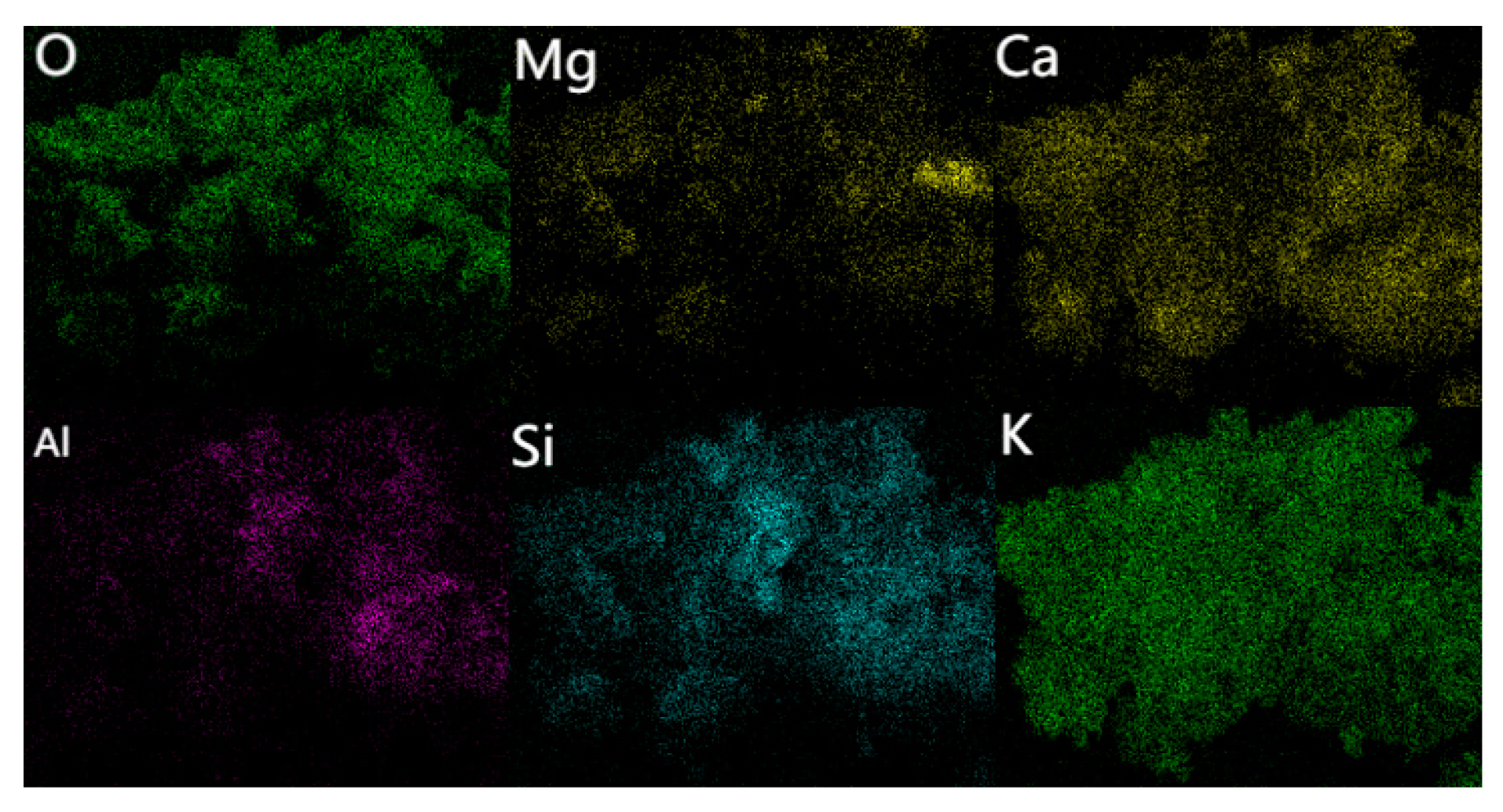
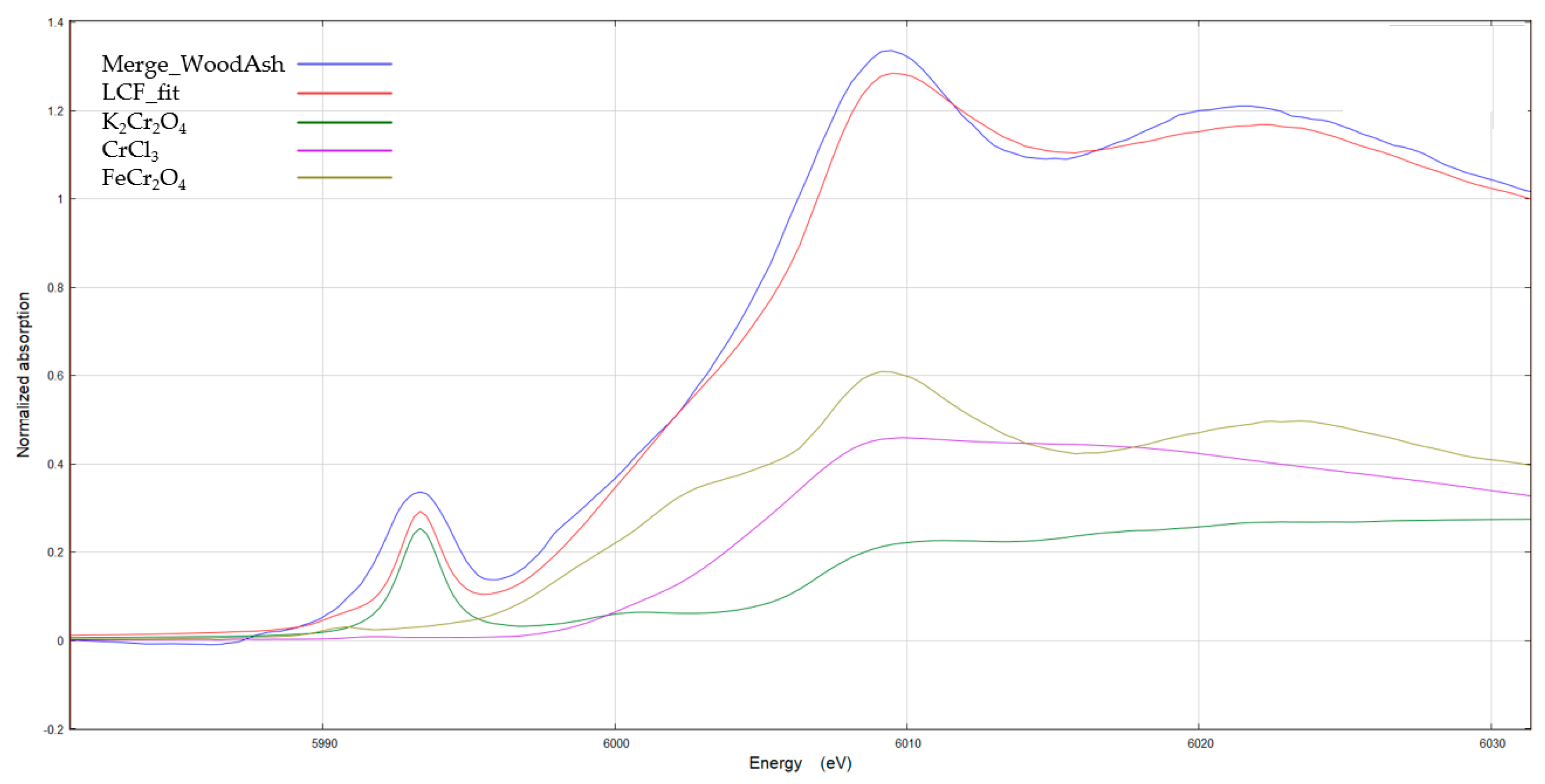
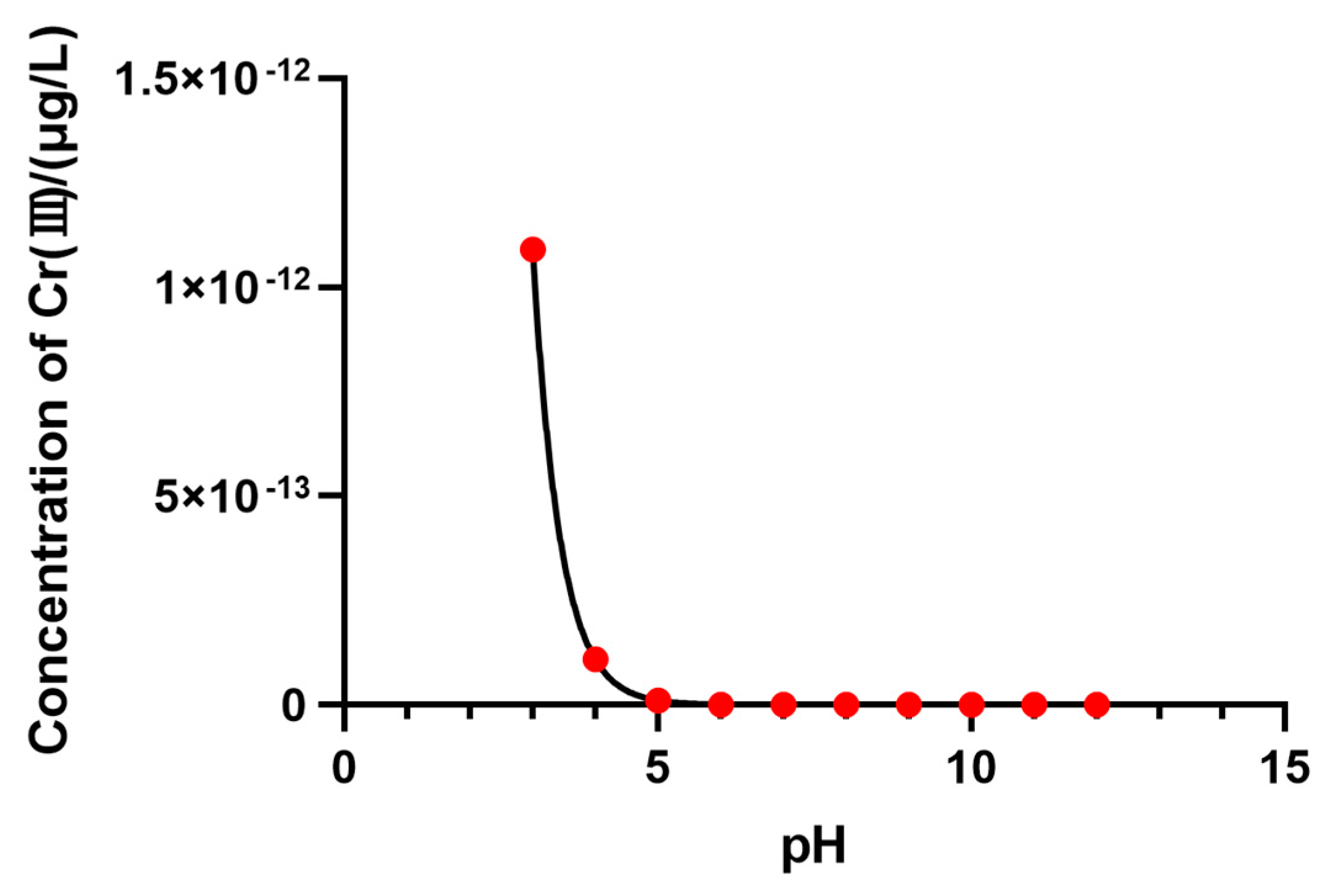
3.3.4. Cu, Cr, and Their Chemical Speciation in Soil Solution
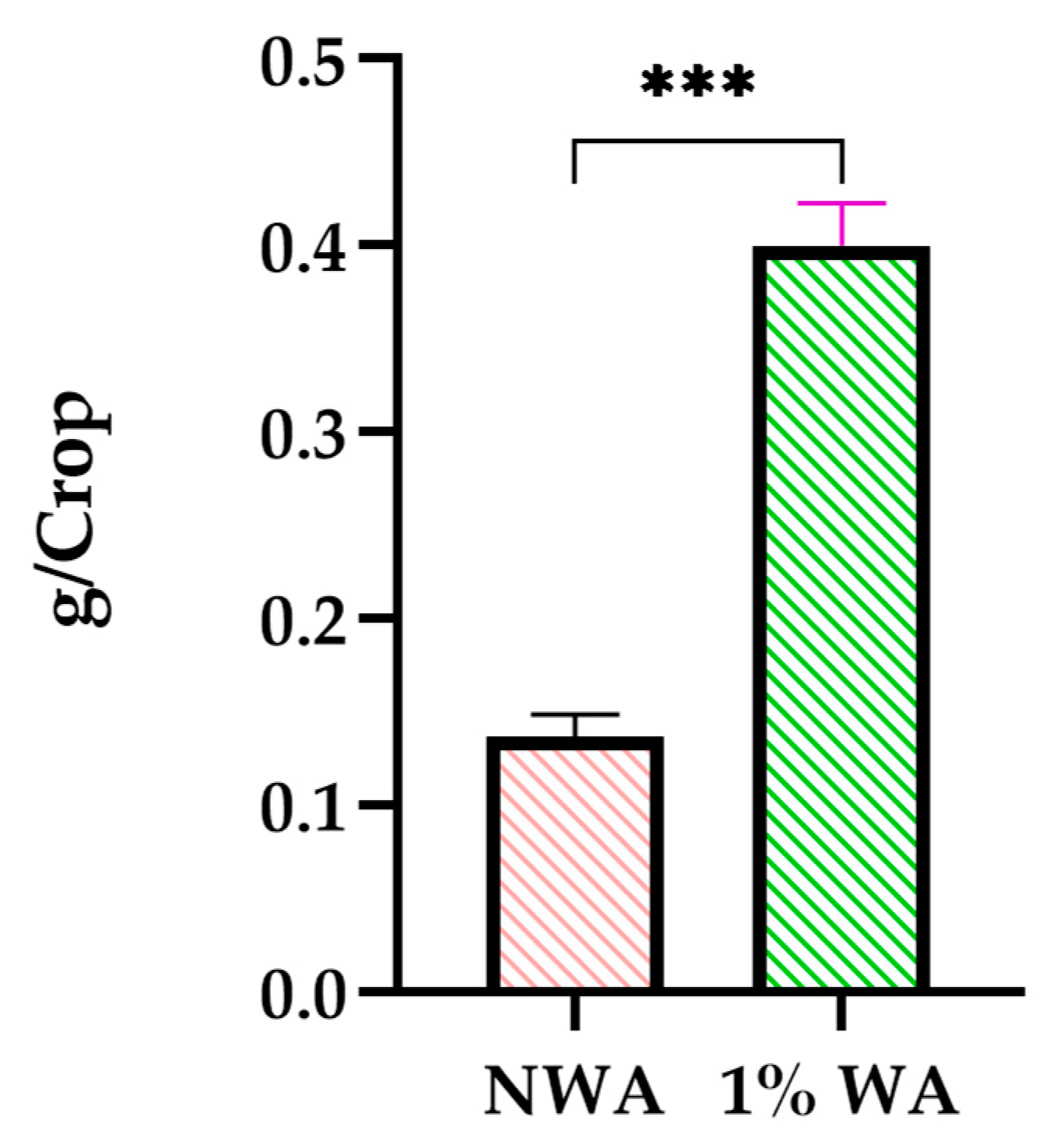
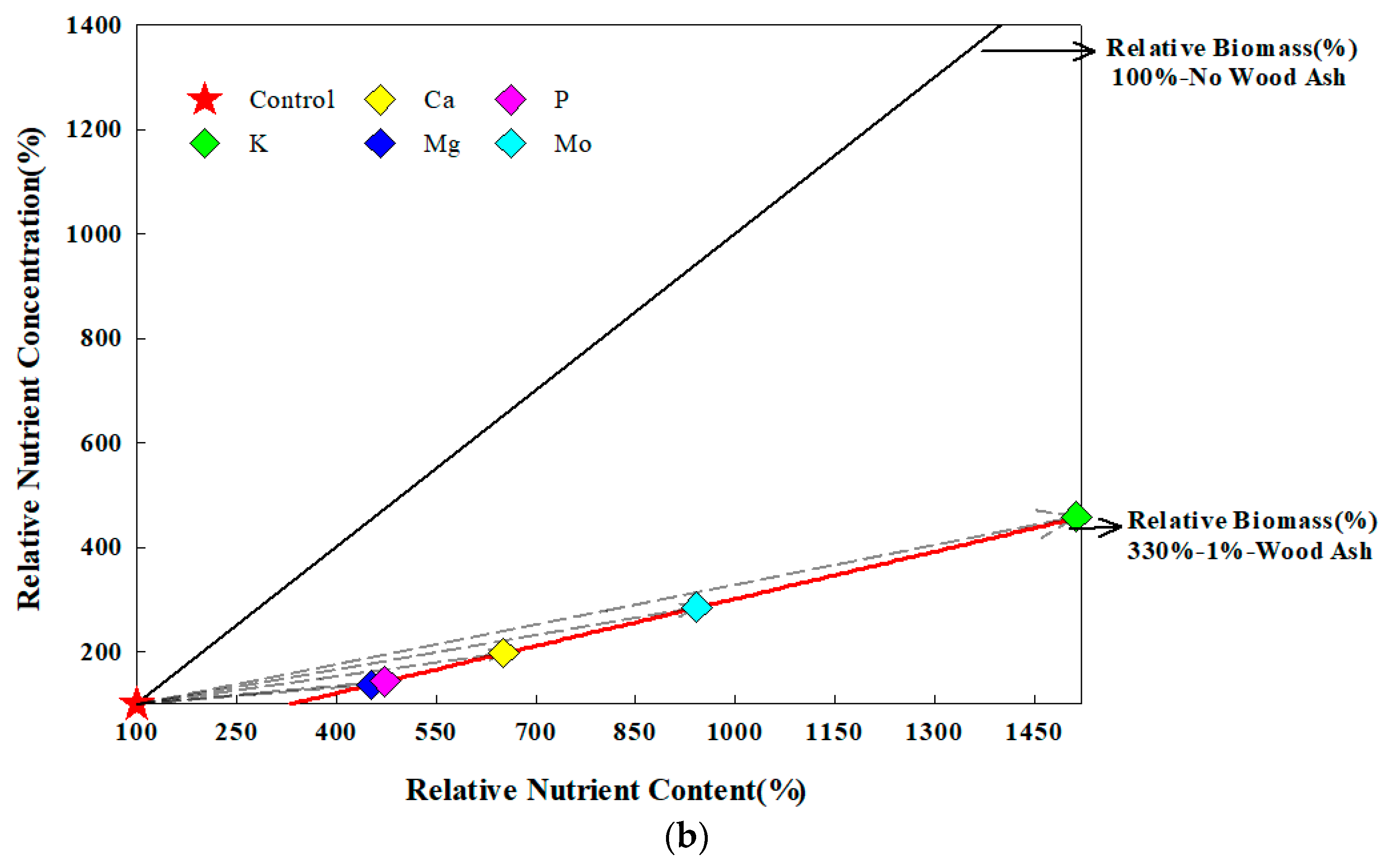
3.4. Crop Growth and Nutrient Vector Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Framework Convention on Climate Change (UNFCCC). Adoption of the Paris Agreement-Paris Agreement text English. 2015. Available online: https://unfccc.int/documents/9064 (accessed on 24 March 2025).
- Szarka, N.; Scholwin, F.; Trommler, M.; Jacobi, H.F.; Eichhorn, M.; Ortwein, A.; Thrän, D. A novel role for bioenergy: A flexible, demand-oriented power supply. Energy 2013, 61, 18–26. [Google Scholar] [CrossRef]
- Zhou, J.; Tabata, T. Research Trends and Future Direction for Utilization of Woody Biomass in Japan. Appl. Sci. 2024, 14, 2205. [Google Scholar] [CrossRef]
- Pei, Y.; Ike, M.; Shiota, K.; Takaoka, M. The impacts of furnace and fuel types on the hazardous heavy metal contents and leaching behavior of woody biomass fly ash. Fuel 2024, 372, 132202. [Google Scholar] [CrossRef]
- Vance, E.D. Land Application of Wood-Fired and Combination Boiler Ashes: An Overview. J. Environ. Qual. 1996, 25, 937–944. [Google Scholar] [CrossRef]
- Clarholm, M. Granulated wood ash and a ‘N-free’ fertilizer to a forest soil effects on P availability. For. Ecol. Manag. 1994, 66, 127–136. [Google Scholar] [CrossRef]
- Tian, H.; Bian, Z.; Shi, H.; Qin, X.; Pan, N.; Lu, C.; Pan, S.; Tubiello, F.N.; Chang, J.; Conchedda, G.; et al. History of anthropogenic Nitrogen inputs (HaNi) to the terrestrial biosphere: A 5arcmin resolution annual dataset from 1860 to 2019. Earth Syst. Sci. Data 2022, 14, 4551–4568. [Google Scholar] [CrossRef]
- Sasaki, I.; Fujiwara, H.; Sasaki, M. Livestock Manure Processing Methods and the Use of Processing by-Products in Japan. 2024. Available online: https://www.jwnet.or.jp/info/assets/files/R05_chousa_chikusanE.pdf (accessed on 24 March 2025).
- Ding, Z.; Ali, E.F.; Almaroai, Y.A.; Eissa, M.A.; Abeed, H.A.A. Effect of Potassium Solubilizing Bacteria and Humic Acid on Faba Bean (Vicia faba L.) Plants Grown on Sandy Loam Soils. J. Soil Sci. Plant Nutr. 2021, 21, 791–800. [Google Scholar] [CrossRef]
- Hannam, K.D.; Venier, L.; Allen, D.; Deschamps, C.; Hope, E.; Jull, M.; Kwiaton, M.M.; Mckenney, D.; Rutherford, P.; Hazlett, P. Wood ash as a soil amendment in Canadian forests: What are the barriers to utilization? Can. J. For. Res. 2018, 48, 442–450. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Song, Y.C.; Li, W.Y.; Feng, J. Ash contents and ash-forming elements of biomass and their significance for solid biofuel combustion. Fuel 2017, 208, 377–409. [Google Scholar] [CrossRef]
- Antonangelo, A.; Neto, J.F.; Crusciol, C.A.C.; Alleoni, L.R.F. Lime and calcium-magnesium silicate in the ionic speciation of an oxisol. Sci. Agric. 2017, 74, 317–333. [Google Scholar] [CrossRef]
- Johansen, J.L.; Nielsen, M.L.; Vestergård, M.; Mortensen, L.H.; Cruz-Paredes, C.; Rønn, R.; Kjøller, R.; Hovmand, M.; Christensen, S.; Ekelund, F. The complexity of wood ash fertilization disentangled: Effects on soil pH, nutrient status, plant growth and cadmium accumulation. Environ. Exp. Bot. 2021, 185, 104424. [Google Scholar] [CrossRef]
- Williams, T.M.; Hollis, C.A.; Smith, B.R. Forest Soil and Water Chemistry following Bark Boiler Bottom Ash Application. J. Environ. Qual. 1996, 25, 955–961. [Google Scholar] [CrossRef]
- Steenari, B.; Lindqvist, O. Stabilisation of biofuel ashes for recycling to forest soil. Biomass-Bioenergy 1997, 13, 39–50. [Google Scholar] [CrossRef]
- Zhan, G.; Erich, M.S.; Ohno, T. Release of trace elements from wood ash by nitric acid. Water Air Soil Pollut. 1996, 88, 297–311. [Google Scholar] [CrossRef]
- James, A.K.; Thring, R.W.; Helle, S.; Ghuman, H.S. Ash management review-applications of biomass bottom ash. Energies 2012, 5, 3856–3873. [Google Scholar] [CrossRef]
- Mayer, E.; Eichermüller, J.; Endriss, F.; Baumgarten, B.; Kirchhof, R.; Tejada, J.; Kappler, A.; Thorwarth, H. Utilization and recycling of wood ashes from industrial heat and power plants regarding fertilizer use. Waste Manag. 2022, 141, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Kindtler, N.L.; Ekelund, F.; Rønn, R.; Kjøller, R.; Hovmand, M.; Vestergård, M.; Christensen, S.; Johansen, J.L. Wood ash effects on growth and cadmium uptake in Deschampsia flexuosa (Wavy hair-grass). Environ. Pollut. 2019, 249, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Lasat, M.M. Phytoextraction of Metals from Contaminated Soil: A Review of Plant/Soil/Metal Interaction and Assessment of Pertinent Agronomic Issues. J. Hazard. Subst. Res. 1999, 2, 5. [Google Scholar] [CrossRef]
- Kinnersley, A.M. The role of phytochelates in plant growth and productivity. Plant Growth Regul. 1993, 12, 207–218. [Google Scholar] [CrossRef]
- Ochecová, P.; Mercl, F.; Košnář, Z.; Tlustoš, P. Fertilization efficiency of wood ash pellets amended by gypsum and superphosphate in the ryegrass growth. Plant Soil Environ. 2017, 63, 47–54. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Kukkola, M.; Moilanen, M.; Arola, M. Long-term effects of ash and N fertilization on stand growth, tree nutrient status and soil chemistry in a Scots pine stand. For. Ecol. Manag. 2006, 235, 116–128. [Google Scholar] [CrossRef]
- Augusto, L.; Bakker, M.R.; Meredieu, C. Wood ash applications to temperate forest ecosystems-Potential benefits and drawbacks. Plant Soil 2008, 306, 181–198. [Google Scholar] [CrossRef]
- Cultivated Soil Classification Committee. Classification of cultivated soils in Japan, third approximation. In Miscellaneous Publication of the National Institute of Agro-Environmental Sciences; JIRCAS: Ibaraki, Japan, 1995; p. 1.
- Okajima, T.; Ohtani, R.; Sumitani, K.; Kawamoto, M. Present Status of SAGA-LS and X-Ray Absorption Spectroscopy on BL11. X-Sen Bunseki No Shinpo 2012, 43, 223–234. [Google Scholar]
- Ravel, B.; Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
- Asaka, O.; Shoda, M. Biocontrol of Rhizoctonia Solani Damping-Off of Tomato with Bacillus Subtilis RB14. Appl. Environ. Microbiol. 1996, 62. [Google Scholar] [CrossRef]
- Makino, T.; Takano, H.; Kamiya, T.; Itou, T.; Sekiya, N.; Inahara, M.; Sakurai, Y. Restoration of cadmium-contaminated paddy soils by washing with ferric chloride: Cd extraction mechanism and bench-scale verification. Chemosphere 2008, 70, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.B., Jr.; Case, V.W. Sampling, Handling, and Analyzing Plant Tissue Samples. Soil Test. Plant Anal. 1990, 3, 389–427. [Google Scholar]
- Ji, L. The high-temperature volatilization of sylvite and solid reaction process between sylvite and minerals. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2019. [Google Scholar] [CrossRef]
- Prasetya, F.A.; Ishizuka, S.; Fukasawa, T.; Ishigami, T.; Sakemi, K.; Fukuda, T.; Fukui, K. Influence of ash species on particle size dependence of water- and citric-acid-soluble potassium concentrations of woody biomass combustion ashes with low potassium content. J. Energy Inst. 2023, 111, 101396. [Google Scholar] [CrossRef]
- Isaac, M.E.; Kimaro, A.A. Diagnosis of Nutrient Imbalances with Vector Analysis in Agroforestry Systems. J. Environ. Qual. 2011, 40, 860–866. [Google Scholar] [CrossRef]
- Gaothobogwe, K.; Ultra, V.; Rantong, G.; Majoni, S.; Manyiwa, T.; Mokgosi, S.; Dineo, O.; Gajaje, K.; Marenga, W.; Sefatlhi, K. Mitigation of Potentially Toxic Elements in Corn (Zea mays) Grown in Farmlands Near Cu-Ni Mine in Central Botswana. Soil Sediment Contam. 2025, 1–27. [Google Scholar] [CrossRef]
- Bourg, A.C.M.; Loch, P.G. Mobilization of Heavy Metals as Affected by pH and Redox Conditions; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Kim, Y.N.; Lee, K.A.; Lee, M.; Kim, K.R. Synergetic effect of complex soil amendments to improve soil quality and alleviate toxicity of heavy metal(loid)s in contaminated arable soil: Toward securing crop food safety and productivity. Environ. Sci. Pollut. Res. 2022, 29, 87555–87567. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils-To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Taupedi, S.B.; Ultra, V.U. Morupule fly ash as amendments in agricultural soil in Central Botswana. Environ. Technol. Innov. 2022, 28, 102695. [Google Scholar] [CrossRef]
- Arinzechi, C.; Dong, C.; Huang, P.; Zhao, P.; Liao, Q.; Li, Q.; Yang, Z. Synergistic mitigation of cadmium stress in rice (Oryzasativa L.) through combined selenium, calcium, and magnesium supplementation. Environ. Geochem. Health 2024, 46, 435. [Google Scholar] [CrossRef] [PubMed]
- Curtin, D.; Peterson, M.E.; Anderson, C.R. pH-dependence of organic matter solubility: Base type effects on dissolved organic C, N, P, and S in soils with contrasting mineralogy. Geoderma 2016, 271, 161–172. [Google Scholar] [CrossRef]
- Xing, J.; Xu, G.; Li, G. Analysis of the complexation behaviors of Cu(II) with DOM from sludge-based biochars and agricultural soil: Effect of pyrolysis temperature. Chemosphere 2020, 250, 126184. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, H. Effect of Common Ions in Agricultural Additives on the Retention of Cd, Cu, and Cr in Farmland Soils. Sustainability 2024, 16, 4870. [Google Scholar] [CrossRef]
- Sutton, R.; Sposito, G. Molecular structure in soil humic substances: The new view. Environ. Sci. Technol. 2005, 39, 9009–9015. [Google Scholar] [CrossRef]
- Sudiono, S.; Yuniarti, M.; Siswanta, D.; Kunarti, E.S.; Triyono, T.; Santosa, S.J. The role of carboxyl and hydroxyl groups of humic acid in removing AuCl4– from aqueous solution. Indones. J. Chem. 2017, 17, 95–104. [Google Scholar] [CrossRef]
- Song, J.; Jin, X.; Wang, X.C.; Jin, P. Preferential binding properties of carboxyl and hydroxyl groups with aluminium salts for humic acid removal. Chemosphere 2019, 234, 478–487. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, Y.; Liu, X.; Miao, T.; Guan, Q.; Yang, R.; Qu, J. Insight into interactions of heavy metals with livestock manure compost-derived dissolved organic matter using EEM-PARAFAC and 2D-FTIR-COS analyses. J. Hazard. Mater. 2021, 420, 126532. [Google Scholar] [CrossRef] [PubMed]
- Kotasâ, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.K.; Crisp, P.T.; Ellis, J.; Baker, R.S. Effect of ph on chromium(VI) species in solution. Talanta 1984, 31, 227–228. [Google Scholar] [CrossRef]
- Makino, T.; Kamewada, K.; Hatta, T.; Takahashi, Y.; Sakura, Y. Determination of optimal chromium oxidation conditions and evaluation of soil oxidative activity in soils. J. Geochem. Explor. 1998, 64, 435–441. [Google Scholar] [CrossRef]
- Gale, N.; Halim, A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef]
- Ferm, A.; Hokkanen, T.; Moilanen, M.; Issakainen, J. Effects of wood bark ash on the growth and nutrition of a Scots pine afforestation in central Finland. Plant Soil 1992, 147, 305–316. [Google Scholar] [CrossRef]
- Vestergård, M.; Bang-Andreasen, T.; Buss, S.M.; Cruz-Paredes, C.; Bentzon-Tilia, S.; Ekelund, F.; Kjøller, R.; Mortensen, L.H.; Rønn, R. The relative importance of the bacterial pathway and soil inorganic nitrogen increase across an extreme wood-ash application gradient. GCB Bioenergy 2018, 10, 320–334. [Google Scholar] [CrossRef]







| Soil | Gray Lowland Soil |
|---|---|
| pH (H2O) | 4.50 ± 0.071 |
| EC | 0.350 ± 0.035 mS/cm |
| CEC | 8.60 ± 0.212 cmolc/kg |
| K+ | 0.028 ± 0 cmolc/kg |
| Ca2+ | 2.13 ± 0 cmolc/kg |
| Mg2+ | 0.370 ± 0 cmolc/kg |
| P2O5 | 15.5 mg ± 0.141/100 g |
| NH4+ | 0.700 mg ± 0.071/100 g |
| NO3− | 19.0 mg ± 0.148/100 g |
| Carbon Content | 3.10 ± 0% |
| Cr | 0.017 ± 0 mg/kg |
| Cd | 0.030 ± 0 mg/kg |
| Element | Unit | Content | |
|---|---|---|---|
| Micro elements | Cd | mg/kg | 2.40 |
| Cr | mg/kg | 160 | |
| As | mg/kg | 22.0 | |
| Cu | mg/kg | 200 | |
| Zn | mg/kg | 160 | |
| Ni | mg/kg | 90.0 | |
| Macro elements | Mg | g/kg | 29.0 |
| P | g/kg | 6.70 | |
| K | g/kg | 190 | |
| Ca | g/kg | 130 | |
| Si | g/kg | 98.0 | |
| Al | g/kg | 16.0 |
| Element | Unit | Content | |
|---|---|---|---|
| Micro elements | Hg | mg/L | 0.0005 |
| Cd | mg/L | 0.0003 | |
| Pb | mg/L | 0.005 | |
| Cr (VI) | mg/L | 2.70 | |
| As | mg/L | 0.840 | |
| Se | mg/L | 0.110 |
| Standards | Weight (%) |
|---|---|
| FeCr2O4 | 58.7 |
| CrCl3 | 8.20 |
| K2CrO4 | 33.1 |
| NWA | 1%-WA | |
|---|---|---|
| Fresh Weight | 1.60 g | 5.60 g |
| Dry Weight | 0.140 g | 0.400 g |
| DryConc. | 8.10 mg/kg | 0.793 mg/kg |
| FreshConc. | 0.709 mg/kg | 0.057 mg/kg |
| NWA | 1%-WA | |
|---|---|---|
| FA1-Cu | 71.4 ± 1.02% | 7.70 ± 3.70% |
| FA2-Cu | 6.15 ± 1.74% | 92.3 ± 3.70% |
| FA1-Cr(III) | 14.2 ± 5.15% | 0.10 ± 0.05% |
| FA2-Cr(III) | 85.8 ± 5.15% | 99.9 ± 0.05% |
| DOC | 5.23 ± 2.15 mg/L | 283 ± 85.8 mg/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jian, C.; Hamamoto, T.; Inoue, C.; Chien, M.-F.; Naganuma, H.; Mori, T.; Sawada, A.; Hidaka, M.; Setoyama, H.; Makino, T. Effects of Wood Ash Fertilizer on Element Dynamics in Soil Solution and Crop Uptake. Agronomy 2025, 15, 1097. https://doi.org/10.3390/agronomy15051097
Jian C, Hamamoto T, Inoue C, Chien M-F, Naganuma H, Mori T, Sawada A, Hidaka M, Setoyama H, Makino T. Effects of Wood Ash Fertilizer on Element Dynamics in Soil Solution and Crop Uptake. Agronomy. 2025; 15(5):1097. https://doi.org/10.3390/agronomy15051097
Chicago/Turabian StyleJian, Chuanzhen, Toru Hamamoto, Chihiro Inoue, Mei-Fang Chien, Hiroshi Naganuma, Takehito Mori, Akihiro Sawada, Masafumi Hidaka, Hiroyuki Setoyama, and Tomoyuki Makino. 2025. "Effects of Wood Ash Fertilizer on Element Dynamics in Soil Solution and Crop Uptake" Agronomy 15, no. 5: 1097. https://doi.org/10.3390/agronomy15051097
APA StyleJian, C., Hamamoto, T., Inoue, C., Chien, M.-F., Naganuma, H., Mori, T., Sawada, A., Hidaka, M., Setoyama, H., & Makino, T. (2025). Effects of Wood Ash Fertilizer on Element Dynamics in Soil Solution and Crop Uptake. Agronomy, 15(5), 1097. https://doi.org/10.3390/agronomy15051097






