Abstract
Tomato yellow leaf curl virus (TYLCV), tomato leaf curl New Delhi virus (ToLCNDV), and tomato chlorosis virus (ToCV) are emerging viruses that cause significant damage to tomato (Solanum lycopersicum). TYLCV and ToLCNDV are single-stranded DNA viruses from the genus Begomovirus, family Geminiviridae, while ToCV is an RNA virus from the genus Crinivirus (family Closteroviridae). These viruses share overlapping geographic ranges, vectors (the whitefly Bemisia tabaci), and host plants, making mixed infections common. This study investigated interactions between TYLCV and ToLCNDV and between ToLCNDV and ToCV in mixed infections of susceptible and TYLCV-resistant tomato genotypes. We evaluated infection, disease development, trans-replication of genome components, and genetic exchange. Our results showed no significant synergistic or antagonistic interactions, complementation, or interference between the viruses. TYLCV resistance in tomato genotypes remained stable. The DNA-B component of ToLCNDV exhibited impaired functionality and was not complemented by TYLCV. No evidence was found that the crinivirus tomato chlorosis virus (ToCV) enhances ToLCNDV infection, suggesting limited interactions despite shared vectors. Genetic exchange was detected in defective DNA (def-DNA) molecules using high-throughput sequencing (HTS), indicating potential genetic interactions between these viruses. These findings suggest that mixed infections do not pose immediate concerns for increased pathogenicity but highlight the ecological implications of genetic exchange, warranting further study of the evolutionary consequences of such interactions in mixed-virus environments.
1. Introduction
The emergence of plant viruses presents a significant global threat to agriculture, leading to substantial damage in economically important crops [1,2]. Whitefly-transmitted viruses, including begomoviruses (Begomovirus, family Geminiviridae) and criniviruses (Crinivirus, family Closteroviridae), have become more prominent in recent decades [3,4]. Their global spread is largely driven by the expansion of cryptic species within the Bemisia tabaci Genn. vector complex, particularly Middle East Asia Minor 1 and Mediterranean species, which have successfully invaded new ecological niches [5,6,7]. Notable examples include single-stranded DNA begomoviruses like tomato yellow leaf curl virus (TYLCV) and tomato leaf curl New Delhi virus (ToLCNDV), as well as the single-stranded RNA crinivirus tomato chlorosis virus (ToCV), all of which severely impact tomato (Solanum lycopersicum L.) production worldwide [4,8,9,10,11].
Begomoviruses are divided into Old World and New World groups, which differ in their geographical distribution, genome organization, and genetic diversity, with Old World viruses generally showing greater genetic variability.
Begomoviruses are a major constraint for vegetable, root, and fiber crops [4,11]. Most Old World begomoviruses, such as TYLCV, are monopartite, containing a single genomic DNA component. However, bipartite begomoviruses like ToLCNDV, which possess two components (DNA-A and DNA-B), are also spreading in this region [12]. The begomovirus genome, approximately 2.6 to 2.8 kb in size, encodes open reading frames (ORFs) arranged bidirectionally from a non-coding intergenic region [13], which contains key elements for replication and transcription [13,14]. This includes the origin of replication nicked by the replication initiation protein, Rep, to initiate virus DNA replication and iterated DNA motifs (iterons) that are specifically recognized by the iteron-related domain in the N-terminal region of the Rep for effective replication [15].
DNA-A of bipartite begomoviruses encodes proteins essential for virus encapsidation, replication, pathogenesis, and transmission: Rep and replication enhancer protein (REn) (AC1 and AC3 ORFs, respectively), transcription activator protein (TrAP, C2 ORF), AV2 precoat protein (AV2 ORF), coat protein (CP, V1 ORF), and protein AC4 (AC4 ORF). DNA-B encodes movement proteins: the nuclear shuttle protein (NSP) and the movement protein (MP) (BC1 and BV1 ORFs, respectively) [13]. Monopartite begomoviruses have a single genomic component that resembles the DNA-A of bipartite begomoviruses [13]. Recently, additional small proteins that can play relevant biological roles have been described in begomoviruses, which implies that the repertoire of proteins might be expanded [16,17,18]. Notably, defective DNAs (def-DNAs), derived from extensive recombination events, are often associated with infections and may influence virus biology [19].
Managing begomovirus-induced damage in tomato plants has primarily depended on genetic resistance, especially through dominant resistance genes like Ty-1, Ty-3, and Ty-2, which restricts viral infections [20,21]. However, the evolution of begomoviruses, frequently driven by recombination and mixed infections, can undermine these resistance strategies [22]. Mixed infections often lead to synergistic interactions, reassortment [23], or recombination, all of which can modify plant–virus interactions and intensify epidemics [24,25,26]. Recombination has been reported in both closely related [27,28,29] and distantly related [30,31] begomoviruses, including both monopartite and bipartite members [23,32]. Additionally, mixed infections of begomoviruses and criniviruses can occur because they share the whitefly vector and host plant and have been linked to pathological alterations [33,34]. These processes can give rise to novel pathogenic variants with potentially severe consequences [27,30,35,36].
Recently, ToLCNDV isolates have emerged in the Mediterranean Basin, some of which are poorly adapted to tomato plants [37,38]. However, mixed infections involving ToLCNDV and TYLCV have been reported to enhance ToLCNDV’s infection capabilities in tomatoes, highlighting the potential for synergistic interactions [39]. Considering the contrasting biological characteristics of ToLCNDV isolates and their varying adaptability to tomato plants, the broader implications of such interactions remain unclear.
Understanding the mechanisms of recombination and trans-replication between TYLCV and ToLCNDV is essential for real-world crop management because these processes can give rise to novel viral variants with enhanced pathogenicity or broader host ranges. Such variants may overcome existing resistance genes deployed in tomato breeding programs and compromise integrated pest management strategies. Monitoring these genetic interactions is therefore key to anticipating viral emergence and improving crop resilience.
In this study, we examined the effects of mixed infections between TYLCV and ToLCNDV in TYLCV-susceptible and TYLCV-resistant tomato plants. Given that begomoviruses and criniviruses frequently co-infect tomato plants due to shared insect vectors, we also assessed whether ToCV could enhance ToLCNDV infection. Additionally, we investigated the potential for genetic exchange between ToLCNDV and TYLCV through reassortment and recombination using high-throughput sequencing (HTS), aiming to enhance our understanding of their ecological and evolutionary dynamics. Our findings contribute to advancing ecosystem-scale analyses of plant–virus interaction networks and informing strategies for managing these emerging viral threats.
2. Materials and Methods
2.1. Plant Material and Experimental Periods
TYLCV-susceptible tomato lines (cv. Moneymaker, cv. Rondeño, and cv. Marmande; IHSM-CSIC seedbank collection) and TYLCV-resistant lines (Ty-1 F1, with heterozygous Ty-1/ty-1 resistance, kindly supplied by M.J. Díez, Universidad Politécnica de Valencia, Spain; and ABL21-5, with homozygous Ty-2/Ty-2 resistance, IHSM-CSIC breeding program) were used. Nicotiana benthamiana D. plants were also included as susceptible controls in the experiments, as they are susceptible to TYLCV, ToLCNDV, and ToCV. Plants were grown in an insect-proof glasshouse under natural lighting with loose temperature control (22–27 °C day, 17–20 °C night).
Mixed-infection experiments were conducted in two independent assays over different periods: TYLCV + ToLCNDV assays were performed from March to June 2016 (Assay 1) and from September to December 2016 (Assay 2). Mixed infections of TYLCV + ToLCNDV DNA-B were conducted from June to July 2017 (Assay 1) and from October to November 2017 (Assay 2). ToCV + ToLCNDV assays were carried out from October to November 2016 (Assay 1) and from April to May 2017 (Assay 2).
2.2. Whiteflies, Virus Isolates, and Virus Inoculation
Virus-free whitefly adult individuals were obtained from a B. tabaci Mediterranean species [6] colony originated from individuals collected in Málaga, Southern Spain, and reared on melon (Cucumis melo L. ANC42, IHSM seedbank collection) plants within insect-proof cages, in an insect-proof glasshouse with loose temperature control (see above) and light supplementation when needed.
Two infectious clones, previously generated from Spanish isolates and described in earlier studies, were used for Agrobacterium tumefaciens-mediated inoculation: the Spanish isolate ES-Alm-Pep-99 of the TYLCV Israel strain (TYLCV-IL, originally described in Israel; GenBank accession number AJ489258) [40] and the Spanish ToLCNDV-ES isolate ES-Al-661-Sq-13 (DNA-A GenBank accession no. KF749223 and DNA-B GenBank accession no. KF749226) [37]. Liquid cultures of A. tumefaciens containing the infectious clones were pelleted and resuspended in agroinoculation buffer (10 mM MgCl2, 10 mM MES pH 5.8, 2.25 mM acetosyringone) to a final OD600 of 1.0. For single infections, inoculation was performed with either the Spanish isolate of TYLCV-IL, ToLCNDV-ES (DNA-A and DNA-B), or ToLCNDV-ES DNA-B alone. In the case of ToLCNDV-ES, the infectious clones containing DNA-A and DNA-B were mixed in a 1:1 volume ratio (v/v) prior to inoculation. For mixed infections, the cultures containing TYLCV-IL and ToLCNDV-ES DNA-A and DNA-B were mixed in equal volumes (1:1:1 ratio, v/v), each adjusted to an OD600 of 1.0. Additionally, co-inoculation treatments included TYLCV-IL with ToLCNDV DNA-B alone, mixed in a 1:1 volume ratio (v/v), both adjusted to OD600 o of 1.0. One hundred µL of the suspension (for both single and mixed infections) was used to agroinoculate each test plant by stem puncture [41]. Mixed infections were performed to explore potential interactions between the two viruses in the host.
For ToLCNDV-ES and TYLCV-IL co-infection in TYLCV-susceptible, TYLCV-resistant tomato plants and the susceptible control N. benthamiana, two different assays were conducted. In Assay 1, two inoculation strategies were employed: simultaneous and sequential inoculation. In the simultaneous approach, plants were inoculated with both TYLCV-IL and ToLCNDV-ES at the two- to three-leaf growth stage using Agrobacterium tumefaciens-mediated inoculation. For the sequential method, plants were first inoculated with TYLCV-IL at the two- to three-leaf growth stage, followed by ToLCNDV-ES inoculation at the four- to five-leaf growth stage. Assay 2 involved simultaneous co-inoculation of both viruses at the two- to three-leaf growth stage, mirroring the simultaneous method in Assay 1. For the co-inoculations of TYLCV-IL with ToLCNDV DNA-B alone, the two assays were performed following the simultaneous approach. Co-inoculations of ToCV and ToLCNDV (DNA-A and DNA-B) were performed sequentially, with ToCV being inoculated first at the three- to four-leaf stage, followed by ToLCNDV-ES at the five- to six-leaf stage. In all experiments, 10 plants per condition were inoculated, except in the experiment that included separate inoculations with TYLCV-IL, ToLCNDV (DNA-A and DNA-B), and ToLCNDV-ES DNA-B, where only 5 plants were used per condition.
For ToCV inoculations, B. tabaci-mediated transmission was used, along with the Spanish isolate Pl-1-2 obtained from a naturally infected tomato plant collected in 1997 in Málaga and maintained at the IHSM on Moneymaker tomato by periodic transmission [42]. This method was chosen because, unlike TYLCV and ToLCNDV, no infectious clone of ToCV is available, and therefore vector-mediated inoculation remains the most biologically relevant and technically feasible approach to achieve consistent ToCV infection. Viruliferous whiteflies were obtained by mass feeding of virus-free B. tabaci adults (48 h acquisition access period, within insect-proof cages) on Moneymaker tomato plants infected with ToCV 30 days before they were used for virus acquisition. Test plants were inoculated by using clip-on cages, with one cage containing 30 viruliferous whiteflies per test plant for a 48 h inoculation access period (IAP). Following the IAP, plants were maintained in an insect-proof glasshouse (see above) and sprayed with insecticide combinations to eliminate whitefly eggs, larval stages, and adults. Treatment routines alternated insecticides with different modes of action to prevent the generation of insecticide resistances, using either pyriproxyfen or spiromesifen combined with either flupyradifurone, spirotetramat, or acetamiprid. Plants were inoculated at the two- to six-leaf growth stage. Mock-inoculated control plants were obtained following the same inoculation procedures but using virus-free whiteflies or virus-free A. tumefaciens.
2.3. Symptom Evaluation and Virus Detection by Tissue Blot Hybridization
TYLCV infection was evaluated using a 0–5 arbitrary symptom severity rating scale adapted from Friedmann et al. [43] as follows: 0 = asymptomatic; 1 = slight yellowing and mosaic on top leaves, no curling; 2 = mild yellowing, mosaic and/or slight curling on top leaves; 3 = moderate yellowing and/or moderate curling on top leaves; 4 = severe yellowing, blistering, and/or severe curling with some leaf size reduction on top leaves of the main stem and/or one branch; 5 = severe yellowing, blistering, very severe curling, leaf deformation and size reduction, and stunting (internode shortening) on top leaves of the main stem and/or one branch. Moreover, virus infection was analyzed by tissue blot hybridization. Fresh cross sections of petioles from the youngest newly emerged leaves of test plants were tissue-blotted onto positively charged nylon membranes (Roche Diagnostics GmbH, Mannheim, Germany). Tissue prints were performed at different time points depending on the experiment: 14, 21, 28, 38, and 48 days post-inoculation (dpi). The final sampling point varied according to the specific viral combination under study and was determined based on the time at which no further differences in hybridization signals were observed relative to the previous time point. In TYLCV and ToLCNDV co-infections, samples were collected up to 48 dpi; in TYLCV and ToLCNDV DNA-B co-inoculations, up to 28 dpi; and in ToCV and ToLCNDV assays, up to 38 dpi. The selected final time point in each case reflects the latest time at which an increase in hybridization signal was observed. Nucleic acids were UV-cross-linked and hybridized with a specific probe. For TYLCV-IL detection, a DIG-labelled DNA probe specific to TYLCV-IL was used [27]. The presence of ToLCNDV-ES DNA-A and DNA-B components was analyzed using a DIG-labelled DNA probe based on the common region and DIG-labelled DNA probes specific to each viral component [37]. ToCV presence was analyzed with a DIG-labelled RNA probe specific to ToCV [44].
2.4. Detection of Recombination Events in Mixed Infections by HTS
To evaluate potential recombination events between TYLCV-IL and ToLCNDV-ES by HTS (Supplementary Figure S1), the youngest apical leaves of co-infected plants confirmed positive for both viruses at 48 dpi were sampled at 90 and 110 dpi and pooled. Total DNA was extracted using a CTAB-based purification method [45] from 5 Rondeño tomato plants (TYLCV-IL + ToLCNDV-ES-Tom sample) and 10 N. benthamiana plants (TYLCV-IL + ToLCNDV-ES-Nb sample). A control sample consisting of a combination of N. benthamiana plants with single infections of TYLCV-IL (5 plants) and ToLCNDV-ES (5 plants) (TYLCV-IL/ToLCNDV-ES-Nb combined sample) was also included. Biological replicates were not included, as the samples were pooled plants. This pooling strategy reflected the variability in mixed infections across the plant populations, enabling the detection of recombination events but not statistical comparisons of viral accumulation.
Viral loads were quantified by qPCR using 2 μL of DNA extract (10 ng/μL), 10 μL of 2× SsoFast EvaGreen supermix (Bio-Rad, Hercules, CA, USA), 1 μL each of 10 μM forward and reverse primers, and 6 μL of ultrapure H2O, in a final volume of 20 μL per reaction. The qPCR conditions consisted of an initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 10 s and 60 °C for 15 s on a LightCycler® 96 system (Roche). Primer pairs used for TYLCV and ToLCNDV quantification were custom-designed using the Primer-Blast tool (NCBI). For TYLCV, the primers were TYLCV_F_2237 (5′-TTGGCAGATTGCTGACCTCC-3′; positions 2237–2256 of AJ489258) and TYLCV_R_2414 (5′-ACCAACGGTTCTTCGACCTG-3′; positions 2395–2414 of AJ489258). For ToLCNDV-ES DNA-A, the primers were ToLCNDV-A_F_1733 (5′-TTGCTTTGCCAGTCACGTTG-3′; positions 1733–1752 of KF749223) and ToLCNDV-A_R_1906 (5′-CAAAACAATGTGGGCGCGTT-3′; positions 1906–1925 of KF749223). For ToLCNDV-ES DNA-B, the primers were ToLCNDV-B_F_2149 (5′-CGACACACCCAATGACACGA-3′; positions 2130–2149 of KF749226) and ToLCNDV-B_R_2333 (5′-TCGTAGAAAAATGAGTGCCGT-3′; positions 2313–2333 of KF749226). Standard curves for TYLCV-IL were generated using a pBSK construct containing a monomeric TYLCV-IL (ES-Alm-Pep-99 isolate; GenBank AJ489258) described by Morilla et al. [46]. For the ToLCNDV-ES standard curve, a pBSK dimer of DNA-A (KF749223) and a pBSK monomer of DNA-B (KF749226) from the ES-Al-661-Sq-13 isolate described by Fortes et al. [47] were employed. Samples and standards were analyzed in triplicate.
To prepare DNA for HTS (Figure 1), circular viral DNA was selectively amplified from 1 μL of total DNA extract using rolling circle amplification (RCA) with φ29 DNA polymerase (TempliPhi kit, GE Healthcare, Chicago, IL, USA), following the manufacturer’s instructions. RCA products were used directly for library preparation. Reactions were performed in duplicate and pooled to minimize random errors introduced by φ29 DNA polymerase. Sequencing was carried out at Macrogen (Seoul, Republic of Korea) using equimolar sequencing libraries prepared with a TruSeq DNA PCR-free kit (550 bp insert; Illumina, San Diego, CA, USA). Libraries were normalized to 4 nM by dilution with 10 mM Tris-HCl (pH 8.5) and sequenced as paired-end 2 × 300 bp reads on an Illumina MiSeq platform.
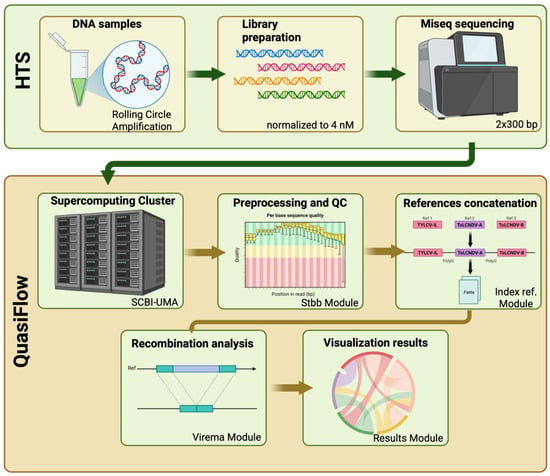
Figure 1.
Overview of the experimental and bioinformatic workflow combining high-throughput sequencing (HTS) and the QuasiFlow pipeline. Viral DNA from infected tomato samples was amplified using rolling circle amplification and subjected to library preparation, followed by Illumina MiSeq sequencing (2 × 300 bp). The resulting reads were analyzed using the QuasiFlow pipeline on the SCBI-UMA supercomputing cluster. QuasiFlow includes quality control and preprocessing (Stbb Module), concatenation and indexing of reference genomes (Index ref. Module), recombination detection (Virema Module), and graphical visualization of the results (Results Module). Created in BioRender, https://BioRender.com/yx5j95p (accessed on 13 April 2025).
Raw reads were preprocessed using QuasiFlow software (version 1.0) [48] (Figure 1) with default settings to remove low-quality, ambiguous, and low-complexity sequences, as well as linkers, adapters, and host-derived sequences. Only reads aligning to viral reference sequences were retained. Sequences shorter than 95 nucleotides or with a PHRED quality score below 26 were discarded.
Cleaned reads were aligned to an artificial viral reference constructed by concatenating the genomic sequences of individual viral components or segments. TYLCV-IL (GenBank AJ489258), ToLCNDV-ES DNA-A (GenBank KF749223), and ToLCNDV-ES DNA-B (GenBank KF749226) were used as reference sequences, joining them end to end to form a single continuous sequence. This strategy was used to achieve the mapping of sequencing reads in the mixed infection, as the software can only process one reference sequence. The Virema Module in QuasiFlow was used to annotate potential recombination events in DNA molecules. Circular representations were generated using CanvasXpress, with annotations added in Inkscape [49].
To validate QuasiFlow’s ability to reliably detect recombinants, we analyzed an RCA-amplified DNA sample (Mm-A) from a previous study on Moneymaker tomato plants co-infected with the Mild strain of tomato yellow leaf curl virus (TYLCV-Mld) and tomato yellow leaf curl Sardinia virus (TYLCSV). This sample was characterized by Garcia-Andres et al. [50], who identified recombinant genome types R1 to R4 between these two viruses. Since only RCA products were available for this sample, DNA quantification was not performed. For analysis, a reference sequence was constructed by concatenating the complete genomic sequences of TYLCV-Mld (GenBank AF071228) and TYLCSV (GenBank Z25751).
2.5. Cloning and Sequencing of def-DNA and Recombinant Molecules
RCA products were obtained from the pooled N. benthamiana samples with mixed infections of TYLCV-IL and ToLCNDV-ES (Supplementary Figure S2). These RCA products were digested with SacI or SalI restriction endonucleases, selected based on the presence of restriction sites identified in the predominant def-DNA molecules detected during the HTS analysis. DNA fragments ranging from 1 to 1.5 kb (SacI digestion) and 0.8 to 1.5 kb (SalI digestion) were purified from agarose gels and ligated into the cloning sites of the pBluescript SK vector (Stratagene, La Jolla, CA, USA). The cloned inserts were sequenced by Macrogen Inc. (Seoul, Republic of Korea) using M13 forward and reverse primers.
The presence of cloned def-DNA molecules was validated by PCR using non-overlapping abutting primers [51] targeting junction/recombinant sites (Supplementary Table S1). Amplification was performed in a T100 Thermal Cycler (Bio-Rad, Hercules, CA, USA) in a 20 μL reaction volume containing NH4 Reaction Buffer (Meridian Bioscience Inc., Cincinnati, OH, USA), 1.5 mM MgCl2, 0.2 mM of each dNTP, 0.2 μM of each primer, 1.5 U of Biotaq DNA Polymerase (Meridian Bioscience Inc.), and 10 ng of total DNA extract as template. The cycling parameters were as follows: initial denaturation at 95 °C for 5 min, followed by 34 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C (for primer pair MA-2854/MA-2855), 55 °C (for primer pairs MA-2856/MA-2857 and MA-2858/MA-2859), or 65 °C (for primer pair MA-2860/MA2861) for 30 s, and extension at 72 °C for 1 min. A final extension was performed at 72 °C for 5 min. The amplified fragments were cloned into a pGEM-T Easy Vector system (Promega Biotech Ibérica, Madrid, Spain) and sequenced using M13 forward and reverse primers.
2.6. Statistical Analyses
Statistical analyses were performed using IBM SPSS version 29.0 (IBM, Armonk, NY, USA). TYLCV symptom severity levels were analyzed via one-way ANOVA followed by Tukey-b post hoc tests. Binary response data (0 = non-infected, 1 = infected) were subjected to generalized linear model (GzLM) analyses using Logit as the link function and a binomial distribution with multiple comparisons performed using LSD tests.
3. Results
3.1. ToLCNDV-ES and TYLCV-IL Co-Infection in TYLCV-Susceptible, TYLCV-Resistant Tomato Plants and N. benthamiana
We examined the interactions between TYLCV-IL and ToLCNDV-ES in both TYLCV-susceptible and TYLCV-resistant tomato genotypes, as well as in N. benthamiana plants.
Consistent with previous findings [29], TYLCV-IL effectively infected TYLCV-susceptible tomato plants, inducing characteristic symptoms (Figure 2 and Figure 3). As already observed for the TYLCV-resistant genotypes used, TYLCV-IL was not detected in plants carrying the Ty-2 resistance gene, suggesting it failed to infect, while it did infect those with the Ty-1 gene although inducing only mild symptoms (Figure 2 and Figure 3). During sequential inoculation in Assay 1, fewer Rondeño plants were infected by TYLCV-IL. However, overall, there were no significant differences in infection rates (GzLM, LSD test, p < 0.05) or symptom severity (ANOVA, Tukey-b test, p < 0.05) between the non-infected and the infected condition across the tested genotypes (Figure 2 and Figure 3).
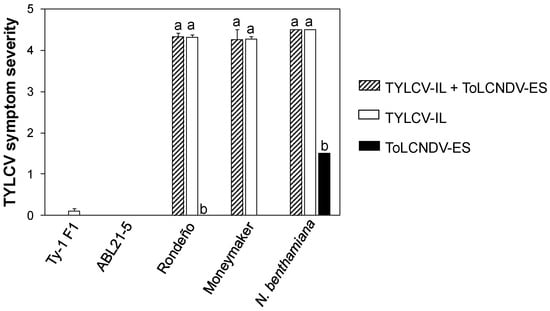
Figure 2.
Symptom severity index in different genotypes inoculated with TYLCV-IL, ToLCNDV-ES, or both viruses. Tomato and Nicotiana benthamiana plants were agroinoculated with infectious clones of tomato yellow leaf curl virus Israel strain (TYLCV-IL) isolate [ES-Alm-Pep-99, GenBank AJ489258], tomato leaf curl New Delhi virus Spain strain (ToLCNDV-ES) isolate [ES-Alm-661-Sq-13; DNA-A: KF749223, DNA-B: KF749226], or both viruses simultaneously (TYLCV-IL + ToLCNDV-ES). The graph shows mean ± standard error of symptom severity scores ranging from 0 (asymptomatic) to 5 (very severe symptoms) recorded at 48 days post-inoculation. Evaluated plant genotypes include two resistant tomato genotypes (Ty-1 F1 and ABL21-5, carrying the Ty-1 and Ty-2 resistance gene, respectively), two susceptible tomato genotypes (Rondeño and Moneymaker), and the susceptible control N. benthamiana. Data represent combined results from two independent biological assays (see Figure 3). No infection was detected in Ty-1 F1 plants inoculated with TYLCV-IL + ToLCNDV-ES or ToLCNDV-ES alone, ABL21-5 plants inoculated with any viral treatment, or Moneymaker plants inoculated with ToLCNDV alone. Bars marked with the same letter are not significantly different (ANOVA, Tukey-b- test, p < 0.05).
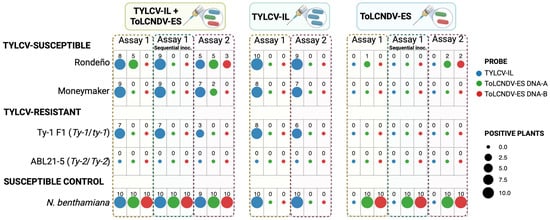
Figure 3.
Detection of TYLCV-IL and ToLCNDV-ES in TYLCV-susceptible and TYLCV-resistant tomatoes and Nicotiana benthamiana plants after single, co-inoculation, and sequential inoculation treatments. Plants of TYLCV-susceptible (cv. Rondeño and Moneymaker) and TYLCV-resistant (Ty-1 F1 [Ty-1/ty1] and ABL21-5 [Ty-2/Ty-2]) tomato genotypes, as well as N. benthamiana, were inoculated with the infectious clone of the Spanish isolates of tomato yellow leaf curl virus strain Israel (TYLCV-IL; isolate ES-Alm-Pep-99, GenBank AJ489258) and/or of tomato leaf curl New Delhi virus strain Spain (ToLCNDV-ES; isolate ES-Alm-661-Sq-13, GenBank KF749223 and KF749226). Agrobacterium-mediated inoculation was performed either singly or simultaneously at the two- to three-leaf growth stage (Assay 1), or sequentially, with TYLCV-IL inoculated first and ToLCNDV-ES at the four- to five-leaf stage (Assay 2). Ten plants were analyzed per condition. The presence of each virus is shown based on tissue blot hybridization of systemic young leaves collected at 48 days post-inoculation using DIG-labelled probes specific to each viral component. The number of positive plants is indicated for each treatment and genotype. Created in BioRender, https://BioRender.com/fgcrwzi (accessed on 13 April 2025).
ToLCNDV-ES exhibited limited ability to infect TYLCV-susceptible tomato genotypes, with systemic infection observed only in a few Rondeño plants, in addition to what is observed in N. benthamiana plants (Figure 3). Simultaneous inoculation with TYLCV-IL slightly increased ToLCNDV-ES infection rates, significantly in the Rondeño genotype (GzLM, LSD test, p < 0.05), suggesting potential functional complementation by TYLCV-IL. All plants testing positive for ToLCNDV-ES were also positive for TYLCV-IL, indicating mixed infections. Plants singly infected with ToLCNDV-ES remained asymptomatic, whereas mixed infections exhibited symptoms similar to those caused by TYLCV-IL alone (Figure 2). Interestingly, ToLCNDV-ES DNA-B was detected less frequently than DNA-A in infected plants.
In TYLCV-resistant tomato plants, ToLCNDV-ES was undetectable regardless of whether inoculation was single or mixed, indicating that there was no complementation of ToLCNDV by TYLCV-IL (plants with the Ty-1 gene) and no overcoming of resistance (plants with the Ty-2 gene) in TYLCV-resistant tomato plants inoculated with TYLCV-IL and ToLCNDV-ES. Conversely, N. benthamiana plants were readily infected by both viruses, either singly or in combination, with no significant differences in symptom expression between single and mixed infections. However, single ToLCNDV-ES infections in N. benthamiana resulted in milder symptoms (Figure 2).
Overall, our findings suggest no significant synergistic interactions between TYLCV-IL and ToLCNDV-ES, except for a slight enhancement of ToLCNDV-ES infection by TYLCV-IL in one TYLCV-susceptible tomato line.
3.2. Absence of Heterologous Trans-Replication of ToLCNDV-ES DNA-B by TYLCV-IL
Previous research has indicated that monopartite begomoviruses can trans-replicate heterologous DNA-B components, potentially modulating plant–virus interactions [36,52,53]. To assess this, we conducted two independent assays involving N. benthamiana and TYLCV-susceptible tomato genotypes, with two assays performed in Rondeño and a single assay in Moneymaker. Our results (Figure 4) show effective TYLCV-IL infections in both plant species regardless of whether they were singly inoculated or co-inoculated with ToLCNDV-ES. However, we did not detect the maintenance of ToLCNDV-ES DNA-B in any co-inoculated plants. Furthermore, the characteristic symptoms induced by TYLCV-IL were consistent across both singly inoculated and co-inoculated plants. These findings suggest that TYLCV-IL does not trans-replicate ToLCNDV-ES DNA-B. A comparative analysis of the iterons in the intergenic region and iteron-related domain of Rep between TYLCV-IL and ToLCNDV-ES revealed significant differences, which may account for the lack of trans-replication observed (Supplementary Figure S3).
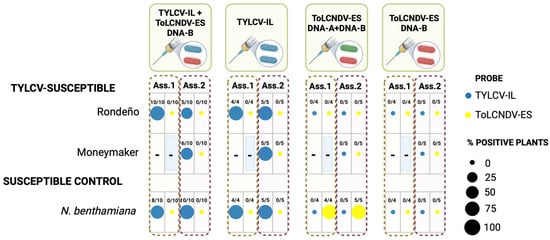
Figure 4.
Maintenance of ToLCNDV-ES DNA-B by TYLCV-IL in TYLCV-susceptible tomato and Nicotiana benthamiana plants. TYLCV-susceptible tomato cultivars (Rondeño and Moneymaker) and N. benthamiana plants were agroinoculated at the two- to three-leaf growth stage with infectious clones of the Spanish isolates of tomato yellow leaf curl virus strain Israel (TYLCV-IL; isolate ES-Alm-Pep-99, GenBank accession AJ489258) and/or tomato leaf curl New Delhi virus strain Spain (ToLCNDV-ES, isolate ES-Alm-661-Sq-13, DNA-A: KF749223, DNA-B: KF749226). Inoculations were performed either singly or simultaneously (co-inoculations) in two independent assays (Assay 1 and Assay 2). Viral presence is shown based on tissue blot hybridization analysis of newly emerged systemic leaves collected at 28 days post-inoculation, which corresponded to the last time point at which differences in hybridization signals were observed for these viral combinations. DIG-labelled DNA probes specific to TYLCV-IL and to ToLCNDV-ES DNA-A, DNA-B, and their common region were used. The number of virus-positive plants over the total number of tested plants is indicated for each treatment. A “–” symbol in the table indicates that the assay was not performed for those specific samples. Created in BioRender.com, https://BioRender.com/73nyeoq (accessed on 14 April 2025).
3.3. Lack of Enhancement of ToLCNDV-ES Infection in Tomato Plants by the Crinivirus ToCV
Some studies have suggested that ToCV may facilitate the infection of distantly related viruses in tomato plants [44]. We therefore investigated whether ToCV could enhance ToLCNDV-ES infection in tomato plants, as mixed infections of these viruses are common due to shared insect vectors. To test this, we performed two independent co-inoculation assays with ToCV and ToLCNDV-ES (Figure 5). Efficient ToCV infections were achieved in both singly and co-inoculated tomato plants, accompanied by characteristic symptoms [10]. However, ToLCNDV-ES rarely infected tomato plants when inoculated alone and did not cause evident symptoms. Co-inoculation with ToCV or pretreatment with virus-free B. tabaci whiteflies did not significantly improve ToLCNDV-ES infection rates in any of the tested tomato genotypes (GzLM, LSD test, p < 0.05). In N. benthamiana plants, both ToCV and ToLCNDV-ES resulted in effective infections when singly or co-inoculated, but no significant increase in symptoms was observed in mixed-infected plants. These results suggest that ToCV does not enhance ToLCNDV-ES infection in tomato plants.
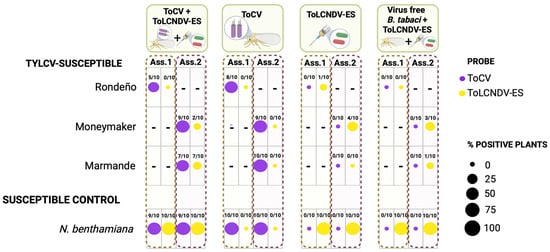
Figure 5.
Interaction between ToCV and ToLCNDV-ES in TYLCV-susceptible tomato and Nicotiana benthamiana plants. TYLCV-susceptible tomato cultivars (Marmande, Rondeño, and Moneymaker) and N. benthamiana plants were inoculated with the Spanish isolates of tomato chlorosis virus (ToCV; isolate Pl-1-2) and/or tomato leaf curl New Delhi virus strain Spain (ToLCNDV-ES; isolate ES-Alm-661-Sq-13, DNA-A: KF749223, DNA-B: KF749226). ToCV was transmitted by viruliferous Bemisia tabaci whiteflies (30 insects per plant, 48 h inoculation access period), while ToLCNDV-ES DNA-A and DNA-B were delivered by Agrobacterium tumefaciens-mediated inoculation of infectious clones. Inoculations were performed either singly or in combination in two independent experiments (Assay 1 and Assay 2). Co-inoculations were performed sequentially with ToCV being inoculated first at the three- to four-leaf stage followed by ToLCNDV-ES at the five- to six-leaf stage. An additional control treatment included plants exposed to virus-free B. tabaci prior to ToLCNDV-ES inoculation. Viral presence is shown based on tissue blot hybridization analysis of newly emerged systemic leaves collected at 38 days post-inoculation, the last time point at which differences in hybridization signals were observed. DIG-labelled RNA probes were used for specific detection of ToCV, while DIG-labelled DNA probes specific to DNA-A and DNA-B components and based on the common region were used for ToLCNDV-ES. The number of virus-positive plants over the total number of tested plants is indicated for each treatment and genotype. A “–” symbol in the table indicates that the assay was not performed for those specific samples. Created in BioRender, https://BioRender.com/ko7uafq (accessed on 15 April 2025).
3.4. Recombination Events Between TYLCV-IL and ToLCNDV-ES in Mixed-Infected Plants
To explore the occurrence of recombination events between TYLCV-IL and ToLCNDV-ES in mixed infections, HTS data were analyzed using QuasiFlow software (version 1.0). Sequencing depths for bulk samples from Rondeño tomato (TYLCV-IL + ToLCNDV-ES-Tom co-infected sample) and N. benthamiana (TYLCV-IL + ToLCNDV-ES-Nb co-infected sample) plants with mixed infections ranged from 4.63 × 106 to 7.41 × 106 filtered reads (Supplementary Table S2). For comparison, similar sequencing depths were obtained for the bulk sample from N. benthamiana plants with single infections of TYLCV-IL or ToLCNDV-ES combined prior to DNA extraction (TYLCV-IL/ToLCNDV-ES-Nb-combined sample) and a control sample from tomato plants co-infected with TYLCV-Mld and TYLCSV (Mm-A sample). These datasets were analyzed for intermolecular and intramolecular recombination events between the two viruses.
Analysis of reads from the TYLCV-Mld + TYLCSV Mm-A sample using QuasiFlow detected intermolecular recombination events equivalent to those identified by García-Andrés et al. [50] (Supplementary Figure S4), confirming the reliability of this method for detecting bona fide recombinants. Additionally, the HTS/QuasiFlow strategy identified new recombination events not previously reported.
In the tomato sample with mixed infections (TYLCV-IL + ToLCNDV-ES-Tom), lower sequence coverage for ToLCNDV-ES DNA-B was observed compared to the N. benthamiana sample (TYLCV-IL + ToLCNDV-ES-Nb) (Figure 6a). This suggests that ToLCNDV-ES DNA-B was less abundant in the tomato plant extract. Supporting this, hybridization results (Figure 3) showed a lower accumulation of ToLCNDV-ES DNA-B compared to DNA-A in tomato plants, as fewer DNA-B-positive plants were detected than DNA-A-positive ones. Viral DNA quantification by qPCR confirmed these observations (Figure 6b). In the mixed-infected tomato sample, TYLCV was more abundant than ToLCNDV-ES DNA-A, with ToLCNDV-ES DNA-B being the least prevalent. Conversely, in N. benthamiana with mixed infections, all viral components were present at higher levels, with TYLCV being the most abundant followed by ToLCNDV-ES DNA-B and DNA-A. In the N. benthamiana combined sample with single infections, TYLCV accumulated to higher levels than ToLCNDV-ES DNA-A and DNA-B. These results suggest that in tomato plants, ToLCNDV-ES DNA-B is less represented compared to TYLCV and DNA-A, a pattern not observed in N. benthamiana.
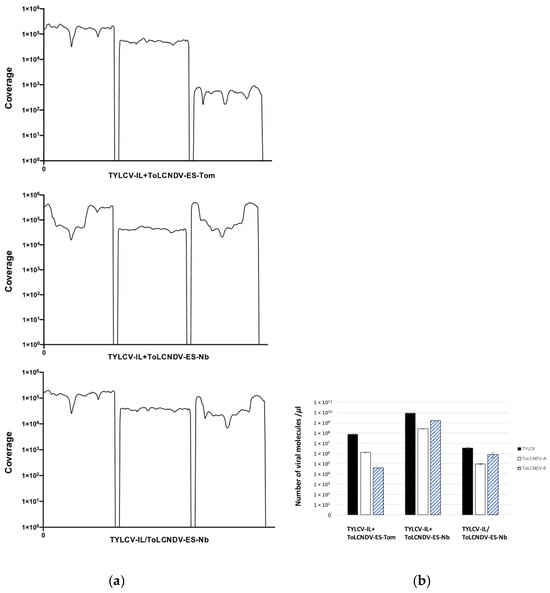
Figure 6.
(a) Coverage plots illustrating the number of nucleotide reads mapped to the genome sequences of tomato yellow leaf curl virus Israel strain (TYLCV-IL) and the DNA-A and DNA-B components of tomato leaf curl New Delhi virus Spain strain (ToLCNDV-ES). Samples were obtained from Solanum lycopersicum (Rondeño cv.) and Nicotiana benthamiana plants subjected to single or mixed infections after inoculation, singly or simultaneously, with the infectious clones of tomato yellow leaf curl virus Israel strain (TYLCV-IL) isolate [ES-Alm-Pep-99] (GenBank AJ489258) and the DNA-A and DNA-B infectious clones of tomato leaf curl New Delhi virus strain Spain (ToLCNDV-ES) isolate [ES-Alm-661-Sq-13] (GenBank accession numbers DNA-A KF749223 and DNA-B KF749226) using Agrobacterium tumefaciens-mediated inoculation. High-throughput sequencing (HTS) was performed on rolling circle amplification (RCA) products from extracts of bulk samples at 90 and 110 days post-inoculation of 5 tomato plants and 10 N. benthamiana plants with mixed infections (TYLCV-IL + ToLCNDV-ES-Tom and TYLCV-IL + ToLCNDV-ES-Nb) or from extracts of bulk samples of 10 N. benthamiana plants with single infections pooled in one sample (TYLCV-IL/ToLCNDV-ES-Nb). (b) Quantification of viral DNA molecules in the DNA extracts (TYLCV-IL + ToLCNDV-ES-Tom, TYLCV-IL + ToLCNDV-ES-Nb, and TYLCV-IL/ToLCNDV-ES-Nb) subjected to RCA and subsequent HTS. Viral loads were quantified by qPCR using custom primers (described in Materials and Methods) for TYLCV-IL and ToLCNDV-ES DNA-A and DNA-B. Standard curves for TYLCV-IL were generated using a pBSK construct containing a monomeric TYLCV-IL (ES-Alm-Pep-99 isolate; GenBank AJ489258) described by Morilla et al. [46]. For ToLCNDV-ES, a pBSK dimer of DNA-A (KF749223) and a pBSK monomer of DNA-B (KF749226) from the ES-Al-661-Sq-13 isolate described by Fortes et al. [47] were employed. Samples and standards were analyzed in triplicate. Statistical comparisons between conditions were not performed due to the pooling of samples, which reflects the variability in mixed infections across the plant populations.
Analysis using QuasiFlow revealed no intermolecular recombination events between TYLCV-IL and ToLCNDV-ES in the tomato sample (Figure 7a). However, intramolecular recombination events were observed in TYLCV-IL, suggesting the presence of putative def-DNAs. In N. benthamiana plants with mixed infections, both TYLCV-IL and ToLCNDV-ES DNA-B exhibited intramolecular recombination events (Figure 7b), indicating the formation of putative def-DNAs. No recombination events were detected for ToLCNDV-ES DNA-A, consistent with findings from single-infection samples (Figure 7c). Interestingly, intermolecular recombination events were identified between TYLCV-IL and ToLCNDV DNA-B but not with ToLCNDV DNA-A (Figure 7b).
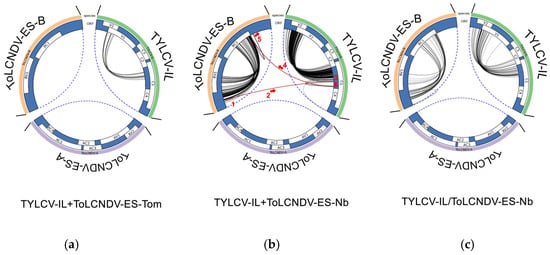
Figure 7.
Circular diagrams showing DNA recombination events detected in Nicotiana benthamiana and Solanum lycopersicum plants infected with tomato yellow leaf curl virus Israel strain (TYLCV-IL) and/or of tomato leaf curl New Delhi virus Spain strain (ToLCNDV-ES). The infectious clones of TYLCV-IL isolate [ES-Alm-Pep-99], (GenBank AJ489258) and the DNA-A and DNA-B infectious clones of ToLCNDV-ES isolate [ES-Alm-661-Sq-13] (GenBank accession numbers DNA-A KF749223 and DNA-B KF749226) were inoculated singly or simultaneously using Agrobacterium tumefaciens-mediated inoculation. Recombination events were identified using QuasiFlow analysis of high-throughput sequencing (HTS) data. Panels show events from rolling circle amplification (RCA) products from extracts of bulk samples of infected plants at 90 and 110 days post-inoculation of (a) mixed infections in Solanum lycopersicum cv. Rondeño (TYLCV-IL + ToLCNDV-ES-Tom mixed infection); (b) mixed infections in N. benthamiana (TYLCV-IL + ToLCNDV-ES-Nb mixed infection); and (c) combined single infections of both viruses in N. benthamiana used as control (TYLCV-IL/ToLCNDV-ES-Nb single infection combined). White boxes indicate open reading frames in the genome components; black lines represent nucleotides involved in the recombination events; dashed blue lines mark genome boundaries. Orange lines highlight recombinant events associated with the To-TYc2 defective DNA between TYLCV-IL and ToLCNDV DNA-B, and red arrows with numbers denote the order of events.
A higher percentage of reads with recombination events was observed in N. benthamiana samples compared to the tomato sample (Supplementary Table S2). Specifically, the tomato sample with mixed infections exhibited approximately 5.92-fold fewer rearrangements than the mixed-infection N. benthamiana sample. Intermediate levels of recombination were detected in the N. benthamiana single-infection sample. Notably, an intermolecular recombination event between ToLCNDV DNA-B and TYLCV-IL in the N. benthamiana mixed-infection sample was supported by 655 reads (indicated by red arrows in Figure 7b), reinforcing the possibility of genetic exchange and the formation of recombinant def-DNA.
To confirm the presence of the putative defective molecules detected in silico, def-DNAs were cloned from RCA products using single-site restriction enzyme digestion followed by cloning and Sanger sequencing (Supplementary Figure S2). Intramolecular recombination events frequently detected in the control N. benthamiana mixed-infection sample were further examined (Supplementary Table S3). As shown in Figure 8a, def-DNA molecules for TYLCV-IL exhibited deletions between the V2 and Rep ORFs. Similarly, def-DNA molecules for ToLCNDV-ES DNA-B showed deletions encompassing significant portions of the BV1 and BC1 ORFs (Figure 8b). Sequences were deposited in GenBank under the following accession numbers: OR699191 to OR699196 for def-DNA molecules of TYLCV-IL, and OR699199 to OR699202, OR699204 to OR699210 for def-DNA molecules of ToLCNDV-ES DNA-B.
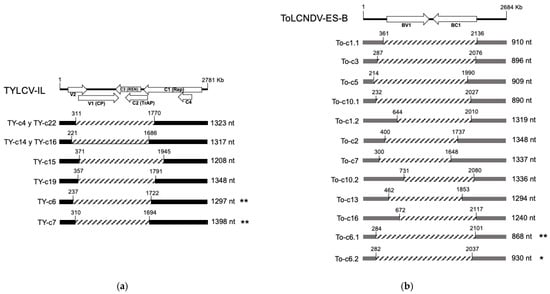
Figure 8.
Bona fide circular defective DNA (def-DNA) molecules cloned from a bulk sample of 10 Nicotiana benthamiana plants infected with the Spanish isolates of tomato yellow leaf curl virus Israel strain (TYLCV-IL) and tomato leaf curl New Delhi virus Spain strain (ToLCNDV-ES) collected at 90 and 110 days post-inoculation. The def-DNAs are represented as linear DNA, starting at the nick site for the initiation of DNA replication located in the intergenic region. Def-DNAs were identified by cloning and sequencing the single-site restriction digestion product from the rolling circle amplification (RCA), which was used in the high-throughput sequencing (HTS) analysis (see Figure 6). (a) Def-DNAs derived from TYLCV-IL and (b) ToLCNDV-ES DNA-B are shown, with conserved regions (black and gray boxes, respectively) and deleted regions (dashed lines). Nucleotide positions of deletions are indicated. Def-DNAs confirmed by PCR amplification directly from the DNA extract using non-overlapping, abutting primers targeting junction/recombinant sites detected in HTS (see Supplementary Figure S2), followed by cloning and sequencing of the cloned fragment, are marked with one asterisk (*), while new sequences detected are marked with two asterisks (**). Linear representations of the genome of each virus are shown at the top of the figures.
The presence of these def-DNAs was confirmed in a number of cases by PCR amplification directly from the DNA extract (Supplementary Figure S2) using non-overlapping abutting primers followed by cloning and sequencing of the amplified fragment (sequences marked with asterisks in Figure 8a,b). Sequence data are available under the following GenBank accession numbers: OR880353 and OR880354 for def-DNA molecules of TYLCV-IL, OR880355 and OR909915 for def-DNA molecules of ToLCNDV-ES DNA-B. Notably, all the def-DNA characterized here are about half the size or smaller than the full genomic DNA, retaining the intergenic/common region necessary for rolling circle replication, suggesting potential replication using viral machinery.
During the cloning and sequencing of def-DNAs from the RCA product, two distinctive recombinant molecules were identified. One def-DNA of ToLCNDV-ES DNA B, measuring 879 base pairs (bp), incorporated a fragment from the C1/Rep ORF of TYLCV-IL (designated as To-TYc2 in Figure 9a), corresponding to the intermolecular recombination event detected via QuasiFlow analysis (red arrows in Figure 7b) and supported by 655 reads. This finding suggests genetic exchange between ToLCNDV-ES and TYLCV-IL. Additionally, a def-DNA of ToLCNDV-ES DNA B, measuring 1306 bp, incorporating a sequence from the N. benthamiana host was detected (designated as To-Nb-c6 in Figure 9b). Further analysis of the HTS data identified approximately 22,000 reads supporting this rearrangement. The N. benthamiana sequence corresponded to a 361-nucleotide fragment located in an intergenic region on scaffold Niben101Scf14297 (positions 176,849–177,209) of the N. benthamiana genome (v1.0.1 “Scaffolds + NrContigs”, database available at https://solgenomics.net/. Sequences were deposited in GenBank under the following accession numbers: OR699190 (ToLCNDV-ES DNA-B-TYLCV-IL recombinant def-DNA) and OR699211 (ToLCNDV-ES DNA-B-N. benthamiana recombinant def-DNA). The presence of both def-DNAs was confirmed in the bulk DNA extract by PCR amplification with non-overlapping abutting primers followed by cloning and sequencing (Supplementary Figure S2). Sequence data are available under the following GenBank accession numbers: OR880356 for ToLCNDV-ES DNA-B-TYLCV-IL recombinant def-DNA and OR880357 for ToLCNDV-ES DNA-B-N. benthamiana recombinant def-DNA.
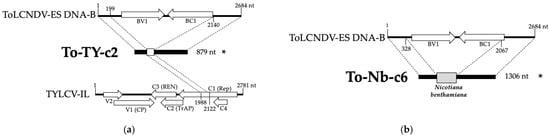
Figure 9.
Recombinant defective DNA (def-DNA) molecules cloned from a Nicotiana benthamiana pooled sample of mixed infections with tomato yellow leaf curl virus Israel strain (TYLCV-IL) and of tomato leaf curl New Delhi virus Spain strain (ToLCNDV-ES) from 10 plants collected at both 90 and 110 days post-inoculation. The def-DNAs are represented as linear molecules, starting at the nick site for DNA replication initiation located in the intergenic region. Def-DNAs were identified by cloning and sequencing the single-site restriction digestion product obtained from the rolling circle amplification (RCA) used in the high-throughput sequencing (HTS) analysis (see Figure 6). These molecules show genetic exchange either (a) between ToLCNDV-ES DNA-B and TYLCV-IL or (b) between ToLCNDV-ES DNA-B and the N. benthamiana genome. Linear representations of the genomes of ToLCNDV-ES and TYLCV-IL are shown at the top and bottom of the figure, respectively. Def-DNAs are illustrated with conserved sequences from ToLCNDV-ES DNA-B (black boxes), TYLCV-IL (open boxes), and N. benthamiana (gray boxes). Nucleotide positions involved in recombination are indicated on the linear genome representations. Clone names and molecule sizes are shown on the left and right, respectively. Asterisks indicate def-DNAs that were confirmed by PCR amplification directly from the DNA extract using non-overlapping abutting primers targeting junction/recombination sites detected by HTS followed by cloning and sequencing.
Although these recombinant def-DNA molecules were detected in N. benthamiana, their formation highlights the potential for similar rearrangements to occur in tomato, especially in mixed infections or under conditions that alter viral replication dynamics. The absence of such molecules in tomato may reflect lower viral accumulation or a more restrictive cellular environment rather than a fundamental inability of the viruses to recombine. Thus, N. benthamiana provides a useful model to explore the patterns of recombination that could be relevant to natural tomato infections.
4. Discussion
Understanding mixed virus infections in plants is essential due to their potential to alter disease dynamics, expand host range, and impact resistance stability. These interactions can be synergistic, where one virus enhances the replication or effects of another, or antagonistic, where one virus suppresses the other, leading to unpredictable outcomes in plant health and disease management [25,30,35,39,44].
In our study, TYLCV-IL and ToLCNDV-ES did not exhibit significant synergistic or antagonistic interactions in tomato plants. Both viruses behaved similarly in susceptible and resistant tomato genotypes, whether in single or mixed infections, except for a slight enhancement of ToLCNDV-ES infection by TYLCV-IL in Rondeño tomato plants, aligning with previous findings [39]. In that study, ToLCNDV-ES, which shows low adaptation to tomato, was found in natural and experimental mixed infections with TYLCV in tomato plants, with an infection ratio of up to 50%. In our experiments, susceptible tomato plants infected with ToLCNDV-ES alone remained asymptomatic. In contrast, mixed infections displayed symptoms similar to those caused by TYLCV-IL alone. This contrasts with other studies in which tomato plants co-infected with TYLCV and other begomoviruses, such as tomato leaf curl Sinaloa virus and tomato yellow mottle virus, developed more severe symptoms than those infected with either virus alone [54]. Although synergistic interactions between begomoviruses [30,34,55], or with other viruses [56,57], have been reported, no synergism was observed between TYLCV-IL and ToLCNDV-ES in our study, in contrast to recent reports [39]. Moreover, resistance-breaking effects observed in mixed infections involving other begomoviruses [25] were not found here. For instance, in chili (Capsicum annuum), synergistic interactions between multiple begomovirus genomic components were shown to break resistance in cultivars previously considered resistant due to suppression of host defense responses [25]. In contrast, the TYLCV resistance provided by the Ty-1 and Ty-2 genes in tomato plants [58] remained effective under mixed infection with TYLCV-IL and ToLCNDV-ES. Therefore, these resistance genes appear to be stable even under the combined challenge posed by both viruses.
Except for the Indian isolate of ToLCNDV in which DNA-A alone can infect tomato, both DNA-A and DNA-B were required for systemic infection of tomato plants, with essential movement functions located in DNA-B [47,59,60]. However, isolates of the ToLCNDV-ES strain that spread to the Mediterranean Basin exhibited impaired ability to infect tomatoes [37,39,61]. Our evidence supports the constraint for DNA-B accumulation in tomatoes, as observed by others [39]. According to our HTS and DNA quantification results, a lower accumulation of ToLCNDV-ES DNA-B occurred in mixed-infected tomato plants than in the more permissive host, N. benthamiana. This likely results in a movement defect that can compromise the pathogenicity of this virus in tomatoes [46]. As DNA was extracted from pooled apical leaves collected at 90 and 110 dpi from five tomato plants and ten N. benthamiana plants, no statistical comparisons between individual replicates were performed. Therefore, the observed differences in DNA-B accumulation between hosts represent general trends across pooled samples rather than statistically validated differences at the individual plant level. Notably, the interaction of ToLCNDV-ES with TYLCV-IL did not fully complement the restrictions of the former virus to infect tomato plants, even though movement functions and modulators of host defense response are present in TYLCV [62,63]. As replication of the ToLCNDV-ES isolate is not impeded [37], other virus–host interaction aspects might be impaired, constraining the infectivity of this virus in tomato plants. The virus coat protein has been shown to be crucial for ToLCNDV pathogenic adaptation in tomatoes and is essential for its infectivity [64]. However, the mechanisms associated with the restriction of ToLCNDV-ES in tomato plants and the existence of host-specific factors for effective complementation of DNA-B by DNA-A require further studies.
The presence of ToCV is a cause for concern, as it is an emerging RNA plant virus worldwide [65] that has been involved in synergistic interactions [44] and shares the B. tabaci vector and tomato host plant with the begomoviruses TYLCV-IL and ToLCNDV-ES. Therefore, these viruses can co-exist in tomato plants, as already observed for TYLCV [66] and other tomato begomoviruses [67]. Synergism between DNA and RNA viruses has been reported [68], which can alter plant–virus interactions and compromise resistance to TYLCV in tomato plants [69]. However, although co-infections of ToCV with TYLCV result in more aggressive disease severity in susceptible tomatoes [33,34] and can enhance the breakdown of resistance provided by the Ty-1 gene, we observed no effect on ToLCNDV-ES interaction with tomato plants. Therefore, the modulation of host defenses by ToCV [70] does not seem to assist ToLCNDV-ES in infecting tomato plants, which could increase the chances of interaction with TYLCV-IL during natural epidemics.
Evidence here and elsewhere shows that, even at low efficiency, ToLCNDV-ES can infect tomatoes and that mixed infection with TYLCV-IL may occur in tomato plants [37,39] and probably in other common hosts. TYLCV-IL is a monopartite begomovirus, and trans-replication of the DNA-B of ToLCNDV-ES might occur in mixed infections with unpredictable phytopathological consequences. In fact, the acquisition of heterologous DNA-Bs by monopartite begomoviruses occurs and can lead to more severe damage to infected plants [36,53,71], even involving ToLCNDV [72]. It should be noted that Rep–iteron compatibility plays a key role in determining efficient replication of geminiviruses [15] and effective reassortment among begomovirus genomic components [36]. Notable differences were observed in the iteron and Rep domains of TYLCV-IL and ToLCNDV-ES (Supplementary Figure S3). Nevertheless, reassortment has been reported despite differences, as observed for the DNA-B of the begomovirus tomato leaf curl Gujarat virus when associated with heterologous DNA-As [30]. Our results support that TYLCV-IL is unable to maintain the DNA-B of ToLCNDV-ES, suggesting that the Rep–iteron differences can constrain trans-replication, as previously observed [55]. This is relevant in terms of the interaction of TYLCV with tomato plants and crop protection because more intense damage in infected plants has been associated with heterologous trans-replication of DNA-B of ToLCNDV by monopartite begomoviruses [36,73]. The observed inability of TYLCV-IL to maintain ToLCNDV-ES DNA-B in the more permissive virus host N. benthamiana [74,75] suggests that strong heterologous recognition constraints exist. These findings indicate that TYLCV-IL is unlikely to acquire the DNA-B component of ToLCNDV-ES, which would have potentially facilitated its evolution towards a bipartite genome structure, enabling a more rapid adaptation to changing environments [53]. However, the presence of def-DNAs and recombination events within mixed infections could suggest that alternative evolutionary paths are possible. The emergence of new viral variants, facilitated by such recombination, could pose a challenge to resistance management strategies. As these recombination events may allow viruses to overcome host resistance, it is crucial to develop adaptive resistance strategies that not only monitor current virus strains but also take into account potential recombinant variants that could threaten the stability of resistance genes such as Ty-1 and Ty-2. The presence of def-DNAs, for example, could promote the development of new virulent variants capable of bypassing existing resistance mechanisms, emphasizing the need for more dynamic management approaches. Moreover, early detection of new viral strains and recombinant variants through advanced diagnostic tools is essential for managing viral resistance effectively. By continuously monitoring the emergence of such variants in field conditions, crop protection strategies could be adapted to prevent the breakdown of resistance genes in susceptible tomato varieties.
Interspecies recombination is a common phenomenon in mixed begomovirus infections, significantly influencing virus evolution, host adaptation, and emergence [31,76]. Such recombination events can occur between monopartite and bipartite begomoviruses [23,32]. In this study, HTS data were analyzed with the QuasiFlow pipeline, and recombination junctions were detected using the Virema Module. This allowed the reconstruction of a broad set of putative recombinant def-DNA molecules, including low-abundance forms. The diversity of these recombination patterns is illustrated in Figure 7. We focused our analysis on the subset of def-DNAs that were experimentally validated through RCA amplification, molecular cloning, and Sanger sequencing. These selected molecules represent well-supported recombinant forms but do not encompass the full diversity detected in silico. Although our HTS analysis did not reveal any full-length recombinant genomes in mixed infections of TYLCV-IL and ToLCNDV-ES, this observation should be interpreted with caution. The limited number of plants analyzed and the use of pooled samples may have masked low-frequency recombinant genomes present in individual plants. Moreover, only a subset of recombinant def-DNA molecules was validated and characterized by cloning and sequencing, so structurally complex or rare recombination events may have gone undetected. Further studies involving individual plant sequencing and deeper HTS analysis could help uncover such events and provide a more comprehensive view of the recombination landscape. Interestingly, we identified numerous DNA rearrangement events indicative of def-DNAs in both single and mixed infections of TYLCV-IL and ToLCNDV-ES. Def-DNAs are subgenomic molecules derived from the parent virus through deletions or rearrangements and are known to occur in begomovirus-infected plants [19,77,78]. Similar def-DNAs have been associated with TYLCV-IL infections in previous studies [79]. Interestingly, these recombination events appeared more frequently in N. benthamiana plants than in tomato plants, suggesting potential differences in virus–host interactions and their impact on genomic rearrangements across different host species and infection scenarios. This aligns with the known permissiveness of N. benthamiana to virus infections [75] and the association between high viral replication rates and increased def-DNA production [77]. Indeed, geminivirus def-DNA production appears to be influenced by the host species, with larger amounts observed in the more permissive N. benthamiana plants [19,79].
The def-DNAs cloned here had approximately half the length of native molecules and contained partial, rearranged fragments with genomic portions of TYLCV-IL and ToLCNDV-ES DNA-B, consistent with previous reports [80]. Surprisingly, we detected no events supporting def-DNAs derived from ToLCNDV-ES DNA-A. In other bipartite begomoviruses, a higher generation of DNA-B-specific def-DNAs was described, which was linked to structural differences between the components [19]. All detected def-DNAs retained genome regions required for viral DNA recognition and replication [14], suggesting they could be maintained during infection. Def-DNAs can be encapsidated, spread within the host plant, and transmitted by insect vectors, thereby persisting in epidemics [79,81]. Moreover, the presence of defective genomes can modulate plant–virus interactions by affecting the onset and persistence of viral infections and disease severity, as observed in both RNA and DNA viruses [19,77,81,82,83].
Interestingly, we detected genetic exchanges between TYLCV-IL and ToLCNDV-ES within def-DNA molecules, supporting the possibility of genetic transfer via these molecules. The biological implications of such genetic exchanges are unpredictable, but they may confer adaptive advantages to the viruses. Additionally, we identified a def-DNA suggesting genetic transfer between the plant host and ToLCNDV-ES, providing further evidence of begomoviruses’ propensity to acquire host genetic material within def-DNA [81]. This phenomenon positions def-DNAs as potential vectors for horizontal gene transfer between plants [79]. Recombinant def-DNA molecules may have significant evolutionary consequences in natural field conditions, as they could act as vehicles for genetic exchange among viruses, host plants, and insect vectors. These molecules may facilitate the acquisition of host genetic material, as seen in some begomovirus infections, thus contributing to viral adaptation to changing environments and new host species. The presence of def-DNA molecules might enhance the persistence of the virus in the field by enabling the spread of defective viral genomes that could still be transmitted by insect vectors, further complicating virus–host dynamics. Additionally, def-DNAs could modulate viral pathogenicity, affecting disease severity and persistence in plant populations. These factors may lead to the emergence of more virulent or resistant viral variants, particularly in areas where multiple begomoviruses coexist and interact.
5. Conclusions
While mixed virus infections can potentially alter plant–virus interactions and lead to resistance breakdown, our study observed no significant changes in pathogenicity resulting from the interaction between TYLCV-IL and ToLCNDV-ES. Furthermore, we did not detect the emergence of recombinant or pseudorecombinant viruses with novel pathogenic characteristics from these mixed infections. However, our findings provide evidence that genetic exchanges between TYLCV-IL and the DNA-B of ToLCNDV-ES can occur within def-DNA molecules of virus populations present in infected plants. The consequences of these genetic exchanges on virus–host interactions, virus ecology, and disease epidemics warrant further investigation. Future studies should also focus on variants better adapted to tomato plants, which appear to be emerging in the ToLCNDV-ES population in the Mediterranean region, to gain a broader understanding of the potential interactions between TYCLV-IL and ToLCNDV-ES.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15051006/s1, Figure S1: General workflow for the extraction and amplification of circular viral genomes from agroinoculated plants; Figure S2: Detailed workflow for the cloning and detection of defective viral DNA molecules (def-DNAs); Figure S3: Proposed iterons and the iteron-related domain (IRD) of the replication-associated protein (Rep); Figure S4: Recombination events identified in a Solanum lycopersicum plant with a mixed infection by isolates of tomato yellow leaf curl virus Mild strain (TYLCV-Mld) and tomato yellow leaf curl Sardinia virus (TYLCSV); Table S1: Non-overlapping, abutting primer pairs for PCR validation of defective DNAs from a Nicotiana benthamiana pooled sample of TYLCV-IL and ToLCNDV-ES mixed infections; Table S2: High-throughput sequencing (HTS) metrics for Nicotiana benthamiana and Solanum lycopersicum plants subjected to co-infections or single infections, analyzed using QuasiFlow software; Table S3: Nucleotide positions involved in deletion events leading to putative defective DNAs.
Author Contributions
Conceptualization, E.M.; methodology, E.M. and I.M.F.; validation, E.M., I.M.F., L.D.-M. and A.G.-P.; formal analysis, I.M.F., L.D.-M. and A.G.-P.; investigation, I.M.F., L.D.-M. and A.G.-P.; resources, E.M.; data curation, I.M.F., L.D.-M. and A.G.-P.; writing and original draft preparation, E.M. and A.G.-P.; writing, review, and editing, E.M., I.M.F., L.D.-M. and A.G.-P.; funding acquisition, E.M. and A.G.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerio de Ciencia e Innovación (MCIN/AEI/10.13039/501100011033), grant numbers PID2019-107657RB-C21 and PID2022-139376OB-C32 to E.M.; by the Consejería de Universidad, Investigación e Innovación, Junta de Andalucía, grant number P18-RT-1249 to E.M.; and by the Consejería de Economía y Conocimiento, Junta de Andalucía co-financed by the Programa Operativo FEDER 2014–2020, grant number UMA18-FEDERJA-178 to A.G.-P. and E.M.
Data Availability Statement
The original data presented in the study are openly available in the NCBI Sequence Read Archive (SRA) under accession number PRJNA1036430.
Acknowledgments
We thank Rafael Fernández-Muñoz (IHSM, UMA-CSIC, Spain) for his help with statistical analyses and Susannah Dale for English editing.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Rybicki, E.P.; Pietersen, G. Plant virus disease problems in the developing world. Adv. Virus Res. 1999, 53, 127–175. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Navas-Castillo, J.; Lopez-Moya, J.J.; Aranda, M.A. Whitefly-transmitted RNA viruses that affect intensive vegetable production. Ann. Appl. Biol. 2014, 165, 155–171. [Google Scholar] [CrossRef]
- Rojas, M.R.; Macedo, M.A.; Maliano, M.R.; Soto-Aguilar, M.; Souza, J.O.; Briddon, R.W.; Kenyon, L.; Rivera Bustamante, R.F.; Zerbini, F.M.; Adkins, S.; et al. World management of geminiviruses. Annu. Rev. Phytopathol. 2018, 56, 637–677. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R. Whitefly infestations: The Christmas Invasion. Nature 2006, 443, 898–900. [Google Scholar] [CrossRef]
- De Barro, P.J. Bemisia tabaci, the capacity to invade. In The Whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) Interaction with Geminivirus-Infected Host Plants; Thompson, W.M.O., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 181–204. [Google Scholar]
- Gilbertson, R.L.; Batuman, O.; Webster, C.G.; Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annu. Rev. Virol. 2015, 2, 67–93. [Google Scholar] [CrossRef]
- Hanssen, I.M.; Lapidot, M.; Thomma, B.P.H.J. Emerging Viral Diseases of Tomato Crops. Mol. Plant-Microbe Interact. 2010, 23, 539–548. [Google Scholar] [CrossRef]
- Moriones, E.; Praveen, S.; Chakraborty, S. Tomato leaf curl New Delhi virus: An emerging virus complex threatening vegetable and fiber crops. Viruses 2017, 9, 264. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Navas-Castillo, J. Tomato chlorosis virus, an emergent plant virus still expanding its geographical and host ranges. Mol. Plant Pathol. 2019, 20, 1307–1320. [Google Scholar] [CrossRef]
- Tatineni, S.; Hein, G.L. Plant viruses of agricultural importance: Current and future perspectives of virus disease management strategies. Phytopathology 2023, 113, 117–141. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Harkins, G.; Lemey, P.; Gray, A.J.A.; Meredith, S.; Lakay, F.; Monjane, A.; Lett, J.-M.; Varsani, A.; et al. The spread of tomato yellow leaf curl virus from the Middle East to the world. PLoS Pathog. 2010, 6, e1001164. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Lett, J.-M.; Martin, D.P.; Roumagnac, P.; Varsani, A.; Zerbini, F.M.; Navas-Castillo, J. ICTV Virus Taxonomy Profile: Geminiviridae 2021. J. Gen. Virol. 2021, 102, 001696. [Google Scholar] [CrossRef] [PubMed]
- Hanley-Bowdoin, L.; Settlage, S.B.; Orozco, B.M.; Nagar, S.; Robertson, D. Geminiviruses: Models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 105–140. [Google Scholar] [CrossRef]
- Arguello-Astorga, G.R.; Ruiz-Medrano, R. An iteron-related domain is associated to Motif 1 in the replication proteins of geminiviruses: Identification of potential interacting amino acid-base pairs by a comparative approach. Arch. Virol. 2001, 146, 1465–1485. [Google Scholar] [CrossRef]
- Gong, P.; Tan, H.; Zhao, S.; Li, H.; Liu, H.; Ma, Y.; Zhang, X.; Rong, J.; Fu, X.; Lozano-Durán, R.; et al. Geminiviruses encode additional small proteins with specific subcellular localizations and virulence function. Nat. Commun. 2021, 12, 4278. [Google Scholar] [CrossRef]
- Zhao, S.; Gong, P.; Ren, Y.; Liu, H.; Li, H.; Li, F.; Zhou, X. The novel C5 protein from tomato yellow leaf curl virus is a virulence factor and suppressor of gene silencing. Stress Biol. 2022, 2, 19. [Google Scholar] [CrossRef]
- Liu, H.; Chang, Z.; Zhao, S.; Gong, P.; Zhang, M.; Lozano-Durán, R.; Yan, H.; Zhou, X.; Li, F. Functional identification of a novel C7 protein of tomato yellow leaf curl virus. Virology 2023, 585, 117–126. [Google Scholar] [CrossRef]
- Patil, B.; Dasgupta, I. Defective interfering DNAs of plant viruses. Crit. Rev. Plant Sci. 2006, 25, 47–64. [Google Scholar] [CrossRef]
- Shahid, M.S.; Ito, T.; Kimbara, J.; Onozato, A.; Natsuaki, K.T.; Ikegami, M. Evaluation of tomato hybrids carrying Ty-1 and Ty-2 loci to Japanese monopartite begomovirus species. J. Phytopathol. 2013, 161, 205–209. [Google Scholar] [CrossRef]
- Prasanna, H.C.; Sinha, D.P.; Rai, G.K.; Krishna, R.; Kashyap, S.P.; Singh, N.K.; Singh, M.; Malathi, V.G. Pyramiding Ty-2 and Ty-3 genes for resistance to monopartite and bipartite tomato leaf curl viruses of India. Plant Pathol. 2015, 64, 256–264. [Google Scholar] [CrossRef]
- Albuquerque, L.; Varsani, A.; Fernandes, F.; Pinheiro, B.; Martin, D.; de Tarso Oliveira Ferreira, P.; Lemos, T.O.; Inoue-Nagata, A.K. Further characterization of tomato-infecting begomoviruses in Brazil. Arch. Virol. 2012, 157, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Tiendrebeogo, F.; Lefeuvre, P.; Hoareau, M.; Harimalala, M.; De Bruyn, A.; Villemot, J.V.; Traoré, V.S.E.; Konaté, G.; Traoré, A.S.; Barro, N.; et al. Evolution of African cassava mosaic virus by recombination between bipartite and monopartite begomoviruses. Virol. J. 2012, 9, 67. [Google Scholar] [CrossRef]
- Vanitharani, R.; Chellappan, P.; Pita, J.S.; Fauquet, C.M. Differential roles of AC2 and AC4 of cassava geminiviruses in mediating synergism and suppression of posttranscriptional gene silencing. J. Virol. 2004, 78, 9487–9498. [Google Scholar] [CrossRef]
- Singh, A.K.; Kushwaha, N.; Chakraborty, S. Synergistic interaction among begomoviruses leads to the suppression of host defense-related gene expression and breakdown of resistance in chilli. Appl. Microbiol. Biotechnol. 2016, 100, 4035–4049. [Google Scholar] [CrossRef] [PubMed]
- Voorburg, C.M.; Yan, Z.; Bergua-Vidal, M.; Wolters, A.-M.A.; Bai, Y.; Kormelink, R. Ty-1, a universal resistance gene against geminiviruses that is compromised by co-replication of a betasatellite. Mol. Plant Pathol. 2020, 21, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Monci, F.; Sanchez-Campos, S.; Navas-Castillo, J.; Moriones, E. A natural recombinant between the geminiviruses Tomato yellow leaf curl Sardinia virus and Tomato yellow leaf curl virus exhibits a novel pathogenic phenotype and is becoming prevalent in Spanish populations. Virology 2002, 303, 317–326. [Google Scholar] [CrossRef]
- Davino, S.; Miozzi, L.; Panno, S.; Rubio, L.; Davino, M.; Accotto, G.P. Recombination profiles between Tomato yellow leaf curl virus and Tomato yellow leaf curl Sardinia virus in laboratory and field conditions: Evolutionary and taxonomic implications. J. Gen. Virol. 2012, 93, 2712–2717. [Google Scholar] [CrossRef]
- Díaz-Pendón, J.; Sánchez-Campos, S.; Fortes, I.; Moriones, E. Tomato yellow leaf curl Sardinia virus, a begomovirus species evolving by mutation and recombination: A challenge for virus control. Viruses 2019, 11, 45. [Google Scholar] [CrossRef]
- Chakraborty, S.; Vanitharani, R.; Chattopadhyay, B.; Fauquet, C.M. Supervirulent pseudorecombination and asymmetric synergism between genomic components of two distinct species of begomovirus associated with severe tomato leaf curl disease in India. J. Gen. Virol. 2008, 89, 818–828. [Google Scholar] [CrossRef]
- Vuillaume, F.; Thebaud, G.; Urbino, C.; Forfert, N.; Granier, M.; Froissart, R.; Blanc, S.; Peterschmitt, M. Distribution of the phenotypic effects of random homologous recombination between two virus species. PLoS Pathog. 2011, 7, e1002028. [Google Scholar] [CrossRef]
- Paz-Carrasco, L.C.; Castillo-Urquiza, G.P.; Lima, A.T.; Xavier, C.A.; Vivas-Vivas, L.M.; Mizubuti, E.S.; Zerbini, F.M. Begomovirus diversity in tomato crops and weeds in Ecuador and the detection of a recombinant isolate of rhynchosia golden mosaic Yucatan virus infecting tomato. Arch. Virol. 2014, 159, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.-c.; Ding, T.-b.; Chu, D. Synergistic effects of a Tomato chlorosis virus and Tomato yellow leaf curl virus mixed infection on host tomato plants and the whitefly vector. Front. Plant Sci. 2021, 12, 672400. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, I.; López-Moya, J.J.; Díaz-Pendón, J.A. Coinfection of tomato plants with tomato yellow leaf curl virus and tomato chlorosis virus affects the interaction with host and whiteflies. Phytopathology 2022, 112, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Pita, J.S.; Fondong, V.N.; Sangare, A.; Otim-Nape, G.W.; Ogwal, S.; Fauquet, C.M. Recombination, pseudorecombination and synergism of geminiviruses are determinant keys to the epidemic of severe cassava mosaic disease in Uganda. J. Gen. Virol. 2001, 82, 655–665. [Google Scholar] [CrossRef]
- Kumari, P.; Singh, A.K.; Sharma, V.K.; Chattopadhyay, B.; Chakraborty, S. A novel recombinant tomato-infecting begomovirus capable of transcomplementing heterologous DNA-B components. Arch. Virol. 2011, 156, 769–783. [Google Scholar] [CrossRef]
- Fortes, M.I.; Sanchez-Campos, S.; Fiallo-Olive, E.; Diaz-Pendón, J.A.; Navas-Castillo, J.; Moriones, E. A novel strain of tomato leaf curl New Delhi virus has spread to the Mediterranean basin. Viruses 2016, 8, 307. [Google Scholar] [CrossRef]
- Ruiz, L.; Simon, A.; Velasco, L.; Janssen, D. Biological characterization of Tomato leaf curl New Delhi virus from Spain. Plant Pathol. 2017, 66, 376–382. [Google Scholar] [CrossRef]
- Vo, T.T.B.; Troiano, E.; Lal, A.; Hoang, P.T.; Kil, E.-J.; Lee, S.; Parrella, G. ToLCNDV-ES infection in tomato is enhanced by TYLCV: Evidence from field survey and agroinoculation. Front. Microbiol. 2022, 13, 954460. [Google Scholar] [CrossRef]
- Morilla, G.; Janssen, D.; Garcia-Andres, S.; Moriones, E.; Cuadrado, I.M.; Bejarano, E.R. Pepper (Capsicum annuum) is a dead-end host for Tomato yellow leaf curl virus. Phytopathology 2005, 95, 1089–1097. [Google Scholar] [CrossRef]
- Monci, F.; Garcia-Andres, S.; Maldonado, J.A.; Moriones, E. Resistance to monopartite begomoviruses associated with the bean leaf crumple disease in Phaseolus vulgaris controlled by a single dominant gene. Phytopathology 2005, 95, 819–826. [Google Scholar] [CrossRef]
- Garcia-Cano, E.; Navas-Castillo, J.; Moriones, E.; Fernandez-Munoz, R. Resistance to Tomato chlorosis virus in Wild Tomato Species that Impair Virus Accumulation and Disease Symptom Expression. Phytopathology 2010, 100, 582–592. [Google Scholar] [CrossRef]
- Friedmann, M.; Lapidot, M.; Cohen, S.; Pilowsky, M. A novel source of resistance to tomato yellow leaf curl virus exhibiting a symptomless reaction to viral infection. J. Am. Soc. Hortic. Sci. 1998, 123, 1004–1007. [Google Scholar] [CrossRef]
- Garcia-Cano, E.; Resende, R.O.; Fernandez-Munoz, R.; Moriones, E. Synergistic interaction between Tomato chlorosis virus and Tomato spotted wilt virus results in breakdown of resistance in tomato. Phytopathology 2006, 96, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Haible, D.; Kober, S.; Jeske, H. Rolling circle amplification revolutionizes diagnosis and genomics of geminiviruses. J. Virol. Methods 2006, 135, 9–16. [Google Scholar] [CrossRef]
- Yamamoto, H.; Wakita, Y.; Kitaoka, T.; Fujishiro, T.; Kesumawati, E.; Koeda, S. Southeast Asian isolate of the Tomato leaf curl New Delhi virus shows higher pathogenicity against tomato and cucurbit crops compared to that of the Mediterranean isolate. Hortic. J. 2021, 90, 314–325. [Google Scholar] [CrossRef]
- Sivalingam, P.N.; Varma, A. Role of betasatellite in the pathogenesis of a bipartite begomovirus affecting tomato in India. Arch. Virol. 2012, 157, 1081–1092. [Google Scholar] [CrossRef]
- Seoane, P.; Díaz-Martínez, L.; Viguera, E.; Claros, M.G.; Grande-Pérez, A. QuasiFlow: A bioinformatic tool for genetic variability analysis from next generation sequencing data. bioRxiv 2022. [Google Scholar] [CrossRef]
- Inkscape Project. Inkscape [Computer Software]. 2020. Available online: https://inkscape.org (accessed on 3 March 2023).
- Garcia-Andres, S.; Tomas, D.M.; Sanchez-Campos, S.; Navas-Castillo, J.; Moriones, E. Frequent occurrence of recombinants in mixed infections of tomato yellow leaf curl disease-associated begomoviruses. Virology 2007, 365, 210–219. [Google Scholar] [CrossRef]
- Briddon, R.W.; Prescott, A.G.; Lunness, P.; Chamberlin, L.C.L.; Markham, P.G. Rapid production of full-length, infectious geminivirus clones by abutting primer PCR (AbP-PCR). J. Virol. Methods 1993, 43, 7–20. [Google Scholar] [CrossRef]
- Blawid, R.; Van, D.T.; Maiss, E. Transreplication of a Tomato yellow leaf curl Thailand virus DNA-B and replication of a DNA− component by Tomato leaf curl Vietnam virus and Tomato yellow leaf curl Vietnam virus. Virus Res. 2008, 136, 107–117. [Google Scholar] [CrossRef]
- Ouattara, A.; Tiendrébéogo, F.; Becker, N.; Urbino, C.; Thébaud, G.; Hoareau, M.; Allibert, A.; Chiroleu, F.; Vernerey, M.S.; Traoré, E.V.; et al. Synergy between an emerging monopartite begomovirus and a DNA-B component. Sci. Rep. 2022, 12, 695. [Google Scholar] [CrossRef] [PubMed]
- Maliano, M.R.; Rojas, M.R.; Macedo, M.A.; Barboza, N.; Gilbertson, R.L. The invasion biology of tomato begomoviruses in Costa Rica reveals neutral synergism that may lead to increased disease pressure and economic loss. Virus Res. 2022, 317, 198793. [Google Scholar] [CrossRef]
- Nogueira, A.M.; Barbosa, T.M.C.; Quadros, A.F.F.; Orílio, A.F.; Bigão, M.C.J.; Xavier, C.A.D.; Ferro, C.G.; Zerbini, F.M. Specific nucleotides in the common region of the begomovirus tomato rugose mosaic virus (ToRMV) are responsible for the negative interference over tomato severe rugose virus (ToSRV) in mixed infection. Viruses 2023, 15, 2074. [Google Scholar] [CrossRef] [PubMed]
- Mascia, T.; Cillo, F.; Fanelli, V.; Finetti-Sialer, M.M.; De Stradis, A.; Palukaitis, P.; Gallitelli, D. Characterization of the interactions between Cucumber mosaic virus and Potato virus Y in Mixed Infections in Tomato. Mol. Plant-Microbe Interact. 2010, 23, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Klap, C.; Luria, N.; Smith, E.; Hadad, L.; Bakelman, E.; Sela, N.; Belausov, E.; Lachman, O.; Leibman, D.; Dombrovsky, A. Tomato brown rugose fruit virus contributes to enhanced Pepino mosaic virus titers in tomato Plants. Viruses 2020, 12, 879. [Google Scholar] [CrossRef]
- Ohnishi, J.; Yamaguchi, H.; Saito, A. Analysis of the Mild strain of tomato yellow leaf curl virus, which overcomes Ty-2 gene-mediated resistance in tomato line H24. Arch. Virol. 2016, 161, 2207–2217. [Google Scholar] [CrossRef]
- Padidam, M.; Beachy, R.N.; Fauquet, C.M. Tomato leaf curl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J. Gen. Virol. 1995, 76, 25–35. [Google Scholar] [CrossRef]
- Saeed, M.; Zafar, Y.; Randles, J.W.; Rezaian, M.A. A monopartite begomovirus-associated DNA beta satellite substitutes for the DNA B of a bipartite begomovirus to permit systemic infection. J. Gen. Virol. 2007, 88, 2881–2889. [Google Scholar] [CrossRef]
- Simon, A.; Ruiz, L.; Velasco, L.; Janssen, D. Absolute quantification of Tomato leaf curl New Delhi virus Spain strain, ToLCNDV-ES: Virus accumulation in a host-specific manner. Plant Dis. 2017, 102, 165. [Google Scholar] [CrossRef]
- Luna, A.P.; Morilla, G.; Voinnet, O.; Bejarano, E.R. Functional analysis of gene-silencing suppressors from tomato yellow leaf curl disease viruses. Mol. Plant-Microbe Interact. 2012, 25, 1294–1306. [Google Scholar] [CrossRef]
- Gover, O.; Peretz, Y.; Mozes-Koch, R.; Maori, E.; Rabinowitch, H.D.; Sela, I. Only minimal regions of tomato yellow leaf curl virus (TYLCV) are required for replication, expression and movement. Arch. Virol. 2014, 159, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.T.B.; Lal, A.; Nattanong, B.; Tabassum, M.; Qureshi, M.A.; Troiano, E. Coat protein is responsible for tomato leaf curl New Delhi virus pathogenicity in tomato. Front. Plant Sci. 2023, 14, 1206255. [Google Scholar] [CrossRef] [PubMed]
- Fiallo-Olivé, E.; Navas-Castillo, J. Tomato chlorosis virus, a promiscuous virus with multiple host plants and whitefly vectors. Ann. Appl. Biol. 2022, 182, 29–36. [Google Scholar] [CrossRef]
- Martinez-Zubiaur, Y.; Fiallo-Olive, E.; Carrillo-Tripp, J.; Rivera-Bustamante, R. First report of tomato chlorosis virus Infecting tomato in single and mixed infections with tomato yellow leaf curl virus in Cuba. Plant Dis. 2008, 92, 836. [Google Scholar] [CrossRef]
- Macedo, M.A.; Inoue-Nagata, A.K.; Silva, T.N.Z.; Freitas, D.M.S.; Rezende, J.A.M.; Barbosa, J.C.; Michereff-Filho, M.; Nascimento, A.R.; Lourenção, A.L.; Bergamin Filho, A. Temporal and spatial progress of the diseases caused by the crinivirus Tomato chlorosis virus and the begomovirus Tomato severe rugose virus in tomatoes in Brazil. Plant Pathol. 2019, 68, 72–84. [Google Scholar] [CrossRef]
- Wege, C.; Siegmund, D. Synergism of a DNA and an RNA virus: Enhanced tissue infiltration of the begomovirus Abutilon mosaic virus (AbMV) mediated by Cucumber mosaic virus (CMV). Virology 2007, 357, 10–28. [Google Scholar] [CrossRef]
- Butterbach, P.; Verlaan, M.G.; Dullemans, A.; Lohuis, D.; Visser, R.G.F.; Bai, Y.; Kormelink, R. Tomato yellow leaf curl virus resistance by Ty-1 involves increased cytosine methylation of viral genomes and is compromised by cucumber mosaic virus infection. Proc. Natl. Acad. Sci. USA 2014, 111, 12942–12947. [Google Scholar] [CrossRef]
- Landeo-Ríos, Y.; Navas-Castillo, J.; Moriones, E.; Cañizares, M. The heterologous expression of the p22 RNA silencing suppressor of the crinivirus Tomato chlorosis virus from Tobacco rattle virus and potato virus X enhances disease severity but does not complement suppressor-defective mutant viruses. Viruses 2017, 9, 358. [Google Scholar] [CrossRef]
- Saunders, K.; Salim, N.; Mali, V.R.; Malathi, V.G.; Briddon, R.; Markham, P.G.; Stanley, J. Characterisation of Sri Lankan cassava mosaic virus and Indian cassava mosaic virus: Evidence for acquisition of a DNA B component by a monopartite Begomovirus. Virology 2002, 293, 63–74. [Google Scholar] [CrossRef]
- Shafiq, M.; Asad, S.; Zafar, Y.; Briddon, R.W.; Mansoor, S. Pepper leaf curl Lahore virus requires the DNA B component of Tomato leaf curl New Delhi virus to cause leaf curl symptoms. Virol. J. 2010, 7, 367. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pandey, P.K.; Banerjee, M.K.; Kalloo, G.; Fauquet, C.M. Tomato leaf curl Gujarat virus, a new begomovirus species causing a severe leaf curl disease of tomato in Varanasi, India. Phytopathology 2003, 93, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.J.; Carter, S.A.; Cole, A.B.; Cheng, N.H.; Nelson, R.S. A natural variant of a host RNA-dependent RNA polymerase is associated with increased susceptibility to viruses by Nicotiana benthamiana. Proc. Natl. Acad. Sci. USA 2004, 101, 6297–6302. [Google Scholar] [CrossRef]
- Hančinský, R.; Mihálik, D.; Mrkvová, M.; Candresse, T.; Glasa, M. Plant viruses infecting solanaceae family members in the cultivated and wild environments: A review. Plants 2020, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Bellido, A.; Hoyer, J.S.; Dubey, D.; Jeannot, R.B.; Duffy, S. Interspecies recombination has driven the macroevolution of cassava mosaic begomoviruses. J. Virol. 2021, 95, e00541-21. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.B. Defective viral genomes: Critical danger signals of viral infections. J. Virol. 2014, 88, 8720–8723. [Google Scholar] [CrossRef]
- Manzoni, T.B.; Lopez, C.B. Defective (interfering) viral genomes re-explored: Impact on antiviral immunity and virus persistence. Future Virol. 2018, 13, 493–503. [Google Scholar] [CrossRef]
- Catoni, M.; Noris, E.; Vaira, A.M.; Jonesman, T.; Matić, S.; Soleimani, R.; Behjatnia, S.A.A.; Vinals, N.; Paszkowski, J.; Accotto, G.P. Virus-mediated export of chromosomal DNA in plants. Nat. Commun. 2018, 9, 5308. [Google Scholar] [CrossRef]
- Patil, B.L.; Dutt, N.; Briddon, R.W.; Bull, S.E.; Rothenstein, D.; Borah, B.K.; Dasgupta, I.; Stanley, J.; Jeske, H. Deletion and recombination events between the DNA-A and DNA-B components of Indian cassava-infecting geminiviruses generate defective molecules in Nicotiana benthamiana. Virus Res. 2007, 124, 59–67. [Google Scholar] [CrossRef]
- Stanley, J.; Saunders, K.; Pinner, M.S.; Wong, S.M. Novel defective interfering DNAs associated with ageratum yellow vein geminivirus infection of Ageratum conyzoides. Virology 1997, 239, 87–96. [Google Scholar] [CrossRef]
- Hillman, B.I.; Carrington, J.C.; Morris, T.J. A defective interfering RNA that contains a mosaic of a plant virus genome. Cell 1987, 51, 427–433. [Google Scholar] [CrossRef]
- Stanley, J.; Frischmuth, T.; Ellwood, S. Defective viral DNA ameliorates symptoms of geminivirus infection in transgenic plants. Proc. Natl. Acad. Sci. USA 1990, 87, 6291–6295. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).