Morphological Diversity and Crop Mimicry Strategies of Weedy Rice Under the Transplanting Cultivation System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Cultivation and Experimental Design
2.3. Traits Investigation
2.3.1. Seedling Morphological Traits
2.3.2. Field Agronomic Traits
Growth-Related Traits
Yield Components
Grain Appearance
2.4. Seed Dormancy and Soil Seedbank Persistence
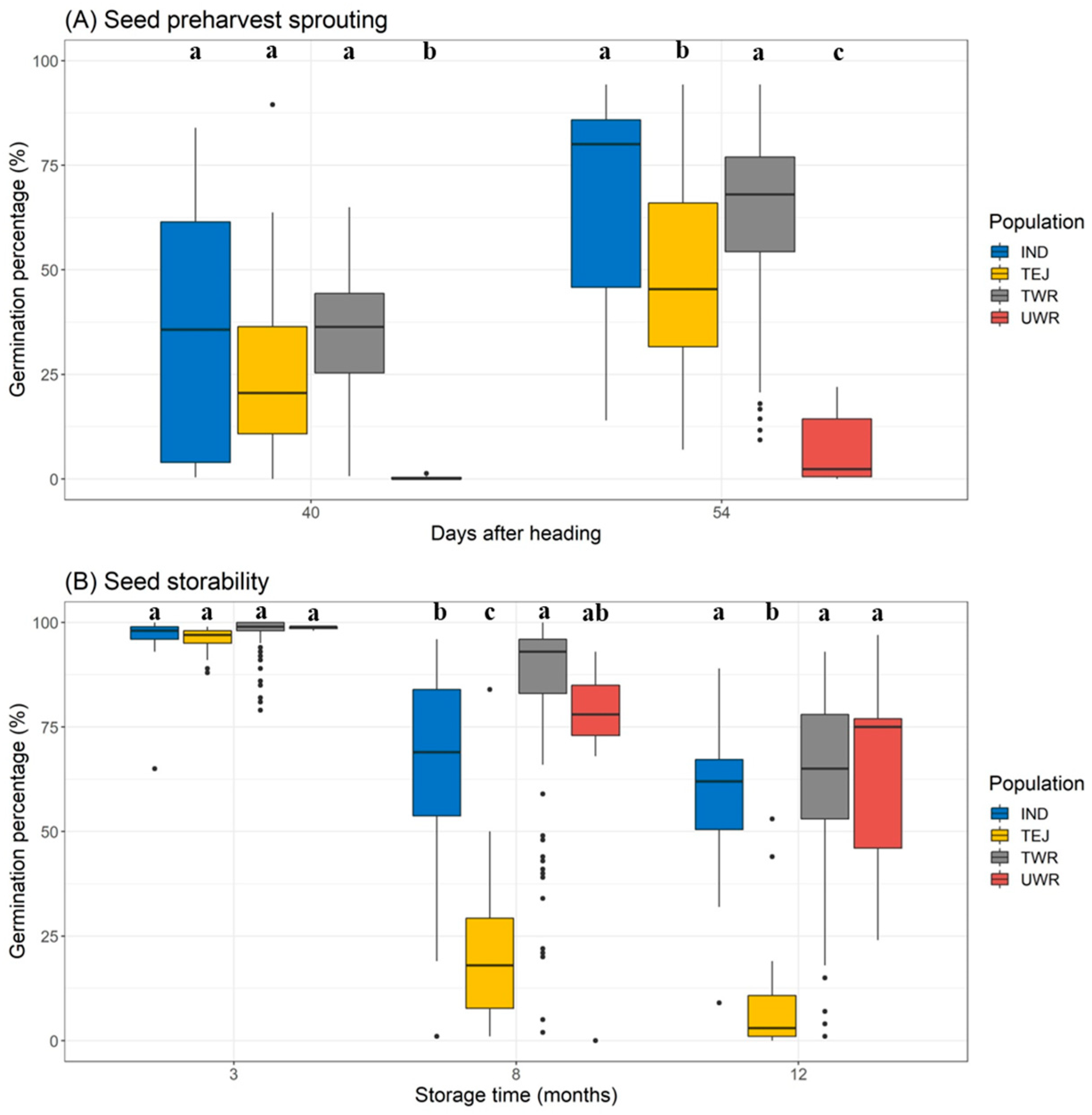
2.4.1. Preharvest Sprouting
2.4.2. Seed Storability
2.4.3. Soil Burial Experiments
2.5. Statistical Analysis
3. Results
3.1. Seedling Vigor and Vegetative Growth Characteristics
3.2. Reproductive Traits and Grain Morphology
3.3. Dormancy and Soil Seedbank Accumulation Potential
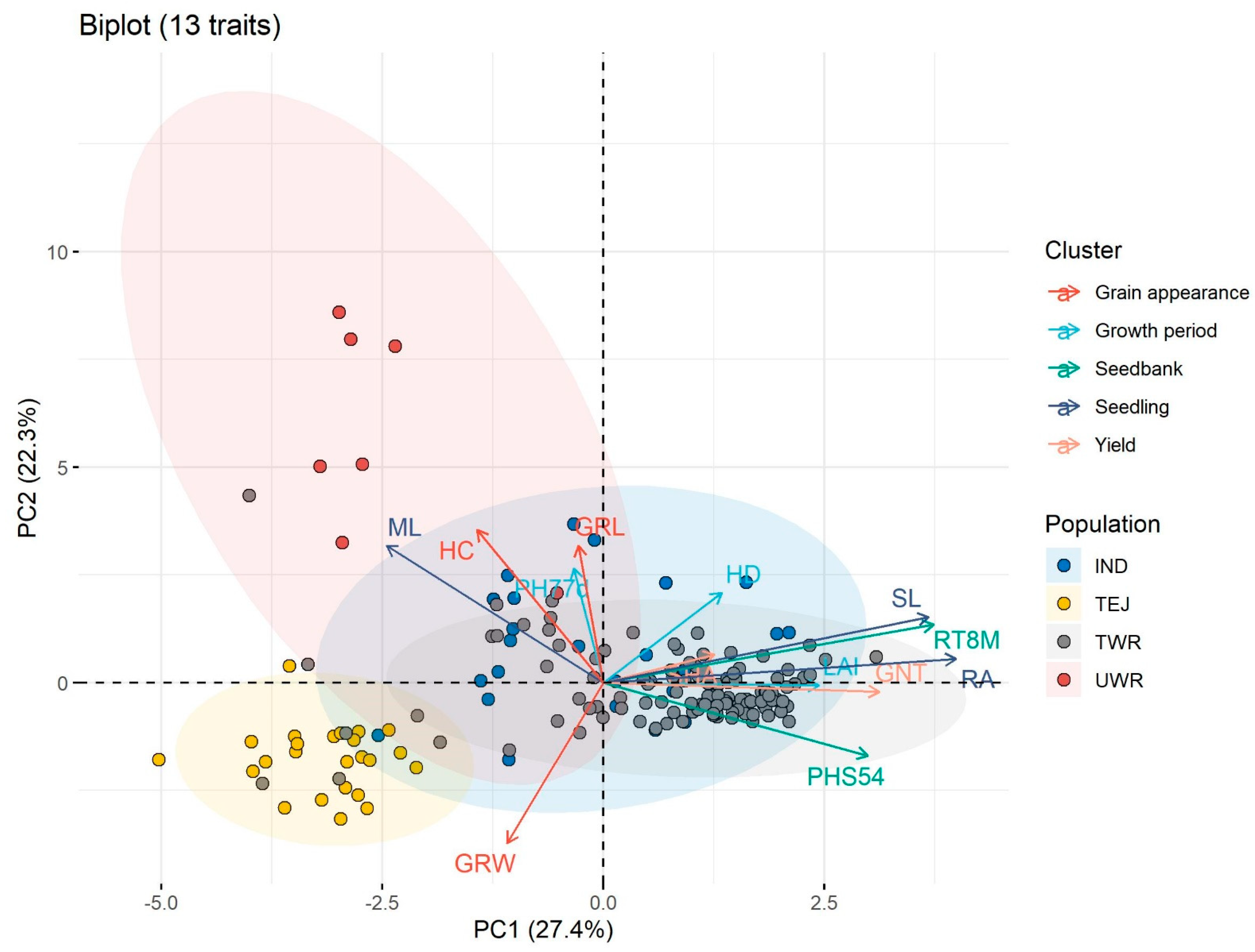
3.4. Morphological Variation and Competitive Traits
3.5. Crop Mimicry Features of Weedy Rice Between Taiwan and U.S.
4. Discussion
4.1. Competitive Advantage Traits Under the Transplant System
4.2. Crop Mimicry and Visual Camouflage of TWR
4.3. Population Dispersal Mechanisms of TWR
4.4. Seedbank Accumulation Dynamics in Subtropical Environments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FAO | Food and Agriculture Organization |
| FER | fertility rate |
| GL | grain length |
| GLW | ratio of grain length to grain width |
| GNT | number of total grains |
| GW | grain width |
| HC | hull color |
| HD | heading date |
| IND | indica cultivar |
| LAI | leaf area index |
| ML | mesocotyl length |
| PCA | principal component analysis |
| PH | plant height |
| PHS | preharvest sprouting rate |
| RA | root surface area |
| SC | pericarp color |
| SHA | seed shattering rate |
| SL | seedling shoot length |
| SN | spike number |
| TARI | Taiwan Agricultural Research Institute |
| TEJ | temperate japonica cultivar |
| TWR | Taiwan weedy rice |
| UWR | U.S. weedy rice |
References
- Wu, D.H.; Gealy, D.R.; Jia, M.H.; Edwards, J.D.; Lai, M.H.; McClung, A.M. Phylogenetic Origin and Dispersal Pattern of Taiwan Weedy Rice. Pest Manag. Sci. 2020, 76, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Qiu, J.; Sun, J.; Song, B.K.; Olsen, K.M.; Fan, L. Weedy Rice, A Hidden Gold Mine in The Paddy Field. Mol. Plant 2022, 15, 566–568. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Gangarani Devi, A.; Dutta, P.; Lamalakshmi, E.; Mohanty, S.; Choudary, A.K.; Das, A.; Sarika, K.; Kumar, S.; et al. Harnessing Weedy Rice as Functional Food and Source of Novel Traits for Crop Improvement. Plant Cell Environ. 2025, 48, 2498–2521. [Google Scholar] [CrossRef] [PubMed]
- Akasaka, M.; Ushiki, J.; Iwata, H.; Ishikawa, R.; Ishii, T. Genetic Relationships and Diversity of Weedy Rice (Oryza sativa L.) and Cultivated Rice Varieties in Okayama Prefecture, Japan. Breed. Sci. 2009, 59, 401–409. [Google Scholar] [CrossRef]
- Juliano, L.M.; Donayre, D.K.M.; Martin, E.C.; Beltran, J.C. Weedy rice: An Expanding Problem in Direct-Seeded Rice in the Philippines. Weed Biol. Manag. 2020, 20, 27–37. [Google Scholar] [CrossRef]
- Shivrain, V.K.; Burgos, N.R.; Gealy, D.R.; Sales, M.A.; Smith, K.L. Gene Flow from Weedy Red rice (Oryza sativa L.) to Cultivated Rice and Fitness of Hybrids. Pest Manag. Sci. Former. Pestic. Sci. 2009, 65, 1124–1129. [Google Scholar] [CrossRef]
- Durand-Morat, A.; Nalley, L.L. Economic Benefits of Controlling Red Rice: A Case Study of the United States. Agronomy 2019, 9, 422. [Google Scholar] [CrossRef]
- Wang, L.; Sun, X.; Peng, Y.; Chen, K.; Wu, S.; Guo, Y.; Zhang, J.; Yang, H.; Jin, T.; Wu, L.; et al. Genomic Insights into the Origin, Adaptive Evolution, And Herbicide Resistance of Leptochloa chinensis, a Devastating Tetraploid Weedy Grass in Rice Fields. Mol. Plant 2022, 15, 1045–1058. [Google Scholar] [CrossRef]
- Kraehmer, H.; Jabran, K.; Mennan, H.; Chauhan, B.S. Global Distribution of Rice Weeds–A Review. Crop Prot. 2016, 80, 73–86. [Google Scholar] [CrossRef]
- Busi, R.; Nguyen, N.K.; Chauhan, B.S.; Vidotto, F.; Tabacchi, M.; Powles, S.B. Can Herbicide Safeners Allow Selective Control of Weedy Rice Infesting Rice Crops? Pest Manag. Sci. 2017, 73, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Fogliatto, S.; Vidotto, F.; Ferrero, A. Morphological Characterisation of Italian Weedy Rice (Oryza sativa) Populations. Weed Res. 2012, 52, 60–69. [Google Scholar] [CrossRef]
- Thurber, C.S.; Reagon, M.; Olsen, K.M.; Jia, Y.; Caicedo, A.L. The Evolution of Flowering Strategies in US Weedy Rice. Am. J. Bot. 2014, 101, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Wongtamee, A.; Maneechote, C.; Pusadee, T.; Rerkasem, B.; Jamjod, S. The Dynamics of Spatial and Temporal Population Genetic Structure of Weedy Rice (Oryza sativa f. spontanea Baker). Genet. Resour. Crop Evol. 2017, 64, 23–39. [Google Scholar] [CrossRef]
- Hoyos, V.; Plaza, G.; Caicedo, A.L. Characterization of the Phenotypic Variability in Colombian Weedy Rice (Oryza spp.). Weed Sci. 2019, 67, 441–452. [Google Scholar] [CrossRef]
- Balbinot, A.; da Rosa Feijó, A.; Fipke, M.V.; Gehrke, V.R.; Agostinetto, D.; Kruse, N.D.; Ziska, L.H.; Camargo, E.R.; de Avila, L.A. Rising Atmospheric CO2 Concentration Affect Weedy Rice Growth, Seed Shattering and Seedbank Longevity. Weed Res. 2022, 62, 277–286. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, W.; Song, X.; Dai, W.; Dai, L.; Zhang, Z.; Qiang, S. Early Flowering and Rapid Grain Filling Determine Early Maturity and Escape from Harvesting in Weedy Rice. Pest Manag. Sci. 2018, 74, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Noldin, J.A.; Chandler, J.M.; McCauley, G.N. Seed Longevity of Red Rice Ecotypes Buried in Soil. Planta Daninha 2006, 24, 611–620. [Google Scholar] [CrossRef]
- Xia, H.B.; Xia, H.; Ellstrand, N.C.; Yang, C.; Lu, B.R. Rapid Evolutionary Divergence and Ecotypic Diversification of Germination Behavior in Weedy Rice Populations. New Phytol. 2011, 191, 1119–1127. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Wu, Y.C.; Wu, B.G.; Li, C.P.; Gealy, D.R.; Wu, D.H. Morphological Diversity Study on Taiwan Weedy Red Rice. J. Agric. Assoc. Taiwan 2017, 18, 161–188. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biologicalimage Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, J.W.; Chung, N.J.; Choi, K.S.; Ahn, S.N. Identification of Molecular Markers for Mesocotyl Elongation in Weedy Rice. Korean J. Breed. Sci. 2012, 44, 238–244. [Google Scholar]
- Galkovskyi, T.; Mileyko, Y.; Bucksch, A.; Moore, B.; Symonova, O.; Price, C.A.; Topp, C.N.; Iyer-Pascuzzi, A.S.; Zurek, P.R.; Fang, S.; et al. GiA Roots: Software for the High Throughput Analysis of Plant Root System Architecture. BMC Plant Biol. 2012, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- International Network for Genetic Evaluation of Rice. Standard Evaluation System for Rice; IRRI, International Rice Research Institute: Los Baños, Philippines, 1996. [Google Scholar]
- Danner, M.; Locherer, M.; Hank, T.; Richter, K.; EnMAP Consortium. Measuring Leaf Area Index (LAI) with the LI-Cor LAI 2200C or LAI-2200 (+2200Clear Kit)–Theory, Measurement, Problems, Interpretation; EnMAP Field Guide Technical Report; GFZ Data Services: Potsdam, Germany, 2015. [Google Scholar]
- Gu, X.Y.; Kianian, S.F.; Hareland, G.A.; Hoffer, B.L.; Foley, M.E. Genetic Analysis of Adaptive Syndromes Interrelated with Seed Dormancy in Weedy Rice (Oryza sativa). Theor. Appl. Genet. 2005, 110, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Whan, A.P.; Smith, A.B.; Cavanagh, C.R.; Ral, J.P.F.; Shaw, L.M.; Howitt, C.A.; Bischof, L. GrainScan: A Low Cost, Fast Method for Grain Size and Colour Measurements. Plant Methods 2014, 10, 23. [Google Scholar] [CrossRef]
- Yadav, S.; Parihar, S.S. Seed Germination and Viability Testing–Principles and Techniques. ICAR Spons. Short Course 2013, 205. [Google Scholar]
- Zhang, Z.; Gao, P.L.; Dai, W.M.; Song, X.L.; Hu, F.; Qiang, S. Effect of Tillage and Burial Depth and Density of Seed on Viability and Seedling Emergence of Weedy Rice. J. Integr. Agric. 2019, 18, 1914–1923. [Google Scholar] [CrossRef]
- Pipatpongpinyo, W.; Korkmaz, U.; Wu, H.; Kena, A.; Ye, H.; Feng, J.; Gu, X.Y. Assembling Seed Dormancy Genes into A System Identified Their Effects on Seedbank Longevity in Weedy Rice. Heredity 2020, 124, 135–145. [Google Scholar] [CrossRef]
- de Mendiburu, F. Agricolae Tutorial (Version 1.3-5); Universidad Nacional Agraria: La Molina, Peru, 2021. [Google Scholar]
- ggplot2: Elegant Graphics for Data Analysis. Available online: https://ggplot2.tidyverse.org (accessed on 20 March 2025).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. CRA Contrib. Packages 2016. [Google Scholar]
- Olajumoke, B.; Juraimi, A.S.; Uddin, M.K.; Husni, M.H.; Alam, M.A. Competitive Ability of Cultivated Rice against Weedy Rice Biotypes: A review. Chil. J. Agric. Res. 2016, 76, 243–252. [Google Scholar] [CrossRef]
- Nadir, S.; Xiong, H.B.; Zhu, Q.; Zhang, X.L.; Xu, H.Y.; Li, J.; Dongchen, W.; Henry, D.; Guo, X.Q.; Khan, S.; et al. Weedy Rice in Sustainable Rice Production. A Review. Agron. Sustain. Dev. 2017, 37, 46. [Google Scholar] [CrossRef]
- Ahmed, Q.N.; Hussain, P.Z.; Othman, A.S. Comparative Study on Vegetative and Reproductive Development between Weedy Rice Morphotypes and Commercial Rice Varieties in Perak, Malaysia. Trop. Life Sci. Res. 2012, 23, 17. [Google Scholar] [PubMed]
- Burgos, N.R.; Norman, R.J.; Gealy, D.R.; Black, H. Competitive N Uptake between Rice and Weedy Rice. Field Crops Res. 2006, 99, 96–105. [Google Scholar] [CrossRef]
- Atwell, B.J.; Wang, H.; Scafaro, A.P. Could Abiotic Stress Tolerance in Wild Relatives of Rice Be Used to Improve Oryza sativa? Plant Sci. 2014, 215, 48–58. [Google Scholar] [CrossRef]
- Delouche, J.C.; Labrada, R. Weedy Rices: Origin, Biology, Ecology and Control; Food & Agriculture Organization: Rome, Italy, 2007; Volume 188. [Google Scholar]
- Svizzero, S.; Ray, A.; Chakraborty, D. Awn Reduction and the Domestication of Asian Rice: A Syndrome or Crop Improvement Trait? Econ. Bot. 2019, 73, 477–488. [Google Scholar] [CrossRef]
- Wedger, M.J.; Pusadee, T.; Wongtamee, A.; Olsen, K.M. Discordant Patterns of Introgression Suggest Historical Gene Flow into Thai Weedy Rice from Domesticated and Wild Relatives. J. Hered. 2019, 110, 601–609. [Google Scholar] [CrossRef]
- Zhan, J.; Lu, X.; Liu, H.; Zhao, Q.; Ye, G. Mesocotyl Elongation, An Essential Trait for Dry-Seeded Rice (Oryza sativa L.): A Review of Physiological and Genetic Basis. Planta 2020, 251, 27. [Google Scholar] [CrossRef]
- Dai, L.; Dai, W.; Song, X.; Lu, B.; Qiang, S. A Comparative Study of Competitiveness between Different Genotypes of Weedy Rice (Oryza sativa) and Cultivated Rice. Pest Manag. Sci. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Wang, H.Q.; Dai, W.M.; Zhang, Z.X.; Li, M.S.; Meng, L.C.; Zhang, Z.; Huan, L.U.; Song, X.L.; Qiang, S. Occurrence Pattern and Morphological Polymorphism of Chinese Weedy Rice. J. Integr. Agric. 2023, 22, 149–169. [Google Scholar] [CrossRef]
- Andres, A.; Fogliatto, S.; Ferrero, A.; Vidotto, F. Growth Variability of Italian Weedy Rice Populations Grown with or Without Cultivated Rice. Crop Sci. 2015, 55, 394–402. [Google Scholar] [CrossRef]
- Dilipkumar, M.; Kumar, V.; Song, B.; Olsen, K.M.; Chuah, T.; Ahmed, S.; Qiang, S. Weedy Rice (Oryza spp.). In Biology and Management of Problematic Crop Weed Species; Academic Press: New York, NY, USA, 2021; pp. 285–309. [Google Scholar]
- Wu, D.; Xie, L.; Sun, Y.; Huang, Y.; Jia, L.; Dong, C.; Shen, E.; Ye, C.Y.; Qian, Q.; Fan, L. A Syntelog-Based Pan-Genome Provides Insights into Rice Domestication and De-Domestication. Genome Biol. 2023, 24, 179. [Google Scholar] [CrossRef] [PubMed]
- Ottis, B.V.; Smith, K.L.; Scott, R.C.; Talbert, R.E. Rice Yield and Quality as Affected by Cultivar and Red Rice (Oryza sativa) density. Weed Sci. 2005, 53, 499–504. [Google Scholar] [CrossRef]
- Rao, A.N.; Brainard, D.C.; Kumar, V.; Ladha, J.K.; Johnson, D.E. Preventive Weed Management in Direct-Seeded Rice: Targeting the Weed Seedbank. Adv. Agron. 2017, 144, 45–142. [Google Scholar]
- Tseng, T.M.; Burgos, N.R.; Shivrain, V.K.; Alcober, E.A.; Mauromoustakos, A. Inter-and Intrapopulation Variation in Dormancy of Oryza sativa (Weedy Red Rice) and Allelic Variation in Dormancy-Linked Loci. Weed Res. 2013, 53, 440–451. [Google Scholar] [CrossRef]
- Ziska, L.H.; Gealy, D.R.; Burgos, N.; Caicedo, A.L.; Gressel, J.; Lawton-Rauh, A.L.; Avila, L.A.; Theisen, G.; Norsworthy, J.; Ferrero, A.; et al. Weedy (Red) Rice: An Emerging Constraint to Global Rice Production. Adv. Agron. 2015, 129, 181–228. [Google Scholar]
- Vidotto, F.; Ferrero, A. Germination Behaviour of Red Rice (Oryza sativa L.) Seeds in Field and Laboratory Conditions. Agronomie 2000, 20, 375–382. [Google Scholar] [CrossRef]
- Batlla, D.; Benech-Arnold, R.L. Predicting Changes in Dormancy Level in Natural Seed Soil Banks. Plant Mol. Biol. 2010, 73, 3–13. [Google Scholar] [CrossRef]
- Nunes, A.L.; Delatorre, C.A.; Merotto Jr, A. Gene Expression Related to Seed Shattering and the Cell Wall in Cultivated and Weedy Rice. Plant Biol. 2014, 16, 888–896. [Google Scholar] [CrossRef]
- Huang, Y.F.; Wu, D.H.; Wang, C.L.; Du, P.R.; Cheng, C.Y.; Cheng, C.C. Survey of rice production practices and perception of weedy red rice (Oryza sativa f. spontanea) in Taiwan. Weed Sci. 2021, 69, 526–535. [Google Scholar] [CrossRef]
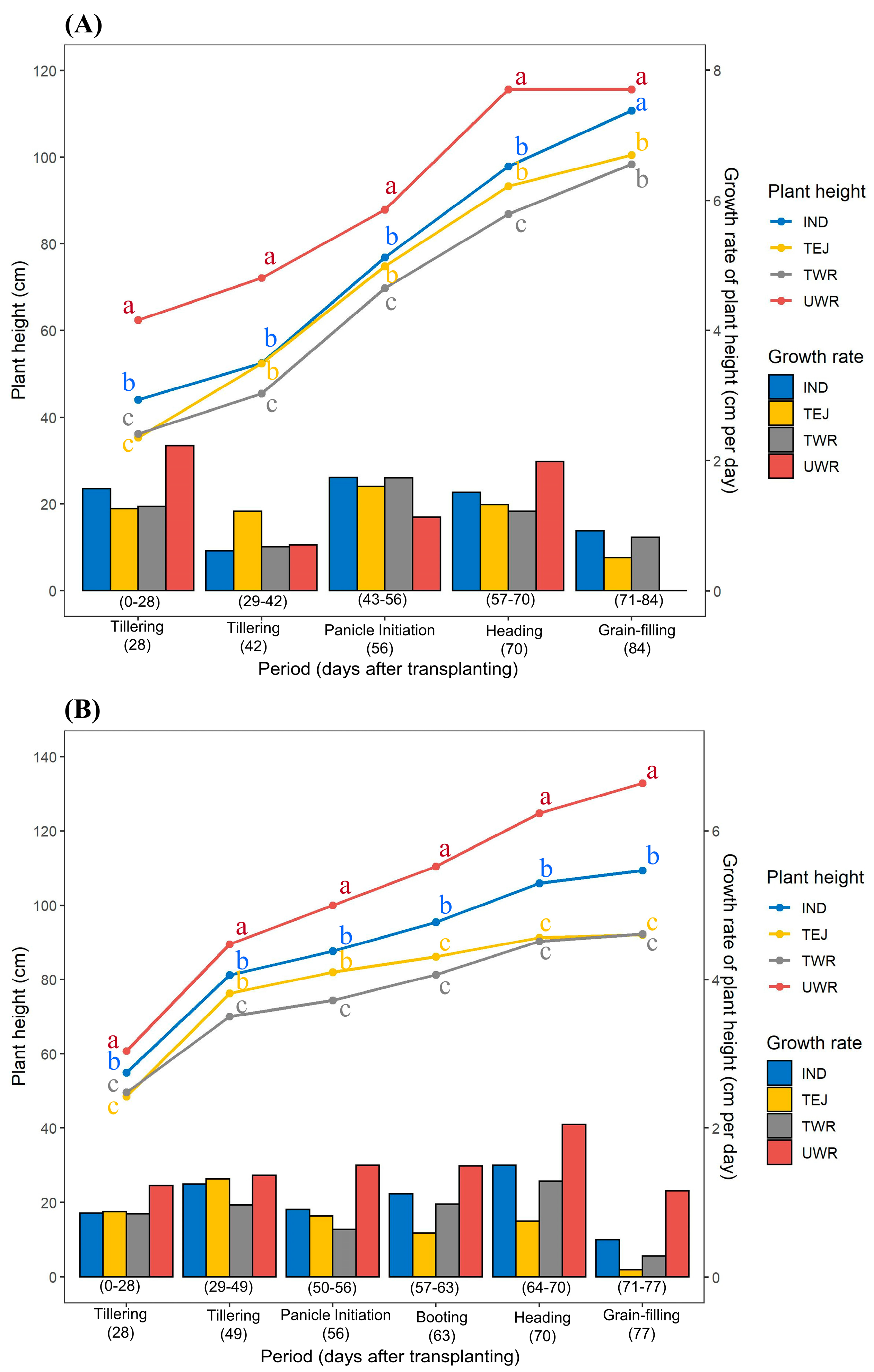
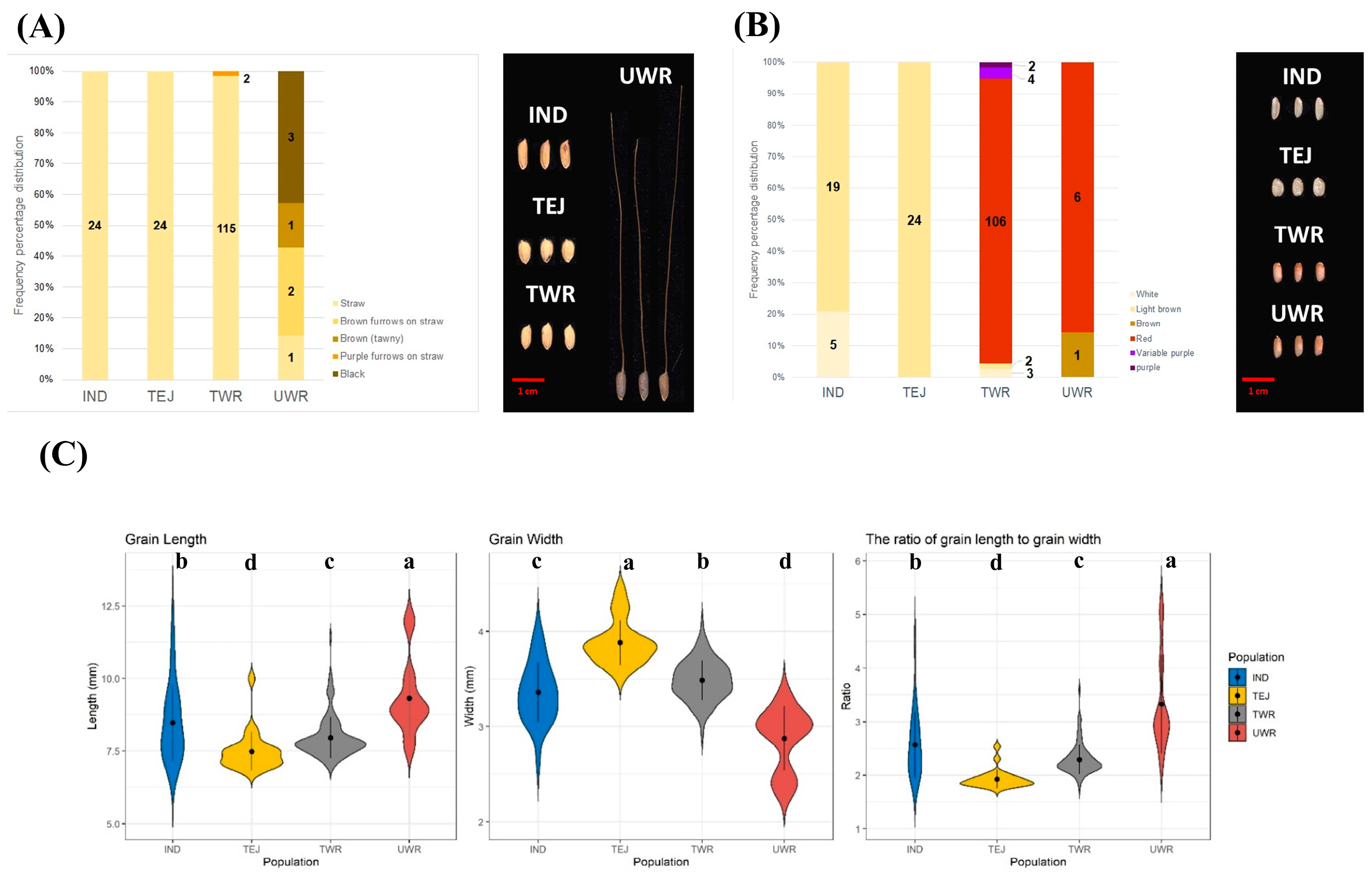
| Pop † | Num. | Seedling Stage | Vegetative Stage | Reproductive Stage | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SL ‡ (mm) | RA (mm2) | ML (mm) | LAI (m2m−2) | HD (Days) | |||||||||||
| 1st Season | 2nd Season | 1st Season | 2nd Season | ||||||||||||
| IND | 24 | 49.5 ± 14.5 | a § | 110.4 ± 20.6 | ab | 5.8 ± 5 | b | 1.8 ± 0.5 | b | 1.9 ± 0.7 | a | 74.9 ± 6.6 | a | 64.5 ± 7.2 | a |
| TEJ | 24 | 27.3 ± 8.2 | b | 68.8 ± 17.6 | c | 4.4 ± 1.9 | b | 1.3 ± 0.4 | c | 1.4 ± 0.6 | b | 66.0 ± 6.5 | b | 56.7 ± 10.1 | b |
| TWR | 117 | 51.1 ± 10.7 | a | 115.9 ± 21.7 | a | 2.4 ± 2.4 | c | 2.0 ± 0.5 | a | 2.1 ± 0.7 | a | 74.0 ± 3.3 | a | 64.7 ± 3.7 | a |
| UWR | 7 | 54.1 ± 9.9 | a | 93.9 ± 26.1 | b | 15.1 ± 4.9 | a | 0.6 ± 0.3 | d | 1.3 ± 0.7 | b | 65.3 ± 5.9 | b | 66.7 ± 7.7 | a |
| Pop † | Num. | SN ‡ | GNT | Fertility (%) | Seed Shattering Rate (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | 1st Season | 2nd Season | ||||||||||
| IND | 24 | 18.8 ± 7.0 | a § | 16.1 ± 3.7 | c | 8029.9 ± 2821.8 | a | 6349.6 ± 1221.2 | b | 82 ± 15 | ab | 80 ± 11 | a | 1.3 ± 0.8 | b | 8.2 ± 6.6 | a |
| TEJ | 24 | 14.1 ± 3.3 | b | 12.1 ± 1.7 | d | 6334.9 ± 2503.1 | b | 4436.2 ± 1063 | c | 85 ± 11 | a | 86 ± 9 | a | 0.5 ± 0.8 | b | 2.8 ± 4.1 | b |
| TWR | 117 | 18.8 ± 4.6 | a | 19.5 ± 5.3 | b | 9013.4 ± 2394.3 | a | 7917.0 ± 2090.1 | a | 86 ± 9 | a | 69 ± 11 | b | 3.0 ± 2.4 | a | 11.9 ± 9.9 | a |
| UWR | 7 | 15.8 ± NA ¶ | ab | 24.3 ± 5.3 | a | 7643.0 ± NA ¶ | 4893.1 ± 2211.8 | bc | 74 ± 16 | b | 79 ± 5 | a | 3.9 ± 3.1 | a | 14.4 ± 9.7 | a | |
| Trial | Depth (cm) | Pop † | Num. | During Burial Trail | Intact Seed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decayed Seed (%) | Germinated Seed (%) | Germinated Seed (%) | Non-Viable Seed (%) | Viable Seed (%) | |||||||||
| Paddy trial 1 | 5 | IND | 24 | 98.99 ± 1.82 | a ‡ | NA | 0.00 ± 0.00 | 0.98 ± 1.8 | b | 0.03 ± 0.15 | b | ||
| TEJ | 24 | 98.71 ± 2.91 | a | NA | 0.00 ± 0.00 | 1.08 ± 2.17 | b | 0.22 ± 0.84 | ab | ||||
| TWR | 117 | 97.98 ± 5.06 | a | NA | 0.00 ± 0.00 | 1.69 ± 4.18 | b | 0.33 ± 1.6 | ab | ||||
| UWR | 7 | 90.94 ± 5.80 | b | NA | 0.00 ± 0.00 | 7.82 ± 4.95 | a | 1.24 ± 1.77 | a | ||||
| 10 | IND | 24 | 96.51 ± 7.13 | a | NA | 0.00 ± 0.00 | 1.74 ± 2.96 | b | 1.75 ± 4.48 | b | |||
| TEJ | 24 | 96.87 ± 11.03 | a | NA | 0.00 ± 0.00 | 0.67 ± 1.37 | b | 2.46 ± 10.04 | b | ||||
| TWR | 117 | 95.49 ± 7.27 | a | NA | 0.00 ± 0.00 | 2.43 ± 4.42 | b | 2.08 ± 4.25 | b | ||||
| UWR | 7 | 29.75 ± 27.46 | b | NA | 0.00 ± 0.00 | 21.48 ± 11.72 | a | 48.77 ± 25.38 | a | ||||
| Paddy trial 2 | 5 | IND | 24 | 97.22 ± 3.78 | a | 0.52 ± 2.34 | b | 0.00 ± 0.00 | 2.24 ± 2.98 | ab | 0.02 ± 0.1 | a | |
| TEJ | 24 | 96.16 ± 5.19 | a | 0.00 ± 0.00 | b | 0.00 ± 0.00 | 3.84 ± 5.19 | b | 0.00 ± 0.00 | a | |||
| TWR | 117 | 97.16 ± 4.23 | a | 0.86 ± 2.06 | b | 0.00 ± 0.00 | 1.96 ± 3.72 | ab | 0.03 ± 0.17 | a | |||
| UWR | 7 | 90.59 ± 8.03 | b | 5.41 ± 6.9 | a | 0.00 ± 0.00 | 4.00 ± 6.32 | a | 0.00 ± 0.00 | a | |||
| 10 | IND | 24 | 98.55 ± 1.46 | a | 1.2 ± 1.28 | ab | 0.00 ± 0.00 | b | 0.23 ± 1.02 | b | 0.02 ± 0.11 | b | |
| TEJ | 24 | 99.13 ± 1.00 | a | 0.85 ± 1.01 | b | 0.00 ± 0.00 | b | 0.02 ± 0.1 | b | 0.00 ± 0.00 | b | ||
| TWR | 117 | 97.79 ± 3.67 | a | 1.88 ± 3.63 | ab | 0.00 ± 0.00 | b | 0.31 ± 0.78 | b | 0.02 ± 0.12 | b | ||
| UWR | 7 | 60.14 ± 28.57 | b | 3.73 ± 4.97 | a | 0.35 ± 0.93 | a | 34.03 ± 26.87 | a | 1.74 ± 3.63 | a | ||
| Upland trial | 5 | IND | 24 | 13.05 ± 9.78 | b | 85.87 ± 12.77 | a | 0.52 ± 1.71 | ab | 0.54 ± 1.67 | a | 0.02 ± 0.1 | a |
| TEJ | 24 | 22.84 ± 14.41 | a | 75.47 ± 17.19 | b | 1.05 ± 2.36 | a | 0.56 ± 1.02 | a | 0.08 ± 0.4 | a | ||
| TWR | 117 | 8.87 ± 8.92 | b | 90.69 ± 9.76 | a | 0.14 ± 0.88 | b | 0.27 ± 0.83 | a | 0.02 ± 0.15 | a | ||
| UWR | 7 | 10.26 ± 4.91 | b | 89.60 ± 4.89 | a | 0.00 ± 0.00 | b | 0.14 ± 0.24 | a | 0.00 ± 0.00 | a | ||
| 10 | IND | 24 | 24.51 ± 12.87 | a | 74.00 ± 14.87 | b | 1.06 ± 2.22 | b | 0.37 ± 1.49 | a | 0.06 ± 0.31 | a | |
| TEJ | 24 | 26.86 ± 17.86 | a | 69.66 ± 21.27 | b | 3.21 ± 5.53 | a | 0.21 ± 0.65 | ab | 0.06 ± 0.3 | a | ||
| TWR | 117 | 14.87 ± 10.54 | b | 84.68 ± 10.77 | a | 0.35 ± 1.24 | b | 0.03 ± 0.16 | b | 0.07 ± 0.57 | a | ||
| UWR | 7 | 26.44 ± 12.96 | a | 73.06 ± 13.19 | b | 0.21 ± 0.39 | b | 0.29 ± 0.4 | ab | 0.00 ± 0.00 | a | ||
| Trait § | Taiwan Weedy Rice † | U.S. Weedy Rice ‡ | ||||
|---|---|---|---|---|---|---|
| Competitive Ability | Crop Mimicry | Seedbank Density | Competitive Ability | Crop Mimicry | Seedbank Density | |
| Seedling vigor (SL) | Positive | Same | ||||
| Seedling vigor (RA) | Positive | Same | ||||
| Seedling vigor (ML) | Negative | Positive | ||||
| Growth (HD) | Positive (pericarp color) | Negative | Positive | Positive (early shattering) | ||
| Growth (LAI) | Positive | Negative | ||||
| Growth (PH in 77d) | Same | Positive (dwarf) | Positive (tall) | Negative | ||
| Yield (SN) | Positive | Same | ||||
| Yield (GNT) | Positive | Positive | Same | |||
| Yield (SHA) | Positive | Positive | ||||
| Grain (GL) | Positive (similar) | Positive (similar) | ||||
| Grain (GW) | Positive (similar) | Positive (similar) | ||||
| Grain (HC) | Positive | Negative | ||||
| Dormancy (PHS54) | Negative | Positive | ||||
| Longevity (RT8M) | Positive | Same | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-T.; Wang, Y.-C.; Du, P.-R.; Li, C.-P.; Wu, D.-H. Morphological Diversity and Crop Mimicry Strategies of Weedy Rice Under the Transplanting Cultivation System. Agronomy 2025, 15, 984. https://doi.org/10.3390/agronomy15040984
Hsu Y-T, Wang Y-C, Du P-R, Li C-P, Wu D-H. Morphological Diversity and Crop Mimicry Strategies of Weedy Rice Under the Transplanting Cultivation System. Agronomy. 2025; 15(4):984. https://doi.org/10.3390/agronomy15040984
Chicago/Turabian StyleHsu, Yi-Ting, Yuan-Chun Wang, Pei-Rong Du, Charng-Pei Li, and Dong-Hong Wu. 2025. "Morphological Diversity and Crop Mimicry Strategies of Weedy Rice Under the Transplanting Cultivation System" Agronomy 15, no. 4: 984. https://doi.org/10.3390/agronomy15040984
APA StyleHsu, Y.-T., Wang, Y.-C., Du, P.-R., Li, C.-P., & Wu, D.-H. (2025). Morphological Diversity and Crop Mimicry Strategies of Weedy Rice Under the Transplanting Cultivation System. Agronomy, 15(4), 984. https://doi.org/10.3390/agronomy15040984







