Abstract
Plant small peptides are critical regulators of various biological processes, including development and stress responses. Polypeptides within the DUF3774 family, known as wound-induced polypeptides (WIPs), have been identified as key players in pattern-triggered immunity (PTI) and defense mechanisms in Arabidopsis. In this study, the genome-wide identification of WIP genes in Glycine max was performed, followed by gene structure correction and validation using second-generation and full-length RNA sequencing data. A total of 31 GmWIP genes were identified and validated, mapped to chromosomes Gm06, Gm12, Gm13, and Gm06_scaffold_301. Phylogenetic analysis grouped these genes into five distinct clusters, with tandem duplication emerging as the primary mechanism for their expansion in the soybean genome. qRT-PCR analysis revealed dynamic and significant changes in GmWIP expression during soybean cyst nematode (SCN) infection in a susceptible soybean cultivar. Remarkably, 90% of the GmWIP genes were downregulated at the early stage of SCN infection (1 dpi), and further corroborated by the pGmWIPs::GUS reporter system. These findings suggest that GmWIP genes may act as regulators in the defense responses of susceptible soybean cultivars, providing a foundation for future functional studies.
1. Introduction
Polypeptides, small molecules typically composed of fewer than 100 amino acids, are formed by linking amino acids via peptide bonds [1]. Over the past few decades, numerous small polypeptide gene families have been identified in plants, including those encoding CLAVATA3 (CLV3), phytosulfokine (PSK), rapid alkalinization factor (RALF), defensin, systemin, and C-terminally encoded peptides (CEPs) [2,3,4,5,6,7]. These polypeptides play vital roles in plant development and responses to biotic and abiotic stresses.
Research has shown that small peptides contribute to critical processes such as root, floral organ, and egg cell development in Arabidopsis. For instance, the activity of the root apical meristem (RAM) is regulated by a feedback loop involving CLE40, a CLV3-related protein, and WUSCHEL-related homeobox 5 (WOX5), which maintains RAM stability [8]. Similarly, the signaling module comprising inflorescence deficient in abscission (IDA) polypeptides and the receptor-like kinases HAESA (HAE) and HAESA-LIKE2 (HSL2) controls floral organ abscission and regulates lateral root (LR) emergence [9,10,11]. Additionally, the cysteine-rich polypeptide egg cell 1 (EC1), expressed in egg cells, primes sperm cell activation during double fertilization [12].
Plants are constantly exposed to environmental and biotic stresses, and small peptides play a role in these processes. Small peptides like CLE25 and CLE9 have been identified as key regulators of drought responses. CLE25 acts as a mobile peptide linking water deficits to abscisic acid (ABA)-mediated dehydration stress tolerance [13], while CLE9 enhances drought tolerance by modulating stomatal closure [14]. In contrast, CAPE1 negatively impacts salt stress tolerance in Arabidopsis [15]. Systemin, a peptide hormone, is crucial for coordinating local and systemic immune responses against insect herbivores [16,17]. Disrupting CLE signaling has been shown to enhance resistance to root-knot nematodes (RKN), and suppressing CLE receptors in transgenic soybean plants improves resistance to soybean cyst nematodes (SCNs, Heterodera glycines) [18,19].
Several wound-induced peptide (WIP) genes also play pivotal roles in plant signaling and immunity. In tomato (Solanum lycopersicum), numerous peptides induced by wounding and MeJA were identified, including CAPE1, which triggered strong antipathogen and minor antiherbivore responses [20]. Recently, regeneration factor1 (REF1) was found to regulate local defense and regeneration mechanisms after wounding [21]. In soybean (Glycine max), WIPs are upregulated in nodules [22]. The overexpression of soybean wound-induced polypeptide genes in Arabidopsis enhances resistance to Pseudomonas syringae pv, and tomato DC3000 promotes pattern-triggered immunity (PTI) and inhibits growth [23].
Soybean is a valuable crop, but SCN infestation causes significant losses, estimated at billions of US dollars annually globally. Recent studies have reported that SCNs occur not only in major soybean-growing regions but also in minor regions across China [24]. Identifying major resistance genes (R-genes) that recognize pathogen effectors is crucial for combatting SCN infection. The DUF3774 family, also known as the wound-induced polypeptide (WIP) family, plays a key role in the signaling pathways involved in plant development and defense against biotic stresses [22,25]. In our previous study, members of the DUF3774 family, identified as wound-induced polypeptides, were shown to respond to SCN infection, potentially playing a role in soybean defense [26].
To explore these polypeptides further, a comprehensive genome-wide identification of soybean WIPs was conducted. Their gene structure was corrected using RNA-seq data, and their expression patterns were analyzed under SCN infection. These findings provide valuable insights and resources for future research on the functional roles of soybean wound-induced polypeptides, particularly in the soybean–SCN interaction.
2. Materials and Methods
2.1. Genome-Wide Identification of GmWIP Genes
The genome, protein sequences, and gff3 annotation files for Glycine max (Wm82.a4.v1), Arabidopsis thaliana (TAIR10), and Glycine soja (Gsoja_509_v1.0) were retrieved from the Phytozome database. Initially, a local protein database was constructed using the soybean protein sequences. The hidden Markov model (HMM) profile of PF12609 was then applied to screen the local database using the BLASTP program with an E-value threshold of <1 × 10−20. Additionally, amino acid sequences of AtWIP proteins were used as queries for local BLASTP searches against the G. max protein database with an E-value threshold of <1 × 10−10. The results from both approaches were merged, and duplicates were removed to obtain a final set of GmWIP candidates.
2.2. Gene Structure Validation and Visualization
To validate the structures of potential GmWIP genes, second-generation RNA-seq data (SRR26596349 and SRR26596344 for roots; SRR19849611 and SRR19849612 from leaves) and full-length RNA-seq data (SRR17876321 and SRR17876325 from roots) were obtained from the NCBI SRA database [27,28,29]. The GmWIP gene structure was checked and corrected using GSAman software v0.84 and the TBtools Target Genome Region Mapping plugin. After checking the gene structure with the RNA-seq data, a corrected gff3 file was created and used to visualize with TBtools [30].
2.3. Conserved Domain and Motif Identification
The sequences were analyzed in the Conserved Domain Database (CDD) of NCBI (https://www.ncbi.nlm.nih.gov/cdd/, accessed on 23 March 2024) to confirm the presence of the DUF3774 domain (PF12609). Conserved motifs were identified using the MEME Suite (https://meme-suite.org/meme/tools/meme, accessed on 23 March 2024), with the maximum number of motifs set to three.
2.4. Phylogenetic Analysis, Physicochemical Properties, Structural Prediction, and Subcellular Localization
The amino acid sequences of the GmWIPs and AtWIPs were aligned using ClustalW, and a phylogenetic tree was constructed via the neighbor-joining (NJ) method in MEGA11, with bootstrap analysis performed using 1000 replicates. Phytochemical properties, including amino acid count, molecular weight, and theoretical isoelectric points, were calculated with the ProtParam tool (https://web.expasy.org/protparam/, accessed on 23 March 2024). Structural predictions for AtWIPs and GmWIPs were performed using Alphafold 2, and the models were retrieved from the Uniprot database (https://www.uniprot.org/, accessed on 25 March 2024). The Plant-mPLoc tool (http://www.csbio.sjtu.edu.cn/bioinf/Cell-PLoc-2, accessed on 25 March 2024) was used to predict the subcellular localization of GmWIPs.
2.5. Chromosomal Distribution, Duplication, and Collinearity Analysis
The GmWIP genes were mapped onto soybean chromosomes using corrected gene structure information. Gene duplication and collinearity analyses within G. max were conducted using MCScanX with an E-value threshold of <1 × 10−3 and a maximum of 10 BLAST (v2.16)hits. Homology analysis between G. max and G. soja was performed using the Dual Synteny Plotter tool to identify conserved syntenic regions.
2.6. Cis-Element Analysis of GmWIP Promoters
To predict cis-acting elements, the 2000 bp upstream promoter regions of the GmWIP genes were extracted. Promoter analysis was performed using the PlantCARE tool (http://plantpan.itps.ncku.edu.tw/index.html, accessed on 25 March 2024), and the results were organized and visualized with TBtools.
2.7. Soybean Cultivation and Nematode Inoculation
The soybean cultivar Williams 82 was used for all experiments. Seeds were surface sterilized in 1% NaClO for 10 min, followed by thorough rinsing with sterile water. The sterilized seeds were germinated in Hoagland nutrient solution-moistened vermiculite at 25 °C. After 10 days, the seedlings were transferred to a PVC tube containing a sand-to-soil mixture (1:1). The soybean cyst nematode (SCN, Heterodera glycines) race 3 (HG 0) was propagated on susceptible soybean (Liaodou 15) in a greenhouse at Shenyang Agricultural University for two months. Cysts were extracted using a 60-mesh sieve (250 μm) and crushed on an 80-mesh sieve (180 μm), and eggs were collected on a 500-mesh sieve (25 μm). The eggs were purified with 35% sucrose, sterilized in 0.1% NaClO for 10 min, rinsed, and incubated in a modified Baermann pan with 3 mM ZnSO4 at 25 °C in the dark for 5 days. Freshly hatched second-stage juveniles (J2s) were suspended in a 0.2% agar solution at 2000 J2/mL, and each soybean root was inoculated with 2000 J2s.
2.8. RNA Extraction, cDNA Synthesis, and qRT-PCR Analysis
SCN-infected and non-infected soybean roots were collected at 1, 5, and 10 days post-inoculation (dpi) for RNA extraction and cDNA synthesis. Total RNA was extracted using the Ultrapure RNA Kit (CWbiotech, Beijing, China). The RNA concentration was assayed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). cDNA was synthesized from 1 μg of total RNA using the SYBR PrimeScript RT Master Mix kit (Takara, Dalian, China). Quantitative real-time PCR (qRT-PCR) was conducted on a CFX Connect Real-Time Quantitative PCR system (Bio-Rad, Hercules, CA, USA) with the following program: 95 °C for 2 min, followed by 40 cycles at 95 °C for 5 s and at 60 °C for 30 s; GmUBI-3 (GenBank accession D28123.1) was used as the internal reference gene, and data quantification was performed using the 2−∆∆Ct method. Each qRT-PCR experiment included three technical and three biological replicates. Log2-transformed GmWIP expression values were used to generate a heatmap.
2.9. Vector Construction, Transgenic Hairy Root Induction, and GUS Histochemical Staining
Soybean gDNA was extracted from soybean roots using a plant genomic DNA kit (CWbiotech, Beijing, China). DNA quality was assayed using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, USA). The promotors of GmWIP1, GmWIP11, and GmWIP25 were cloned and inserted into the pNINC2GUS to construct the pGmWIP::GUS reporter system. Recombinant vectors were introduced into Agrobacterium rhizogenes K599 to generate transgenic soybean hairy roots, as described by Qi et al. [31]. The hairy roots were inoculated with 500 second-stage SCN juveniles and harvested at 1, 5, and 10 dpi for GUS activity analysis using a ready-to-use GUS staining solution (Phygene, Fuzhou, China). The nematodes were stained with acid fuchsin, and images were captured using an Olympus DP80 microscope (Olympus, Tokyo, Japan).
3. Results
3.1. Identification of Wound-Induced Polypeptide Genes in Soybean
In this study, soybean WIP genes (GmWIPs) were identified using two distinct genome-wide scanning approaches. First, the HMM profile of PF12609 was applied to search soybean protein sequences, identifying 38 potential GmWIP genes (gene IDs are provided in Supplementary Material S1). Next, the amino acid sequences of AtWIP1, AtWIP2, AtWIP3, AtWIP4, and AtWIP5 were used to perform a BLAST (v2.16) search against soybean protein sequences, identifying 25 GmWIP genes. After removing redundant entries, 36 unique GmWIP genes were obtained.
To validate the gene structure and annotations of these GmWIPs, data from four second-generation RNA-seq datasets (SRR26596349 and SRR26596344 from roots, SRR19849611 and SRR19849612 from leaves) and full-length RNA-seq datasets (SRR17876321 and SRR17876325 from roots) were utilized. Using GSAman software (v0.8.4) and the Target Genome Region Mapping plugin in TBtools (v2.210), five genes (Glyma.03G212500.1, Glyma.04G147900.1, Glyma.12G107300.1, Glyma.15G276600.1, and Glyma.18G202300.1) with incorrect annotations were identified. These five genes were excluded from further analysis. Ultimately, 31 candidate GmWIP genes were identified and renamed GmWIP1 to GmWIP31 (Table 1 and Supplementary Material S1).

Table 1.
Physicochemical properties of GmWIP proteins.
The physicochemical properties of the GmWIPs were calculated using ProtParam. The amino acid lengths of the GmWIPs ranged from 69 to 106, with molecular weights between 7778.84 and 11,629.23 Da and theoretical pI values ranging from 7.78 to 10.44. Except for GmWIP4, GmWIP10, GmWIP19, and GmWIP29, the remaining GmWIPs were predicted to localize in the chloroplast (Table 1).
Using GmWIP amino acid sequences to query the CDD, all 31 GmWIP genes were confirmed to belong to the DUF3774 family (Figure S1). Chromosomal mapping of the GmWIP genes was performed using corrected annotations in the gff3 file. As shown in Figure 1, nine GmWIP genes (GmWIP1−GmWIP9) were located on chromosome Gm06, nine (GmWIP10−GmWIP18) on Gm12, 11 (GmWIP19−GmWIP29) on Gm13, and two (GmWIP30−GmWIP31) on Gm06_scaffold_301.
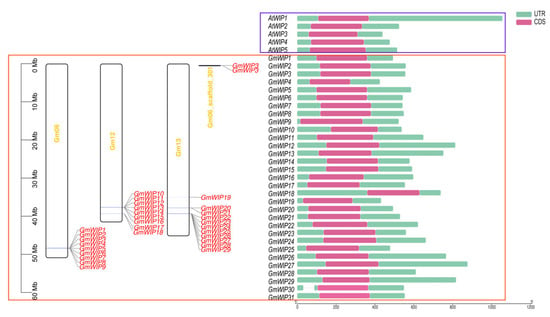
Figure 1.
Chromosomal distribution and gene structure of the GmWIP genes. The GmWIP genes are located on chromosomes Gm06, Gm12, Gm13, and Gm06_scaffold301. The gene structures of the five AtWIP genes and 31 GmWIP genes are represented within blue and red boxes, respectively. Non-coding regions (UTRs) are indicated by green boxes, while coding sequences are shown in purple boxes. The x-axis represents the length of the GmWIP genes from 5′ to 3′.
To examine the structural components of the GmWIP genes, their exon−intron organization was analyzed using the corrected gff3 file. Except for GmWIP30, which contained one intron, all the GmWIP and AtWIP genes were found to contain one 5′ UTR, one 3′ UTR, and one exon (Figure 1).
3.2. Conserved Motifs, Classification, and Phylogenetic Analysis of GmWIPs
To explore the conserved regions within the GmWIP proteins, motif analysis was performed using the MEME tool, identifying three unique conserved motifs (designated as Motifs 1–3; Figure 2). The lengths of these conserved motifs ranged from 21 to 27 amino acids. For example, Motif 1, comprising 21 amino acid residues, was present across all AtWIPs and GmWIPs (Figure 2), while AtWIP1, AtWIP2, GmWIP4, GmWIP10, GmWIP16, GmWIP22, and GmWIP29 did not contain Motifs 2 and 3 (Figure 2).
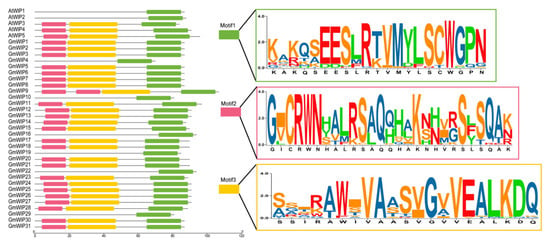
Figure 2.
Conserved motif prediction of wound-induced polypeptides. Three distinct conserved motifs were identified using the MEME tool, represented by colored boxes. Different colored letters indicate various amino acid residues, and the size of the letters corresponds to the frequency of amino acid occurrence. The x-axis shows the length of the wound-induced polypeptides (5′ to 3′).
For protein classification, a phylogenetic tree was constructed using the NJ method, with 1000 bootstrap replicates based on the amino acid sequences of the 31 identified GmWIP and five AtWIP proteins. The phylogenetic tree grouped the GmWIP and AtWIP proteins into five distinct clades (groups I–V) according to the tree’s topology (Figure 3).
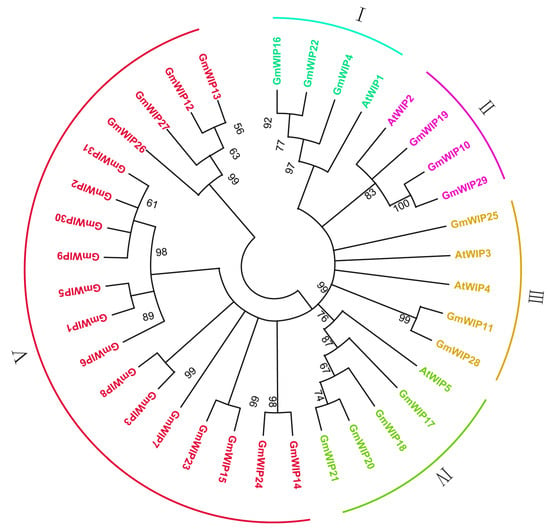
Figure 3.
Phylogenetic tree of wound-induced polypeptides from Arabidopsis thaliana and Glycine max. The phylogenetic tree was constructed using the amino acid sequences of five AtWIPs and 31 GmWIPs, analyzed with ClustalW and the NJ method in MEGA11. The tree is based on 1000 bootstrap replicates, with Roman numerals on the outer edges representing different groups.
This classification aligned with the results of conserved motif analysis and AlphaFold 2 structure predictions (Figure 2 and Figure S2). For instance, in clade II, GmWIP10, GmWIP19, GmWIP29, and AtWIP29 exhibited close evolutionary relationships and shared similar structural characteristics (Figure 2 and Figure S2). Similar patterns were observed in other clades; clade IV included GmWIP17, GmWIP18, GmWIP20, GmWIP21, and AtWIP5, while clade V comprised 18 GmWIPs with analogous structures (Figure 2 and Figure S2).
3.3. Duplication and Collinearity Analysis
To investigate the expression mechanisms of the GmWIP gene family, a collinearity alignment within the soybean genome was conducted. This analysis revealed that 29 GmWIP genes are located within syntenic blocks on soybean chromosomes (Figure 4 and Supplementary Material S2).
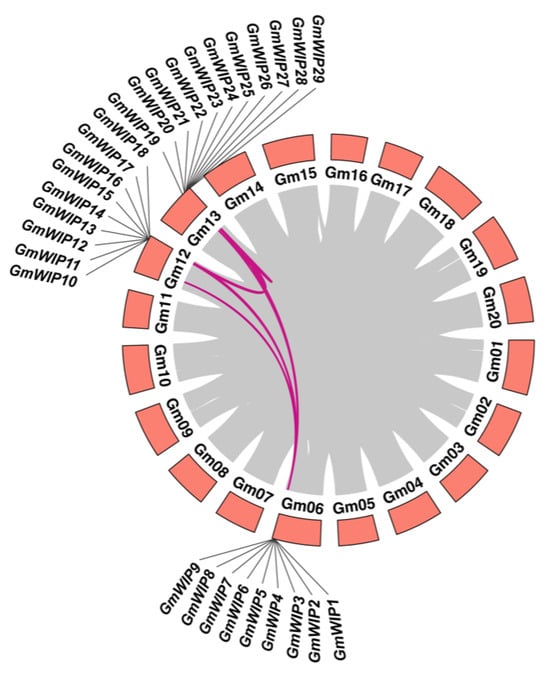
Figure 4.
Gene duplication and collinearity analysis of GmWIP gene pairs. The gray lines represent all duplicated genes in the Glycine max genome, while the purple lines indicate duplicated GmWIP gene pairs. Chromosome numbers are shown at the bottom of each chromosome, with renamed gene IDs displayed at the top.
Approximately 61% (19 of 31) of the GmWIP genes originated from tandem duplication events (Supplementary Material S2), forming six tandemly duplicated gene clusters containing two to five genes each. Specifically, three clusters contained two genes each (GmWIP12/GmWIP13, GmWIP17/GmWIP18, GmWIP30/GmWIP31), one cluster contained three genes (GmWIP1, GmWIP2, GmWIP3), and two clusters contained five genes each (GmWIP5/GmWIP6/GmWIP7/GmWIP8/GmWIP9 and GmWIP23/GmWIP24/GmWIP25/GmWIP26/GmWIP27). In addition to tandem duplication, 39% (12 of 31) of the GmWIP genes were involved in WGD or segmental duplication events. These findings suggest that tandem duplication was the primary driver of GmWIP gene expansion within the soybean genome. Collinearity analysis identified 40 orthologous gene pairs between G. max and G. soja (Figure 5 and Supplementary Material S3). These results indicate that WIP gene family members are highly conserved between the two species, reflecting their evolutionary stability and functional importance.
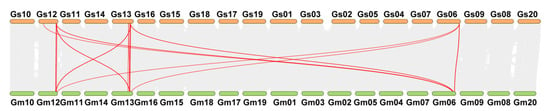
Figure 5.
Collinearity analysis of the wound-induced polypeptide gene family between Glycine soja and Glycine max. The gray lines represent collinear blocks between G. soja and G. max. The red lines highlight syntenic gene pairs within the wound-induced polypeptide family. Gs represents Glycine soja, and Gm represents Glycine max. Chromosome numbers are indicated.
3.4. Expression Patterns of GmWIP Genes Under SCN Infection
To examine the expression profiles of the GmWIP genes, non-infected and SCN-infected roots were collected at 1 dpi (migration stage), 5 dpi (syncytium formation stage), and 10 dpi (syncytium maintenance stage) for qRT-PCR analysis. The results revealed dynamic expression trends in the GmWIP genes in response to SCN infection across the different parasitic stages (Figure 6). Based on their qRT-PCR values, genes with similar expression patterns were grouped into six major clades in the heatmap (Figure 6A).
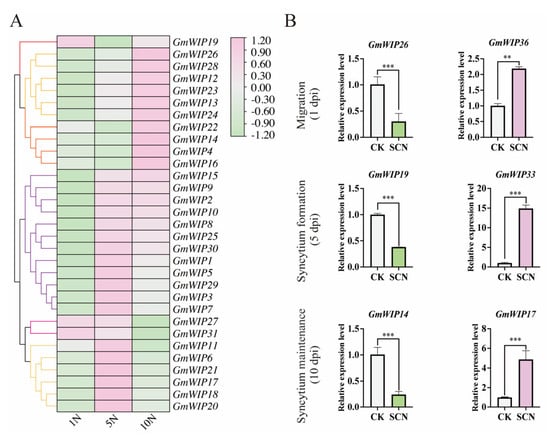
Figure 6.
Expression analysis of GmWIP genes under soybean cyst nematode infection. (A) Heatmap of GmWIP gene expression at different stages of soybean cyst nematode infection. Log2-transformed qRT-PCR values were used to create the heatmap, with genes exhibiting similar expression patterns clustered together. 1N, 5N, and 10N present the relative expression level of soybean roots inoculated with nematodes compared with non-nematode-infected roots at 1 dpi, 5 dpi, and 10 dpi. (B) The most significantly downregulated and upregulated GmWIP genes at different SCN infection stages (1, 5, and 10 dpi). “**” and “***” represent p < 0.01 and p < 0.001, respectively.
Several notable expression patterns were observed. For instance, GmWIP19 was upregulated at 1 dpi, downregulated at 5 dpi, and then upregulated again at 10 dpi and was not clustered with other GmWIP genes. Conversely, GmWIP15, GmWIP9, GmWIP2, and GmWIP10 were downregulated at 1 dpi but upregulated at both 5 and 10 dpi. GmWIP6, GmWIP21, GmWIP17, and GmWIP18 were upregulated at 5 dpi but downregulated at 1 and 10 dpi (Figure 6A). Interestingly, most GmWIP genes, except GmWIP19, GmWIP27, and GmWIP31, were downregulated at 1 dpi (Figure 6A).
Comparative analysis with non-infected roots revealed the most significant expression changes for GmWIP23 (0.30-fold downregulation) and GmWIP31 (2.18-fold upregulation) at 1 dpi. At 5 dpi, GmWIP16 (0.38-fold downregulation) and GmWIP7 (7.71-fold upregulation) exhibited the most significant changes, while at 10 dpi, GmWIP11 (0.24-fold downregulation) and GmWIP17 (4.88-fold upregulation) showed the highest regulatory shifts (Figure 6B).
3.5. Cis-Acting Elements of GmWIP Promoters and GUS Activity Under SCN Infection
To explore regulatory elements within the GmWIP promoters, approximately 2000 bp upstream of the genomic sequences were analyzed. In total, 16 types of cis-acting elements were identified in the promoters of both GmWIP and AtWIP genes, including those associated with plant development (e.g., cell cycle regulation and circadian control), hormone response (e.g., abscisic acid, MeJA, salicylic acid, gibberellin, and auxin responsiveness), light responsiveness, and stress response (e.g., defense and stress responsiveness, low-temperature responsiveness, MYB binding for drought inducibility, and wound response). Additionally, MYB binding sites involved in the regulation of flavonoid biosynthetic genes were identified (Figure 7, Supplementary Material S5).
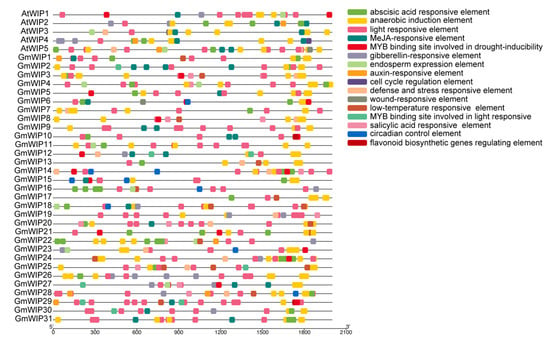
Figure 7.
Cis-acting elements in the promoters of wound-induced polypeptide genes. The cis-acting elements in the upstream 2000 bp region of the AtWIP and GmWIP gene promoters were predicted using PlantCARE. Different colored boxes represent various cis-acting elements. The x-axis shows the length of the promoter regions of the wound-induced polypeptides (5′ to 3′).
To further study the expression patterns of GmWIP genes, the promotors of GmWIP1, GmWIP11, and GmWIP25 were cloned to construct pGmWIPs::GUS reporter systems. In uninfected roots, strong GUS expression was observed in pGmUBI (positive control) roots, weak expression in pGmWIP1 roots, strong expression in pGmWIP11 roots, and expression localized to vascular tissues in pGmWIP25 roots.
Under SCN infection, pGmUBI roots exhibited strong GUS expression at 1, 5, and 10 dpi. For pGmWIP1, weak GUS expression was observed at 1 dpi, with stronger expression at 5 and 10 dpi. Strong GUS expression was also detected in pGmWIP11 and pGmWIP25 roots at all three stages of infection (1, 5, and 10 dpi) (Figure 8).
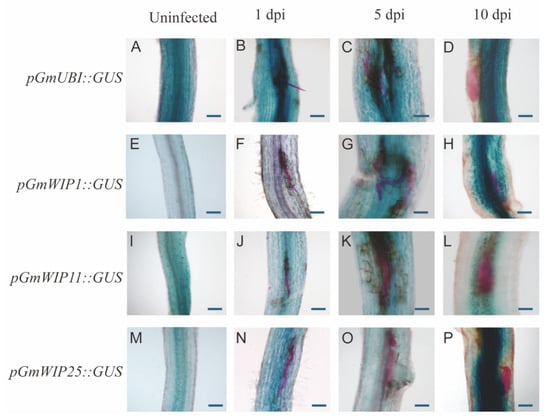
Figure 8.
Expression patterns of pGmWIPs in uninfected and SCN-infected roots. The promoters of GmWIP1, GmWIP11, and GmWIP25 were cloned and fused to the pNINC2GUS vector to construct the pGmWIP::GUS reporter system. Recombinant plasmids were introduced into Agrobacterium rhizogenes strain K599 for the generation of transgenic soybean hairy roots. pGmUBI was used as a positive control at 1 dpi, 5 dpi and 10 dpi (A–D). Weak GUS activity was observed in uninfected roots (E) and SCN-infected roots at 1 dpi (F), while strong activity was seen at 5 dpi (G) and 10 dpi (H) for pGmWIP1. Strong GUS expression was observed in pGmWIP11 and pGmWIP25 in uninfected roots (I,M) at 1 dpi (J,N), 5 dpi (K,O), and 10 dpi (L,P). Nematodes were stained red with acid fuchsin. Bars = 100 μm.
4. Discussion
Wound-induced polypeptides are a family of short peptides found across eukaryotes, typically ranging from 81 to 97 amino acids in length. Although commonly annotated as wound-induced, the evidence supporting this classification is limited. In the soybean genome, 31 GmWIP genes were identified and found to be distributed across chromosomes Gm06, Gm12, Gm13, and Gm06_scaffold_301 (Table 1). A previous study by Li et al. (2013) reported 38 WIP genes in the soybean genome [22], consistent with our HMM search results (Supplementary Material S1).
In this study, a comprehensive genome-wide analysis of GmWIPs was performed. Initial searches using HMM and BLAST (v2.16) identified 36 candidate GmWIPs (Supplementary Material S1). However, gene structure visualization revealed annotation errors in several candidates (e.g., Glyma.03G117200.1, Glyma.04G147900.1, Glyma.06G299000.1, Glyma.13G301500.1, Glyma.12G107300.1, Glyma.12G218400.1, Glyma.15G276600.1, Glyma.18G202300.1) (Supplementary Material S1). To address these inaccuracies, second-generation RNA-seq data from soybean roots (SRR26596349 and SRR26596344) and leaves (SRR19849611 and SRR19849612) and full-length RNA-seq data from roots (SRR17876321 and SRR17876325) were utilized [27,28,29]. Using GSAman software (v0.8.4) and the Target Genome Region Mapping plugin in TBtools, Glyma.03G212500.1, Glyma.04G147900.1, Glyma.12G107300.1, Glyma.15G276600.1, and Glyma.18G202300.1 were excluded from further analysis. The remaining GmWIP genes were validated through conserved motif and domain analyses (Figure S1), revealing peptide lengths ranging from 69 to 106 amino acids (Table 1). These GmWIPs displayed a high degree of similarity to their Arabidopsis orthologues in terms of gene structure, conserved motifs, and three-dimensional configurations (Figure 1, Figure 2 and Figure S2).
In Arabidopsis thaliana, five WIP genes (AtWIPs) are clustered on a single chromosome. These genes exhibit substantial sequence homology to the GmWIPs located on soybean chromosomes Gm12 and Gm13, indicating a shared evolutionary origin. Duplication analysis revealed that WIP gene expansion in the soybean genome occurred primarily through tandem duplication (Figure 4 and Supplementary Material S2). Notably, in Figure 3, none of the GmWIP genes in clade V had an A. thaliana ortholog, which may suggest that these genes are either specific to G. max or have undergone lineage-specific expansion, divergence, or neofunctionalization. Moreover, all the genes involved in tandem duplication events, except GmWIP17 and GmWIP18, belonged to the same subfamily (Figure 3 and Supplementary Material S2). These findings highlight the significant evolutionary role of tandem duplication in the expansion of the GmWIP gene family.
In plants, small yet powerful polypeptides act as peptide hormones, playing pivotal roles in intercellular and extracellular communication. This signaling, facilitated by small molecules, is crucial for regulating plant growth and development. Examples include systemin, RALF, and CLE peptides, which serve as key regulators of development and stress responses [32,33]. Recent studies have highlighted the role of plant peptides as critical signaling molecules in stress responses, addressing challenges such as mechanical wounding, pathogen infection, nutrient deficiencies, drought, and high salinity [34].
Notably, wound signals can induce the expression of several polypeptides, including REF1, systemin, plant elicitor peptide 1 (Pep1), and CAPE1 [6,20,21,35]. Among these, Pep1 has been shown to elicit damage-induced immunity in Arabidopsis, while CAPE1 activates anti-herbivore and anti-pathogen defenses under wounding stress in tomato [20,35]. In a study by Yu et al. (2018), the overexpression of GmWIP genes in Arabidopsis was found to enhance plant resistance to Pst DC3000 by boosting PTI responses [23]. Similarly, in the authors’ previous study, several GmWIPs were identified to be differentially expressed in response to SCN infection and Bacillus treatment [26]. In this study, further dynamic expression patterns of GmWIPs were observed during SCN infection (Figure 6). Based on these findings, the functions of GmWIPs are hypothesized to be analogous to CLE peptides, which are known to play critical roles in soybean nodulation and responses to cyst nematodes [19,36].
In plant–pathogen interactions, wound-induced polypeptides respond to the infection of plant pathogens. Potato virus Y (PVY) infection significantly downregulates the wound-induced protein DUF3774 (Soltu.DM.02G018790) [25]. In Arabidopsis, the expression of AtWIP1 and AtWIP2 is significantly suppressed at 12 and 24 h following inoculation with Pst DC3000. However, their expression is significantly upregulated in response to flg22 treatment, suggesting that these genes play a critical role in PTI [23]. Similarly, with the exception of GmWIP29, GmWIP27, and GmWIP31, the majority of GmWIP genes are downregulated at 1 dpi. These findings show that the members of DUF3774 may play roles in the early stages of plant pathogen infection. In our study, we also used the pGmWIP::GUS reporter system, which demonstrated low GmWIP expression at 1 dpi (Figure 8). These findings support the hypothesis that GmWIPs are integral components of the soybean PTI system in the early stages of soybean–SCN interaction.
Plants have developed sophisticated mechanisms to detect wounds and activate appropriate defense responses. Wound signaling also triggers the production of damage-associated molecular patterns (DAMPs), which further amplify defense responses [37]. Plant-parasitic nematodes infect host roots by using their stylet to penetrate cell walls and secreting cell wall-degrading enzymes. These enzymes are thought to facilitate DAMP production, which targets pattern recognition receptors (PRRs) and activates the downstream signaling pathways involved in plant defense [38,39].
Recent research has shown that RKN infections increase the expression of polygalacturonase-inhibiting proteins, a marker of DAMP production, while concurrently reducing cyst nematode infections. However, this DAMP-triggered immunity does not inhibit RKN infections [40]. In this study, GmWIPs were downregulated at 1 dpi in susceptible soybean cultivars during the SCN migration stage. This suggests that SCN may suppress GmWIP expression to subvert the host immune system and facilitate infection. These findings emphasize the need for further research to elucidate the role of GmWIPs in the basal resistance of soybean against SCNs.
5. Conclusions
Through comprehensive genome-wide identification and RNA-seq analysis, 31 GmWIP genes were shown to be distributed across chromosomes Gm06, Gm12, Gm13, and scaffold Gm06_scaffold_301. qRT-PCR and a pGmWIPs::GUS reporter system revealed dynamic expression patterns in these GmWIPs during SCN parasitism. These results suggest a potential role for GmWIPs in soybean PTI and defense responses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15040957/s1, Supplementary Material S1: GmWIP gene information; Supplementary Material S2: Duplication analysis of GmWIP genes; Supplementary Material S3: Collinearity analysis of WIP genes between Glycine max and Glycine soja; Supplementary Material S4: Primers used in this study; Supplementary Material S5: Regulatory elements on GmWIP promoters; Figure S1: Conserved domain identification of wound-induced polypeptides; Figure S2: Structure prediction of AtWIPs and GmWIPs.
Author Contributions
Conceptualization: W.K. and P.L., Data curation: W.K., Z.S. and J.X. Formal analysis: W.K., Z.S. and J.X. Funding acquisition: W.K., Z.S., J.X. and N.Q. Investigation: W.K., N.Q. and P.L. Methodology: W.K., N.Q. and P.L. Project administration: Z.S. and J.X. Resources: W.K., Z.S. and J.X., Software: W.K. and N.Q. Supervision: W.K. and P.L. Validation: N.Q. and P.L. Visualization: W.K. and N.Q. Writing—original draft: W.K. and N.Q. Writing—review & editing: W.K., Z.S., J.X., N.Q. and P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Liaoning Provincial Doctoral Research Start-up Fund (2021-BS-280), the Doctoral Start-up Foundation of Shenyang Normal University (BS202414), and the National Natural Science Foundation of China (32402324). Funding was also received from the Basic Research Project of Liaoning Provincial Department of Education (LJKQZ202223670), the Basic Research Project of Liaoning Provincial Department of Education (LJ212411035015), and the National Natural Science Foundation of China (42107447).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors would like to thank all the reviewers who participated in the review during the preparation of this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Abbreviations
| SCN | soybean cyst nematode |
| WIP | wound-induced polypeptide |
| qRT-PCR | quantitative real-time PCR |
References
- Wang, W.; Liu, Z.; Liu, Y.; Su, Z.; Liu, Y. Plant polypeptides: A review on extraction, isolation, bioactivities and prospects. Int. J. Biol. Macromol. 2022, 207, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.C.; Brand, U.; Running, M.P.; Simon, R.; Meyerowitz, E.M. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 1999, 283, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Matsubayashi, Y.; Hanai, H.; Sakagami, Y. Phytosulfokine-α, a Peptide Growth Factor Found in Higher Plants: Its Structure, Functions, Precursor and Receptors. Plant Cell Physiol. 2000, 41, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Pearce, G.; Moura, D.S.; Stratmann, J.; Ryan, C.A., Jr. RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc. Natl. Acad. Sci. USA 2001, 98, 12843–12847. [Google Scholar] [CrossRef]
- Broekaert, W.F.; Terras, F.; Cammue, B.; Osborn, R.W. Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995, 108, 1353. [Google Scholar] [CrossRef]
- Ryan, C.A.; Pearce, G. Systemin: A polypeptide signal for plant defensive genes. Annu. Rev. Cell Dev. Biol. 1998, 14, 1–17. [Google Scholar] [CrossRef]
- Ohyama, K.; Ogawa, M.; Matsubayashi, Y. Identification of a biologically active, small, secreted peptide in Arabidopsis by in silico gene screening, followed by LC-MS-based structure analysis. Plant J. 2008, 55, 152–160. [Google Scholar] [CrossRef]
- Stahl, Y.; Wink, R.H.; Ingram, G.C.; Simon, R. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Curr. Biol. 2009, 19, 909–914. [Google Scholar] [CrossRef]
- Butenko, M.A.; Patterson, S.E.; Grini, P.E.; Stenvik, G.-E.; Amundsen, S.S.; Mandal, A.; Aalen, R.B. Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell 2003, 15, 2296–2307. [Google Scholar] [CrossRef]
- Stenvik, G.-E.; Tandstad, N.M.; Guo, Y.; Shi, C.-L.; Kristiansen, W.; Holmgren, A.; Clark, S.E.; Aalen, R.B.; Butenko, M.A. The EPIP peptide of INFLORESCENCE DEFICIENT IN ABSCISSION is sufficient to induce abscission in Arabidopsis through the receptor-like kinases HAESA and HAESA-LIKE2. Plant Cell 2008, 20, 1805–1817. [Google Scholar] [CrossRef]
- Kumpf, R.P.; Shi, C.-L.; Larrieu, A.; Stø, I.M.; Butenko, M.A.; Péret, B.; Riiser, E.S.; Bennett, M.J.; Aalen, R.B. Floral organ abscission peptide IDA and its HAE/HSL2 receptors control cell separation during lateral root emergence. Proc. Natl. Acad. Sci. USA 2013, 110, 5235–5240. [Google Scholar] [CrossRef] [PubMed]
- Sprunck, S.; Rademacher, S.; Vogler, F.; Gheyselinck, J.; Grossniklaus, U.; Dresselhaus, T. Egg cell–secreted EC1 triggers sperm cell activation during double fertilization. Science 2012, 338, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A small peptide modulates stomatal control via abscisic acid in long-distance signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shi, X.; Zhang, Y.; Wang, J.; Yang, J.; Ishida, T.; Jiang, W.; Han, X.; Kang, J.; Wang, X. CLE9 peptide-induced stomatal closure is mediated by abscisic acid, hydrogen peroxide, and nitric oxide in Arabidopsis thaliana. Plant Cell Environ. 2019, 42, 1033–1044. [Google Scholar] [CrossRef]
- Chien, P.-S.; Nam, H.G.; Chen, Y.-R. A salt-regulated peptide derived from the CAP superfamily protein negatively regulates salt-stress tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 5301–5313. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Lin, J. Systemin-mediated long-distance systemic defense responses. New Phytol. 2020, 226, 1573–1582. [Google Scholar] [CrossRef]
- Pearce, G.; Strydom, D.; Johnson, S.; Ryan, C.A. A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 1991, 253, 895–897. [Google Scholar] [CrossRef]
- Nakagami, S.; Notaguchi, M.; Kondo, T.; Okamoto, S.; Ida, T.; Sato, Y.; Higashiyama, T.; Tsai, A.Y.-L.; Ishida, T.; Sawa, S. Root-knot nematode modulates plant CLE3-CLV1 signaling as a long-distance signal for successful infection. Sci. Adv. 2023, 9, eadf4803. [Google Scholar] [CrossRef]
- Guo, X.; Chronis, D.; De La Torre, C.M.; Smeda, J.; Wang, X.; Mitchum, M.G. Enhanced resistance to soybean cyst nematode Heterodera glycines in transgenic soybean by silencing putative CLE receptors. Plant Biotechnol. J. 2015, 13, 801–810. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Lee, C.-Y.; Cheng, K.-T.; Chang, W.-H.; Huang, R.-N.; Nam, H.G.; Chen, Y.-R. Quantitative peptidomics study reveals that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell 2014, 26, 4135–4148. [Google Scholar] [CrossRef]
- Yang, W.; Zhai, H.; Wu, F.; Deng, L.; Chao, Y.; Meng, X.; Chen, Q.; Liu, C.; Bie, X.; Sun, C.; et al. Peptide REF1 is a local wound signal promoting plant regeneration. Cell 2024, 187, 3024–3038.e14. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Yu, G.; Luo, L. A wound-induced small polypeptide gene family is upregulated in soybean nodules. Chin. Sci. Bull. 2013, 58, 1003–1009. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Y.; Liu, Y.; Li, N.; Yan, J.; Luo, L. Wound-induced polypeptides improve resistance against Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. Biochem. Biophys. Res. Commun. 2018, 504, 149–156. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Li, X.; Zhao, L.; Chen, S. First report of the soybean cyst nematode, Heterodera glycines, on soybean in Zhejiang, eastern China. Plant Dis. 2009, 93, 319. [Google Scholar] [CrossRef]
- Glushkevich, A.; Spechenkova, N.; Fesenko, I.; Knyazev, A.; Samarskaya, V.; Kalinina, N.O.; Taliansky, M.; Love, A.J. Transcriptomic reprogramming, alternative splicing and RNA methylation in potato (Solanum tuberosum L.) plants in response to potato virus Y infection. Plants 2022, 11, 635. [Google Scholar] [CrossRef]
- Kang, W.; Duan, Y.; Lei, P. Transcriptomic changes in soybean underlying growth promotion and defense against cyst nematode after Bacillus simplex Sneb545 treatment. Gene 2024, 898, 148080. [Google Scholar] [CrossRef]
- Kang, W.; Zhu, X.; Wang, Y.; Chen, L.; Duan, Y. Transcriptomic and metabolomic analyses reveal that bacteria promote plant defense during infection of soybean cyst nematode in soybean. BMC Plant Biol. 2018, 18, 86. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Sun, A.; Wang, L.; Ren, C.; Liu, J.; Gao, X. Transcriptome analysis reveals key drought-stress-responsive genes in soybean. Front. Genet. 2022, 13, 1060529. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, Y.; Qin, R.; Jiang, D.; Chang, D.; Tian, Z.; Li, C.; Wang, C. Full-length transcriptional analysis of the same soybean genotype with compatible and incompatible reactions to Heterodera glycines reveals nematode infection activating plant defense response. Front. Plant Sci. 2022, 13, 866322. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Qi, N.; Yan, J.; Lei, P.; Kang, W.; Liu, X.; Xuan, Y.; Fan, H.; Wang, Y.; Yang, N.; Chen, L.; et al. Transcriptome Analysis of GmPUB20A Overexpressing and RNA-Interferencing Transgenic Hairy Roots Reveals Underlying Negative Role in Soybean Resistance to Cyst Nematode. J. Agric. Food Chem. 2023, 71, 18059–18073. [Google Scholar] [CrossRef] [PubMed]
- Grienenberger, E.; Fletcher, J.C. Polypeptide signaling molecules in plant development. Curr. Opin. Plant Biol. 2015, 23, 8–14. [Google Scholar] [CrossRef]
- Ryan, C.A.; Pearce, G.; Scheer, J.; Moura, D.S. Polypeptide hormones. Plant Cell 2002, 14 (Suppl. S1), S251–S264. [Google Scholar] [CrossRef]
- Chen, Y.L.; Fan, K.T.; Hung, S.C.; Chen, Y.R. The role of peptides cleaved from protein precursors in eliciting plant stress reactions. New Phytol. 2020, 225, 2267–2282. [Google Scholar] [CrossRef]
- Hander, T.; Fernández-Fernández, Á.D.; Kumpf, R.P.; Willems, P.; Schatowitz, H.; Rombaut, D.; Staes, A.; Nolf, J.; Pottie, R.; Yao, P.; et al. Damage on plants activates Ca2+-dependent metacaspases for release of immunomodulatory peptides. Science 2019, 363, eaar7486. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.W.; Lee, Y.W.; Hwang, C.H. Soybean nodule-enhanced CLE peptides in roots act as signals in GmNARK-mediated nodulation suppression. Plant Cell Physiol. 2011, 52, 1613–1627. [Google Scholar] [CrossRef]
- Vega-Muñoz, I.; Duran-Flores, D.; Fernández-Fernández, Á.D.; Heyman, J.; Ritter, A.; Stael, S. Breaking bad news: Dynamic molecular mechanisms of wound response in plants. Front. Plant Sci. 2020, 11, 610445. [Google Scholar] [CrossRef]
- Seong, S.-Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef]
- Gillet, F.-X.; Bournaud, C.; Antonino de Souza Júnior, J.D.; Grossi-de-Sa, M.F. Plant-parasitic nematodes: Towards understanding molecular players in stress responses. Ann. Bot. 2017, 119, 775–789. [Google Scholar] [CrossRef]
- Shah, S.J.; Anjam, M.S.; Mendy, B.; Anwer, M.A.; Habash, S.S.; Lozano-Torres, J.L.; Grundler, F.M.; Siddique, S. Damage-associated responses of the host contribute to defence against cyst nematodes but not root-knot nematodes. J. Exp. Bot. 2017, 68, 5949–5960. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).