Abstract
Weedy rice, also known as red rice, mainly originates from the de-domestication of cultivated rice and is a vicious weed in paddy fields around the world. Its red seeds are rich in oxidized proanthocyanidins (OPAs). This study investigates whether OPA content varies among different weedy rice strains, whether these variations are possibly related to their adaptation to the local environment, and whether the change in OPA content could affect seed germination. A total of 202 weedy rice accessions from 69 populations across China were collected. Their OPA content, Rc/Rd genotypes, and seed germination percentage on the second, third, and seventh day were detected, respectively. Using bivariate Pearson’s two-tailed correlation analysis and generalized linear mixed models, our results showed that the content of OPAs varied widely among the different strains of weedy rice and were significantly correlated with local environment (latitude) and Rc/Rd genotypes but not with seed germination percentage on the second, third, and seventh day. Thus, the content of OPAs in Chinese weedy rice seeds is closely related to its ecological adaptability. These findings provide insights into the effect of OPA content on the ecological adaptability of weedy rice, which is beneficial to the control and germplasm resource utilization of weedy rice.
1. Introduction
Rice pericarps may exhibit up to seven different colors, including white, light brown, spotted brown, brown, red, variable purple, and purple, due to the accumulation of oxidized proanthocyanidins (OPAs) and anthocyanins [1,2]. The red color of rice’s pericarp is primarily due to OPA accumulation [3,4,5,6].
Weedy rice is also called red rice because its pericarp color is usually red, caused by the deposition of OPAs [7]. Weedy rice, which is de-domesticated from cultivated rice, is becoming a kind of malignant weed in paddy fields all over the world [7]. Weedy rice can significantly decrease the yield and quality of cultivated rice [8,9,10]. In comparison with cultivated crops, weedy rice benefits from greater light, water, and nutrient availability due to its more extensive morphological diversity, high survival rate, and substantial reproductive advantage [11,12].
Latitude has significantly influenced the climatic environment of the Chinese mainland, resulting in less rainfall, lower temperatures, and a lower annual accumulated temperature in northern China, while in southern China, there is more rainfall, higher temperatures, and a higher annual accumulated temperature. This has formed the distribution of rice cultivation in China, where japonica rice is mainly planted in the northern regions and indica rice is mainly planted in the southern regions [13]. As weedy rice in China mainly comes from the de-domestication of cultivated rice, this has also led to a situation whereby in northern China, weedy rice is mainly of the japonica type, while in southern China, it is mainly of the indica type [14,15]. Is the change in the content of OPAs related to its adaptability to the environment?
The Rc and Rd genes are the key genes for regulating the red color of the weedy rice pericarp [2,6,16]. The Rc gene encodes a basic helix–loop–helix (BHLH) protein that regulates the PA synthesis pathway. The Rd gene encodes dihydro-flavonol-4-reductase (DFR), a key enzyme in the PA synthesis pathway [17]. Although the RcRd, Rcrd, and rcRd genotypes have red, brown, and white pericarps, respectively, only the RcRd genotype can accumulate OPAs in caryopsis [16,17]. Do different Rc/Rd genotypes of weedy rice significantly affect the changes in the content of OPAs in their seeds?
Non-oxidized PAs are colorless and stored in the vacuoles of seed coat endothelial cells during the early stages of seed development [18]. As the seeds mature, colorless non-oxidized PAs can be transported to the apoplastic space of seed coat endothelial cells via membrane vesicles or other mechanisms, where they undergo oxidative brown polymerization and further cross-linkage with other cell wall components via apoplastic polyphenol oxidases [19]. After Arabidopsis seeds mature, their coat is made up entirely of dead tissue and is colored brownish by OPAs [19]. Artificially adding non-oxidized PAs to Arabidopsis seeds reduced their germination percentage gradually [20,21]. Non-oxidizing PAs inhibited seed germination by promoting ABA biosynthesis rather than inhibiting its degradation [21]. Does the accumulation of OPAs in mature seeds affect the germination of weedy rice seeds in China?
Based on rice planting regionalization in China (Wang et al., 2023 [22]), we randomly selected 202 weedy rice strains from 69 rice populations to investigate the relationship between OPA content and Rc/Rd genotypes, latitude, and seed germination. Although earlier studies have evaluated the genetic basis of pigmentation, its ecological implications remain unexplored. The present research aims to answer the following questions: (a) Does the content of OPAs vary among different weedy rice strains? (b) Is the variation in OPA content related to adaptation to the local environment, such as latitude? (c) Does the change in OPA content affect seed germination?
2. Materials and Methods
2.1. Weedy Rice Strains Collected Across China
Three strains of weedy rice were randomly selected from 69 populations of weedy rice, with 5 missing during the experiment, and a total of 202 weedy rice accessions were preserved [22] (Figure 1). According to the harm degree of weedy rice in Chinese provinces and the sampling range that can basically cover the rice-producing areas in China, 69 weedy rice populations were selected [22]. Latitude information was recorded. All weedy rice strains were planted in the rice paddy field at Nanjing Agricultural University (Nanjing, Jiangsu Province, China) to mitigate the impact of maternal effects, environmental factors, seed lifespan, and storage on the seeds. Each strain was planted in three rows with a line distance of 30 cm and individual spacing of 20 cm. The field was managed conventionally. Each strain was harvested 50 days after heading. The seeds were dried at ambient temperature for one month until the water content was approximately 12% and then stored in a −20 °C freezer [23].
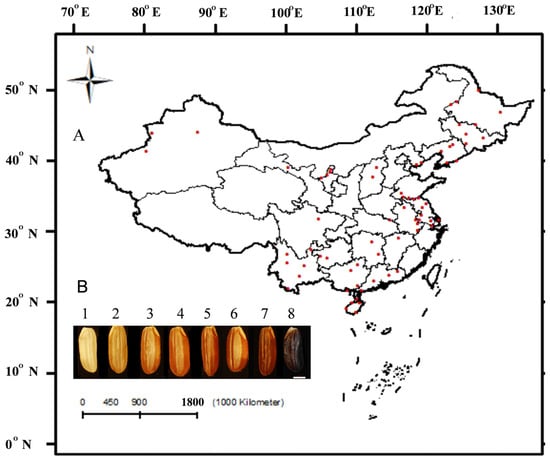
Figure 1.
Sampling sites of 69 weedy rice populations, including 202 strains across China, and the caryopsis color of weedy rice. Note: (A) The red dots on the map represent the 69 sample sites. (B) The caryopsis color of weedy rice. The scale value is 2 mm. Samples (1–8) are from Jiangxi Nanchang (WRL-5201), Yunnan Xishuangbanna (WRL-8553), Yunnan Xishuangbanna (WRL-8554), Hunan Chenzhou (WRL-8623), Anhui Xuancheng (WRL-8191), Guangdong Meizhou (WRL-7375), Guangdong Maoming (WRL-5425), and Jilin Songyuan (WRL-2906), respectively.
2.2. Determination of Rc/Rd Genotypes
Polyacrylamide gel electrophoresis was used to measure the Rc/Rd allele of 202 weedy rice strains randomly selected from 69 populations across China (Figure 2). Seedling leaves were collected when 3–4 leaves emerged from each strain. DNA was extracted using the SDS method and stored at −20 °C [24,25]. Rc is a dominant allele, and rc is a recessive allele with a 14 bp deletion. The Rc and rc alleles were analyzed using RED4 primers [26], which had the following sequences: RED4-F: TTACAGGGGAGCAGAAACA and RED4-R: TCTTCTCCTCTCTTTCAGCAC. The PCR reaction mixture included 4 μL of a 2 × PCR mix, 4.5 μL of ddH2O, 0.25 μL of RED4-F, 0.25 μL of RED4-R, and 1 μL of the DNA template. The 2 × PCR Mix (Nanjing Jitian Biotechnology Co., Ltd., Nanjing, China) consisted of 0.2 mmol·L−1 dNTPs, 100 mmol·L−1 KCl, 20 mmol·L−1 Tris-HCl (pH 8.5), 5 U/100 μL Taq polymerase, and 3 mmol·L−1 MgCl2. The PCR conditions were as follows: 5 min of pre-denaturation at 94 °C, followed by 30 cycles of 94 °C denaturation for 45 s, 55 °C annealing for 45 s, and 72 °C extension for 1 min, followed by an 8 min 72 °C final extension and 10 min of 4 °C cooling.
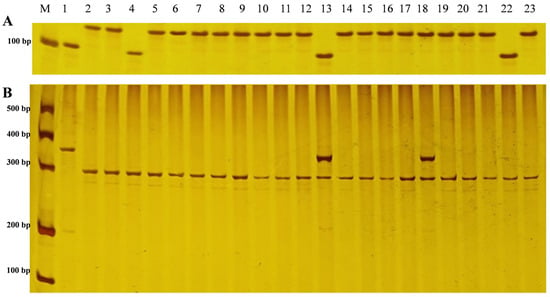
Figure 2.
Rc/Rd alleles of weedy rice strains using polyacrylamide gel electrophoresis. Note: (A) represents the Rc/rc alleles. The Rc allele fragment was 118 bp in size, but the rc allele was 104 bp in size. (B) represents the Rd/rd alleles. The Rdp-tagged Rd allele and the Rdp-tagged rd allele had a size of 294 bp and 346 bp, respectively. M. 500 bp DNA marker; 1. Nipponbare (control sample, rcrcrdrd); 2. Anhui maanshang (WRL-4661, RcRcRdRd); 3. Gansu zhangye (WRL-4320, RcRcRdRd); 4. Guangdong Zhaoqing (WRL-5164, rcrcRdRd); 5. Guangxi qinzhou (WRL-6454, RcRcRdRd); 6. Guizhou anshun (WRL-731, RcRcRdRd); 7. Hainan east (WRL-1531, RcRcRdRd); 8. Hebei Qinhuangdao (WRL-2943, RcRcRdRd); 9. Henan xinyang (WRL-4596, RcRcRdRd); 10. Heilongjiang qiqihar (WRL-1830, RcRcRdRd); 11. Hunan yiyang (WRL-1387, RcRcRdRd); 12. Jilin Tonghua (WRL-1940, RcRcRdRd); 13. Jiangsu yancheng (WRL-3171 rcrcRdrd); 14. Jiangxi nanchang (WRL-5200, RcRcRdRd); 15. Liaoning tieling (WRL-111, RcRcRdRd); 16. Ningxia shizuishan (WRL-3784, RcRcRdRd); 17. Shandong yutai (WRL-3483, RcRcRdRd); 18. Shanxi Taiyuan (WRL-4557, RcRcRdrd); 19. Shanghai Chongming (WRL-3552, RcRcRdRd); 20. Sichuan Chengdu (WRL-810, RcRcRdRd); 21. Xinjiang Aksu (WRL-3990, RcRcRdRd); 22. Yunnan Yuanjiang (WRL-7798, rcrcRdRd); 23. Zhejiang Jiaxing (WRL-6330, RcRcRdRd).
The Rd/rd alleles were detected through cleaved amplified polymorphic sequencing (CAPS) using Rdp-F: CGTGCGAATCCAACACAA and Rdp-R: CTTGACCACCTCGTTCTC [27]. The PCR reaction mixture included 4.5 μL of a 2 × PCR mix, 4 μL of ddH2O, 0.25 μL of Rdp-F, 0.25 μL of Rdp-R, and 1 μL of DNA. The PCR conditions were as follows: 5 min of pre-denaturation at 94 °C; 30 cycles of 94 °C denaturation for 30 s, 54 °C annealing for 30 s and 72 °C extension for 30 s; and 7 min of 72 °C final extension and 10 min of 4 °C cooling. The 20 μL digestion reaction included 2 μL of 0.1% BSA, 2 μL of 10 × TaqI buffer, 1 μL of TaqI endonuclease (Takara), 10 μL of ddH2O, and 5 μL of the PCR product. The digestion temperature was 65 °C, and the incubation time was 1 h.
The Rc allele fragment was 118 bp in size, but the rc allele was 104 bp in size due to a 14 bp deletion, and 6% polyacrylamide gel and silver staining were used. After TaqI enzyme digestion, the Rdp-tagged Rd allele and the Rdp-tagged rd allele had a size of 294 bp and 346 bp, respectively [28]. For the Rc/Rd allele, Oryza sativa Nipponbare (a rice cultivar for the international reference genome, rcrcrdrd) was used as an internal reference.
2.3. Determination of OPA Content
The vanillin method was used to determine OPAs [4,16,29]. Ninety plump seeds from each strain were dehulled with a hulling machine, milled, and filtered through a 40-mesh screen. The seed powder (<0.5 g) was defatted with 10 mL of hexane at room temperature and then centrifuged at 10,000× g for 10 min. We discarded the supernatant and allowed the pellet to dry at room temperature in a fume hood. PAs were extracted by incubating the dried pellet in 10 mL of 80% methanol for 15 h at 4 °C, followed by centrifugation at 10,000 g for 15 min. The supernatant containing the PAs was collected for further analysis. We used three replicates for each sample. Approximately 1 mL of the supernatant was mixed with 2.5 mL of 1% vanillin aldehyde–methanol (w/v) and 2.5 mL of 25% sulfuric acid–methanol (v/v) in a 10 mL conical tube. After 20 min of incubation in a 30 °C water bath, we measured the mixture’s absorbance using a spectrophotometer at a wavelength of 500 nm. PA content was determined using the equivalent (+)-catechin (CAE) to generate a standard curve. PA-free Oryza sativa Nipponbare (a rice cultivar for the international reference genome) was used as an internal reference, and PA content was expressed as equivalent CAE/g sample powder [4,16].
2.4. Seed Germination
Ninety plump rice seeds were separated into three groups and placed on Petri dishes with two layers of filter paper each. The samples were germinated using 6 mL of distilled water. The dishes were subsequently wrapped in plastic bags and placed in incubators (SPX-400-GBH; Shanghai Yuejin Company, Shanghai, China) at a constant temperature of 28 °C and a light–dark cycle of 16:8 h [14].
A radicle protruding more than 3 mm from the hull indicated favorable germination (Gu et al., 2003 [23]). Seed germination percentage on the 2nd day, 3rd day, and 7th day were determined as SG2, SG3, and SG7, respectively [30] using these Formulas (1)–(3) [14]:
SG3 = (number of seeds germinated by Day 2/total seed number) × 100%;
SG3 = (number of seeds germinated by Day 3/total seed number) × 100%;
SG7 = (number of seeds germinated by Day 7/total seed number) × 100%;
2.5. Data Analysis
SPSS 18.0 (https://www.ibm.com/products/spss-statistics, 15 October,2024, IBM Corporation, Armonk, NY, USA) was used for bivariate Pearson’s two-tailed correlation analysis (α = 0.05) with generalized linear mixed models (OPAs as the custom target; seed germination, genotype, and latitude as the fixed effect). ArcGIS10.8 (https://www.esri.com/en-us/home, 15 October 2024, Environmental Systems Research Institute, Inc. (Esri), Redlands, CA, USA) was used to map the geological data. Other diagrams were created using Origin 8.0 (https://www.originlab.com/Index.aspx, 15 October 2024, OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. Analysis of Rc/Rd Alleles
The proportion of the Rc dominant allele was 87.8% (Figure 2A). The proportion of the Rd dominant allele was 98.2% (Figure 2B). The 202 strains included 163 with the RcRcRdRd genotype, 20 with the RcrcRdRd genotype, 12 with the rcrcRdRd genotype, 3 with the RcRcRdrd genotype, 3 with the RcrcRdrd genotype, and 1 with the rcrcRdrd genotype.
The proportion of genotypes carrying Rc and Rd dominant alleles was 93.5%. Compared to the other five genotypes (RcrcRdRd, RcRcRdrd, RcrcRdrd, rcrcRdRd, and rcrcRdrd), the proportion of RcRcRdRd was 81.5%.
3.2. Statistical Analysis of OPA Content
The OPA content in mature seeds from the 202 weedy rice strains followed a normal distribution, with a minimum of 0 and a maximum of 67.01 mg CAE/g (Figure 3A). OPAs were exclusively detected in all genotypes carrying Rc and Rd dominant alleles (Figure 3B).
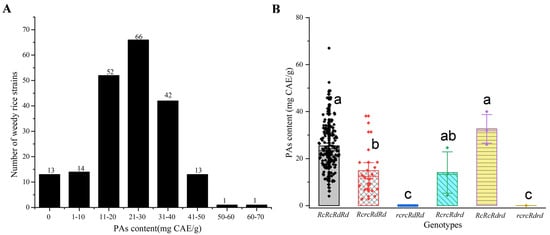
Figure 3.
OPA content and Rc/Rd genotypes of the 202 weedy rice strains. Note: (A) Distribution of OPA content in the 202 weedy rice lines. The number on the black column represents the number of weedy rice strains in the content range. (B) OPA content in mature seeds of Rc/Rd genotypes detected in the 202 weedy rice strains. Dots represent the number of weedy rice strains. Vertical bars indicate the ± standard deviation of the mean. Lowercase letters (a, b, c) indicate significant differences (p < 0.05) according to Tukey’s HSD test.
3.3. The Influence of OPAs on Rc/Rd Genotypes, Latitude, and Seed Germination
Using bivariate Pearson’s two-tailed correlation analysis, the statistical results showed that the variations in proanthocyanidin (OPA) content in the seeds of weedy rice across China had no significant correlation with seed germination percentage on the second (r = −0.078, p = 0.415), third (r = −0.018, p = 0.851), and seventh days (r = −0.011, p = 0.876), respectively (Figure 4A–C), while OPA content had a significant correlation with Rc/Rd genotypes (r = −0.051, p = 0.000) and latitude (r = 0.242, p = 0.000) (Figure 5A,B).
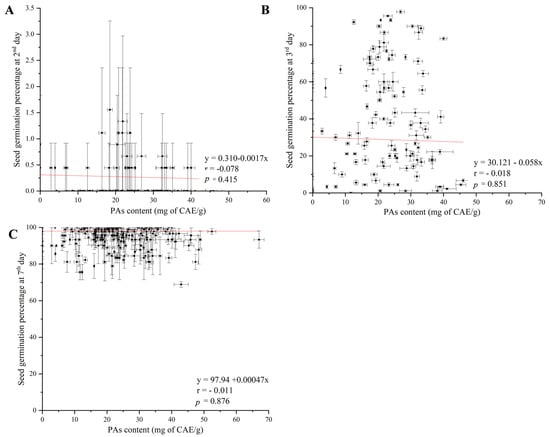
Figure 4.
Pearson correlation between OPA content and seed germination percentage on the 2nd, 3rd, and 7th days. Note: (A) OPA content with seed germination percentage on the 2nd day; (B) OPA content with seed germination percentage on the 3rd day; (C) OPA content with seed germination percentage on the 7th day. Vertical bars indicate the ± standard deviation of the mean. Red line is trend line.
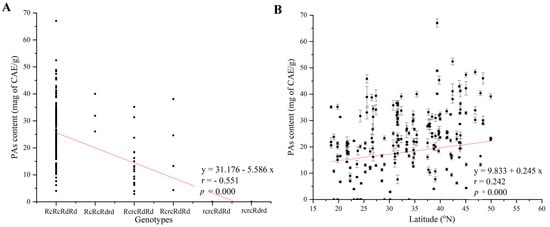
Figure 5.
Pearson correlation between OPA content and Rc/Rd genotypes as well as latitude. Note: (A) OPA content with Rc/Rd genotypes; (B) OPA content with latitude. Vertical bars indicate ± the standard deviation of the mean. Red line is trend line.
Using generalized linear mixed models, the statistical results showed that OPA content had no significant effect on seed germination percentage on the second, third, and seventh days (Table 1). OPA content had a significant effect on Rc/Rd genotypes (F = 15.153, p = 0.000) and latitude (F = 5.469, p = 0.021) (Table 1).

Table 1.
Relationship among OPA content, latitude, Rc/Rd genotypes, and seed germination percentage using generalized linear mixed models.
4. Discussion
4.1. Significant Differences in OPA Content Among Weedy Rice Strains Across China
We determined the Rc/Rd allele and OPA content in 202 weedy rice accessions from 69 populations across China (Figure 2 and Figure 3). The OPA content in mature weedy rice seeds could only be detected when both Rc and Rd dominant alleles were present. This is consistent with previous results [16]. The present phylogenetic results support the hypothesis that weedy rice in China was de-domesticated from cultivated rice [31,32]. We found that the proportion of the Rc allele in weedy rice exceeded 87.8%, whereas the proportion of the Rd allele was as high as 98.2%. This implies that cultivated rice containing the Rd allele might be more prone to mutate and de-domesticate into weedy rice. These genotypes with Rc and Rd dominant alleles may have relative fitness advantages over other genotypes, including the ability to synthesize PAs in seeds, which leads to a gradual dominance of the Rc and Rd allele ratio [16,33].
The OPA content in mature seeds from the 202 weedy rice accessions followed a normal distribution, with a minimum of 0 and a maximum of 67.01 mg CAE/g. OPA content exhibited a typical multigene-regulated pattern [34]. According to several studies, PAs, potent antioxidants that neutralize free radicals, effectively lower the risk of cardiovascular disease and prevent the spread of breast cancer [1,35,36,37,38]. PAs benefit the human diet and affect the quality of foods like wine, fruit juice, and tea [19,39,40]. Thus, weedy rice accessions can serve as good genetic resources for cultivating rice with high PA content [41,42,43].
A comparative analysis of eight traditional red rice cultivars from Sri Lanka revealed that the bran layer’s OPAs contribute to remarkable phytochemical enhancements, exhibiting over sevenfold higher antioxidant capacity than three white rice cultivars [41]. These nutritional advantages have gained substantial scientific and consumer attention, particularly as global dietary patterns increasingly emphasize food quality and health-promoting properties in response to rising living standards and heightened health awareness.
4.2. The Variation in OPA Content Was Significantly Affected by the Latitude of the Sampling Site
Bivariate Pearson’s two-tailed correlation and generalized linear mixed model analysis both showed that the content change of OPAs in the seeds of weedy rice in China is significantly affected by latitude (Figure 5B). Latitude has a profound impact on the environmental changes in the Chinese mainland, and it has even led to the north–south planting distribution of the indica/japonica subspecies of cultivated rice [13]. This may enable Chinese weedy rice, which has undergone de-domestication from cultivated rice, to adapt to the local environment by regulating the changes in the content of OPAs in its seeds.
Some studies have shown that OPAs may play an important role in the survival of mature seeds after falling into local fields [20,22,44,45]. In addition, PAs in mature seeds may provide UV protection, repel pests and large herbivores, and resist aging, predation, and microbial decay [2,22,46,47].
4.3. The Change in OPA Content Had No Significant Effect on Seed Germination
Bivariate Pearson’s two-tailed correlation and generalized linear mixed model analysis both showed that OPA content had no significant effect on seed germination percentage on the second, third, and seventh day (Figure 4, Table 1). Seed germination is regulated by complex interactions between genetic, hormonal, and environmental factors [48]. Dormancy-related quantitative trait loci (QTLs), such as qSD7-1 and qSD12, have been identified, with qSD7-1 encoding a protein kinase involved in abscisic acid (ABA) sensitivity [49]. The Sdr4 gene, homologous to cultivated rice, acts as a central regulator by interacting with ABA and gibberellin (GA) metabolism genes to suppress germination [50]. Additionally, allelic variations in OsVP1 (Viviparous-1) enhance dormancy by promoting ABA accumulation [51].
During seed development, the co-expression of Rc and Rd alleles promotes non-oxidized PA synthesis, which boosts abscisic acid (ABA) production [16]. However, with seed ripening, the unoxidized PAs are transported from the vacuole to the outer wall and become oxidized PAs, potentially losing their ability to stimulate ABA synthesis [19]. This may partly explain why, despite the differences in the content of OPAs, the mature seeds of weedy rice in temperate regions exhibit no dormancy or only have a weak dormancy characteristic [7,52,53]. Further research is needed to determine how OPA variation affects the survivability of weedy rice under various environmental stress conditions.
5. Conclusions
Our results indicate that there is a wide range of variation in the content of OPAs among different varieties of weedy rice. This variation is significantly correlated with Rc/Rd genotypes and latitude (an important environmental factor), but it has no significant effect on seed germination. These findings suggest that OPA variation could be closely related to their ecological adaptability. In the next step, new techniques and methods, such as genome-wide association analysis and gene editing, can be used to further explore the function of OPAs in the fitness of weedy rice.
Author Contributions
Methodology, Y.Y., J.C. and X.Y.; investigation, Y.Y., J.C., B.R. and Q.Q.; resources, S.Q.; writing—original draft, Z.S., X.S. and W.D.; writing—review and editing, W.D.; supervision, W.D.; project administration, W.D.; funding acquisition, W.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the National Natural Science Foundation of China (Grant No. 32071656), construction of the biodiversity station in Santai Mountain (SJ2023F108), the China Transgenic Organism Research and Commercialization Project (Grant No. 2016ZX08011-001), and the National Natural Science Foundation of China (Grant No. 30800604). No competing interests exist regarding the presentation of information. All authors have read and approved the manuscript.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lu, Y.; Zuo, Z.; Yang, Z. Toward breeding pigmented rice balancing nutrition and yield. Trends Plant Sci. 2024, 29, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Wu, D.; Fang, Y.; Ye, C.; Zhu, Q.-H.; Wei, X.; Fan, L. Population genomic analysis unravels the evolutionary roadmap of pericarp color in rice. Plant Commun. 2024, 5, 100778. [Google Scholar] [CrossRef] [PubMed]
- Oki, T.; Masuda, M.; Kobayashi, M.; Nishiba, Y.; Furuta, S.; Suda, I.; Sato, T. Polymeric procyanidins as radical-scavenging components in red-hulled rice. J. Agric. Food Chem. 2002, 50, 7524–7529. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Tang, F.; Huang, Y.; Xu, F.; Chen, Y.; Tong, C.; Chen, H.; Bao, J. Analysis of Genotype × Environment Interactions for Polyphenols and Antioxidant Capacity of Rice by Association Mapping. J. Agric. Food Chem. 2014, 62, 5361–5368. [Google Scholar] [CrossRef]
- Sweeney, M.T.; Thomson, M.J.; Pfeil, B.E.; Mccouch, S. Caught red-handed: Rc encodes a basic helix-loop-helix protein conditioning red pericarp in rice. Plant Cell 2006, 18, 283–294. [Google Scholar] [CrossRef]
- Xia, D.; Zhou, H.; Wang, Y.; Li, P.; Fu, P.; Wu, B.; He, Y. How rice organs are colored: The genetic basis of anthocyanin biosynthesis in rice. Crop J. 2021, 9, 598–608. [Google Scholar] [CrossRef]
- Delouche, J.C.; Burgos, N.R.; Gealy, D.R.; de San Martín, G.Z.; Labrada, R.; Larinde, M.; Rosell, C. Weedy Rices—Origin, Biology, Ecology and Control; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2007. [Google Scholar]
- Dai, L.; Dai, W.M.; Song, X.L.; Lu, B.R.; Qiang, S. A comparative study of competitiveness between different genotypes of weedy rice (Oryza sativa) and cultivated rice. Pest Manag. Sci. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Gressel, J.; Valverde, B.E. A strategy to provide long-term control of weedy rice while mitigating herbicide resistance transgene flow, and its potential use for other crops with related weeds. Pest Manag. Sci. 2009, 65, 723–731. [Google Scholar] [CrossRef]
- Kanapeckas, K.L.; Tseng, T.; Vigueira, C.C.; Ortiz, A.; Bridges, W.C.; Burgos, N.R.; Fischer, A.J.; Lawton-Rauh, A. Contrasting patterns of variation in weedy traits and unique crop features in divergent populations of US weedy rice (Oryza sativa sp.) in Arkansas and California. Pest Manag. Sci. 2018, 74, 1404–1415. [Google Scholar] [CrossRef]
- Burgos, N.R.; Norman, R.J.; Gealy, D.R.; Black, H. Competitive N uptake between rice and weedy rice. Field Crops Res. 2006, 99, 96–105. [Google Scholar] [CrossRef]
- Nakabayashi, K.; Leubner-Metzger, G. Seed dormancy and weed emergence: From simulating environmental change to understanding trait plasticity, adaptive evolution, and population fitness. J. Exp. Bot. 2021, 72, 4181–4185. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.Y.; Zhang, S.J.; Ford-Lloyd, B.V.; Jin, X.; Wu, Y.; Yan, H.X.; Liu, P.; Yang, X.; Lu, B.R. Latitudinal distribution and differentiation of rice germplasm: Its implications in breeding. Crop Sci. 2011, 51, 1050–1058. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, S.D.; Kong, M.Y.; Chao, J.; Chen, X.F.; Yang, J.L.; Shi, Z.; Qiang, S.; Song, X.; Dai, W.-M. Better performance of germination in hyperosmotic solutions in conspecific weedy rice than cultivated rice. J. Syst. Evol. 2019, 57, 519–529. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, W.; Wu, C.; Song, X.; Qiang, S. Genetic diversity and origin of Japonica- and Indica-like rice biotypes of weedy rice in the Guangdong and Liaoning provinces of China. Genet. Resour. Crop Evol. 2012, 59, 399–410. [Google Scholar] [CrossRef]
- Furukawa, T.; Maekawa, M.; Oki, T.; Suda, I.; Iida, S.; Shimada, H.; Takamure, I.; Kadowaki, K. Rc and Rd genes are involved in proanthocyanidin synthesis in rice pericarp. Plant J. 2007, 49, 91–102. [Google Scholar] [CrossRef]
- Nakai, K.; Inagaki, Y.; Nagata, H.; Miykzaki, C.; Iida, S. Molecular characterization of the gene for dihydroflavonol 4-reductase of Japonica rice varieties. Plant Biotechnol. 1998, 15, 221–225. [Google Scholar] [CrossRef][Green Version]
- Abrahams, S.; Lee, E.; Walker, A.R.; Tanner, G.J.; Larkin, P.J.; Ashton, A.R. The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J. 2003, 35, 624–636. [Google Scholar] [CrossRef]
- Zhao, J.; Pang, Y.; Dixon, R.A. The mysteries of proanthocyanidin transport and polymerization. Plant Physiol. 2010, 153, 437–443. [Google Scholar] [CrossRef]
- Debeaujon, I.; Léon-Kloosterziel, K.M.; Koornneef, M. Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol. 2000, 122, 403–414. [Google Scholar] [CrossRef]
- Jia, L.G.; Wu, Q.Y.; Ye, N.H.; Liu, R.; Shi, L.; Xu, W.F.; Zhi, H.; Bin Rahman, A.N.M.R.; Xia, Y.; Zhang, J. Proanthocyanidins inhibit seed germination by maintaining a high level of abscisic acid in Arabidopsis thaliana F. J. Integr. Plant Biol. 2012, 54, 663–673. [Google Scholar] [CrossRef]
- Wang, H.; Dai, W.; Zhang, Z.; Meng-Shuo, L.; Meng, L.; Zhang, Z.; Lu, H.; Song, X.-L.; Qiang, S. Occurrence pattern and morphological polymorphism of Chinese weedy rice. J. Integr. Agric. 2023, 22, 149–169. [Google Scholar] [CrossRef]
- Gu, X.; Zongxiang, C.; Foley, M.E. Inheritance of Seed Dormancy in Weedy Rice. Crop Sci. 2003, 43, 835–843. [Google Scholar] [CrossRef]
- Dai, W.M.; Zhang, K.Q.; Wu, J.R.; Wang, L.; Duan, B.W.; Zheng, K.L.; Cai, R.; Zhuang, J.-Y. Validating a segment on the short arm of chromosome 6 responsible for genetic variation in the hull silicon content and yield traits of rice. Euphytica 2008, 160, 317–324. [Google Scholar] [CrossRef]
- Lu, Y.J.; Zheng, K.L. A simple method for isolation of rice DNA. Chin. J. Rice Sci. 1992, 6, 47–48. [Google Scholar]
- Li, X.Y.; Qiang, S.; Song, X.L.; Cai, K.; Sun, Y.N.; Shi, Z.H.; Dai, W.-M. Allele types of Rc gene of weedy rice from Jiangsu Province, China. Rice Sci. 2014, 21, 252–261. [Google Scholar] [CrossRef]
- Neff, M.M.; Neff, J.D.; Chory, J.; Pepper, A.E. dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: Experimental applications in Arabidopsis thaliana genetics. Plant J. 1998, 14, 387–392. [Google Scholar] [CrossRef]
- Lim, S.; Ha, S. Marker development for the identification of rice seed color. Plant Biotechnol. Rep. 2013, 7, 391–398. [Google Scholar] [CrossRef]
- Sun, B.S.; Ricardo-Da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Seeds; Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Li, L.F.; Li, Y.L.; Jia, Y.; Caicedo, A.L.; Olsen, K.M. Signatures of adaptation in the weedy rice genome. Nat. Genet. 2017, 49, 811–814. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, Y.; Mao, L.; Ye, C.; Wang, W.; Zhang, J.; Yu, Y.; Fu, F.; Wang, Y.; Qian, F.; et al. Genomic variation associated with local adaptation of weedy rice during de-domestication. Nat. Commun. 2017, 8, 15323. [Google Scholar] [CrossRef]
- Gu, X.Y.; Foley, M.E.; Horvath, D.P.; Anderson, J.V.; Feng, J.H.; Zhang, L.H.; Mowry, C.R.; Ye, H.; Suttle, J.C.; Kadowaki, K.I.; et al. Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics 2011, 189, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.M.; Zhang, K.Q.; Duan, B.W.; Zheng, K.L.; Zhuang, J.Y.; Cai, R. Genetic dissection of silicon content in different organs of rice. Crop Sci. 2005, 45, 1345–1352. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Ou, K.Q.; Gu, L.W. Absorption and metabolism of proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Pintha, K.; Yodkeeree, S.; Limtrakul, P. Proanthocyanidin in red rice inhibits MDA-MB-231 breast cancer cell invasion via the expression control of invasive proteins. Biol. Pharm. Bull. 2015, 38, 571–581. [Google Scholar] [CrossRef]
- Qi, Q.Q.; Chu, M.J.; Yu, X.T.; Xie, Y.N.; Li, Y.L.; Du, Y.M.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and proanthocyanidins: Chemical Structures, food Sources, bioactivities, and product development. Food Rev. Int. 2023, 39, 4581–4609. [Google Scholar] [CrossRef]
- Bhupenchandra, I.; Chongtham, S.K.; Gangarani Devi, A.; Dutta, P.; Lamalakshmi, E.; Mohanty, S.; Choudhary, A.K.; Das, A.; Sarika, K.; Kumar, S.; et al. Harnessing weedy rice as functional food and source of novel traits for crop improvement. Plant Cell Environ. 2024, 48, 2498–2521. [Google Scholar] [CrossRef]
- Shirley, B.W. Flavonoids in seeds and grains: Physiological function, agronomic importance and the genetics of biosynthesis. Seed Sci. Res. 1998, 8, 415–422. [Google Scholar] [CrossRef]
- Gunaratne, A.; Wu, K.; Li, D.Q.; Bentota, A.; Corke, H.; Cai, Y.Z. Antioxidant activity and nutritional quality of traditional red-grained rice varieties containing proanthocyanidins. Food Chem. 2013, 138, 1153–1161. [Google Scholar] [CrossRef]
- Kong, M.Y.; He, X.T.; Yin, Z.D.; Chen, X.S.; Zhang, Y.J.; Shi, Z.H.; Song, X.; Qiang, S.; Dai, W. Removing harmful pericarp character of weedy rice as the first step of domestication towards direct-seeding rice using CRISPR/Cas9-Targeted mutagenesis. Agronomy 2023, 13, 1130. [Google Scholar] [CrossRef]
- Tang, L.; Ma, D.R.; Xu, Z.J.; Deng, H.F.; Chen, W.F.; Yuan, L.P. Utilization of weedy rice for development of japonica hybrid rice (Oryza sativa L.). Plant Sci. 2011, 180, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.G.; Pang, L.H.; Jiang, X.Q.; Lu, B.R. Impact of soil burial depths on survival of weedy rice seeds: Implications for weed management. Agronomy 2024, 14, 1281. [Google Scholar] [CrossRef]
- Vargas, A.A.M.; Agostinetto, D.; Carlos, F.S.; Cereza, T.V.; Ulguim, A.D.R. Survival and distribution of weedy rice seedbank after twenty-two years of different rice cropping systems. Ciência Rural 2023, 53, e20210787. [Google Scholar] [CrossRef]
- Ghotbzadeh, S.; Gianinetti, A. A response of the imbibed dormant red rice caryopsis to biotic challenges involves extracellular pH increase to elicit superoxide production. Seed Sci. Res. 2018, 28, 261–271. [Google Scholar] [CrossRef]
- Long, R.L.; Gorecki, M.J.; Renton, M.; Scott, J.K.; Colville, L.; Goggin, D.E.; Commander, L.E.; Westcott, D.A.; Cherry, H.; Finch-Savage, W.E. The ecophysiology of seed persistence: A mechanistic view of the journey to germination or demise. Biol. Rev. Camb. Philos. Soc. 2015, 90, 31–59. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, H.; Chen, T.; Ding, L.; Zhang, L.; Ding, X.; Zhang, J.; Qian, Q.; Xiang, Y. Sdr4 dominates pre-harvest sprouting and facilitates adaptation to local climatic condition in Asian cultivated rice. J. Integr. Plant Biol. 2022, 64, 1246–1263. [Google Scholar] [CrossRef]
- Gu, X.Y.; Kianian, S.F.; Foley, M.E. Isolation of three dormancy QTLs as Mendelian factors in rice. Heredity 2006, 96, 93–99. [Google Scholar] [CrossRef][Green Version]
- Sugimoto, K.; Takeuchi, Y.; Ebana, K.; Miyao, A.; Hirochika, H.; Hara, N.; Ishiyama, K.; Kobayashi, M.; Ban, Y.; Hattori, T.; et al. Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. USA 2010, 107, 5792–5797. [Google Scholar] [CrossRef]
- Chen, W.; Wang, W.; Lyu, Y.; Wu, Y.; Huang, P.; Hu, S.; Wei, X.; Jiao, G.; Sheng, Z.; Tang, S.; et al. OsVP1 activates Sdr4 expression to control rice seed dormancy via the ABA signaling pathway. Crop J. 2021, 9, 68–78. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.J.; Sun, X.X.; He, X.T.; Yang, J.L.; Chen, X.F.; Shi, Z.; Xiao-Ling, S.; Qiang, S.; Dai, W. Weedy rice de-domesticated from cultivated rice has evolved strong resistance to seed ageing. Weed Res. 2021, 61, 396–405. [Google Scholar] [CrossRef]
- Xia, H.B.; Xia, H.; Ellstrand, N.C.; Yang, C.; Lu, B.R. Rapid evolutionary divergence and ecotypic diversification of germination behavior in weedy rice populations. N. Phytol. 2011, 191, 1119–1127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).