Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
2.2. Measurement and Calculation Methods
2.3. Soluble Protein Content Detection
2.4. Real-Time Quantitative PCR Analysis
2.5. Metabolites Extraction
2.6. UHPLC-MS/MS Analysis
2.7. Precision and Accuracy
3. Results
3.1. Changes of Nitrogen Accumulation of Soybean Plants Under Low-P Stress
3.2. 15N Abundance of Soybean Plants Under Low-P Stress
3.3. Changes in Nodule Nitrogen Fixation Accumulation and Ratio of Nodule Nitrogen Fixation of Soybean Plants Under Low-P Stress
3.4. Effect of Low-P Stress on Soluble Protein Content of Soybean
3.5. Effect of Low-P Stress on PTs of Soybean
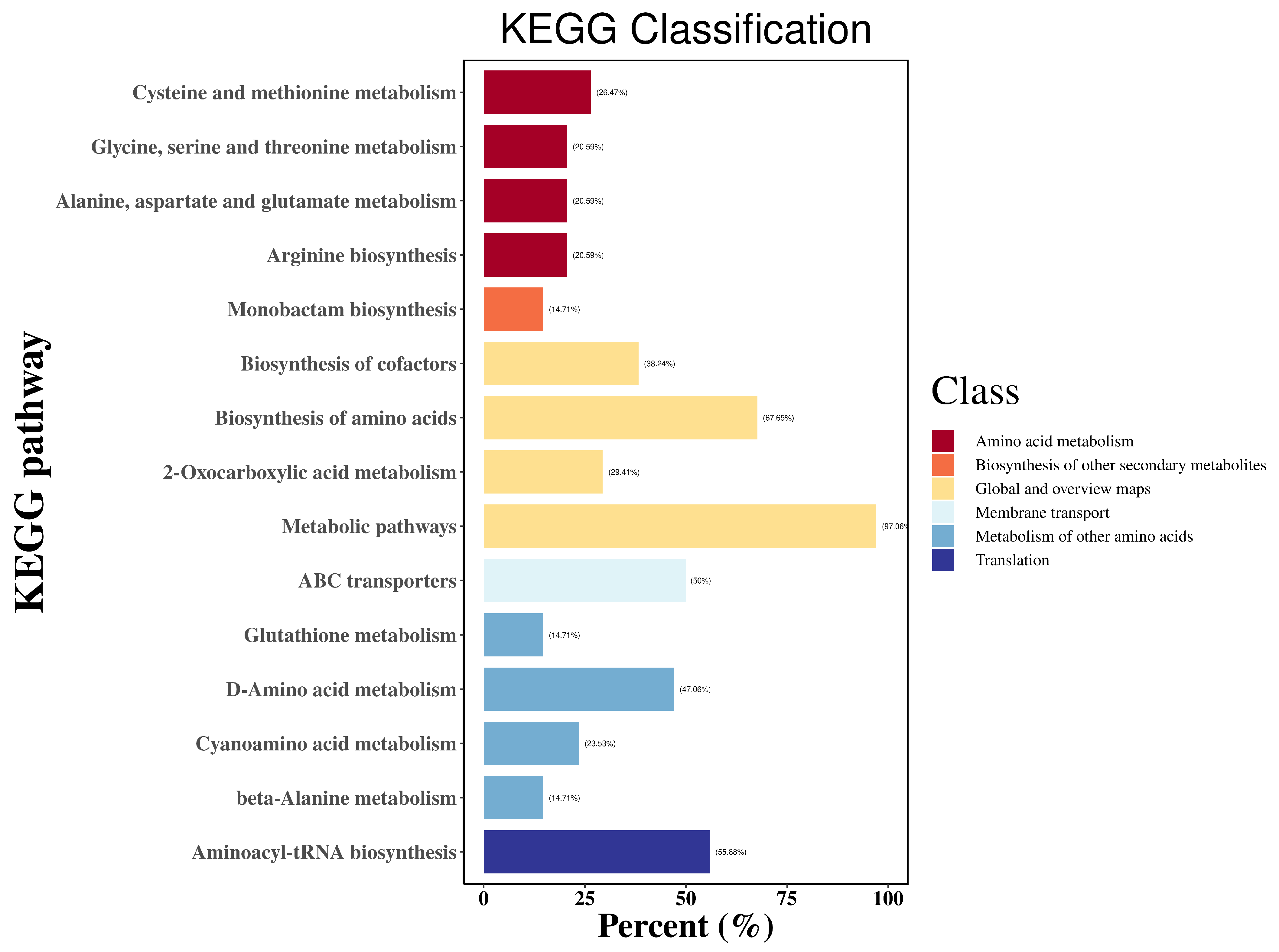
3.6. Effect of Low-P Stress on Amino Acid Metabolism in Soybean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duan, Z.B.; Zhang, M.; Liang, S.; Fan, L. Natural allelic variation of GmST05 controlling seed size and quality in soybean. Plant Biotechnol. J. 2022, 20, 1807–1818. [Google Scholar] [CrossRef]
- Lu, J.L. Plant Nutrition; China Agricultural University: Beijing, China, 2003; pp. 35–36. [Google Scholar]
- Deng, L.; Zhang, C.; Yuan, K.; Gao, Y.; Pan, Y.W.; Ge, X.L.; He, Y.X.; Yuan, Y.; Lu, Y.; Zhang, X.X.; et al. Prioritizing natural-selection signals from the deep-sequencing genomic data suggests multi-variant adaptation in Tibetan highlanders. Natl. Sci. Rev. 2019, 6, 1201–1222. [Google Scholar] [CrossRef]
- King, K.E.; Lauter, N.; Lin, S.F.; Scott, M.P.; Shoemaker, R.C. Evaluation and QTL mapping of phosphorus concentration in soybean seed. Euphytica 2013, 189, 261–269. [Google Scholar] [CrossRef]
- Chen, L.; Liao, H. Engineering crop nutrient efficiency for sustainable agriculture. J. Integr. Plant Biol. 2017, 59, 710–735. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Gao, Z.; Zhou, H.W.; He, Y.; Liu, Y.X.; Lai, Y.L.; Zheng, J.K.; Li, X.X.; Liao, H. GmPTF1 modifies root architecture responses to phosphate starvation primarily through regulating GmEXPB2 expression in soybean. Plant J. 2021, 107, 525–543. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Efficiency of soil and fertilizer phosphorus use: Reconciling changing concepts of soil phosphorus behaviour with agronomic information. Exp. Agric. 2009, 45, 128–135. [Google Scholar] [CrossRef]
- Sun, M.; Li, P.C.; Wang, N.; Zheng, C.S.; Sun, X.Z.; Dong, H.L.; Han, H.M.; Feng, W.N.; Shao, J.J.; Zhang, Y.F. Soil available phosphorus deficiency reduces boll biomass and lint yield by affecting sucrose metabolism in cotton-boll subtending leaves. Agronomy 2022, 12, 1065. [Google Scholar] [CrossRef]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Sulieman, S.; Van Ha, C.; Schulze, J.; Tran, L.S. Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J. Exp. Bot. 2013, 64, 2701–2712. [Google Scholar] [CrossRef]
- Tsvetkova, G.E.; Georgiev, G.I. Effect of phosphorus nutrition on the nodulation, nitrogen fixation and nutrient-use efficiency of Bradyrhizobium Japonicum-soybean (Glycine max l. merr.) symbiosis. Bulg. J. Plant Physiol. 2003, 3, 315–335. [Google Scholar]
- Isaac, M.E.; Harmand, J.M.; Drevon, J.J. Growth and nitrogen acquisition strategies of Acacia senegal seedlings under exponential phosphorus additions. J. Plant Physiol. 2010, 168, 776–781. [Google Scholar] [CrossRef]
- Maistry, P.M.; Cramer, M.D.; Chimphango, S.B. N and P colimitation of N2-fixing and N-supplied fynbos legumes from the Cape Floristic Region. Plant Soil 2013, 373, 217–228. [Google Scholar] [CrossRef]
- Sharma, P.; Kumari, A. Approaches to Enhance Abiotic and Biotic Stress Tolerance in Leguminous Crops and Microgreens; Mathur, P., Gupta, A., Eds.; Springer: Cham, Switzerland, 2025. [Google Scholar] [CrossRef]
- Qi, W.D. Functional Analysis of GmG3PT3 Involvement in Soybean Responses to Phosphorus Deficiency; South China Agricultural University: Guangzhou, China, 2017. [Google Scholar]
- Cai, B.Y.; Ge, J.P.; Zu, W. Soluble protein content in leaves and seeds of different soybean genotypes as affected by different phosphorus supplies. J. Plant Nutr. Fertil. 2007, 13, 1185–1188. [Google Scholar]
- Yang, L.X.; Wang, Y.L.; Huang, J.Y.; Zhu, J.G.; Yang, H.J.; Liu, G.; Dong, G.C.; Hu, J. Seasonal changes in the effects of free-air CO2 enrichment (FACE) on phosphorus uptake and utilization of rice at three levels of nitrogen fertilization. Field Crops Res. 2007, 102, 141–150. [Google Scholar] [CrossRef]
- Chu, S.S.; Zhang, X.Q.; Yu, K.Y.; Lv, L.L.; Sun, C.Y.; Liu, X.Q.; Zhang, J.Y.; Jiao, Y.Q.; Zhang, D. Genome-wide analysis reveals dynamic epigenomic differences in soybean response to low-phosphorus stress. Int. J. Mol. Sci. 2020, 21, 6817. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, J.; Trigueros, M.; Rojas-Triana, M.; Fernández, M.; Albar, J.P.; Bustos, R.; Paz-Ares, J.; Rubio, V. Proteomics identifies ubiquitin-proteasome targets and new roles for chromatin-remodeling in the Arabidopsis response to phosphate starvation. J. Proteom. 2013, 94, 1–22. [Google Scholar] [CrossRef]
- Jiang, W.Z.; He, P.M.; Zhou, M.; Lu, X.; Chen, K.; Liang, C.Y.; Tian, J. Soybean responds to phosphate starvation through reversible protein phosphorylation. Plant Physiol. Biochem. 2021, 167, 222–234. [Google Scholar] [CrossRef]
- Liang, C.Y.; Tian, J.; Liao, H. Proteomics dissection of plant responses to mineral nutrient deficiency. Proteomics 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and Environmental Regulation of Root Development. Annu. Rev. Plant Biol. 2019, 70, 465–488. [Google Scholar] [CrossRef]
- Chen, L.Y.; Qin, L.; Zhao, J.; Liao, H. Advances in Pht1 Phosphate Transporter Family Genes in Legumes. Soybean Sci. 2015, 34, 1057–1065. [Google Scholar]
- Song, H.N.; Yin, Z.T.; Chao, M.N.; Ning, L.H.; Zhang, D.; Yu, D.Y. Functional properties and expression quantitative trait loci for phosphate transporter GmPT1 in soybean. Plant Cell Environ. 2014, 37, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Reid, R.J.; Ayling, S.M. Phosphorus uptake by plants: From soil to cell. Plant Physiol. 1998, 116, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Wang, J.X.; Zhao, J.; Tian, J.; Liao, H. Control of phosphate homeostasis through gene regulation in crops. Curr. Opin. Plant Biol. 2014, 21, 59–66. [Google Scholar] [CrossRef]
- Mitsukawa, N.; Okumura, S.; Shirano, Y.; Sato, S.; Kato, T.; Harashima, S.; Shibata, D. Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc. Natl. Acad. Sci. USA 1997, 94, 7098–7102. [Google Scholar]
- Yang, X.J.; Finnegan, P.M. Regulation of phosphate starvation responses in higher plants. Ann. Bot. 2010, 105, 513–526. [Google Scholar] [CrossRef]
- Tian, J.; Venkatachalam, P.; Liao, H.; Yan, X.L.; Raghothama, K. Molecular cloning and characterization of phosphorus starvation responsive genes in common bean (Phaseolus vulgaris L.). Planta 2007, 227, 51–65. [Google Scholar] [CrossRef]
- Grønlund, M.; Albrechtsen, M.; Johansen, I.E.; Hammer, E.C.; Nielsen, T.H.; Jakobsen, I. The interplay between P uptake pathways in mycorrhizal peas: A combined physiologyogical and gene-silencing approach. Physiol. Plant. 2013, 149, 234–248. [Google Scholar] [CrossRef]
- Volpe, V.; Dellaglio, E.; Giovannetti, M.; Ruberti, C.; Costa, A.; Genre, A.; Guether, M.; Bonfante, P. An AM-induced, MYB-family gene of Lotusjaponicus (LjMAMI) affects root growth in an AM-independent manner. Plant J. 2013, 73, 442–455. [Google Scholar] [CrossRef]
- Wang, F.; Cui, P.J.; Tian, Y.; Huang, Y.; Wang, H.F.; Liu, F.; Chen, Y.F. Maize ZmPT7 regulates Pi uptake and redistribution which is modulated by phosphorylation. Plant Biotechnol. J. 2020, 18, 2406–2419. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, M.; Liang, R.S.; Shi, X.Y.; Chen, L.L.; Hu, X.; Wang, S.C.; Dai, X.L.; Qu, H.Y.; Li, H.H.; et al. OsWRKY21 and OsWRKY108 function redundantly to promote phosphate accumulation through maintaining the constitutive expression of OsPHT1;1 under phosphate-replete conditions. New Phytol. 2021, 229, 1598–1614. [Google Scholar] [CrossRef]
- Wang, S.C.; Xu, T.T.; Chen, M.; Geng, L.Y.; Huang, Z.Y.; Dai, X.L.; Qu, H.Y.; Zhang, J.; Li, H.H.; Gu, M.; et al. The transcription factor OsWRKY10 inhibits phosphate uptake via suppressing OsPHT1;2 expression under phosphate-replete conditions in rice. J. Exp. Bot. 2022, 74, 1074–1089. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.R.; Dai, X.L.; Qu, H.Y.; Men, Q.; Liu, J.Y.; Yu, L.; Gu, M.; Xu, G.H. The rice phosphate transporter OsPHT1;7 plays a dual role in phosphorus redistribution and anther development. Plant Physiol. 2022, 188, 2272–2288. [Google Scholar] [CrossRef]
- Noike, Y.; Okamoto, I.; Tada, Y. Root epidermis-specific expression of a phosphate transporter TaPT2 enhances the growth of transgenic Arabidopsis under Pi-replete and Pi-depleted conditions. Plant Sci. 2023, 327, 111540. [Google Scholar] [CrossRef]
- Wang, P.F.; Li, G.Z.; Li, G.W.; Yuan, S.S.; Wang, C.Y.; Xie, Y.X.; Guo, T.C.; Kang, G.Z.; Wang, D.W. TaPHT1;9-4B and its transcriptional regulator TaMYB4-7D contribute to phosphate uptake and plant growth in bread wheat. New Phytol. 2021, 231, 1968–1983. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.L.; Cao, H.R.; Zhao, J.; Bai, S.; Peng, W.T.; Li, J.; Sun, L.L.; Chen, L.Y.; Lin, Z.H.; Shi, C.; et al. A natural uORF variant confers phosphorus acquisition diversity in soybean. Nat. Commun. 2022, 13, 3796. [Google Scholar] [CrossRef]
- Luan, M.D.; Zhao, F.G.; Sun, G.F.; Xu, M.; Fu, A.; Lan, W.Z.; Luan, S. A SPX domain vacuolar transporter links phosphate sensing to homeostasis in Arabidopsis. Mol. Plant 2022, 15, 1590–1601. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, H.Y.; Chu, S.S.; Li, H.Y.; Chi, Y.J.; Triebwasser, F.D.; Lv, H.Y.; Yu, D.Y. Integrating QTL mapping and transcriptomics identifies candidate genes underlying QTLs associated with soybean tolerance to low-phosphorus stress. Plant Mol. Biol. 2017, 83, 137–150. [Google Scholar] [CrossRef]
- Medhi, A.K.; Dhar, S.; Roy, A. Effect of different growth regulators and phosphorus levels on nodulation, yield and quality components in green gram. Indian J. Plant Physiol. 2014, 19, 74–78. [Google Scholar] [CrossRef]
- Ran, Q.X.; Pang, J.Y.; Dong, R.; He, J. Enhanced seed yield, essential amino acids and unsaturated fatty acids in soybean seeds with phosphorus fertilizer supply. J. Food Compos. Anal. 2024, 125, 105813. [Google Scholar] [CrossRef]
- Hernández, G.; Ramírez, M.; Valdés-López, O.; Tesfaye, M.; Graham, M.A.; Czechowski, T.; Schlereth, A.; Wandrey, M.; Erban, A.; Cheung, F.; et al. Phosphorus stress in common bean: Root transcript and metabolic responses. Plant Physiol. 2007, 144, 752–767. [Google Scholar] [CrossRef]
- Le roux, M.R.; Khan, S.; Vapentine, A.J. Nitrogen and carbon costs of soybean and lupin root systems during phosphate starvation. Symbiosis 2009, 48, 102–109. [Google Scholar] [CrossRef]
- Yao, Y.B.; Yuan, H.M.; Wu, G.W.; Yan, J.; Zhao, D.S.; Chen, S.; Kang, Q.H.; Ma, C.M.; Gong, Z.P. Nitrogen fixation capacity and metabolite responses to phosphorus in soybean nodules. Symbiosis 2022, 88, 21–35. [Google Scholar] [CrossRef]
- Yao, Y.B.; Yuan, H.M.; Wu, G.W.; Ma, C.M.; Gong, Z.P. Proteome Analysis of the Soybean Nodule Phosphorus Response Mechanism and Characterization of Stress-Induced Ribosome Structural and Protein Expression Changes. Front. Plant Sci. 2022, 13, 908889. [Google Scholar] [CrossRef]
- Zhou, M.; Zhu, S.N.; Mo, X.H.; Guo, Q.; Li, Y.X.; Tian, J.; Liang, C.Y. Proteomic analysis dissects molecular mechanisms underlying plant responses to phosphorus deficiency. Cells 2022, 11, 651. [Google Scholar] [CrossRef] [PubMed]
- Lazali, M.; Bargaz, A.; Carlsson, G.; Ounane, S.M.; Drevon, J.J. Discrimination against 15N among recombinant inbred lines of Phaseolus vulgaris L. contrasting in phosphorus use efficiency for nitrogen fixation. J. Plant Physiol. 2014, 171, 199–204. [Google Scholar] [CrossRef]
- Høgh-Jensen, H.; Schjoerring, J.K.; Soussana, J.F. The influence of phosphorus deficiency on growth and nitrogen fixation of white clover plants. Ann. Bot. 2002, 90, 745–753. [Google Scholar] [CrossRef]
- Desnos, T. Root branching responses to phosphate and nitrate. Curr. Opin. Plant Biol. 2007, 11, 82–87. [Google Scholar] [CrossRef]
- Rotaru, V.; Sinclair, T.R. Interactive influence of phosphorus and iron on nitrogen fixation by soybean. Environ. Exp. Bot. 2009, 66, 94–99. [Google Scholar] [CrossRef]
- Chen, L.Y.; Qin, L.; Zhou, L.L.; Li, X.X.; Chen, Z.C.; Liao, H. A nodule-localized phosphate transporter GmPT7 plays an important role in enhancing symbiotic N2 fixation and yield in soybean. New Phytol. 2019, 221, 2013–2025. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Yang, A.H.; Kong, L.J.; Wang, H.Y.; Ao, X. Proteome characterization of two contrasting soybean genotypes in response to different phosphorus treatments. AoB Plants 2021, 13, lab019. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, X.M.; Dong, S.K. Biochemical characterization and metabolic reprogramming of amino acids in Soybean roots under drought stress. Physiol. Plant. 2024, 176, e14319. [Google Scholar] [CrossRef] [PubMed]
- Tawaraya, K.; Horie, R.; Shinano, T.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of soybean root exudates under phosphorus deficiency. Soil Sci. Plant Nutr. 2014, 60, 679–694. [Google Scholar]
- Zhang, L. Root exudates and soil fertility. Bull. Biol. 2000, 35, 17. [Google Scholar] [CrossRef]
- Mo, X.H. Phosphate Starvation Responsive Protein Phosphatase GmHAD1-2 Regulates Flavonol Biosynthesis and Lateral Root Development in Soybean; South China Agricultural University: Guangzhou, China, 2019. [Google Scholar] [CrossRef]
- Shi, G.Y. Physiological Mechanism and Proteomics of Soybean Root Response to Low Phosphorus Stress; Guizhou University: Guiyang, China, 2021. [Google Scholar] [CrossRef]
- Yao, Y.B.; Yuan, H.M.; Liu, D.D.; Cheng, L.L. Response of soybean root exudates and related metabolic pathways to low phosphorus stress. PLoS ONE 2024, 19, e0314256. [Google Scholar] [CrossRef]
- Keitaro, T.; Ryota, H.; Saki, S.; Tadao, W.; Kazuki, S.; Akira, O. Metabolite profiling of root exudates of common bean under phosphorus deficiency. Metabolites 2014, 4, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Hernández, G.; Valdés-López, O.; Ramírez, M.; Goffard, N.; Weiller, G.; Aparicio-Fabre, R.; Fuentes, S.I.; Erban, A.; Kopka, J.; Udvardi, M.K.; et al. Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol. 2009, 151, 1221–1238. [Google Scholar] [CrossRef]
- Lundberg, P.; Lundquist, P.O. Primary metabolism in N2 fixing Alnus incanaFrankia symbiotic root nodules studied with 15N and 31P nuclear magnetic resonance spectroscopy. Planta 2004, 219, 661–672. [Google Scholar]
- Yao, Y.B. Multi-omics analysis of carbon and nitrogen metabolism pathways in soybean nodules under phosphorus stress. Soybean Sci. 2024, 43, 624–631. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, M.; Yang, G.; Sun, M.; Yang, A.; Sun, C.; Zhao, H.; Ao, X. Root morphology, nitrogen metabolism and amino acid metabolism in soybean under low phosphorus stress. Sci. Rep. 2024, 14, 28583. [Google Scholar] [CrossRef]

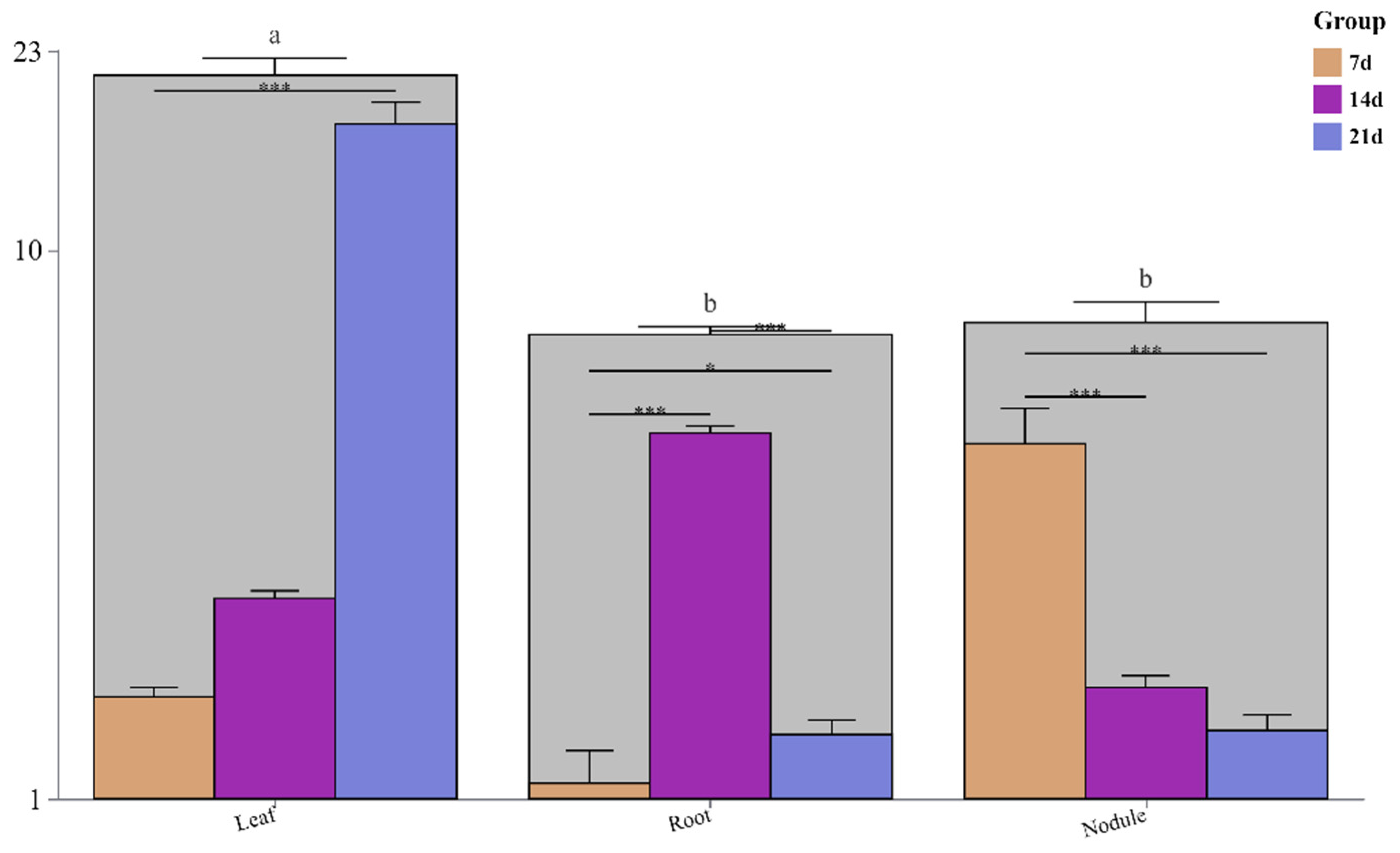


| Treatments | Aboveground | Root | Nodule | ||||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 7 days | P1 | 23.98 ± 0.21 * | 31.78 ± 1.67 * | 9.53 ± 0.10 * | 9.54 ± 0.10 * | 1.22 ± 0.00 * | 1.19 ± 0.08 * |
| P31 | 39.49 ± 0.47 | 66.90 ± 1.82 | 10.73 ± 0.16 | 10.73 ± 0.16 | 5.39 ± 0.12 | 8.82 ± 0.34 | |
| 14 days | P1 | 29.78 ± 0.49 * | 47.68 ± 1.02 * | 16.74 ± 0.01 * | 9.94 ± 0.24 * | 1.53 ± 0.00 * | 2.56 ± 0.05 * |
| P31 | 90.78 ± 2.70 | 92.42 ± 1.97 | 19.60 ± 0.15 | 14.17 ± 0.13 | 11.20 ± 0.46 | 8.49 ± 0.06 | |
| 21 days | P1 | 48.26 ± 3.11 * | 46.48 ± 0.75 * | 18.96 ± 0.96 * | 17.09 ± 0.21 * | 1.23 ± 0.12 * | 2.67 ± 0.14 * |
| P31 | 143.23 ± 7.02 | 225.48 ± 3.62 | 37.99 ± 0.08 | 44.90 ± 2.57 | 26.82 ± 0.21 | 24.64 ± 0.30 | |
| 28 days | P1 | 75.03 ± 2.98 * | 63.15 ± 0.12 * | 25.38 ± 1.12 * | 20.02 ± 1.04 * | 2.23 ± 0.04 * | 2.67 ± 0.17 * |
| P31 | 248.56 ± 14.83 | 325.61 ± 8.05 | 66.41 ± 1.26 | 76.50 ± 2.38 | 33.59 ± 1.18 | 24.87 ± 1.04 | |
| 35 days | P1 | 102.52 ± 0.27 * | 97.70 ± 3.49 * | 41.52 ± 1.04 * | 22.74 ± 1.28 * | 3.65 ± 0.35 * | 2.69 ± 0.23 * |
| P31 | 403.80 ± 14.93 | 476.66 ± 2.02 | 92.52 ± 2.31 | 90.20 ± 1.73 | 37.02 ± 0.39 | 36.22 ± 0.68 | |
| 42 days | P1 | 186.66 ± 0.59 * | 175.45 ± 1.29 * | 53.08 ± 1.68 * | 58.64 ± 0.16 * | 6.69 ± 0.04 * | 2.92 ± 0.21 * |
| P31 | 425.70 ± 2.87 | 464.67 ± 5.73 | 89.55 ± 6.04 | 140.15 ± 1.13 | 40.09 ± 0.65 | 30.95 ± 1.67 | |
| 49 days | P1 | 302.11 ± 0.88 * | 271.70 ± 5.51 * | 92.58 ± 0.19 * | 87.96 ± 4.19 * | 11.23 ± 0.07 * | 5.58 ± 0.23 * |
| P31 | 446.68 ± 1.03 | 684.25 ± 7.95 | 117.42 ± 1.36 | 108.26 ± 2.85 | 46.79 ± 0.77 | 25.94 ± 1.39 | |
| Treatments | Aboveground | Root | Nodule | ||||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 7 days | P1 | 1.89 ± 0.01 | 1.91 ± 0.00 | 2.11 ± 0.04 * | 2.02 ± 0.01 | 0.91 ± 0.00 * | 1.02 ± 0.02 * |
| P31 | 1.91 ± 0.02 | 1.94 ± 0.02 | 1.80 ± 0.00 | 1.81 ± 0.05 | 1.21 ± 0.01 | 0.78 ± 0.00 | |
| 14 days | P1 | 2.08 ± 0.00 * | 2.17 ± 0.02 | 2.22 ± 0.02 | 2.33 ± 0.05 | 1.00 ± 0.03 * | 1.18 ± 0.05 * |
| P31 | 1.84 ± 0.01 | 2.00 ± 0.04 | 2.12 ± 0.04 | 2.13 ± 0.01 | 0.62 ± 0.00 | 0.78 ± 0.03 | |
| 21 days | P1 | 2.31 ± 0.00 * | 2.28 ± 0.04 * | 2.29 ± 0.03 * | 2.23 ± 0.03 * | 1.01 ± 0.04 * | 1.21 ± 0.00 * |
| P31 | 1.33 ± 0.06 | 1.30 ± 0.03 | 1.71 ± 0.02 | 1.76 ± 0.01 | 0.51 ± 0.03 | 0.44 ± 0.00 | |
| 28 days | P1 | 2.42 ± 0.00 * | 2.31 ± 0.03 * | 2.40 ± 0.00 * | 2.35 ± 0.03 * | 1.28 ± 0.02 * | 1.06 ± 0.01 * |
| P31 | 1.17 ± 0.02 | 1.55 ± 0.03 | 1.61 ± 0.01 | 2.00 ± 0.00 | 0.50 ± 0.00 | 0.53 ± 0.02 | |
| 35 days | P1 | 2.45 ± 0.02 * | 2.43 ± 0.01 * | 2.44 ± 0.01 * | 2.44 ± 0.03 * | 1.00 ± 0.03 * | 1.40 ± 0.01 * |
| P31 | 1.20 ± 0.01 | 1.60 ± 0.00 | 1.75 ± 0.00 | 1.98 ± 0.03 | 0.57 ± 0.01 | 0.59 ± 0.04 | |
| 42 days | P1 | 2.27 ± 0.08 * | 2.58 ± 0.02 * | 2.47 ± 0.05 * | 2.63 ± 0.00 * | 0.80 ± 0.01 * | 1.08 ± 0.04 * |
| P31 | 1.23 ± 0.00 | 1.72 ± 0.01 | 1.68 ± 0.00 | 2.07 ± 0.00 | 0.55 ± 0.00 | 0.53 ± 0.00 | |
| 49 days | P1 | 2.20 ± 0.05 * | 2.38 ± 0.06 * | 2.41 ± 0.03 * | 2.49 ± 0.04 * | 0.77 ± 0.03 * | 0.89 ± 0.08 * |
| P31 | 1.18 ± 0.01 | 1.72 ± 0.06 | 1.66 ± 0.02 | 2.06 ± 0.06 | 0.46 ± 0.00 | 0.53 ± 0.03 | |
| Treatments | Aboveground | Root | Nodule | ||||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 7 days | P1 | 10.26 ± 0.17 * | 11.76 ± 0.00 * | 5.86 ± 0.11 | 3.18 ± 0.02 * | 0.89 ± 0.00 * | 0.79 ± 0.06 * |
| P31 | 16.67 ± 0.51 | 24.11 ± 0.15 | 5.42 ± 0.02 | 4.33 ± 0.13 | 3.42 ± 0.06 | 6.54 ± 0.26 | |
| 14 days | P1 | 10.99 ± 0.11 * | 13.54 ± 0.71 * | 5.50 ± 0.09 * | 2.31 ± 0.11 * | 1.07 ± 0.01 * | 1.57 ± 0.01 * |
| P31 | 40.34 ± 1.59 | 31.33 ± 0.79 | 7.01 ± 0.22 | 4.22 ± 0.09 | 9.10 ± 0.37 | 6.32 ± 0.15 | |
| 21 days | P1 | 14.56 ± 0.79 * | 11.49 ± 0.50 * | 5.81 ± 0.11 * | 4.53 ± 0.11 * | 0.86 ± 0.10 * | 1.61 ± 0.08 * |
| P31 | 85.36 ± 1.55 | 128.9 ± 0.50 | 18.31 ± 0.28 | 18.81 ± 0.86 | 22.70 ± 0.43 | 21.08 ± 0.19 | |
| 28 days | P1 | 20.06 ± 0.71 * | 15.03 ± 0.75 * | 6.94 ± 0.29 * | 4.50 ± 0.03 * | 1.37 ± 0.04 * | 1.73 ± 0.12 * |
| P31 | 160.14 ± 7.94 | 159.62 ± 7.75 | 34.13 ± 0.87 | 26.14 ± 0.80 | 28.49 ± 0.97 | 20.55 ± 1.02 | |
| 35 days | P1 | 26.56 ± 0.75 * | 19.61 ± 1.10 * | 10.80 ± 0.82 * | 4.46 ± 0.02 * | 2.54 ± 0.21 * | 1.44 ± 0.11 * |
| P31 | 256.88 ± 7.70 | 225.39 ± 2.39 | 43.48 ± 1.31 | 31.36 ± 1.50 | 30.63 ± 0.50 | 29.12 ± 0.02 | |
| 42 days | P1 | 58.44 ± 1.28 * | 26.11 ± 1.54 * | 13.38 ± 0.37 * | 7.83 ± 0.82 * | 5.07 ± 0.00 * | 1.88 ± 0.09 * |
| P31 | 267.45 ± 1.61 | 200.22 ± 4.51 | 44.03 ± 1.14 | 44.77 ± 0.50 | 33.43 ± 0.59 | 25.55 ± 1.31 | |
| 49 days | P1 | 101.24 ± 4.32 * | 58.84 ± 6.80 * | 24.97 ± 1.04 * | 15.85 ± 0.32 * | 8.62 ± 0.05 * | 3.96 ± 0.31 * |
| P31 | 287.07 ± 2.21 | 295.93 ± 11.39 | 58.63 ± 1.56 | 34.78 ± 1.55 | 40.28 ± 0.54 | 21.48 ± 1.43 | |
| Treatments | Aboveground | Root | Nodule | ||||
|---|---|---|---|---|---|---|---|
| 2022 | 2023 | 2022 | 2023 | 2022 | 2023 | ||
| 7 days | P1 | 42.79 ± 0.32 | 37.01 ± 0.14 | 36.05 ± 1.49 * | 33.37 ± 0.56 * | 72.41 ± 0.16 * | 66.49 ± 0.56 * |
| P31 | 42.18 ± 0.78 | 36.09 ± 0.74 | 45.57 ± 0.16 | 40.40 ± 1.88 | 63.53 ± 0.34 | 74.16 ± 0.08 | |
| 14 days | P1 | 36.93 ± 0.21 * | 28.35 ± 0.88 * | 32.85 ± 0.61 | 23.36 ± 1.77 * | 69.86 ± 1.05 * | 61.27 ± 1.67 * |
| P31 | 44.42 ± 0.42 | 33.97 ± 1.59 | 35.80 ± 1.43 | 29.72 ± 0.25 | 81.29 ± 0.06 | 74.39 ± 1.30 | |
| 21 days | P1 | 30.20 ± 0.03 * | 24.76 ± 1.47 * | 30.76 ± 0.94 * | 26.54 ± 1.02 * | 69.54 ± 1.26 * | 60.23 ± 0.13 * |
| P31 | 59.78 ± 1.87 | 57.20 ± 1.14 | 48.20 ± 0.84 | 41.95 ± 0.48 | 84.63 ± 0.93 | 85.57 ± 0.26 | |
| 28 days | P1 | 26.75 ± 0.11 * | 23.80 ± 1.15 * | 27.35 ± 0.05 * | 22.60 ± 1.02 * | 61.31 ± 0.77 * | 64.94 ± 0.61 * |
| P31 | 64.50 ± 0.65 | 48.96 ± 1.16 | 51.38 ± 0.34 | 34.17 ± 0.01 | 84.83 ± 0.07 | 82.56 ± 0.67 | |
| 35 days | P1 | 25.90 ± 0.70 * | 20.04 ± 0.41 * | 26.13 ± 0.56 * | 19.75 ± 1.02 * | 69.88 ± 0.93 * | 53.78 ± 0.57 * |
| P31 | 63.65 ± 0.44 | 47.44 ± 0.31 | 46.98 ± 0.24 | 34.72 ± 0.99 | 82.73 ± 0.48 | 80.45 ± 1.45 | |
| 42 days | P1 | 31.32 ± 0.24 * | 14.87 ± 0.77 * | 25.31 ± 1.51 * | 13.35 ± 0.07 * | 75.71 ± 0.46 * | 64.44 ± 1.51 * |
| P31 | 62.83 ± 0.04 | 43.17 ± 0.44 | 49.14 ± 0.19 | 31.94 ± 0.09 | 83.37 ± 0.12 | 82.56 ± 0.21 | |
| 49 days | P1 | 33.52 ± 1.51 * | 21.57 ± 2.00 * | 26.97 ± 1.08 * | 17.88 ± 1.47 * | 76.73 ± 0.96 * | 70.75 ± 2.67 * |
| P31 | 64.27 ± 0.34 | 43.30 ± 2.16 | 49.91 ± 0.75 | 32.25 ± 2.28 | 86.09 ± 0.27 | 82.67 ± 1.09 | |
| Pathway | Compounds | Upregulated | Downregulated | |
|---|---|---|---|---|
| P1_Leaf_7d-P31_Leaf_7d | 37 | 26 | 15 | 11 |
| P1_Leaf_14d-P31_Leaf_14d | 37 | 26 | 17 | 9 |
| P1_Leaf_21d-P31_Leaf_21d | 37 | 26 | 19 | 7 |
| P1_Root_7d-P31_Root_7d | 37 | 26 | 18 | 8 |
| P1_Root_14d-P31_Root_14d | 37 | 26 | 14 | 12 |
| P1_Root_21d-P31_Root_21d | 37 | 26 | 13 | 13 |
| P1_Nodule_7d-P31_Nodule_7d | 37 | 26 | 18 | 8 |
| P1_Nodule_14d-P31_Nodule_14d | 37 | 26 | 19 | 7 |
| P1_Nodule_21d-P31_Nodule_21d | 37 | 26 | 12 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Liu, X. Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans. Agronomy 2025, 15, 836. https://doi.org/10.3390/agronomy15040836
Yao Y, Liu X. Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans. Agronomy. 2025; 15(4):836. https://doi.org/10.3390/agronomy15040836
Chicago/Turabian StyleYao, Yubo, and Xinlei Liu. 2025. "Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans" Agronomy 15, no. 4: 836. https://doi.org/10.3390/agronomy15040836
APA StyleYao, Y., & Liu, X. (2025). Responses of Nitrogen Metabolism Pathways to Low-Phosphorus Stress: Decrease in Nitrogen Accumulation and Alterations in Protein Metabolism in Soybeans. Agronomy, 15(4), 836. https://doi.org/10.3390/agronomy15040836






