Abstract
One of the most persistent and damaging diseases in olive trees is olive knot disease. This disease is caused by an infection by the Gram-negative phytopathogenic bacterium Pseudomonas savastanoi pv. savastanoi that is notoriously difficult to control. The increasing demand for eco-friendly and sustainable agricultural solutions has driven research into plant-based agents. This study investigated the antibacterial properties of essential oils (EOs) and their constituents, olive mill wastewater (OMWW), the phenolic compound hydroxytyrosol (HTyr), and algae and garlic extracts, as well as copper-based and plant-stimulating commercial products against P. savastanoi pv. savastanoi, a significant olive tree pathogen. Antibacterial activity was determined using the Kirby–Bauer disc diffusion and broth microdilution methods. The EOs derived from Thymus vulgaris (thyme) and Origanum compactum (oregano), and their key components thymol and carvacrol, exhibited the strongest antibacterial efficacy. Conversely, the OMWW, plant-stimulating products, and algae and garlic extracts showed limited to no antibacterial activity in vitro, with their antibacterial properties determined using the disc diffusion method. While the EOs were highly effective in vitro, regardless of the testing method, their efficacy in bacterial growth inhibition was strain- and concentration-dependent, possibly highlighting some metabolic or genetic variability in the target pathogen, even though the MIC values against all tested strains of P. savastanoi pv. savastanoi were equal. Bacterial membrane disruption and the consequent leakage of metabolites were determined as the modes of action of carvacrol and oregano EO. Carvacrol also promoted plant growth in lettuce without significant phytotoxic effects, although minor necrotic lesions were observed in young olive leaves at higher concentrations, presenting these agents as potential next-generation green bactericides.
1. Introduction
In agriculture, olives (Olea europaea L.) are a hallmark of the Mediterranean region, celebrated for the numerous health benefits associated with olive oil consumption. However, olive cultivation is frequently challenged by various diseases that threaten orchard productivity. Among these, olive knot disease stands out as one of the most persistent and economically damaging. This disease affects olive trees worldwide and is considered chronic and capable of causing substantial economic losses through both direct and indirect damage to host plants [1]. Olive knot disease is caused by infection with the Gram-negative bacterial pathogen Pseudomonas savastanoi pv. savastanoi, a member of the Pseudomonas syringae species complex. Given the limited options for plant-protective solutions against P. savastanoi pv. savastanoi—typically with reliance on the preventive application of copper-based products and antibiotics, the latter of which are prohibited in many agricultural areas—and the scarcity of effective bactericidal compounds, there is an urgent need for innovative solutions. The implementation of alternative molecules for integrated and organic disease management is crucial to ensure sustainability in olive cultivation. The negative consequences of excessive pesticide use have driven increased interest in alternative approaches to plant protection.
Regulatory frameworks, such as the EU Directive 2009/128/EC [2], aim to reduce pesticide use and the associated risks by 50% across the European Union by 2030, aligning with initiatives like the Farm to Fork and Biodiversity Strategies. In this context, plant metabolites have emerged as promising candidates for biopesticides, offering a potential balance between high crop yields and sustainable agroecosystems. However, despite their promise, it remains a significant challenge to develop plant-based products that are both highly effective and commercially viable. While some biopesticides, including plant- and microorganism-based compounds, are already available, they are often not registered explicitly as plant protection products. Additionally, their effectiveness in commercial settings is often limited when compared to that of the conventional plant protection solutions [3,4]. Essential oils (EOs) have emerged as highly promising alternative options due to their complex mixtures of bioactive compounds, particularly terpenoids, which are well documented for their antibacterial properties [5]. Their lipophilic nature facilitates their penetration through microbial cell membranes, leading to toxicity and eventual cell death [6]. Consequently, EOs and their components are particularly effective against Gram-negative bacterial pathogens [7,8,9,10,11,12]. However, the chemical composition of EOs can vary significantly due to factors such as climate, geographical location, cultivation practices, harvest timing, plant genetics, and physiological characteristics [13]. Among plant species, those in the Lamiaceae family are frequently highlighted for their antibacterial potential.
The in vitro antibacterial potential of plant-based treatments, including their strain susceptibility and inhibitory concentrations, can vary widely due to several factors. These include the testing methods employed, the solubility of the active components in the growth medium, and the lack of a universally optimised testing protocol for plant pathogenic microorganisms. Notably, broth dilution methods are often regarded as the “gold standard” for antibacterial testing [14,15]. In addition, variations in the results may arise due to differences in bacterial strain or pathovar susceptibility within the same species, such as those observed in P. savastanoi [7]. Plant secondary metabolites exert their antimicrobial effects through diverse mechanisms. These include cytotoxicity, cell lysis, cytoplasmic coagulation, leakage of cellular metabolites and ions, the disruption of the proton motive force and fatty acid composition of membranes, increased membrane permeability, alterations in intra- and extracellular ATP and ATPase enzyme activity, and interference with membrane proteins as well [5,6,7,11]. Another crucial mechanism of action is the disruption of bacterial communication, known as quorum sensing (QS), which inhibits key virulence factors such as proteolytic activity and biofilm formation [7,11]. Some studies have shown that the antibacterial compound carvacrol to be an effective plant growth-promoter. Therefore, it can be evaluated further as a plant-stimulating compound for plant development [16].
Besides EOs and terpenoids, plant waste such as olive mill wastewater (OMWW) is considered a candidate in plant protection due to its antibacterial activity caused by the concentrated phenolic compounds in its chemical profile. However, significant variability in the concentration of total phenolic compounds in OMWW obtained from fruit processing has been documented in the literature, particularly across different olive cultivars or filtration methods [17]. This suggests that the antimicrobial activity of OMWW may differ depending on the olive variety processed. For instance, OMWW from four cultivars—"Frantoio”, “Mission”, “Taggiasca”, and “Picual”—has been shown to exhibit antibacterial properties against human pathogens such as Staphylococcus aureus, Escherichia coli, P. aeruginosa, and Listeria monocytogenes [17,18,19]. However, research has shown that even very low concentrations of the bioactive compounds from OMWW can inhibit pathogen growth in vitro. For example, one study demonstrated that OMWW extract was more effective than standard copper treatments against certain phytopathogens [20]. Hydroxytyrosol (HTyr) is a phenolic compound found in OMWW, and it has antimicrobial activity against numerous plant pathogenic bacterial and fungal species, including the bacteria R. radiobacter and P. savastanoi pv. savastanoi, which are known to induce gall formation in host plants and are notoriously difficult to control [21].
The primary objective of this study was to evaluate the susceptibility of P. savastanoi pv. savastanoi to the antibacterial effects of EOs derived from species within the Lamiaceae family, including Mentha, Salvia, Thymus, and Origanum. Additionally, this study assessed the antibacterial activity of specific active compounds in these EOs that contributed to their chemotypes, as well as other plant-based treatments, such as OMWW, HTyr, plant-stimulating products, and copper-based commercial products. By evaluating the antibacterial potential and mechanisms of action of these bioactive compounds across varying concentrations, this study aimed to identify their effectiveness in vitro and the longevity of their antibacterial properties to provide critical insights for developing plant protection products. These products must balance antibacterial efficacy with minimal phytotoxicity. The aim was to determine the effects of the most potent antibacterial treatments on olive plants and lettuce. Parameters such as fresh and dry leaf weight in both plant types, as well as root weight in the lettuce, were measured to determine the treatments’ stimulating or detrimental effects on herbaceous and woody plant species.
2. Materials and Methods
2.1. Preparation of the Bacterial Cultures
The reference strain of the bacterium P. savastanoi pv. savastanoi, CFBP 5075 (Italy), was obtained from the National Institute of Agricultural Research, INRA (Paris, France). The strains coded as A1-1 and I7 L were isolated from symptomatic olive tissue from orchards located in Slovenian and Croatian Istria, from the varieties “Arbequina” and “Leccino”, respectively. To maintain the bacterial cultures, stock cultures (stored at −80 °C with 20% glycerol) were prepared by sub-culturing them on solid King’s B (KB) growth medium (King Medium B, Pseudomonas Agar F, Biolife, Milano, Italy). The plates were incubated at 27 °C for 24–48 h.
2.2. Susceptibility of Pseudomonas savastanoi pv. savastanoi to Antimicrobials
The antibacterial susceptibility of P. savastanoi pv. savastanoi was evaluated using the disc diffusion method, following the Kirby–Bauer protocol [22]. Solid KB growth medium was inoculated with 100 μL of a bacterial suspension, with the bacterial density adjusted to 108 CFU/mL according to the McFarland Equivalence Turbidity Standard 0.5 (REMELTM, Thermo Fisher Scientific Inc., Waltham, MA, USA). Sterile antibiotic discs (Ø 6 mm; Whatman AA Discs, PLC, Cytiva, China) were aseptically placed onto the plates, and 15 μL of the antimicrobials (pure, filtered, or diluted in sterile distilled water) was pipetted onto the discs under a laminar flow (NUVE LN 090, Ankara, Turkey). This included testing its response to the EOs and their constituents, commercial copper-based products Neoram WG (Gowan Crop Protection, Harpenden, UK), Nordox 75 WG (Syngenta, Basel, Switzerland) and Amaline Flow (Nufarm GmbH & Co KG, Linz, Austria), and two plant stimulants Tora Micro Blue (Agrochem Maks d.o.o., Zagreb, Croatia) and Phylgreen (Rovensa Next, Madrid, Spain). The antibacterial effects of the OMWW treatments were evaluated based on pH adjustments to the filtered solutions. In addition, the antibacterial potential was determined for the phenol HTyr and the aqueous fresh garlic extract. The concentrations of the commercial copper-based and plant-stimulating products were tested at the recommended dosages for use in fruit orchards. Sterile distilled water (SDW) was used as the negative control. The antibiotic tetracycline (Fisher Scientific, Hampton, NH, USA) served as the reference treatment. Before application, the treatment suspensions were thoroughly vortexed to ensure homogeneity. The diffusion of the antimicrobials was enhanced by incubating the plates at +4 °C for two hours before transferring them into an incubator at 27 °C in the dark for 24 h [23]. After incubation for 24 h, the diameter of the inhibition zones was measured using a digital calliper, and the results of at least five disc measurements were interpreted in millimetres.
2.3. Preparation of the Antibacterial Treatments
2.3.1. Essential Oils and Their Constituents
The susceptibility of the reference strain of P. savastanoi pv. savastanoi was determined for five different EOs and their constituents. For this experiment, aliquots of 15 µL of the treatments were applied to sterile antibiotic discs placed in aseptic conditions on KB agar medium. The testing included the concentrations of the treatments presented in Table 1. The treatments used for this experiment were commercial EOs of thyme (Thymus vulgaris L.), oregano (Origanum compactum Benth.), peppermint (Mentha × piperita L.), sweet marjoram (Origanum majorana L.), and sage (Salvia officinalis L.) purchased from Pranarom, Ath, Belgium. The EO constituents used for determination of the susceptibility of P. savastanoi pv. savastanoi were the most concentrated compounds in the chemical profiles of the listed EOs. The antibacterial activity was determined for DL-menthol (peppermint), carvacrol (oregano), and (−)-terpinen-4-ol (sweet marjoram), purchased from Thermo Fisher Scientific Inc. (Waltham, MA, USA); thymol (thyme) (VWR International, Leuven, Belgium); and α,β-thujone (sage) purchased from Sigma Aldrich (Burlington, MA, USA).

Table 1.
The list of antimicrobials selected for testing the susceptibility of Pseudomonas savastanoi pv. savastanoi reference strain CFBP 5075 (Italy).
2.3.2. Copper-Based and Plant-Stimulating Products
Along with the plant-based antimicrobials, the testing included three commercial copper-based products registered as fungicides/bactericides that are used in fruit orchards as plant protection chemicals. These products differ based on the active ingredients in their formulations. The active ingredient in Nordox 75 WG is copper(I) oxide (Nordox AS, Ostensjoveien, Oslo, Norway); in Neoram WG, it is copper oxychloride (Gowan Crop Protection Limited, Herpenden, Hertfordshire, UK); and in Amaline Flow, it is tribasic copper sulphate + zoxamide (Nufram GmbH & Co. KG, Linz, Austria). Aliquots of 15 µL of these products at the concentrations presented in Table 1 were applied to the discs.
Plant-stimulating products were submitted to testing due to the potential antimicrobial side effects caused by their content of antibacterial compounds, such as the copper(II) carbonate soluble in water present in the Tora Micro Blue product (AGROCHEM-MAKS, Zagreb, Croatia) and the seaweed Ascophyllum nodosum (L.) Le Jolis (genus Ascophyllum, family Fucaceae) contained in Phylgreen (Tradecorp, Albacete, Spain) as a bio-based plant-stimulating product, which exhibited antibacterial activity in the recent study [24]. These products were tested at the concentrations suggested by the manufacturers for use in orchards by diluting them in SDW to obtain the concentrations listed in Table 1.
2.3.3. Plant Extracts—Garlic, OMWW, and the Phenol HTyr
The extracts of OMWW were obtained after fruit processing of the olive varieties “Istarska bjelica” (T1), “Leccino” (T2), “Rosinjola” (T3), “Buža” (T4), and “Buža puntoža” (T5). The OMWW samples were stored at +4 °C for nine days after their collection and prior to filtration. The preparation of the OMWW treatments was described in detail in our previous study [25]. Briefly, filtration of the OMWW involved centrifugation of the samples at 4000 rpm for 10 min at a temperature of 4 °C. In addition, the OMWW was divided into two fractions based on the pH adjustments. The first fraction of the OMWW included its use only as filtered samples (T1–T5), whilst the other fraction included the acidification of the samples with HCl solution to obtain a final pH of 2 (T1A–T5A) [26,27]. The phenol HTyr was tested as a chemical frequently connected with the antibacterial effect of OMWW. For this purpose, pure HTyr was purchased from Sigma Aldrich (Merck, Darmstadt, Germany). The phenol was dissolved in SDW to obtain concentrations of 2.50 (for broth dilution) and 1.25 mg/mL (for disc diffusion), respectively.
The aqueous extract of fresh garlic bulbs was prepared following the methodology described in our previous study [25]. In brief, 20 mg of fresh garlic cloves was shredded and emerged in 100 mL of SDW. The extract was sealed with parafilm M and wrapped in aluminum foil and kept at room temperature for 24 h, after which step it was filtered through 0.22 µm sterile antibiotic filters twice using a syringe.
2.4. The Susceptibility of Three Different Bacterial Strains to Antimicrobials
Further testing was conducted of the treatments to which the reference strain of P. savastanoi pv. savastanoi showed susceptibility. This testing also included the most effective copper-based preparation, Nordox 75 WG, with the active ingredient copper(I) oxide at a concentration of 2.0 mg/mL, against two additional strains of P. savastanoi pv. savastanoi, namely A1–1 and I7 L. The antibacterial activity of the EOs and their compounds was evaluated by applying these treatments at a concentration of 50 mg/mL, diluted in SDW. Along with the plant-based treatments, the antibiotic tetracycline was used as a reference at a concentration of 0.50 mg/mL. These concentrations were chosen to prevent the formation of excessively dense suspensions during the determination of the MIC [24]. For the testing, aliquots of 15 µL were applied to sterile discs to determine the susceptibility of P. savastanoi pv. Savastanoi, as is listed in Table 1.
2.5. Determination of the Minimal Inhibitory Concentrations (MICs) of Antimicrobials Against Different Strains of P. savastanoi pv. savastanoi
Due to the observed susceptibility of the reference strain CFBP 5075 of P. savastanoi pv. savastanoi to selected chemicals, further MIC testing was conducted using the concentrations specified in Table 2. The MIC determination was performed using the broth microdilution method (BM) in polystyrene 96-well plates following the Clinical and Laboratory Standards Institute (CLSI) guidelines [28]. For this purpose, pure colonies of P. savastanoi pv. savastanoi were collected from the culture plates and transferred into liquid KB (KBL) medium prepared using the ingredients per litre as follows: 20 g of bacteriological peptone (Biolife, Viale Monza, Milano, Italy), 1.5 g of K2HPO4 (VWR International bvba, Galdenaaksabaan, Leuven, Belgium), MgSO4 × 7H2O (GRAM MOL, Zagreb, Croatia), and 10 mL of glycerol (GRAM MOL, Zagreb, Croatia). The bacterial suspensions were incubated overnight in an incubator–shaker at 26–27 °C and 80 rpm. The bacterial density was adjusted to approximately 106 CFU/mL using spectrophotometric measurements, specifically an optical density of 0.15 at 600 nm (OD600) [29]. For the BM, the final treatment volume was 400 µL (200 µL of the treatment diluted in KBL medium and 200 µL of an overnight bacterial culture adjusted to a density of 106 CFU/mL). The treatment concentrations were adjusted to the levels listed in Table 2 upon the addition of the bacterial suspension. To minimise potential evaporation of the treatments, the plates were closed with a lid and covered with aluminum foil to prevent potential photodegradation of the treatments during their incubation.

Table 2.
Treatments with concentrations used for determination of antibacterial activity and minimal inhibitory concentrations (MICs) of antimicrobials against Pseudomonas savastanoi pv. savastanoi.
The MIC was defined as the lowest treatment concentration that showed no visible bacterial growth after 24 h of incubation at 26–27 °C. To validate the MIC values, INT salt (iodonitrotetrazolium violet, 95%, Alfa Aesar GmbH & Co. KG, Thermo Fisher Scientific Inc., Waltham, MA, USA) was added to the wells [30,31,32]. A total volume of 5 µL per well of 0.04% (w/v) INT salt diluted in SDW was used. The presence of colour changes after 2 h of incubation in the wells treated with INT salt indicated bacterial viability.
The controls included treatments without bacteria (only KB medium) and bacterial suspensions without antimicrobials. The treatments excluded from the MIC determination due to the lack of susceptibility of P. savastanoi pv. savastanoi included the commercial product Phylgreen and the OMWW solutions. The EOs and their components were tested against different P. savastanoi pv. savastanoi strains, including the reference strain CFBP 5075 (Italy), A1–1 (Slovenia), and I7 L (Croatia), to determine possible differences in their susceptibility to these treatments.
2.6. The Inhibition of Growth and the Growth Kinetics of P. savastanoi pv. savastanoi Treated with Plant-Based Antimicrobials
The inhibition of bacterial growth was estimated using the BM with spectrophotometric readings of the experimental treatments set up in sterile 96-well plates with lids (DELTALAB, S.L., Barcelona, Spain). Absorbance readings were made using a TECAN Infinite 200 PRO spectrophotometer (Männedorf, Switzerland) at a 600 nm wavelength (optical density; OD600). For the BM, the total volume of the treatments was 400 µL per well, as previously described. The inhibition of bacterial growth was calculated using the formula
where A600 represents the absorbance of the control (bacterial suspensions without the antimicrobial treatment), and B600 represents the absorbance value of the specific treatment. The KBL medium was used as a blank reading for all of the treatments, including the negative control.
Inhibition (%) = [(A600 − B600)/A600] × 100,
2.7. The Growth Kinetics of P. savastanoi pv. savastanoi Treated with EOs and Their Constituents
To determine the time at which the bacterial growth was the most inhibited after exposure to the most effective treatments (the thyme and oregano EOs and thymol and carvacrol compounds), a growth kinetics assay was performed according to the guidelines provided by [28], with a few modifications. The determined MIC of the antimicrobials, as well as concentrations equal to ½ of MIC and 2× MIC, was screened for the experiment. For the test, the concentrations were diluted in sterile saline (1:9 v/v) up to a total volume of 1.0 mL, and aliquots of 400 μL were added into the wells in 96-well plates at various time points after exposure to the treatment (0, 1, 2, 4, 6, and 24 h). After 24 h from each time point, the bacterial growth in the wells was recorded based on the optical density using a spectrophotometric reading at 600 nm (TECAN Infinite 200 PRO spectrophotometer; Männedorf, Switzerland). A bacterial culture without treatment served as the negative control.
2.8. The Mechanism of Action of Oregano EO and Carvacrol Against P. savastanoi pv. savastanoi
Due to the strong antibacterial activity observed, the mechanisms of action of oregano EO and its concentrated component, carvacrol, were evaluated at ½ MIC, MIC, and 2× MIC. The MIC values for the oregano EO were 0.31, 0.63, and 1.25 mg/mL, while for carvacrol, these values were 1.25, 2.50, and 5.00 mg/mL, respectively. The effects on the leakage of metabolites, such as proteins and nucleic acids, from P. savastanoi pv. savastanoi were used as indicators of cell wall membrane disruption. The methodology followed was based on slightly modified versions of the methods described by Miksusanti et al. [33] and Elcocks et al. [31]. For this evaluation, the reference strain P. savastanoi pv. savastanoi (CFBP 5075) was cultured on solid KB agar medium and incubated for 24 h at 27 °C. Post-incubation, several colonies were transferred into 150 mL of the KBL medium and further incubated in an incubator–shaker at 80 rpm. The overnight bacterial culture was adjusted to approximately 106 CFU/mL in 10 mL of KBL and distributed into centrifuge tubes in triplicate for each treatment. The tubes were centrifuged three times at 4800 rpm for 15 min each. After each centrifugation, the supernatant was discarded, and the pellet was resuspended in fresh sterile KBL medium. The treatments were then transferred into glass tubes, bringing the total volume to 3.0 mL. These tubes were placed in an incubator–shaker at 160 rpm for two hours. To obtain pure filtrates, the treatments were filtered through sterile antibiotic filters (Ø 0.22 µm) mounted onto sterile syringes. The leakage of metabolites was determined by measuring the absorbance at 260 nm (nucleic acids) and 280 nm (proteins) using a DeNovix spectrophotometer/fluorometer (DeNovix Inc., Wilmington, DE, USA). The effect of the treatments on bacterial metabolites was represented by the ratio of the absorbance values of the treatments compared to those of the negative control (without antimicrobials).
2.9. The Effect of Oregano EO and Carvacrol on Lettuce and Olive Plants
To evaluate the effects of oregano EO and its concentrated ingredient, carvacrol, a greenhouse experiment was conducted on lettuce (Lactuca sativa L. cv. Quefir) at the Institute of Agriculture and Tourism, Poreč, Croatia (coordinates: 45°13′17″ N; 13°36′9″ E). The experiments with the lettuce and olive plants were set up in late May and early June 2023, respectively. The treatments were applied foliarly to young lettuce plants at the four-leaf stage, including a total of 15 plants per treatment. Oregano EO was applied at concentrations of 0.63 and 1.25 mg/mL, while carvacrol was applied at 2.50 and 5.0 mg/mL concentrations prepared in SDW, which were the concentrations equivalent to the MIC and 2× MIC determined in vitro against P. savastanoi pv. savastanoi. SDW served as the negative control. Lettuce seeds were initially sown in polystyrene trays filled with a mixture of substrate and vermiculite (80:20). Post-germination, the seedlings were transplanted into individual pots containing approximately 0.50 L of the substrate. The pots were placed into plastic bags filled to one-third of their volume with water to maintain adequate moisture and prevent treatment runoff. Each plant received 15 mL of the treatment suspension. No visible phytotoxic effects were observed, and the plant biomass (fresh and dry weights of the shoots and roots), plant height, and root length were measured 14 days after application.
In addition, the effects of carvacrol at concentrations of 1.25 and 2.50 mg/mL were tested on the woody species olive (Olea europaea L.) using 2–3-year-old Leccino cv. plants grown in a greenhouse [34]. The plants were irrigated via a drip irrigation system to minimise runoff. Eight olive plants were included in the experiment, with foliar treatments applied using a hand garden sprayer. A total volume of 1 L per treatment was used to ensure complete coverage of the plant. Visual symptoms of phytotoxicity were monitored, and symptomatic leaves were collected 14 days post-treatment. The fresh and dry weights of the symptomatic leaves were recorded, alongside those of the control treatments using only distilled water.
2.10. Data Analysis
2.10.1. Antibacterial Activity of the Plant-Based Treatments
For determination of the effect of the concentration of the treatments and the specific strain responses, the data obtained on the inhibition of bacterial growth were subjected to a two-way ANOVA in the statistical software R version 4.2.2 [35]. All of the quantitative in vitro antimicrobial tests included at least three replicates. The effect of the treatments on the growth of P. savastanoi pv. savastanoi was determined for the treatments considering the bacterial strain as one factor and the concentration as the second factor. Negative values for the percentage of inhibition (% < 0) were excluded from the analysis due to interference from overly dense antimicrobial suspensions at 600 nm readings. Significant differences in the means were determined based on a p value ≤ 0.05. To control the false discovery rate, the p values were adjusted using the Benjamini–Hochberg correction. This adjustment enabled the identification of the most effective concentration for each antimicrobial. For multiple comparisons of the means, the contrast function from the multcomp package (v1.4-16) [36] was applied.
2.10.2. The Antibacterial Mechanisms of Action and In Vivo Effect of Oregano EO and Carvacrol on the Lettuce and Olive Plants
The data on the in vivo effect (length and weight) of the antimicrobials applied to the bacteria and lettuce and olive plants, respectively, were subjected to a one-way ANOVA using the statistical software STATISTICA 12.5 (StatSoft Inc., Tulsa, OK, USA). Significant variations in the means were determined using the post hoc Tukey’s HSD test based on a p value threshold of ≤0.05.
3. Results
3.1. The Susceptibility of Pseudomonas savastanoi pv. savastanoi to the Antimicrobials
The in vitro application of the treatments (Table 3) showed that the reference strain of P. savastanoi pv. savastanoi was susceptible to a total of 18 treatments, including the reference antibiotic treatment. All of the copper-based products effectively inhibited the growth of P. savastanoi pv. savastanoi, with the highest susceptibility observed at the recommended concentration of 2.0 mg/mL of Nordox 75 WG, which contained the active ingredient copper(I) oxide, producing inhibition zones of up to 24 mm. Considering the undiluted EOs, the effect against P. savastanoi pv. savastanoi was highest for the thyme and oregano treatments (exceeding 20 mm on average). The other EO treatments were less effective. The EO components showed similar results, with a high effect observed for carvacrol, followed by that of α,β-thujone and (−)-terpinen-4-ol, the most concentrated components of the oregano, sage, and sweet marjoram EOs. The plant-based extracts indicated that P. savastanoi pv. savastanoi was not susceptible to the filtered OMWW, regardless of pH adjustments. Similarly, no antibacterial effect was observed with the plant-stimulating product Phylgreen, while Tora Micro Blue exhibited some antibacterial activity (9.0 mm on average). The HTyr solution inhibited bacterial growth by 7.3–17.7 mm. Notably, a comparable antibacterial effect to that of the copper-based treatments was observed with the garlic extract, producing inhibition zones up to 18.9 mm.

Table 3.
The susceptibility of the Pseudomonas savastanoi pv. savastanoi reference strain CFBP 5075 to the antimicrobials. The zone of inhibition of bacterial growth around the discs (including discs with Ø = 6.0 mm) is presented as the range of at least five disc measurements per treatment.
The application of the treatments (Table 4) showed that the different strains of P. savastanoi pv. savastanoi were susceptible to a total of eight treatments, including the reference antibiotic treatment, with low variability in the measured inhibition halos. When the EOs were applied at a concentration of 50 mg/mL, an effect against P. savastanoi pv. savastanoi was determined only for the thyme and oregano treatments (an average ranging from 7.76 to 9.69 mm). The other EO treatments were not effective. The EO components showed similar results, with a high effect observed for thymol, carvacrol, and then α,β-thujone, as the most concentrated components of the thyme, oregano, and sage EOs. The HTyr solution inhibited the bacterial growth by 7.03–9.57 mm on average, depending on bacterial strain. Notably, a comparable antibacterial effect to that of the copper-based treatments was observed using the aqueous garlic extract for all strains, producing inhibition zones of up to 17.80 mm on average.

Table 4.
The susceptibility test of three different strains of Pseudomonas savastanoi pv. savastanoi to a concentration of 50 mg/mL of the EOs and their constituents, along with the other antimicrobials, expressed as the mean and standard deviation value for at least five measurements (including a diameter of the disc Ø = 6 mm) of the halo zone around the antibiotic discs.
3.2. Minimal Inhibitory Concentrations (MICs) of the Antimicrobials
The MICs in the treatments using the BM with the addition of INT salt are presented in Table 5. Overall, the MIC values varied depending on the treatment applied, without variation observed among the strains of P. savastanoi pv. savastanoi. The EOs from thyme and oregano exhibited MIC values of 1.25 and 0.63 mg/mL, respectively, across all of the bacterial strains. Among the individual EO components, thymol displayed an MIC of 5.0 mg/mL, whereas carvacrol demonstrated a lower MIC of 2.50 mg/mL. In contrast, the phenol HTyr and the garlic extract exhibited no detectable antimicrobial activity against the tested strains within the tested concentration range. Among the copper-based products, two out of three—Neoram WG and Amaline Flow—were effective at their initial concentrations. Nordox 75 WG, on the other hand, demonstrated an MIC value of 1.0 mg/mL, with this being half of the recommended concentration against P. savastanoi pv. savastanoi.

Table 5.
Minimal inhibitory concentrations (MICs) of antimicrobials against Pseudomonas savastanoi pv. savastanoi strains (CFBP 5075, A1-1, and I7 L) determined using iodonitrotetrazolium (INT) salt in 96-well microtiter plates.
3.3. Inhibition of the Growth of Three Different Strains of Pseudomonas savastanoi pv. savastanoi Treated with Plant-Based Antimicrobials
According to the results of the two-way ANOVA, the variations in the growth inhibition of P. savastanoi pv. savastanoi observed are contingent upon two key factors: the bacterial strain and the concentration of the applied antimicrobial (Table S1). Conversely, the growth inhibition predominantly relies on the concentration of the EO constituents. In summary, the EOs exhibit higher rates of growth inhibition, exceeding 60% at lower doses. The concentration-dependent nature of the increased activity against P. savastanoi pv. savastanoi strains is more accurately described by the constituents of the EOs. Complete growth inhibition of P. savastanoi pv. savastanoi was achieved with the treatments with oregano EO at concentrations of 10 and 5.0 mg/mL against the Croatian and Slovenian strains, whilst no significant difference was observed up to 0.63 mg/mL or 1.25 mg/mL against the reference strain (Figure 1). Nevertheless, the bacterial growth inhibition remained approximately 60% or higher up to a dose of 10 mg/mL. Considering the EO constituents, the application of carvacrol resulted in the highest rates of bacterial growth inhibition against P. savastanoi pv. savastanoi, reaching up to 100%. This effect persisted at up to 1.25 mg/mL against the reference strain, with no significant difference observed when a lower dose of 0.63 mg/mL was applied. A similar pattern was observed for the other strains, where lower concentrations demonstrated significantly weaker activity.
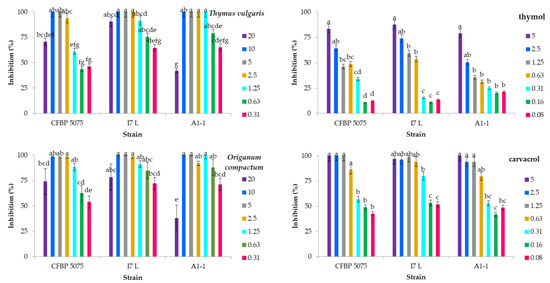
Figure 1.
The percentage of growth inhibition for three strains of Pseudomonas savastanoi pv. savastanoi was assessed following the application of seven serially diluted concentrations of essential oils (EOs) and EO components. The growth inhibition was determined based on optical density measurements (OD600) after 24 h of incubation. The bars represent the concentrations of the antimicrobials, with the highest concentration on the left and the lowest on the right. Significant differences between means (p ≤ 0.05) are indicated by different letters within each antimicrobial treatment. The tested strains include CFBP 5075 (reference strain, Italy), I7 L (Croatian strain, Istria), and A1-1 (Slovenian strain, Istria).
3.4. The Growth Kinetics of Pseudomonas savastanoi pv. savastanoi Treated with Plant-Based Antimicrobials
The growth kinetics of the reference strain P. savastanoi pv. savastanoi was determined for the ½ MIC, MIC, and 2× MIC of two effective EOs and two EO components over a 224 h incubation period, with time points of 0, 1, 2, 4, 6, and 24 h (Figure 2). All of the tested EOs caused the absence of bacterial growth throughout the observed period compared to that in the negative control without treatment. A slight increase in OD600 was recorded at the 4 h time point for the thyme treatment at a concentration of 0.63 mg/mL. The application of the EO components inhibited the bacterial growth across all of the treatments after 24 h of incubation. By 24 h, all of the treatments significantly reduced the bacterial growth relative to that of the control.
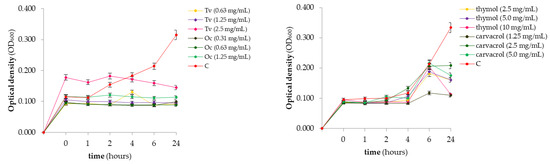
Figure 2.
Illustration of the growth kinetic curves of Pseudomonas savastanoi pv. savastanoi when treated with ½ MIC, MIC, and 2× MIC of the plant-based antimicrobial agents. Tv—Thymus vulgaris EO (thyme); Oc—Origanum compactum EO (oregano); C—negative control (bacterial suspension without antimicrobials).
3.5. The Leakage of Intercellular Metabolites of P. savastanoi pv. savastanoi Treated with Origanum compactum EO and Carvacrol
Absorbance readings of the filtrate from oregano EO and its concentrated compound, carvacrol, confirmed that their mechanisms of action targeted intercellular metabolites, specifically proteins and nucleic acids (Figure 3). Bacterial cell membrane disruption as the mode of action was more pronounced with the application of carvacrol. Both treatments showed an increase in the absorbance ratio with the application of higher concentrations. However, carvacrol exhibited the strongest effect, with the absorbance at 280 nm being more than 7 and 21 times higher and that at 260 nm being 4 and 14 times higher at concentrations corresponding to ½ MIC, MIC, and 2× MIC, respectively. Oregano EO caused weaker metabolite leakage, with increases in the absorbance of more than two and four times at 280 nm and one and two times at 260 nm at all tested concentrations, respectively.
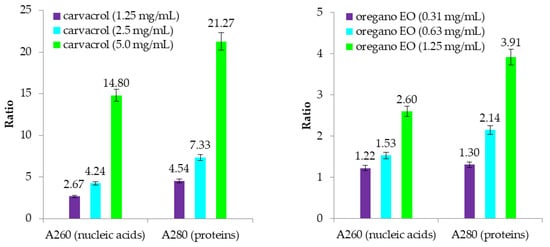
Figure 3.
Ratio of the absorbance readings for the treatments to those for the non-treated control.
3.6. The Effect of Oregano EO and Carvacrol on the Lettuce and Olive Plants
The effects of the oregano EO and carvacrol suspensions were evaluated based on the morphological characteristics of the lettuce plants two weeks after their application (Figure 4; Figure S1).
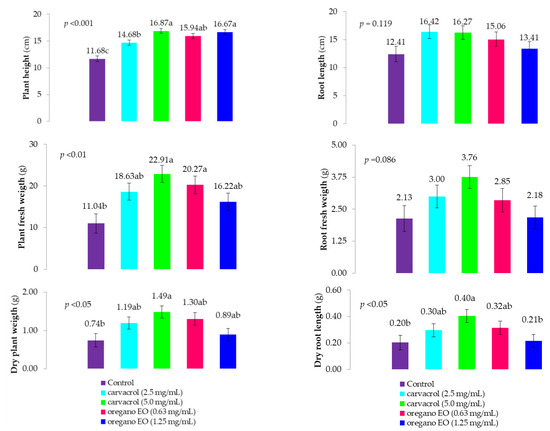
Figure 4.
Effect of carvacrol and oregano EO (Origanum compactum) application on morphological parameters and plant biomass two weeks after application to lettuce plants. Different letters above the bars represent the statistical difference between the treatments within each parameter at a significance level of p < 0.05.
Significant variations were observed in the plants’ height, fresh and dry weights, and dry root weight (Table S2). The highest values for all parameters were recorded when carvacrol was applied at a concentration of 5.0 mg/mL. All of the treatments significantly differed from the negative control in terms of plant height (11.68 cm on average). The tallest plants were observed in the carvacrol treatment (16.87 cm on average) when it was applied at the highest concentration of 5.0 mg/mL, while neither concentration of O. compactum significantly differed from carvacrol, showing plant heights of 15.94 and 16.67 cm on average, respectively. Fresh plant weight was significantly affected by all of the treatments, with the application of 5.0 and 0.63 mg/mL of carvacrol and oregano EO, respectively, yielding the highest weights of 22.91 and 20.27 g on average. Treatment with carvacrol and oregano EO at concentrations of 2.50 and 1.25 mg/mL, respectively, did not differ significantly from the other treatments except the control. A similar pattern was observed for dry plant weight, where the highest values were recorded with the application of carvacrol at the highest concentration, which significantly differed only from the control treatment. The root parameters were less affected by the treatments. Root length ranged from 12.40 (control) to 16.42 cm (carvacrol = 2.5 mg/mL) on average, with no significant differences among the treatments. Fresh root weight showed slight increases across the treatments, but these differences were not statistically significant, ranging from 2.13 g (control) to 3.76 g (carvacrol = 5.0 mg/mL) on average. In contrast, significant differences were noted in dry root weight, with the highest value observed with the application of carvacrol at a concentration of 5.0 mg/mL (0.40 g on average). Carvacrol (2.50 mg/mL) and oregano EO (1.25 mg/mL) did not significantly differ from carvacrol (5.0 mg/mL), though they did not show significant differences compared to the control either, where the average dry root weight was 0.20 g.
According to the ANOVA, despite the observed visual damage, no significant differences were determined for leaf weight 14 days after the application of the treatments (Table S2). The average fresh and dry weights of the leaves were both higher than 4.00 g and 1.70 g, respectively (Figure 5).
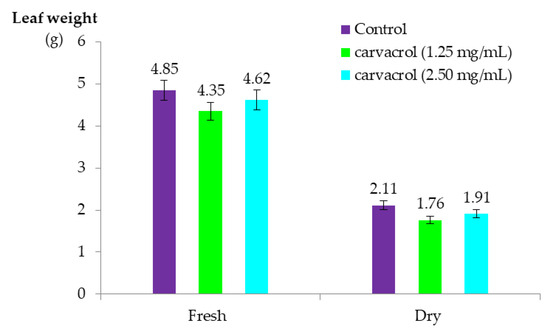
Figure 5.
Fresh and dry weights of olive leaves collected 14 days after application of carvacrol suspension in greenhouse experiment.
4. Discussion
Encouraged by the growing need for effective, eco-friendly, and sustainable agricultural solutions, this study aimed to investigate the antibacterial properties of various plant-based treatments. These included EOs and their constituents, OMWW, the phenolic compound HTyr, and algae and garlic extracts, as well as copper-based and plant-stimulating commercial products available on the market. The results indicated that EOs and their individual compounds have the greatest potential for the development of next-generation green bactericides. The most effective treatments inhibited bacterial growth at low concentrations, such as 0.63 mg/mL, with the highest antibacterial activity observed for the EOs derived from thyme and oregano, as well as their individual compounds, such as thymol and carvacrol. In addition to the EOs, all of the bacterial strains tested were evaluated for their susceptibility to copper-based treatments. These strains were isolated from olive orchards where copper-based products are routinely applied at least twice per growing season, in accordance with integrated plant protection recommendations [37]. The copper-based commercial products were effective against P. savastanoi pv. savastanoi, regardless of the active ingredient. However, copper(I) oxide exhibited slightly stronger antibacterial activity compared to that of the other active ingredients. These results indicate that the tested strains did not exhibit resistance to these active ingredients.
Furthermore, the P. savastanoi pv. savastanoi strains did not exhibit susceptibility to the commercial product Phylgreen, which is based on algae, or to OMWW, regardless of the adjusted pH of the extracts. The lack of antibacterial activity of the OMWW may be attributed to the low concentration of phenolic compounds in the extracts, as well as the presence of sugars, which may have antagonised the antibacterial properties of the phenolics [17]. Additionally, the ability of bacteria to degrade phenolic compounds may have contributed to these results [38]. The antibacterial activity of HTyr, a phenol frequently associated with the antibacterial properties of OMWW, was insufficient in its effectiveness against P. savastanoi pv. savastanoi. A similar result was observed for the garlic extract. The bacterial strains were susceptible to the aqueous fresh garlic extract, with the inhibition zones comparable to those observed in the copper-based treatments when tested via antibacterial disc diffusion. However, the MIC was not detectable for the highest concentration in the microdilution testing, and this lack of an effect was likely due to the dilution of the volatile sulphur-containing compounds in garlic known for their antibacterial activity [39,40]. The lack of an effect observed with the seaweed A. nodosum product (Phylgreen) may have been due to the low concentrations applied in this study, which might not have been sufficient to demonstrate antibacterial activity. This finding is supported by [24], who reported effective inhibitory concentrations against E. coli ranging from 11.50 to 23.0 mg/mL, which are significantly higher concentrations compared to those recommended for use in orchards.
However, all of the EOs and their individual constituents were effective when applied in pure form without dilution. The high antibacterial efficacy of the thyme and oregano EOs, as well as the compounds thymol and carvacrol, supports the previous literature indicating that these plant-derived compounds can effectively inhibit the growth of various phytopathogenic bacterial species, including those from the Pseudomonas genus [7,10,11,41]. The absence of antibacterial activity observed with the other treatments in diluted form, such as the peppermint and sage EOs, as well as sweet marjoram, and their respective constituents (DL-menthol and (−)-terpinen-4-ol), may be attributed to the doses used, which may not have been high enough to inhibit bacterial growth at the tested density (≈108 CFU/mL). Considering the naturally lower bacterial densities in orchards [42,43], these treatments could still be promising candidates for biopesticide formulations, particularly for use in orchards, where the densities of pathogenic bacterial populations are low. Furthermore, the lack of antibacterial effects observed in the disc diffusion testing may have been due to the limited diffusion capacity of these compounds through the solid growth medium. A standardised protocol for testing the antimicrobial effects of plant-derived products is still being refined in many laboratories [15,44].
Despite these variations, the inhibitory potential of the EOs against P. savastanoi pv. savastanoi exhibited strain- and concentration-dependent effects. Slight differences in the antibacterial activity of the plant-derived compounds against various strains of a single bacterial species have previously been reported. The study in [7] demonstrated that the antimicrobial effects of EOs and their constituents within P. savastanoi species can be pathovar-specific. Interestingly, the effect against P. savastanoi pv. savastanoi was significantly higher than that against P. savastanoi pv. nerii, which are closely related pathovars of P. savastanoi. These findings are consistent with the results of the present study, which further emphasises the strain-level diversity. It is hypothesised that some strains may carry genes conferring resistance to antimicrobials, influenced by the genetic plasticity of bacteria [45,46], which could account for the slight variation in the susceptibility of P. savastanoi pv. savastanoi strains observed. Notably, the concentration dependency was determined using spectrophotometric optical density readings. At higher concentrations, some of the tested agents interfered with the readings, leading to artificially higher optical density values than those of the negative control, which in turn resulted in an apparent reduction in bacterial growth inhibition [24], which may have contributed to the observed differences for the bacterial strains. However, the treatments with the thyme and oregano EOs resulted in complete bacterial growth inhibition. A recent study by Tardugno et al. [47] also demonstrated a great antimicrobial effect against P. savastanoi pv. savastanoi when using EOs derived from Thymus and Origanum species, which contain thymol and carvacrol as their main components. Our study of the duration and timing of the antibacterial effects over a 224 h period showed that both the thyme and oregano EOs rapidly inhibited bacterial growth, with effects observed as early as at 1 h of exposure across all of the concentrations tested. This effect remained consistent throughout the testing period. Among the EO compounds, carvacrol and thymol exhibited rapid action compared to the non-treated control, with the effect lasting up to 24 h. After a 6 h exposure period, the EO compounds showed some regrowth of the bacterial cultures based on the optical density readings. However, the bacterial populations remained at lower densities compared to those in the non-treated control. Furthermore, the literature supports the strong antibacterial activity of EOs composed primarily of carvacrol against various bacterial species, pathovars, and strains [7,48,49], and our findings corroborate this. Carvacrol exhibited the strongest inhibition rates (>90%) against all of the strains tested, suggesting its potential for future use in controlling bacterial plant diseases.
The high efficacy of terpenic phenols, particularly carvacrol, aligns with other reports indicating that these compounds exhibit stronger antibacterial effects than other terpene functional groups [5,7,43,50,51]. Secondary plant metabolites can cause the leakage of metabolites and ions from the cytoplasm of the cells, increase membrane permeability, and disrupt membrane proteins in many target microorganisms, pests, and plants [7,11]. The disruption of the bacterial cell membrane was confirmed as the mode of action of the oregano EO and carvacrol, with a notably stronger effect on metabolite leakage when carvacrol was applied alone. This effect intensified with higher concentrations against P. savastanoi pv. savastanoi. The weaker effect of the oregano EO could be attributed to interactions between carvacrol and other components within the oil. Nevertheless, a concentration-dependent effect on metabolite leakage was observed, particularly at higher concentrations, suggesting that stronger antibacterial effects may require higher concentrations of the EO. This emphasises the need for further investigation into the interactions among the complex components in EOs and their impact on efficacy. Interestingly, compounds like carvacrol have been shown to inhibit plasmid-mediated antibiotic resistance in bacteria, highlighting their potential application in controlling bacterial strains resistant to the current bactericides [6].
Given the significant antibacterial activity of the oregano EO and carvacrol in vitro, this study aimed to evaluate whether the effective antibacterial concentrations of these treatments could be harmful to plants in a greenhouse setting, as such treatments could be phytotoxic and are already included in bio-herbicide formulations available on the market [52]. Foliar application of these treatments at the defined concentrations did not result in visible injuries to the lettuce plants during the 14-day experimental period. On the contrary, the treatments appeared to stimulate plant growth. Compared to the control, all of the treatments positively influenced their morphometric properties, such as plant height and weight. Notably, carvacrol, when applied individually, had the most pronounced effect, although the differences between treatments were not statistically significant. However, a clear difference was observed compared to the untreated plants. The treatments did not significantly impact root weight or length, except for some differences in the dry matter weight of the roots, particularly with the highest concentration of carvacrol (5.0 mg/mL), compared to that in the control. Interestingly, while the oregano EO generally showed a lower effect on promoting plant height with increasing concentrations, this contrasted with carvacrol, with which higher concentrations were more effective. This discrepancy may be attributed to interactions between the compounds in the EO which could have interfered with or inhibited the effect of carvacrol in the mixture. Given that carvacrol stimulated lettuce growth and affected leaf weight, its effect at varying concentrations was also tested on a woody species, the olive. In this case, phytotoxic injuries were observed on the young leaves of the Olea europaea cv. “Leccino”. These injuries were characterised by small necrotic lesions visible only with the application of a higher dosage of carvacrol. While these lesions were visible, no significant differences were found in the fresh or dry weight of the leaves between the treatments and the control, suggesting that the olive plants did not experience significant long-term damage due to the treatment after 14 days. These injuries may have resulted from interference with the cuticular wax and disruption of the leaf cell membranes, causing damage in areas where the treatment droplets landed. To mitigate such damage, one potential solution could be the use of adjuvants or the application of fine, mist-sized droplets to reduce the risk of phytotoxicity. This research aligns with findings from Saghrouchni et al. [16] which demonstrated the strong antimicrobial and plant-growth-promoting properties of carvacrol when used on ryegrass in greenhouse settings. Moreover, in the foliar application of oregano EO [41], the phytotoxicity of the applied treatment in vivo followed a dose-dependent pattern, with a safe threshold observed at a 0.5% solution of EO, below which no adverse effects on the plants were detected. The latter studies support the findings of this research, where both antibacterial and plant-stimulatory effects were achieved using carvacrol, though some injuries were still observed in the plants at concentrations greater than the MIC.
Further research should focus on gaining a deeper understanding of the mechanisms of action of plant-derived agents, considering the diversity between herbaceous and woody plant species. Additionally, developing stable formulations and effective application methods for field use is crucial. Standardising the dosages to balance stimulatory and potentially damaging effects will be essential to avoid phytotoxicity, which is commonly observed in studies examining the herbicidal properties of these agents. To overcome volatility issues and improve the stability of EOs, plant extracts, phenols, and other bioactive compounds, encapsulating these compounds into nanoparticles is proposed. Nanoparticle encapsulation ensures controlled, long-term release and enhances the stability of the compounds by reducing their evaporation and preventing photo- and thermal degradation [53,54,55]. Moreover, encapsulation can reduce the effective concentrations needed compared to those of free forms of EOs or extracts, indicating improved efficacy against microorganisms [56].
5. Conclusions
The EOs, particularly those derived from thyme and oregano, and their key constituents, such as thymol and carvacrol, demonstrated the highest and rapid antibacterial activity against P. savastanoi pv. savastanoi. The other plant-based treatments, such as OMWWS, garlic extract, and a seaweed-based product, showed a limited effect or the absence of antibacterial activity at the tested concentrations. The antibacterial activity of the EOs and their constituents was both strain- and concentration-dependent, reflecting the diversity within P. savastanoi pv. savastanoi populations.
Carvacrol and oregano EO caused bacterial membrane disruption and metabolite leakage. However, the efficacy of EOs may be reduced due to interactions among their components or their evaporation during application. These treatments not only exhibited strong antibacterial effects but also stimulated plant growth in lettuce without visible phytotoxicity. However, higher concentrations of carvacrol caused minor necrotic lesions on young olive leaves, highlighting the importance of optimising the dosages to balance efficacy and safety. Further studies should focus on standardising EO-based formulations, understanding their interactions with different plant species, and developing application techniques to minimise their phytotoxicity. Encapsulation and the use of adjuvants offer promising solutions for improving the stability and efficiency of these plant-derived treatments in agricultural settings.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15040819/s1. Table S1: The effect of different concentrations of plant-based antibacterials on inhibition of the growth of different strains of Pseudomonas savastanoi pv. savastanoi—results of the two-way ANOVA; Table S2: The effect of the Origanum compactum EO and carvacrol treatments on lettuce and olive plants in the greenhouse experiment—results of the ANOVA. Figure S1: Phenotype of lettuce plants 14 days after application of oregano’s EO (Origanum compactum) and carvacrol in greenhouse experiment.
Author Contributions
Conceptualisation: L.K. and S.G. Methodology: L.K., J.L. and E.Đ. Validation: J.L., E.Đ., L.K. and S.G. Formal analysis: J.L. and L.K. Investigation: L.K. Resources: S.G. Data curation: J.L. and L.K. Writing—original draft preparation: L.K. Writing—review and editing: E.Đ. and S.G. Visualisation: L.K. Supervision: S.G. and E.Đ. Project administration: S.G. Funding acquisition: S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Croatian Science Foundation under project number HRZZ-UIP-2020-02-7413.
Data Availability Statement
The data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Mirik, M.; Aysan, Y.; Sahin, F. Characterization of Pseudomonas savastanoi pv. savastanoi strains isolated from several host plants in Turkey and report of fontanesia as a new host. J. Plant Pathol. 2011, 93, 263–270. [Google Scholar]
- EU Directive 2009/128/EC. Directive 2009/128/EC of the European Parliament and of the Council Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides. Available online: https://www.fao.org/faolex/results/details/en/c/LEX-FAOC113943/ (accessed on 14 October 2024).
- Broniarek-Niemiec, A.; Børve, J.; Puławska, J. Control of Bacterial Canker in Stone Fruit Trees by Chemical and Biological Products. Agronomy 2023, 13, 1166. [Google Scholar] [CrossRef]
- Ayilara, M.S.; Adeleke, B.S.; Akinola, S.A.; Fayose, C.A.; Adeyemi, U.T.; Gbadegesin, L.A.; Omole, R.K.; Johnson, R.M.; Uthman, Q.O.; Babalola, O.O. Biopesticides as a promising alternative to synthetic pesticides: A case for microbial pesticides, phytopesticides, and nanobiopesticides. Front. Microbiol. 2023, 14, 1040901. [Google Scholar] [CrossRef] [PubMed]
- Wińska, K.; Mçczka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Camele, I.; Grul’ova, D.; Elshafie, H.S. Chemical Composition and Antimicrobial Properties of Mentha × piperita cv. ‘Kristinka’ Essential Oil. Plants 2021, 10, 1567. [Google Scholar] [CrossRef]
- Bozkurt, I.A.; Soylu, S.; Kara, M.; Soylu, E.M. Chemical Composition and Antibacterial Activity of Essential Oils Isolated from Medicinal Plants against Gall Forming Plant Pathogenic Bacterial Disease Agents. KSU J. Agric. Nat. 2020, 23, 1474–1482. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Touj, N.; Hammami, I.; Dridi, K.; Al-Ayed, A.S.; Hamdi, N. Chemical Composition and in vivo Efficacy of the Essential Oil of Mentha piperita L. in the Suppression of Crown Gall Disease on Tomato Plants. J. Oleo Sci. 2019, 68, 419–426. [Google Scholar] [CrossRef]
- Muthee Gakuubi, M.; Wagacha, J.M.; Dossaji, S.F.; Wanzala, W. Chemical Composition and Antibacterial Activity of Essential Oils of Tagetes minuta (Asteraceae) against Selected Plant Pathogenic Bacteria. Int. J. Microbiol. 2016, 2016, 7352509. [Google Scholar] [CrossRef]
- Benali, T.; Bouyahya, A.; Habbadi, K.; Zengin, G.; Khabbach, A.; Achbani, E.H.; Hammani, K. Chemical composition and antibacterial activity of the essential oil and extracts of Cistus ladaniferus subsp. ladanifer and Mentha suaveolens against phytopathogenic bacteria and their ecofriendly management of phytopathogenic bacteria. Biocatal. Agric. Biotechnol. 2020, 28, 101696. [Google Scholar] [CrossRef]
- Grul’ová, D.; Caputo, L.; Elshafie, H.S.; Baranová, B.; De Martino, L.; Sedlák, V.; Gogal’ová, Z.; Poráčová, B.; Camele, I.; De Feo, V. Thymol Chemotype Origanum vulgare L. Essential Oil as a Potential Selective Bio-Based Herbicide on Monocot Plant Species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; González-García, V.; Palacio-Bielsa, A.; Lorenzo-Vidal, B.; Buzón-Durán, L.; Martín-Gil, J.; Martín-Ramos, P. Antibacterial Activity of Ginko biloba Extracts against Clavibacter michiganensis subsp. michiganensis, Pseudomonas spp., and Xanthomonas vesicatoria. Horticulturae 2023, 9, 461. [Google Scholar] [CrossRef]
- Schollenberger, M.; Staniek, T.M.; Paduch-Cichal, E.; Dasiewicz, B.; Gadomska-Gajadhur, A.; Mirzwa-Mróz, E. The activity of essential oils obtained from species and interspecies hybrids of the Mentha genus against selected plant pathogenic bacteria. Acta Sci. Pol. Hortorum Cultus 2018, 17, 167–174. [Google Scholar] [CrossRef]
- Wenzler, E.; Maximos, M.; Asempa, T.E.; Biehle, L.; Schuetz, A.N.; Hirsch, E.B. Antimicrobial susceptibility testing: An updated primer for clinicians in the era of antimicrobial resistance: Insight from the Society of Infectious Diseases Pharmacists. Pharmacoteraphy 2023, 43, 264–278. [Google Scholar] [CrossRef]
- Eloff, J.N. Avoiding pitfalls in determining antimicrobial activity of plant extracts and publishing the results. BMC Complement. Altern. Med. 2019, 22, 106. [Google Scholar] [CrossRef]
- Saghrouchni, H.; Barnossi, A.E.; Mssillou, I.; Lavkor, I.; Ay, T.; Kara, M.; Alarfaj, A.A.; Hirad, A.H.; Nafidi, H.A.; Bourhia, M.; et al. Potential of carvacrol as plant growth-promotor and green fungicide against fusarium wilt disease of perennial ryegrass. Front. Plant Sci. 2023, 14, 973207. [Google Scholar] [CrossRef]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, V.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef]
- Caballero-Guerrero, B.; Garrido-Fernández, A.; Fermoso, F.G.; Rodríguez-Gutierrez, G.; Fernández-Prior, M.A.; Reinhard, C.; Nyström, L.; Benítez-Cabello, A.; Nóe Arroyo-López, F. Antimicrobial effects of treated olive mill waste on foodborne pathogens. LWT Food Sci. Technol. 2022, 164, 113628. [Google Scholar] [CrossRef]
- Medina, E.; Romero, C.; Santos, B.; Castro, A.; García Romero, F.; Brenes, M. Antimicrobial Activity of Olive Solutions from stored Alpeorujo against Plant Pathogenic Microorganisms. J. Agric. Food Chem. 2011, 59, 6927–6932. [Google Scholar] [CrossRef]
- Capasso, R.; Evidente, A.; Schivo, L.; Orru’, G.; Marcialis, M.A.; Cristinzio, G. Antibacterial polyphenols from olive oil mill waste waters. J. Appl. Bacteriol. 1995, 79, 393–398. [Google Scholar] [CrossRef]
- Pannucci, E.; Caracciolo, R.; Romani, A.; Cacciola, F.; Dugo, P.; Bernini, R.; Varvaro, L.; Santi, L. An hydroxytyrosol enriched extract from olive mill wastewater exerts antioxidant activity and antimicrobial activity on Pseudomonas savastanoi pv. savastanoi and Agrobacterium tumefaciens. Nat. Prod. Res. 2019, 35, 2677–2684. [Google Scholar] [CrossRef]
- Hudzicki, J. Kirby-Bauer Disk Diffusion Susceptibility Test Protocol; American Society for Microbiology: Washington, DC, USA, 2009; pp. 1–23. [Google Scholar]
- Vardar-Vardar-Unlü, G.; Candan, F.; Sökmen, A.; Daferera, D.; Polissiou, M.; Sökmen, M.; Dönmez, E.; Tepe, B. Antimicrobial and antioxidant activity of the essential oil and methanol extracts of Thymus pectinatus Fisch. et Mey. Var. pectinatus (Lamiaceae). J. Agric. Food Chem. 2003, 51, 63–67. [Google Scholar] [CrossRef]
- Hejna, M.; Dell’Anno, M.; Liu, Y.; Rossi, L.; Aksmann, A.; Pogorzelski, G.; Jóźwik, A. Assessment of the antibacterial and antioxidant activities of seeweed–derived extracts. Sci. Rep. 2024, 14, 21044. [Google Scholar] [CrossRef]
- Košćak, L.; Lamovšek, J.; Košćak, L.; Lamovšek, J.; Lukić, M.; Kovačević, T.K.; Đermić, E.; Goreta Ban, S.G.; Major, N.; Godena, S. Varietal Susceptibility of Olive to Pseudomonas savastanoi pv. savastanoi and Antibacterial Potential of Plant-Based Agents. Microorganisms 2024, 12, 1301. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetič Vodopivec, B. Ultrasonic Extraction of Phenols from Olive Mill Wastewater: Comparison with Conventional Methods. J. Agric. Food Chem. 2011, 59, 2725–12731. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic composition and antimicrobial activity of Algerian olive products and by-products. Food Sci. Technol. 2018, 98, 323–328. [Google Scholar] [CrossRef]
- CLSI. Methods for Determining Bactericidal Activity of Antimicrobial Agents; Wayne, P.A., Ed.; Approved Guideline. CLSI Document M26-A; Clinical Laboratory Standards Institute: Wayne, PA, USA, 1999. [Google Scholar]
- Liu, Q.; Yang, J.; Ahmed, W.; Wan, X.; Wei, L.; Ji, G. Exploiting the antibacterial mechanism of phenazine substances from Lysobacter antibioticus 13-6 against Xanthomonas oryzae pv. oryzicola. J. Microbiol. 2022, 60, 496–510. [Google Scholar] [CrossRef]
- Mombeshora, M.; Chi, G.F.; Mukanganyama, S. Antibiofilm Activity of Extract and a Compound Isolated from Triumfetta welwitschii against Pseudomonas aeruginosa. Biochem. Res. Int. 2021, 2021, 9946183. [Google Scholar] [CrossRef]
- Elcocks, E.R.; Spencer-Phillips, P.T.N.; Adukwu, E.C. Rapid bactericidal effect of cinnamon bark essential oil against Pseudomonas aeruginosa. J. Appl. Microbiol. 2019, 128, 1025–1037. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef]
- Miksusanti; Jenie, B.S.L.; Priosoeryanto, B.P.; Syarief, R.; Rekso, G.T. Mode of action temu kunci (Kaempferia pandurata) essential oil on E. coli k1.1 cell determined by leakage of material cell and salt tolerance assays. HAYATI J. Biosci. 2008, 15, 56–60. [Google Scholar] [CrossRef]
- Obanor, F.O.; Jaspers, M.V.; Jones, E.E.; Walter, M. Greenhouse and field evaluation of fungicides for control of olive leaf spot in New Zealand. Crop Prot. 2008, 27, 1335–1342. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 9 January 2025).
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Barić, B. Integrirana zaštita maslina od bolesti i štetnika unutar integrirane proizvodnje. Pomol. Croat. 2006, 12, 87–92. [Google Scholar]
- Mandal, S.M.; Chakraborty, D.; Dey, S. Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal. Behav. 2010, 5, 359–368. [Google Scholar] [CrossRef]
- Novy, P.; Kloucek, P.; Rondevaldova, J.; Havlik, J.; Kourimska, L.; Kokoska, L. Thymoquinone vapor significantly affects the results of Staphylococcus aureus sensitivity tests using the standard broth microdilution method. Fitoterapia 2014, 94, 102–107. [Google Scholar] [CrossRef]
- Reiter, J.; Levina, N.; van der Linden, M.; Gruhlke, M.; Martin, C.; Slusarenko, A.J. Diallylthiosulfinate (Allicin), a Volatile Antimicrobial from Garlic (Allium sativum), Kills Human Lung Pathogenic Bacteria, Including MDR Strains, as a Vapor. Molecules 2017, 22, 1711. [Google Scholar] [CrossRef]
- Tarakanov, R.I.; Dzhalilov, F.S.U. Using of Essential Oils and Plant Extracts against Pseudomonas savastanoi pv. glycinea and Curtobacterium flaccumfaciens pv. flaccumfaciens on Soybean. Plants 2022, 11, 2989. [Google Scholar] [CrossRef]
- Quesada, J.M.; Penyalver, R.; Pérez-Panadés, J.; Salcedo, C.I.; Carbonell, E.A.; López, M.M. Dissemination of Pseudomonas savastanoi pv. savastanoi populations and subsequent appearance of olive knot disease. Plant Pathol. 2010, 59, 262–269. [Google Scholar] [CrossRef]
- Baldassarre, F.; Schiavi, D.; Ciarroni, S.; Tagliavento, V.; De Stradis, A.; Vergaro, V.; Suranna, G.P.; Balestra, G.M.; Ciccarella, G. Thymol-Nanoparticles as Effective Biocides against the Quarantine Pathogen Xyllela fastidiosa. Nanomaterials 2023, 13, 1285. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Knežević, M.; Abram, M. Challenges to antimicrobial susceptibility testing of plant-derived polyphenolic compounds. Arch. Ind. Hyg. Toxicol. 2020, 71, 300–311. [Google Scholar] [CrossRef]
- Nagy, J.K.; Móricz, Á.M.; Böszörményi, A.; Ambrus, Á.; Schwarczinger, I. Antibacterial effect of essential oils and their components against Xanthomonas arboricola pv. pruni revealed by microdilution and direct bioautographic assays. Front. Cell. Infect. Microbiol. 2023, 13, 1204027. [Google Scholar] [CrossRef]
- Ashraf, S.; Chatha, M.A.; Ejaz, W.; Janjua, H.A.; Hussain, I. Lysozyme-coated silver nanoparticles for differentiating bacterial strains on the basis of antibacterial activity. Nanoscale Res. Lett. 2014, 9, 565. [Google Scholar] [CrossRef] [PubMed]
- Tardugno, R.; Serio, A.; Pellati, F.; D’Amato, S.; Chaves López, C.; Bellardi, M.G.; Di Vito, M.; Savini, V.; Paparella, A.; Benvenuti, S. Lavandula × intermedia and Lavandula angustifolia essential oils: Phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2019, 33, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Bouchekouk, C.; Zohra Kara, F.; Tail, G.; Saidi, F.; Benabdelkader, T. Essential oil composition and antibacterial activity of Pteridium aquilinum (L.) Kuhn. Biol. Futur. 2019, 70, 56–61. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Caparrotta, S.; Comparini, D.; Marone, E.; Kimmenfield, R.; Luzzietti, L.; Taiti, C.; Mancuso, S. Correlation between VOC fingerprinting and antimicrobial activity of several essential oils extracted by plant resins against A. tumefaciens and P. savastanoi. Flavour. Fragr. J. 2019, 5, 377–387. [Google Scholar] [CrossRef]
- Camele, I.; Elshafie, H.S.; Caputo, L.; De Feo, V. Anti-quorum Sensing and Antimicrobial Effect of Mediterranean Plant Essential Oils against Phytopathogenic Bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar] [CrossRef]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial Activity of Some Essential Oils—Present Status and Future Perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Vinceković, M.; Maslov Bandić, L.; Jurić, S.; Jalšenjak, N.; Caić, A.; Živičnjak, E.; Ðermić, E.; Karoglan, M.; Osrečak, M.; Topolovec-Pintarić, S. The enhancement of bioactive potential in Vitis vinifera leaves by application of microspheres loaded with biological and chemical agents. J. Plant Nutr. 2019, 42, 543–558. [Google Scholar] [CrossRef]
- Jurić, S.; Sopko Stracenski, K.; Król-Kilińska, Z.; Žutić, I.; Uher, S.F.; Ðermić, E.; Vinceković, M. The enhancement of plant secondary metabolites content in Lactuca sativa L. by encapsulated bioactive agents. Sci. Rep. 2020, 10, 3737. [Google Scholar] [CrossRef]
- Melchionna Albuquerque, P.; Gomes Azevedo, S.; Pereira de Andrade, C.; Corrêa de Souza D’Ambros, N.; Martins Pérez, M.T.; Manzato, L. Biotechnological Applications of Nanoencapsulated Essential Oils: A Review. Polymers 2022, 14, 5495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).