Enhancing Soybean Physiology and Productivity Through Foliar Application of Soluble Monoammonium Phosphate
Abstract
1. Introduction
2. Materials and Methods
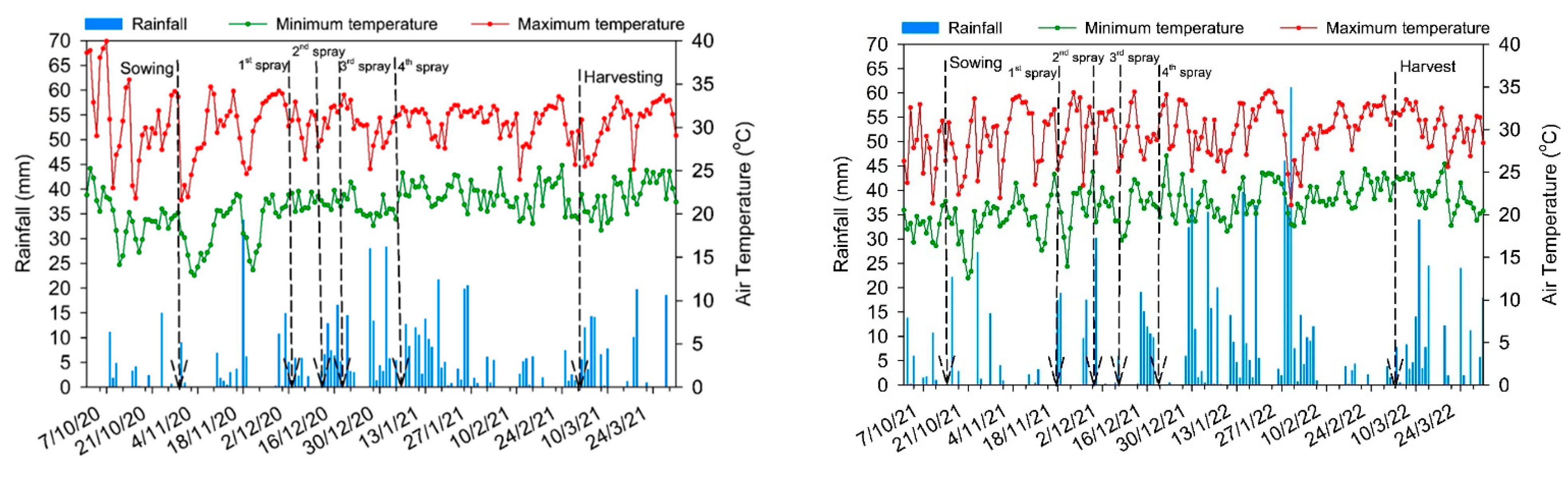
2.1. Site Description
2.2. Experimental Design and Treatment Descriptions
- (I)
- Control (no treatment);
- (II)
- Foliar spraying of soluble MAP at the V4 vegetative phenological stage [30];
- (III)
- Foliar spraying of soluble MAP at the V6 vegetative phenological stage [30];
- (IV)
- Foliar spraying of soluble MAP at the R1 reproductive phenological stage [30];
- (V)
- Foliar spraying of soluble MAP at the R3 reproductive phenological stage [30];
- (VI)
- Foliar spraying of soluble MAP in all phenological stages—V4, V6, R1, and R3.
2.3. Management Practices
2.4. Nutritional Analyses (Crop Nutrition)
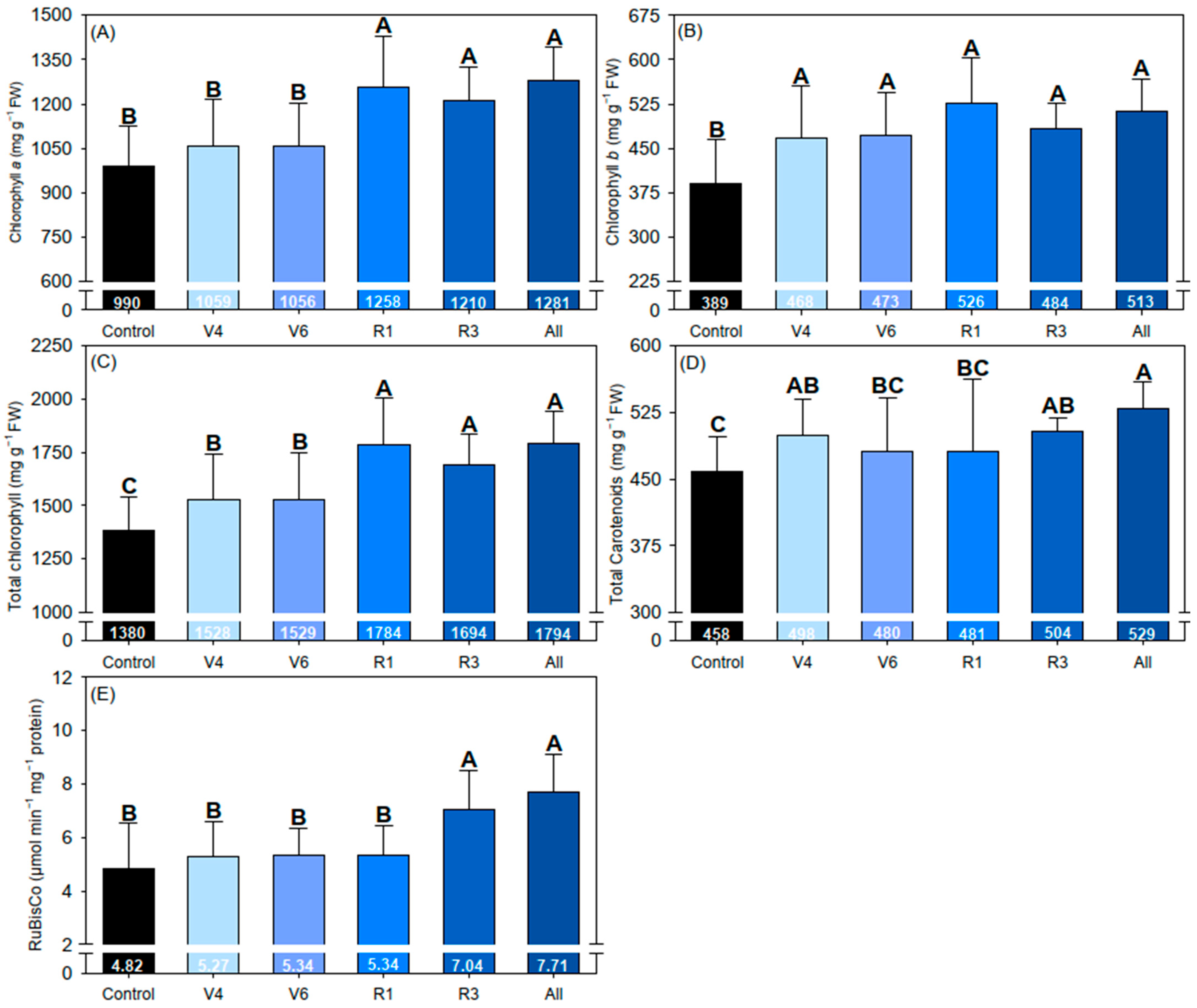
2.5. Photosynthetic Pigments and Enzyme
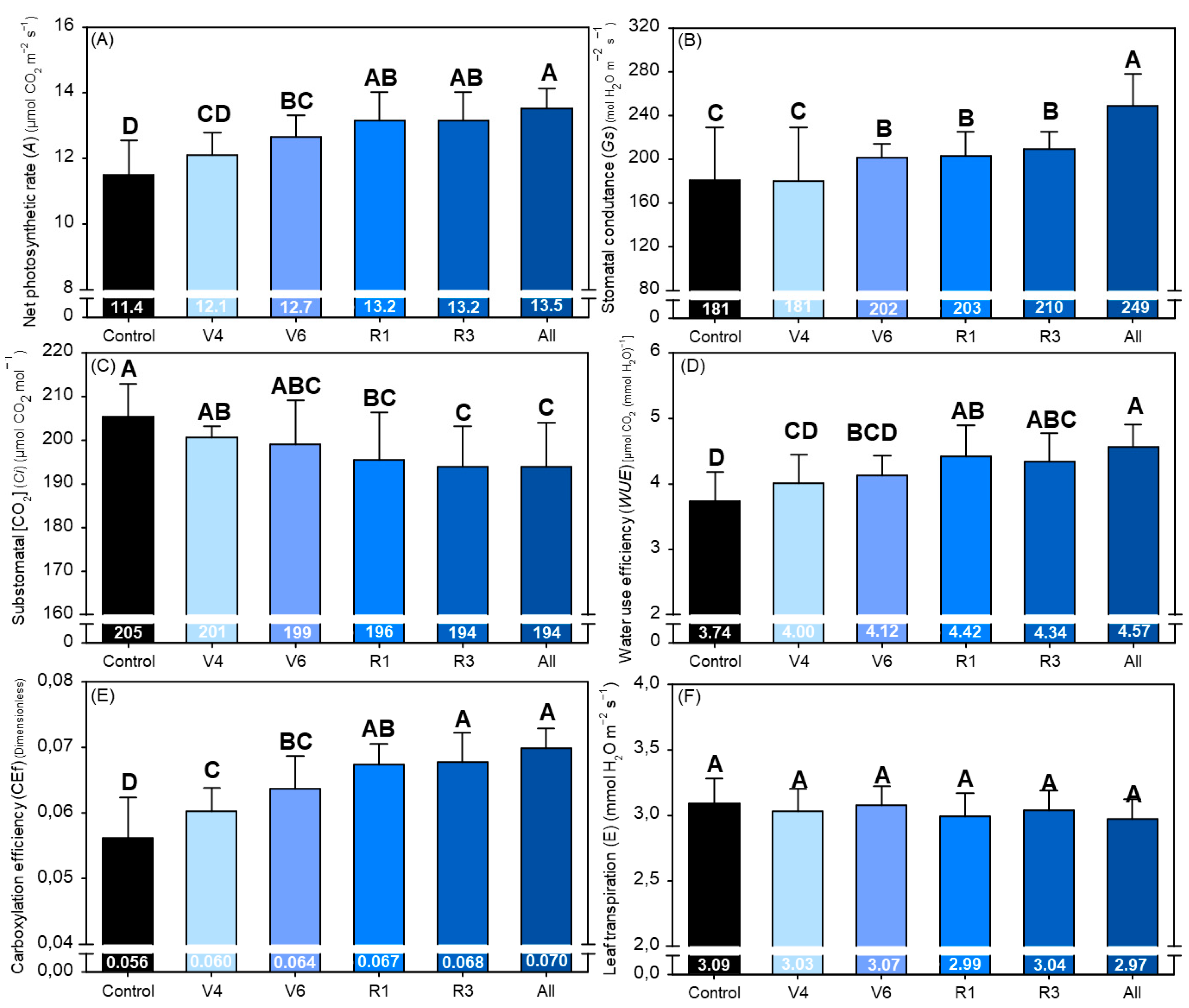
2.6. Gas Exchange Parameters
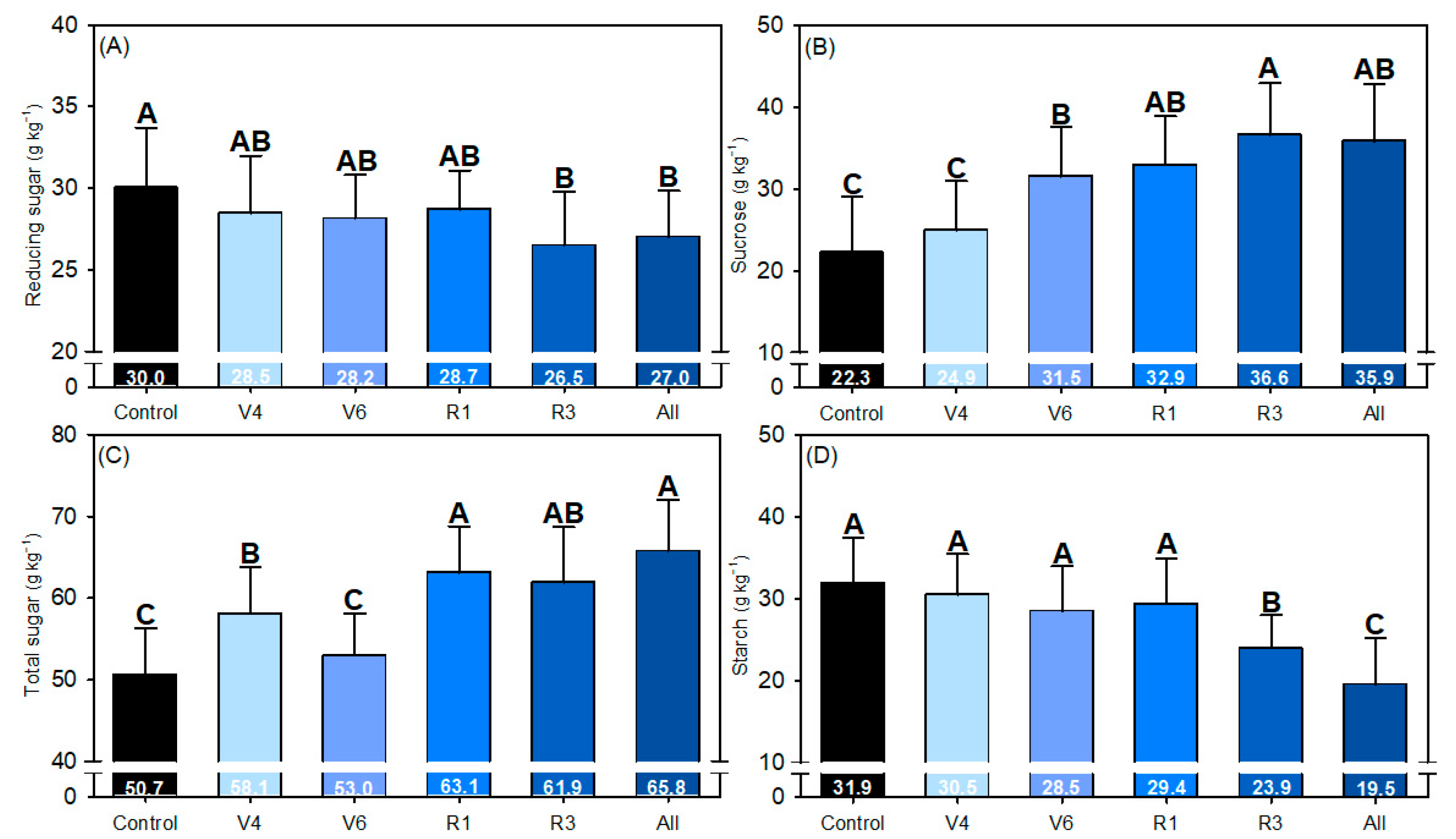
2.7. Total Soluble Sugar Concentration
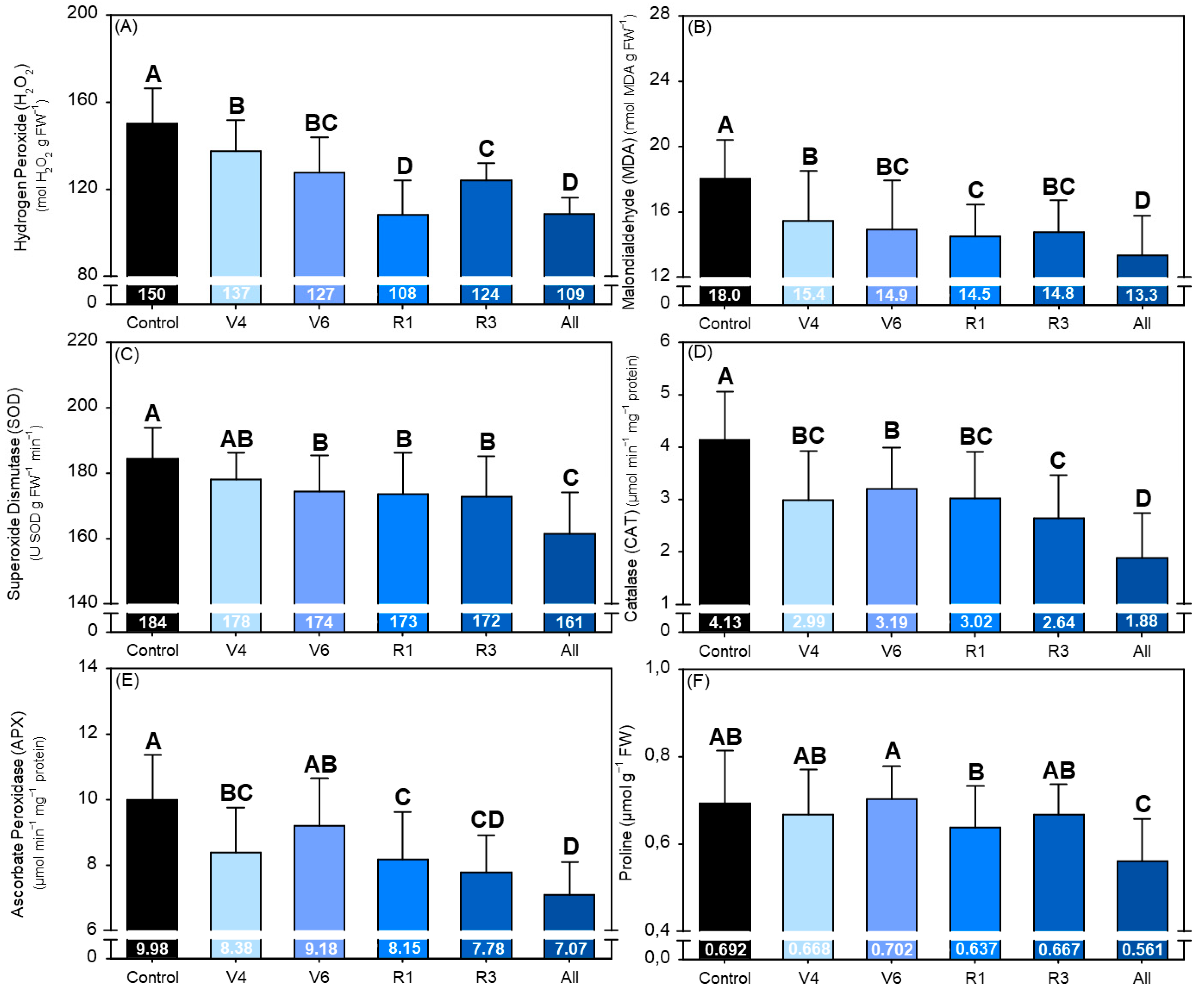
2.8. Oxidative Stress and Antioxidant Enzymes
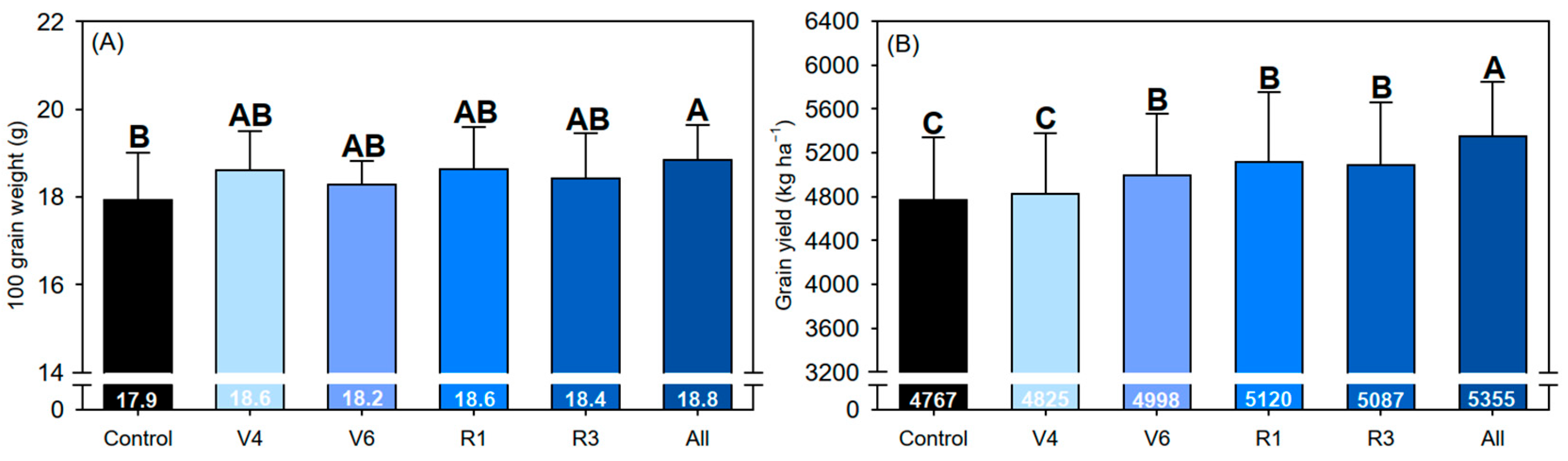
2.9. Agronomic Parameters and Grain Yield
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MAP | Monoammonium phosphate |
| A | Net photosynthetic rate |
| gs | Stomatal conductance |
| Ci | Internal CO2 concentration in the substomatal cavity |
| E | Transpiration |
| WUE | Water use efficiency |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| APX | Ascorbate peroxidase |
References
- USDA. Available online: https://www.usda.gov/sites/default/files/documents/USDA-Agricultural-Projections-to-2030.pdf (accessed on 19 January 2025).
- CONAB. Acompanhamento Da Safra Brasileira—Quinto Levantamento Safra 2024/25; National Supply Company (CONAB): Brasília, Brazil, 2025; Volume 8.
- Fageria, N.K.; Filho, M.P.B.; Moreira, A.; Guimarães, C.M. Foliar Fertilization of Crop Plants. J. Plant Nutr. 2009, 32, 1044–1064. [Google Scholar] [CrossRef]
- Oliveira, S.L.; Crusciol, C.A.C.; Rodrigues, V.A.; Galeriani, T.M.; Portugal, J.R.; Bossolani, J.W.; Moretti, L.G.; Calonego, J.C.; Cantarella, H. Molybdenum Foliar Fertilization Improves Photosynthetic Metabolism and Grain Yields of Field-Grown Soybean and Maize. Front. Plant Sci. 2022, 13, 887682. [Google Scholar]
- Guelfi, D.R.; Chagas, W.F.T.; Lacerda, J.R.; Chagas, R.M.R.; de Souza, T.L.; Andrade, A.B. Monoammonium Phosphate Coated with Polymers and Magnesium for Coffee Plants. Ciência E Agrotecnologia 2018, 42, 261–270. [Google Scholar] [CrossRef]
- Rodrigues, V.A.; Crusciol, C.A.C.; Bossolani, J.W.; Portugal, J.R.; Moretti, L.G.; Bernart, L.; Vilela, R.G.; Galeriani, T.; Lollato, R.P. Foliar Nitrogen as Stimulant Fertilization Alters Carbon Metabolism, Reactive Oxygen Species Scavenging, and Enhances Grain Yield in a Soybean–Maize Rotation. Crop Sci. 2021, 61, 3687–3701. [Google Scholar] [CrossRef]
- Rengel, Z.; Cakmak, I.; White, P.J. Marschner’s Mineral Nutrition of Plants; Academic Press: Cambridge, MA, USA, 2022; ISBN 0323853528. [Google Scholar]
- Moretti, L.G.; Lazarini, E.; Bossolani, J.W.; Parente, T.L.; Caioni, S.; Araujo, R.S.; Hungria, M. Can Additional Inoculations Increase Soybean Nodulation and Grain Yield? Agron. J. 2018, 110, 715–721. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Momesso, L.; Garcia, A.; Kuramae, E.E.; Hungria, M. Bacterial Consortium and Microbial Metabolites Increase Grain Quality and Soybean Yield. J. Soil. Sci. Plant Nutr. 2020, 20, 1923–1934. [Google Scholar] [CrossRef]
- Bossolani, J.W.; dos Santos, F.L.; Meneghette, H.H.A.; Sanches, I.R.; Moretti, L.G.; Parra, L.F.; Lazarini, E. Soybean in Crop Rotation with Maize and Palisade Grass Intercropping Enhances the Long-Term Effects of Surface Liming in No-till System. J. Soil. Sci. Plant Nutr. 2021, 21, 119–130. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Kuramae, E.E.; Bossolani, J.W.; Moreira, A.; Costa, N.R.; Alves, C.J.; Pascoaloto, I.M.; Rondina, A.B.L.; Hungria, M. Effects of Growth-Promoting Bacteria on Soybean Root Activity, Plant Development, and Yield. Agron. J. 2020, 112, 418–428. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Moretti, L.G.; Aquino, G.S. Phosphorus, Potassium and Sulfur Interactions in Soybean Plants on a Typic Hapludox. Commun. Soil Sci. Plant Anal. 2018, 49, 405–415. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Moretti, L.G. Yield, Yield Components, Soil Chemical Properties, Plant Physiology, and Phosphorus Use Efficiency in Soybean Genotypes. Commun. Soil Sci. Plant Anal. 2017, 48, 2464–2476. [Google Scholar] [CrossRef]
- Ahmad, Z.; Waraich, E.A.; Rehman, M.Z.U.; Ayub, M.A.; Usman, M.; Alharby, H.; Bamagoos, A.; Barutçular, C.; Raza, M.A.; Çiğ, F.; et al. Foliar Application of Phosphorus Enhances Photosynthesis and Biochemical Characteristics of Maize under Drought Stress. Phyton 2021, 90, 503–514. [Google Scholar]
- Hudina, M.; Stampar, F. Effect of Phosphorus and Potassium Foliar Fertilization on Fruit Quality of Pears. In Proceedings of the International Symposium on Foliar Nutrition of Perennial Fruit Plants 594, Meran, Italy, 10–15 September 2001; pp. 487–493. [Google Scholar]
- Moreira, A.; Fageria, N.K.; Garcia, A.G.Y. Effect of Liming on the Nutritional Conditions and Yield of Alfalfa Grown in Tropical Conditions. J. Plant Nutr. 2011, 34, 1107–1119. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Souza, L.G.M.; Bruno, I.P. Bioavailability of Nutrients in Seeds from Tropical and Subtropical Soybean Varieties. Commun. Soil Sci. Plant Anal. 2016, 47, 888–898. [Google Scholar] [CrossRef]
- Moreira, A.; Sfredo, G.J.; Moraes, L.A.C.; Fageria, N.K. Agronomic Efficiency of Two Types of Lime and Phosphate Fertilizer Sources in Brazilian Cerrado Soils Cultivated with Soybean. Commun. Soil Sci. Plant Anal. 2014, 45, 2319–2330. [Google Scholar] [CrossRef]
- Viveiros, J.; Moretti, L.G.; Alves Filho, I.; Pacola, M.; Jacomassi, L.M.; Rodrigues, V.A.; Jamal, A.; Bossolani, J.W.; Portugal, J.R.; Carbonari, C.A.; et al. Can Foliar Application of Soluble Monoammonium Phosphate Effectively Alleviate Herbicide-Induced Oxidative Stress in Key Crops? Front. Plant Sci. 2025, 16, 1504244. [Google Scholar] [CrossRef]
- Viveiros, J.; Moretti, L.G.; Pacola, M.; Jacomassi, L.M.; de Souza, F.M.; Rodrigues, V.A.; Bossolani, J.W.; Portugal, J.R.; Carbonari, C.A.; Crusciol, C.A.C. Foliar Application of Phosphoric Acid Mitigates Oxidative Stress Induced by Herbicides in Soybean, Maize, and Cotton Crops. Plant Stress. 2024, 13, 100543. [Google Scholar] [CrossRef]
- Fageria, N.K.; Nascente, A.S. Management of Soil Acidity of South American Soils for Sustainable Crop Production; Elsevier: Amsterdam, The Netherlands, 2014; Volume 128, ISBN 9780128021392. [Google Scholar]
- Moreira, A.; Moraes, L.A.C.; de Melo, T.R.; Heinrichs, R.; Moretti, L.G. Management of Copper for Crop Production. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2022; Volume 173, pp. 257–298. ISBN 9780323989558. [Google Scholar]
- Santos, L.O.; Moraes, L.A.C.; Petineli, R.; Moretti, L.G.; Moreira, A. Yield, Yield Components, Soil Fertility, and Nutritional Status of Soybean as Influenced by Limestone and Copper Interactions. J. Plant Nutr. 2020, 43, 2445–2454. [Google Scholar] [CrossRef]
- Fernández, V.; Brown, P.H. From Plant Surface to Plant Metabolism: The Uncertain Fate of Foliar-Applied Nutrients. Front. Plant Sci. 2013, 4, 289. [Google Scholar]
- Hussein, H.A.Z.; Awad, A.A.M.; Beheiry, H.R. Improving Nutrients Uptake and Productivity of Stressed Olive Trees with Mono-Ammonium Phosphate and Urea Phosphate Application. Agronomy 2022, 12, 2390. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes Gonçalves, J.L.; Sparovek, G. Köppen’s Climate Classification Map for Brazil. Meteorologische Zeitschrift 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Unicamp Center of Meteorological and Climatic Research Applied to Agriculture. Municipalities Climate of São Paulo State: Botucatu, Brazil. Available online: www.cpa.unicamp.br (accessed on 19 January 2025).
- Jahn, R.; Blume, H.P.; Asio, V.B.; Spaargaren, O.; Schad, P. Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA—Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Fehr, W.R.; Caviness, C.E. Iowa State University Cooperative Extension Service, Special Report 80; Iowa State University of Science and Technology: Ames, IA, USA, 1977. [Google Scholar]
- Moretti, L.G.; Crusciol, C.A.C.; Leite, M.F.A.; Momesso, L.; Bossolani, J.W.; Costa, O.Y.A.; Hungria, M.; Kuramae, E.E. Diverse Bacterial Consortia: Key Drivers of Rhizosoil Fertility Modulating Microbiome Functions, Plant Physiology, Nutrition, and Soybean Grain Yield. Environ. Microbiome 2024, 19, 50. [Google Scholar] [CrossRef]
- Moretti, L.G.; Crusciol, C.A.C.; Bossolani, J.W.; Calonego, J.C.; Moreira, A.; Garcia, A.; Momesso, L.; Kuramae, E.E.; Hungria, M. Beneficial Microbial Species and Metabolites Alleviate Soybean Oxidative Damage and Increase Grain Yield during Short Dry Spells. Eur. J. Agron. 2021, 127, 126293. [Google Scholar] [CrossRef]
- Embrapa Soybean Production Technologies. Production Systems 17; Embrapa Soybean: Londrina, Brazil, 2020; Available online: https://www.embrapa.br/en/soja (accessed on 24 January 2025).
- Ambrosano, E.J.; Tanaka, R.T.; Mascarenhas, H.A.A.; van Raij, B.; Quaggio, J.A.; Cantarella, H. Leguminosas e Oleaginosas. In Recomendações de Adubação e Calagem para o Estado de São Paulo; van Raij, B., Cantarella, H., Quaggio, J.A., Furlani, A.M.C., Eds.; Instituto Agronômico: Campinas, Brazil, 1997; pp. 187–203. [Google Scholar]
- Malavolta, E.; Vitti, G.C.; Oliveira, S.A. Evaluation of the Nutritional Status of Plants: Principles and Applications, 2nd ed.; POTAFOS: Piracicaba, Brazil, 1997. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1987; Volume 148, pp. 350–382. ISBN 0076-6879. [Google Scholar]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as Well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar]
- Reid, C.D.; Tissue, D.T.; Fiscus, E.L.; Strain, B.R. Comparison of Spectrophotometric and Radioisotopic Methods for the Assay of Rubisco in Ozone-treated Plants. Physiol. Plant 1997, 101, 398–404. [Google Scholar]
- Nelson, N. A Photometric Adaptation of the Somogyi Method for the Determination of Glucose. J. Biol. Chem. 1944, 3, 375–380. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The Effect of Drought and Ultraviolet Radiation on Growth and Stress Markers in Pea and Wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Alas, R.M.; Smith, R.J.; Lea, P.J. Response of Antioxidant Enzymes to Transfer from Elevated Carbon Dioxide to Air and Ozone Fumigation, in the Leaves and Roots of Wild-type and a Catalase-deficient Mutant of Barley. Physiol. Plant 1998, 104, 280–292. [Google Scholar]
- Gratao, P.L.; Monteiro, C.C.; Peres, L.E.P.; Azevedo, R.A. The Isolation of Antioxidant Enzymes from Mature Tomato (Cv. Micro-Tom) Plants. HortScience 2008, 43, 1608–1610. [Google Scholar]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591. [Google Scholar] [CrossRef]
- Levene, H. Robust Tests for Equality of Variances. In Contributions to Probability and Statistics: Essays in …; Olkin, I., Ghurye, S.G., Hoeffding, W., Madow, W.G., Mann, H.B., Eds.; Stanford University Press: Redwood City, CA, USA, 1960; Volume 69, pp. 278–292. [Google Scholar]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of Shoot and Root Dry Matter and Carbohydrates in Bean Plants Suffering from Phosphorus, Potassium and Magnesium Deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in Phloem Export of Sucrose in Leaves in Response to Phosphorus, Potassium and Magnesium Deficiency in Bean Plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar]
- Fageria, N.K.; Stone, L.F. Micronutrient Deficiency Problems in South America. In Micronutrient Deficiencies in Global Crop Production; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 245–266. ISBN 9781402068607. [Google Scholar]
- Fernández, V.; Gil-Pelegrín, E.; Eichert, T. Foliar Water and Solute Absorption: An Update. Plant J. 2021, 105, 870–883. [Google Scholar] [CrossRef]
- Moreira, A.; Moraes, L.A.C.; Schroth, G.; Becker, F.J.; Mandarino, J.M.G. Soybean Yield and Nutritional Status Response to Nitrogen Sources and Rates of Foliar Fertilization. Agron. J. 2017, 109, 629–635. [Google Scholar] [CrossRef]
- Eichert, T.; Fernández, V. Uptake and Release of Elements by Leaves and Other Aerial Plant Parts. In Marschner’s Mineral Nutrition of Plants; Elsevier: Amsterdam, The Netherlands, 2023; pp. 105–129. [Google Scholar]
- Cantarella, H.; Quaggio, J.; Mattos, D., Jr.; Boaretto, R.; van Raij, B. Bulletin 100: Fertilization and Liming Recommendations for the State of São Paulo; Cantarella, H., Quaggio, J.A., Mattos, D., Jr., Boaretto, R.M., van Raij, B., Eds.; Agronomic Institute: Campinas, Brazil, 2022; Volume 1. [Google Scholar]
- Ruiz-Navarro, A.; Fernández, V.; Abadía, J.; Abadía, A.; Querejeta, J.I.; Albaladejo, J.; Barberá, G.G. Foliar Fertilization of Two Dominant Species in a Semiarid Ecosystem Improves Their Ecophysiological Status and the Use Efficiency of a Water Pulse. Environ. Exp. Bot. 2019, 167, 103854. [Google Scholar] [CrossRef]
- Henningsen, J.N.; Görlach, B.M.; Fernández, V.; Dölger, J.L.; Buhk, A.; Mühling, K.H. Foliar P Application Cannot Fully Restore Photosynthetic Capacity, P Nutrient Status, and Growth of P Deficient Maize (Zea mays L.). Plants 2022, 11, 2986. [Google Scholar] [CrossRef]
- Fiedor, L.; Kania, A.; Myśliwa-Kurdziel, B.; Orzeł, Ł.; Stochel, G. Understanding Chlorophylls: Central Magnesium Ion and Phytyl as Structural Determinants. Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 1491–1500. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Ying, Y.Q.; Zhang, Y.B.; Bi, Y.F.; Wang, A.K.; Du, X.H. Alleviation of Drought Stress in Phyllostachys Edulis by N and P Application. Sci. Rep. 2018, 8, 228. [Google Scholar] [CrossRef]
- Maheshwari, C.; Garg, N.K.; Hassan, M.; Tyagi, A. An Insight: Impact of Reduced Rubisco on Plant Physiology and Biochemistry. Indian J. Agric. Sci. 2021, 91, 16–20. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar]
- Farooq, M.A.; Niazi, A.K.; Akhtar, J.; Saifullah; Farooq, M.; Souri, Z.; Karimi, N.; Rengel, Z. Acquiring Control: The Evolution of ROS-Induced Oxidative Stress and Redox Signaling Pathways in Plant Stress Responses. Plant Physiol. Biochem. 2019, 141, 353–369. [Google Scholar] [PubMed]
- Cunningham, C. Characterization of Dry Spells in Southeastern Brazil during the Monsoon Season. Int. J. Climatol. 2020, 40, 4609–4621. [Google Scholar] [CrossRef]

| Attributes | Values | Unit/Extractant |
|---|---|---|
| pH | 5.2 | CaCl2 |
| Soil organic matter | 24 | g dm−3 |
| Phosphorus | 25 | mg dm−3 |
| Sulfur | 14 | mg dm−3 |
| Potential acidity [H + Al+3] | 31 | mmolc dm−3 |
| Potassium | 3.9 | mmolc dm−3 |
| Calcium | 38 | mmolc dm−3 |
| Magnesium | 11 | mmolc dm−3 |
| Cation exchange capacity | 79 | CECpH 7.0 |
| Base saturation (BS) | 55 | % |
| Iron | 13 | mg dm−3 |
| Copper | 1.6 | mg dm−3 |
| Manganese | 15 | mg dm−3 |
| Zinc | 3.0 | mg dm−3 |
| Boron | 0.6 | mg dm−3 |
| Clay | 520 | g kg−1 |
| Sandy | 360 | g kg−1 |
| Silt | 120 | g kg−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, V.A.; Moretti, L.G.; Alves Filho, I.; Pacola, M.; Viveiros, J.; Jacomassi, L.M.; Oliveira, S.L.; Jamal, A.; Galeriani, T.M.; Campos, M.d.; et al. Enhancing Soybean Physiology and Productivity Through Foliar Application of Soluble Monoammonium Phosphate. Agronomy 2025, 15, 818. https://doi.org/10.3390/agronomy15040818
Rodrigues VA, Moretti LG, Alves Filho I, Pacola M, Viveiros J, Jacomassi LM, Oliveira SL, Jamal A, Galeriani TM, Campos Md, et al. Enhancing Soybean Physiology and Productivity Through Foliar Application of Soluble Monoammonium Phosphate. Agronomy. 2025; 15(4):818. https://doi.org/10.3390/agronomy15040818
Chicago/Turabian StyleRodrigues, Vitor Alves, Luiz Gustavo Moretti, Israel Alves Filho, Marcela Pacola, Josiane Viveiros, Lucas Moraes Jacomassi, Sirlene Lopes Oliveira, Amine Jamal, Tatiani Mayara Galeriani, Murilo de Campos, and et al. 2025. "Enhancing Soybean Physiology and Productivity Through Foliar Application of Soluble Monoammonium Phosphate" Agronomy 15, no. 4: 818. https://doi.org/10.3390/agronomy15040818
APA StyleRodrigues, V. A., Moretti, L. G., Alves Filho, I., Pacola, M., Viveiros, J., Jacomassi, L. M., Oliveira, S. L., Jamal, A., Galeriani, T. M., Campos, M. d., Portugal, J. R., Bossolani, J. W., & Crusciol, C. A. C. (2025). Enhancing Soybean Physiology and Productivity Through Foliar Application of Soluble Monoammonium Phosphate. Agronomy, 15(4), 818. https://doi.org/10.3390/agronomy15040818







