Moderate Nitrogen Management Enhancing Maize Lodging Resistance by Reducing Pathogen Infection and Expansion of Stalk Rot
Abstract
1. Introduction
2. Materials and Methods
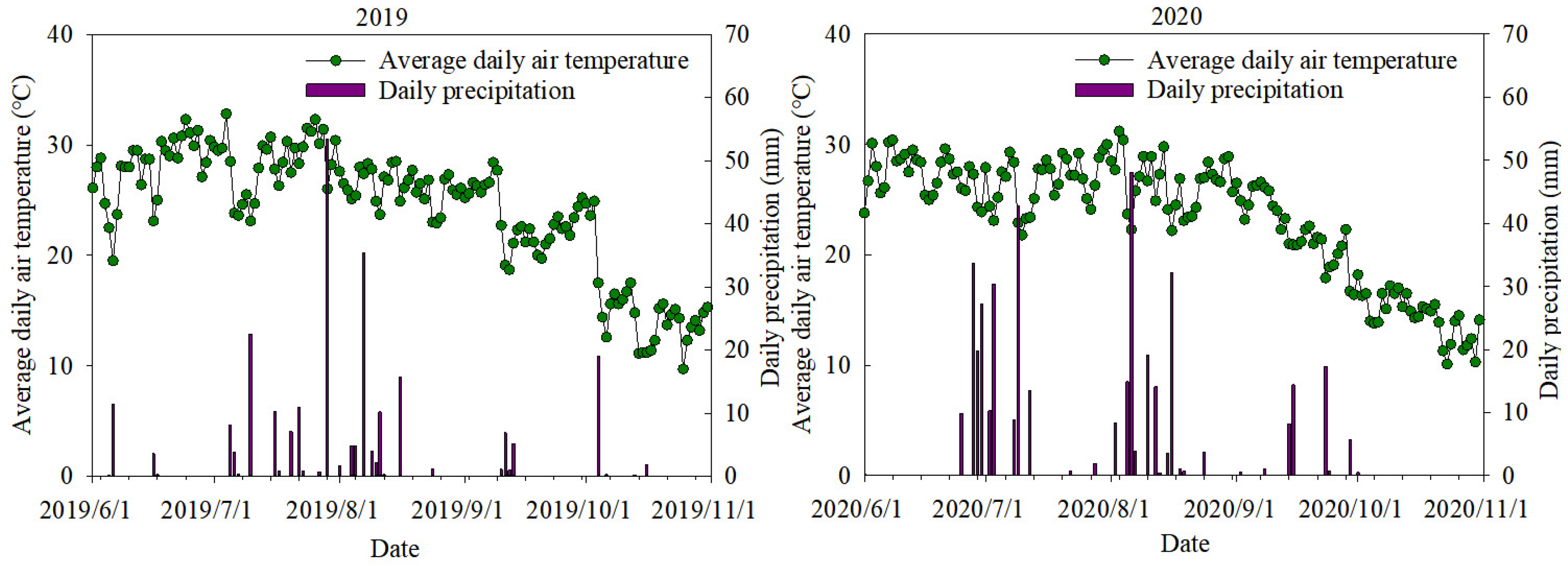
2.1. Experimental Site Description
2.2. Experimental Material and Design
2.3. Measuring Items and Methods
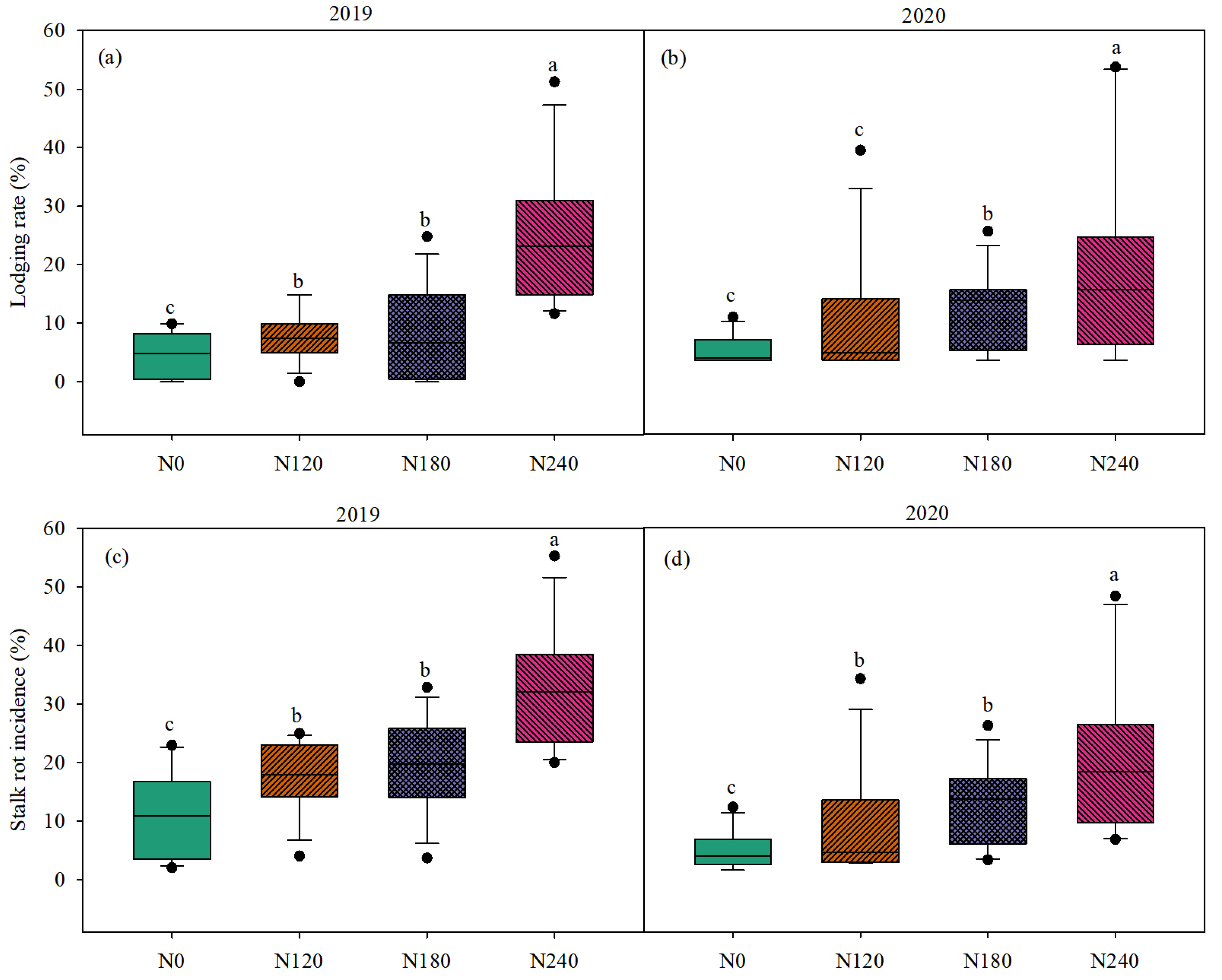
2.3.1. Natural Stalk Lodging Rate and Stalk Rot Incidence
2.3.2. Morphological Characteristics of Basal Third Internode
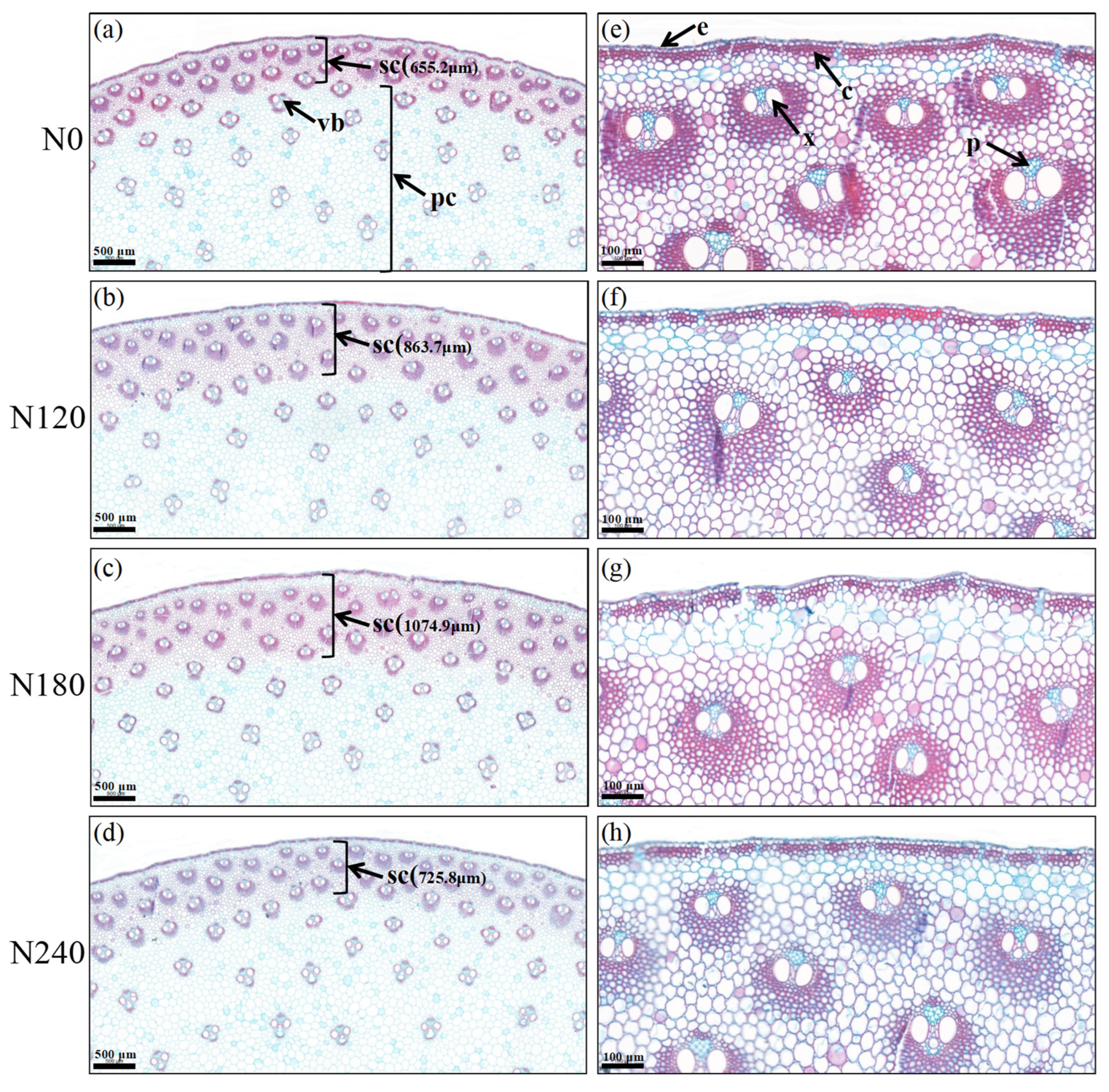
2.3.3. Histochemical Staining of Transverse
2.3.4. Mechanical Property
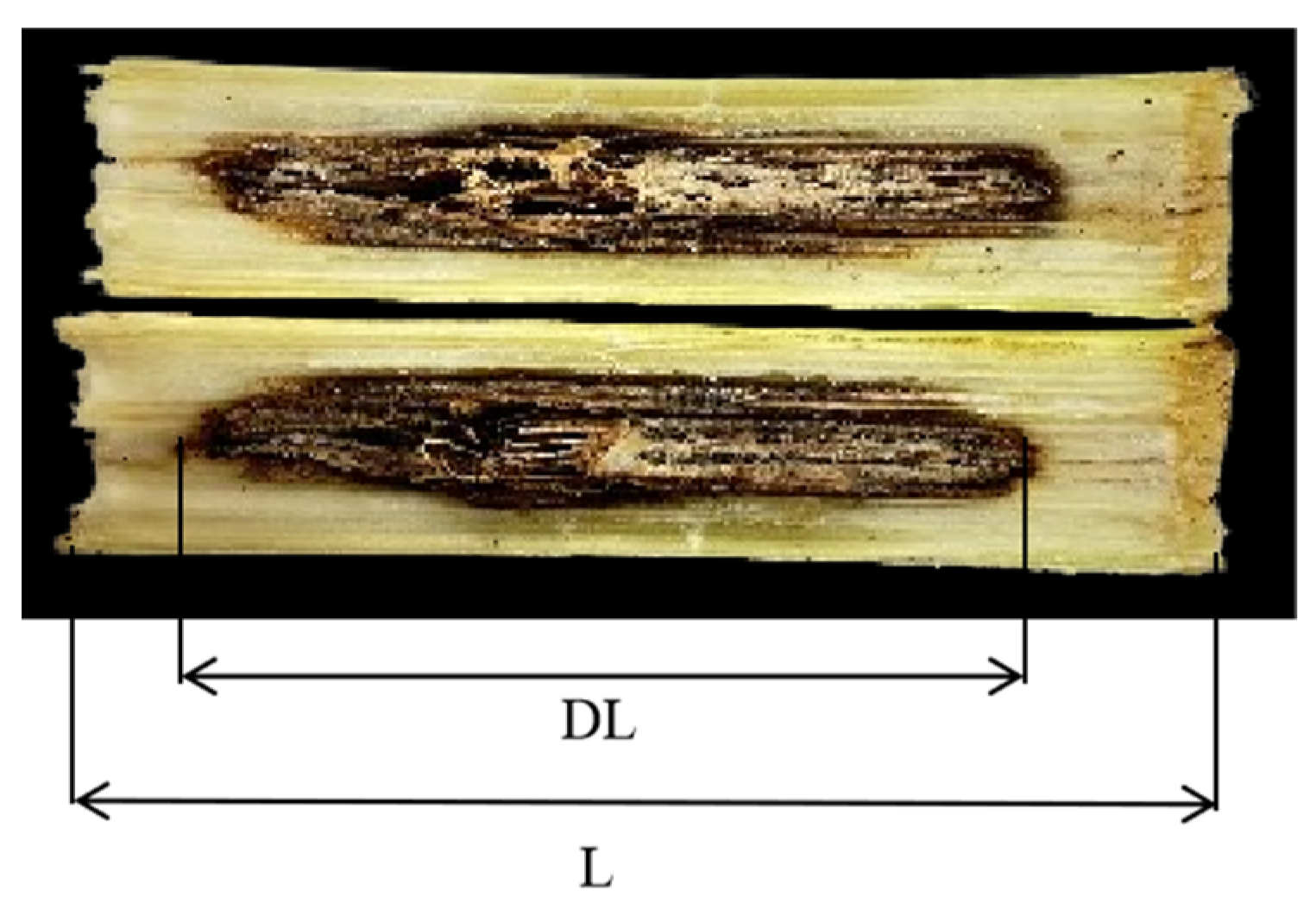
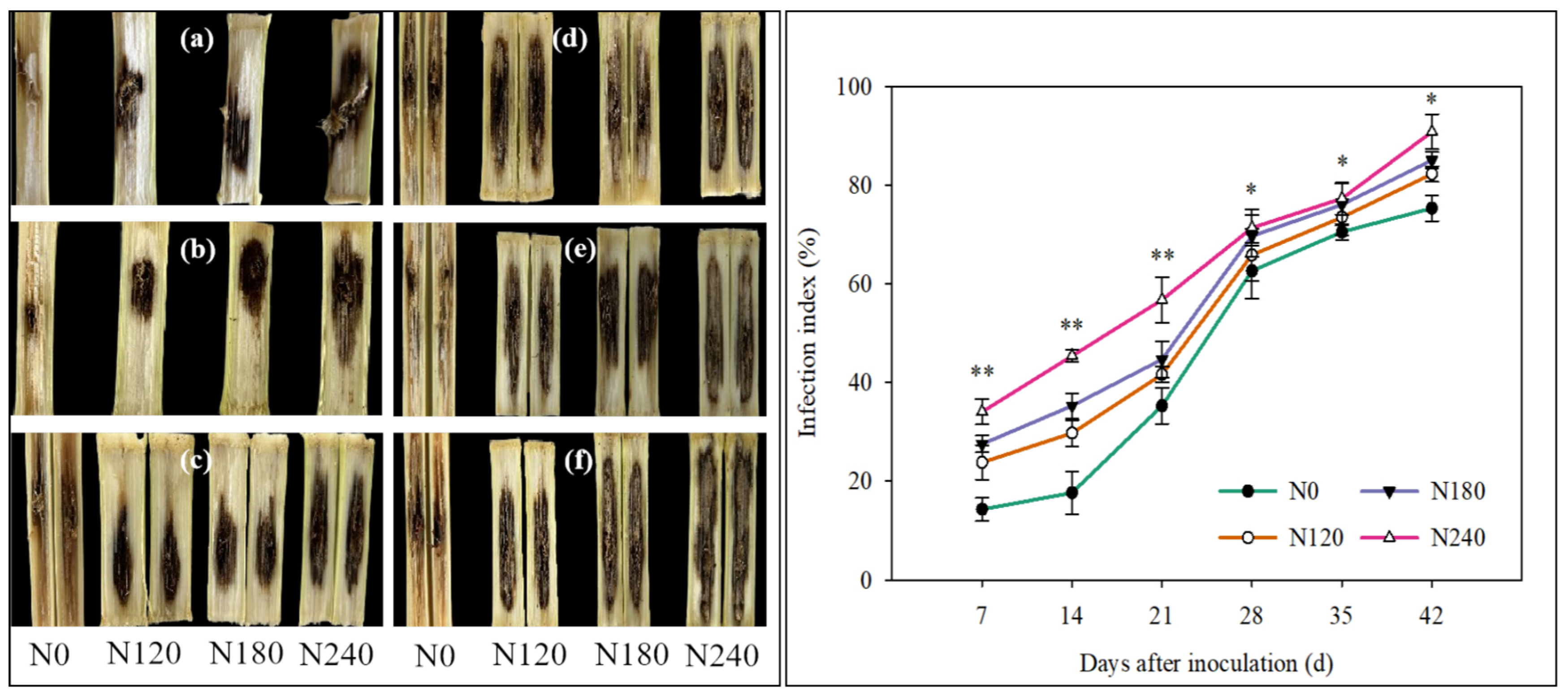
2.3.5. Infection Index
2.4. Statistical Analysis
3. Results
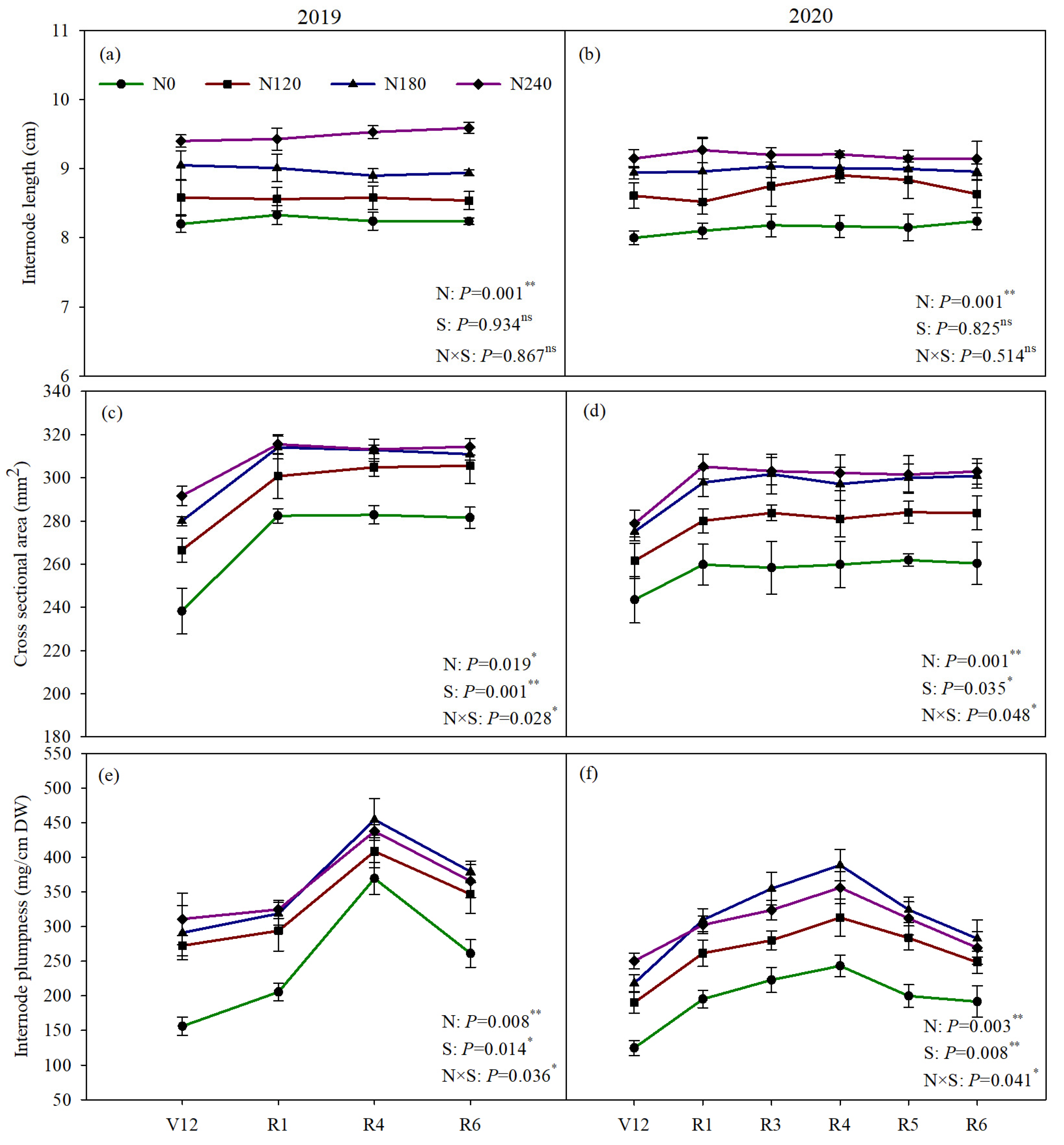
3.1. The Morphological Parameters of Basal Third Internodes
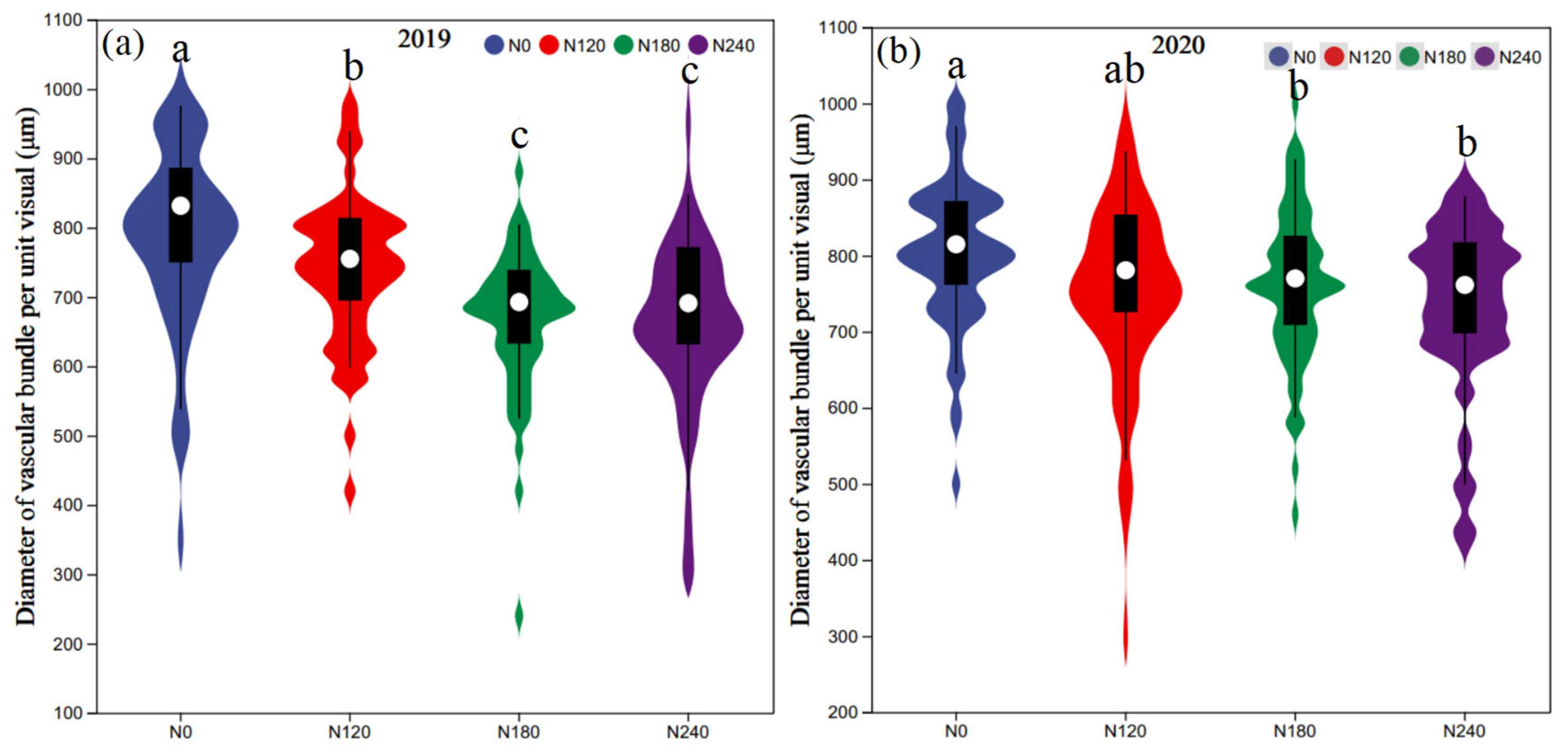
3.2. The Anatomical Structure of Basal Third Internodes
3.3. The Mechanical Strength of Basal Third Internodes
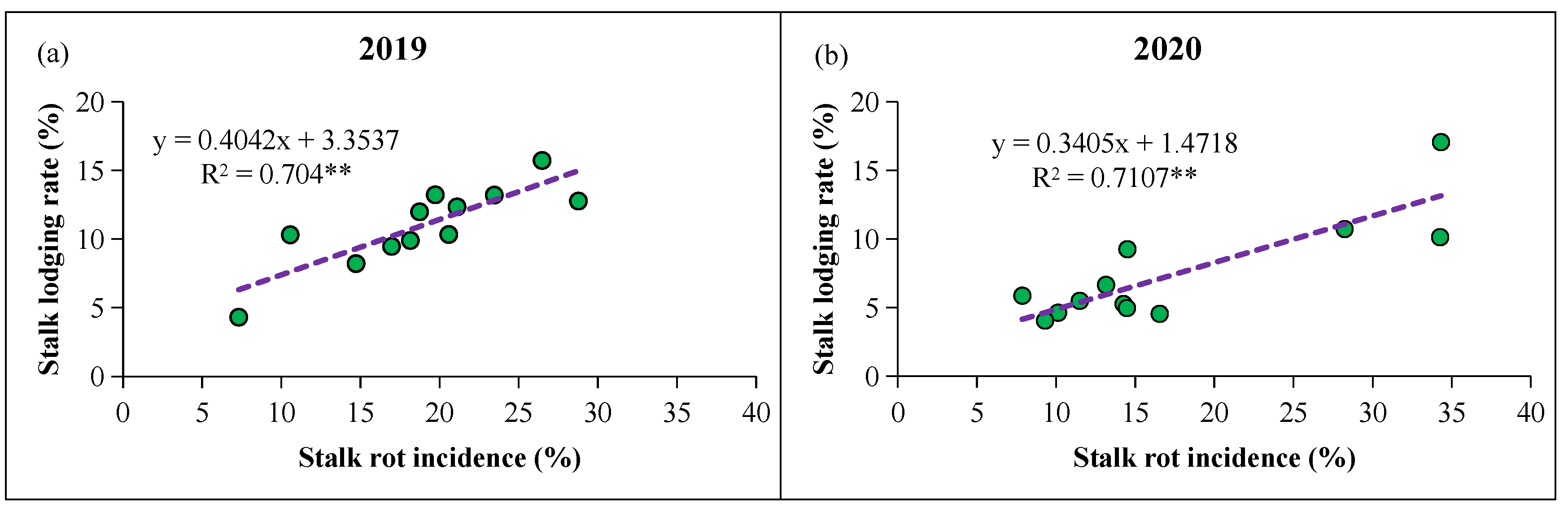
3.4. Stalk Lodging Rate and Stalk Rot Incidence and Relationship Between Them
3.5. Infection Process and Infection Index Under Different N Rates
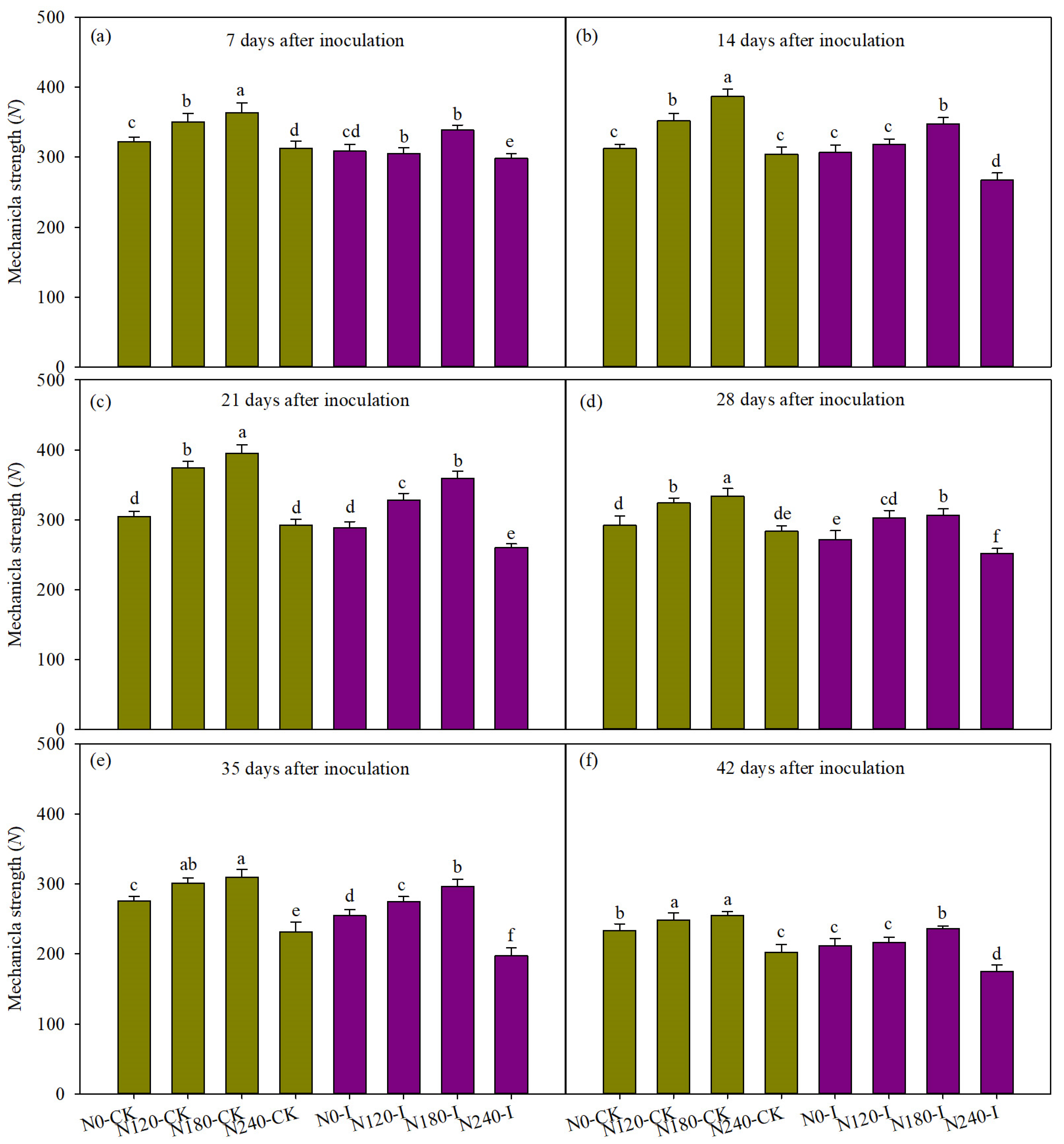
3.6. The Mechanical Strength of Basal Third Internodes Inoculated by Pathogen Under Different N Rates
3.7. Grain Yield Under Different N Application Levels
4. Discussion
4.1. Moderately Reducing N Optimized Basal Internodes Morphogenesis and Achieved Stable Grain Yield
4.2. Moderately Reducing N Enhanced Resistance to Pathogen Infection and Reduced the Lodging Rate
4.3. Moderately Reducing N Improved Resistance to Pathogen Expansion and Maintained Relatively High Stalk Mechanical Strength
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, X.P.; Cui, Z.L.; Vitousek, P.M.; Cassman, K.G.; Matson, P.A.; Bai, J.S.; Meng, Q.F.; Hou, P.; Yue, S.C.; Romheld, V.; et al. Integrated soil-crop system management for food security. Proc. Natl. Acad. Sci. USA 2011, 108, 6399–6404. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Clark, M. Food, Agriculture & the Environment: Can We Feed the World & Save the Earth? Daedalus 2015, 144, 8–23. [Google Scholar]
- Cui, Z.L.; Zhang, H.Y.; Chen, X.P.; Zhang, C.C.; Ma, W.Q.; Huang, C.D.; Zhang, W.F.; Mi, G.H.; Miao, Y.X.; Li, X.L.; et al. Pursuing sustainable productivity with millions of smallholder farmers. Nature 2018, 555, 363–366. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhang, S.; Lv, Y.J.; Ning, F.F.; Cao, Y.B.; Liao, S.H.; Wang, P.; Huang, S.B. Optimizing ear-plant height ratio to improve kernel number and lodging resistance in maize (Zea mays L.). Field Crops Res. 2022, 276, 108376. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, C.W.; Zhang, Z.; Wang, Z.X.; Ming, B.; Xue, J.; Yang, H.Y.; Xu, H.G.; Meng, L.; Cui, N.B.; et al. Evaluating how lodging affects maize yield estimation based on UAV observations. Front. Plant Sci. 2023, 13, 979103. [Google Scholar] [CrossRef]
- Li, S.Y.; Ma, W.; Peng, J.Y.; Chen, Z.M. Study on yield loss of summer maize due to lodging at the big flare stage and grain filling stage. Sci. Agric. Sin. 2015, 48, 3952–3964. [Google Scholar]
- Sun, Q.; Gu, X.H.; Chen, L.P.; Qu, X.Z.; Zhang, S.; Zhou, J.P.; Pan, Y.C. Hyperspectral estimation of maize (Zea mays L.) yield loss under lodging stress. Field Crops Res. 2023, 302, 109042. [Google Scholar] [CrossRef]
- Huang, Z.F.; Ming, B.; Hou, L.Y.; Xue, J.; Wang, K.R.; Xie, R.Z.; Hou, P.; Wang, Z.G.; Ma, D.L.; Gao, J.L.; et al. Improving maize quality from mechanical grain harvesting by matching maize varieties with accumulated temperature in northeast China. J. Sci. Food Agric. 2023, 103, 5061–5069. [Google Scholar] [CrossRef]
- Kong, F.L.; Liu, F.; Li, X.L.; Yin, P.J.; Lan, T.Q.; Feng, D.J.; Song, B.; Lei, E.; Li, Z.; Wang, X.L.; et al. Ecological factors regulate stalk lodging within dense planting maize. Field Crops Res. 2024, 317, 109529. [Google Scholar] [CrossRef]
- Ma, D.L.; Xie, R.Z.; Liu, X.; Niu, X.K.; Hou, P.; Wang, K.R.; Lu, Y.L.; Li, S.K. Lodging-related stalk characteristics of maize varieties in China since the 1950s. Crop Sci. 2014, 54, 2805–2814. [Google Scholar] [CrossRef]
- Zhang, P.; Gu, S.C.; Wang, Y.Y.; Yang, R.M.; Yan, Y.; Zhang, S.; Sheng, D.C.; Cui, T.; Huang, S.B.; Wang, P. Morphological and mechanical variables associated with lodging in maize (Zea mays L.). Field Crops Res. 2021, 269, 108178. [Google Scholar]
- Sekhon, R.S.; Joyner, C.N.; Ackerman, A.J.; McMahan, C.S.; Cook, D.D.; Robertson, D.J. Stalk bending strength is strongly associated with maize stalk lodging incidence across multiple environments. Field Crops Res. 2020, 249, 107737. [Google Scholar]
- Xue, J.; Gao, S.; Hou, L.Y.; Li, L.L.; Ming, B.; Xie, R.Z.; Wang, K.R.; Hou, P.; Li, S.K. Physiological influence of stalk rot on maize lodging after physiological maturity. Agronomy 2021, 11, 2271. [Google Scholar] [CrossRef]
- Robertson, D.J.; Brenton, Z.W.; Kresovich, S.; Cook, D.D. Maize lodging resistance: Stalk architecture is a stronger predictor of stalk bending strength than chemical composition. Biosyst. Eng. 2022, 219, 124–134. [Google Scholar]
- Huang, G.M.; Zhang, Y.; Gu, S.H.; Wen, W.L.; Lu, X.J.; Guo, X.Y. Identifying key factors influencing maize stalk lodging resistance through wind tunnel simulations with machine learning algorithms. Artif. Intell. Agric. 2025; in press, corrected proof. [Google Scholar]
- Ghorbani, M.; Amirahmadi, E. Biochar and soil contributions to crop lodging and yield performance—A meta-analysis. Plant Physiol. Biochem. 2024, 215, 109053. [Google Scholar]
- Khokhar, M.; Hooda, K.S.; Singh, V. Open access post flowering stalk rot complex of maize present status and future prospects. Maydica 2014, 59, 226–242. [Google Scholar]
- Wang, Q.; Xue, J.; Chen, J.G.; Fan, Y.H.; Zhang, G.Q.; Xie, R.Z.; Ming, B.; Hou, P.; Wang, K.R.; Li, S.K. Key indicators affecting maize stalk lodging resistance of different growth periods under different sowing dates. J. Integr. Agric. 2020, 19, 2419–2428. [Google Scholar] [CrossRef]
- Goszczyska, T.; Botha, W.J.; Venter, S.N.; Coutinho, T.A. Isolation and identification of the causal agent of brown stalk rot, a new disease of maize in South Agrica. Plant Dis. 2007, 91, 643–773. [Google Scholar]
- Yu, C.J.; Saravanakumar, K.; Xia, H.; Gao, J.X.; Fu, K.H.; Sun, J.N.; Dou, K.; Chen, J. Occurrence and virulence of Fusarium spp. Associated with stalk rot of maize in North-East China. Physiol. Mol. Plant Pathol. 2017, 98, 1–8. [Google Scholar]
- Wang, W.; Wang, B.; Sun, X.F.; Qi, X.B.; Zhao, C.H.; Chang, X.L.; Khaskheli, M.L.; Gong, G.S. Symptoms and pathogens diversity of corn Fusarium sheath rot in Sichuan Province, China. Sci. Rep. 2021, 11, 2835. [Google Scholar]
- Zhang, P.; Gu, S.C.; Wang, Y.Y.; Xu, C.C.; Zhao, Y.T.; Liu, X.L.; Wang, P.; Huang, S.B. The relationships between maize (Zea mays L.) lodging resistance and yield formation depend on dry matter allocation to ear and stem. Crop J. 2023, 11, 258–268. [Google Scholar]
- Zhai, J.; Zhang, Y.M.; Zhang, G.Q.; Tian, M.; Xie, R.Z.; Ming, B.; Hou, P.; Wang, K.R.; Xue, J.; Li, S.K. Effects of nitrogen fertilizer management on stalk lodging resistance traits in summer maize. Agriculture 2022, 12, 162. [Google Scholar] [CrossRef]
- Ahmad, I.; Batyrbek, M.; Ikram, K.; Ahmad, S.; Kamran, M.; Khan, R.S.; Hou, F.J.; Han, Q.F. Nitrogen management improves lodging resistance and production in maize (Zea mays L.) at a high plant density. J. Integr. Agric. 2023, 2, 417–433. [Google Scholar]
- Liu, F.; Zhou, F.; Wang, X.L.; Zhan, X.X.; Guo, Z.X.; Liu, Q.L.; Wei, G.; Lan, T.Q.; Feng, D.J.; Kong, F.L.; et al. Optimizing nitrogen management enhances stalk lodging resistance and grain yield in dense planting maize by improving canopy light distribution. Eur. J. Agric. 2023, 148, 126871. [Google Scholar]
- Sun, Q.; Liu, X.G.; Yang, J.; Wang, H.Q.; Fu, C.X.; Li, W.X. MicroRNA528 affects lodging resistance of maize by regulating lignin biosynthesis under N-luxury conditions. Mol. Plant 2018, 11, 806–814. [Google Scholar]
- Li, B.; Gao, F.; Ren, B.Z.; Dong, S.T.; Liu, P.; Zhao, B.; Zhang, J.W. Lignin metabolism regulates lodging resistance of maize hybrids under varying planting density. J. Integr. Agric. 2021, 20, 2077–2089. [Google Scholar]
- Cater, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhan, X.X.; Kong, F.L.; Lium, Q.L.; Lan, T.Q.; Liu, Y.Q.; Xu, J.Z.; Ou, Q.; Chen, L.; Kessel, G.; Kempenaar, C.; et al. Maize basal internode development significantly affects stalk lodging resistance. Field Crops Res. 2022, 286, 108611. [Google Scholar]
- Liu, C.; Zheng, S.; Gui, J.S.; Fu, C.J.; Yu, H.S.; Song, D.L.; Shen, J.H.; Qin, P.; Liu, X.M.; Han, B.; et al. Shortened basal intenodes encodes a gibberenllin 2-oxidase and contributes to lodging resistance in rice. Mol. Plant 2018, 11, 288–299. [Google Scholar]
- Kong, E.Y.; Liu, D.C.; Guo, X.L.; Yang, W.L.; Sun, J.Z.; Li, X.; Zhan, K.H.; Cui, D.Q.; Lin, J.X.; Zhang, A.M. Anatomical and chemical characteristics associated with lodging resistance in wheat. Crop J. 2013, 1, 43–49. [Google Scholar]
- Zhu, M.C.; Lin, C.H.; Jiang, Z.R.; Yan, F.Y.; Li, Z.Y.; Tang, X.N.; Yang, F.; Ding, Y.F.; Li, W.W.; Liu, Z.H.; et al. Uniconazole enhances lodging resistance by increasing structural carbohydrate and sclerenchyma cell wall thickness of japonica rice (Oryza sativa L.) under shading stress. Environ. Exp. Bot. 2023, 206, 105145. [Google Scholar]
- Li, W.J.; He, P.; Jin, J.Y. Effect of potassium on ultrastructure of maize stalk pith and young root and their relation to stalk rot resistance. Agric. Sci. China 2019, 9, 1467–1474. [Google Scholar]
- Kamran, M.; Ahmad, I.; Wang, H.Q.; Wu, X.R.; Xu, J.; Liu, T.N.; Ding, R.X.; Han, Q.F. Mepiquat choloride application increases lodging resistance of maize by enhancing stem physical strength and lignin biosynthesis. Field Crops Res. 2018, 224, 148–159. [Google Scholar]
- Ali, S.; Li, Z.Z.; Zhang, X.; Xi, Y.L.; Shaik, M.R.; Khan, M. How do novel plant growth regulators and cultivation models strategies affect mechanical strength, lodging resistance and maize productivity in semi-arid regions? Agric. Water Manag. 2024, 295, 108790. [Google Scholar]
- Wu, W.; Ma, B.L. Understanding the trade-off between lodging resistance and seed yield, and developing some non-destructive methods for predicting crop lodging risk in canola production. Field Crops Res. 2022, 288, 108691. [Google Scholar]
- Li, Z.; Gao, G.D.; Xu, L.S.; Wang, Z.K.; Wang, C.Y.; Yang, T.H.; Kuai, J.; Wang, B.; Xu, Z.H.; Zhao, J.; et al. Reducing nitrogen application at high planting density enhances secondary cell wall formation and decreases stem lodging in rapeseed. Eur. J. Agron. 2024, 156, 127162. [Google Scholar]
- Li, Z.J.; Liu, F.C.; Wu, W. Optimizing nitrogen management strategies to minimise lodging risk while sustaining high seed yield in rapeseed. Eur. J. Agron. 2023, 142, 126671. [Google Scholar]
- Wu, W.F.; Li, X.Y.; Chen, S.C.; Jin, B.J.; Wu, C.Y.; Li, G.; Sun, C.L.; Zhou, Y.G.; Lin, X.Y. Nitrogen fertilization modulates rice phyllosphere functional genes and pathogens through fungal communities. Sci. Total Environ. 2024, 929, 172622. [Google Scholar] [PubMed]
- Guo, C.; Wang, B.B.; Yang, Y.; Wang, C.M.; Zhou, T.W.; Li, M.Q.; Duan, X.C. Advances in studies of maize stalk rot. J. Plant Genet. Resour. 2019, 20, 1118–1128. [Google Scholar]
- Xue, J.; Gao, S.; Fan, Y.H.; Li, L.L.; Ming, B.; Wang, K.R.; Xie, R.Z.; Hou, P.; Li, S.K. Traits of plant morphology, stalk mechanical strength, and biomass accumulation in the selection of lodging-resistance maize cultivars. Eur. J. Agron. 2020, 117, 126073. [Google Scholar]
- Sun, J.J.; Wang, Y.Z.; Zhang, X.R.; Cheng, Z.Q.; Song, Y.H.; Li, H.M.; Wang, N.; Liu, S.; Cao, Z.J.; Li, H.X.; et al. Transcriptomic and metabolomic analyses reveal the role of phenylalanine metabolism in the maize response to stalk rot caused by Fusarium proliferatum. Int. J. Mol. Sci. 2024, 25, 1492. [Google Scholar] [CrossRef]
- Molina, A.; Sánchez-Vallet, A.; Jordá, L.; Carrasco-López, C.; Rodríguez-Herva, J.J.; López-Solanilla, E. Plant cell walls: Source of carbohydrate based signals in plant-pathogen interactions. Curr. Opin. Plant Biol. 2024, 82, 102630. [Google Scholar]
| Soil Organic Matter (g kg−1) | Total N (g kg−1) | Total K (g kg−1) | Available N (mg kg−1) | Available P (mg kg−1) | Available K (mg kg−1) |
|---|---|---|---|---|---|
| 16.1 | 1.27 | 2.2 | 75.7 | 32.1 | 107.0 |
| Years | Treatments | Number of Vascular Bundles in Sclerenchyma | Number of Vascular Bundles in Parenchyma | Total Number of Vascular Bundles |
|---|---|---|---|---|
| 2019 | N0 | 43.14 ± 0.6 c | 22.28 ± 1.4 a | 65.18 ± 0.5 b |
| N120 | 48.23 ± 1.2 b | 20.35 ± 0.7 ab | 68.08 ± 0.9 a | |
| N180 | 51.32 ± 2.3 b | 19.19 ± 0.6 b | 70.31 ± 1.2 a | |
| N240 | 55.04 ± 0.8 a | 16.27 ± 1.1 c | 71.42 ± 1.5 a | |
| 2020 | N0 | 42.09 ± 0.9 d | 26.52 ± 2.1 a | 68.51 ± 0.8 a |
| N120 | 46.37 ± 1.1 c | 22.37 ± 1.5 b | 68.37 ± 2.4 a | |
| N180 | 49.06 ± 1.5 b | 20.26 ± 1.4 b | 69.61 ± 1.6 a | |
| N240 | 53.34 ± 1.2 a | 15.17 ± 0.9 c | 68.57 ± 1.7 a | |
| ANOVA (p-value) | ||||
| Y (Year) | 0.651 ns | 0.066 ns | 0.199 ns | |
| N (N) | 0.036 * | 0.025 * | 0.494 ns | |
| Y × N | 0.142 ns | 0.089 ns | 0.760 ns | |
| Years | N Rates | Mechanical Strength (N) | |||||
|---|---|---|---|---|---|---|---|
| V12 | R1 | R3 | R4 | R5 | R6 | ||
| 2019 | N0 | 191.1 ± 6.9 c | 341.0 ± 10.0 ab | - | 375.5 ± 13.8 c | - | 269.0 ± 13.8 b |
| N120 | 231.5 ± 12.8 b | 337.0 ± 8.6 b | - | 389.9 ± 10.1 b | - | 287.0 ± 13.4 a | |
| N180 | 251.8 ± 11.3 a | 347.5 ± 6.9 a | - | 415.7 ± 11.1 a | - | 295.9 ± 9.4 a | |
| N240 | 226.4 ± 10.7 b | 321.2 ± 13.4 c | - | 364.4 ± 10.4 c | - | 259.9 ± 6.4 b | |
| 2020 | N0 | 193.1 ± 10.5 d | 279.0 ± 12.9 c | 321.7 ± 7.8 c | 304.7 ± 7.7 c | 291.6 ± 13.8 b | 233.4 ± 9.5 b |
| N120 | 262.6 ± 5.2 b | 328.8 ± 10.2 ab | 350.0 ± 12.9 b | 374.6 ± 9.2 b | 323.6 ± 7.4 a | 248.5 ± 10.0 a | |
| N180 | 290.6 ± 15.3 a | 336.6 ± 3.1 a | 363.1 ± 14.7 a | 395.0 ± 12.6 a | 333.8 ± 10.5 a | 254.9 ± 5.7 a | |
| N240 | 240.3 ± 3.8 c | 303.4 ± 8.9 b | 312.6 ± 10.2 c | 292.0 ± 8.5 c | 283.5 ± 7.6 b | 202.3 ± 11.1 c | |
| ANOVA (p-value) | |||||||
| Y (Year) | 0.082 ns | 0.144 ns | - | 0.065 ns | - | 0.044 * | |
| N (N) | 0.024 * | 0.013 * | 0.001 ** | 0.032 * | 0.003 ** | 0.049 * | |
| Y × N | 0.065 ns | 0.246 ns | - | 0.109 ns | - | 0.073 ns | |
| Years | N Rates | Harvested Ear Numbers (104 hm−2) | Kernel Number per Ear | 1000-Kernel Weight (g) | Grain Yield (kg ha−1) |
|---|---|---|---|---|---|
| 2019 | N0 | 7.14 ± 0.12 a | 412.94 ± 11.42 c | 296.93 ± 8.92 c | 8155.07 ± 94.27 c |
| N120 | 7.13 ± 0.08 a | 422.85 ± 9.34 b | 312.86 ± 9.29 b | 8572.72 ± 42.03 b | |
| N180 | 7.31 ± 0.01 a | 444.53 ± 9.12 a | 319.73 ± 4.26 a | 9193.28 ± 54.31 a | |
| N240 | 7.24 ± 0.02 a | 447.19 ± 5.83 a | 310.48 ± 5.87 b | 9058.65 ± 71.50 a | |
| 2020 | N0 | 7.21 ± 0.13 a | 443.95 ± 13.44 c | 270.91 ± 3.86 c | 8089.86 ± 92.57 c |
| N120 | 7.29 ± 0.09 a | 484.30 ± 6.23 b | 291.47 ± 7.31 b | 8419.88 ± 81.46 b | |
| N180 | 7.44 ± 0.04 a | 495.72 ± 4.87 a | 302.91 ± 5.35 a | 9040.59 ± 95.66 a | |
| N240 | 7.31 ± 0.08 a | 498.93 ± 5.40 a | 300.22 ± 6.86 a | 8943.01 ± 62.55 a | |
| ANOVA (p-value) | |||||
| Y (Year) | 0.047 * | 0.015 * | 0.052 ns | 0.431 ns | |
| N (N) | 0.055 | 0.044 * | 0.034 * | 0.042 * | |
| Y × N | 0.073 ns | 0.125 ns | 0.082 ns | 0.236 ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Lv, L.; Cui, Y.; Shi, Y.; Zhang, J. Moderate Nitrogen Management Enhancing Maize Lodging Resistance by Reducing Pathogen Infection and Expansion of Stalk Rot. Agronomy 2025, 15, 787. https://doi.org/10.3390/agronomy15040787
Zheng M, Lv L, Cui Y, Shi Y, Zhang J. Moderate Nitrogen Management Enhancing Maize Lodging Resistance by Reducing Pathogen Infection and Expansion of Stalk Rot. Agronomy. 2025; 15(4):787. https://doi.org/10.3390/agronomy15040787
Chicago/Turabian StyleZheng, Mengjing, Lihua Lv, Yongzeng Cui, Yueling Shi, and Jingting Zhang. 2025. "Moderate Nitrogen Management Enhancing Maize Lodging Resistance by Reducing Pathogen Infection and Expansion of Stalk Rot" Agronomy 15, no. 4: 787. https://doi.org/10.3390/agronomy15040787
APA StyleZheng, M., Lv, L., Cui, Y., Shi, Y., & Zhang, J. (2025). Moderate Nitrogen Management Enhancing Maize Lodging Resistance by Reducing Pathogen Infection and Expansion of Stalk Rot. Agronomy, 15(4), 787. https://doi.org/10.3390/agronomy15040787





