Genetic Diversity and Distinctiveness of Common Beans (Phaseolus vulgaris L.) Between Landraces and Formal Cultivars Supporting Ex Situ Conservation Policy: The Borlotti Case Study in Northern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Phenotyping of Plant Material
2.3. DNA Extraction and Genotyping with ddRAD Sequencing
2.4. SNP Calling Procedure
2.5. SNP Calling Procedure and Clustering Analysis
3. Results
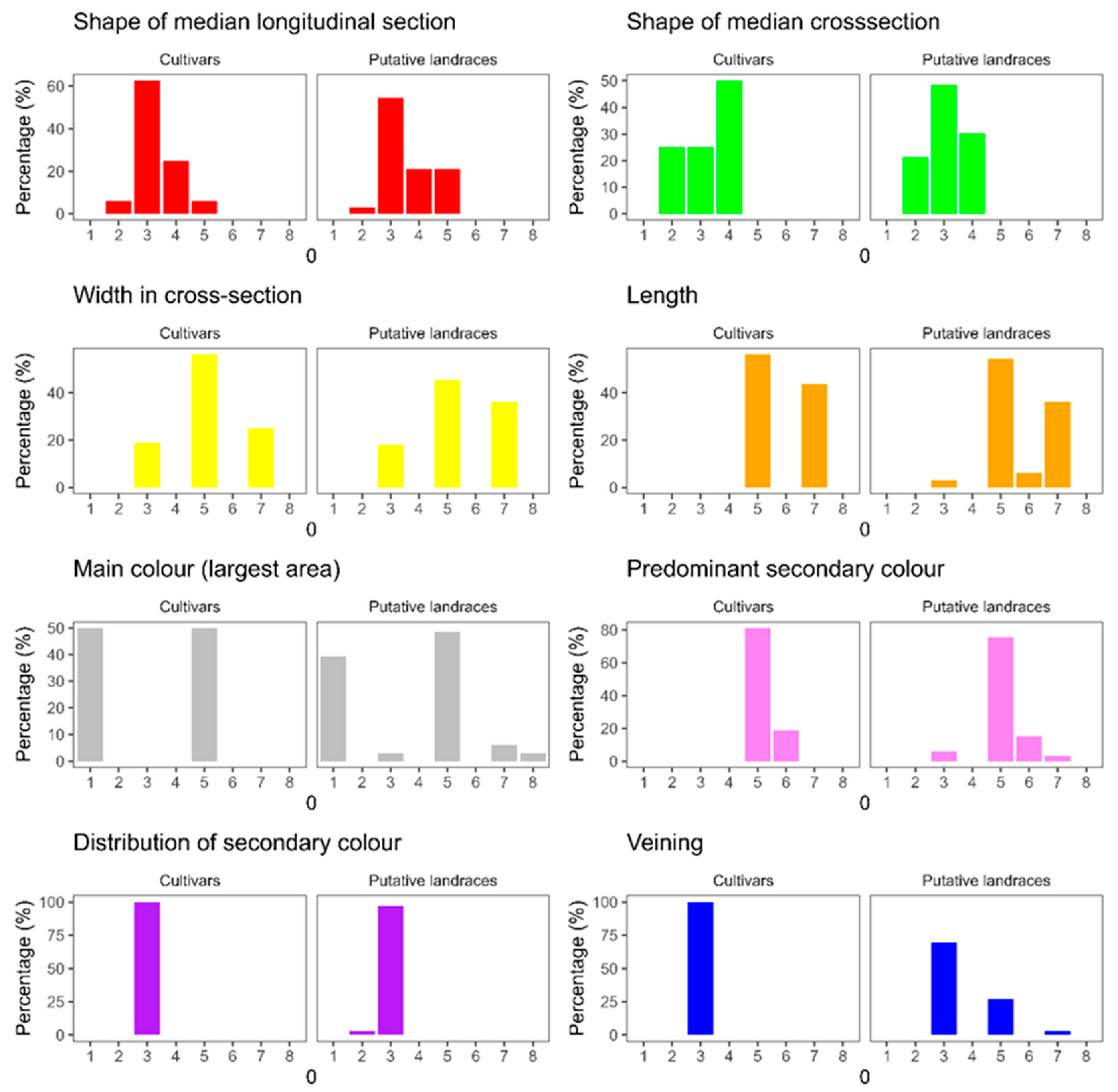
3.1. Morphological Characterization
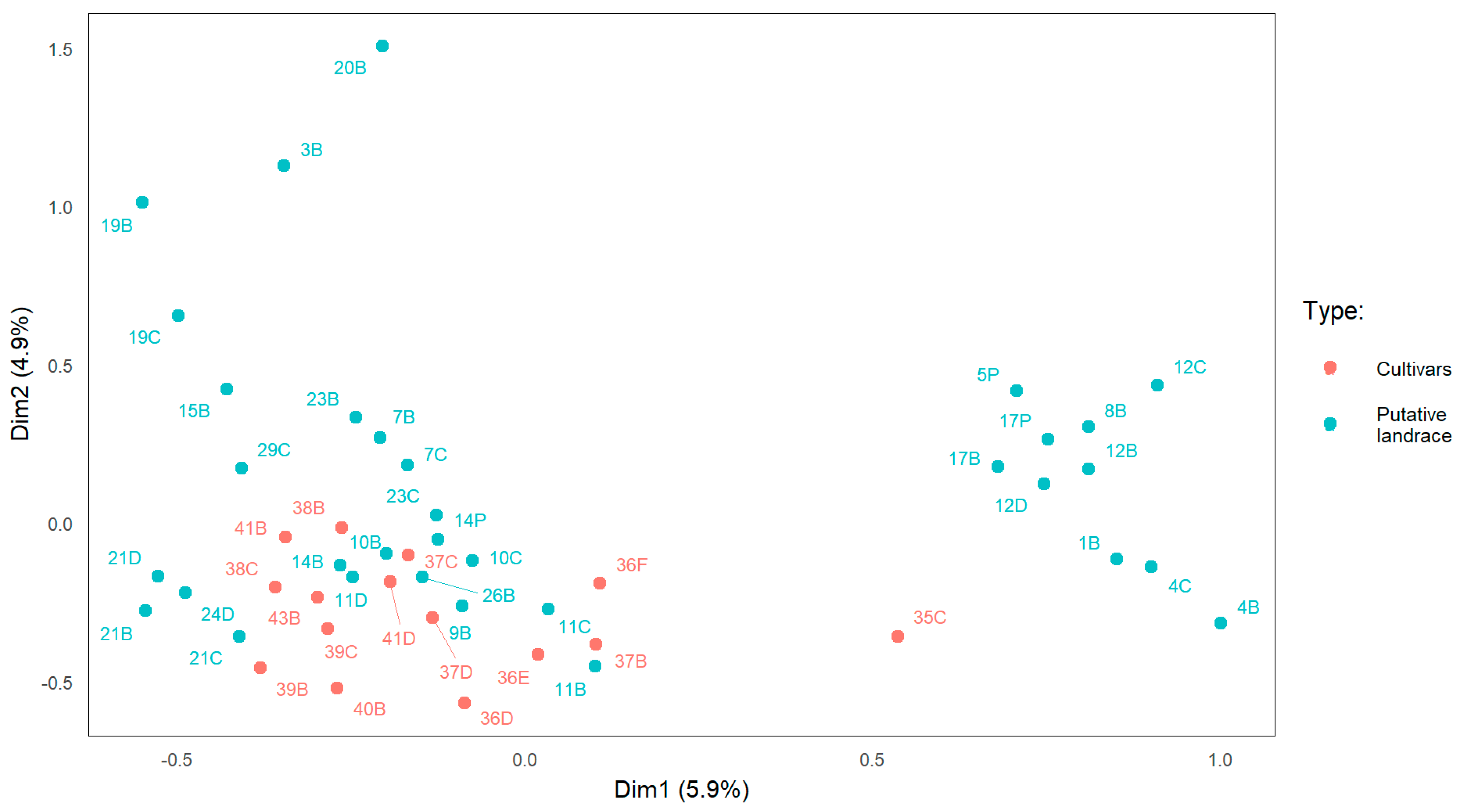
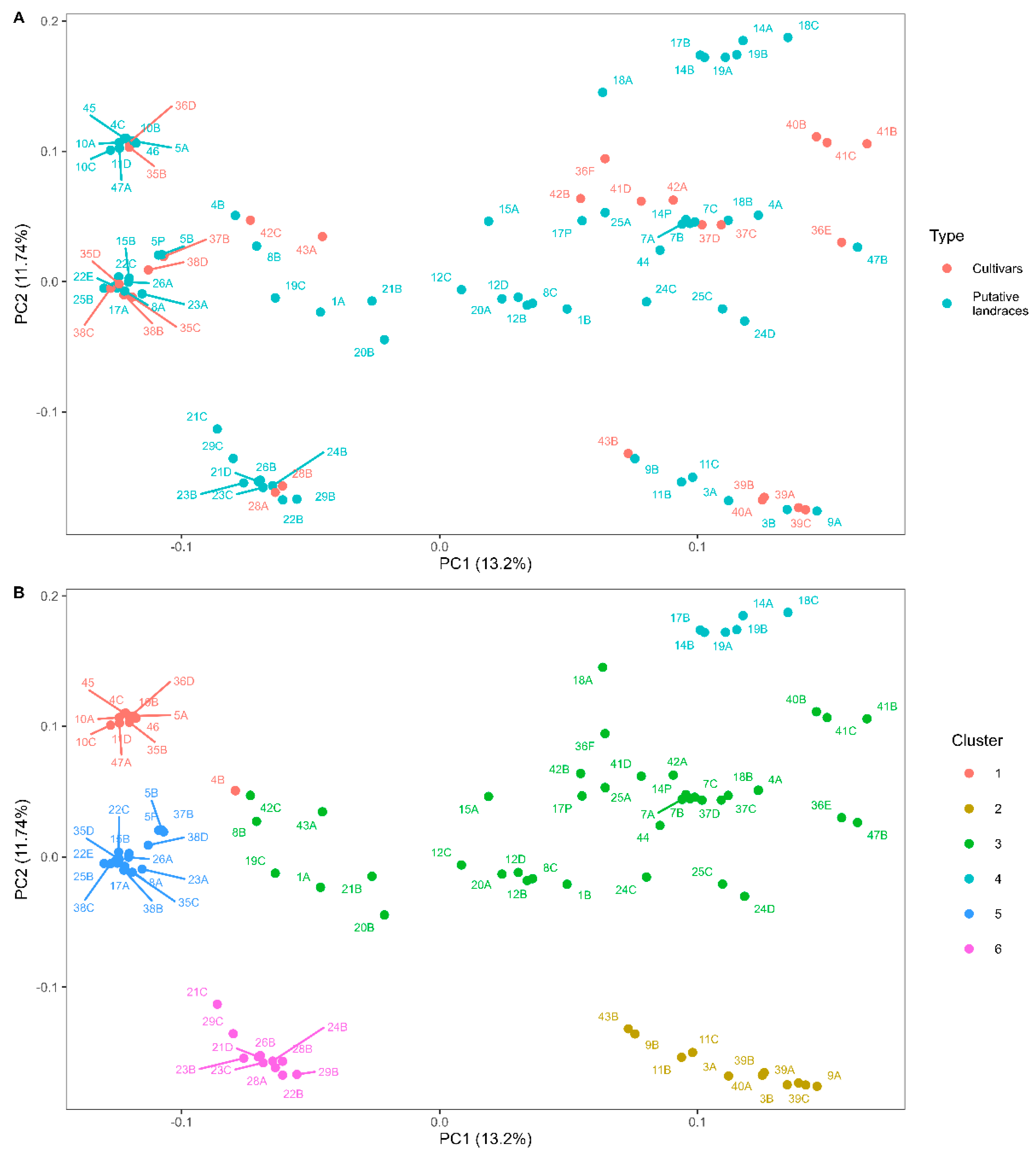
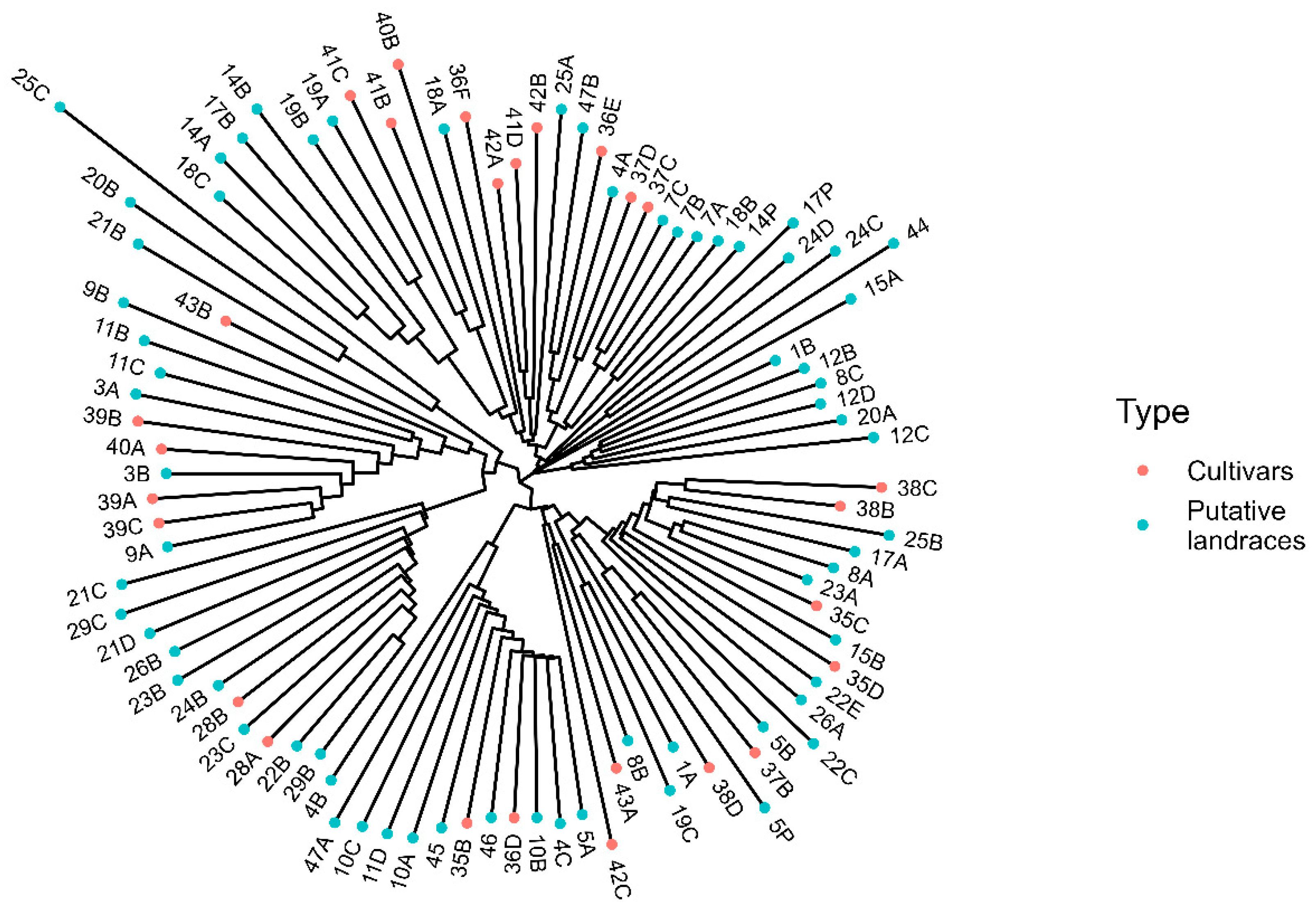
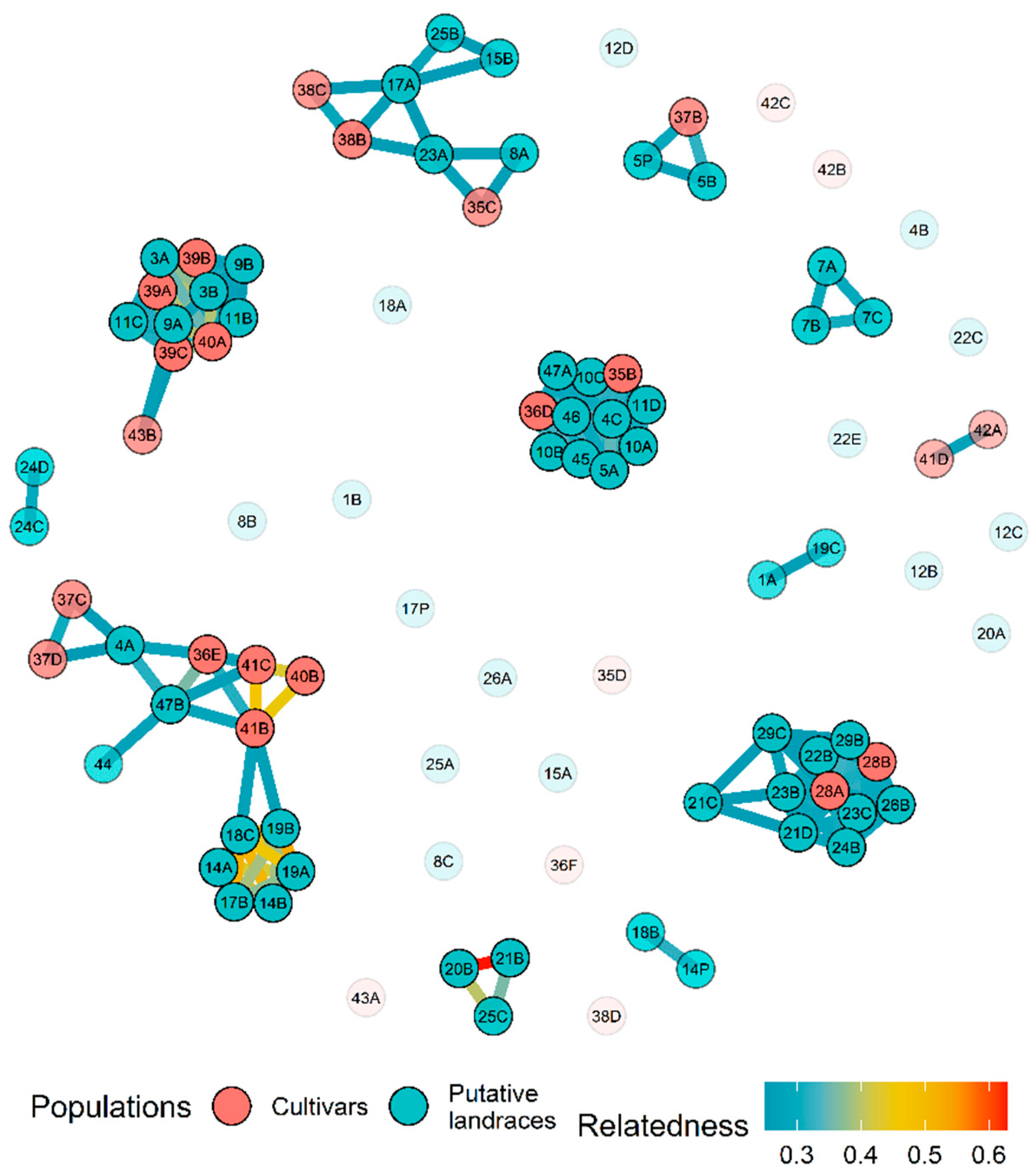
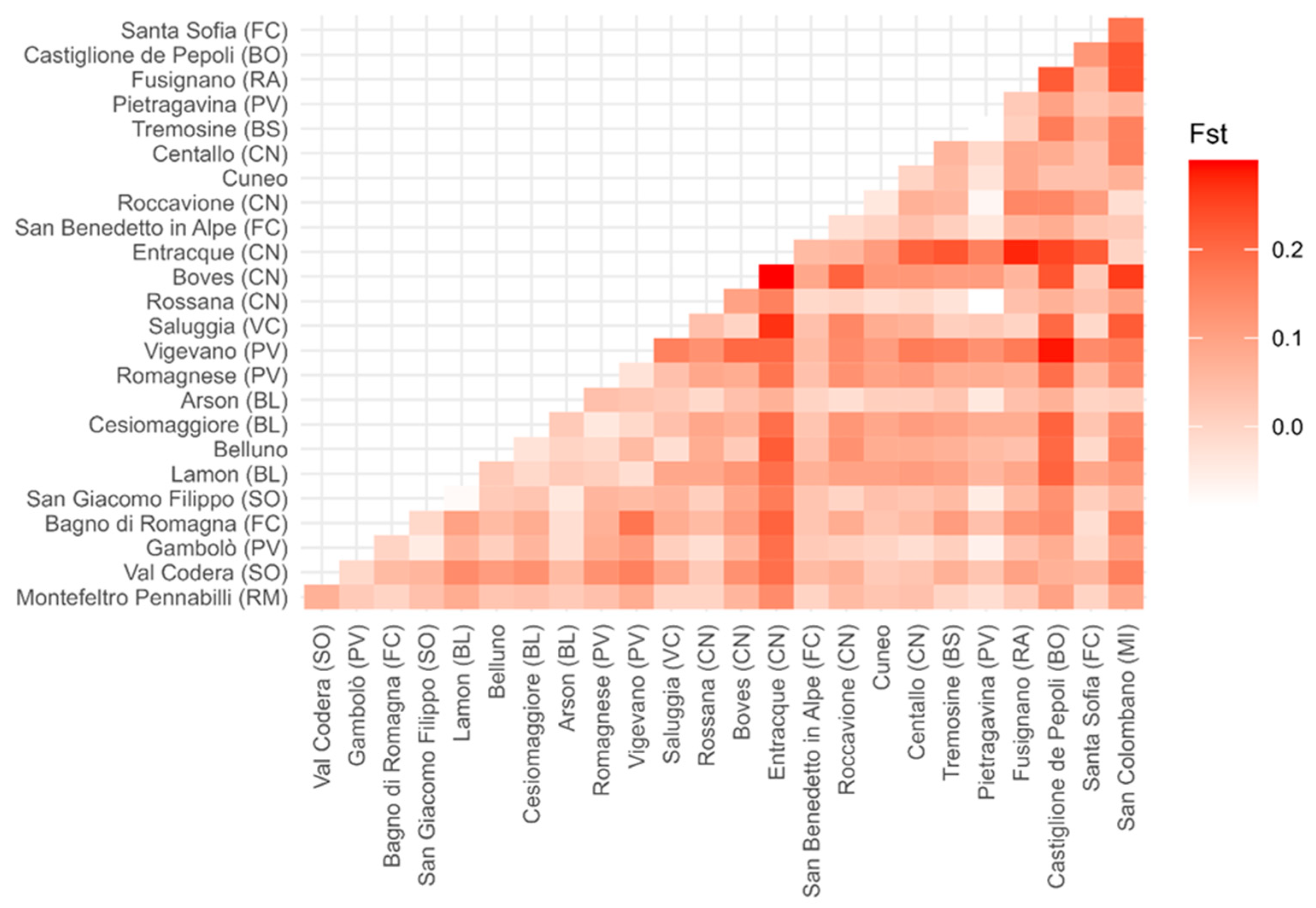
3.2. Genetic Diversity Analysis
4. Discussion
4.1. Morphological Characterization
4.2. Genetic Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, S.P.; Gutierrez, J.A.; Molina, A.; Urea, C.; Gepts, P. Genetic diversity in cultivated common bean: II. Marker-based analysis of morphological and agronomic traits. Crop Sci. 1991, 31, 23–29. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Loi, L. Italian common bean landraces: History, genetic diversity and seed quality. Diversity 2010, 2, 837–862. [Google Scholar] [CrossRef]
- Bellucci, E.; Benazzo, A.; Xu, C.; Bitocchi, E.; Rodriguez, M.; Alseekh, S.; Di Vittori, V.; Gioia, T.; Neumann, K.; Cortinovis, G.; et al. Selection and adaptive introgression guided the complex evolutionary history of the European common bean. Nat. Commun. 2023, 14, 1908. [Google Scholar] [CrossRef] [PubMed]
- Bitocchi, E.; Rau, D.; Bellucci, E.; Rodriguez, M.; Murgia, M.L.; Gioia, T.; Santo, D.; Nanni, L.; Attene, G.; Papa, R. Beans (Phaseolus ssp.) as a model for understanding crop evolution. Front. Plant Sci. 2017, 8, 722. [Google Scholar] [CrossRef]
- Perale, M. Milacis Cultus Aperire Paramus, 1st ed.; AiCS: Lamon, Italy, 2001. [Google Scholar]
- Myers, J.R.; Formiga, A.K.; Janick, J. Iconography of beans and related legumes following the columbian exchange. Front. Plant Sci. 2022, 13, 851029. [Google Scholar] [CrossRef]
- Sanson, S. Dalla conservazione alla valorizzazione: La biodiversità coltivata come strategia di sviluppo sostenibile del territorio. In Montagne di Cibo. Studi e Ricerche in Terra Bellunese; Provincia di Belluno: Belluno, Italy, 2013. [Google Scholar]
- Turchi, A. Orticoltura Pratica; Edizioni Agricole: Bologna, Italy, 1962. [Google Scholar]
- Turchi, A.; Turchi, F. Orticoltura Pratica; Edizioni Agricole: Bologna, Italy, 1997. [Google Scholar]
- Ranalli, P.; Parisi, B. Fagiolo e Fagiolino. Coltivazione, Scelta Delle Cultivar e Post-Raccolta; Edagricole Calderini: Bologna, Italy, 2018. [Google Scholar]
- Parisi, B.; Campion, B. Il Fagiolo; Prom: Montanaso Lombardo, LO, Italy, 2010. [Google Scholar]
- Comes, O. Del fagiolo comune: Storia, filogenesi, qualità sospettata tossicità e sistemazione delle sue razze ovunque coltivate. Atti Ist. Incoragg Napoli 1910, 61, 75–145. [Google Scholar]
- Šajgalík, M.; Ondreičková, K.; Hauptvogel, P.; Mihálik, D.; Glasa, M.; Kraic, J. Higher effectiveness of new common bean (Phaseolus vulgaris L.) germplasm acquisition by collecting expeditions associated with molecular analyses. Sustainability 2019, 11, 5270. [Google Scholar] [CrossRef]
- Corrado, G. Food history and gastronomic traditions of beans in Italy. J. Ethn. Foods. 2022, 9, 6. [Google Scholar] [CrossRef]
- De Luca, D.; Cennamo, P.; Del Guacchio, E.; Di Novella, R.; Caputo, P. Conservation and genetic characterisation of common bean landraces from Cilento region (southern Italy): High differentiation in spite of low genetic diversity. Genetica 2018, 146, 29–44. [Google Scholar] [CrossRef]
- Landoni, M.; Bertoncini, A.; Ghidoli, M.; Rossi, G.; Cassani, E.; Locatelli, S.; Balconi, C.; Pilu, R. PGRFA management of outcrossing plants propagated by seed: From on-farm to ex situ conservation and some italian maize case studies. Agronomy 2024, 14, 1030. [Google Scholar] [CrossRef]
- Camacho-Villa, T.C.; Maxted, N.; Scholten, M.; Ford-Lloyd, B. Defining and identifying crop landraces. Plant Genet. Resour. 2005, 3, 373–384. [Google Scholar] [CrossRef]
- Pringle, J.S. The concept of the cultivar. Arboric. Urban For. 1975, 1, 30–34. [Google Scholar]
- Canella, M.; ·Ardenghi, N.M.G.; Müller, J.V.; Rossi, G.; Guzzon, F. An updated checklist of plant agrobiodiversity of northern Italy. Genet. Resour. Crop Evol. 2022, 69, 2159–2178. [Google Scholar] [CrossRef]
- Spataro, G.; Negri, V. The European seed legislation on conservation varieties: Focus, implementation, present and future impact on landrace on farm conservation. Genet. Resour. Crop Evol. 2013, 60, 2421–2430. [Google Scholar] [CrossRef]
- FAO. 1983. Available online: https://www.fao.org/documents/card/en?details=10d11795-0962-5b45-8b15-463e8679a39c (accessed on 4 February 2024).
- Lupton, F.G.H. History of wheat breeding. In Wheat Breeding; Springer: Dordrecht, The Netherlands, 1987. [Google Scholar]
- Klaedtke, S.M.; Caproni, L.; Klauck, J.; de la Grandville, P.; Dutartre, M.; Stassart, P.M.; Chable, V.; Negri, V.; Raggi, L. Short-term local adaptation of historical common bean (Phaseolus vulgaris L.) varieties and implications for in situ management of bean diversity. Int. J. Mol. Sci. 2017, 18, 493. [Google Scholar] [CrossRef] [PubMed]
- Catarcione, G.; Paolacci, A.R.; Alicandri, E.; Gramiccia, E.; Taviani, P.; Rea, R.; Costanza, M.T.; De Lorenzis, G.; Puccio, G.; Mercati, F.; et al. Genetic diversity and population structure of common bean (Phaseolus vulgaris L.) landraces in the Lazio region of Italy. Plants 2023, 12, 744. [Google Scholar] [CrossRef]
- Fiore, M.C.; Raimondo, F.M.; Mercati, F.; Digangi, I.; Sunseri, F.; Scialabba, A. Preserving biodiversity in marginal rural areas: Assessment of morphological and genetic variability of a sicilian common bean germplasm collection. Plants 2020, 9, 989. [Google Scholar] [CrossRef]
- Schröder, S.; Mamidi, S.; Lee, R.; McKain, M.; McClean, P.; Osorno, J. Optimization of genotyping by sequencing (GBS) data in common bean (Phaseolus vulgaris L.). Mol. Breed. 2016, 36, 1. [Google Scholar] [CrossRef]
- Lioi, L.; Zuluaga, D.L.; Pavan, S.; Sonnante, G. Genotyping-by-Sequencing reveals molecular genetic diversity in italian common bean landraces. Diversity 2019, 11, 154. [Google Scholar] [CrossRef]
- Pootakham, W. Genotyping by Sequencing (GBS) for genome-wide SNP identification in plants. Methods Mol. Biol. 2023, 2638, 1–8. [Google Scholar] [CrossRef]
- Radhika Ramya, A.; Rajesh, V.; Jyothi, P.; Swathi, G. A review on in situ and ex situ conservation strategies for crop germplasm. Int. J. Appl. Biol. Pharm. 2014, 5, 267–273. [Google Scholar]
- Dulloo, M.E.; Hunter, D.; Borelli, T. Ex situ and in situ conservation of agricultural biodiversity: Major advances and research needs. Not. Bot. Horti Agrobot. Cluj-Napoca 2010, 38, 123–135. [Google Scholar]
- Engels, J.M.M.; Ebert, A.W. A critical review of the current global ex situ conservation system for plant agrobiodiversity. I. History of the development of the global system in the context of the political/legal framework and its major conservation components. Plants 2021, 10, 1557. [Google Scholar] [CrossRef]
- Zaccardelli, M.; Pentangelo, A.; Tripodi, P. Characterization of bean (Phaseolus vulgaris L.) ecotype “Fagiolo occhio nero di Oliveto Citra” using agronomic, biochemical and molecular approaches. Pak. J. Biol. Sci. 2013, 16, 901–910. [Google Scholar] [CrossRef][Green Version]
- Nicoletto, C.; Zanin, G.; Sambo, P.; Dalla Costa, L. Quality assessment of typical common bean genotypes cultivated in temperate climate conditions and different growth locations. Sci. Hortic. 2019, 256, 108599. [Google Scholar] [CrossRef]
- Sica, P.; Scariolo, F.; Galvao, A.; Battaggia, D.; Nicoletto, C.; Maucieri, C.; Palumbo, F.; Franklin, D.; Cabrera, M.; Borin, M.; et al. Molecular hallmarks, agronomic performances and seed nutraceutical properties to exploit neglected genetic resources of common beans grown by organic farming in two contrasting environments. Front. Plant Sci. 2021, 12, 674985. [Google Scholar] [CrossRef]
- Eastwood, R.J.; Tambam, B.B.; Aboagye, L.M.; Akparov, Z.I.; Aladele, S.E.; Allen, R.; Amri, A.; Anglin, N.L.; Araya, R.; Arrieta-Espinoza, G.; et al. Adapting agriculture to climate change: A synopsis of coordinated national crop wild relative seed collecting programs across five continents. Plants 2022, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Grigorieva, E.; Livenets, A.; Stelmakh, E. Adaptation of Agriculture to Climate Change: A Scoping Review. Climate 2023, 11, 202. [Google Scholar] [CrossRef]
- Protocol for Tests on Distinctness, Uniformity and Stability Phaseolus vulgaris L. French Bean, CPVO-TP/012/4. Available online: https://cpvo.europa.eu/sites/default/files/documents/phaseolus_vulgaris.pdf (accessed on 4 January 2024).
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef]
- FastQC v0.11.5. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 4 February 2024).
- Phytozome Website. Available online: https://phytozome-next.jgi.doe.gov/info/Aofficinalis_V1_1 (accessed on 4 February 2024).
- GATK Genome Analysis Toolkit. Available online: https://gatk.broadinstitute.org/hc/en-us/articles/360037499012?id=3225 (accessed on 4 February 2024).
- GATK Best-Practices-Workflows for Germplasm Variant Calling. Available online: https://gatk.broadinstitute.org/hc/en-us/sections/360007226651-Best-Practices-Workflows (accessed on 4 February 2024).
- R Core Team 2020. Available online: https://www.eea.europa.eu/mobile/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 25 April 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Yu, G.; Smith, D.K.; Zhu, H.; Guan, Y.; Tsan-Yuk Lam, T. GGTREE: An R package for visualization and annotation ofphylogenetic trees with their covariates and otherassociated data. Methods Ecol. Evol. 2017, 8, 28–36. [Google Scholar]
- Mijangos, J.L.; Gruber, B.; Berry, O.; Pacioni, C.; Georges, A. dartR v2: An accessible genetic analysis platform for conservation, ecology and agriculture. Methods Ecol. Evol. 2022, 13, 2150–2158. [Google Scholar] [CrossRef]
- Lioi, L.; Piergiovanni, A.R.; Soressi, G.P.; Nigro, C.; Tamietti, G.; Turina, M.; Campion, B. Caratterizzazione, selezione, risanamento e valutazione di cultivar locali di fagiolo comune (Phaseolus vulgaris L.). Italus Hortus 2007, 14, 31–40. [Google Scholar]
- Sinkovič, L.; Pipan, B.; Sinkovič, E.; Meglič, V. Morphological Seed Characterization of Common (Phaseolus vulgaris L.) and Runner (Phaseolus coccineus L.) Bean Germplasm: A Slovenian Gene Bank Example. Biomed. Res. Int. 2019, 16, 6376948. [Google Scholar] [CrossRef]
- Delfini, J.; Moda-Cirino, V.; dos Santos Neto, J.; Ruas, P.M.; Sant’Ana, G.C.; Gepts, P.; Gonçalves, L.S.A. Population structure, genetic diversity and genomic selection signatures among a Brazilian common bean germplasm. Sci. Rep. 2021, 11, 2964. [Google Scholar] [CrossRef]
- Angioi, S.A.; Rau, D.; Attene, G.; Nanni, L.; Bellucci, E.; Logozzo, G.; Negri, V.; Spagnoletti Zeuli, P.L.; Papa, R. Beans in Europe: Origin and structure of the European landraces of Phaseolus vulgaris L. Theor. Appl. Genet. 2010, 121, 829–843. [Google Scholar] [CrossRef]
- Vidak, M.; Šatović, Z.; Liber, Z.; Grdiša, M.; Gunjača, J.; Kilian, A.; Carović-Stanko, K. Assessment of the Origin and Diversity of Croatian Common Bean Germplasm Using Phaseolin Type, SSR and SNP Markers and Morphological Traits. Plants 2021, 10, 665. [Google Scholar] [CrossRef]
- Lee, Z.; Kim, S.; Choi, S.J.; Joung, E.; Kwon, M.; Park, H.J.; Shim, J.S. Regulation of Flowering Time by Environmental Factors in Plants. Plants 2023, 12, 3680. [Google Scholar] [CrossRef]
- Yan, F.H.; Zhang, L.P.; Cheng, F.; Yu, D.M.; Hu, J.Y. Accession-specific flowering time variation in response to nitrate fluctuation in Arabidopsis thaliana. Plant Divers. 2020, 43, 78–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Viteri, D.M.; Linares, A.M. Inheritance of coloured stripes on the flower standard and their association with seed coat colour in common bean. Can. J. Plant Sci. 2019, 99, 961–965. [Google Scholar] [CrossRef]
- Sadohara, R.; Long, Y.; Izquierdo, P.; Urrea, C.A.; Morris, D.; Cichy, K. Seed coat colour genetics and genotype × environment effects in yellow beans via machine-learning and genome-wide association. Plant Genome 2022, 15, e20173. [Google Scholar] [CrossRef]
- Blanca, J.; Pons, C.; Montero-Pau, J.; Sanchez-Matarredona, D.; Ziarsolo, P.; Fontanet, L.; Fisher, J.; Plazas, M.; Casals, J.; Rambla, J.L.; et al. European traditional tomatoes galore: A result of farmers’ selection of a few diversity-rich loci. J. Exp. Bot. 2022, 73, 3431–3445. [Google Scholar] [CrossRef] [PubMed]
- Stagnati, L.; Soffritti, G.; Desiderio, F.; Lanubile, A.; Zambianchi, S.; Marocco, A.; Rossi, G.; Busconi, M. The Rediscovery of Traditional Maize Agrobiodiversity: A Study Case from Northern Italy. Sustainability 2022, 14, 12110. [Google Scholar] [CrossRef]
- Santamaria, P.; Ronchi, L. Varietà da conservazione in Italia: Lo stato dell’arte per le specie orticole. Italus Hortus 2016, 23, 29–44. [Google Scholar]
- Giupponi, L.; Pedrali, D.; Leoni, V.; Rodari, A.; Giorgi, A. The Analysis of Italian Plant Agrobiodiversity Databases Reveals That Hilly and Sub-Mountain Areas Are Hotspots of Herbaceous Landraces. Diversity 2021, 13, 70. [Google Scholar] [CrossRef]
- Casañas, F.; Simó, J.; Casals, J.; Prohens, J. Toward an Evolved Concept of Landrace. Front. Plant Sci. 2017, 8, 145. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Sun, X.; Caiji, Z.; Yang, J.; Cui, D.; Cao, G.; Ma, X.; Han, B.; Xue, D.; et al. Influence of ethnic traditional cultures on genetic diversity of rice landraces under on-farm conservation in southwest China. J. Ethnobiol. Ethnomed. 2016, 12, 51. [Google Scholar] [CrossRef]
- Stagnati, L.; Martino, M.; Soffritti, G.; Lanubile, A.; Ravasio, A.; Marocco, A.; Rossi, G.; Busconi, M. Microsatellite and morphological characterization of three Rostrato di Val Chiavenna (Sondrio, Italy) maize (Zea mays L.) accessions. Genet. Resour. Crop Evol. 2021, 68, 3025–3038. [Google Scholar] [CrossRef]
- McLean-Rodríguez, F.D.; Costich, D.E.; Camacho-Villa, T.C.; Pè, M.E.; Dell’Acqua, M. Genetic diversity and selection signatures in maize landraces compared across 50 years of in situ and ex situ conservation. Heredity 2021, 126, 913–928. [Google Scholar] [CrossRef]
- Santalla, M.; De Ron, A.M.; De La Fuente, M. Integration of genome and phenotypic scanning gives evidence of genetic structure in Mesoamerican common bean (Phaseolus vulgaris L.) landraces from the southwest of Europe. Theor. Appl. Genet. 2010, 120, 1635–1651. [Google Scholar]
- Ariani, A.; Berny Mier y Teran, J.C.; Gepts, P. Genome-wide identification of SNPs and copy number variation in common bean (Phaseolus vulgaris L.) using genotyping-by-sequencing (GBS). Mol. Breed. 2016, 36, 87. [Google Scholar] [CrossRef]
- Campa, A.; Murube, E.; Ferreira, J.J. Genetic diversity, population structure, and linkage disequilibrium in a Spanish common bean diversity panel revealed through genotyping-by-sequencing. Genes 2018, 9, E518. [Google Scholar] [CrossRef] [PubMed]
- Pavan, S.; Curci, P.L.; Zuluaga, D.L.; Blanco, E.; Sonnante, G. Genotyping-by-sequencing highlights patterns of genetic structure and domestication in artichoke and cardoon. PLoS ONE 2018, 13, e0205988. [Google Scholar] [CrossRef]
- Alipour, H.; Bihamta, M.R.; Mohammadi, V.; Peyghambari, S.A.; Bai, G.; Zhang, G. Genotyping-by-Sequencing (GBS) Revealed Molecular Genetic Diversity of Iranian Wheat Landraces and Cultivars. Front Plant Sci. 2017, 8, 1293. [Google Scholar] [CrossRef] [PubMed]
- Peringottillam, M.; Kunhiraman Vasumathy, S.; Selvakumar, H.K.K.; Alagu, M. Genetic diversity and population structure of rice (Oryza sativa L.) landraces from Kerala, India analysed through genotyping-by-sequencing. Mol. Genet. Genomics 2022, 297, 169–182. [Google Scholar] [CrossRef]
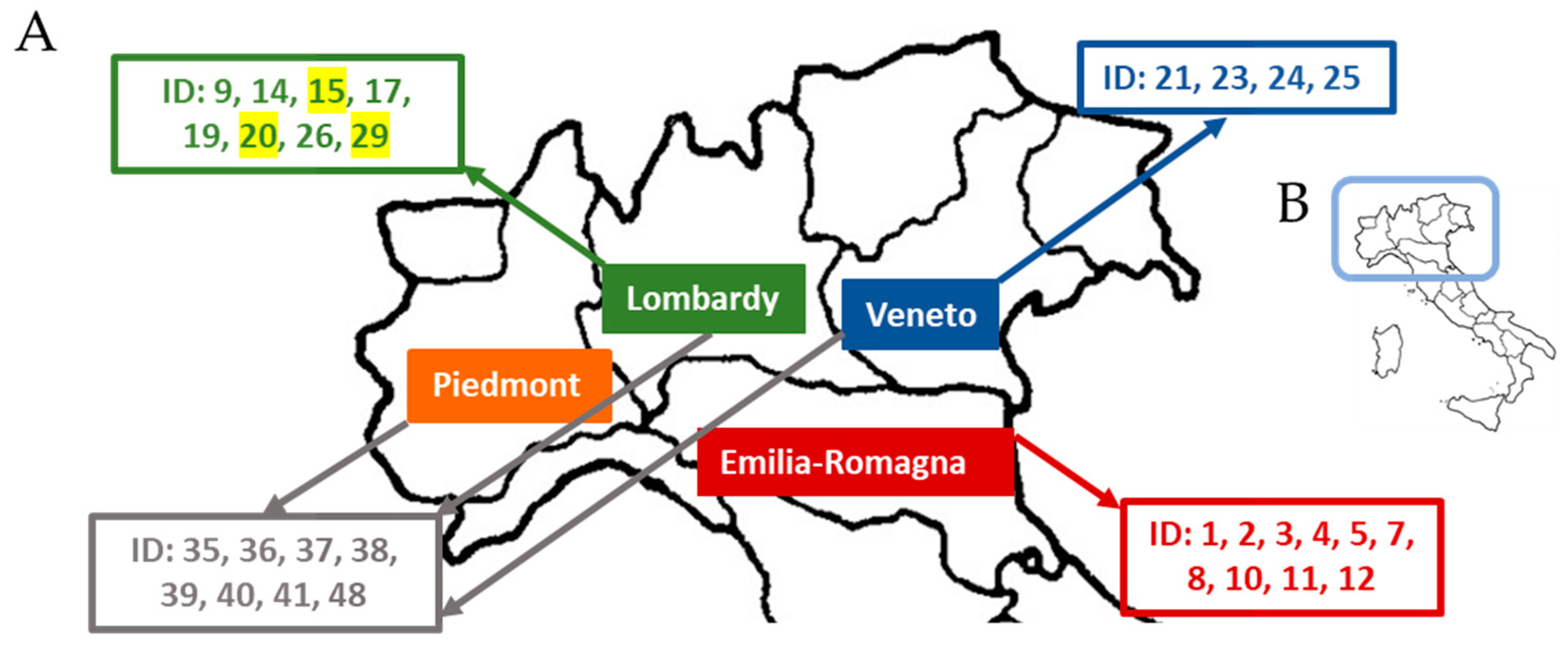
| ID | Bank Code | Accession Name | Borlotti | Region | Type | GBS | Phenotyping |
|---|---|---|---|---|---|---|---|
| 1 | 2241 | Borlotto | Yes | Emilia–Romagna | putative landrace | 2 | 1 |
| 3 | 2285 | Borlotto | Yes | Emilia–Romagna | putative landrace | 2 | 1 |
| 4 | 2249 | Borlotto | Yes | Emilia–Romagna | putative landrace | 3 | 2 |
| 5 | 3074 | Borlotto | Yes | Emilia–Romagna | putative landrace | 3 | 1 |
| 7 | 1804 | Brunetto | Yes* | Emilia–Romagna | putative landrace | 3 | 2 |
| 8 | 1358 | Borlotto | Yes | Emilia–Romagna | putative landrace | 3 | 1 |
| 9 | 2663 | Borlotto | Yes | Lombardy | putative landrace | 2 | 1 |
| 10 | 2885 | Borlotto | Yes | Emilia–Romagna | putative landrace | 3 | 2 |
| 11 | 2891 | Borlotto | Yes | Emilia–Romagna | putative landrace | 3 | 2 |
| 12 | 2884 | Borlotto | Yes | Emilia–Romagna | putative landrace | 3 | 3 |
| 14 | 1828 | Borlotto | Yes | Lombardy | putative landrace | 3 | 2 |
| 15 | 1817 | Dorato di Codera | No | Lombardy | putative landrace | 2 | 1 |
| 17 | 2401 | Borlotto | Yes | Lombardy | putative landrace | 3 | 2 |
| 18 | 1824 | Borlotto | Yes | Lombardy | putative landrace | 3 | N/A |
| 19 | 2468 | Borlotto della Valchiavenna | Yes | Lombardy | putative landrace | 3 | 2 |
| 20 | 1913 | Fagiolo di San Giacomo Filippo | No | Lombardy | putative landrace | 2 | 1 |
| 21 | 1785 | Fagiolo di Lamon | Yes* | Veneto | putative landrace | 3 | 3 |
| 22 | 1789 | Fagiolo di Lamon della vallata bellunese | Yes* | Veneto | putative landrace | 3 | N/A |
| 23 | 1781 | Fagiolo “bala rossa” | Yes* | Veneto | putative landrace | 3 | 2 |
| 24 | 1922 | Spagnolet di Lamon | Yes* | Veneto | putative landrace | 3 | 1 |
| 25 | 2171 | Spagnolet di Lamon | Yes* | Veneto | putative landrace | 3 | N/A |
| 26 | 2825 | Borlotto | Yes | Lombardy | putative landrace | 2 | 1 |
| 28 | 3080 | Borlotto | Yes | Lombardy | cultivar | 2 | N/A |
| 29 | 3078 | Anellino d’Oltrepò | No | Lombardy | putative landrace | 2 | 1 |
| 35 | 3988 | Fagiolo di Saluggia | Yes* | Veneto | cultivar | 3 | 1 |
| 36 | 3989 | Rossano | Yes* | Piedmont | cultivar | 3 | 3 |
| 37 | VLA042 | Sanguigno | Yes* | N/A | cultivar | 3 | 3 |
| 38 | VLA046 | Regina rossa di Boves | Yes* | Piedmont | cultivar | 3 | 2 |
| 39 | VLA047 | Borlotto di Entraque | Yes | Piedmont | cultivar | 3 | 2 |
| 40 | VLA050 | Regina precoce di Roccavione | Yes* | Piedmont | cultivar | 2 | 1 |
| 41 | VLA054 | Borlotto gigante | Yes | Piedmont | cultivar | 3 | 2 |
| 42 | VLA056 | Regina rossa di Centallo | Yes* | Piedmont | cultivar | 3 | N/A |
| 43 | N/A | Billò | Yes* | Piedmont | cultivar | 2 | 1 |
| 44 | 3458 | Fagiolo del Belesi (rosso) | Yes* | Lombardy | putative landrace | 1 | N/A |
| 45 | 3535 | Borlotto della Nina Laini | Yes | Lombardy | putative landrace | 1 | N/A |
| 46 | 3457 | Borlotto della Nina Laini | Yes | Lombardy | putative landrace | 1 | N/A |
| 47 | 2410 | Borlotto nano di Pietragavina | Yes | Lombardy | putative landrace | 2 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losa, A.; Sala, T.; Toppino, L.; Fricano, A.; Rossi, G.; Gipli, V.; Landoni, M. Genetic Diversity and Distinctiveness of Common Beans (Phaseolus vulgaris L.) Between Landraces and Formal Cultivars Supporting Ex Situ Conservation Policy: The Borlotti Case Study in Northern Italy. Agronomy 2025, 15, 786. https://doi.org/10.3390/agronomy15040786
Losa A, Sala T, Toppino L, Fricano A, Rossi G, Gipli V, Landoni M. Genetic Diversity and Distinctiveness of Common Beans (Phaseolus vulgaris L.) Between Landraces and Formal Cultivars Supporting Ex Situ Conservation Policy: The Borlotti Case Study in Northern Italy. Agronomy. 2025; 15(4):786. https://doi.org/10.3390/agronomy15040786
Chicago/Turabian StyleLosa, Alessia, Tea Sala, Laura Toppino, Agostino Fricano, Graziano Rossi, Valerio Gipli, and Michela Landoni. 2025. "Genetic Diversity and Distinctiveness of Common Beans (Phaseolus vulgaris L.) Between Landraces and Formal Cultivars Supporting Ex Situ Conservation Policy: The Borlotti Case Study in Northern Italy" Agronomy 15, no. 4: 786. https://doi.org/10.3390/agronomy15040786
APA StyleLosa, A., Sala, T., Toppino, L., Fricano, A., Rossi, G., Gipli, V., & Landoni, M. (2025). Genetic Diversity and Distinctiveness of Common Beans (Phaseolus vulgaris L.) Between Landraces and Formal Cultivars Supporting Ex Situ Conservation Policy: The Borlotti Case Study in Northern Italy. Agronomy, 15(4), 786. https://doi.org/10.3390/agronomy15040786







