Abstract
Herbivore-induced plant volatiles (HIPVs) serve as powerful defense mechanisms that help plants mitigate pest-induced stress. Geraniol is a HIPV released by tea leaves in response to damage inflicted by tea green leafhoppers. In order to investigate whether the release of geraniol is a defensive mechanism of tea plants against infestation by tea green leafhoppers, our study explored the effects of geraniol on tea green leafhoppers, including the selectivity of tea green leafhoppers’ response to geraniol, survival and reproductive parameters, as well as alterations in endophytes and the transcriptome. The findings indicated that while geraniol did not exhibit strong repellent or lethal effects on tea green leafhoppers, it significantly reduced the egg-laying and hatching rates. Through 16S rRNA microbial sequencing, we found that geraniol treatment significantly altered the composition of endophytic microbial communities in tea green leafhoppers, potentially affecting their metabolic functions. Transcriptome analysis further showed that genes associated with energy metabolism, such as glutamate dehydrogenase, were significantly upregulated in response to geraniol, suggesting that tea green leafhoppers may enhance energy metabolism to counteract geraniol-induced stress. Additionally, the downregulation of antimicrobial peptide-related signaling pathways suggests that geraniol may weaken the immune capacity of tea green leafhoppers, potentially reducing their resistance to pathogens. These findings indicate that the strategic application of geraniol could be a promising approach to controlling tea green leafhopper populations. This study enhances our understanding of the insect-resistant mechanisms of HIPVs and provides new insights into environmentally sustainable pest management strategies for tea plantations.
1. Introduction
Tea, a nonalcoholic beverage, is second only to water in terms of global consumption. Due to the significant economic value of tea trees, they are widely cultivated, including in Hainan Province, a tropical region of China. Their cultivation is affected by various pests, including tea geometrid Ectropis obliqua hypulina (Wehrli, 1943), tea tussock moth Euproctis pseudoconspersa (Strand, 1923) (Lepidoptera), tea green leafhopper Empoasca onukii (Matsuda, 1952), tea aphid Toxoptera aurantii (Boyer de Fonscolombe, 1841) (Hemiptera), and yellow tea thrips Scirtothrips dorsalis (Hood, 1919) (Thysanoptera) [1,2,3,4,5]. Among these, the tea green leafhopper is the most widespread pest in tea plantations and has been reported in tea-producing regions of China, Japan, India, and Vietnam [6,7]. Both nymphs and adults feed on young stems and leaves, and female individuals lay eggs on tender stems, hindering branch growth. In severe infestations, leaf edges and tips turn reddish-brown and may wither [8]. Tea production losses due to this pest typically range from 20% to 30%, but in extreme cases, yield reductions can reach 50% or more [9]. The prevention and control of tea green leafhoppers is of great significance to the tea industry.
Currently, chemical control is the primary method for managing tea green leafhoppers. However, excessive insecticide use has led to significant resistance in these pests [10], necessitating increased pesticide application, which exacerbates the problem of pest resistance. Additionally, pesticide residues in tea frequently exceed safety limits [11,12], lowering tea quality and posing risks to human health. Developing environmentally friendly pest management strategies to reduce pesticide reliance is therefore crucial. A promising approach involves investigating the regulation of insect physiological behavior through herbivore-induced plant volatiles (HIPVs), which are gaining attention as potential tools for sustainable pest control [13,14,15].
When herbivorous insects feed on plants, the plants release volatile compounds as part of their induced defense response. These volatiles can directly repel pests or attract their natural enemies. Some HIPVs also induce defense mechanisms in neighboring plants, priming them against potential pest attacks [16,17,18]. In addition, it has been reported that certain HIPVs are capable of disrupting the homeostasis of the insect gut microbiota [19,20]. HIPVs with insect-resistant properties primarily include fatty acids, terpenes, and heterocyclic compounds. Upon herbivore attack, glycosidic precursors of fatty acid-derived volatiles, known as green leaf volatiles (GLVs), rapidly hydrolyze, releasing free GLVs as the first line of defense. Among these, 3-hexenol and cis-3-hexenyl acetate are the most studied insect-resistant fatty acid volatiles. In corn, these compounds serve as signaling molecules, triggering increased terpene volatile production in nearby plants to enhance insect resistance [21]. Indole is a heterocyclic HIPV with insect-repellent properties. In rice, its release is proportional to the severity of Spodoptera frugiperda (J.E.Smith, 1797) infestation, and indole-treated rice exhibits significantly enhanced resistance to S. frugiperda larvae [22]. Additionally, indole emitted by pest-damaged plants can elevate jasmonic acid levels in adjacent plants, further strengthening their insect resistance [18]. Terpenoids are the most extensively studied insect-resistant HIPVs. For instance, volatile compounds released by Vaccinium species following Lymantria dispar (Linnaeus, 1758) infestation reduce gypsy moth feeding by 70% [23]. In corn, Diabrotica virgifera (LeConte, 1868) feeding induces the release of (E)-β-caryophyllene, which attracts natural enemies of the pest [24]. Another terpene HIPV, (3E)-4,8-dimethylnon-1,3,7-triene, disrupts host plant recognition by Spodoptera littoralis (Boisduval, 1833) [25]. One study also showed that DMNT could also significantly disrupt the microbiota in the insect midgut and directly protect plants from the damage of the diamondback moth (Plutella xylostella, Linnaeus, 1767) [19].
When tea trees are fed on by herbivorous insects, they release a large number of volatile compounds as part of their defense response [26]. For example, volatiles induced by cicada damage attract the natural enemy Stethynium empoascae (Subba Rao, 1966) [27,28]. Our previous study demonstrated that feeding by tea green leafhoppers significantly increases geraniol emission from tea leaves [29]. Geraniol is a common essential oil in plants with antimicrobial activity [30]. It exhibits insecticidal properties. For instance, a concentration of 0.026 mg/cm2 is lethal to Rhyzopertha dominica (Fabricius, 1792) and Lasioderma serricorne (Fabricius, 1792) [31]. In addition, our earlier study found that geraniol significantly inhibits the growth of Acinetobacter johnsonii (Bouvet & Grimont, 1986), a gut microorganism by tea green leafhoppers [29]. Based on the above research, it was postulated that geraniol induced by the infestation of tea green leafhoppers may actively participate in the defense of tea plants against pests. This might occur through its impact on the endophytic communities within the pests. Concurrently, it was hypothesized that the expression of certain resistance-related genes in the tea green leafhopper was likewise impacted by the stress exerted by geraniol [19]. This study investigated the effects of geraniol on the physiological behavior of tea green leafhoppers and found that it significantly reduced egg-laying in females and markedly suppressed egg hatching, potentially acting as a natural population regulator of this pest. Furthermore, we examined changes in the endophyte community and transcriptomic responses of tea green leafhoppers to geraniol exposure, providing deeper insights into its molecular mechanisms. This research will provide a better understanding of the biological and genetic effects of geraniol on the tea green leafhopper, which may contribute to the development of environmentally friendly methods for controlling this pest in tea plantations.
2. Materials and Methods
2.1. Insects
Adult tea green leafhoppers were collected from a tea garden in Jianfengling (108°51′ E, 18°44′ N) National Forest Park, Ledong City, Hainan Province. Field-collected insects were captured using a net, and then they were placed in an insect-rearing cage (25 × 25 × 50 cm) filled with tender tea tree leaves and brought back to the laboratory. Subsequently, they were reared indoors on tender branches of Camellia sinensis var. Hainan dayezhong in March 2024. The laboratory conditions were maintained at a temperature of 23.5 °C ± 2 °C, relative humidity of 60% ± 5%, and a photoperiod of 14 h light:10 h dark (L:D).
2.2. Selectivity of Tea Green Leafhoppers to Geraniol
The selectivity assay was conducted in Y-tubes according to Mu et al. [32]. This experiment was performed in June 2024. Geraniol (purity: ≥98%, Product S24471, Shanghai yuanye Bio-Technology, Shanghai, China) was prepared in a series of concentrations (10%, 1%, 0.1%, 0.01%, and 0.001%) using mineral oil as a solvent. Prior to testing, the gas flow rate in both arms of the Y-tube was adjusted to 100 mL/min. Air was blown through the system for 10 min to ensure that the Y-tube was saturated with the volatile odor source. Adult tea green leafhoppers were introduced into the base port of the Y-tube by using a finger-shaped tube. Before releasing each insect, air was blown for 30 s. A response was recorded when a leafhopper entered one of the Y-tube arms and moved at least 5 cm upwind. If no response was observed within 10 min, the trial was excluded from the analysis. Ten adult leafhoppers were tested for each odor source, with each insect used for only one trial. After testing every 5 leafhoppers, the inner and outer walls of the Y-tube were wiped with a cotton ball soaked in absolute ethanol and then air dried. The positions of the odor source and control bottles on the two arms of the Y-tube were then swapped to eliminate any potential bias caused by geometric asymmetry. The bioassay was conducted between 9:00 a.m. and 4:00 p.m., when tea green leafhoppers exhibit peak activity. Environmental conditions were maintained at a temperature of 25 ± 3 °C, relative humidity of 70% ± 5%, and an illumination level of 32,000–36,000 lx.
2.3. Determination of the Effect of Geraniol Treatment on the Survival Rate of Tea Green Leafhoppers
A 10% (w/v) geraniol (a mixture containing 10% geraniol in water) was placed in a 15 mL centrifuge tube with a small hole in the cap [20], and the tube was positioned inside a covered 500 mL plastic cup. Water was used as the control. In total, 20 adult tea green leafhoppers were introduced into each plastic cup. After 5 days, the survival rate of adult leafhoppers was recorded. This experiment was conducted in July 2024, with three replicates.
2.4. Determination of the Total Number of Eggs Laid by Tea Green Leafhoppers
According to the method used in previous studies, a 10% (w/v) geraniol solution (a mixture containing 10% geraniol in water) was placed in a 15 mL centrifuge tube with a small hole in the cap [32], and the tube was positioned inside a lidded 10 × 25-cm plastic jar. Water was used as the control. The total number of eggs laid by tea green leafhoppers was determined following a previously described protocol [33]. Three clean, egg-free tender tea branches cultivated in floral foam were placed in the jar. Three male and three female tea green leafhoppers, which had emerged three days prior and had not yet reached sexual maturity, were introduced into the jar for rearing. Every 2–3 days, the tender branches were removed and examined under a blue light flashlight to count the number of eggs. Fresh, egg-free tea branches were then introduced into the jar. This process continued until no additional eggs were detected or all adult leafhoppers had died. The total number of eggs laid was summed, and the average number of eggs per female was calculated. The experiment was conducted in quadruplicate in July 2024.
2.5. Determination of the Egg Hatching Rate of Tea Green Leafhoppers
A 10% (w/v) geraniol solution (a mixture containing 10% geraniol in water) was placed in a 15 mL centrifuge tube with a small hole in the cap, and the tube was positioned inside an acrylic plastic insect-rearing cage. Water was used as the control. Tea green leafhoppers were allowed to lay eggs on tender, egg-free tea branches in the cage for 24 h. The branches containing eggs were then transferred to plastic jars containing 10% geraniol. The number of successfully hatched eggs was recorded daily until the branches had completely withered. The egg hatching rate was calculated. The experiment was conducted in triplicate in August 2024.
2.6. Determination of Endophytes of Tea Green Leafhoppers
A 10% (w/v) geraniol solution (a mixture containing 10% geraniol in water) was placed in a 15 mL centrifuge tube with a small hole in the cap, and the tube was positioned inside a 10 × 25 cm plastic jar. Water was used as the control. Five newly emerged adult tea green leafhoppers were introduced into the jar and fed with tender tea branches for 7 days. After the feeding period, the surviving leafhoppers were collected. To minimize the interference of surface bacteria as much as possible, in accordance with the requirements of Biomarker Technologies Company, they were surface-disinfected with 75% alcohol for 2 min and then preserved at −80°C. The experiment was conducted in six replicates, with 10 tea green leafhopper samples (two jars) collected per replicate. The collected samples were sent to Biomarker Technologies (Beijing, China) for 16S rRNA micro-biome sequencing in October 2024. For 16S rRNA microbiome data, Metastats and random forest analyses were performed on the Biomarker Microbial Diversity Analysis Platform. Metastats analysis used R packet Metastats 2009 and random forest analysis used R packet Random Forest 4.6-10 [34,35]. The PCA analysis was carried out using the Quantitative Insights into Microbial Ecology (QIIME) method at the Operational Taxonomic Unit (OTU) level.
2.7. Determination of the Transcriptome of Tea Green Leafhoppers
A 10% (w/v) geraniol solution (a mixture containing 10% geraniol in water) was placed in a 15 mL centrifuge tube with a small hole in the cap, and the tube was positioned inside a covered 500 mL plastic cup. Water was used as the control. In total, 20 newly emerged adult tea green leafhoppers were introduced into the cup and fed with tea tree leaves for 12 h. After the feeding period, the leafhoppers were collected, preserved at −80 °C, and sent to Biomarker Technologies (Beijing, China) for transcriptome sequencing in August 2024. The experiment was conducted in quadruplicate. Transcriptome data were screened and analyzed (Gene Ontology enrichment analysis and Kyoto Encyclopedia of Genes and Genomes enrichment analysis) on the transcriptome analysis platform of Biomarker Technologies (Beijing, China) [36,37,38,39,40]. The PCA analysis was conducted using the ggplot2 (two-dimensional PCA) method.
3. Results
3.1. Geraniol Treatment Reduced the Oviposition and Hatching Rates of Tea Green Leafhoppers Without Affecting Their Selectivity and Survival
Most pest-induced volatiles have a strong repellent effect on insects. To assess whether tea green leafhoppers exhibit selectivity toward geraniol, we tested their response to concentrations of 10%, 1%, 0.1%, 0.01%, and 0.001% geraniol. The results indicated that tea green leafhoppers did not exhibit a preference for any of these concentrations (Figure 1a). To further investigate the physiological effects of geraniol on tea green leafhoppers, we analyzed its impact on adult survival, egg-laying behavior, and egg hatching rates. Our findings showed that the exposure to 10% geraniol for 5 days did not significantly affect the survival rate of adult leafhoppers (Figure 1b). However, geraniol treatment significantly reduced the number of eggs laid by female adults (Figure 1c). In the control group, the mean total number of eggs laid per female adult reached 27, whereas in the geraniol-treated group, this number dropped to 9. More notably, control group, egg hatching peaked on days 7–8 after oviposition, whereas in the geraniol-treated group, hatching did not increase beyond 5% (Figure 1e).
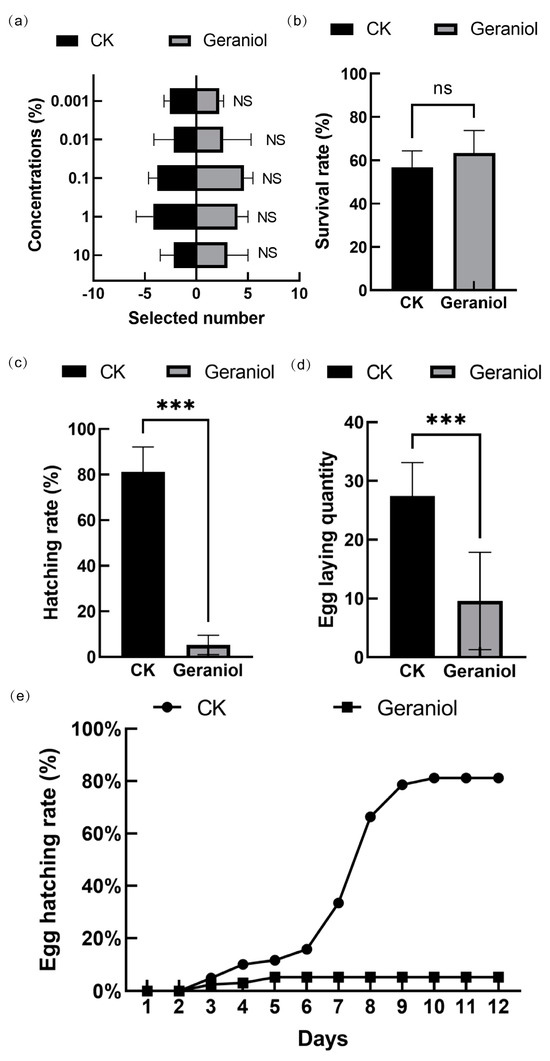
Figure 1.
(a) Selectivity of movement of tea green leafhopper to different concentrations of geraniol; (b) Effect of 10% geraniol on the survival rate of tea green leafhopper; (c) Effects of 10% geraniol on the total number of eggs laid by female adults; (d) Effect of 10% geraniol on the egg hatching rate; (e) Effect of 10% geraniol on the cumulative hatching proportion. CK, water treatment. The t-test method was used for the significance test. The error bars represent the standard deviation. *** p < 0.001; ns p > 0.05.
3.2. Effect of Geraniol Treatment on the Endophyte Community of Tea Green Leafhoppers
3.2.1. Geraniol Treatment Altered the Endophyte Community of Tea Green Leafhoppers
At the phylum level, the dominant bacterial groups in both the geraniol-treated and control tea green leafhoppers were Proteobacteria, Firmicutes, and Actinobacteria. Compared with the control group, the relative abundance of Proteobacteria and Actinobacteria decreased in the geraniol-treated group, while Firmicutes abundance increased (Figure 2a). At the genus level, excluding unclassified genera, the most abundant genera in the control group were Pacuibacter, Pantoea, and Acinetobacter, whereas in the geraniol-treated group, the dominant genera were Pacuibacter, Exiguobacterium, and Lactobacillus. Compared with the control group, the relative abundances of Pacuibacter, Pantoea, Acinetobacter, Nocardioides, Saccharopolyspora, and Wolbachia decreased in the geraniol-treated group, whereas those of Exiguobacterium, Lactobacillus, and Bacteroides increased (Figure 2b).
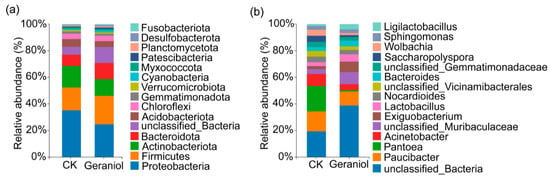
Figure 2.
Effects of 10% geraniol on the endophytes of tea green leafhopper at the phylum and genus levels. (a) Differences at the phylum level; (b) Differences at the genus level. CK, water treatment.
To assess differences in endophyte abundance between the treatment and control groups, species abundance data were analyzed using the Metastats method. At the phylum level, 17 phyla were identified, with significant differences in the abundance observed in 16. At the order level, 74 orders were detected, of which 71 exhibited significant differences in the abundance between the two groups. Notably, the relative abundance of Pseudomonadales was significantly lower in the geraniol-treated group (Figure 3a). At the genus level, 376 genera were identified across both groups, with significant differences found in 365. Among these, the relative abundances of Acinetobacter and Staphylococcus were significantly reduced in the geraniol-treated group (Figure 3b,c).
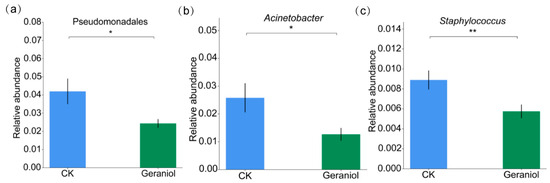
Figure 3.
Metastats analysis of the effect of 10% geraniol on the endophytes of tea green leafhopper. (a) Differences in relative abundance of Pseudomonadales; (b) Differences in relative abundance of Acinetobacter; (c) Differences in relative abundance of Staphylococcus. CK, water treatment. The t-test method was used for the significance test. The error bars represent the standard deviation. * p < 0.05, ** p < 0.01.
3.2.2. Geraniol Treatment Altered the Alpha and Beta Diversity of Endophytes in Tea Green Leafhoppers
Alpha diversity was analyzed to assess differences in bacterial populations between the treatment and control groups. The Chao and Ace indices, which are indicative of species richness, were significantly higher in the geraniol-treated group than in the control group, indicating a notable increase in endophyte species richness following geraniol treatment (Figure 4a,b) [41]. The Simpson index, which accounts for both species rich-ness and evenness, was significantly higher in the control group than in the geraniol-treated group. This suggests that geraniol treatment led to a reduction in endophyte diversity in tea green leafhoppers (Figure 4c) [41]. To further evaluate changes in beta diversity, principal component analysis (PCA) was performed. The results showed a clear separation between the geraniol-treated and control groups, indicating that geraniol treatment significantly altered the composition of endophytic communities in tea green leafhoppers (Figure 4d).
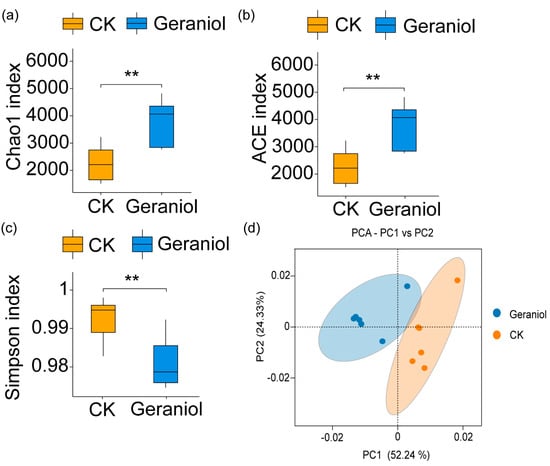
Figure 4.
Effects of 10% geraniol on alpha diversity and beta diversity of the tea green leafhopper endophytes. (a) Chao index analysis; (b) Ace index analysis; (c) Simpson index analysis; (d), Beta diversity analysis. CK, water treatment. The t-test method was used for the significance test. The error bars represent the maximum and minimum values of the data within the group. ** p < 0.01. The upper line represents the first quartile of the data within the group, the lower line represents the third quartile of the data within the group, and the middle line represents the median.
3.2.3. Analysis of Key Differential Endophytic Flora of Tea Green Leafhoppers
Random forest analysis achieves the classification of samples by constructing multiple decision trees and obtains characteristic species that have an important impact on the differences between samples. It can identify the key species that can distinguish the differences between the two groups of samples. These species cause the largest decreases in mean Gini index. At the phylum level, excluding unclassified bacteria, Bacteroidetes was the key phylum demarcating the treatment group from the control group (Figure 5a). Other key differentiating phyla included Proteobacteria, Fibrobacterota, Deferribacterota, and Actinobacteriota. Among these, Proteobacteria and Actinobacteria were particularly significant, as they not only had high relative abundances in the endophytic bacterial community but also exhibited notable differences between the two groups.
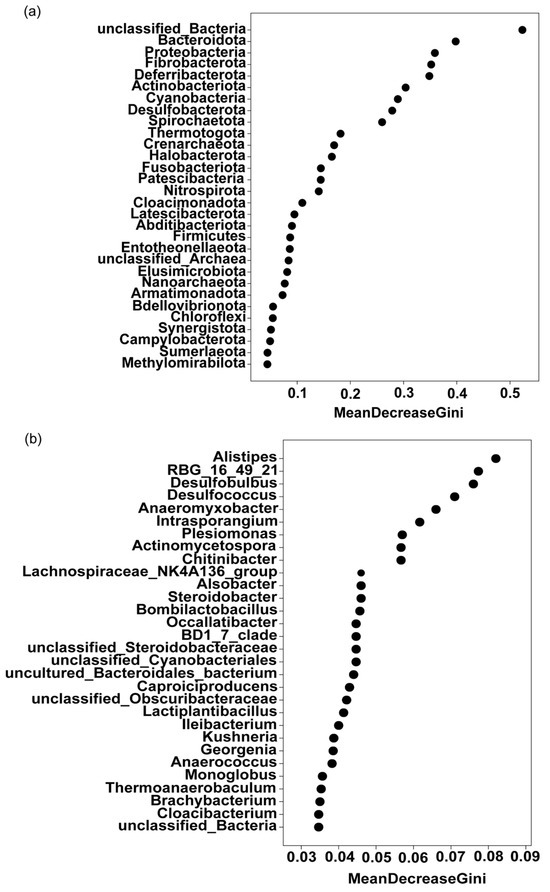
Figure 5.
Random forest analysis of the effects of 10% geraniol on the phylum and genus levels of endophytic bacteria in tea green leafhopper. (a) Effect at the phylum level; (b) Effect at the genus level. Black circles refer to the MeanDecreaseGini. MeanDecreaseGini refers to the mean decrease in the Gini index.
At the genus level, Alistipes was the primary differentiating genus between the treatment and control groups (Figure 5b). Other key differentiating genera included RBG_16_49_21, Desulfobulbus, Desulfococcus, Anaeromyxobacter, and Intrasporangium (Figure 5b). The bacterial communities of these genera contributed significantly to the differences between the treatment and control groups.
3.3. Geraniol Treatment Activated the Expression of Defense-Related Genes in Tea Green Leafhoppers
3.3.1. Transcriptome Quality of Tea Green Leafhoppers
Transcriptome sequencing and quality control analysis yielded 56.78 GB of raw data, with the Q30 base percentage exceeding 93.34% for all samples. After assembling the raw sequences, 59,544 unigenes were obtained, with a total length of 167,126,283 bp. The N50 value of the unigenes was 1709, indicating high assembly integrity. PCA was performed to assess gene expression patterns and sample relationships. The results showed clear separation between the geraniol-treated and control groups, indicating significant transcriptomic differences (Figure 6a). A total of 922 differentially expressed genes (DEGs) were identified, with 539 upregulated and 383 downregulated in the geraniol-treated group compared with the control group (Figure 6b).
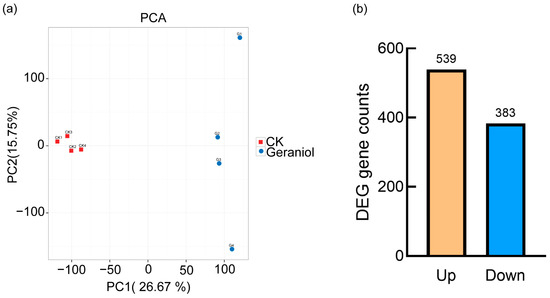
Figure 6.
(a) Principal component analysis of the 10% geraniol treatment group and control group; (b) Up-regulated and down-regulated genes in the 10% geraniol treatment group compared to control group. CK, water treatment.
3.3.2. Transcriptome Analysis of Tea Green Leafhoppers
Enrichment and comparative analysis of the DEGs (Differentially Expressed Genes) with the GO (Gene Ontology) and KEGG (Kyoto Encyclopedia of Genes and Genomes) databases to identify the functions exerted by the differentially expressed genes were conducted first (Figure 7). Then, genes with significant changes were screened based on FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values (Figure 8).
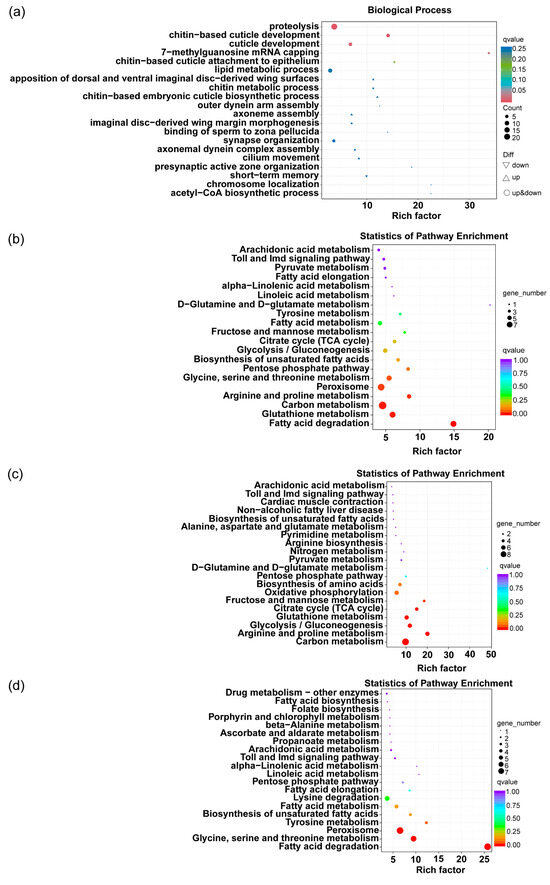
Figure 7.
(a) GO enrichment analysis of differential gene expression in tea green leafhopper; (b) KEGG enrichment analysis of differential gene expression in tea green leafhopper; (c) enrichment map of the main up-regulated differential gene pathways in the 10% geraniol treatment group compared to control group; (d) enrichment map of the main down-regulated differential gene pathways in the 10% geraniol treatment group compared to control group. The “Rich factor” represents the ratio of the proportion of genes annotated to a specific pathway among the differentially expressed genes to the proportion of genes annotated to that pathway among all genes. A q-value less than 0.05 indicated a significant difference. The lower the q value, the higher the level of confidence.
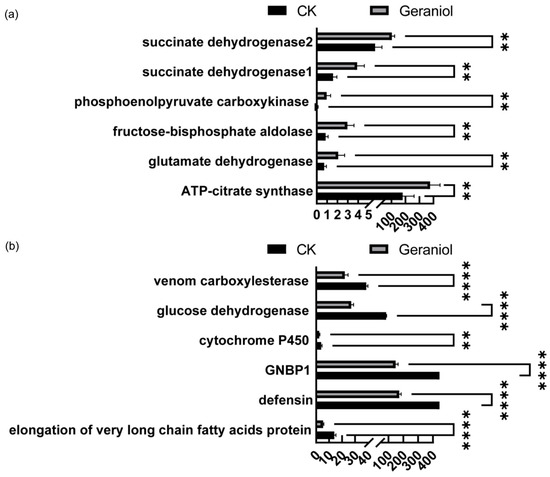
Figure 8.
(a) The differences in FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values of the major upregulated genes compared with the water treatment group.; (b) The differences in FPKM values of the major downregulated genes compared with the water treatment group. CK, water treatment. The t-test method was used for the significance test. The error bars represent the standard deviation. ** p < 0.01, **** p < 0.0001.
DEGs in tea green leafhoppers following geraniol treatment were analyzed for functional enrichment using the Gene Ontology (GO) database (Figure 7a). The highest number of DEGs were associated with proteolysis, chitin-based cuticle development, and cuticle development, all of which showed significant differences between the treatment and control groups. KEGG enrichment analysis was performed on all differentially expressed genes through the transcriptome analysis platform of Biomarker Technologies (Figure S1). The pathways associated with the physiological metabolism of insects were screened from KEGG enrichment analysis to determine if the metabolism-related genes of the tea green leafhopper respond to the geraniol treatment. These pathways included the phagosome pathway, carbon metabolism, fatty acid degradation, glutathione metabolism, glycine, serine, and threonine metabolism, lysine metabolism, arginine and proline metabolism, fatty acid metabolism, glycolysis, oxidative phosphorylation, the tricarboxylic acid (TCA) cycle, the pentose phosphate pathway, arachidonic acid metabolism, drug metabolism, fructose and mannose metabolism, pyruvate metabolism, tyrosine metabolism, and the Toll and Imd signaling pathways. As a result, 57 differentially expressed genes in these pathways were obtained. Most DEGs were enriched in carbon metabolism and the peroxisome metabolic pathway (Figure 7b). Among these 57 DEGs, 33 were downregulated and 24 were upregulated. The upregulated genes were predominantly associated with carbon metabolism, followed by arginine and proline metabolism, glycolysis, and other metabolic pathways (Figure 7c). Among the downregulated genes, those involved in fatty acid degradation; glycine, serine, and threonine metabolism; and peroxisome metabolic pathways were predominant (Figure 7d).
Based on the FPKM values, expression of the following genes underwent remarkable alterations subsequent to geraniol treatment. The genes showing major upregulated genes included ATP citrate synthase, glutamate dehydrogenase, fructose bisphosphate aldolase, phosphoenolpyruvate carboxykinase, and succinate dehydrogenase (Figure 8a). The genes showing major downregulated genes included elongation of very long-chain fatty acid protein, defensin, gram-negative bacteria-binding protein 1 (GNBP1), cytochrome P450, venom carboxylesterase, and glucose dehydrogenase (Figure 8b).
4. Discussion
HIPVs serve as a crucial defense mechanism against herbivorous insects. Research on pest-induced volatiles has primarily focused on their role in regulating interactions across three trophic levels: plants, pests, and natural enemies. Numerous studies have reported the pest-repellent effects of HIPVs, natural enemies attracted toward them, and their ability to trigger anti-insect responses in neighboring plants. For instance, in tobacco, volatiles induced by Heliothis virescens (Fabricius, 1777) exhibit a repellent effect on female H. virescens moths [42]. Geraniol is a volatile compound released by tea leaves in response to feeding by tea green leafhoppers. To assess its potential repellency or attractiveness, we tested tea green leafhopper selectivity across different geraniol concentrations. However, our results indicated that geraniol neither attracted nor repelled the insects. Previous studies have shown that plant essential oils possess broad-spectrum insecticidal properties, exerting both lethal and sublethal effects on various insects and mites [43,44]. To investigate whether geraniol had similar effects, we fumigated tea green leafhoppers with 10% geraniol. The survival rate did not differ significantly between the treatment and control groups, indicating that 10% geraniol does not exert lethal effects on tea green leafhoppers. Other studies have demonstrated that plant volatiles can influence insect oviposition and egg hatching. For example, basil plant volatiles significantly reduce the number of eggs laid by Tuta absoluta (Meyrick, 1917) on nearby tomato plants [45]. Similarly, aromatic plants have been shown to inhibit oviposition in Bemisia tabaci (Gennadius, 1889) [46], while certain essential oils suppress both oviposition and egg hatching in Planococcus citri (Risso, 1813) [47]. These findings align with our results, which show that geraniol affects both the oviposition amount and egg hatching rate of female tea green leafhoppers. Based on this, the development of a geraniol-based sustained-release system could serve as a promising strategy to reduce egg-laying and hatching in tea green leafhoppers, thereby slowing population growth and mitigating infestations in tea plantations.
To further elucidate the molecular mechanisms underlying the effects of geraniol on tea green leafhoppers, we conducted 16S rRNA microbiome and transcriptome analyses. Endophytes play a crucial role in insects, forming mutualistic relationships over long-term evolution. These bacteria contribute to various physiological processes, including nutrient absorption, reproductive metabolism regulation, and the breakdown of plant defense compounds [48,49]. Our results revealed significant alterations in the endophytic microbiota composition of tea green leafhoppers following geraniol treatment. The increase in the Chao index and ACE index suggested that, when subjected to geraniol treatment, tea green leafhoppers had maintained the stability of their bodies by increasing the number of species endophytes within. The decrease in the Simpson index suggested that, following geraniol treatment, the number of certain microbial groups in tea green leafhoppers had increased significantly as an adaptation to the threat posed by geraniol. It was likely that the geraniol treatment had enabled some species that were not initially dominant to grow rapidly and emerge as dominant ones, or had strengthened the position of the originally dominant species. This had caused the community structure to develop in an unbalanced way, thus reducing the Simpson index.
In both the treatment and control groups, the dominant microbial phyla were Proteo-bacteria and Firmicutes, consistent with previous findings on the microbiota of other piercing-sucking insects, such as aphids (Sitobion miscanthi Takahashi, 1921 and Acyrthosiphon pisum Harris, 1776) and rice planthoppers (Laodelphax striatellus Fallén, 1826 and Sogatella furcifera Horváth, 1899) [50,51,52]. Additionally, studies have shown that plant volatiles can disrupt the endophytic microbiota of P. xylostella (Linnaeus, 1767) [53], aligning with our observation that geraniol significantly altered the endophytic community in tea green leafhoppers. The bacterial community of Pseudomonadales in tea green leafhoppers plays a key role in carbohydrate, amino acid, and lipid metabolism [54]. The reduction in Pseudomonadales abundance in the geraniol-treated group may have impaired these metabolic functions in tea green leafhoppers. Endophytes also contribute to insect detoxification metabolism. For instance, in brown planthoppers, the relative abundances of Acinetobacter, Arsenophonus, Staphylococcus, and Wolbachia positively correlated with the expression of detoxification-related genes [55]. In our study, Acinetobacter and Staphylococcus abundances were significantly reduced in the geraniol-treated group. Transcriptome analysis further revealed a significant downregulation of CYP450, suggesting that these bacterial genera may influence tea green leafhopper detoxification metabolism by modulating CYP450 expression.
When insects encounter environmental stress, they regulate gene expression to adapt to the changing conditions. For instance, the exposure to plant volatile organic compounds induced the expression of CYP450 detoxification genes in Helicoverpa armigera (Hübner, 1808) larvae, enhancing their resistance to environmental stressors [56]. In this study, geraniol treatment significantly upregulated the expression of succinate dehydrogenase and glutamate dehydrogenase. These enzymes serve as potential insecticidal targets. Succinate dehydrogenase, a mitochondrial enzyme closely associated with respiratory metabolism. It can catalyze the oxidation of succinate to fumarate and directly transfer the resultant reducing equivalents to the respiratory chain [57]. The activation of succinate dehydrogenase gene expression by geraniol treatment indicates that tea green leafhoppers may prepare for further respiratory metabolism as a mechanism to cope with geraniol-induced stress. Insect glutamate dehydrogenase may play a critical role in regulating amino acid utilization for energy production [58]. The significant upregulation of glutamate dehydrogenase in the geraniol-treated group indicates an increase in energy metabolism, which may also contribute to tea green leafhoppers’ resistance to geraniol exposure. Conversely, glucose dehydrogenase was among the most significantly downregulated genes following geraniol treatment. Previous transcriptomic studies in Nasonia vitripennis (Walker, 1836) have shown that glucose dehydrogenase is involved in sperm storage and release, as well as ovarian metabolic activity [59]. This suggests that the reduced fecundity of female tea green leafhoppers observed in our study may be linked to the downregulation of glucose dehydrogenase expression. The Toll and Imd signaling pathways regulate the expression of antimicrobial peptide genes, which are crucial for insect immune defense against fungal and bacterial infections [60]. Transcriptome analysis revealed significant downregulation of GNBP1 and defensin genes in the Toll and Imd signaling pathways, indicating a decrease in antimicrobial peptide immunity. This result provides evidence that geraniol treatment compromises the immune function of tea green leafhoppers.
5. Conclusions
Our study demonstrates that the treatment with 10% geraniol significantly reduces both the oviposition amount and egg hatching rates in tea green leafhoppers. Geraniol treatment also led to substantial changes in the composition of the endophytic microbiota. The reduced abundance of Pseudomonadales suggests a potential disruption in the metabolic functions of endophytic bacteria. Transcriptome analysis revealed an increased expression of genes related to respiration and energy metabolism, such as glutamate dehydrogenase, indicating that tea green leafhoppers may enhance energy metabolism to counteract geraniol-induced stress. Further analysis suggested that the suppression of egg-laying in female tea green leafhoppers may be linked to the downregulation of glucose dehydrogenase expression. Additionally, the observed decrease in the expression of antimicrobial peptide-related signaling pathway-related genes suggests that geraniol may impair the immune function of tea green leafhoppers. These findings provide valuable insights into the ecological functions of pest-induced plant volatiles and fresh perspectives for the integrated management of tea green leafhoppers.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/agronomy15040782/s1, Figure S1: Functional enrichment of tea green leafhopper genes compared with KEGG database.
Author Contributions
Conceptualization, J.T. and Y.Z.; methodology, Y.Z. and H.S.; study design, J.T., H.H., J.S., Y.Z. and H.S.; project administration, J.S. and Y.Z.; resources, S.M. (Shiqin Mo) and S.M. (Shiliang Mo); investigation, J.T., H.H, S.M. (Shiqin Mo) and S.M. (Shiliang Mo); literature search, J.T., H.H. and H.S.; figures, J.T. and H.H.; data analysis, J.T., H.H., S.M. (Shiqin Mo) and S.M. (Shiliang Mo); data collection, J.T., H.H., S.M. (Shiliang Mo) and S.M. (Shiqin Mo); writing—original draft preparation, J.T. and Y.Z.; writing—review and editing, J.T., J.S. and Y.Z.; funding acquisition, Y.Z.; supervision, Y.Z., J.S. and H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (grant number 32260785), Hainan Provincial Natural Science Foundation of China (grant number 323RC521).
Data Availability Statement
Data can be provided upon formal request to the authors.
Acknowledgments
We are grateful to Boan Zong and Haiqiang Liang for their assistance in harvesting of tea branches.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, J.; Lin, G.; Batool, K.; Zhang, S.; Chen, M.; Xu, J.; Wu, J.; Jin, L.; Gelbic, I.; Xu, L.; et al. Alimentary Tract Transcriptome Analysis of the Tea Geometrid, Ectropis oblique (Lepidoptera: Geometridae). J. Econ. Entomol. 2018, 111, 1411–1419. [Google Scholar] [CrossRef]
- Wakamura, S.; Yasuda, T.; Mochizuki, F. Mating behavior of the tea tussock moth, Euproctis pseudoconspersa (strand) (Lepidoptera: Lymantriidae). Appl. Entomol. Zool. 1996, 31, 619–621. [Google Scholar] [CrossRef]
- Zhang, R.; Lun, X.; Zhang, Y.; Zhao, Y.; Xu, X.; Zhang, Z. Characterization of Ionotropic Receptor Gene EonuIR25a in the Tea Green Leafhopper, Empoasca onuki Matsuda. Plants 2023, 12, 2034. [Google Scholar] [CrossRef]
- Lu, C.; Shen, N.; Jiang, W.; Xie, B.; Zhao, R.; Zhou, G.; Zhao, D.; He, Y.; Chen, W. Different Tea Germplasms Distinctly Influence the Adaptability of Toxoptera aurantii (Hemiptera: Aphididae). Insects 2023, 14, 695. [Google Scholar] [CrossRef]
- Hazarika, L.K.; Bhuyan, M.; Hazarika, B.N. Insect pests of tea and their management. Annu. Rev. Entomol. 2009, 54, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Kanu, U.C.; Wang, Z.; Qiu, C.; Wen, Q.; Li, X.; Qiu, D.; Gan, Y.; Mao, R. Redefining the tea green leafhopper: Empoasca onukii Matsuda (Hemiptera: Cicadellidae) as a vital asset in premium tea production. Life 2025, 15, 133. [Google Scholar] [CrossRef]
- Roy, C.; Naskar, S.; Ghosh, S.; Rahaman, P.; Mahanta, S.; Sarkar, N.; Chaudhuri, R.K.; Babu, A.; Roy, S.; Chakraborti, D. Sucking pest management in tea (Camellia sinensis (L.) Kuntze) cultivation: Integrating conventional methods with bio-control strategies. Crop Prot. 2024, 183, 106759. [Google Scholar] [CrossRef]
- Jin, S.; Chen, Z.M.; Backus, E.A.; Sun, X.L.; Xiao, B. Characterization of EPG waveforms for the tea green leafhopper, Empoasca vitis Gothe (Hemiptera: Cicadellidae), on tea plants and their correlation with stylet activities. J. Insect Physiol. 2012, 58, 1235–1244. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Wang, D.; Wu, G.; Wang, Q. Effects of Artemisia argyi volatiles on the behavior of the tea green leafhopper (Empoasca onukii) in tea plantations. Chin. J. Appl. Entomol. 2022, 59, 773–784. [Google Scholar] [CrossRef]
- Wei, Q.; Mu, X.-C.; Yu, H.-Y.; Niu, C.-D.; Wang, L.-X.; Zheng, C.; Chen, Z.; Gao, C.-F. Susceptibility of Empoasca vitis (Hemiptera: Cicadellidae) populations from the main tea-growing regions of China to thirteen insecticides. Crop Prot. 2017, 96, 204–210. [Google Scholar] [CrossRef]
- Pu, X.-Y.; Feng, M.-G.; Shi, C.-H. Impact of three application methods on the field efficacy of a Beauveria bassiana-based mycoinsecticide against the false-eye leafhopper, Empoasca vitis (Homoptera: Cicadellidae) in the tea canopy. Crop Prot. 2005, 24, 167–175. [Google Scholar] [CrossRef]
- Ye, G.-Y.; Xiao, Q.; Chen, M.; Chen, X.-x.; Yuan, Z.-J.; Stanley, D.W.; Hu, C. Tea: Biological control of insect and mite pests in China. Biol. Control. 2014, 68, 73–91. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.M.; Khan, Z.R.; Pickett, J.A. The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 2007, 52, 375–400. [Google Scholar] [CrossRef]
- Szendrei, Z.; Rodriguez-Saona, C. A meta-analysis of insect pest behavioral manipulation with plant volatiles. Entomol. Exp. Appl. 2010, 134, 201–210. [Google Scholar] [CrossRef]
- Song, H.; Dong, Z.; Li, L.; Lu, Z.; Li, C.; Yu, Y.; Men, X. Relationships among the feeding behaviors of a mirid bug on cotton leaves of different ages and plant biochemical substances. J. Insect Sci. 2021, 21, 15. [Google Scholar] [CrossRef]
- Liu, C.M.; Matsuyama, S.; Kainoh, Y.A.-O. Synergistic effects of volatiles from host-infested plants on host-searching behavior in the parasitoid wasp Lytopylus rufipes (Hymenoptera:Braconidae). J. Chem. Ecol. 2019, 45, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Erb, M.; Veyrat, N.; Robert, C.A.M.; Xu, H.; Frey, M.; Ton, J.; Turlings, T.C.J. Indole is an essential herbivore-induced volatile priming signal in maize. Nat. Commun. 2015, 6, 6273. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Huang, S.; Jiang, T.; Wang, C.; Tao, Z.; He, C.; Tang, Q.; Li, P. Volatile DMNT directly protects plants against Plutella xylostella by disrupting the peritrophic matrix barrier in insect midgut. eLife 2021, 10, e63938. [Google Scholar] [CrossRef]
- Gasmi, L.; Martínez-Solís, M.; Frattini, A.; Ye, M.; Collado, M.C.; Turlings, T.C.J.; Erb, M.; Herrero, S. Can Herbivore-Induced Volatiles Protect Plants by Increasing the Herbivores’ Susceptibility to Natural Pathogens? Appl. Environ. Microbiol. 2018, 85, e01468-18. [Google Scholar] [CrossRef]
- von Mérey, G.; Veyrat, N.; Mahuku, G.; Valdez, R.L.; Turlings, T.C.J.; D’Alessandro, M. Dispensing synthetic green leaf volatiles in maize fields increases the release of sesquiterpenes by the plants, but has little effect on the attraction of pest and beneficial insects. Phytochemistry 2011, 72, 1838–1847. [Google Scholar] [CrossRef]
- Ye, M.A.-O.; Glauser, G.A.-O.; Lou, Y.A.-O.; Erb, M.A.-O.; Hu, L.A.-O. Molecular dissection of early defense signaling underlying volatile-mediated defense regulation and herbivore resistance in rice. Plant Cell 2019, 31, 687–698. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.R.; Rodriguez-Saona, L.E.; Frost, C.J. Herbivore-induced volatiles in the perennial shrub, vaccinium corymbosum, and their role in inter-branch signaling. J. Chem. Ecol. 2009, 35, 163–175. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Zakir, A.; Bengtsson, M.; Sadek, M.M.; Hansson, B.S.; Witzgall, P.; Anderson, P. Specific response to herbivore-induced de novo synthesized plant volatiles provides reliable information for host plant selection in a moth. J. Exp. Biol. 2013, 216, 3257–3263. [Google Scholar] [CrossRef] [PubMed]
- Naskar, S.; Roy, C.; Ghosh, S.; Mukhopadhyay, A.; Hazarika, L.K.; Chaudhuri, R.K.; Roy, S.; Chakraborti, D. Elicitation of biomolecules as host defense arsenals during insect attacks on tea plants (Camellia sinensis (L.) Kuntze). Appl. Microbiol. Biotechnol. 2021, 105, 7187–7199. [Google Scholar] [CrossRef]
- Wang, M.; Han, S.; Wu, Y.; Lin, J.; Zhou, J.; Han, B. Tea green leafhopper-induced synomone attracts the egg parasitoids, mymarids to suppress the leafhopper. Pest Manag. Sci. 2023, 79, 3785–3795. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Naseem, T.; Holopainen, J.K.; Liu, T.; Zhang, J.; Zhang, F. Tritrophic interactions among arthropod natural enemies, herbivores and plants considering volatile blends at different scale levels. Cells. 2023, 12, 251. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Yang, Z. Characterization of terpene synthase from tea green leafhopper being involved in formation of geraniol in tea (Camellia sinensis) leaves and potential effect of geraniol on insect-derived endobacteria. Biomolecules 2019, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- de Lira, M.H.P.; de Andrade Júnior, F.P.; Moraes, G.F.Q.; da Silva Macena, G.; de Oliveira Pereira, F.; Lima, I.O. Antimicrobial activity of geraniol: An integrative review. J. Essent. Oil Res. 2020, 32, 187–197. [Google Scholar] [CrossRef]
- Ramadan, G.R.M.; Abdelgaleil, S.A.M.; Shawir, M.S.; El-bakary, A.S.; Zhu, K.Y.; Phillips, T.W. Terpenoids, DEET and short chain fatty acids as toxicants and repellents for Rhyzopertha dominica (Coleoptera: Bostrichidae) and Lasioderma serricorne (Coleoptera: Ptinidae). J. Stored Prod. Res. 2020, 87, 101610. [Google Scholar] [CrossRef]
- Mu, D.; Cui, L.; Ge, J.; Wang, M.X.; Liu, L.F.; Yu, X.P.; Zhang, Q.H.; Han, B.Y. Behavioral responses for evaluating the attractiveness of specific tea shoot volatiles to the tea green leafhopper, Empoaca vitis. Insect Sci. 2012, 19, 229–238. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, M.; Chen, Z. The relative preference of Empoasca onukii (Hemiptera: Cicadellidae) for oviposition on twenty-four tea cultivars. J. Econ. Entomol. 2022, 115, 1521–1530. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. Public Libr. Sci. Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Binns, D.; Chang, H.Y.; Fraser, M.; Li, W.; McAnulla, C.; McWilliam, H.; Maslen, J.; Mitchell, A.; Nuka, G.; et al. InterProScan 5: Genome-scale protein function classification. Bioinformatics 2014, 30, 1236–1240. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Eddy, S.R. Profile hidden Markov models. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The protein families database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Wu, S.; Zhang, Z.; Li, Y. Global Geographic Diversity and Distribution of the Myxobacteria. Microbiol. Spectr. 2021, 9, e0001221. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Mescher, M.C.; Tumlinson, J.H. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature 2001, 410, 577–580. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal properties of several essential oils on the house fly (Musca domestica L.). Phytother. Res. 2008, 22, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Yarou, B.B.; Bawin, T.; Boullis, A.; Heukin, S.; Lognay, G.; Verheggen, F.J.; Francis, F. Oviposition deterrent activity of basil plants and their essentials oils against Tuta absoluta (Lepidoptera: Gelechiidae). Environ. Sci. Pollut. Res. Int. 2018, 25, 29880–29888. [Google Scholar] [CrossRef] [PubMed]
- Wagan, T.; He, Y.; Long, M.; Chakira, H.; Zhao, J.; Hua, H. Effectiveness of aromatic plant species for repelling and preventing oviposition of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). J. Appl. Entomol. 2018, 142, 287–295. [Google Scholar] [CrossRef]
- Erdemir, T.; Erler, F. Repellent, oviposition-deterrent and egg-hatching inhibitory effects of some plant essential oils against citrus mealybug, Planococcus citri Risso (Hemiptera: Pseudococcidae). J. Plant Dis. Prot. 2017, 124, 473–479. [Google Scholar] [CrossRef]
- Douglas, A.E. Nutritional interactions in insect-microbial symbioses: Aphids and their symbiotic bacteria buchnera. Annu. Rev. Entomology. 1998, 43, 17–37. [Google Scholar] [CrossRef]
- Boone, C.K.; Keefover-Ring, K.; Mapes, A.C.; Adams, A.S.; Bohlmann, J.; Raffa, K.F. Bacteria associated with a tree-killing insect reduce concentrations of plant defense compounds. J. Chem. Ecol. 2013, 39, 1003–1006. [Google Scholar] [CrossRef]
- Li, T.; Xiao, J.H.; Wu, Y.Q.; Huang, D.W. Diversity of bacterial symbionts in populations of Sitobion miscanthi (Hemiptera: Aphididae) in China. Environ. Entomol. 2014, 43, 605–611. [Google Scholar] [CrossRef]
- Xu, T.T.; Chen, J.; Jiang, L.Y.; Qiao, G.A.-O. Diversity of bacteria associated with Hormaphidinae aphids (Hemiptera: Aphididae). Insect Sci. 2021, 28, 165–179. [Google Scholar] [CrossRef]
- Duan, X.Z.; Sun, J.T.; Wang, L.T.; Shu, X.H.; Guo, Y.; Keiichiro, M.; Zhu, Y.X.; Bing, X.L.; Hoffmann, A.A.; Hong, X.A.-O. Recent infection by Wolbachia alters microbial communities in wild Laodelphax striatellus populations. Microbiome 2020, 8, 104. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Q.; Gurr, G.M.; Vasseur, L.; Han, S.; You, M. Gut bacteria mediated adaptation of diamondback moth, Plutella xylostella, to secondary metabolites of host plants. mSystems 2023, 8, e00826-23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Huang, X.-Y.; Zi, H.-B.; Gao, T.; Ji, R.-J.; Sheng, J.; Zhi, D.; Zhang, Y.-L.; Gong, C.-M.; et al. Altitude as a key environmental factor shaping microbial communities of tea green leafhoppers (Matsumurasca onukii). Microbiol. Spectr. 2023, 11, e0100923. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, Y.; Cai, T.; Deng, X.; Liu, C.; Li, J.; He, S.; Li, J.; Wan, H. Antibiotics increased host insecticide susceptibility via collapsed bacterial symbionts reducing detoxification metabolism in the brown planthopper, Nilaparvata lugens. J. Pest Sci. 2021, 94, 757–767. [Google Scholar] [CrossRef]
- Wu, C.; Ding, C.; Chen, S.; Wu, X.; Zhang, L.; Song, Y.; Li, W.; Zeng, R.A.-O. Exposure of Helicoverpa armigera larvae to plant volatile organic compounds induces cytochrome P450 monooxygenases and enhances larval tolerance to the insecticide methomyl. Insects 2021, 12, 238. [Google Scholar] [CrossRef]
- Hederstedt, L.; Rutberg, L. Succinate dehydrogenase–A comparative review. Microbiol. Rev. 1981, 45, 542–555. [Google Scholar] [CrossRef]
- Bond, P.A.; Sang, J.H. Glutamate dehydrogenase of Drosophila larvae. J. Insect Physiol. 1968, 14, 341–359. [Google Scholar] [CrossRef]
- Pannebakker, B.A.; Trivedi, U.; Blaxter, M.A.; Watt, R.; Shuker, D.M. The transcriptomic basis of oviposition behaviour in the parasitoid Wasp Nasonia vitripennis. PLoS ONE 2013, 8, e68608. [Google Scholar] [CrossRef]
- Tanji, T.; Hu, X.; Weber, A.N.R.; Ip, Y.T. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol. Cell. Biol. 2007, 27, 4578–4588. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).