Health Risk Assessment of Tetracyclines Contamination in Soil-Cabbage (Brassica campestris L. ssp. chinensis) System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Seeds and Soils
2.2. Pot Experiment
2.3. Risk Assessment
2.3.1. Health Risk Assessment
2.3.2. Ecological Risk Assessment
2.4. Statistical Analysis
3. Results
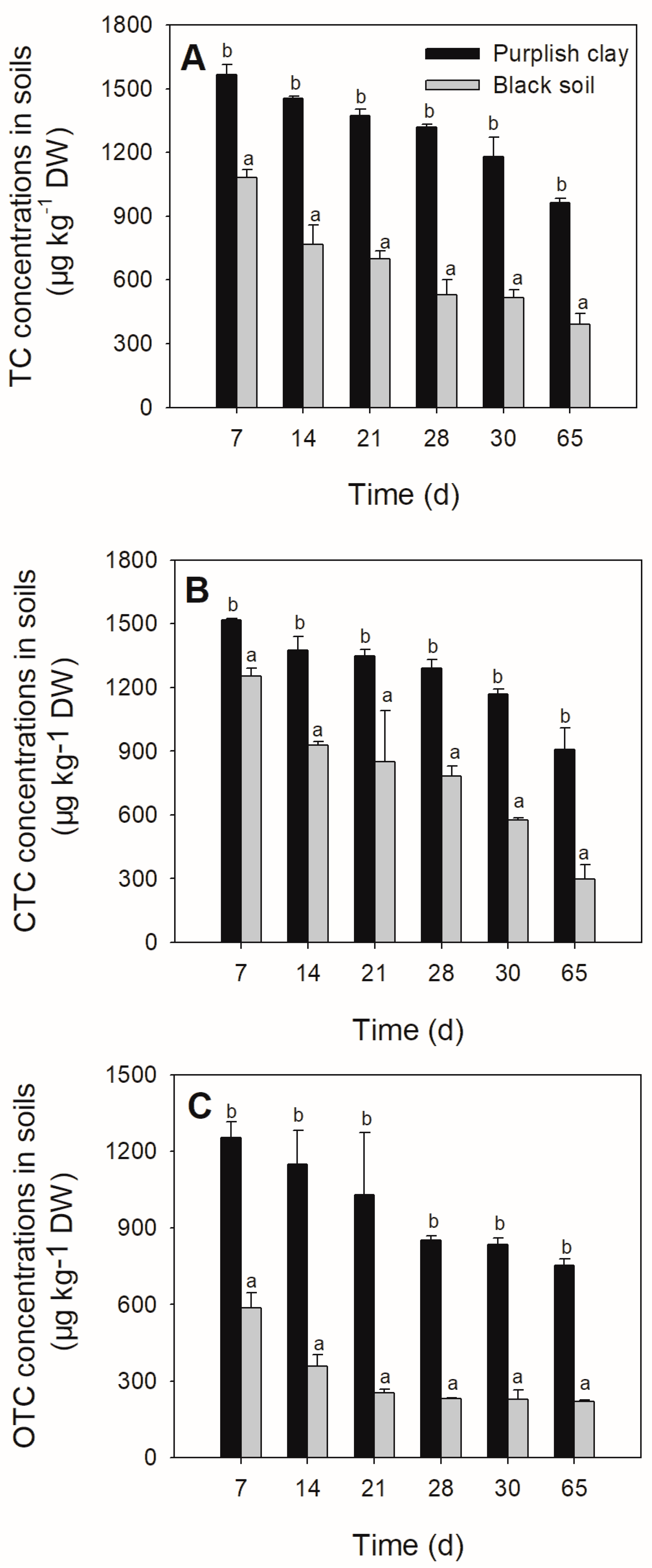
3.1. The Degradation of Tetracyclines in the Soils
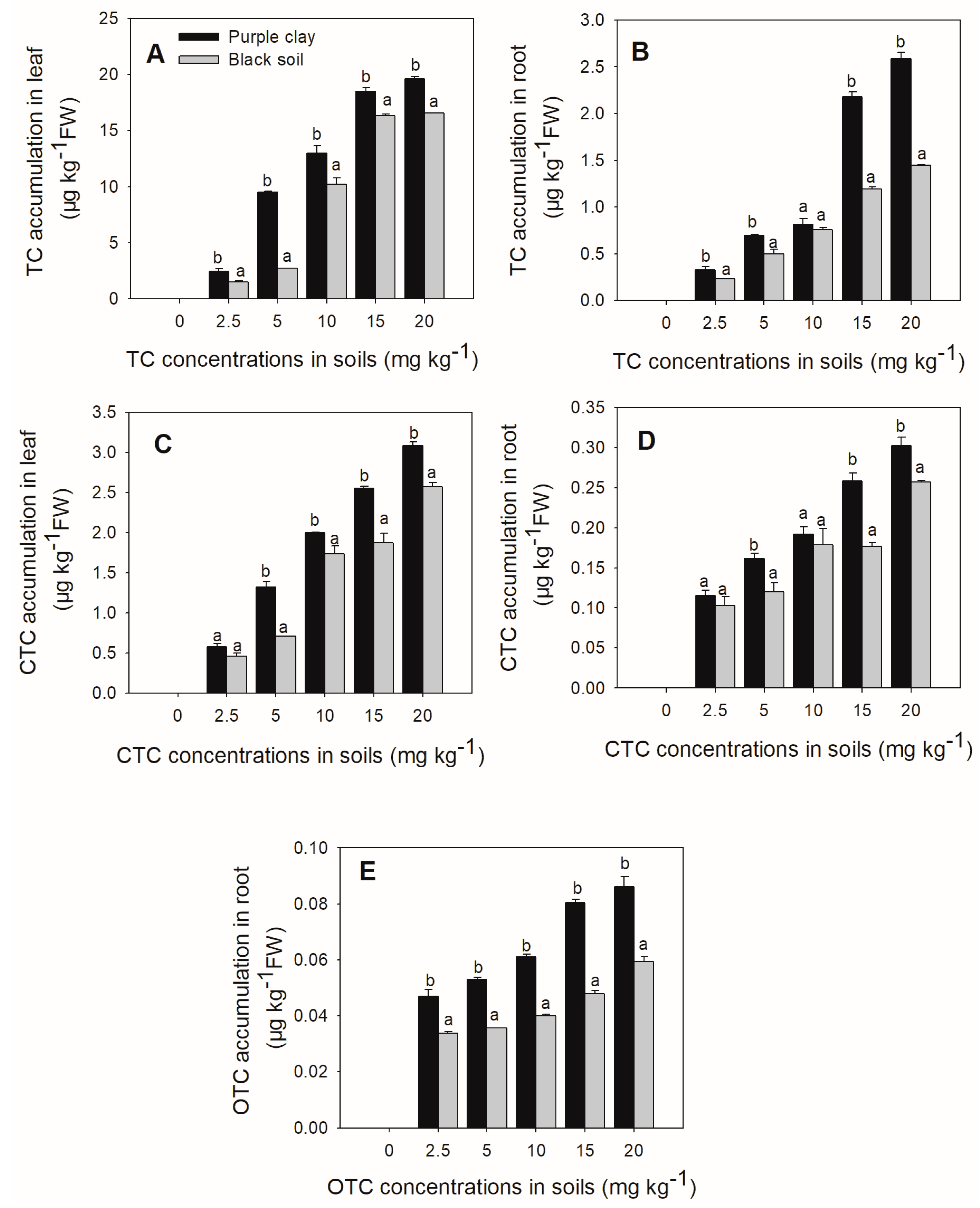
3.2. Accumulation of Tetracyclines in Chinese Cabbage
3.3. The Average Daily Dose of Tetracyclines in the Soil–Cabbage Ecosystem
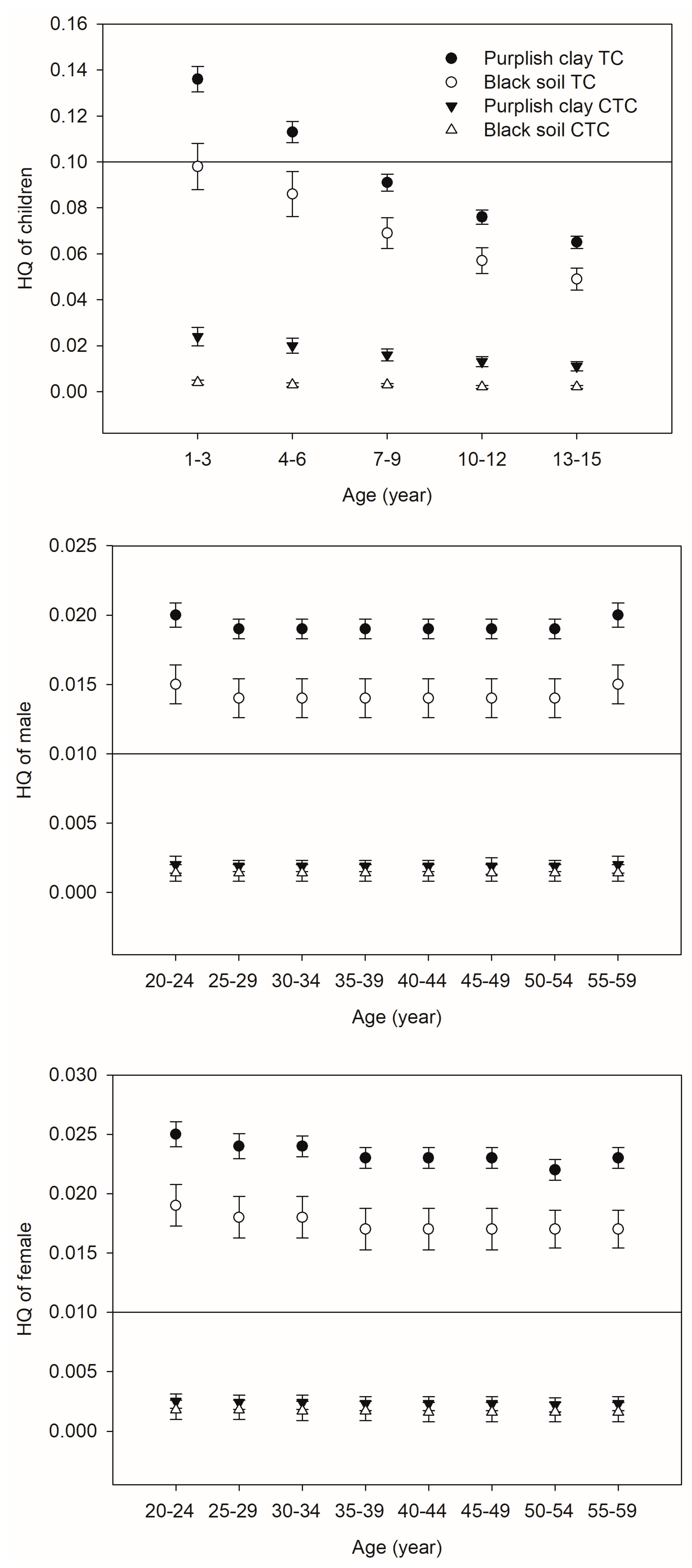
3.4. Health Risk Assessment for Tetracyclines in Vegetable
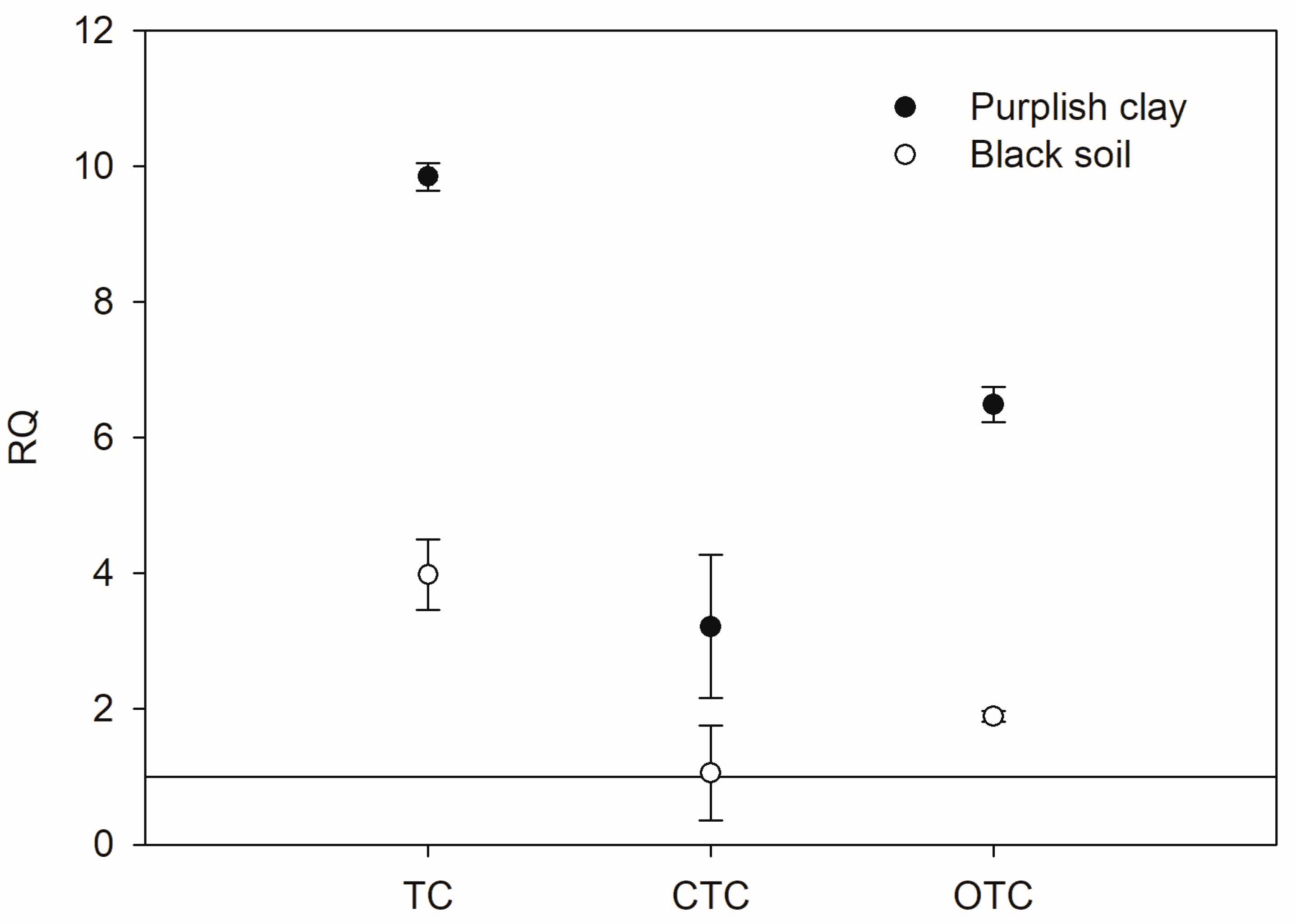
3.5. Ecological Risk Assessment for Tetracyclines in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.G.; Johnson, T.A.; Su, J.Q.; Qiao, M.; Guo, G.X.; Stedtfeld, R.D.; Hashsham, S.A.; Tiedje, J.M. Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc. Nat. Acad. Sci. USA 2013, 110, 3435–3440. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, K.; Moudgil, P.; Soni, D.; Jadhav, V.J. Occurrence and risk assessment of tetracycline residues in layer eggs in Haryana, India. J. Food Protect. 2025, 88, 100449. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Huang, X.Y.; Gu, J.Y.; Chen, C.; Long, X.X.; Zeng, Q.Y. Distribution characteristics and risk assessment of tetracycline antibiotics (TCs) in soil-vegetable system with soil fertilized with animal manure. Environ. Sci. 2023, 44, 4440–4447. (In Chinese) [Google Scholar]
- Liu, M.J.; Yang, B.G.; Xu, X.P.; Ding, W.C.; Chuan, L.M.; He, P.; Zhou, W. Construction and verification of Chinese cabbage nutrient expert system based on yield response and agronomic efficiency. J. Plant Nutr. Fertil. 2025, 31, 156–166. (In Chinese) [Google Scholar]
- Wang, J.J. Production status and development countermeasures of Chinese cabbage in China. China Fruit Veg. 2020, 40, 80–82. [Google Scholar]
- Li, Y.W.; Wu, X.L.; Mo, C.H.; Tai, Y.P.; Huang, X.P.; Xiang, L. Investigation of sulfonamide, tetracycline, and quinolone antibiotics in vegetable farmland soil in the Pearl river delta area, southern China. J. Agric. Food Chem. 2011, 59, 7268–7276. [Google Scholar] [CrossRef]
- Gu, J.; Chen, C.; Huang, X.; Mo, J.; Xie, Q.; Zeng, Q. Occurrence and risk assessment of tetracycline antibiotics in soils and vegetables from vegetable fields in pearl river delta, South China. Sci. Total Environ. 2021, 776, 145959. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emissionand fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.; Gudda, F.O.; Ling, W. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessmen. Chemosphere 2020, 255, 127006. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, Y.; Shi, M.; Qiu, T.; Gao, M.; Tian, S.; Wang, X. Effect of antibiotic type and vegetable species on antibiotic accumulation in soil-vegetable system, soil microbiota, and resistance genes. Chemosphere 2021, 263, 128099. [Google Scholar] [CrossRef]
- Chi, S.L.; Wang, W.Z.; Xu, W.H.; Li, T.; Li, Y.H.; Zhang, C.L. Effects of tetracycline antibiotics on growth and characteristics of enrichment and transformation in two vegetables. Environ. Sci. 2018, 39, 935–943. (In Chinese) [Google Scholar]
- Liu, D.; Lu, L.; Wang, M.; Hussain, B.; Tian, S.; Luo, W.; Zhou, J.; Yang, X. Tetracycline uptake by Chinese cabbage grown on contaminated soils and its toxicity in human liver cell line HL-7702. Environ. Pollut. 2019, 253, 312–321. [Google Scholar] [PubMed]
- Du, L.; Liu, W.K. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar]
- Blackwell, P.A.; Kay, P.; Boxall, B.A. The dissipation and transport of veterinary antibiotics in a sandy loam soil. Chemosphere 2007, 67, 292–299. [Google Scholar]
- Murray, A.K.; Stanton, I.; Gaze, W.H.; Snape, J. Dawning of a new era: Environmental risk assessment of antibiotics and their potential to select for antimicrobial resistance. Water Res. 2021, 200, 117233. [Google Scholar]
- Halling-Srensen, B.; Sengelv, G.; Tjrnelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and accumulative pattern of TCs and sulfonamides in edible vegetables of cucumber, tomato, and lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar]
- Prosser, R.S.; Sibley, P.K. Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation. Environ. Int. 2015, 75, 223–233. [Google Scholar]
- Heaton, P.C.; Fenwick, S.R.; Brewer, D.E. Association between tetracycline or doxycycline and hepatotoxicity: A population based case-control study. J. Clin. Pharm. Ther. 2007, 32, 483–487. [Google Scholar]
- Singh, A.; Pratap, S.G.; Raj, A. Occurrence and dissemination of antibiotics and antibiotic resistance in aquatic environment and its ecological implications: A review. Environ. Sci. Pollut. R. 2024, 31, 47505–47529. [Google Scholar]
- Gong, W.; Guo, L.; Huang, C.; Xie, B.; Jiang, M.; Zhao, Y.; Zhang, H.; Wu, Y.X.; Liang, H. A systematic review of antibiotics and antibiotic resistance genes (args) in mariculture wastewater: Antibiotics removal by microalgal-bacterial symbiotic system (mbss), args characterization on the metagenomic. Sci. Total Environ. 2024, 930, 172601. [Google Scholar]
- China Report Hall. Analysis of Development Trends and Future Investment Research Report on Chinese Cabbage Industry from 2024 to 2029. Available online: https://www.chinabgao.com/freereport/97663.html (accessed on 14 October 2024).
- Xiao, W.; Yang, X.; He, Z.; Rafiq, M.T.; Hou, D.; Li, T. Model for evaluation of the phytoavailability of chromium (Cr) to rice (Oryza sativa L.) in representative Chinese soils. J. Agric. Food Chem. 2013, 61, 2925–2932. [Google Scholar] [PubMed]
- Feng, Q. Research on Risk Assessment and Detection Methods of Major Antibiotics and Heavy Metal Residues in Honey from Zhejiang Province. Ph.D. Thesis, Zhejiang University of Technology, Hangzhou, China, 2013. (In Chinese). [Google Scholar]
- Xiang, L.; Wu, X.L.; Jiang, Y.N.; Yan, Q.Y.; Li, Y.W.; Huang, X.P.; Cai, Y.Q.; Mo, C.H. Occurrence and risk assessment of tetracycline antibiotics in soil from organic vegetable farms in a subtropical city, south China. Environ. Sci. Pollut. R. 2016, 23, 13984–13995. [Google Scholar]
- Georgakakos, C.B.; Hicks, B.J.; Walter, M.T. Farmer perceptions of dairy farm antibiotic use and transport pathways as determinants of contaminant loads to the environment. J. Environ. Manag. 2021, 281, 111880. [Google Scholar]
- Karci, A.; Balcioglu, I.A. Investigation of the tetracycline, sulfonamide, and fluoroquinolone antimicrobial compounds in animal manure and agricultural soils in Turkey. Sci. Total Environ. 2009, 407, 4652–4664. [Google Scholar]
- Hu, X.G.; Zhou, Q.X.; Luo, Y. Occurrence and source analysis of typical veterinary antibiotics in manure, soil, vegetables and groundwater from organic vegetable bases, northern China. Environ. Pollut. 2010, 158, 2992–2998. [Google Scholar]
- Li, C.; Chen, J.Y.; Wang, J.H.; Ma, Z.H.; Han, P.; Luan, Y.X.; Lu, A.X. Occurrence of antibiotics in soils and manures from greenhouse vegetable production bases of Beijing, China and an associated risk assessment. Sci. Total Environ. 2015, 521, 101–107. [Google Scholar]
- Bao, S.D. Soil Agro-Chemistrical Analysis; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Tan, K.H. Soil Sampling, Preparation, and Analysis; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Chaturvedi, R.; Sankar, K. Laboratory Manual for the Physicochemical Analysis of Soil, Water and Plant; Wildlife Institute of India: Dehradun, India, 2006.
- Hendershot, W.H.; Duquette, M. A simple barium chloride method for determining cation exchange capacity and exchangeable cations. Soil Sci. Soc. Am. J. 1986, 50, 605–608. [Google Scholar]
- Rashid, A.; Ryan, J.; Estefan, G. Soil and Plant Analysis Laboratory Manual; International Center for Agricultural Research in the Dry Areas (ICARDA): Aleppo, Syria, 2001. [Google Scholar]
- Day, P.R. Particle fractionation and particle-size analysis. In Methods of Soil Analysis, Agronomy Monograph No. 9; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1965; pp. 545–567. [Google Scholar]
- Liu, D.; Lu, L.L.; Luo, W.J.; Tian, S.K.; Yang, X.E. Degradation of tetracyclines in soils and their effects on root growth of Chinese cabbages (Brassica campestris L.). Acta Scien. Circum. 2017, 37, 1957–1966. (In Chinese) [Google Scholar]
- Kumar, K.; Gupta, S.C.; Baidoo, S.K.; Chander, Y.; Rosen, C.J. Antibiotic uptake by plants from soil fertilized with animal manure. J. Environ. Qual. 2005, 34, 2082–2085. [Google Scholar]
- Yang, Y.; Owino, A.A.; Gao, Y.; Yan, X.; Xu, C.; Wang, J. Occurrence, composition and risk assessment of antibiotics in soils from Kenya, Africa. Ecotoxicology 2016, 25, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Ecology and Environment of the People’s Republic of China. Notice on Issuing the Framework Guidelines for Technical Methods of Environmental Risk Assessment of Chemical Substances (Trial) [EB/OL]. 10 May 2020. Available online: https://www.mee.gov.cn/xxgk2018/xxgk/xxgk05/201909/t20190910733204.html (accessed on 10 May 2020). (In Chinese)
- Chu, T.S.; Wang, B.X.; Meng, T.; Lai, S.X.; Yang, Z.L. Estimation on farmland carrying capacity for livestock and poultry manure based on farmland soil antibiotics ecological risk values. J. China Agric. Univ. 2021, 26, 138–151. (In Chinese) [Google Scholar]
- Li, X.J. Study on the Adsorption and Migration of Tetracycline Antibiotics in Soil. Ph.D. Thesis, Chongqing University, Chongqing, China, 2022. (In Chinese). [Google Scholar]
- Li, Z.J.; Fan, F.F.; Long, J. Effects of soil temperature on degradation of oxytetracycline in soils. Res. J. Chem. Environ. 2013, 17, 58–64. [Google Scholar]
- Li, C.N.; Awasthi, M.K.; Liu, J.; Yao, T. Veterinary tetracycline residues: Environmental occurrence, ecotoxicity, and degradation mechanism. Environ. Res. 2025, 266, 120417. [Google Scholar]
- Strotmann, U.; Thouand, G.; Pagga, U.; Gartiser, S.; Heipieper, H.J. Toward the future of OECD/ISO biodegradability testing-new approaches and developments. Appl. Microbiol. Biotechnol. 2023, 107, 2073–2095. [Google Scholar] [CrossRef]
- Strotmann, U.; Durand, M.J.; Thouand, G.; Eberlein, C.; Heipieper, H.J.; Gartiser, S.; Pagga, U. Microbiological toxicity tests using standardized ISO/OECD methods—current state and outlook. Appl. Microbiol. Biotechnol. 2024, 108, 454. [Google Scholar]
- Masciandaro, G.; Macci, C.; Peruzzi, E.; Ceccanti, B.; Doni, S. Organic matter-microorganism-plant in soil bioremediation: A synergic approach. Rev. Environ. Sci. Biotechnol. 2013, 12, 399–419. [Google Scholar] [CrossRef]
- Pollard, A.T.; Morra, M.J. Fate of tetracycline antibiotics in dairy manure amended soils. Environ. Rev. 2018, 26, 102–112. [Google Scholar] [CrossRef]
- He, D.C.; Wu, G.Y.; Xu, Z.C.; Fang, J.D.; Zhang, S.K. Uptake of selected tetracycline antibiotics by pak choi and radish from manure-amended soils. J. Agro-Environ. Sci. 2014, 33, 1095–1099. (In Chinese) [Google Scholar]
- Withgott, J. Ubiquitous herbieide emaseulates frogs. Science 2002, 296, 447–448. [Google Scholar] [CrossRef]
- Kang, D.H.; Gupta, S.; Rosen, C.; Fritz, V.; Rohwer, C. Antibiotic uptake by vegetable crops from manure-applied soils. J. Agric. Food Chem. 2013, 61, 9992–10001. [Google Scholar] [PubMed]
| Parameters | Purplish Clay | Black Soil |
|---|---|---|
| pH | 5.43 ± 0.02 b | 5.98 ± 0.02 a |
| Organic Matter (g kg−1) | 47.09 ± 0.23 b | 60.57 ± 1.05 a |
| Total Nitrogen (g kg−1) | 2.35 ± 0.01 b | 3.03 ± 0.05 a |
| Available Phosphorus (mg kg−1) | 71.76 ± 1.70 b | 85.27 ± 0.53 a |
| Available Potassium (mg kg−1) | 332.83 ± 22.02 b | 655.55 ± 13.82 a |
| Cation Exchange Capacity (cmol kg−1) | 20.20 ± 1.41 b | 34.0 ± 2.51 a |
| Sand (%) | 11.40 ± 0.26 b | 20.60 ± 1.54 a |
| Silt (%) | 73.0 ± 2.41 a | 60.20 ± 2.21 b |
| Clay (%) | 15.60 ± 1.17 b | 19.20 ± 1.24 a |
| Tetracycline (mg kg−1) | ND | ND |
| Chlortetracycline (mg kg−1) | ND | ND |
| Oxytetracycline (mg kg−1) | ND | ND |
| Age (Year) | Weight (kg) | ADD (μg kg−1 Day−1) | |||||
|---|---|---|---|---|---|---|---|
| Purplish Clay TC | Black Soil TC | Purplish Clay CTC | Black Soil CTC | Purplish Clay OTC | Black Soil OTC | ||
| 1–3 | 10 | 0.780 ± 0.0315 | 0.581 ± 0.057 | 0.136 ± 0.020 | 0.022 ± 0.005 | - | - |
| 4–6 | 12 | 0.650 ± 0.0263 | 0.484 ± 0.056 | 0.113 ± 0.016 | 0.019 ± 0.004 | - | - |
| 7–9 | 15 | 0.520 ± 0.0209 | 0.387 ± 0.038 | 0.090 ± 0.013 | 0.015 ± 0.003 | - | - |
| 10–12 | 18 | 0.434 ± 0.0175 | 0.323 ± 0.032 | 0.076 ± 0.011 | 0.012 ± 0.003 | - | - |
| 13–15 | 21 | 0.372 ± 0.0150 | 0.277 ± 0.027 | 0.065 ± 0.010 | 0.011 ± 0.003 | - | - |
| Age(Year) | Gender | Weight (kg) | ADD (μg kg−1 Day−1) | |||||
|---|---|---|---|---|---|---|---|---|
| Purplish Clay TC | Black Soil TC | Purplish Clay CTC | Black Soil CTC | Purplish Clay OTC | Black Soil OTC | |||
| 20–24 | Male | 67.2 | 0.115 ± 0.005 | 0.085 ± 0.008 | 0.020 ± 0.003 | 0.014 ± 0.003 | - | - |
| Female | 53.8 | 0.143 ± 0.006 | 0.107 ± 0.010 | 0.025 ± 0.003 | 0.018 ± 0.004 | - | - | |
| 25–29 | Male | 70.4 | 0.110 ± 0.004 | 0.081 ± 0.008 | 0.019 ± 0.002 | 0.014 ± 0.003 | - | - |
| Female | 55.3 | 0.140 ± 0.006 | 0.104 ± 0.010 | 0.024 ± 0.003 | 0.018 ± 0.004 | - | - | |
| 30–34 | Male | 71.4 | 0.108 ± 0.004 | 0.080 ± 0.008 | 0.019 ± 0.002 | 0.014 ± 0.003 | - | - |
| Female | 56.8 | 0.136 ± 0.005 | 0.101 ± 0.010 | 0.024 ± 0.003 | 0.017 ± 0.004 | - | - | |
| 35–39 | Male | 71.5 | 0.108 ± 0.004 | 0.080 ± 0.008 | 0.019 ± 0.002 | 0.014 ± 0.003 | - | - |
| Female | 57.8 | 0.134 ± 0.005 | 0.099 ± 0.010 | 0.023 ± 0.003 | 0.017 ± 0.004 | - | - | |
| 40–44 | Male | 71.2 | 0.108 ± 0.004 | 0.081 ± 0.008 | 0.019 ± 0.002 | 0.014 ± 0.003 | - | - |
| Female | 59.0 | 0.131 ± 0.005 | 0.097 ± 0.010 | 0.023 ± 0.003 | 0.016 ± 0.004 | - | - | |
| 45–49 | Male | 71.2 | 0.108 ± 0.004 | 0.081 ± 0.008 | 0.019 ± 0.003 | 0.014 ± 0.003 | - | - |
| Female | 59.7 | 0.129 ± 0.005 | 0.096 ± 0.010 | 0.023 ± 0.003 | 0.016 ± 0.004 | - | - | |
| 50–54 | Male | 70.6 | 0.109 ± 0.004 | 0.081 ± 0.008 | 0.019 ± 0.002 | 0.014 ± 0.003 | - | - |
| Female | 60.4 | 0.128 ± 0.005 | 0.095 ± 0.009 | 0.022 ± 0.003 | 0.016 ± 0.004 | - | - | |
| 55–59 | Male | 69.1 | 0.112 ± 0.005 | 0.083 ± 0.008 | 0.020 ± 0.003 | 0.014 ± 0.003 | - | - |
| Female | 59.4 | 0.130 ± 0.005 | 0.097 ± 0.009 | 0.023 ± 0.003 | 0.016 ± 0.004 | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Shohag, M.J.I.; Qiu, W.; Lu, L.; Wang, Y.; Yang, X. Health Risk Assessment of Tetracyclines Contamination in Soil-Cabbage (Brassica campestris L. ssp. chinensis) System. Agronomy 2025, 15, 768. https://doi.org/10.3390/agronomy15040768
Liu D, Shohag MJI, Qiu W, Lu L, Wang Y, Yang X. Health Risk Assessment of Tetracyclines Contamination in Soil-Cabbage (Brassica campestris L. ssp. chinensis) System. Agronomy. 2025; 15(4):768. https://doi.org/10.3390/agronomy15040768
Chicago/Turabian StyleLiu, Di, Md. Jahidul Islam Shohag, Weiwen Qiu, Lingli Lu, Yuyan Wang, and Xiaoe Yang. 2025. "Health Risk Assessment of Tetracyclines Contamination in Soil-Cabbage (Brassica campestris L. ssp. chinensis) System" Agronomy 15, no. 4: 768. https://doi.org/10.3390/agronomy15040768
APA StyleLiu, D., Shohag, M. J. I., Qiu, W., Lu, L., Wang, Y., & Yang, X. (2025). Health Risk Assessment of Tetracyclines Contamination in Soil-Cabbage (Brassica campestris L. ssp. chinensis) System. Agronomy, 15(4), 768. https://doi.org/10.3390/agronomy15040768








