Citrus Greening Disease Infection Reduces the Energy Flow Through Soil Nematode Food Webs
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Experimental Design
2.2. Nematode Extraction and Identification
2.3. Data Analysis
2.4. Statistical Analyses
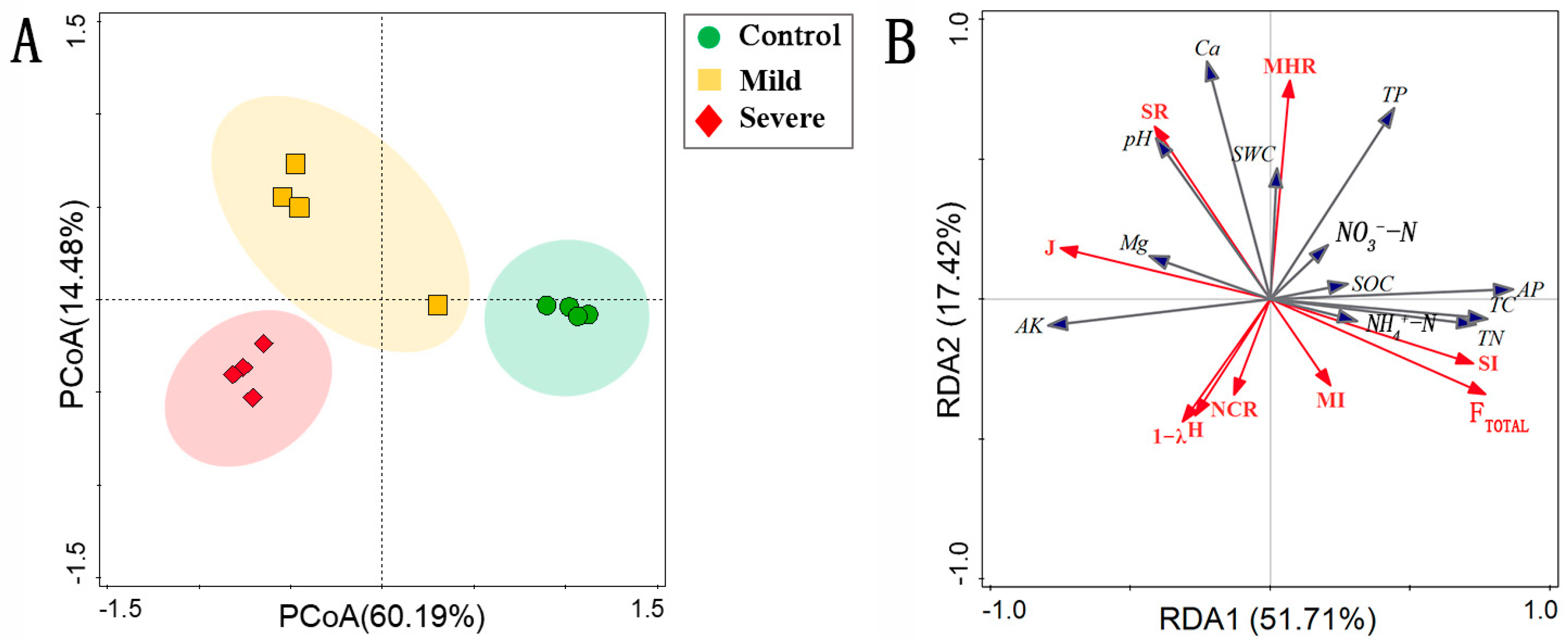
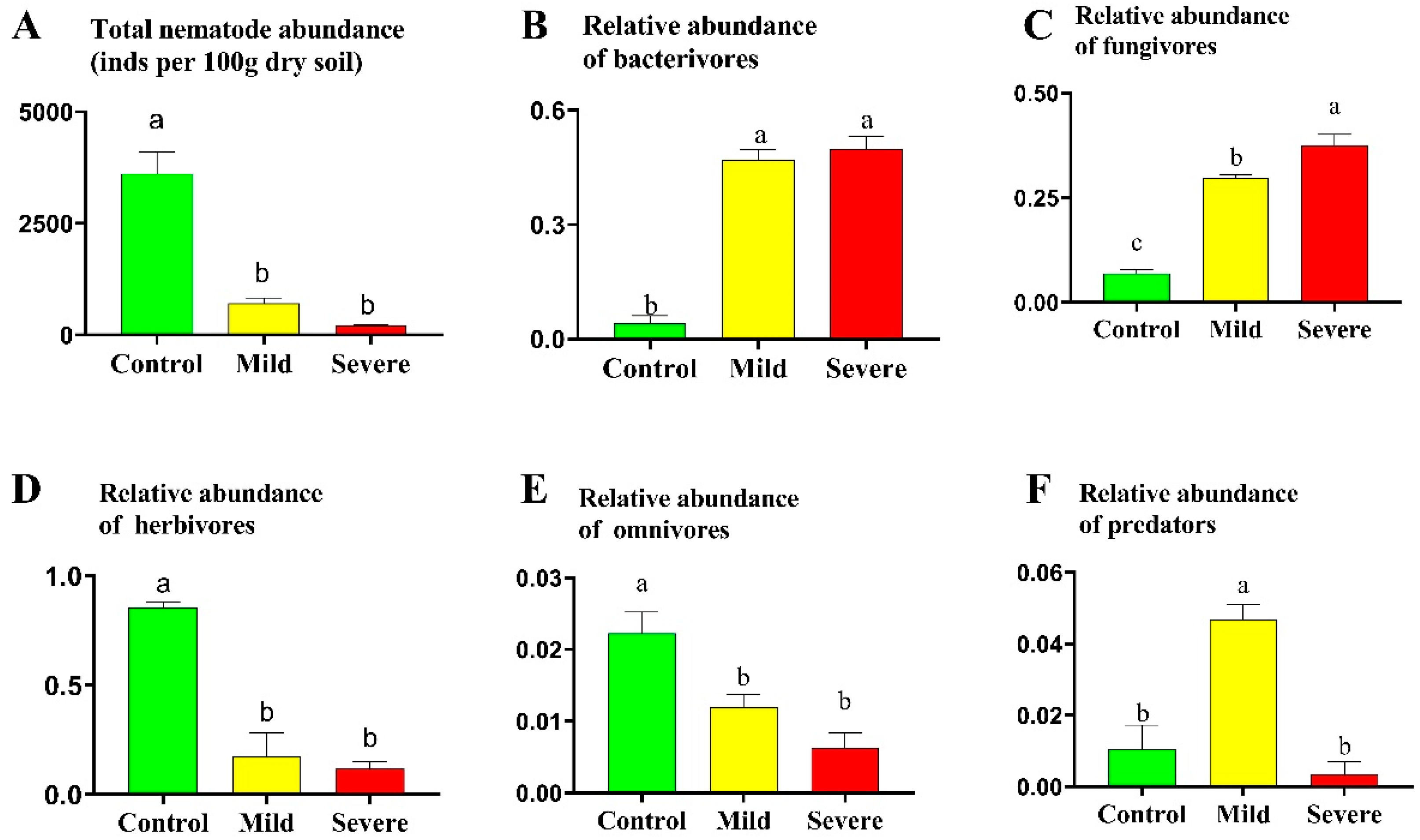
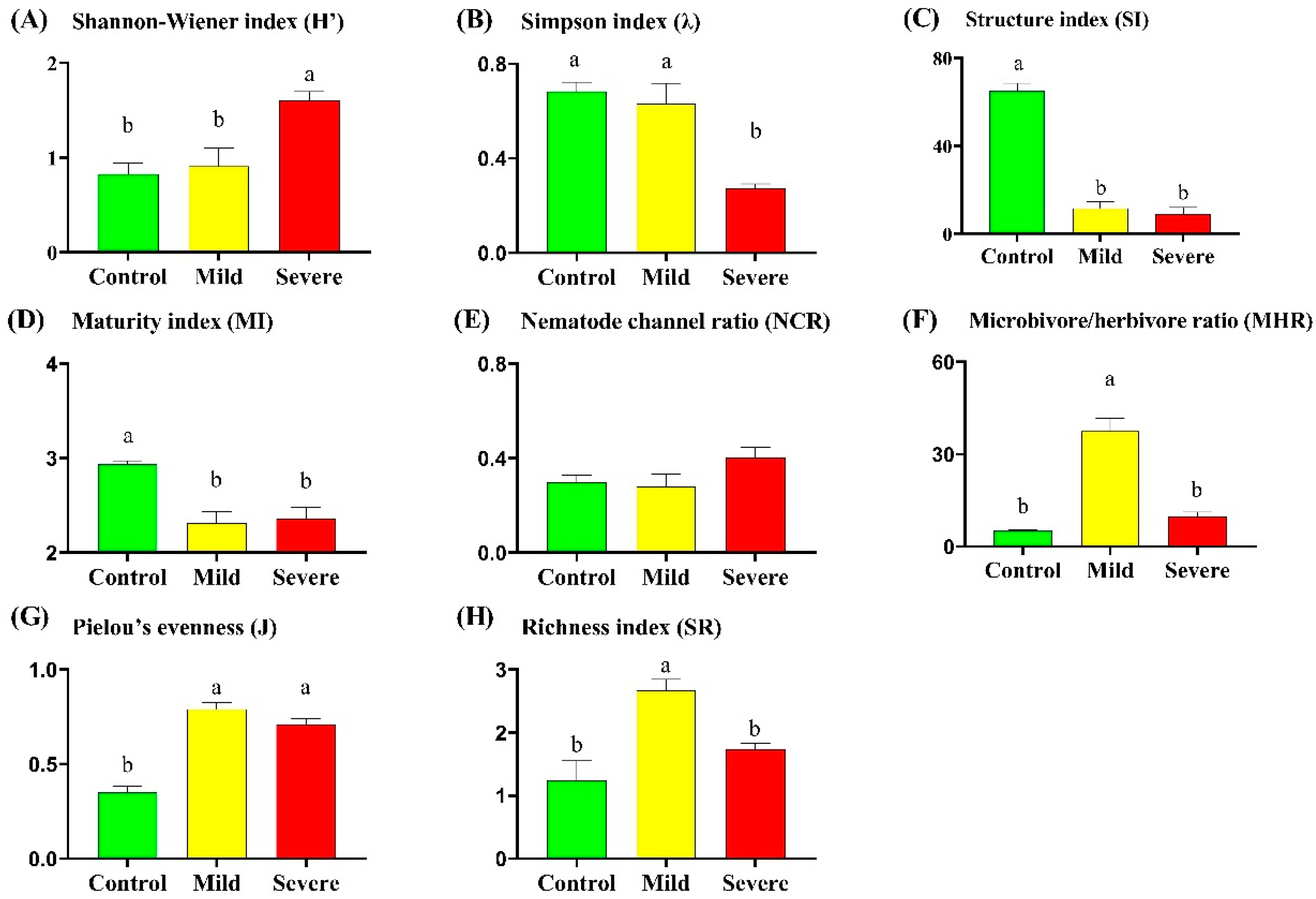
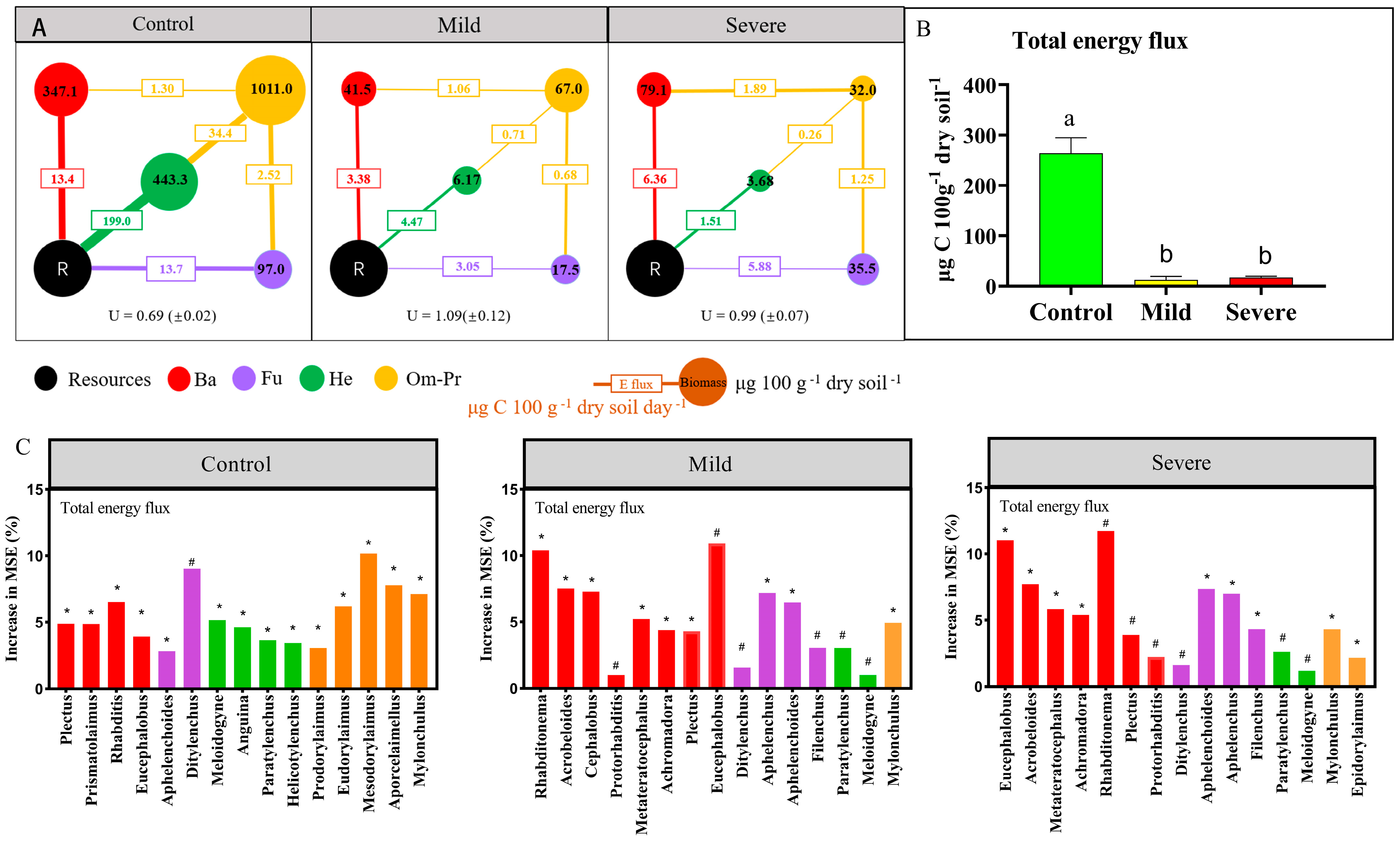
3. Results
3.1. Soil Nematode Communities
3.2. Energy Fluxed of Nematode Food Webs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloemlimited bacterium of greening disease of citrus is a member of the a subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Teixeira, D.d.C.; Saillard, C.; Eveillard, S.; Danet, J.L.; Costa, P.I.d.; Ayres, A.J.; Bove, J. ‘Candidatus Liberibacter americanus’, associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. Int. J. Syst. Evol. Microbiol. 2005, 55, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Blaustein, R.A.; Lorca, G.L.; Teplitski, M. Challenges for managing Candidatus Liberibacter spp.(Huanglongbing disease pathogen): Current control measures and future directions. Phytopathology 2018, 108, 424–435. [Google Scholar] [CrossRef]
- Kim, J.S.; Sagaram, U.S.; Burns, J.K.; Li, J.L.; Wang, N. Response of sweet orange (Citrus sinensis) to ‘Candidatus Liberibacter asiaticus’ infection: Microscopy and microarray analyses. Phytopathology 2009, 99, 50–57. [Google Scholar] [CrossRef]
- Shin, K.; Ascunce, M.S.; Narouei-Khandan, H.A.; Sun, X.; Jones, D.; Kolawole, O.O.; Goss, E.M.; van Bruggen, A.H.C. Effects and side effects of penicillin injection in huanglongbing affected grapefruit trees. Crop Prot. 2016, 90, 106–116. [Google Scholar] [CrossRef]
- Pérez-Zarate, L.A.; Osorio-Acosta, F.; Ortega-Arenas, L.D.; Martínez-Hernández, A.; Villanueva-Jiménez, J.A. Huanglongbing (HLB) de los Cítricos: Impacto Económico y Variabilidad genética del patógeno en México. Agro-Divulg. 2024, 4. [Google Scholar] [CrossRef]
- Trivedi, P.; He, Z.; Van Nostrand, J.D.; Albrigo, G.; Zhou, J.; Wang, N. Huanglongbing alters the structure and functional diversity of microbial communities associated with citrus rhizosphere. ISME J. 2012, 6, 363–383. [Google Scholar] [CrossRef]
- Ritz, K.; Trudgill, D.L. Utility of nematode community analysis as an integrated measure of the functional state of soils: Perspectives and challenges. Plant Soil 1999, 212, 1–11. [Google Scholar] [CrossRef]
- Bongers, T.; Bongers, M. Functional diversity of nematodes. Appl. Soil Ecol. 1998, 10, 239–251. [Google Scholar] [CrossRef]
- Yeates, G.W.; Wardle, D.A.; Watson, R.N. Responses of soil nematode populations, community structure, diversity and temporal variability to agricultural intensification over a seven-year period. Soil Biol. Biochem. 1999, 31, 1721–1733. [Google Scholar] [CrossRef]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161. [Google Scholar]
- Sánchez-Moreno, S.; Nicola, N.L.; Ferris, H.; Zalom, F.G. Effects of agricultural management on nematode-mite assemblages: Soil food web indices as predictors of mite community composition. Appl. Soil Ecol. 2009, 41, 15. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Neher, D.A. Soil nematode genera that predict specific types of disturbance. Appl. Soil Ecol. 2013, 64, 135–141. [Google Scholar] [CrossRef]
- Gao, D.; Liu, S.; Gao, F.; Ning, C.; Wu, X.; Yan, W.; Smith, A. Response of soil micro-food web to nutrient limitation along a subtropical forest restoration. Sci. Total Environ. 2024, 909, 168349. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, D.; Liu, S.; Yan, W.; Zhao, J. Forest restoration increases energy flow through the fungal channel and decreases energy flow through the herbivorous channel in soil micro-food webs. Soil Biol. Biochem. 2024, 198, 109561. [Google Scholar] [CrossRef]
- Gao, D.; Wang, F.; Li, J.; Yu, S.; Li, Z.; Zhao, J. Soil nematode communities as indicators of soil health in different land use types in tropical area. Nematology 2020, 22, 595–610. [Google Scholar] [CrossRef]
- Liu, T.; Chen, X.; Hu, F.; Ran, W.; Shen, Q.; Li, H.; Whalen, J.K. Carbon-rich organic fertilizers to increase soil biodiversity: Evidence from a meta-analysis of nematode communities. Agric. Ecosyst. Environ. 2016, 232, 199–207. [Google Scholar] [CrossRef]
- Barnes, A.D.; Jochum, M.; Lefcheck, J.S.; Eisenhauer, N.; Scherber, C.; O’Connor, M.I.; de Ruiter, P.; Brose, U. Energy flux: The link between multitrophic biodiversity and ecosystem functioning. Trends Ecol. Evol. 2018, 33, 186–197. [Google Scholar] [CrossRef]
- Majdi, N.; Traunspurger, W. Free-living nematodes in the freshwater food web: A review. J. Nematol. 2015, 47, 28. [Google Scholar] [PubMed]
- Tighe, M.; Edwards, M.M.; Cluley, G.; Lisle, L.; Wilson, S.C. Colorimetrically determining total antimony in contaminated waters and screening for antimony speciation. J. Hydrol. 2018, 563, 84–91. [Google Scholar] [CrossRef]
- Kowalenko, C.G.; Babuin, D. Interference Problems with Phosphoantimonylmolybdenum Colorimetric Measurement of Phosphorus in Soil and Plant Materials. Commun. Soil Sci. Plant Anal. 2007, 38, 12. [Google Scholar] [CrossRef]
- Viglierchio, D.R.; Schmitt, R.V. On the methodology of nematode extraction from field samples: Baermann funnel modifications. J. Nematol. 1983, 15, 438. [Google Scholar] [PubMed]
- Zhao, J.; Wang, K. Methods for cleaning turbid nematode suspensions collected from different land-use types and soil types. Soil Ecol. Lett. 2021, 4, 429–434. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; de Goede, R.G.M.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera: An outline for soil ecologists. Nematology 1993, 25, 315. [Google Scholar]
- Wasilewska, L. The role of namatodes in agroecosystems. Zesz. Probl. Postępów Nauk Rol. 1989, 358. [Google Scholar]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Migunova, V.D.; Ackermann, M.; Ruess, L.; Scheu, S. Changes in plant species richness induce functional shifts in soil nematode communities in experimental grassland. PLoS ONE 2018, 6, e24087. [Google Scholar] [CrossRef]
- Thakur, M.P.; Reich, P.B.; Fisichelli, N.A.; Stefanski, A.; Cesarz, S.; Dobies, T.; Rich, R.L.; Hobbie, S.E.; Eisenhauer, N. Nematode community shifts in response to experimental warming and canopy conditions are associated with plant community changes in the temperate-boreal forest ecotone. Oecologia 2014, 175, 713–723. [Google Scholar] [CrossRef]
- Bongers, T. The maturity index: An ecological measure of environmental disturbance based on nematode species composition. Oecologia 1990, 83, 14–19. [Google Scholar] [CrossRef]
- Ferris, H.; Bongers, T.; Goede, R.G.M.d. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Neher, D.A.; Lee, C.C. Nematode communities and microbial biomass in soils with annual and perennial crops. Appl. Soil Ecol. 1994, 1, 17–28. [Google Scholar] [CrossRef]
- Gao, D.; Moreira-Grez, B.; Wang, K.; Zhang, W.; Xiao, S.; Wang, W.; Chen, H.; Zhao, J. Effects of ecosystem disturbance on nematode communities in calcareous and red soils: Comparison of taxonomic methods. Soil Biol. Biochem. 2021, 155, 108162. [Google Scholar] [CrossRef]
- Ferris, H. Form and function: Metabolic footprints of nematodes in the soil food web. Eur. J. Soil Biol. 2010, 46, 97–104. [Google Scholar] [CrossRef]
- Schwarz, B.; Barnes, A.D.; Thakur, M.P.; Brose, U.; Ciobanu, M.; Reich, P.B.; Rich, R.L.; Rosenbaum, B.; Stefanski, A.; Eisenhauer, N. Warming alters energetic structure and function but not resilience of soil food webs. Nat. Clim. Change 2017, 7, 895–900. [Google Scholar] [CrossRef]
- Wan, B.; Liu, T.; Gong, X.; Zhang, Y.; Li, C.; Chen, X.; Hu, F.; Griffiths, B.S.; Liu, M. Energy flux across multitrophic levels drives ecosystem multifunctionality: Evidence from nematode food webs. Soil Biol. Biochem. 2022, 169, 108656. [Google Scholar] [CrossRef]
- Topalović, O.; Geisen, S. Nematodes as suppressors and facilitators of plant performance. New Phytol. 2023, 238, 2305–2312. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Shao, Y.; Xu, G.; Fu, S. Effects of vegetation removal on soil properties and decomposer organisms. Soil Biol. Biochem. 2011, 43, 954–960. [Google Scholar] [CrossRef]
- Bai, X.; Wu, J.; Zhang, B.; Zhao, H.; Tian, F.; Wang, B. Metagenomics reveals functional profiles of soil nitrogen and phosphorus cycling under different amendments in saline-alkali soil. Environ. Res. 2025, 267, 120686. [Google Scholar] [CrossRef]
- Hussain, M.; Bano, S.; Qasim, M.; Gao, J.; Arthurs, S.P.; Wang, L.; Qiu, D.; Mao, R. Association between temperature and reproductive fitness of Diaphorina citri infected with Candidatus Liberibacter asiaticus. Agronomy 2022, 12, 815. [Google Scholar] [CrossRef]
- da Graça, J.V.; Douhan, G.W.; Halbert, S.E.; Keremane, M.L.; Lee, R.F.; Vidalakis, G.; Zhao, H. Huanglongbing: An overview of a complex pathosystem ravaging the world’s citrus. J. Integr. Plant Biol. 2016, 58, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Padhi, E.M.; Maharaj, N.; Lin, S.-Y.; Mishchuk, D.O.; Chin, E.; Godfrey, K.; Foster, E.; Polek, M.; Leveau, J.H.; Slupsky, C.M. Metabolome and microbiome signatures in the roots of citrus affected by huanglongbing. Phytopathology 2019, 109, 2022–2032. [Google Scholar] [CrossRef] [PubMed]
- Reiter, B.; Pfeifer, U.; Schwab, H.; Sessitsch, A. Response of endophytic bacterial communities in potato plants to infection with Erwinia carotovora subsp. atroseptica. Appl. Environ. Microbiol. 2002, 68, 2261–2268. [Google Scholar] [CrossRef]
- Ahmed, A.; Liu, Y.; He, P.; He, P.; Wu, Y.; Munir, S.; He, Y. Bacillus quorum quenching shapes the citrus mycobiome through interkingdom signaling. Sci. Total Environ. 2024, 956, 177074. [Google Scholar] [CrossRef]
- Ma, W.; Pang, Z.; Huang, X.; Xu, J.; Pandey, S.S.; Li, J.; Achor, D.S.; Vasconcelos, F.N.; Hendrich, C.; Huang, Y. Citrus Huanglongbing is a pathogen-triggered immune disease that can be mitigated with antioxidants and gibberellin. Nat. Commun. 2022, 13, 529. [Google Scholar] [CrossRef]
- Mohan, S.; Kiran Kumar, K.; Sutar, V.; Saha, S.; Rowe, J.; Davies, K.G. Plant root-exudates recruit hyperparasitic bacteria of phytonematodes by altered cuticle aging: Implications for biological control strategies. Front. Plant Sci. 2020, 11, 763. [Google Scholar] [CrossRef]
- Li, G.; Liu, T.; Whalen, J.K.; Wei, Z. Nematodes: An overlooked tiny engineer of plant health. Trends Plant Sci. 2024, 29, 52–63. [Google Scholar] [CrossRef]
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Limburg, K.E. Increasing complexity and energy flow in models of food webs. Ecol. Model. 1985, 29, 5–25. [Google Scholar] [CrossRef]
- Weitere, M.; Erken, M.; Majdi, N.; Arndt, H.; Norf, H.; Reinshagen, M.; Traunspurger, W.; Walterscheid, A.; Wey, J.K. The food web perspective on aquatic biofilms. Ecol. Monogr. 2018, 88, 543–559. [Google Scholar] [CrossRef]
| Index Category | Food Wed Carbon Flux | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FTotal | U | Fba-op | Ffu-op | Fhe-op | Fba | Ffu | Fhe | ||||||||||
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | ||
| Soil physico-chemical properties | SWC | 0.150 | 0.641 | −0.138 | 0.668 | 0.001 | 0.998 | −0.179 | 0.577 | −0.167 | 0.605 | 0.107 | 0.742 | −0.146 | 0.652 | −0.197 | 0.540 |
| pH | −0.150 | 0.641 | 0.352 | 0.262 | −0.280 | 0.378 | −0.547 | 0.065 | −0.434 | 0.159 | −0.356 | 0.256 | −0.506 | 0.093 | −0.454 | 0.138 | |
| SOC | 0.244 | 0.445 | −0.284 | 0.371 | −0.242 | 0.449 | −0.067 | 0.836 | −0.017 | 0.958 | −0.125 | 0.699 | −0.009 | 0.978 | −0.020 | 0.950 | |
| AK | −0.561 | 0.058 | 0.580 | <0.05 | −0.340 | 0.279 | −0.632 | <0.05 | −0.577 | 0.050 | −0.424 | 0.170 | −0.631 | <0.05 | −0.589 | <0.05 | |
| AP | 0.418 | 0.176 | −0.669 | <0.05 | 0.326 | 0.302 | 0.493 | 0.103 | 0.608 | <0.05 | 0.656 | <0.05 | 0.462 | 0.131 | 0.599 | <0.05 | |
| NH4+-N | 0.413 | 0.182 | −0.356 | 0.256 | −0.249 | 0.436 | 0.331 | 0.293 | 0.428 | 0.165 | 0.258 | 0.418 | 0.334 | 0.289 | 0.383 | 0.219 | |
| NO3+-N | −0.277 | 0.384 | −0.022 | 0.946 | −0.087 | 0.788 | −0.336 | 0.286 | −0.123 | 0.704 | −0.065 | 0.841 | −0.352 | 0.261 | −0.125 | 0.699 | |
| TN | 0.858 | <0.001 | −0.672 | <0.05 | −0.074 | 0.820 | 0.701 | <0.05 | 0.817 | <0.001 | 0.526 | 0.079 | 0.754 | <0.05 | 0.805 | <0.05 | |
| TC | 0.866 | <0.001 | −0.691 | <0.05 | −0.171 | 0.596 | 0.609 | <0.05 | 0.838 | <0.001 | 0.390 | 0.211 | 0.670 | <0.05 | 0.837 | <0.001 | |
| TP | 0.247 | 0.440 | −0.445 | 0.147 | 0.180 | 0.575 | 0.054 | 0.867 | 0.214 | 0.504 | 0.194 | 0.546 | 0.043 | 0.894 | 0.210 | 0.513 | |
| Ca | −0.205 | 0.522 | 0.135 | 0.676 | 0.017 | 0.959 | −0.517 | 0.085 | −0.455 | 0.137 | −0.216 | 0.500 | −0.503 | 0.096 | −0.466 | 0.127 | |
| Mg | −0.441 | 0.151 | 0.115 | 0.722 | 0.323 | 0.307 | −0.208 | 0.518 | −0.488 | 0.108 | −0.003 | 0.992 | −0.265 | 0.406 | −0.501 | 0.097 | |
| Nematode Ecological indices | H | −0.324 | 0.305 | 0.202 | 0.529 | 0.058 | 0.857 | −0.271 | 0.393 | −0.417 | 0.177 | −0.177 | 0.581 | −0.262 | 0.411 | −0.413 | 0.182 |
| λ | 0.383 | 0.219 | −0.239 | 0.454 | −0.053 | 0.869 | 0.274 | 0.389 | 0.444 | 0.148 | 0.208 | 0.517 | 0.272 | 0.392 | 0.439 | 0.152 | |
| SI | 0.892 | <0.001 | −0.763 | <0.05 | −0.147 | 0.648 | 0.880 | <0.001 | 0.886 | <0.001 | 0.382 | 0.220 | 0.916 | <0.001 | 0.876 | <0.001 | |
| NCR | −0.202 | 0.529 | 0.296 | 0.351 | 0.238 | 0.456 | −0.324 | 0.305 | −0.181 | 0.574 | 0.286 | 0.367 | −0.296 | 0.350 | −0.174 | 0.589 | |
| MHR | −0.358 | 0.253 | 0.023 | 0.944 | 0.349 | 0.266 | −0.322 | 0.307 | −0.339 | 0.281 | −0.073 | 0.822 | −0.383 | 0.219 | −0.337 | 0.284 | |
| MI | 0.717 | <0.05 | −0.345 | 0.273 | −0.272 | 0.393 | 0.616 | <0.05 | 0.701 | <0.05 | 0.270 | 0.395 | 0.668 | <0.05 | 0.696 | <0.05 | |
| J | −0.891 | <0.001 | 0.816 | <0.05 | −0.066 | 0.838 | −0.870 | <0.001 | −0.896 | <0.001 | −0.564 | 0.056 | −0.897 | <0.001 | −0.887 | <0.001 | |
| SR | −0.632 | <0.05 | 0.617 | <0.05 | 0.025 | 0.938 | −0.863 | <0.001 | −0.761 | <0.05 | −0.353 | 0.260 | −0.870 | <0.001 | −0.762 | <0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Li, Z.; Zhao, J. Citrus Greening Disease Infection Reduces the Energy Flow Through Soil Nematode Food Webs. Agronomy 2025, 15, 635. https://doi.org/10.3390/agronomy15030635
Wang M, Li Z, Zhao J. Citrus Greening Disease Infection Reduces the Energy Flow Through Soil Nematode Food Webs. Agronomy. 2025; 15(3):635. https://doi.org/10.3390/agronomy15030635
Chicago/Turabian StyleWang, Mengqiang, Zhilei Li, and Jie Zhao. 2025. "Citrus Greening Disease Infection Reduces the Energy Flow Through Soil Nematode Food Webs" Agronomy 15, no. 3: 635. https://doi.org/10.3390/agronomy15030635
APA StyleWang, M., Li, Z., & Zhao, J. (2025). Citrus Greening Disease Infection Reduces the Energy Flow Through Soil Nematode Food Webs. Agronomy, 15(3), 635. https://doi.org/10.3390/agronomy15030635







