Abstract
Climate change and rising soil salinization pose significant challenges to agriculture and food security, particularly in coastal regions. Halophytes, salt-tolerant plants thriving in saline environments, offer promising solutions, as they show resilience to extreme conditions and provide nutritional and ecological benefits. This study investigates the potential of edible halophytes in the Mediterranean Basin, focusing on Tuscany’s salt marshes. A combination of literature reviews and field surveys identified 24 edible species among 60 recorded halophytes. These plants, mainly from the Amaranthaceae family, ranging from seasonal therophytes to perennial geophytes, display diverse life forms and adaptive strategies for saline habitats. The study highlights their nutritional and economic value, with edible parts such as leaves and shoots rich in essential minerals and antioxidants. Fieldwork and geospatial analyses revealed distinct patterns of species distribution, emphasizing the role of halophytes in maintaining ecosystem resilience and offering sustainable agricultural opportunities in degraded lands. By mapping their distribution and analyzing biodiversity indices, this research underscores the importance of conserving halophyte species as genetic resources and advancing their integration into agricultural systems. The findings provide a basis for developing innovative approaches to enhance food security in saline-affected areas.
1. Introduction
Soil salinization is a major issue of climate change, closely linked to rising temperatures and negatively affecting crop growth, soil fertility, and quality, with significant potential consequences for food security [1]. Salinization induces osmotic stress in plants, reducing water absorption and increasing toxic sodium and chloride levels. Unfortunately, conventional crops, such as maize and beans, are highly sensitive to saline stress, leading to reduced yields and further soil degradation [2].
Climate change is exacerbating ecosystem degradation and is posing unprecedented threats to agriculture and crop productivity [3,4], endangering food security on a global scale [5,6]. Among the many consequences of this warming trend, soil salinization has emerged as a critical issue. Soil salinization is a major consequence of climate change, particularly in coastal areas affected by sea-level rise. The accumulation of soluble salts, primarily sodium chloride, in the soil disrupts soil structure and fertility, as well as its natural balance [7], reducing crop yields and threatening global food security [1].
Halophytes are salt-tolerant plants that naturally thrive in soils with salinity levels comparable to that of seawater (up to 500 mM) [8]. These plants are not only resilient to extreme environmental conditions but also offer significant nutritional potential, as they are rich in vitamins, minerals, and antioxidants [9].
Due to their resilience and quality, as well as their unique ability to complete their life cycles under harsh conditions, halophytes represent a promising solution to enable or expand agricultural production into marginal and degraded lands, where conventional crops fail due to high saline stress [2], with diverse applications in food, biofuels, and medicinal products and a global market value estimated between USD 200 and 600 per hectare [10]. However, despite the growing global interest, only a small number of halophyte species have been incorporated into commercial cultivation systems. Currently, most halophytes used as food are still hand-harvested from wild, self-seeding plants [11].
Currently, more than 6% of the world’s land is affected by salinization, with approximately 30.7 million hectares impacted in the European Union alone, particularly in Mediterranean areas, where it contributes to desertification [12]. In the Mediterranean, sea levels have been rising since 1880, with coastal areas experiencing the increased salinization of groundwater and farmlands [13,14,15]. The IPCC AR6 projects that by 2150, around 19,000 km of Mediterranean coastal plains will be exposed to marine flooding and salinization [15]. These impacts will further degrade farmland, threaten coastal ecosystems, and jeopardize food security in the region.
Italy, with approximately 3.2 million hectares affected by salinization [16], is a hotspot for land degradation [17,18]. Recent studies indicate a high risk of salinization in various coastal areas, both on the mainland and on islands [15,16,17,18,19].
In the context of ongoing climate change, the potential of halophytes for agriculture is particularly significant. To fully harness the benefits of these highly specialized plants, it is essential to identify and protect wild populations in diverse brackish areas, both as a direct resource and as a reservoir of genetic biodiversity.
This study aims to contribute to the knowledge of Italian edible halophytes in the Mediterranean Basin and their potential as new crops for human food production in the context of rising sea levels and increasing soil salinization.
To achieve this, we reviewed the literature on edible halophyte species found in the main brackish areas of the Tuscany Region in central Italy. The information obtained was complemented by field surveys conducted to assess their actual presence and abundance. Finally, using the combined data, we developed QGIS interactive maps as a tool for mapping and monitoring the status and distribution of edible halophyte species across the region.
2. Materials and Methods
2.1. Study Area
The study areas are typical salt marshes located in Tuscany, an administrative region in Central Italy, covering a surface area of approximately 23,000 km2 and characterized by complex geological and topographical features [20]. The central part of the region is mostly hilly (66.5%), while only 8.4% of the territory is flat, and 25.1% is made of mountains. Bordered to the west by the Mediterranean Sea and to the east by the Apennine Mountains, which form an arcuate chain running from northwest to southeast, Tuscany’s coastline stretches for around 442 km, including sandy beaches, cliffs, and rocky shores [21].
The bioclimatic area of the region can be considered subhumid meso-Mediterranean [22], with a climate ranging from arid thermo-Mediterranean to supra-temperate hyper-humid.
The Tuscany Region is known for its rich floristic biodiversity, which reflects the diverse ecological conditions present across its landscapes, including coastal, mountainous, and wetland areas. This rich biodiversity, which includes numerous threatened species, is described in numerous floristic inventories [23,24,25,26,27,28,29,30].
The brackish areas of the Tuscany Region are distributed along almost the entire coastal sector of the region, both continental and insular [31,32]. In the present work, we focused on the seven accessible and more extensive continental brackish areas of the Region. These areas are distributed from the Lame di Fuori in the North (43°41′08.1″ N 10°17′24.3″ E) to Orbetello Lagoon in the South (42°27′58.5″ N 11°12′57.7″ E) and cover a total surface of about 4800 ha (Figure 1).
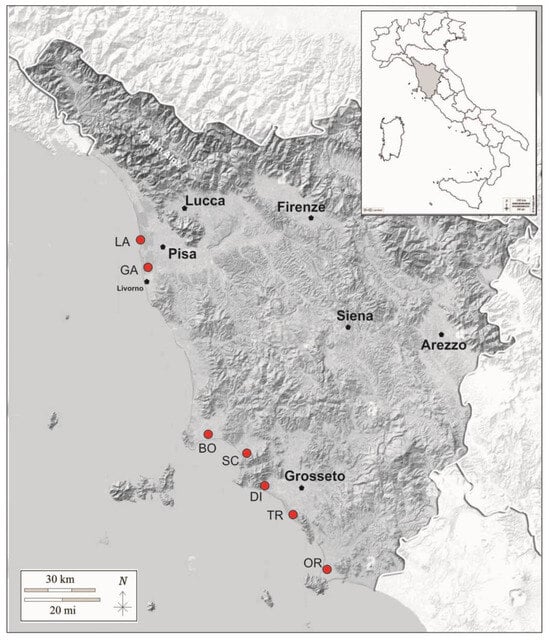
Figure 1.
Red dots, geographical location of the investigated salt marshes in Tuscany (Italy). LA, Lame-Colmate del Bozzone; GA, Galanchio-Cornacchiaia; BO, Orti-Bottagone; SC, Scarlino; OR, Orbetello; DI, Diaccia Botrona; TR, Trappola.
The selected areas provide an important ecosystem service as buffer zones between inland non-saline soils and marine ecosystems, occupying ancient marshy depressions behind coastal dunes. Following land reclamation efforts after the Second World War, these zones were partially drained and modified, and now consist of loamy–clayey soils. In these areas, the salinity levels are influenced by their distance from the sea and the reclamation canals directly connected to it. In some cases, such as in Orbetello, salt marshes are in direct contact with the lagoon, making them susceptible to seawater flooding. In other areas, typically 1–2 km from the coast, salinity is mainly influenced by the intrusion of saline groundwater (https://www.appenninosettentrionale.it/itc/?page_id=2113, accessed on 10 February 2025).
The combination of past human intervention and natural coastal processes continues to shape these unique habitats, which support a blend of terrestrial and aquatic species. Their geomorphology is defined by the seasonal alternation of wet and dry periods, which contributes to the dynamic salinity gradients found in the soils, with seasonal fluctuations driven by variations in freshwater input and evaporation rates. The average salinity values recorded for the studied areas ranges from 4–8 (strongly saline soils) to 8–16 dS/m (very strongly saline soils) [33].
Based on the data from the nearby thermo-pluviometric stations of Bocca d’Arno (for Lame di Fuori and Galanchio), Follonica (for Orti-Bottagone and Scarlino), Alberese (for Diaccia Botrona and Trappola), and Orbetello, the annual average temperature for the period 2015-2024, ranges from 15.93 to 17.03 °C, depending on the station. The analysis of the available data reveals a significant increase in the recorded average temperature values in August over the same decade across the four stations. These values range from 23.05 to 26.48 °C. Total annual rainfall displays a significant gradient, with values ranging from 501.69 mm to 915.27 mm (http://www.sir.toscana.it/consistenza-rete, accessed on 9 February 2025).
2.2. Literature Survey
A comprehensive literature review regarding the flora and vegetation of the selected brackish areas was conducted using a literature search on the Scopus and Web of Science databases, which are widely recognized as the most reliable and reputable sources for systematic reviews of peer-reviewed scientific research [34]. The search was performed using the following keyword combinations: “Saline” AND “Italy”; “Tuscany” OR “Saltwater areas”; “Halophytes” AND “Ecosystem services”; “Sustainable agriculture”, “Saline agriculture”, “Saltmarshes”, “Flora”, “Vegetation survey”.
The literature search spanned from January 1996 to July 2024, focusing on studies addressing the ecological, agricultural, and socio-economic aspects of saline environments, particularly in the coastal regions of Italy. This timeframe was selected to capture recent developments in saline agriculture and ecosystem services, ensuring a comprehensive understanding of the topic. The chosen keywords targeted research exploring the intricate interactions between saline environments and native halophytic flora, with a particular emphasis on edible species and their potential contributions to sustainable agriculture and ecosystem resilience.
2.3. Field Monitoring
To establish and/or update a census of the halophytic species present in the main Tuscan salt marshes we conducted repeated floristic monitoring investigations from April 2023 to September 2024. Additionally, data from WikiplantbaseToscana [35] were used as a proxy for the regional distribution and rarity of the species.
Species were classified according to the methods of Pignatti [36] and Pignatti et al. [37] and aligned with the updated nomenclature based on the work of Bartolucci et al. [38] and Galasso et al. [39]. Taxa were identified by their binomial names and compiled into a floristic list organized by family. Each species was categorized according to its life form and chorotype, as described by Pignatti et al. [37].
Species were identified as halophytic on the base of their occurrence in reference habitats for terrestrial halophytes, as defined in the Habitats Interpretation Manual of the European Union, version EUR 28 (accessed on 22 August 2024). Additionally, occurrence data were cross-referenced with the eHaloph digital database [40], from which salinity tolerance values were extracted and compared with those in current literature. For this study, among the halophytes of the salt marshes of Tuscany, we selected the species with known edible value, both traditionally and in the literature [41,42,43,44,45].
2.4. Data Processing
The distribution of edible halophytes in the brackish areas was assessed by integrating data from previous vegetation studies with the field surveys. This approach allowed us to calculate the average distribution and abundance of each species, providing a comprehensive overview of the incidence of different halophytes in each area. The derived indices are based on the Braun-Blanquet floristic-sociological approach, with the addition of the “r” 156 class (rare) [46]. For the multivariate analysis, the cover values were transformed according to the method proposed by van Der Maarel [47]. A biodiversity analysis of the areas was performed by comparing Shannon’s index (H′), calculated from the distribution and abundance values, when available, and using the following formula:
where pi represents the proportion of the coverage of each species relative to the total coverage in the area. Higher H′ values indicate greater species diversity and a more even distribution of individuals across species.
H′ = −Σpi × ln(pi)
2.5. QGIS Interactive Maps
The distribution of edible halophytes in the selected Tuscany’s salt marshes was mapped using QGIS software v. 3.24.1 (QGIS Association, http://www.qgis.org), utilizing the Web Map Service of the Tuscany Region (https://www.regione.toscana.it/-/geoscopio-wms, accessed on 20 August 2024). The presence of each species is reported on the interactive map by a georeferenced symbol, each corresponding to its occurrence within the perimeter of the brackish area inserted into a map based on the WGS84 (EPSG:4326) standard. Each salt marsh area was assigned a unique identification number, and its surface area was calculated. Species presence, along with photographs and references to literature, were recorded in attribute tables. These tables include the toponym related to an identification number, ranging from 1 to 7, ordered geographically from the northernmost to the southernmost areas along the coast.
2.6. Statistical Analysis
Data of species abundance and H index were analyzed by two-way ANOVA, with plant species and the area as main factors and species abundance as a dependent variable. Means were separated by Tukey’s HSD post hoc tests (p ≤ 0.05). Differences in halophyte species diversity among Tuscany’s salt marshes were processed by the Kruskal–Wallis test. Medians were separated by Dunn–Bonferroni pairwise comparisons. Statistical analyses were performed using PSPP software v. 2.0.1 (Free Software Foundation, Inc., GNU Project, Boston, MA, USA).
3. Results and Discussion
3.1. Literature Survey
A comprehensive literature review provides a solid basis for future research on the sustainable use of halophytes in degraded or high-salinity regions, especially considering the challenges posed by climate change and sea-level rise. This research is critical for understanding the full potential of these species, both as sustainable food sources and in their role in enhancing ecosystem resilience, particularly in areas facing increasing salinization due to climate change.
The bibliographic research, spanning from January 1996 to July 2024 and consulting over 400 articles, focused on the interactions between saline environments and native halophytic flora. Special emphasis was placed on edible species and the ecosystem services they provide, including biodiversity conservation, carbon sequestration, and coastal protection.
The reviewed studies highlighted key insights into the potential of halophytes as alternative food sources and examined various aspects of brackish zones. These aspects include their surface area and biodiversity, with particular focus on the role of halophytic species in maintaining ecosystem stability and resilience. The ecosystem services offered by these wetlands include coastal protection, carbon sequestration, nutrient cycling, and water purification. Salt marshes have been identified as critical natural buffers against storm surges and coastal erosion, an essential ecosystem function that links their ecological functions to broader social and economic benefits. [48,49].
From the consulted literature, 17 studies focused specifically on the flora and vegetation of the brackish areas along the Tuscan continental coast [30,31,32,43,50,51,52,53,54,55,56,57,58,59,60,61,62]. These studies describe the areas as hosting a specialized halophytic flora that forms intricate mosaics of low meadows. These meadows are dominated by halophytic and salt-tolerant species, which are well adapted to withstand periodic flooding and variable saline conditions [32,51].
The vegetation within these ecosystems is predominantly composed of herbaceous angiosperms, with a high tolerance to salinity. The distribution of the species follows environmental gradients such as salinity, oxygen availability, and pH, forming clearly delineated zones [63]. This zonation highlights the adaptive strategies employed by the flora in these challenging habitats and underlines the importance of these ecosystems in supporting biodiversity and providing valuable ecosystem services.
3.2. Field Surveys and Data Processing
Field surveys in the areas investigated, along with the analysis of the mentioned literature, have confirmed the presence of 60 halophytes. This allowed us to draw up a floristic list, where each species is categorized within its family and is accompanied by its biological form, chorotype, and conservation status, if available (Table S1—Supplementary Material).
These taxa are distributed across 13 families and 42 genera. The most representative genera in terms of the number of species is Salicornia, with four of the eight taxa (including species and subspecies) accepted for the Italian flora.
Figure 2 shows a clear predominance of Poaceae (17 species) and Amaranthaceae (14 species). Together, these two families account for 52% of the total species diversity. These families dominate saline environments due to their well-developed tolerance mechanisms, including osmotic regulation, salt excretion, and efficient water-use strategies. Their ability to thrive under high salinity and seasonal water fluctuations highlights their adaptive advantage and ecological role in maintaining biodiversity and ecosystem functionality [64,65,66].
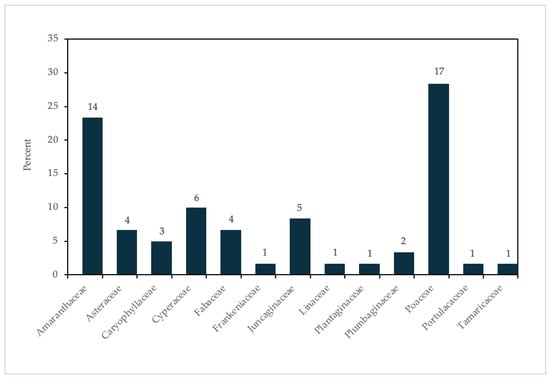
Figure 2.
Distribution of halophyte species across families listed in the studied areas. Numbers above bars indicate the number of species.
Families with moderate representation include Fabaceae (six species), Linaceae (five species), Asteraceae (four species), and Frankeniaceae (four species). Fabaceae, for instance, benefits from symbiosis with nitrogen-fixing bacteria, enabling survival in nutrient-poor, saline soils [67]. Secondary adaptations, such as reduced transpiration surfaces and specialized root structures, have also been observed in Asteraceae and Linaceae [68].
In contrast, minor families such as Juncaginaceae, Plantaginaceae, Plumbaginaceae, Portulacaceae, and Tamaricaceae are represented by only 1–2 species each. Their limited representation likely reflects their specialization for narrow ecological niches or restricted distributions within saline environments [69]. For example, species in Plumbaginaceae often thrive in coastal salt marshes with extreme salinity.
This distribution pattern underscores the ecological significance of Poaceae and Amaranthaceae as key contributors to the resilience and functionality of saline ecosystems. In the context of climate change, where increasing salinity, drought, and sea-level rise pose significant threats to these habitats, the halophytic species provides critical insights into plant adaptations and informs conservation strategies. Furthermore, these species may offer promising models for developing salt-tolerant crops and promoting sustainable agriculture in saline-affected regions [70].
The analysis of the biological forms highlights the predominance of scapose therophytes, with almost 30%, followed by rhizomatous geophytes and caespitose hemicryptophytes, with 20% and 10%, respectively. Among chamaephytes, the suffruticose and succulent forms show the highest presence, together accounting for 10% (Figure 3). These data reflect diverse strategies for surviving in these saline and variable environments. The dominance of scapose therophytes indicates a strategy to quickly complete their life cycle in favorable conditions, avoiding the prolonged stress of salinity or drought. Meanwhile, the presence of rhizomatous geophytes indicates a good adaptation of the rhizomes to saline soil and a preference for vegetative spread, which is an important strategy for colonizing unstable or stressful environments like salt marshes.
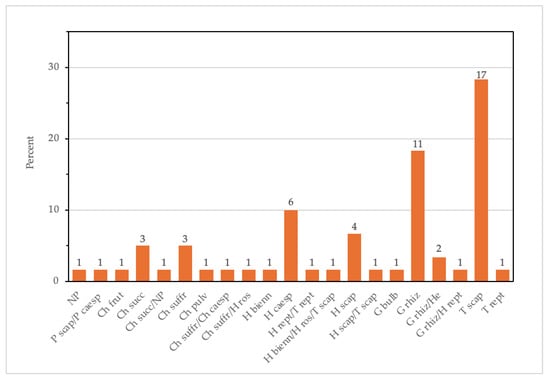
Figure 3.
Bar plot graph of the life forms of halophyte species in the studied areas. The numbers above the bars represent the number of species. T, therophytes; H, hemicryptophytes; G, geophytes; Ch, chamaephytes; NP, nano-phanerophytes; P, phanerophytes.
The distribution of the different biological forms highlights different evolutionary strategies that enable halophytic plants to thrive in challenging and variable environments, providing insights into the resilience and functionality of these ecosystems. The prevalence of therophytes and hemicryptophytes highlights the importance of fugitive and intermediate strategies for survival in Mediterranean saline habitats [71,72]. Therophytes avoid unfavorable periods by completing their life cycles during the most suitable seasons, while hemicryptophytes and geophytes utilize morphological adaptations (basal buds and underground organs such as rhizomes) to endure seasonal fluctuations [72]. The limited presence of chamaephytes indicates their specialization, with specific physiological adaptations, in ecological niches characterized by extreme aridity and persistent salinity.
The chorological spectrum, derived by grouping the various chorotypes according to the methods of Pignatti [36], indicates that most species exhibit a broad and Euro-Mediterranean distribution (Figure 4). This finding suggests that the halophytes of Tuscany display a high degree of ecological tolerance and adaptability to the diverse environmental characteristics of the Mediterranean region. The Euro-Mediterranean chorotype reflects their capacity to thrive across a wide range of Mediterranean habitats, ranging from saline coastal marshes to inland dry steppe-like environments, often defined by heterogeneous climatic conditions, varying soil salinity, and diverse ecological contexts. These habitats are typically characterized by hot, dry summers and mild, rainy winters—conditions that have favored the evolution of specific mechanisms, such as osmotic adjustments and salt-excreting tissues, to cope with high salinity and seasonal water fluctuations.
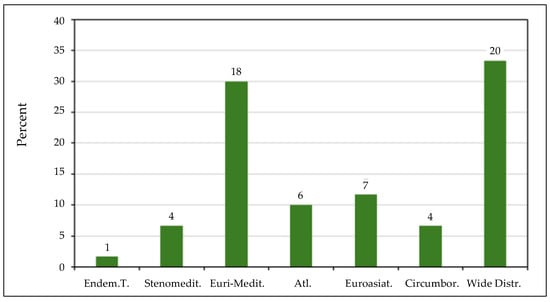
Figure 4.
Bar plot of the main chorotypes of the halophyte species listed in the studied salt marshes. The numbers above the bars represent the number of species. Endem.T., Endemic of Tuscany; Stenomedit., Stenomediterranean; Euri-Medit., Euro-Mediterranean; Atl., Atlantic; Euroasiat, Euroasiatic; Circumbor., Circumboreal; Wide distr., Wide distribution.
Moreover, this distribution pattern may indicate an evolutionary history shaped by effective dispersal mechanisms and the ability to colonize analogous environments throughout the Mediterranean Basin. However, despite their adaptability, increasing climatic variability and anthropogenic pressures may pose significant challenges to the resilience and long-term persistence of these species. In the context of climate change, halophytes could play a key role as bioindicators of ecosystem resilience and serve as models for understanding plant responses to increasing salinity, prolonged drought, and temperature variability. Under climatic changing conditions, the ecological versatility and competitiveness of halophytes make these species particularly relevant for the conservation and management of Mediterranean ecosystems.
Table 1 lists the halophytic species, with documented ethnobotanical and edible uses. These species were assessed for their maximum salinity tolerance, specific plant parts used for edible purposes, and presence/absence in the surveyed sites, providing a comprehensive understanding of their regional distribution and ecological roles. In addition, their biological forms and chorotypes were analyzed to explore potential correlations with their distribution and ecological adaptability.
Overall, we identified 24 edible halophytic species, representing approximately 38% of the total halophytes recorded in the study areas. Most of these species belong to the Amaranthaceae family (12 species), while other families are represented by only 1–2 species each.
In this case, the most represented genus is Salicornia, with a total of four species, two of which are present in all the analyzed areas (Table 1). Salicornia is a genus with a rich history of both edible and non-edible applications. The food use of the species of this genus is not entirely new, as historical accounts document its consumption as a salt substitute. Today, Salicornia is celebrated as a gourmet ingredient: its tender aerial parts are consumed fresh in salads, transformed into pickles, or processed into beverages and other culinary products. This renewed interest in its edible potential has only gained momentum in recent years, marking a significant shift in its perception and usage.
The analysis of life forms and chorotypes reveals a trend that aligns with the distribution observed in the complete checklist of halophytes recorded in the study areas. Therophytes, such as Atriplex spp., Suaeda maritima, Salicornia perennans, and S. procumbens, dominate the list (Figure 5). Their prevalence highlights adaptive strategies to short, seasonal growth cycles in saline habitats, a characteristic consistent with Grime’s strategy theory [73]. In contrast, chamaephytes and hemicryptophytes, including Limonium narbonense, Salicornia fruticosa, S. perennis, and Plantago coronopus, represent perennial life forms that persist in extreme environments through dormant phases or reduced metabolic activity. Additionally, geophytes, such as Bolboschoenus maritimus, utilize underground structures for survival, showcasing resilience against salinity and flooding [2].
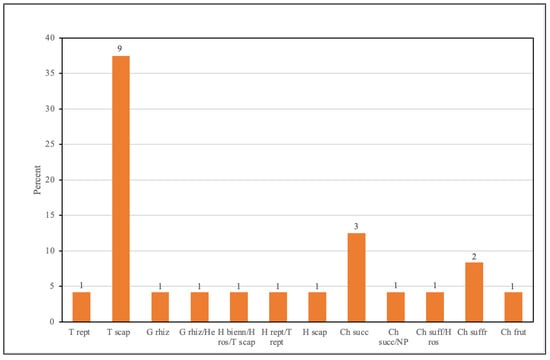
Figure 5.
Life-forms of edible halophyte species in Tuscany salt marshes. The numbers above the bars represent the number of species. T, therophytes; H, hemicryptophytes; G, geophytes; Ch, chamaephytes; NP, nano-phanerophytes; P, phanerophytes.
In terms of chorological distribution, most edible species exhibit a Euri-Mediterranean affinity (Figure 6), reflecting a strong Mediterranean influence combined with broader temperate tolerance [74]. Species such as Salicornia perennis and Halimione portulacoides, classified as Mediterranean–Atlantic or Circumboreal, display a wider ecological amplitude, thriving in both coastal and inland saline environments [75]. The presence of cosmopolitan species, such as Suaeda maritima and Portulaca oleracea, underscores their adaptability to saline and disturbed habitats on a global scale, supporting their resilience and ability to colonize diverse environments [75].
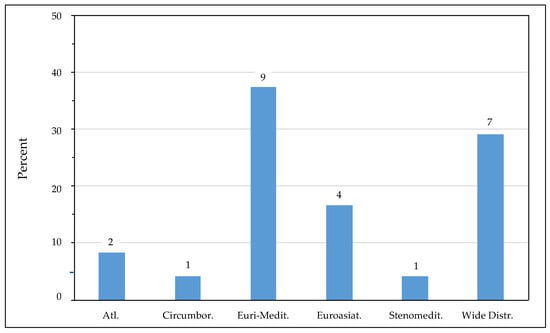
Figure 6.
Main chorotypes of the edible halophyte species listed in Tuscany salt marshes. The numbers above the bars represent the number of species. Atl., Atlantic; Circumbor., Circumboreal; Euri-medit., Euro-Mediterranean; Euroasiat, Euroasiatic; Steno-medit., Stenomediterranean; Wide Distr., Wide Distribution.

Table 1.
Edible halophytes of Tuscany salt marshes (La, Lame-Colmate del Bozzone; Ga, Galanchio-Cornacchiaia; Bo, Orti-Bottagone; Sc, Scarlino; Or, Orbetello; Di, Diaccia Botrona; Tr, Trappola) with their conservation status (Cs), life form, chorotype, and edible parts (* newly found species).
Table 1.
Edible halophytes of Tuscany salt marshes (La, Lame-Colmate del Bozzone; Ga, Galanchio-Cornacchiaia; Bo, Orti-Bottagone; Sc, Scarlino; Or, Orbetello; Di, Diaccia Botrona; Tr, Trappola) with their conservation status (Cs), life form, chorotype, and edible parts (* newly found species).
| Family/Plant Name | Cs | Life Form | Chorotype | Presence/Absence | Edible Parts | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| La | Ga | Bo | Sc | Or | Di | Tr | |||||
| Amaranthaceae | |||||||||||
| Arthrocaulon macrostachyum (Moric.) Piirainen and G.Kadereit | Ch succ | Medit.-Macaron. | * | * | * | * | Shoots [76] | ||||
| Atriplex littoralis L. | T scap | Europ. | * | * | * | Leaves, whole plant [77] | |||||
| Atriplex patula L. subsp. patula | T scap | Subcosmop. | * | * | * | Leaves [78] | |||||
| Atriplex prostrata Boucher ex DC. | T scap | Paleotemp. | * | * | * | * | * | * | * | Leaves, whole plant [79] | |
| Beta vulgaris L. subsp. maritima (L.) Arcang.* | LC | H scap | Euri-Medit. | * | * | Aerial parts [80] | |||||
| Halimione portulacoides (L.) Aellen | Ch frut/P rept | Circumbor. | * | * | * | * | * | * | Leaves [81] | ||
| Halocnemum cruciatum (Forssk.) Tod | Ch succ/NP | S-Medit | * | Shoots [82] | |||||||
| Salicornia fruticosa (L.) L. | Ch succ | Euri-Medit./Afric. | * | * | * | Shoots [83] | |||||
| Salicornia perennans Willd. subsp. perennans | T scap | Euri-Medit. | * | * | * | * | * | * | * | Shoots [84] | |
| Salicornia perennis Mill. | LC | Ch succ | Medit.-At. (Euri-) | * | * | * | * | * | * | * | Shoots [85] |
| Salicornia procumbens Sm. Subsp. procumbens | T scap | Steno-Medit | * | * | Shoots [86] | ||||||
| Soda inermis Fourr. | T scap | Paleotemp. | * | * | * | * | * | Leaves [87] | |||
| Suaeda maritima (L.) Dumort. | T scap | Cosmop. | * | * | * | * | * | * | * | Leaves [88] | |
| Suaeda vera J.F. Gmel. | NP | Cosmop. | * | * | Leaves [87] | ||||||
| Asteraceae | |||||||||||
| Artemisia caerulescens L. | Ch suffr | Euri-Medit. | * | * | * | * | * | * | Leaves, flowers [89] | ||
| Limbarda crithmoides (L.) Dumort. subsp. longifolia (Arcang.) Greuter | Ch suffr | Medit.-Atl. (Steno-) | * | * | * | * | * | * | * | Leaves [90] | |
| Caryophyllaceae | |||||||||||
| Spergularia marina (L.) Besser | LC | T rept | Subcosmop. | * | * | * | * | * | * | * | Leaves, aereal part [91] |
| Cyperaceae | |||||||||||
| Bolboschoenus maritimus (L.) Palla | G rhiz | Cosmop. | * | * | * | * | Seeds, root [92] | ||||
| Fabaceae | |||||||||||
| Trifolium resupinatum L. | H rept/T rept | Paleotemp. | * | * | Leaves, flowers [93] | ||||||
| Trifolium squamosum L. | T scap | Euri-Medit. | * | * | Leaves, flowers [93] | ||||||
| Plantago coronopus L. | H bienn/H ros/T scap | Euri-Medit. | * | * | * | * | * | * | * | Leaves [94] | |
| Plumbaginaceae | |||||||||||
| Limonium narbonese Mill. | Ch suff/H ros | Euri-Medit. | * | * | * | * | * | * | * | Leaves [95] | |
| Poaceae | |||||||||||
| Phragmites australis L. | LC | G rhiz/He | Subcosmop. | * | * | * | * | * | * | Leaves, young shoots [96] | |
| Portulacaceae | |||||||||||
| Portulaca oleracea L. | LC | T scap | Subcosmop. | * | * | Leaves, stems, flowers, seeds [97] | |||||
The identified species exhibit significant nutritional and gastronomic potential. Leaves and young shoots are the most consumed parts, particularly in species such as Atriplex spp., Salicornia spp., Suaeda maritima, Limonium narbonense, and Plantago coronopus. Traditionally consumed raw or cooked, these parts are rich in essential minerals and antioxidants [98]. The aerial parts of Beta vulgaris subsp. maritima and Portulaca oleracea are notable for their high nutritional value, including the presence of omega-3 fatty acids and vitamins [98]. Less commonly, roots and seeds of Bolboschoenus maritimus have been utilized, emphasizing their ethnobotanical importance [75].
Annual therophytes, such as Atriplex spp. and Salicornia spp., often provide young shoots and leaves that can be harvested quickly, coinciding with their seasonal growth cycles. In contrast, perennial chamaephytes and hemicryptophytes, including Limonium narbonense and Plantago coronopus, offer consistent yields of edible parts such as leaves, even in harsh conditions. This reflects the functional role of life forms in determining resource availability and sustainability for local populations.
The salt marshes and coastal saline areas surveyed in this study represent valuable reservoirs of edible plant resources. These ecosystems not only contribute to local biodiversity but also hold potential for sustainable exploitation of halophyte species as food sources. As climate change increases the salinization of agricultural lands, understanding and promoting the cultivation of edible halophytes may provide innovative solutions for food security in saline environments.
The two-way ANOVA, performed on abundance data (Table S2—Supplementary Material), indicated a significant effect of the area (F = 5.10, df = 4, p < 0.001) and of species (F = 13.41, df = 21, p < 0.001) on the species abundance, with an interaction effect (F = 5.62, df = 84, p < 0.001) (Figure 7). Halimione portulacoides was the most abundant species (8%), standing out significantly from all the others. Species such as Salicornia perennis, Phragmites australis, Salicornia procumbens, Limonium narbonense, and Arthrocaulon machrostachium exhibited intermediate abundances ranging between 5 and 3%, while species such as Atriplex littoralis, Portulaca oleracea, and Trifolium squamosum were detected sporadically and showed significantly lower abundances (Table S2—Supplementary Material). These results highlight a clear dominance of H. portulacoides in the Tuscany salt marshes, likely due to its high tolerance to salinity and its ability to occupy specific ecological niches in highly dynamic environments. Halimione portulacoides, an obligate halophyte and perennial shrub, is highly valued for its multifaceted uses, ranging from human nutrition to traditional medicine, where it has been employed as a natural remedy for diabetes [99]. Its young shoots are edible and appreciated for their salty flavor, making them a sought-after ingredient in various culinary preparations [81]. Beyond its gastronomic appeal, the aerial parts of this species are rich in antioxidant and bioactive compounds, offering promising applications in medical and pharmaceutical research [43]. Ecologically, H. portulacoides thrives in saline environments, forming dense monospecific stands or coexisting with Salicornia perennans, particularly in the drainage systems of the area under study. The significant presence of this species also suggests its crucial role in maintaining the ecological stability of these ecosystems, providing habitats for other species and contributing to nutrient cycling.
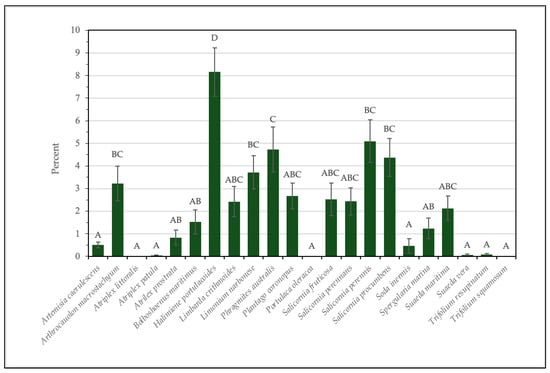
Figure 7.
Mean abundance of different edible halophyte species in Tuscany salt marshes. Bars represent standard errors. Different letters indicate significant difference among values (Dunn–Bonferroni pairwise comparison).
The significant interaction between area and species underscores how species distribution is strongly influenced by the specific characteristics of individual habitats, such as salinity, soil texture, and hydrological regime. This demonstrates the importance of adopting tailored management strategies for each area to preserve biodiversity and promote the resilience of these ecosystems, which are highly sensitive to climate change.
The distribution of abundances, with a few dominant species and many species of lower abundance, is typical of ecosystems characterized by extreme environmental conditions. Despite their lower abundances, species such as Limonium narbonense and Soda inermis could play key ecological roles, for instance, as indicators of ecosystem health. In this context, further exploration of the ecological dynamics of these species could provide valuable insights for the conservation and management of salt marsh areas.
The statistical analyses of the diversity data, based on the Shannon index (H), show a significant difference among the five areas surveyed (χ2 = 39.67; df = 4; p < 0.001). The areas with the highest diversity indexes of plant species were the Lame-Colmate del Bozzone and the Scarlino areas, while a lower diversity of halophyte species was recorded in the Galanchio-Cornacchiaia, Orti-Bottagone, Scarlino, Orbetello, Diaccia Botrona, and Trappola areas (Figure 8).
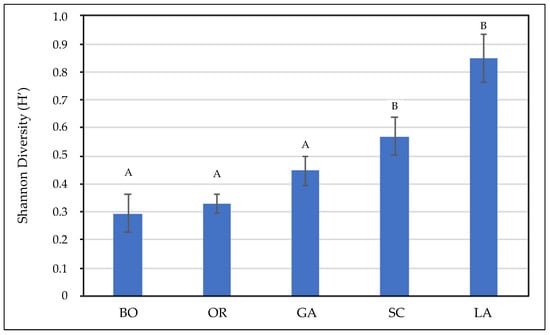
Figure 8.
Diversity indexes for the edible halophytes species in Tuscany salt marshes (La, Lame-Colmate del Bozzone; Ga, Galanchio-Cornacchiaia; Bo, Orti-Bottagone; Sc, Scarlino; Or, Orbetello; Di, Diaccia Botrona; Tr, Trappola). Bars represent standard errors. Different letters indicate significant difference among values (Dunn–Bonferroni pairwise comparison).
In this study, the presence and distribution of edible halophytes in the salt marshes were reported in digital maps generated by QGIS (Supplementary Material). Symbols, ranging from dark red to light blue, provide a clear visual representation of each species’ salt tolerance, based on reports in the literature [40,43]. Specifically, symbols in warmer colors (from dark red to yellow) indicate species with a higher tolerance to elevated salinity, whereas symbols in cooler colors (from green to light blue) represent species that tolerate lower soil salinity levels.
Each species is linked to an attribute table that includes the toponym of the brackish area where it was found, its corresponding abundance index, relevant references, a photo, and a link to a data sheet. These data sheets contain detailed information about the properties and uses of the species, survey data, and photographs collected during fieldwork (see, for example, Figure 9).
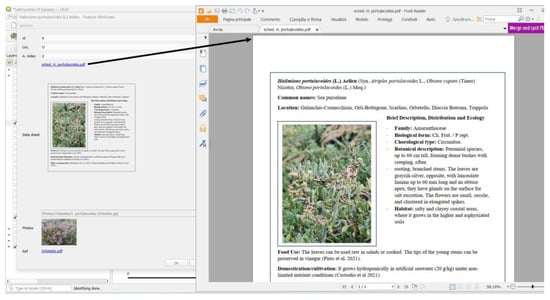
Figure 9.
Data sheet of the species Halimione portulacoides (L.) Aellen, containing the identification number of the area, the identification letter of the area, the abundance index, the link to all the collected information about the species, the photo collected in the area.
Using the “identify feature” function, a window displaying photos, data sheets, references, and other attachments is activated (see, for example, Figure 10).
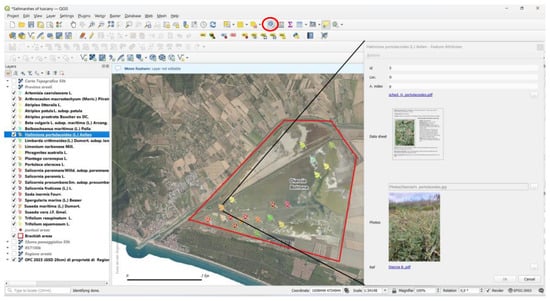
Figure 10.
Graphical user interface of QGIS, highlighting the “identify feature” tool in the top-right corner with a red circle. Activating this tool allows users to click on features and open the attribute table, which contains links to all attachments.
This digital database can serve as a versatile and powerful tool for monitoring the status and distribution of halophyte species across the territory. Furthermore, being accessible on mobile devices allows for the direct upload of new spatial and photographic data during field surveys.
4. Conclusions
The agricultural potential of halophytes remains largely untapped, with most species still being hand-harvested from the wild. This study provides crucial insights into their distribution, biodiversity, and potential as genetic resources, offering a foundation for expanding their use in sustainable agriculture. By examining Tuscany’s salt marshes, we identified 24 edible halophyte species, highlighting their resilience, nutritional value, and potential for agricultural integration. Geospatial analysis underscored their ecological roles and contributions to maintaining biodiversity in degraded lands.
Italy alone has 3.2 million hectares of salinized land. Given the acceleration of soil salinization in Mediterranean coastal areas due to climate change, halophytes represent a viable solution for restoring productivity in marginal areas and mitigating the adverse effects of salinization. However, to fully harness the benefits of halophytes, future efforts should focus on their conservation, cultivation, and commercialization. Scaling up agricultural practices, developing market opportunities, and establishing supportive policy will be key to unlocking their potential. By leveraging their unique properties and environmental benefits, halophytes may offer a promising pathway toward sustainable agriculture, enhanced food security, and resilient ecosystems in the Mediterranean Basin and beyond.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15030634/s1. Table S1, halophytes recorded in the studied areas; Table S2, mean abundances of the edible halophytic species; GIS-processed material for the interactive maps, complete with descriptive sheets for the various species.
Author Contributions
Conceptualization, T.L. and I.V.; methodology, T.L., A.B. and I.V.; software, T.L. and S.B.; validation, T.L., S.B. and I.V.; formal analysis, T.L., S.B. and I.V.; investigation, T.L., A.B. and I.V.; resources, T.L., A.B. and I.V.; data curation, T.L., S.B. and I.V.; writing—original draft preparation, T.L., S.B. and I.V.; writing—review and editing, T.L., S.B. and I.V.; visualization, T.L., S.B., A.B. and I.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Pisa, PRA 2020/2021 Project “HALOphytes grown in saline Water for the production of Innovative ready-to eat salad–HALOWIN” (code PRA_2020_43).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We acknowledge the use of ChatGTP (https://chatgpt.com/) to identify improvements in the writing style.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zaman, M.; Shahid, S.A.; Heng, L.; Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Byerlee, D. A Window of Opportunities for Poor Farmers: Investing for Long-Term Food Supply. Dev. Outreach 2008, 10, 9–12. [Google Scholar] [CrossRef]
- Shrestha, S. Effects of Climate Change in Agricultural Insect Pest. Acta Sci. Agric. 2019, 3, 74–80. [Google Scholar] [CrossRef]
- Byerlee, D. Policies to Promote Cereal Intensification in Ethiopia: A Review of Evidence and Experience. In IFPRI Discussion Paper. 707; International Food Policy Research Institute (IFPRI): Washington, DC, USA, 2007. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: Synthesis Report; Working Group Contribution to the Fourth Assessment Report of Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2007. [Google Scholar]
- Stavi, I. Food Security among Dryland Pastoralists and Agropastoralists: The Climate, Land-Use Change, and Population Dynamics Nexus. Anthr. Rev. 2022, 9, 299–323. [Google Scholar] [CrossRef]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt Tolerance and Crop Potential of Halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Duarte, B. Nutritional Valuation and Food Safety of Endemic Mediterranean Halophytes Species Cultivated in Abandoned Salt Pans under a Natural Irrigation Scheme. Estuar. Coast. Shelf Sci. 2022, 265, 107733. [Google Scholar] [CrossRef]
- Negacz, K.; Vellinga, P. The Emergence of a Governance Landscape for Saline Agriculture. In Halt Soil Salinization, Boost Soil Productivity. In Proceedings of the Global Symposium on Salt-Affected Soils, Rome, Italy, 20 October 2022; pp. 284–285. [Google Scholar]
- Corrêa, R.C.G. Halophytes for Future Horticulture: The Case of Small-Scale Farming in the Mediterranean Basin. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Springer Nature: Berlin, Germany, 2021; pp. 2367–2393. [Google Scholar]
- Anzidei, M. Coastal Structure, Sea-Level Changes and Vertical Motion of the Land in the Mediterranean. Geol. Soc. Lond. Spec. Publ. 2014, 388, 453–479. [Google Scholar] [CrossRef]
- Meli, M.; Camargo, C.M.; Olivieri, M.; Slangen, A.B.; Romagnoli, C. Sea-Level Trend Variability in the Mediterranean during the 1993–2019 Period. Front. Mar. Sci. 2023, 10, 1150488. [Google Scholar] [CrossRef]
- Vecchio, A.; Anzidei, M.; Serpelloni, E. Sea Level Rise Projections up to 2150 in the Northern Mediterranean Coasts. Environ. Res. Lett. 2024, 19, 014050. [Google Scholar] [CrossRef]
- Canfora, L.; Salvati, L.; Benedetti, A.; Dazzi, C.; Lo Papa, G. Saline soils in Italy: Distribution, ecological processes and socioeconomic issues. Riv. Econ. Agrar. 2017, 72, 63–77. [Google Scholar]
- Salvati, L. Towards a Process-Based Evaluation of Land Vulnerability to Soil Degradation in Italy. Ecol. Indic. 2011, 11, 1216–1227. [Google Scholar] [CrossRef]
- Costantini, E.A.; Dazzi, C. The Soils of Italy; Springer: Berlin, Germany, 2013; ISBN 94-007-5642-9. [Google Scholar]
- Dazzi, C. Saline Waters and Soil Quality [Italy]. Ital. J. Agron. 2006, 1, 467–474. [Google Scholar] [CrossRef]
- Costantini, E.; Urbano, F.; Aramini, G.; Barbetti, R.; Bellino, F.; Bocci, M.; Bonati, G.; Fais, A.; L’Abate, G.; Loj, G. Rationale and Methods for Compiling an Atlas of Desertification in Italy. Land Degrad. Dev. 2009, 20, 261–276. [Google Scholar] [CrossRef]
- Raspini, F.; Bianchini, S.; Ciampalini, A.; Del Soldato, M.; Montalti, R.; Solari, L.; Tofani, V.; Casagli, N. Persistent Scatterers Continuous Streaming for Landslide Monitoring and Mapping: The Case of the Tuscany Region (Italy). Landslides 2019, 16, 2033–2044. [Google Scholar] [CrossRef]
- Stragapede, F. LE COSTE: Caratteristiche, tendenze evolutive, erosione e interventi di difesa. In Monografie di geologia ambientale; Edizione SIGEA: Milano, Italy, 2023. [Google Scholar]
- Pesaresi, S.; Biondi, E.; Casavecchia, S. Bioclimates of Italy. J. Maps 2017, 13, 955–960. [Google Scholar] [CrossRef]
- Sposimo, P.; Castelli, C. La Biodiversità in Toscana. Specifiee Habitat in Pericolo; Archivio Del Repertorio Naturalistico Toscano (RENATO): Firenze, Italy, 2005. [Google Scholar]
- Selvi, F. Diversity, Geographic Variation and Conservationof the Serpentine Flora of Tuscany (Italy). Biodivers. Conserv. 2007, 16, 1423–1439. [Google Scholar] [CrossRef]
- Foggi, B.; Viciani, D.; Baldini, R.M.; Carta, A.; Guidi, T. Conservation Assessment of the Endemic Plants of the Tuscan Archipelago, Italy. Oryx 2015, 49, 118–126. [Google Scholar] [CrossRef]
- Bedini, G.; Garbari, F.; Peruzzi, L. Chrobase. It–Chromosome Numbers for the Italian Flora. 2016. Available online: https://italianbotanist.pensoft.net/article/8818/ (accessed on 1 January 2025).
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E. An Updated Checklist of the Vascular Flora Native to Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Ardenghi, N.; Banfi, E.; Celesti-Grapow, L.; Albano, A.; Alessandrini, A.; Bacchetta, G.; Ballelli, S. An Updated Checklist of the Vascular Flora Alien to Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2018, 152, 556–592. [Google Scholar] [CrossRef]
- Orsenigo, S.; Fenu, G.; Gargano, D.; Montagnani, C.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Carta, A.; Castello, M. Red List of Threatened Vascular Plants in Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 310–335. [Google Scholar] [CrossRef]
- Fiaschi, T.; Bonari, G.; Frignani, F.; Gizzi, G.; Landi, M.; Magrini, S.; Quilghini, G.; Pafumi, E.; Scoppola, A.; Angiolini, C. Vascular Flora of the Isthmus of Feniglia (Southern Tuscany, Italy). Ital. Bot. 2024, 17, 77–101. [Google Scholar] [CrossRef]
- Bertacchi, A.; Lombardi, T.; Saggese, A.; Lazzeri, V. The Vegetation of a Relict Salt Marsh Area in the Pisan Coast in the Context of Brackish Wetlands of Tuscany. Plant Sociol. 2021, 58, 41–53. [Google Scholar] [CrossRef]
- Gennai, M.; Angiolini, C.; Bertacchi, A.; Gabellini, A.; Sarmati, S.; Viciani, D.; Foggi, B. Studying Local Species Assemblages of Salt-Affected Vegetation for Monitoring Natura 2000 Habitats. Plant Sociol. 2022, 59, 1–10. [Google Scholar] [CrossRef]
- Ungaro, F.; Calzolari, C.; Fantappiè, M.; Napoli, R.; Barbetti, R.; Tarocco, P.; Staffilani, F.; Puddu, R.; Fanni, S.; Ragazzi, F. National Mapping and Reporting of Salt-Affected Soils of Italy; Food and Agriculture Organization: FAO, Rome, 2024. [Google Scholar] [CrossRef]
- Singh, A. A Review of Wastewater Irrigation: Environmental Implications. Resour. Conserv. Recycl. 2021, 168, 105454. [Google Scholar]
- Bedini, G.; Peruzzi, L. Wikiplantbase# Toscana-Verso Un Catalogo Collaborativo, Online e Gratuito Delle Piante Vascolari Di Toscana. In Riunione scientifica del Gruppo per la Floristica, Società Botanica Italiana, 18-19/10/2013, Roma; ITA: Rome, Italy, 2013. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Bologna, Italy, 1982; Volume 13, ISBN 88-506-2449-2. [Google Scholar]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia; Edagricole: Milano, Italy, 2017; Volume 2019, pp. 1–4. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D. A Second Update to the Checklist of the Vascular Flora Native to Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Alessandrini, A.; Ardenghi, N.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D. A Second Update to the Checklist of the Vascular Flora Alien to Italy. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2024, 158, 297–340. [Google Scholar] [CrossRef]
- Santos, J.; Al-Azzawi, M.; Aronson, J.; Flowers, T.J. eHALOPH a Database of Salt-Tolerant Plants: Helping Put Halophytes to Work. Plant Cell Physiol. 2016, 57, e10. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M. Ethnobotany, Nutritional Traits, and Healthy Properties of Some Halophytes Used as Greens in the Mediterranean Basin. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Springer Nature: Berlin/Heidelberg, Germany, 2020; pp. 1–19. [Google Scholar]
- Ortuño, J.A.; Verde, A.; Fajardo, J.; Rivera, D.; Obón, C.; Alcaraz, F. Halophytes in Arts and Crafts: Ethnobotany of Glassmaking. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Springer: Berlin/Heidelberg, Germany, 2021; pp. 2675–2706. [Google Scholar]
- Lombardi, T.; Ventura, I.; Bertacchi, A. Floristic Inventory of Ethnobotanically Important Halophytes of North-Western Mediterranean Coastal Brackish Areas, Tuscany, Italy. Agronomy 2023, 13, 615. [Google Scholar] [CrossRef]
- Farzana, T.; Guo, Q.; Rahman, M.S.; Rose, T.J.; Barkla, B.J. Salinity and Nitrogen Source Affect Productivity and Nutritional Value of Edible Halophytes. PLoS ONE 2023, 18, e0288547. [Google Scholar] [CrossRef]
- Hussain, W.; Abbas, Q.; Saleem, S.; Khan, S.W.; Shah, M.A. Assessment of Floristic Diversity and Traditional Knowledge from the Selected Mountainous Valleys of District Gilgit, Gilgit-Baltistan, Pakistan. Ethnobot. Res. Appl. 2024, 27, 1–22. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge Der Vegetationskunde; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 3-7091-4078-1. [Google Scholar]
- Van der Maarel, E. Transformation of Cover-Abundance Values in Phytosociology and Its Effects on Community Similarity. Vegetatio 1979, 39, 97–114. [Google Scholar]
- Costanza, R.; Cumberland, J.H.; Daly, H.; Goodland, R.; Norgaard, R.B. An Introduction to Ecological Economics; CRC Press: Boca Raton, FL, USA, 1997; ISBN 1-00-304084-5. [Google Scholar]
- Assessment, M.E. Ecosystems and Human Well-Being: Wetlands and Water; World Resources Institute: Washington, DC, USA, 2005; ISBN 1-56973-597-2. [Google Scholar]
- Sforzi, S.; Selvi, F. Flora Vascolare Della Palude “Diaccia Botrona”(Castiglione Della Pescaia, Grosseto). Atti Soc. Toscana Sci. Nat. Resid. Pisa Mem. Ser. B 1999, 106, 99–114. [Google Scholar]
- Cutini, M.; Agostinelli, E.; Acosta, T.; Molina, J. Coastal Salt-marsh Zonation in Tyrrhenian Central Italy and Its Relationship with Other Mediterranean Wetlands. Plant Biosyst. 2010, 144, 1–11. [Google Scholar] [CrossRef]
- Viciani, D.; Lombardi, L. La Vegetazione Del Padule Di Orti-Bottagone (Piombino, Toscana Meridionale) e La Sua Importanza Botanica Ai Fini Conservazionistici. Parlatorea 2001, 5, 101–118. [Google Scholar]
- Viciani, D.; Gabellini, A.; Biagini, P. La Vegetazione Del Padule Di Scarlino (Con Note Illustrative Della Carta Della Vegetazione, Scala 1: 12.000); S.EL.CA.: Firenze, Italy, 2001; pp. 1–48. [Google Scholar]
- Arrigoni, P.V. La Flora Vascolare Del Parco Della Maremma (Toscana, Italia Centrale). Webbia 2003, 58, 151–240. [Google Scholar] [CrossRef]
- Andreucci, F. La Vegetazione Alofila Della Laguna Di Orbetello (Toscana, Grosseto). Fitosociologia 2004, 41, 31–49. [Google Scholar]
- Sani, A.; Tomei, P.E. La Vegetazione Psammofila Del Litorale Di San Rossore (Toscana Settentrionale) e La Sua Importanza Conservazionistica. Parlatorea 2006, 8, 99–119. [Google Scholar]
- Bertacchi, A.; Lombardi, T.; Tomei, P. Le Aree Umide Salmastre Della Tenuta Di San Rossore (PI): Zonazione e Successione Delle Specie Vegetali in Relazione Alla Salinità Del Suolo; INTER NOS, Volume 1, Pisa, Italia: ETS; 2007; pp. 1–14. Available online: https://www.researchgate.net/publication/283301704_Le_aree_umide_salmastre_della_Tenuta_di_San_Rossore_PI_zonazione_e_successione_delle_specie_vegetali_in_relazione_alla_salinita_del_suolo (accessed on 1 February 2025).
- Londi, G.; Biagini, P.; Campedelli, T.; Mini, L.; Tellini Florenzano, G. Storia Ed Ecologia Del Padule Di Scarlino; Comune di Scarlino: Scarlino, Italy, 2007. [Google Scholar]
- Selvi, F. A Critical Checklist of the Vascular Flora of Tuscan Maremma (Grosseto Province, Italy). Flora Mediterr. 2010, 20, 47–139. [Google Scholar]
- Viciani, D.; Foggi, B.; Ferretti, G. The Mediterranean Salt Steppes (Order Limonietalia Br.-Bl. & O. Bolòs 1958) in Tuscany (Central Italy). Acta Bot. Gall. 2012, 159, 85–96. [Google Scholar]
- Angiolini, C.; Landi, M.; Pieroni, G.; Frignani, F.; Finoia, M.G.; Gaggi, C. Soil Chemical Features as Key Predictors of Plant Community Occurrence in a Mediterranean Coastal Ecosystem. Estuar. Coast. Shelf Sci. 2013, 119, 91–100. [Google Scholar] [CrossRef]
- Biondi, E.; Casavecchia, S.; Estrelles, E.; Soriano, P. Halocnemum M. Bieb. Vegetation in the Mediterranean Basin. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2013, 147, 536–547. [Google Scholar]
- Taramelli, A.; Valentini, E.; Piedelobo, L.; Righini, M.; Cappucci, S. Assessment of State Transition Dynamics of Coastal Wetlands in Northern Venice Lagoon, Italy. Sustainability 2021, 13, 4102. [Google Scholar] [CrossRef]
- Rozema, J.; Schat, H. Salt Tolerance of Halophytes, Research Questions Reviewed in the Perspective of Saline Agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef]
- Flowers, T.J.; Galal, H.K.; Bromham, L. Evolution of Halophytes: Multiple Origins of Salt Tolerance in Land Plants. Funct. Plant Biol. 2010, 37, 604–612. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Soussi, M.; Khadri, M.; Pliego, L.; Ocaña, A.; Lluch, C. Effects of Salt Stress on the Symbiosis Cicer arietinum L.-Mesorhizobium cicero; European Association for Grain Legume Research: Villadolid, Spain, 1998. [Google Scholar]
- Flowers, T.J.; Colmer, T.D. Plant Salt Tolerance: Adaptations in Halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef]
- Grigore, M.-N.; Toma, C. Anatomical Adaptations of Halophytes. A Rev. Class. Lit. Recent 2017, 338, 9–36. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Adamson, R.S. The Classification of Life-Forms of Plants. Bot. Rev. 1939, 5, 546–561. [Google Scholar] [CrossRef]
- Cain, S.A. Life-Forms and Phytoclimate. Bot. Rev. 1950, 16, 1–32. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the Existence of Three Primary Strategies in Plants and Its Relevance to Ecological and Evolutionary Theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Hot-Spots Analysis for Conservation of Plant Biodiversity in the Mediterranean Basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Zohary, D. The Origin of Cultivated Cereals and Pulses in the Near East. Chromosomes Today 1973, 4, 307–320. [Google Scholar]
- Weber, M.; Knoy, C.; Kindscher, K.; Brown, R.C.; Niemann, S.; Chapman, J. Identification of Medicinally Active Compounds in Prairie Plants by HPLC Coupled to Electron Impact-Mass Spectrometry. Am. Lab. 2007, 39, 9. [Google Scholar]
- Luczaj, L.; Pieroni, A.; Tardío, J.; Pardo-de-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild Food Plant Use in 21st Century Europe: The Disappearance of Old Traditions and the Search for New Cuisines Involving Wild Edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Pericin, C. Piante Selvatiche Commestibili Dell’Istria; Centro di Ricerche Storiche Rovigno: Rovinj, Croatia, 2020. [Google Scholar]
- Nedelcheva, A. An Ethnobotanical Study of Wild Edible Plants in Bulgaria. EurAsian J. BioSciences 2013, 7, 77–94. [Google Scholar] [CrossRef]
- Tan, A.; Adanacıoglu, N.; Karabak, S.; Aysar, N.; Tan, A.S.; Aykas, L. Sea Beets [Beta vulgaris Subsp. maritima (L.) Arcang.] Wild Edible Beets and Home Garden Beets of Turkey. ANADOLU Ege Tarımsal Araştırma Enstitüsü Derg. 2017, 27, 54–61. [Google Scholar]
- Pires, E.d.O., Jr.; Di Gioia, F.; Rouphael, Y.; Ferreira, I.C.; Caleja, C.; Barros, L.; Petropoulos, S.A. The Compositional Aspects of Edible Flowers as an Emerging Horticultural Product. Molecules 2021, 26, 6940. [Google Scholar] [CrossRef]
- Nasernakhaei, F.; Zahraei, M. Halocnemum strobilaceum (Pall.) M. Bieb.: A Review of Its Botany, Phytochemistry, Pharmacology and Ethnobotany. J. Med. Plants 2021, 20, 1–12. [Google Scholar] [CrossRef]
- Farhat, M.B.; Beji-Serairi, R.; Selmi, S.; Saidani-Tounsi, M.; Abdelly, C. Salicornia fruticosa L. and Portulaca oleracea L. Antioxidants as Affected by Domestic Cooking Processes. Int. J. Gastron. Food Sci. 2022, 27, 100462. [Google Scholar] [CrossRef]
- Loconsole, D.; Cristiano, G.; De Lucia, B. Glassworts: From Wild Salt Marsh Species to Sustainable Edible Crops. Agriculture 2019, 9, 14. [Google Scholar] [CrossRef]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional Characterization and Storage Ability of Salicornia ramosissima and Sarcocornia perennis for Fresh Vegetable Salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Lopes, M.; Silva, A.S.; Séndon, R.; Barbosa-Pereira, L.; Cavaleiro, C.; Ramos, F. Towards the Sustainable Exploitation of Salt-Tolerant Plants: Nutritional Characterisation, Phenolics Composition, and Potential Contaminants Analysis of Salicornia ramosissima and Sarcocornia perennis alpini. Molecules 2023, 28, 2726. [Google Scholar] [CrossRef] [PubMed]
- Ríos, S.; Obon, C.; Martínez-Francés, V.; Verde, A.; Ariza, D.; Laguna, E. Halophytes as Food: Gastroethnobotany of Halophytes. In Handbook of Halophytes: From Molecules to Ecosystems Towards Biosaline Agriculture; Springer Nature: Berlin, Germany, 2020; pp. 1–36. [Google Scholar]
- Pornpitakdamrong, A.; Sudjaroen, Y. Seablite (Suaeda maritima) Product for Cooking, Samut Songkram Province, Thailand. Food Nutr. Sci. 2014, 5, 850–856. [Google Scholar]
- Łuczaj, Ł.; Jug-Dujaković, M.; Dolina, K.; Vitasović-Kosić, I. Plants in Alcoholic Beverages on the Croatian Islands, with Special Reference to Rakija Travarica. J. Ethnobiol. Ethnomed. 2019, 15, 51. [Google Scholar] [CrossRef]
- Iltaf, M.; Niaz, S.I.; Majeed, M.K.; Saleem, M.; Shah, M.; Ali, M.; Shakeel Abbas, S.; Amin, A. DFT, GC-MS Analysis and Biological Evaluation of Limbarda crithmoides L. Dumort Essential Oil; an Important Edible Halophyte Grown in Pakistan. Nat. Prod. Res. 2024, 1–8. [Google Scholar] [CrossRef]
- Ismail, C.; Baraka, A.; Abdallah, R.; El-Dien, O.; Mostafa, D. Spergularia marina: A Potential Source of a Novel Calcium Channel Blocker with Antihypertensive and Diuretic Activities. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 506–517. [Google Scholar]
- Wollstonecroft, M.M.; Ellis, P.R.; Hillman, G.C.; Fuller, D.Q. Advances in Plant Food Processing in the Near Eastern Epipalaeolithic and Implications for Improved Edibility and Nutrient Bioaccessibility: An Experimental Assessment of Bolboschoenus maritimus (L.) Palla (Sea Club-Rush). Veg. Hist. Archaeobotany 2008, 17, 19–27. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J. Trifolium Species–the Latest Findings on Chemical Profile, Ethnomedicinal Use and Pharmacological Properties. J. Pharm. Pharmacol. 2016, 68, 845–861. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. The Medicinal Potential of Plants from the Genus Plantago (Plantaginaceae). Ind. Crop. Prod. 2016, 83, 213–226. [Google Scholar] [CrossRef]
- González-Orenga, S.; Grigore, M.-N.; Boscaiu, M.; Vicente, O. Constitutive and Induced Salt Tolerance Mechanisms and Potential Uses of Limonium Mill. Species. Agronomy 2021, 11, 413. [Google Scholar] [CrossRef]
- Velez-Gavilan, J. Phragmites australis (Common Reed). CABI Compend. 2024. [Google Scholar] [CrossRef]
- Mattera, M.; Pilla, N.; Aguzzi, A.; Gabrielli, P.; Di Lena, G.; Durazzo, A.; Lucarini, M. Portulaca oleracea L.: Literature Quantitative Research Analysis. Nat. Prod. Res. 2024, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Sagi, M. Halophyte Crop Cultivation: The Case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Ali, B.; Musaddiq, S.; Iqbal, S.; Rehman, T.; Shafiq, N.; Hussain, A. The Therapeutic Properties, Ethno Pharmacology and Phytochemistry of Atriplex Species: A Review. Pak. J. Biochem. Biotechnol. 2021, 2, 49–64. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).