Abstract
Rosemary (Salvia rosmarinus (L.)) is an herb associated with various pharmacological benefits and exhibits antioxidant effects contributing to improved health. This study aimed to investigate the impact of different LED light conditions on the biological activity of rosemary, with a focus on enhancing its functional properties for agricultural applications. The aerial parts of rosemary grown under red light exhibited the highest growth rate. Additionally, the highest 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical scavenging activities (87.72 ± 0.60% and 17.16 ± 0.65%, respectively) were detected in the red light-treated group rather than in the other treatment groups. Comparably, red light treatment induced the highest total phenolic and total flavonoid contents, measuring 126.72 ± 1.47 mg∙GAE/g and 21.02 ± 1.61 mg∙QE/g, respectively, in rosemary. High-performance liquid chromatography revealed that rosmarinic acid was the predominant phenolic compound in the aerial parts of rosemary grown under red light. These findings suggest that optimizing light conditions can be an effective strategy for improving the functional properties of rosemary, providing insights into its potential application in smart farming and sustainable agricultural practices.
1. Introduction
Herbs have been used in therapeutic and culinary applications for centuries. Moreover, the diverse organic compounds produced by these plants play vital roles in the perfume, cosmetic, and pharmaceutical industries [1]. The growing interest in the benefits of herbs encourages extensive research on herb-derived bioactive compounds, which highlights their potential role in preventing and treating various diseases [2]. Rosemary, an evergreen perennial plant belonging to the Lamiaceae family, is native to the Mediterranean region and is cultivated worldwide [3]. Cultivation of rosemary involves easy and manageable processes, which make it accessible to beginners. Its leaves and stems are widely used in various applications, including tea, aromatherapy, spices, and perfumes [4]. In recent years, concerns have been raised regarding the potentially harmful effects of the excessive use of artificial additives in food and cosmetics, highlighting the need for research to develop safe alternative additives. Owing to its low toxicity, it poses minimal safety concerns when used as an additive [5]. Rosemary is well known for its anti-inflammatory, antimicrobial, moisturizing, and soothing properties [6]. Additionally, it helps improve insulin resistance, supports blood sugar regulation, and contributes to cardiovascular health [7].
Population aging is rapidly accelerating with increasing life expectancies, advances in medical technologies, and declining birth rates [8]. The antioxidant capacity of the body gradually declines with age, reducing the ability to effectively eliminate reactive oxygen species (ROS) and leading to increased oxidative stress. Oxidative stress damages cells and DNA, accelerates the aging process, and increases the risk of various diseases, including chronic diseases, inflammation, neurodegenerative disorders, and cardiovascular conditions [9]. Owing to the ability to activate or deactivate various receptors, proteins, ions, and other signaling molecules, ROS play a crucial role in cellular physiological and pathological processes by influencing redox homeostasis [10]. However, excessive ROS generated through metabolic processes or external factors, such as radiation and toxic substances, can lead to oxidative stress that disrupts cellular functions and signaling pathways [11]. Flavonoids and phenolic acids play crucial roles in enhancing the resistance to oxidation [12]. Notably, phenolic acids, including rosmarinic, gallic, caffeic, p-coumaric, and ferulic acids, are abundant in plants [13]. These phenolic acids exert protective effects against various health conditions, including cancer, bacterial infections, and inflammation [14].
Plant factories enable the stable production of high-quality crops throughout the year by artificially controlling environmental factors such as light, temperature, humidity, and carbon dioxide concentration. The controlled internal environment in this system allows plant growth rates that are two to four times higher than those for conventional outdoor cultivation, facilitating large-scale production in limited spaces [15]. Light is the most critical regulatory factor for plant photosynthesis, and the photosynthetic efficacy and plant growth are significantly influenced by the wavelength and intensity of light [15,16]. The spectral composition of light closely interacts with the physiological responses of plants and crucially regulates plant growth and development [16]. Cultivation methods that utilize artificial lighting present an important research focus associated with the enhancement of the pharmacological efficacy of plants [17].
However, the pharmacological efficacy of rosemary grown under different artificial light sources remains rarely explored. Therefore, in this study, we aimed to evaluate the effects of different artificial light sources on the accumulation of bioactive compounds in the aerial parts of rosemary by comparative analysis of the ROS scavenging activity and total phenolic content, total flavonoid content, and levels of the phenolic compound in plants grown under white, blue, red, and green lights. The goal of this study was to optimize light conditions that can support the growth of rosemary with enhanced bioactive properties.
2. Materials and Methods
2.1. Plant Material and Sample Extraction
The rosemary used in this study was sourced from the Donggubak Flower and Succulent Botanical Garden (Chuncheon, Republic of Korea). Before the LED light treatment, rosemary plants were grown for 6 weeks under controlled environmental conditions in a growth chamber. The plants were cultivated in soil-filled pots with a spacing of 15 cm. During this period, the temperature was maintained at 24 °C and the relative humidity at 50%; the light source was standard fluorescent light with a photoperiod of 16 h light/8 h dark. For each light source, three rosemary pots were used and evaluated as replicate experimental values. Rosemary plants were grown under four different light sources (white, red, blue, and green lights) for four weeks; subsequently, the aerial parts were dried at 60 °C (Figure 1). The dried aerial parts of the rosemary were finely ground and extracted at room temperature for 72 h using 70% ethanol at a ratio of 1:5 (w/v). The extract was filtered through filter paper (Whatman No. 42, ashless, GE Healthcare, Piscataway, NJ, USA) and concentrated under reduced pressure at 45 °C using a rotary evaporator (EYELA N-1110, Tokyo, Japan). The final extract (10 mg/mL) was used for further analyses.
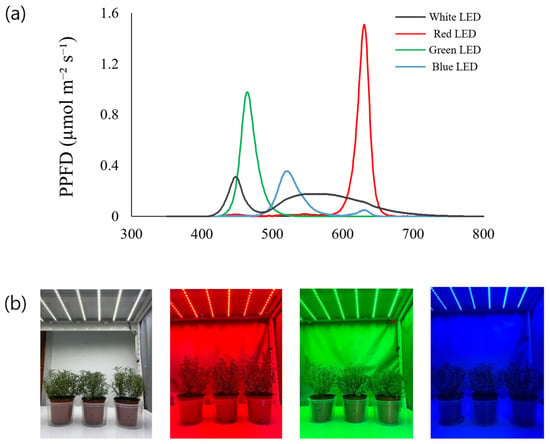
Figure 1.
Biomass effect of rosemary grown under four different LED light sources. (a) Wavelengths of four colored LED lights (white, red, green, blue), (b) rosemary plants grown under four different LED light sources.
2.2. DPPH Radical Scavenging Assay
DPPH free radical scavenging activity was measured following a method reported by Kalpoutzakis with modifications [18]. The extracts were diluted to concentrations of 10, 25, 50, and 100 μg/mL, and 10 µL of 0.15 mM DPPH solution was added to each. DPPH was used in the experiment, using methanol as a solvent. The mixtures were incubated in the dark at room temperature for 30 min. The absorbance of the mixture was recorded at 517 nm using a UV spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The results of the DPPH radical scavenging activity assay were expressed as inhibition rates (%).
DPPH inhibition rate (%) = [1 − (Sample OD − Sample blank)/(−)control average)] × 100
2.3. ABTS Radical Scavenging Assay
The ABTS radical scavenging activity was measured using a method reported by Mansoori with modifications [19]. A solution of ABTS radicals was prepared by mixing 7.4 mM ABTS and 2.6 mM potassium persulfate at a 1:1 ratio, followed by incubation in the dark for 20 h. The samples were diluted to concentrations of 10, 25, 50, and 100 µg/mL. Next, 100 µL of each sample was mixed with 190 µL of ABTS solution and incubated at room temperature for 10 min. The antioxidant activity was evaluated based on the absorbance of the mixture at 734 nm. The ABTS radical scavenging activity results are expressed as inhibition rates (%).
2.4. Total Phenolic Content
Total phenolic content was measured using the Folin–Ciocalteu colorimetric method with modifications [20]. A 100 µL aliquot of the sample, diluted to 1000 µg/mL, was mixed with 50 µL of 2 N Folin–Ciocalteu phenol reagent and allowed to react for 3 min. Subsequently, 30 µL of 20% Na2CO3 was added, and the mixture was incubated for 15 min, followed by the addition of 1 mL of distilled water. The mixture was centrifuged at room temperature for 2 min and the absorbance of the supernatant was measured at 723 nm. The TPC (total phenolic content) was determined considering gallic acid as a standard, and the results were calculated based on the calibration curve (y = 0.0041x + 0.1089, R2 = 0.9961).
2.5. Total Flavonoid Content
Total flavonoid content was determined using a method described by Ayele with modifications [21]. A 500 µL aliquot of the sample, diluted to 1000 µg/mL, was mixed with 100 µL of 10% aluminum nitrate and 100 µL of 1 M potassium acetate, followed by incubation for 40 min; next, the absorbance was measured at 415 nm. The total flavonoid content was determined using quercetin as a standard, and the results were calculated based on a calibration curve (y = 0.0147x + 0.1328, R2 = 0.9933).
2.6. Analysis of Phenolic Compounds
The extract was diluted in 70% EtOH at a concentration of 10,000 µg/mL, filtered, and used for the experiment. Six standard compounds were used: protocatechuic, caffeic, p-coumaric, gallic, rosmarinic, and ferulic acids. All standard compounds were diluted to concentrations of 10, 25, 50, 100, and 200 µg/mL for the analyses. All phenolic acids were analyzed using a high-performance liquid chromatography (HPLC) system (Agilent 1260 series, Agilent Technologies Inc., Santa Clara, CA, USA). The levels of protocatechuic acid, caffeic acid, p-coumaric acid, and ferulic acid were analyzed using an HC-18 column (4.6 × 250 mm, 5 µm, Agilent Technologies Inc., Santa Clara, CA, USA) with the column temperature set at 25 °C. The injection volume was 10 µL, and the mobile phases consisted of HPLC-grade water containing 0.1% formic acid (Solvent A) and acetonitrile (Solvent B). The wavelength was set to 288 nm and the flow rate was maintained at 0.5 mL/min. The gradient elution of the solvents was programmed as follows: 5–15% B (5 min), 15–50% B (40 min), 50–70% B (2 min), 70–100% B (1 min), 100% B (7 min), 100–5% B (1 min), and 5% B (9 min). The gallic acid and rosmarinic acid contents were analyzed using a C18 column (4.6 × 150 mm, 5 µm, Agilent Technologies Inc., Santa Clara, CA, USA) with the column temperature set to 40 °C. The injection volume was 20 µL, and the flow rate was maintained at 1.5 mL/min. The mobile phase consisted of HPLC-grade water with 0.5% acetic acid (Solvent A) and methanol with 0.5% acetic acid (Solvent B), and the wavelength was set to 280 nm. The gradient elution of the solvents was programmed as follows: 0–20% B (0–0.01 min), 20–60% B (0.01–2 min), 60–80% B (2–15 min), 100% B (15–30 min), 100–10% B (30–35 min), and 10–0% B (35–40 min).
2.7. Statistical Analysis
All experiments were performed in triplicate, and the results are presented as the mean ± standard deviation. Statistical analyses were conducted using IBM SPSS Statistics version 26. One-way analysis of variance (ANOVA) was used to evaluate the significance of differences between groups, and Duncan’s multiple range test was performed considering a significance level of p < 0.05.
3. Results and Discussion
3.1. Characterization of Growth of Rosemary Treated with LED Lights
The total length of the aerial parts of rosemary plants was measured at 7-day intervals to assess the effects of LED light on growth. The length of the aerial parts of rosemary grown under white and blue light increased by approximately 1 cm over 4 weeks, whereas the length increased by approximately 2 cm in the green light treatment group. Contrastingly, rosemary plants treated with red light grew from 14.5 cm to 18.9 cm, exhibiting an increase of approximately 4 cm, which reflected faster plant growth than that associated with other light treatments (Figure 2). Previous reports indicated that red and blue lights enhance photosynthetic efficiency, increase chlorophyll content, and promote the accumulation of total flavonoids and phenolic compounds in a few plant species [22]. The results of this study reflected faster growth of the aerial parts in rosemary grown under red and green light than in the white and blue light-treated counterparts. Comparably, previous reports demonstrate that red light can promote the growth of wheat seedlings, and Rehmannia glutinosa grown under blue and red light exhibit significantly greater growth than those cultivated under fluorescent light [23]. Additionally, the combination of red and blue light was reported to be effective in promoting chlorophyll accumulation and fruit yield in strawberries [24]. Similarly, the results of the present study demonstrate a positive effect of red light on the growth of rosemary aerial parts, suggesting that red light may also play a crucial role in other physiological responses.
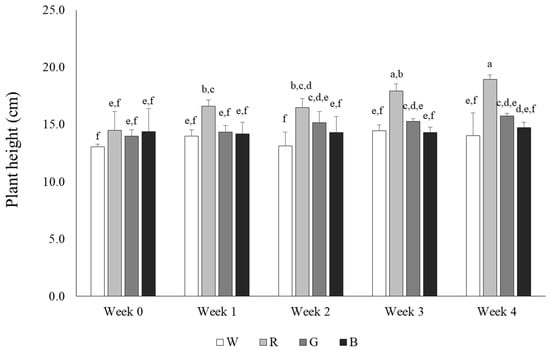
Figure 2.
Differences in rosemary growth under four different light sources. W: LED—white, R: LED—red, G: LED—green, B: LED—blue. Values represent means of data obtained from three independent experiments (p < 0.05). Significance was indicated with different letters according to statistical analysis.
3.2. Effect of LED Lights on the ROS Scavenging Activity in Rosemary
To evaluate the antioxidant activities of the aerial parts of rosemary plants grown under different light sources, DPPH and ABTS radical scavenging activities were analyzed. The DPPH radical scavenging assay revealed the highest activity (87.72 ± 0.60%) in plants grown under red light, and the lowest activity (75.11 ± 0.84%) was observed in plants grown under green light (Figure 3a). Similarly, the ABTS radical scavenging activity revealed the highest value (17.16 ± 0.65%) in the red light-treated group, followed by the green, blue, and white light-treated counterparts presenting a descending order (Figure 3b); these differences were statistically significant. This study aimed to identify the optimal light conditions by comparing the antioxidant activity of the aerial parts of rosemary under different light sources. Similar to the highest ABTS and DPPH radical scavenging activities detected in the red light-treated group, the highest total phenolic and flavonoid contents were recorded in the red light-treated group. Moreover, previous studies have reported that red light-treated strawberries exhibit high DPPH and ABTS radical scavenging activities, which is consistent with the present study findings, in which rosemary aerial parts grown under red light showed the highest antioxidant activity [24].
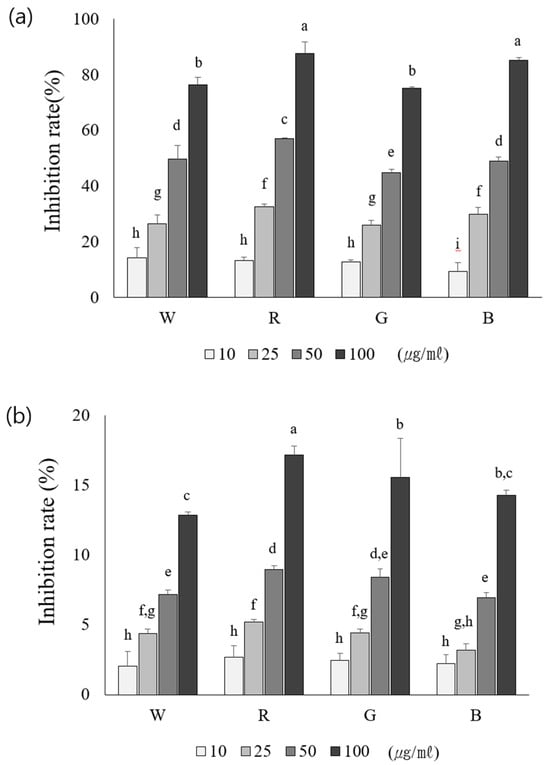
Figure 3.
Comparison of antioxidant activity using DPPH (a) and ABTS (b) radical scavenging assays in extracts obtained from rosemary grown under four different LED light sources. W: LED—white, R: LED—red, G: LED—green, B: LED—blue. Values represent means of data obtained from three independent experiments (p < 0.05). Significance was indicated with different letters according to statistical analysis.
3.3. Comparative Analyses of Total Phenolic and Flavonoid Contents Varying with Types of LED Light
Figure 4a shows the total phenolic content in the aerial parts of rosemary plants grown under four different light sources. The red light treatment induced the highest total phenolic content [126.72 ± 1.47 mg GAE (gallic acid equivalent)/g], whereas the lowest level of total phenolics was observed in the white light-treated group (82.10 ± 1.33 mg GAE/g). The total phenolic content significantly differed among plants exposed to different light treatments. Moreover, plants grown under red light exhibited the highest total flavonoid content [21.02 ± 1.61 mg QE (quercetin equivalents)/g], followed by the blue, green, and white light-treated counterparts presenting different flavonoid levels in descending order (Figure 4b). This result was similar to the variations in the total phenolic content. Additionally, the total flavonoid content in the aerial parts of rosemary significantly differed with different light treatments. A previous report revealed that exposure to red light for 16 h per day led to increased phenolic accumulation in garlic compared to the blue, green, and white light-treated counterparts [25]. Blue and red light increased the total phenolic and flavonoid content in pea sprouts; furthermore, blue light positively influenced the effect on ABTS-reducing power [26]. Exposure to red light enhanced flavonoid concentration in wheat sprouts [27], whereas jewel orchids exhibited a reduced flavonoid level under red light. Blue light negatively affected flavonoid biosynthesis. However, the combination of red and blue lights enhanced the total flavonoid content, suggesting a synergistic effect of these light sources [28]. However, these findings highlighting the positive effects of the combination of red and blue light on flavonoid accumulation were not addressed in the present study. Further research can validate how the combination of light sources influences antioxidant activity in rosemary.
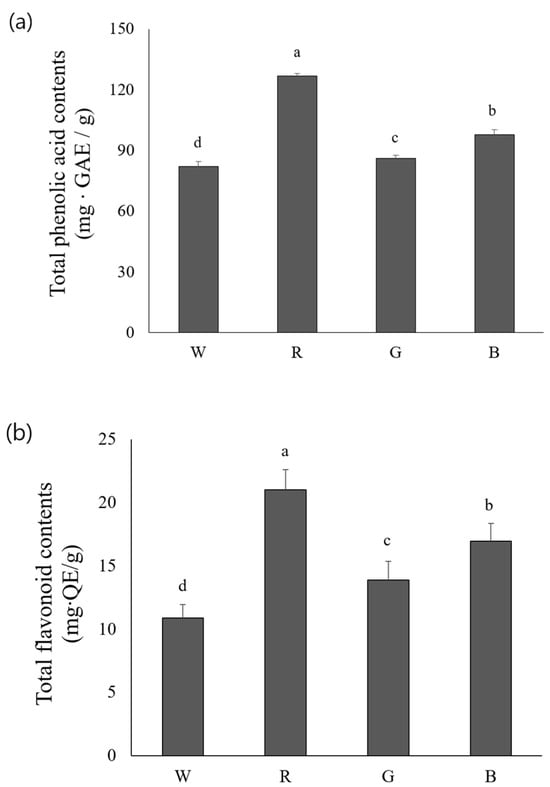
Figure 4.
Comparison of total phenol content (a) and total flavonoid content (b) in extracts obtained from rosemary grown under four different LED light sources. W: LED—white, R: LED—red, G: LED—green, B: LED—blue. Values represent means of data obtained from three independent experiments (p < 0.05). Significance was indicated with different letters according to statistical analysis.
3.4. Comparative Analysis of Phenolic Compounds in Rosemary Plants Treated with Different LED Lights
HPLC analysis using six phenolic standards revealed that rosmarinic acid was the most abundant compound in the aerial parts of rosemary plants, with the highest concentration observed in the red light-treated group. Protocatechuic acid represented the most abundant phenolic compound (7.34 ± 3.21 µg/mL) under blue light conditions, while p-coumaric acid exhibited the highest level (4.52 ± 0.16 µg/mL) under white light treatment; the remaining phenolic compounds exhibited the highest concentrations in the red light-treated group (Table 1). Among the six phenolic compounds analyzed in the aerial parts of rosemary plants, the highest levels of caffeic acid, gallic acid, rosmarinic acid, and ferulic acid were detected in the red light-treated group. Similarly, wheat sprouts grown under red light showed an increased accumulation of ferulic and p-coumaric acids on days 8 and 12, respectively [29]. Moreover, the growth of basil was promoted under red light and the synthesis of phenolic compounds increased. However, the synthesis of rosmarinic and gallic acids is significantly increased by blue light exposure [30]. These results may be attributed to the differences in the sensitivity of photoreceptors among plant species and variations in growth stages. Considering that each light source may have distinct effects on the synthesis of specific phenolic compounds, further research is required to optimize the synthesis of phenolic compounds in rosemary using suitable light conditions.

Table 1.
Analysis of phenolic compounds in extracts of rosemary grown under four different LED light sources.
The observed increase in total phenolic and flavonoid content under red light suggests that red wavelengths play a critical role in secondary metabolite biosynthesis. This could be attributed to the stimulation of specific photoreceptors, such as phytochromes, which regulate phenolic biosynthesis pathways. Previous studies have also reported enhanced bioactive compound accumulation under red light in crops such as strawberries and wheat [25,29]. However, unlike these studies, our findings highlight a significant impact on rosmarinic acid accumulation, indicating that rosemary may have species-specific responses to light treatments.
The results of this study have significant agronomic implications, particularly in controlled environment agriculture and vertical farming systems. The use of red LED light to enhance bioactive compound production in rosemary suggests that optimizing light conditions could improve the functional value of medicinal plants in smart farming systems. Furthermore, LED technology offers energy-efficient solutions compared to conventional lighting, making it a sustainable approach for large-scale cultivation. Future research should explore the economic feasibility of implementing specific LED treatments in commercial production and investigate the combined effects of red and blue light for further optimization.
Although our study demonstrates the beneficial effects of red LED light on rosemary, further research is needed to determine the optimal light spectra combinations for maximizing plant secondary metabolite production. The combined effects of red and blue light, which have been shown to enhance photosynthetic efficiency in other crops, should be investigated. Additionally, future studies should evaluate how these light treatments perform in real-world greenhouse or open-field settings, where natural light conditions vary. Exploring the scalability of LED-based growth systems in commercial agronomy will be crucial for integrating this technology into sustainable agricultural practices.
4. Conclusions
In conclusion, this study elucidates the variable growth rates and antioxidant activities of the aerial parts of rosemary grown under different light sources. Red light treatment significantly increased the antioxidant content of rosemary, providing fundamental information required to enhance the applicability of this plant in the food and cosmetic industries. These findings potentially contribute to a comprehensive knowledge of the effects of light sources on plant growth processes and facilitate the effective determination of optimal light conditions for plant growth. Further research on the combined effects of different light sources on physiological traits and antioxidant components in rosemary can potentially help establish optimized cultivation environments, thereby maximizing its industrial potential.
Author Contributions
Conceptualization, E.S.S.; methodology, formal analysis, J.P., J.W.S., D.Y.H., H.J.C., M.J.K., J.K.N., and S.K.K.; investigation, E.S.S.; writing, E.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mostafa, S.; Wang, Y.; Zeng, W.; Jin, B. Floral scents and fruit aromas: Functions, compositions, biosynthesis, and regulation. Front. Plant Sci. 2022, 13, 860157. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; Vostinaru, O.; Rigano, D.; de Aguiar Andrade, E.H. Bioactive compounds present in essential oils: Advances and pharmacological applications. Front. Pharmacol. 2023, 14, 1130097. [Google Scholar] [CrossRef]
- Hammer, M.; Junghanns, W. Rosmarinus officinalis L.: Rosemary. In Medicinal, Aromatic and Stimulant Plants; Springer: Berlin/Heidelberg, Germany, 2020; pp. 501–521. [Google Scholar]
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An ancient plant with uses in personal healthcare and cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Hosseinzadeh, H. Toxicity and safety of rosemary (Rosmarinus officinalis): A comprehensive review. Naunyn-Schm Arch. Pharm. 2024, 398, 9–23. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Camargo, S.E.A.; De Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Kabubii, Z.N.; Mbaria, J.M.; Mathiu, P.M.; Wanjohi, J.M.; Nyaboga, E.N. Diet supplementation with rosemary (Rosmarinus officinalis L.) leaf powder exhibits an antidiabetic property in streptozotocin-induced diabetic male wistar rats. Diabetol. 2024, 5, 12–25. [Google Scholar] [CrossRef]
- Kudo, S.; Mutisya, E.; Nagao, M. Population aging: An emerging research agenda for sustainable development. Soc. Sci. 2015, 4, 940–966. [Google Scholar] [CrossRef]
- Yang, J.; Luo, J.; Tian, X.; Zhao, Y.; Li, Y.; Wu, X. Progress in understanding oxidative stress, aging, and aging-related diseases. Antioxidants 2024, 13, 394. [Google Scholar] [CrossRef]
- Iliadis, S.; Papanikolaou, N.A. Reactive oxygen species mechanisms that regulate protein–protein interactions in cancer. Inter. J. Mol. Sci. 2024, 25, 9255. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as natural phenolic compounds and their role in therapeutics: An overview. Fut. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Phenolic acids of plant origin—A review on their antioxidant activity in vitro (o/w emulsion systems) along with their in vivo health biochemical properties. Foods 2020, 9, 534. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and their role in oxidative stress-induced human diseases: Insights into protective effects, antioxidant potentials and mechanism (s) of action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Liu, J.; Van Iersel, M.W. Photosynthetic physiology of blue, green, and red light: Light intensity effects and underlying mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light-quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of light on secondary metabolite biosynthesis in medicinal plants. Fron Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef]
- Kalpoutzakis, E.; Chatzimitakos, T.; Athanasiadis, V.; Mitakou, S.; Aligiannis, N.; Bozinou, E.; Gortzi, O.; Skaltsounis, L.A.; Lalas, S.I. Determination of the total phenolics content and antioxidant activity of extracts from parts of plants from the greek island of crete. Plants 2023, 12, 1092. [Google Scholar] [CrossRef]
- Mansoori, A.; Singh, N.; Dubey, S.K.; Thakur, T.K.; Alkan, N.; Das, S.N.; Kumar, A. Phytochemical characterization and assessment of crude extracts from Lantana camara L. for antioxidant and antimicrobial activity. Front. Agro 2020, 2, 582268. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Pappas, V.M.; Palaiogiannis, D.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Pulsed electric field-based extraction of total polyphenols from Sideritis raiseri using hydroethanolic mixtures. Oxygen 2022, 2, 91–98. [Google Scholar] [CrossRef]
- Ayele, D.T.; Akele, M.; Melese, A. Analysis of total phenolic contents, flavonoids, antioxidant and antibacterial activities of Croton macrostachyus root extracts. BMC Chem. 2022, 16, 30. [Google Scholar] [CrossRef]
- Seo, J.M.; Arasu, M.V.; Kim, Y.B.; Park, S.U.; Kim, S.J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 2015, 177, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, X.; Zhang, S.; Zhang, Y.; Chen, L.; Zheng, W.; Xue, X. Effects of light quality on growth, nutritional characteristics, and antioxidant properties of winter wheat seedlings (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 978468. [Google Scholar] [CrossRef]
- Raiciu, A.D.; Livadariu, O.; Maximilian, C.; Crețu, A.M. The assessment of the effect induced by LED-s irradiation on garlic sprouts (Allium sativum L.). Roman. Biotechnol. Lett. 2018, 23, 14187–14191. [Google Scholar]
- Choi, H.G.; Moon, B.Y.; Kang, N.J. Effects of LED light on the production of strawberry during cultivation in a plastic greenhouse and in a growth chamber. Sci. Hort. 2015, 189, 22–31. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Hu, T.; Zhang, S.; Zhang, Y.; Zhao, T.; Yu, H.; Kang, Y. The influence of light-emitting diodes on the phenolic compounds and antioxidant activities in pea sprouts. J. Funct. Foods 2016, 25, 459–465. [Google Scholar] [CrossRef]
- Raiciu, D.; Livadariu, O.; Maximilian, C.; Bira, A. The evaluation of the effect of LED-s irradiation on wheat sprouts (Triticum aestivum L.). Roman. Biotechnol. Lett. 2020, 25, 1615–1620. [Google Scholar] [CrossRef]
- Gam, D.T.; Khoi, P.H.; Ngoc, P.B.; Linh, L.K.; Hung, N.K.; Anh, P.T.L.; Thu, N.T.; Hein, N.T.T.; Khanh, T.D.; Ha, C.H. LED Lights promote growth and flavonoid accumulation of Anoectochilus roxburghii and are linked to the enhanced expression of several related genes. Plants 2020, 9, 1344. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.M.; Ha, T.W.; Park, C.H.; Kim, N.S.; Ye, H.J.; Chun, S.W.; Kim, C.S.; Park, S.U. Effects of LED lights on expression of genes involved in phenylpropanoid biosynthesis and accumulation of phenylpropanoids in wheat sprout. Agronomy 2019, 9, 307. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. Microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).