Biochar and Ammonium Nitrate Synergies: Enhancing Nitrogen Availability and Maize Growth in Oxisols
Abstract
1. Introduction
2. Materials and Methods
2.1. Pyrolysis and Biochar Production
2.2. Soils and Experimental Conditions
2.3. Soil and Solution Sampling
2.4. Maize Growth and N Nutritional Status
2.5. Statistical Analysis
3. Results
3.1. Soil Available Nitrogen
3.2. Nitrogen in Soil Solution
3.3. Maize Nutrition and Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Initial | Feedstock | Dystroferric Clayey Red Latosol | Red-Yellow Medium-Textured Latosol | ||||
| FD | BC | BC + NAM | FD | BC | BC + NAM | ||
| Bam | 6.18 ± 0.06 | 6.23 ± 0.05 | 6.27 ± 0.04 | 6.13 ± 0.05 | 6.29 ± 0.04 | 6.12 ± 0.03 | |
| SC | 6.16 ± 0.07 | 6.15 ± 0.07 | 6.06 ± 0.03 | 6.24 ± 0.03 | 6.23 ± 0.05 | 6.20 ± 0.03 | |
| CM | 6.51 ± 0.05 | 6.22 ± 0.04 | 6.26 ± 0.03 | 6.73 ± 0.06 | 6.52 ± 0.05 | 6.43 ± 0.02 | |
| SCa | 6.45 ± 0.05 | 6.42 ± 0.01 | 6.36 ± 0.06 | 6.48 ± 0.05 | 6.58 ± 0.02 | 6.44 ± 0.03 | |
| OS | 6.19 ± 0.06 | 6.54 ± 0.03 | |||||
| NAM | 6.02 ± 0.01 | 6.12 ± 0.02 | |||||
| Residual | Feedstock | Dystroferric clayey Red Latosol | Red-Yellow medium-texture Latosol | ||||
| FD | BC | BC + NAM | FD | BC | BC + NAM | ||
| Bam | 6.13 ± 0.05 | 6.02 ± 0.04 | 5.84 ± 0.04 | 6.02 ± 0.04 | 6.15 ± 0.04 | 6.23 ± 0.02 | |
| SC | 5.96 ± 0.03 | 5.88 ± 0.02 | 5.99 ± 0.06 | 6.43 ± 0.03 | 6.37 ± 0.06 | 6.35 ± 0.03 | |
| CM | 6.58 ± 0.04 | 6.22 ± 0.06 | 6.18 ± 0.02 | 7.14 ± 0.05 | 6.70 ± 0.05 | 6.79 ± 0.02 | |
| SCa | 6.19 ± 0.05 | 6.13 ± 0.04 | 6.11 ± 0.07 | 6.74 ± 0.05 | 6.69 ± 0.03 | 6.62 ± 0.01 | |
| OS | 5.99 ± 0.06 | 6.13 ± 0.03 | |||||
| NAM | 5.86 ± 0.06 | 6.25 ± 0.04 | |||||
| Initial | Feedstock | Dystroferric Clayey Red Latosol | Red-Yellow Medium-Textured Latosol | ||||
| FD | BC | BC + NAM | FD | BC | BC + NAM | ||
| Bam | 6.42 ± 0.07 | 6.49 ± 0.05 | 6.69 ± 0.10 | 5.63 ± 0.06 | 5.78 ± 0.04 | 5.44 ± 0.03 | |
| SC | 6.01 ± 0.11 | 6.27 ± 0.10 | 6.58 ± 0.18 | 5.24 ± 0.03 | 5.60 ± 0.02 | 5.48 ± 0.03 | |
| CM | 7.32 ± 0.02 | 7.00 ± 0.05 | 7.21 ± 0.06 | 7.12 ± 0.06 | 6.40 ± 0.06 | 6.63 ± 0.03 | |
| SCa | 6.80 ± 0.07 | 6.88 ± 0.07 | 7.11 ± 0.03 | 6.99 ± 0.08 | 7.12 ± 0.03 | 6.86 ± 0.03 | |
| OS | 6.49 ± 0.07 | 5.77 ± 0.07 | |||||
| NAM | 6.65 ± 0.05 | 5.40 ± 0.02 | |||||
| 15 days after sowing | Feedstock | Dystroferric clayey Red Latosol | Red-Yellow medium-textured Latosol | ||||
| FD | BC | BC + NAM | FD | BC | BC + NAM | ||
| Bam | 6.62 ± 0.11 | 6.34 ± 0.17 | 6.69 ± 0.05 | 6.69 ± 0.15 | 7.10 ± 0.09 | 7.13 ± 0.10 | |
| SC | 7.26 ± 0.15 | 6.54 ± 0.19 | 6.34 ± 0.06 | 7.19 ± 0.05 | 7.06 ± 0.08 | 6.92 ± 0.02 | |
| CM | 7.86 ± 0.08 | 6.53 ± 0.16 | 6.58 ± 0.02 | 7.50 ± 0.08 | 7.40 ± 0.08 | 7.16 ± 0.13 | |
| SCa | 7.95 ± 0.10 | 6.43 ± 0.13 | 6.59 ± 0.13 | 7.73 ± 0.13 | 7.46 ± 0.10 | 7.79 ± 0.14 | |
| OS | 6.14 ± 0.01 | 7.05 ± 0.12 | |||||
| NAM | 6.08 ± 0.07 | 7.12 ± 0.02 | |||||
References
- ABRELPE. Panorama Dos Resíduos Sólidos No Brasil 2020; ABRELPE: São Paulo, Brazil, 2020. [Google Scholar]
- Masunga, R.H.; Uzokwe, V.N.; Mlay, P.D.; Odeh, I.; Singh, A.; Buchan, D.; De Neve, S. Nitrogen Mineralization Dynamics of Different Valuable Organic Amendments Commonly Used in Agriculture. Appl. Soil. Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]
- Sharma, B.; Vaish, B.; Monika; Singh, U.K.; Singh, P.; Singh, R.P. Recycling of Organic Wastes in Agriculture: An Environmental Perspective. Int. J. Environ. Res. 2019, 13, 409–429. [Google Scholar] [CrossRef]
- de Oliveira Paiva, I.; de Morais, E.G.; Jindo, K.; Silva, C.A. Biochar N Content, Pools and Aromaticity as Affected by Feedstock and Pyrolysis Temperature. Waste Biomass Valorization 2024, 15, 3599–3619. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Lang, T.; Jensen, A.D.; Jensen, P.A. Retention of Organic Elements during Solid Fuel Pyrolysis with Emphasis on the Peculiar Behavior of Nitrogen. Energy Fuels 2005, 19, 1631–1643. [Google Scholar] [CrossRef]
- De Morais, E.G.; Silva, C.A.; Gao, S.; Melo, L.C.A.; Lago, B.C.; Teodoro, J.C.; Guilherme, L.R.G. Empirical Correlation between Electrical Conductivity and Nitrogen Content in Biochar as Influenced by Pyrolysis Temperature. Nitrogen 2024, 5, 288–300. [Google Scholar] [CrossRef]
- Ahmad, Z.; Mosa, A.; Zhan, L.; Gao, B. Biochar Modulates Mineral Nitrogen Dynamics in Soil and Terrestrial Ecosystems: A Critical Review. Chemosphere 2021, 278, 130378. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, Y.; Guo, L.; Wu, B.; Yan, B.; Wang, Y.; Liu, H.; Li, G.; Wang, Y.; Wang, H. Optimal Effects of Combined Application of Nitrate and Ammonium Nitrogen Fertilizers with a Ratio of 3:1 on Grain Yield and Water Use Efficiency of Maize Sowed in Ridge–Furrow Plastic Film Mulching in Northwest China. Agronomy 2022, 12, 2943. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Van Zwieten, L.; Bolan, N.; Dong, D.; Quin, B.F.; Meng, J.; Li, F.; Wu, F.; Wang, H.; et al. A Critical Review of Biochar-Based Nitrogen Fertilizers and Their Effects on Crop Production and the Environment. Biochar 2022, 4, 36. [Google Scholar] [CrossRef]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How Biochar Works, and When It Doesn’t: A Review of Mechanisms Controlling Soil and Plant Responses to Biochar. GCB Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of Biochar on Soil Available Inorganic Nitrogen: A Review and Meta-Analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Wang, T.; Camps Arbestain, M.; Hedley, M.; Bishop, P. Chemical and Bioassay Characterisation of Nitrogen Availability in Biochar Produced from Dairy Manure and Biosolids. Org. Geochem. 2012, 51, 45–54. [Google Scholar] [CrossRef]
- Almendros, G.; Knicker, H.; González-Vila, F.J. Rearrangement of Carbon and Nitrogen Forms in Peat after Progressive Thermal Oxidation as Determined by Solid-State 13C- and 15N-NMR Spectroscopy. Org. Geochem. 2003, 34, 1559–1568. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock Choice, Pyrolysis Temperature and Type Influence Biochar Characteristics: A Comprehensive Meta-Data Analysis Review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Jindo, K.; Audette, Y.; Higashikawa, F.S.; Silva, C.A.; Akashi, K.; Mastrolonardo, G.; Sánchez-Monedero, M.A.; Mondini, C. Role of Biochar in Promoting Circular Economy in the Agriculture Sector. Part 1: A Review of the Biochar Roles in Soil N, P and K Cycles. Chem. Biol. Technol. Agric. 2020, 7, 15. [Google Scholar] [CrossRef]
- Schellekens, J.; Silva, C.A.; Buurman, P.; Rittl, T.F.; Domingues, R.R.; Justi, M.; Vidal-Torrado, P.; Trugilho, P.F. Molecular Characterization of Biochar from Five Brazilian Agricultural Residues Obtained at Different Charring Temperatures. J. Anal. Appl. Pyrolysis 2018, 130, 249–255. [Google Scholar] [CrossRef]
- Costantini, M.; Bacenetti, J. Soybean and Maize Cultivation in South America: Environmental Comparison of Different Cropping Systems. Clean. Environ. Syst. 2021, 2, 100017. [Google Scholar] [CrossRef]
- Lopes, A.S.; Guilherme, L.R.G. A Career Perspective on Soil Management in the Cerrado Region of Brazil. Adv. Agron. 2016, 137, 1–72. [Google Scholar] [CrossRef]
- Cassim, B.M.A.R.; Lisboa, I.P.; Besen, M.R.; Otto, R.; Cantarella, H.; Inoue, T.T.; Batista, M.A. Nitrogen: From Discovery, Plant Assimilation, Sustainable Usage to Current Enhanced Efficiency Fertilizers Technologies–A Review. Rev. Bras. Cienc. Solo 2024, 48, e0230037. [Google Scholar] [CrossRef]
- Harty, M.A.; McDonnell, K.P.; Whetton, R.; Gillespie, G.; Burke, J.I. Comparison of Ammonia-N Volatilization Losses from Untreated Granular Urea and Granular Urea Treated with NutriSphere-N. Soil. Use Manag. 2024, 40, e12891. [Google Scholar] [CrossRef]
- Motasim, A.M.; Samsuri, A.W.; Nabayi, A.; Akter, A.; Haque, M.A.; Abdul Sukor, A.S.; Adibah, A.M. Urea Application in Soil: Processes, Losses, and Alternatives—A Review. Discov. Agric. 2024, 2, 42. [Google Scholar] [CrossRef]
- Elwan, M.W.M.; Abd El-Hamed, K.E. Influence of Nitrogen Form, Growing Season and Sulfur Fertilization on Yield and the Content of Nitrate and Vitamin C of Broccoli. Sci. Hortic. 2011, 127, 181–187. [Google Scholar] [CrossRef]
- Soinne, H.; Keskinen, R.; Räty, M.; Kanerva, S.; Turtola, E.; Kaseva, J.; Nuutinen, V.; Simojoki, A.; Salo, T. Soil Organic Carbon and Clay Content as Deciding Factors for Net Nitrogen Mineralization and Cereal Yields in Boreal Mineral Soils. Eur. J. Soil. Sci. 2021, 72, 1497–1512. [Google Scholar] [CrossRef]
- Asibi, A.E.; Chai, Q.; Coulter, J.A. Mechanisms of Nitrogen Use in Maize. Agronomy 2019, 9, 775. [Google Scholar] [CrossRef]
- De Faria, I.K.P.; Vieira, J.L.V.; Tenelli, S.; de Almeida, R.E.M.; Campos, L.J.M.; da Costa, R.V.; Zavaschi, E.; de Almeida, R.F.; de Mello e Silva Carneiro, L.; Otto, R. Optimal Plant Density and Nitrogen Rates for Improving Off-Season Corn Yields in Brazil. Sci. Agric. 2019, 76, 344–352. [Google Scholar] [CrossRef]
- Ciampitti, I.A.; Lemaire, G. From Use Efficiency to Effective Use of Nitrogen: A Dilemma for Maize Breeding Improvement. Sci. Total Environ. 2022, 826, 154125. [Google Scholar] [CrossRef]
- Marzi, M.; Shahbazi, K.; Kharazi, N.; Rezaei, M. The Influence of Organic Amendment Source on Carbon and Nitrogen Mineralization in Different Soils. J. Soil. Sci. Plant Nutr. 2020, 20, 177–191. [Google Scholar] [CrossRef]
- Sediyama, M.A.N.; Santos, M.R.; Vidigal, S.M.; Salgado, L.T. Produtividade e Exportação de Nutrientes Em Beterraba Cultivada Com Cobertura Morta e Adubação Orgânica. Rev. Bras. Eng. Agrícola Ambient. 2011, 15, 883–889. [Google Scholar] [CrossRef]
- Mota, C.P.; Silva, C.A. Biochar–Nitrogen Composites: Synthesis, Properties, and Use as Fertilizer for Maize. AppliedChem 2024, 4, 157–173. [Google Scholar] [CrossRef]
- Castejón-del Pino, R.; Sánchez-Monedero, M.A.; Sánchez-García, M.; Cayuela, M.L. Fertilization Strategies to Reduce Yield-Scaled N2O Emissions Based on the Use of Biochar and Biochar-Based Fertilizers. Nutr. Cycl. Agroecosyst 2024, 129, 491–501. [Google Scholar] [CrossRef]
- Puga, A.P.; Grutzmacher, P.; Cerri, C.E.P.; Ribeirinho, V.S.; de Andrade, C.A. Biochar-Based Nitrogen Fertilizers: Greenhouse Gas Emissions, Use Efficiency, and Maize Yield in Tropical Soils. Sci. Total Environ. 2020, 704, 135375. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, S.; Yin, M.; He, Z.; Bi, D. Co-Pyrolysis of Cellulose with Urea and Chitosan to Produce Nitrogen-Containing Compounds and Nitrogen-Doped Biochar: Product Distribution Characteristics and Reaction Path Analysis. J. Anal. Appl. Pyrolysis 2023, 169, 105795. [Google Scholar] [CrossRef]
- Yang, H.I.; Lou, K.; Rajapaksha, A.U.; Ok, Y.S.; Anyia, A.O.; Chang, S.X. Adsorption of Ammonium in Aqueous Solutions by Pine Sawdust and Wheat Straw Biochars. Environ. Sci. Pollut. Res. 2018, 25, 25638–25647. [Google Scholar] [CrossRef]
- Zhang, H.; Voroney, R.P.; Price, G.W. Effects of Temperature and Activation on Biochar Chemical Properties and Their Impact on Ammonium, Nitrate, and Phosphate Sorption. J. Environ. Qual. 2017, 46, 889–896. [Google Scholar] [CrossRef]
- Puga, A.P.; de Almeida Queiroz, M.C.; Ligo, M.A.V.; Carvalho, C.S.; Pires, A.M.M.; de Oliveira Santos Marcatto, J.; Alberto de Andrade, C. Nitrogen Availability and Ammonia Volatilization in Biochar-Based Fertilizers. Arch. Agron. Soil. Sci. 2020, 66, 992–1004. [Google Scholar] [CrossRef]
- De Morais, E.G.; Silva, C.A.; Gao, S.; Melo, L.C.A.; Benevenute, P.A.N.; Lago, B.C.; Teodoro, J.C.; Guilherme, L.R.G. Rapid Adsorption of Ammonium on Coffee Husk and Chicken Manure-Derived Biochars: Mechanisms Unveiled by Chemical Speciation, Physical, and Spectroscopic Approaches. Sustainability 2025, 17, 1616. [Google Scholar] [CrossRef]
- Pan, J.; Ma, J.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of Biochar Application for Enhanced Anaerobic Digestion: A Review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef]
- Bremner, J.M.; Keeney, D.R. Determination and Isotope-Ratio Analysis of Different Forms of Nitrogen in Soils: 3. Exchangeable Ammonium, Nitrate, and Nitrite by Extraction-Distillation Methods. Soil. Sci. Soc. Am. J. 1966, 30, 577–582. [Google Scholar] [CrossRef]
- Dos Santos, H.G.; Jacomine, P.K.T.; Anjos, L.H.C.D.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; de Oliveira, J.B.; Cunha, T.J.F. Sistema Brasileiro de Classificação de Solos; Embrapa Solos: Brasilia, Brazil, 2018. [Google Scholar]
- Novais, R.F.; Neves, J.C.L.; Barros, N.F. Ensaio Em Ambiente Controlado. In Métodos de Pesquisa Em Fertilidade do Solo; Oliveira, A.J., Ed.; Embrapa-SEA: Brasilia, Brazil, 1991; pp. 189–253. [Google Scholar]
- Do Carmo, D.L.; Silva, C.A.; de Lima, J.M.; Pinheiro, G.L. Electrical Conductivity and Chemical Composition of Soil Solution: Comparison of Solution Samplers in Tropical Soils. Rev. Bras. Cienc. Solo 2016, 40, e0140795. [Google Scholar] [CrossRef]
- Kjeldahl, J. Neue Methode Zur Bestimmung Des Stickstoffs in Organischen Körpern. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Ferreira, E.B.; Cavalcanti, P.P.; Nogueira, D.A. ExpDes: An R Package for ANOVA and Experimental Designs. Applied Mathematics 2014, 5, 2952–2958. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, Y.; Ma, J.; Jiang, J.; You, X.; Lv, R.; Zhou, S.; Pan, C.; Liu, B.; Xu, Q.; et al. How Does Biochar Influence Soil Nitrification and Nitrification-Induced N2O Emissions? Sci. Total Environ. 2024, 908, 168530. [Google Scholar] [CrossRef]
- Yuan, D.; Wang, G.; Hu, C.; Zhou, S.; Clough, T.J.; Wrage-Mönnig, N.; Luo, J.; Qin, S. Electron Shuttle Potential of Biochar Promotes Dissimilatory Nitrate Reduction to Ammonium in Paddy Soil. Soil. Biol. Biochem. 2022, 172, 108760. [Google Scholar] [CrossRef]
- Hu, C.; Wang, X.; Li, J.; Luo, L.; Liu, F.; Wu, W.; Xu, Y.; Li, H.; Tan, B.; Zhang, G. Trends in the Research on Soil Nitrogen Leaching from Farmland: A Bibliometric Analysis (2014–2023). Clim. Smart Agric. 2024, 1, 100026. [Google Scholar] [CrossRef]
- Yang, X.; Lu, Y.; Ding, Y.; Yin, X.; Raza, S.; Tong, Y. Optimising Nitrogen Fertilisation: A Key to Improving Nitrogen-Use Efficiency and Minimising Nitrate Leaching Losses in an Intensive Wheat/Maize Rotation (2008–2014). Field Crops Res. 2017, 206, 1–10. [Google Scholar] [CrossRef]
- Autret, B.; Guillier, H.; Pouteau, V.; Mary, B.; Chenu, C. Similar Specific Mineralization Rates of Organic Carbon and Nitrogen in Incubated Soils under Contrasted Arable Cropping Systems. Soil. Tillage Res. 2020, 204, 104712. [Google Scholar] [CrossRef]
- Morais, E.G.; Silva, C.A.; Maluf, H.J.G.M.; de Oliveira Paiva, I.; de Paula, L.H.D. Effects of Compost-Based Organomineral Fertilizers on the Kinetics of NPK Release and Maize Growth in Contrasting Oxisols. Waste Biomass Valorization 2022, 14, 2299–2321. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Z.; Tian, D.; Wang, J.; Wang, B.; Chen, H.Y.H.; Quan, Q.; Chen, W.; Yang, J.; Meng, C.; et al. Global Variations and Controlling Factors of Soil Nitrogen Turnover Rate. Earth Sci. Rev. 2020, 207, 103250. [Google Scholar] [CrossRef]
- Braos, L.B.; Carlos, R.S.; Kuhnen, F.; Ferreira, M.E.; Mulvaney, R.L.; Khan, S.A.; Cruz, M.C.P. da Predicting Soil Nitrogen Availability for Maize Production in Brazil. Nitrogen 2022, 3, 555–568. [Google Scholar] [CrossRef]
- Adriaanse, F.G.; Human, J.J. Effect of Time of Application and Nitrate: Ammonium Ratio on Maize Grain Yield, Grain N Concentration and Soil Mineral N Concentration in a Semi-Arid Region. Field Crops Res. 1993, 34, 57–70. [Google Scholar] [CrossRef]
- Ravazzolo, L.; Trevisan, S.; Forestan, C.; Varotto, S.; Sut, S.; Dall’Acqua, S.; Malagoli, M.; Quaggiotti, S. Nitrate and Ammonium Affect the Overall Maize Response to Nitrogen Availability by Triggering Specific and Common Transcriptional Signatures in Roots. Int. J. Mol. Sci. 2020, 21, 686. [Google Scholar] [CrossRef]
- Guo, S.; Zhou, Y.; Shen, Q.; Zhang, F. Effect of Ammonium and Nitrate Nutrition on Some Physiological Processes in Higher Plants-Growth, Photosynthesis, Photorespiration, and Water Relations. Plant Biol. 2007, 9, 21–29. [Google Scholar] [CrossRef]
- de Carvalho, T.A.; Puga, A.P.; Moreno Pires, A.M.; Vieira Ligo, M.A.; de Andrade, C.A. Residual Effect of Nitrogen Fertilizers Formulated with Biochar. Hortic. Int. J. 2019, 3, 315–318. [Google Scholar] [CrossRef]
- Freitas, A.M.; Nair, V.D.; Harris, W.G. Biochar as Influenced by Feedstock Variability: Implications and Opportunities for Phosphorus Management. Front. Sustain. Food Syst. 2020, 4, 510982. [Google Scholar] [CrossRef]
- de Morais, E.G.; Silva, C.A. Novel Slow-Release NPK Biochar-Based Fertilizers with Acidulated Apatite: Evaluation of the Fertilization Value in a Short-Term Experiment. J. Soil. Sci. Plant Nutr. 2023, 23, 4937–4954. [Google Scholar] [CrossRef]
- Phillips, C.L.; Meyer, K.M.; Garcia-Jaramillo, M.; Weidman, C.S.; Stewart, C.E.; Wanzek, T.; Grusak, M.A.; Watts, D.W.; Novak, J.; Trippe, K.M. Towards Predicting Biochar Impacts on Plant-Available Soil Nitrogen Content. Biochar 2022, 4, 9. [Google Scholar] [CrossRef]
- Nguyen, V.-T.; Vo, T.-D.-H.; Tran, T.; Nguyen, T.-N.; Le, T.-N.-C.; Bui, X.-T.; Bach, L.-G. Biochar Derived from the Spent Coffee Ground for Ammonium Adsorption from Aqueous Solution. Case Stud. Chem. Environ. Eng. 2021, 4, 100141. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 13143–13151. [Google Scholar]
- Naseri, A.; Alirezalu, A.; Noruzi, P.; Alirezalu, K. The Effect of Different Ammonium to Nitrate Ratios on Antioxidant Activity, Morpho-Physiological and Phytochemical Traits of Moldavian Balm (Dracocephalum Moldavica). Sci. Rep. 2022, 12, 16841. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.-S.; Hur, J. Biochar-Induced Priming Effects in Soil via Modifying the Status of Soil Organic Matter and Microflora: A Review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef]
- Resh, H.M. Hydroponic Food Production; CRC Press: New York, NY, USA, 2022; ISBN 9781003133254. [Google Scholar]
- Karthik, R.; Dhaker, D.; Raising, L.P. Performance of Cereals under Need Based Nitrogen Management Strategies: A Review. Agric. Rev. 2021, 43, 320–326. [Google Scholar] [CrossRef]
- Silva, L.; Conceição, L.A.; Lidon, F.C.; Maçãs, B. Remote Monitoring of Crop Nitrogen Nutrition to Adjust Crop Models: A Review. Agriculture 2023, 13, 835. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, Z.; Xu, L.; Buyong, F.; Chay, T.C.; Li, Z.; Cai, Y.; Hu, B.; Zhu, Y.; Wang, X. Modified Biochar: Synthesis and Mechanism for Removal of Environmental Heavy Metals. Carbon. Res. 2022, 1, 8. [Google Scholar] [CrossRef]
- Zhang, X.; Ward, B.B.; Sigman, D.M. Global Nitrogen Cycle: Critical Enzymes, Organisms, and Processes for Nitrogen Budgets and Dynamics. Chem. Rev. 2020, 120, 5308–5351. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira da Silva, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient Availability and Leaching in an Archaeological Anthrosol and a Ferralsol of the Central Amazon Basin: Fertilizer, Manure and Charcoal Amendments. Plant Soil. 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Liu, X.; Hu, B.; Chu, C. Nitrogen Assimilation in Plants: Current Status and Future Prospects. J. Genet. Genom. 2022, 49, 394–404. [Google Scholar] [CrossRef]
- Liu, S.; He, Z.; Dong, Q.; Zhao, A.; Huang, F.; Bi, D. Comparative Assessment of Water and Organic Acid Washing Pretreatment for Nitrogen-Rich Pyrolysis: Characteristics and Distribution of Bio-Oil and Biochar. Biomass Bioenergy 2022, 161, 106480. [Google Scholar] [CrossRef]
- Shi, J.; Fan, X.; Tsang, D.C.W.; Wang, F.; Shen, Z.; Hou, D.; Alessi, D.S. Removal of Lead by Rice Husk Biochars Produced at Different Temperatures and Implications for Their Environmental Utilizations. Chemosphere 2019, 235, 825–831. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H.; Huang, H.; Xiao, R.; Li, R.; Zhang, Z. Influence of Temperature and Residence Time on Characteristics of Biochars Derived from Agricultural Residues: A Comprehensive Evaluation. Process Saf. Environ. Prot. 2020, 139, 218–229. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W.; Steiner, C.; Das, K.C.; Ahmedna, M.; Rehrah, D.; Watts, D.W.; Busscher, W.J.; et al. Characterization of Designer Biochar Produced at Different Temperatures and Their Effects on a Loamy Sand. Ann. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of Pyrolysis Temperature and Manure Source on Physicochemical Characteristics of Biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef]
- Murchie, E.H.; Kefauver, S.; Araus, J.L.; Muller, O.; Rascher, U.; Flood, P.J.; Lawson, T. Measuring the Dynamic Photosynthome. Ann. Bot. 2018, 122, 207–220. [Google Scholar] [CrossRef]
- Sales, C.R.G.; Molero, G.; Evans, J.R.; Taylor, S.H.; Joynson, R.; Furbank, R.T.; Hall, A.; Carmo-Silva, E. Phenotypic Variation in Photosynthetic Traits in Wheat Grown under Field versus Glasshouse Conditions. J. Exp. Bot. 2022, 73, 3221–3237. [Google Scholar] [CrossRef]
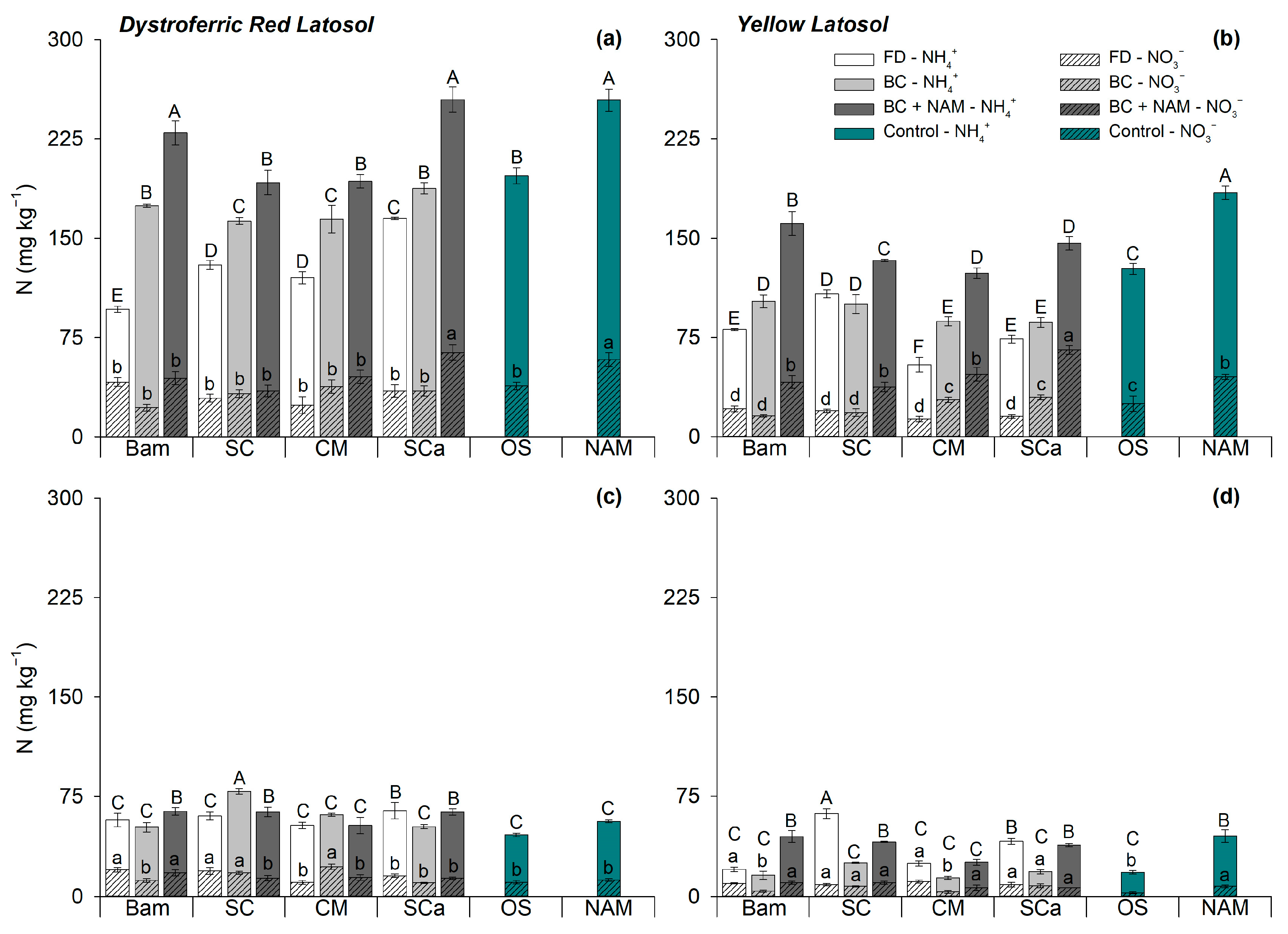

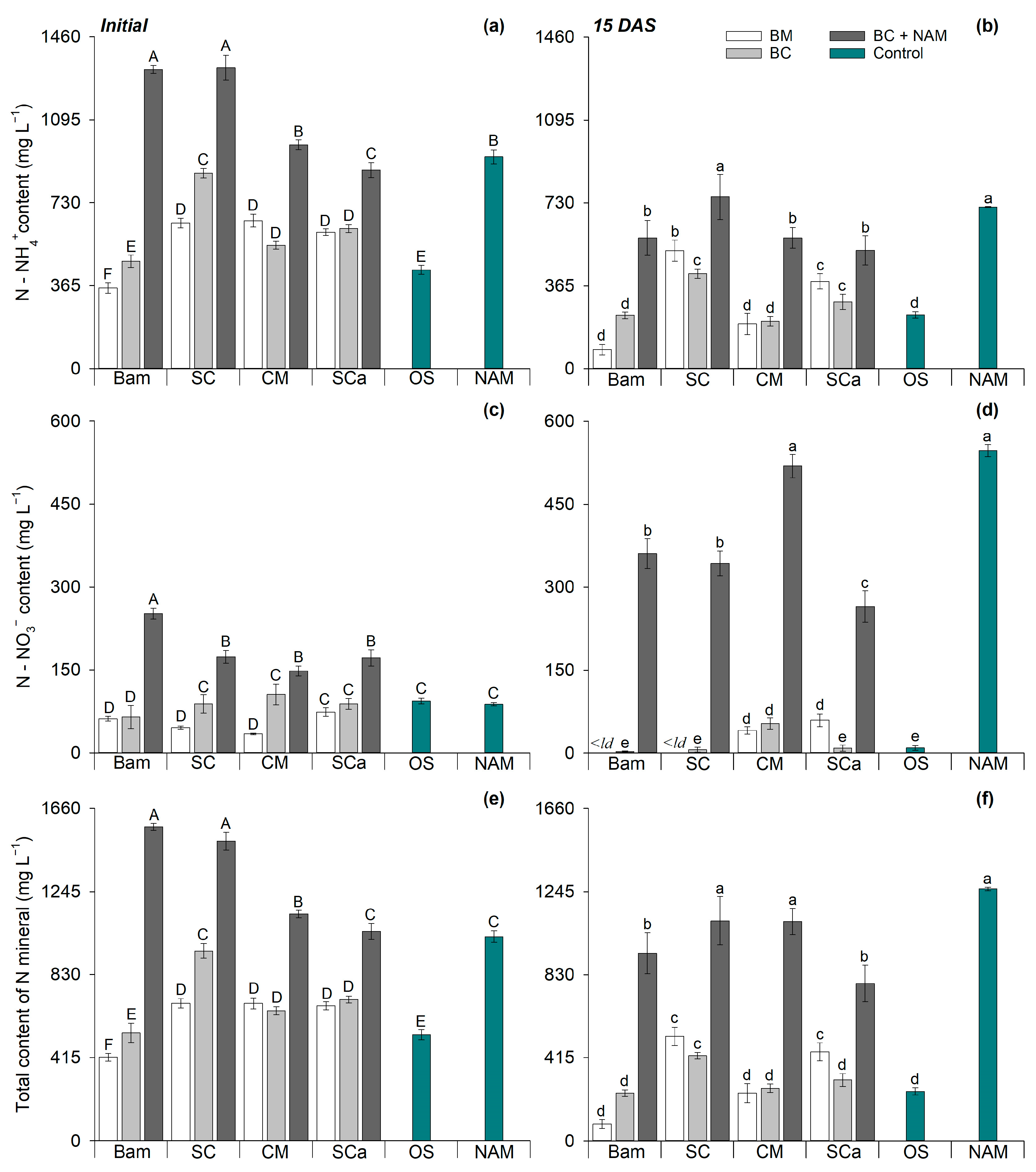
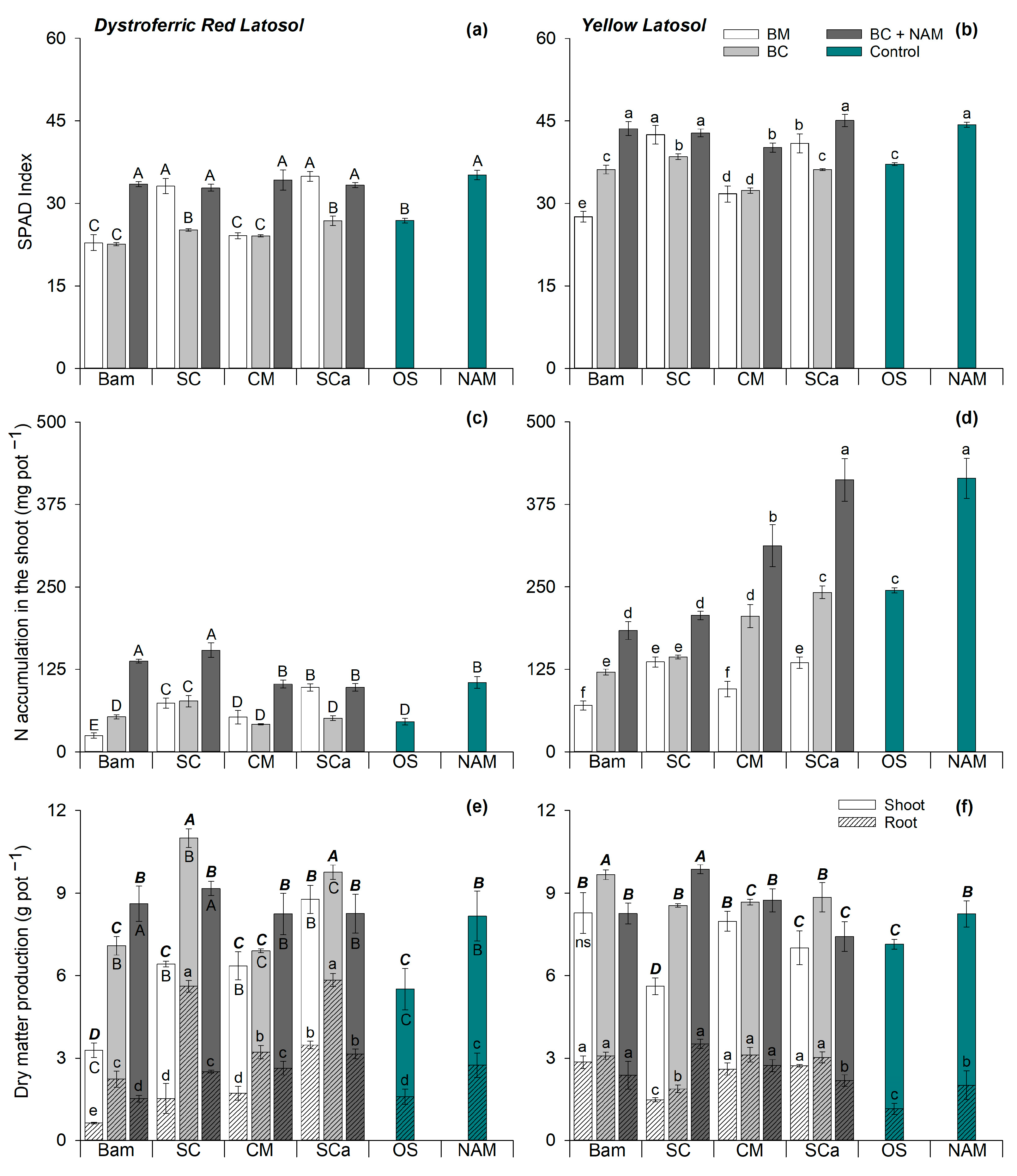
| N Source | pH | EC (mS cm−1) | Total C (g kg−1) | Total N (g kg−1) | N-NH4+ (mg kg−1) | N-NO3− (mg kg−1) |
|---|---|---|---|---|---|---|
| Bam | 6.6 | 1.1 | 479 | 4 | 78.7 | 65.0 |
| Bam300 | 7.2 | 0.5 | 772 | 5 | 78.7 | 34.2 |
| SC | 5.3 | 25.6 | 336 | 41 | 160.7 | 44.5 |
| SC300 | 8.3 | 31.3 | 430 | 49 | 2889.9 | 136.8 |
| CM | 7.5 | 7.5 | 250 | 33 | 1607.4 | 2384.7 |
| CM300 | 9.6 | 6.6 | 309 | 37 | 253.1 | 147.1 |
| SCa | 7.9 | 7.8 | 435 | 89 | 745.6 | 547.2 |
| SCa300 | 9.4 | 8.3 | 472 | 75 | 242.8 | 218.9 |
| Parameters | LVd | LVa |
|---|---|---|
| pH (1 g of soil: 2.5 mL of water) | 4.3 | 5.8 |
| EC (mS cm−1) | 0.16 | 0.25 |
| Total carbon (g kg−1) | 46 | 11 |
| Total nitrogen (g kg−1) | 4.5 | 1.3 |
| Clay (g kg−1) | 750 | 230 |
| Silt (g kg−1) | 110 | 25 |
| Sand (g kg−1) | 140 | 745 |
| Acronym of Treatment | Description of Treatments Applied | N Input (mg pot−1) |
|---|---|---|
| Bam | Bamboo (feedstock) (2.7 g pot−1) | 10.8 |
| Bam300 | Bamboo-derived biochar (2.7 g pot−1) | 13.5 |
| Bam300 + N | Bamboo-derived biochar (2.7 g pot−1) plus ammonium nitrate (300 mg kg−1 of N) | 253.5 |
| SC | Sunflower cake (feedstock) (2.7 g pot−1) | 110.7 |
| SC300 | Sunflower cake-derived biochar (2.7 g pot−1) | 132.3 |
| SC300 + N | Sunflower cake-derived biochar (2.7 g pot−1) plus ammonium nitrate (300 mg kg−1 of N) | 372.3 |
| CM | Chicken manure (feedstock) (2.7 g pot−1) | 89.1 |
| CM300 | Chicken manure-derived biochar (2.7 g pot−1) | 99.9 |
| CM300 + N | Chicken manure-derived biochar (2.7 g pot−1) plus ammonium nitrate (300 mg kg−1 of N) | 339.9 |
| SCa | Shrimp carcass (feedstock) (2.7 g pot−1) | 240.0 |
| SCa300 | Shrimp carcass-derived biochar (2.7 g pot−1) | 202.5 |
| SCa300 + N | Shrimp carcass-derived biochar (2.7 g pot−1) plus ammonium nitrate (300 mg kg−1 of N) | 442.5 |
| OS | Without the application of ammonium nitrate, biochar, and/or feedstock. | 0 |
| NAM | Only application of ammonium nitrate (300 mg kg−1 of N) | 240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, I.d.O.; Morais, E.G.d.; Silva, C.A. Biochar and Ammonium Nitrate Synergies: Enhancing Nitrogen Availability and Maize Growth in Oxisols. Agronomy 2025, 15, 633. https://doi.org/10.3390/agronomy15030633
Paiva IdO, Morais EGd, Silva CA. Biochar and Ammonium Nitrate Synergies: Enhancing Nitrogen Availability and Maize Growth in Oxisols. Agronomy. 2025; 15(3):633. https://doi.org/10.3390/agronomy15030633
Chicago/Turabian StylePaiva, Igor de Oliveira, Everton Geraldo de Morais, and Carlos Alberto Silva. 2025. "Biochar and Ammonium Nitrate Synergies: Enhancing Nitrogen Availability and Maize Growth in Oxisols" Agronomy 15, no. 3: 633. https://doi.org/10.3390/agronomy15030633
APA StylePaiva, I. d. O., Morais, E. G. d., & Silva, C. A. (2025). Biochar and Ammonium Nitrate Synergies: Enhancing Nitrogen Availability and Maize Growth in Oxisols. Agronomy, 15(3), 633. https://doi.org/10.3390/agronomy15030633






