Evaluating the Level of Total Mercury Present in the Soils of a Renowned Tea Production Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Overview of the Study Area
2.2. Sample Collection and Analysis
2.3. Evaluation Method
2.3.1. Soil Accumulation Index Method
2.3.2. Positive Matrix Factorization (PMF) Model
2.3.3. Health Risk Assessment Methods
2.4. Statistical Analysis Methods
3. Results and Discussion
3.1. The Distribution Characteristics and Pollution Evaluation of Total Mercury in Soil of Tea Garden
3.2. Distribution of Total Mercury Content in Tea
3.3. Source Apportionment of Total Mercury in Tea Garden Soil
3.4. Health Risk Assessment of Total Mercury in Soil of Tea Garden
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aschner, M. Mercury toxicity. J. Pediatr. 2001, 138, 450–451. [Google Scholar] [CrossRef] [PubMed]
- Zahir, F.; Rizwi, S.; Haq, S.; Khan, R. Low dose mercury toxicity and human health. Environ. Toxicol. Pharmacol. 2005, 20, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Guo, G.; Yan, Z. Status and environmental management of soil mercury pollution in China: A review. J. Environ. Manage 2021, 277, 111442. [Google Scholar] [CrossRef]
- Xu, J.; Bravo, A.; Lagerkvist, A.; Bertilsson, S.; Sjöblom, R.; Kumpiene, J. Sources and remediation techniques for mercury contaminated soil. Environ. Int. 2015, 74, 42–53. [Google Scholar] [CrossRef]
- Yu, H.; Li, J.; Luan, Y. Meta-analysis of soil mercury accumulation by vegetables. Sci. Rep. 2018, 8, 1261. [Google Scholar] [CrossRef]
- Cao, H.; Qiao, L.; Zhang, H.; Chen, J. Exposure and risk assessment for aluminium and heavy metals in Puerh tea. Sci. Total. Environ. 2010, 408, 2777–2784. [Google Scholar] [CrossRef]
- Colapinto, C.; Arbuckle, T.; Dubois, L.; Fraser, W. Is there a relationship between tea intake and maternal whole blood heavy metal concentrations? J. Expo. Sci. Environ. Epidemiol. 2016, 26, 503–509. [Google Scholar] [CrossRef]
- Krebs, D. The role of ground vegetation in the uptake of mercury and methylmercury in a forest ecosystem. Plant. Soil. 2003, 253, 445–455. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Li, X.; Zhang, J.; Gong, Y.; Yang, S.; Ye, M.; Yu, X.; Ma, J. Status of mercury accumulation in agricultural soils across China (1976–2016). Ecotoxicol. Environ. Saf. 2020, 197, 110564. [Google Scholar] [CrossRef]
- Bailey, E.; Gray, J.; Theodorakos, P. Mercury in vegetation and soils atabandoned mercury mines in southwestern Alaska, USA. Geochem. Explor. Environ. Anal. 2002, 2, 275–285. [Google Scholar] [CrossRef]
- Rimondi, V.; Gray, J.; Costagliola, P.; Vaselli, O.; Lattanzi, P. Concentration, distribution, and translocation of mercury and methylmercury in mine-waste, sediment, soil, water, and fish collected near the Abbadia San Salvatore mercury mine, Monte Amiata district, Italy. Sci. Total. Environ. 2012, 414, 318–327. [Google Scholar] [CrossRef]
- Song, Z.; Wang, C.; Ding, L.; Chen, M.; Hu, Y.; Li, P.; Zhang, L.; Feng, X. Soil Mercury Pollution Caused by Typical Anthropogenic Sources in China: Evidence from Stable Mercury Isotope Measurement and Receptor Model Analysis. J. Clean. Prod. 2020, 288, 125687. [Google Scholar] [CrossRef]
- Driscoll, C.; Mason, R.; Chan, H.; Jacob, D.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Feng, X.; Li, P.; Fu, X.; Wang, X.; Zhang, H.; Lin, C. Mercury pollution in China: Implications on the implementation of the Minamata Convention. Environ. Sci. Process Impacts 2022, 24, 634–648. [Google Scholar] [CrossRef]
- Pribil, M.; Rimondi, V.; Costagliola, P.; Lattanzi, P.; Rutherford, D. Assessing mercury distribution using isotopic fractionation of mercury processes and sources adjacent and downstream of a legacy mine district in Tuscany, Italy. Appl. Geochem. 2020, 117, 104600. [Google Scholar] [CrossRef]
- Yevugah, L.; Darko, G.; Bak, J. Does mercury emission from small-scale gold mining cause widespread soil pollution in Ghana? Environ. Pollut. 2021, 284, 116945. [Google Scholar] [CrossRef]
- Feng, Z.; Deng, L.; Guo, Y.; Guo, G.; Wang, L.; Zhou, G.; Huan, Y.; Liang, T. The spatial analysis, risk assessment and source identification for mercury in a typical area with multiple pollution sources in southern China. Environ. Geochem. Health 2023, 45, 4057–4069. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Aamir, M.; Liu, K.; Yang, F.; Liu, W. Status of mercury accumulation in agricultural soil across China: Spatial distribution, temporal trend, influencing factor and risk assessment. Environ. Pollut. 2018, 240, 116e124. [Google Scholar] [CrossRef]
- Xia, J.; Wang, J.; Zhang, L.; Anderson, C.; Wang, X.; Zhang, H.; Dai, Z.; Feng, X. Screening of native low mercury accumulation crops in a mercurypolluted mining region: Agricultural planning to manage mercury risk in farming communities. J. Clean. Prod. 2020, 262, 121324. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Y.; Hu, K.; Chen, J.; Djomo, S.; Yang, X.; Knudsen, M. Economic, environmental, and emergy analysis of China's green tea production. Sustain. Prod. Consum. 2021, 28, 269–280. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Li, Z.; Wang, Y.; Yang, Y.; Liu, M.; Li, D.; Sun, G.; Zeng, B. Concentrations, leachability, and health risks of mercury in green tea from major production areas in China. Ecotoxicol. Environ. Saf. 2022, 232, 113279. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, H.; Guo, Z.; Lu, P.; Song, F.; Liu, L.; Liu, J.; Rose, N.; Wang, F. Positive matrix factorization on source apportionment for typical pollutants in different environmental media: A review. Environ. Sci. Process Impacts 2020, 22, 239–255. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xu, H.; Zhao, H.; Gao, J.; Liang, D.; Li, Y.; Wang, W.; Feng, Y.; Shi, G. Source apportionment of fine particulate matter at a megacity in China, using an improved regularization supervised PMF model. Sci. Total. Environ. 2023, 879, 163198. [Google Scholar] [CrossRef] [PubMed]
- Norris, G.; Duval, R.; Brown, S.; Bai, S. EPA Positive Matrix Factorization (PMF) 5.0 Fundamentals and User Guide; U.S. Environmental Protection Agency: Washington, DC, USA, 2014; pp. 1–136. [Google Scholar]
- Li, F.; Zhang, J.; Jiang, W.; Liu, C.; Zhang, Z.; Zhang, C.; Zeng, G. Spatial health risk assessment and hierarchical risk management for mercury in soils from a typical contaminated site, China. Environ. Geochem. Health 2017, 39, 923–934. [Google Scholar] [CrossRef]
- Xu, X.; Han, J.; Pang, J.; Wang, X.; Lin, Y.; Wang, Y.; Qiu, G. Methylmercury and inorganic mercury in Chinese commercial rice: Implications for overestimated human exposure and health risk. Environ. Pollut. 2020, 258, 113706. [Google Scholar] [CrossRef]
- Wei, M.; Pan, A.; Ma, R.; Wang, H. Distribution characteristics, source analysis and health risk assessment of heavy metals in farmland soil in Shiquan County, Shaanxi Province. Process Saf. Environ. Prot. 2023, 171, 225–237. [Google Scholar] [CrossRef]
- Guan, X.; Yang, J. Research on Chinese tea consumption and its corresponding enlightenments. J. Tea. Sci. 2014, 40, 75–79. [Google Scholar]
- JECFA. The Joint FAO/WHO Expert Committee on Food Additives Seventy Second Meeting: Summary and Conclusions; JECFA: Geneva, Switzerland, 2010; pp. 1–16. [Google Scholar]
- Huang, X.; Hu, J.; Qin, F.; Quan, W.; Cao, R.; Fan, M.; Wu, X. Heavy metal pollution and ecological assessment around the Jinsha Coal-Fired Power Plant (China). Int. J. Environ. Res. Public Health 2017, 14, 1589. [Google Scholar] [CrossRef]
- GB 15618-2018; Soil Environmental Quality—Risk Control Standard for Soil Contamination of Agricultural Land. Ministry of Ecology and Environment of the People’s Republic of China; State Administration for Market Regulation: Beijing, China, 2018.
- Qu, R.; Han, G.; Liu, M.; Zhang, Q. Vertical distribution and contamination of soil mercury in karst catchment, southwest China: Land-use type influence. Clean-Soil Air Water 2021, 49, 1–8. [Google Scholar] [CrossRef]
- Zeng, S.; Wang, X.; Yuan, W.; Luo, J.; Wang, D. Mercury accumulation and dynamics in montane forests along an elevation gradient in Southwest China. J. Environ. Sci. 2022, 34, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Guo, J.; Wang, L.; Wu, Q. Vertical distribution characteristics of soil mercury and its formation mechanism in permafrost regions: A case study of the Qinghai-Tibetan plateau. J. Environ. Sci. 2022, 113, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Brodziak-Dopierała, B.; Fischer, A.; Szczelina, W.; Stojko, J. The content of mercury in herbal dietary supplements. Biol. Trace Elem. Res. 2018, 185, 236–243. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, L.; Liu, H. Soil mercury distribution and pollution in urban and suburbs of Guilin. Geochimica 2000, 29, 94–99. [Google Scholar] [CrossRef]
- Li, J.; Liu, M.; Zhang, W.; Tian, Y. Evaluation and source analysis on heavy metal pollution of vegetable garden soil in the urban-rural area of Fushun city. Adv. Mater. Res. 2012, 518–523, 2121–2129. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Meng, W.; Liu, N.; Wu, P. Accumulation and source apportionment of heavy metal(loid)s in agricultural soils based on GIS, SOM and PMF: A case study in superposition areas of geochemical anomalies and zinc smelting, Southwest China. Process. Saf. Environ. Prot. 2022, 159, 964–977. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, R.; Li, Y.; Peng, Y.; Wen, X.; Ni, X. Distribution, accumulation, and potential risks of heavy metals in soil and tea leaves from geologically different plantations. Ecotoxicol. Environ. Saf. 2020, 195, 110475. [Google Scholar] [CrossRef]
- Li, Y.; Chang, M.; Ding, S.; Wang, S.; Ni, D.; Hu, H. Monitoring and source apportionment of trace elements in PM2.5: Implications for local air quality management. J. Environ. Manage. 2017, 196, 16–25. [Google Scholar] [CrossRef]
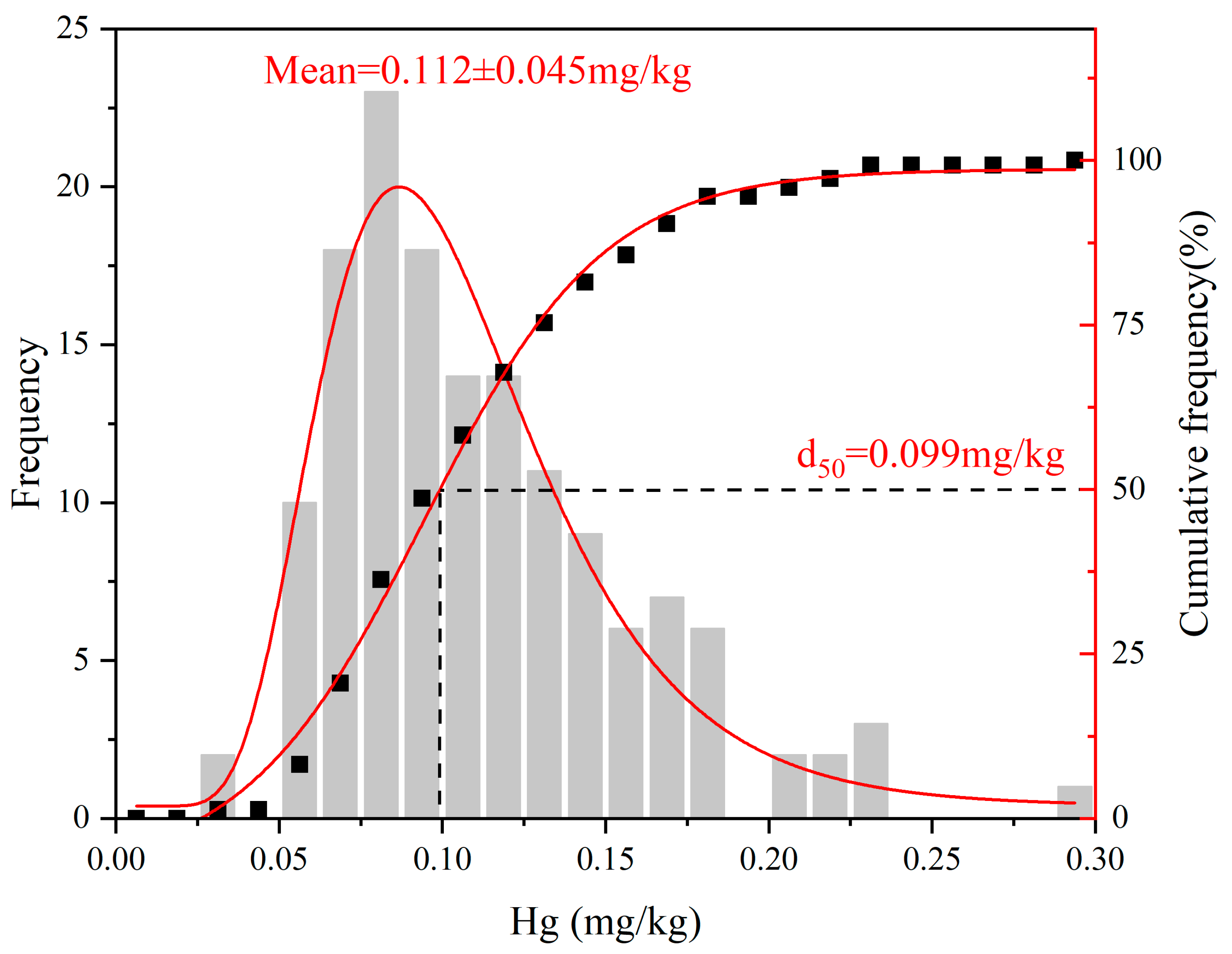

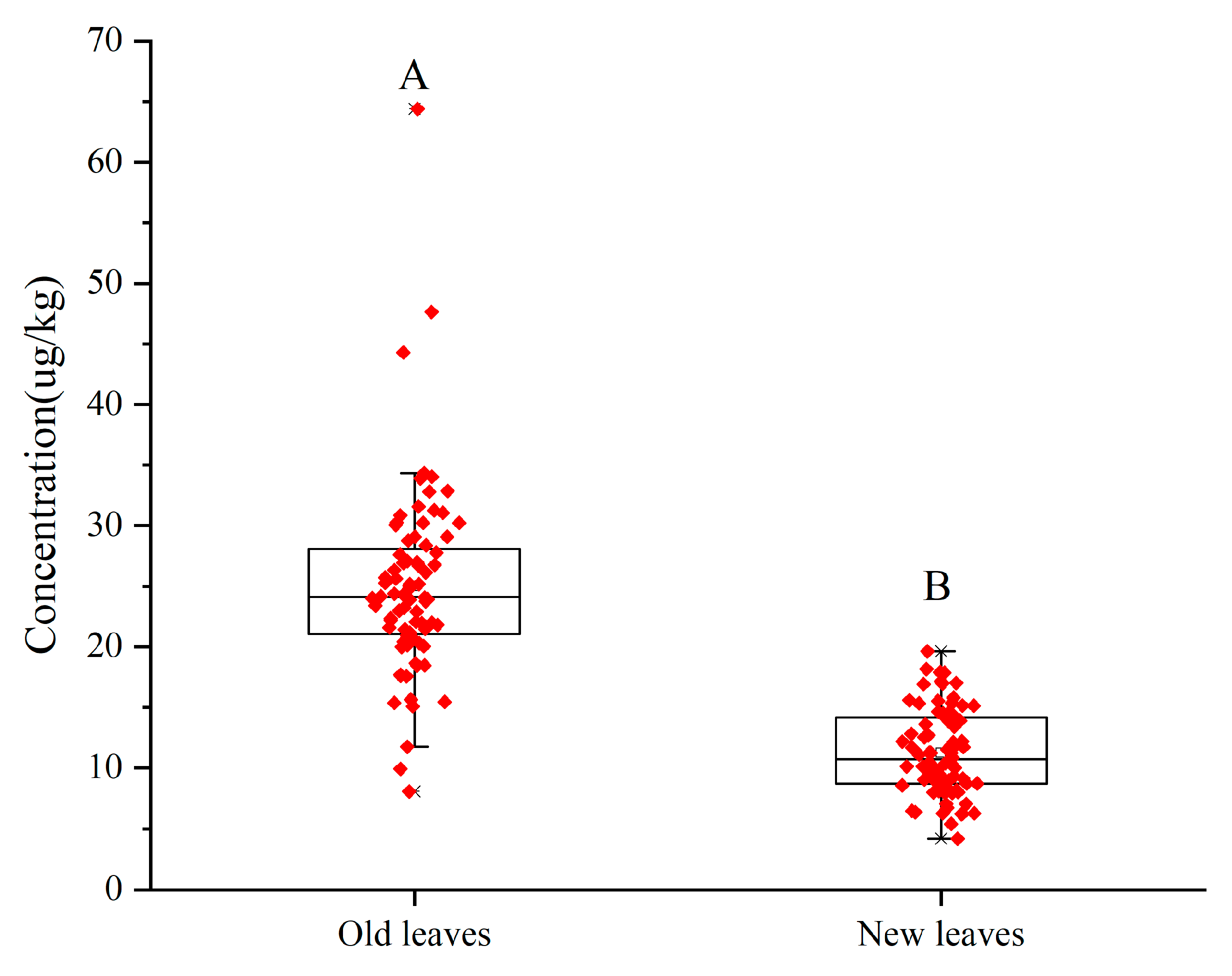

| Area | Samples | Min | Max | Med |
|---|---|---|---|---|
| YC | 49 | −1.06 | 1.29 | −0.39 |
| AX | 49 | −1.19 | 0.88 | −0.01 |
| DH | 18 | −2.28 | 0.94 | −0.09 |
| NA | 30 | −1.75 | 0.47 | −0.53 |
| Classification | Pollution Degree | Cumulative Index | Samples | Proportion(%) |
|---|---|---|---|---|
| Level 1 | pollution-free | I ≤ 0 | 99 | 67.81 |
| Level 2 | No pollution to medium pollution | 0 < I ≤ 1 | 46 | 31.51 |
| Level 3 | medium pollution | 1 < I ≤ 2 | 1 | 0.68 |
| Element | Source Component Spectrum (mg/kg) | Source Contribution Rate (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 6 | |
| Hg | 0.000 | 0.008 | 0.011 | 0.081 | 0.012 | 0.000 | 0.2 | 6.7 | 9.9 | 72.4 | 10.7 | 0.0 |
| Cu | 10.02 | 0.00 | 0.39 | 1.87 | 0.00 | 0.00 | 81.6 | 0.0 | 3.2 | 15.2 | 0.0 | 0.0 |
| As | 0.18 | 0.72 | 8.18 | 0.00 | 0.00 | 0.93 | 1.8 | 7.2 | 81.7 | 0.0 | 0.0 | 9.3 |
| Cd | 0.006 | 0.000 | 0.002 | 0.006 | 0.000 | 0.029 | 14.5 | 0.0 | 4.4 | 13.6 | 0.0 | 67.5 |
| Ni | 0.07 | 10.65 | 0.57 | 0.49 | 0.00 | 1.15 | 0.6 | 82.4 | 4.4 | 3.8 | 0.0 | 8.9 |
| Zn | 7.31 | 1.90 | 1.92 | 7.76 | 16.52 | 32.31 | 10.8 | 2.8 | 2.8 | 11.5 | 24.4 | 47.7 |
| Pb | 0.00 | 2.12 | 0.00 | 0.00 | 48.22 | 8.40 | 0.0 | 3.6 | 0.0 | 0.0 | 82.1 | 14.3 |
| Min | Max | Mean | Std. | |
|---|---|---|---|---|
| PDI (μg/kg bw/day) | 0.013 | 0.157 | 0.060 | 0.024 |
| HQHg (%) | 0.023 | 0.275 | 0.104 | 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Xie, R.; Liu, L.; Huang, Z. Evaluating the Level of Total Mercury Present in the Soils of a Renowned Tea Production Region. Agronomy 2025, 15, 435. https://doi.org/10.3390/agronomy15020435
Xu J, Xie R, Liu L, Huang Z. Evaluating the Level of Total Mercury Present in the Soils of a Renowned Tea Production Region. Agronomy. 2025; 15(2):435. https://doi.org/10.3390/agronomy15020435
Chicago/Turabian StyleXu, Jinghua, Ruijia Xie, Liping Liu, and Zhiqun Huang. 2025. "Evaluating the Level of Total Mercury Present in the Soils of a Renowned Tea Production Region" Agronomy 15, no. 2: 435. https://doi.org/10.3390/agronomy15020435
APA StyleXu, J., Xie, R., Liu, L., & Huang, Z. (2025). Evaluating the Level of Total Mercury Present in the Soils of a Renowned Tea Production Region. Agronomy, 15(2), 435. https://doi.org/10.3390/agronomy15020435





