Abstract
Iron (Fe) deficiency is a pervasive agricultural concern on a global scale. Intercropping plays a pivotal role in activating soil nutrient cycling and crop nutrient uptake and utilization. This study integrates plant physiology, soil physicochemical determination, high-throughput sequencing, and metabolomics techniques to conduct pot experiments using field-collected soils with soybean and maize plants. This study aims to investigate the mechanisms through which microorganisms in a soybean–maize intercropping system regulate Fe deficiency adaptation. The results revealed that intercropping enhances the resilience of soybean and maize in Fe-deficient environments, facilitates nutrient absorption by plants, and enriches soil nutrient content. Moreover, intercropping fostered more intricate microbial interactions in comparison to monocropping. The dominant microorganisms in the rhizosphere of intercropped soybean and maize included genera Microbacterium, Sphingomonas, Shinella, and Rhizobium. Microbacterium, Sphingomonas, Shinella, and Rhizobium have the potential to produce Fe chelators or enhance plant Fe absorption. Additionally, intercropping notably modified the composition of root exudates derived from soybean and maize. The soybean and maize rhizosphere exhibited significant enrichment with oleamide, coumestrol, glycitein, and daidzein. Coumestrol may have an effect of promoting Fe absorption, and it is significantly positively correlated with the genus Nakamurella in the maize rhizosphere and the genus Pirellula in the soybean rhizosphere. Consequently, these findings suggested that the rhizosphere of intercropped soybean and maize significantly enriches specific microbial communities and root exudates, thereby enhancing microecosystem stability and improving plant tolerance to Fe deficiency.
1. Introduction
Intercropping, as a widely employed agricultural practice, entails the simultaneous cultivation of diverse plant species within the same field [1], which is primarily adopted to achieve sustainable intensification and enhance agricultural quality. Therefore, intercropping serves as an efficient land utilization strategy and a sustainable agricultural practice [2]. Numerous studies have consistently demonstrated that intercropping systems exhibit enhanced utilization of light and heat alongside improved nitrogen use efficiency, ultimately leading to elevated grain yields [3]. Due to the interspecies facilitation in the rhizosphere, particularly between gramineae and leguminosae, intercropping systems exhibit enhanced benefits within resource-limited agricultural contexts [4]. The intercropping of legumes and cereals has proven its potential to increase nitrogen facilitation and yield through nitrogen fixation [5]. The intercropping of legumes and maize enhances the fertility status of rhizosphere soil primarily through alterations in enzyme activities and shifts in the composition of soil microbial flora [6]. Therefore, the intercropping of soybean and maize is a viable and sustainable agronomic approach.
Iron (Fe) is a crucial micronutrient for plant survival. However, the solubilizing capacity and bioavailability of Fe are notably limited in alkaline and calcareous soils, thereby impeding the absorption and utilization of Fe in crops [7]. Fe deficiency is a prevalent nutritional limitation associated with the development of leaf chlorosis in plants growing in calcareous and/or alkaline soils [8]. Plants have developed two strategies in response to Fe limitation: a reduction-based strategy employed by non-grasses (Strategy I), where plants enhance the reduction of Fe (III) to Fe (II) and subsequently transport it into the root epidermis through the Fe-regulated transporter [9]. In contrast, graminaceous monocots adopt a chelation strategy (Strategy II), which involves biosynthesis and secretion of phytosiderophores (PSs) from the mugineic acid family and then chelates Fe (III) from the rhizosphere to form chelates Fe (III)-PSs [10]. These complexes are subsequently internalized by yellow stripe-like family transporters in the roots [4]. Intercropping of soybean and maize enhances Fe nutrition in soybean, thereby significantly promoting symbiotic nitrogen fixation within the nodules [11]. However, the mechanisms by which intercropping regulates Fe uptake remain inadequately elucidated.
The rhizosphere refers to the soil volume surrounding plant roots, which is significantly influenced by root function and serves as a site for biogeochemical processes driven by active microorganisms [12]. Planting patterns, fertilization conditions, and root exudates can all exert influences on the biogeochemical processes occurring in the rhizosphere soil [13]. Soil microorganisms play a crucial role in nutrient retention, driving nutrient recycling, maintaining soil structure, and facilitating the conversion of organic carbon [14,15]. Intercropping generally leads to an increase in both the abundance and diversity of microorganisms, consequently enhancing soil enzyme activity and improving crop quality [16]. The intercropping systems of potatoes and legumes have the potential to elevate dehydrogenase activity and microbial biomass carbon in smallholder farms [17]. The concentrations of available phosphorus and soil organic matter are higher in intercropped soybean and maize rhizosphere soil, while the stability of the microorganism community structure in the soybean rhizosphere is enhanced [18]. Legume-based intercropping systems can augment both symbiotic and non-symbiotic beneficial populations, thereby enhancing rhizobia community diversity [19]. Therefore, further research is warranted to elucidate the impact of intercropping on soil microbiota architecture and diversity.
To counteract infection and confer tissue-specific resistance, plants release a diverse array of biologically active compounds into the rhizosphere, including primary metabolites such as sugars, amino acids, and carboxylic acids, alongside an extensive repertoire of secondary metabolites [20]. Root exudates play a pivotal role in the initial stages of plant colonization by serving as essential sources of metabolites that function as nutrients, chemoattractants, or antimicrobials for soil microbes [21]. Furthermore, root exudates dynamically alter throughout plant growth and development, thereby shaping microbial communities with distinct diversity and functionalities [22]. The quantity of root exudates is intricately linked to microbial biomass, and together, they influence root interactions [22]. In intercropping systems, the release of root exudates, such as carboxylates, phosphatases, and phytosiderophores from crop plant roots and soil microbes, may enhance the solubility of phosphorus, Fe, and zinc for improved nutrient acquisition by crops [23]. Thus, it is crucial to investigate the microbial community and root exudates in regulating plant environmental adaptation within intercropping systems.
This study employed a range of physiological, biochemical, high-throughput sequencing, and metabolomics techniques to investigate the mechanisms underlying Fe uptake and transport in the soil-plant system during soybean and maize intercropping. The research aims to establish a theoretical and scientific foundation for the regulation of plants under varying soil Fe content conditions while providing insights and solutions for mitigating plant Fe deficiency.
2. Materials and Methods
2.1. Experimental Design and Plant Materials
Approximately 2500 kg of Fe-deficient soil (a pH value of 7.9 and an Fe content of 2.87 mg/kg) was collected from 0 to 20 cm of depth in Jingyuan County, Gansu Province, China (104.71° E, 36.54° N). Subsequently, soybean and maize pot experiments were conducted from May to September 2021 at the Institute of Soil and Water Conservation, Northwest A&F University. The recovered soil was air-dried and sieved into pots with a diameter of 30 cm. Each pot was filled with 11 kg of soil and simultaneously mixed with 2 g of urea and 2 g of phosphate fertilizer. A total of 216 pots were utilized for planting. The Glycine max L. cv. Zhonghuang 13 and Zea mays L. cv. Zhengdan 958 were used. The planting patterns consist of M (monoculture) and I (intercropping). The Fe concentration in the soil was set up by adding Fe (III)-EDTA to establish distinct Fe gradients: Fe1 (Fe deficiency, soil effective iron content of 3 mg·kg−1), Fe2 (Fe sufficiency, soil effective iron content of 5 mg·kg−1), and Fe3 (Fe excess, soil effective iron content of 8 mg·kg−1). The harvest time is based on the growth cycle of soybean and includes three phases: P1 (the flowering stage), P2 (the grain stage), and P3 (the maturity stage).
2.2. Plant Physiological Indicators Determination
We used a portable photosynthesis instrument (Li-6400, LiCor, Lincoln, NE, USA) to measure the net photosynthetic rate of soybean and maize fully unfolded leaves at 9 to 11 a.m. The photosynthetic instrument’s air temperature, effective photosynthetic radiation, air relative humidity, and CO2 concentration settings were maintained at 25 °C, 800 µmol m−2 s−1, 70%, and 400 µmol mol−1. The chlorophyll content of the uppermost fully expanded leaves of soybean and maize plants was assessed using the SPAD-502PLUS chlorophyll instrument (Konica Minolta, Kumamoto, Japan) at 10 a.m. [24].
2.3. Plant Element Content Determination
We collected roots, stems, leaves, root nodules, pods, seeds of soybean, and roots, stems, and leaves of maize at different periods. Samples of different plant tissues were dried using a drying cabinet (DGX-9143B) and weighed with an electronic balance (AL-204). Whole plant Fe content was measured using a flame spectrophotometer (Hitachi Z2000, Hitachi, Chiyoda, Japan) [25]. Plant full nitrogen content was measured using a Kjeldahl nitrogen meter (KjeltecTM 8400, FOSS, Hillerød, Denmark) [26]. Plant organic carbon is measured using a digestion process [27]. Whole plant phosphorus was measured using an ultraviolet spectrophotometer (UV-1900, Shimadzu, Kyoto, Japan) [28].
2.4. Collection of Plant and Soil Samples
A total of 36 treatments were collected across 3 distinct Fe concentration gradients (Fe1, Fe2, Fe3) during 3 periods (P1, P2, P3), as well as under 2 planting patterns (intercropping, monocropping), with 8 replicates for each treatment. The entire soybean plant was carefully extracted from the pot. After removing large soil clumps, the root zone (RZ) soil was collected by gently shaking the roots. Subsequently, the roots were excised and placed into a sterile 50 mL centrifuge tube. Phosphoric acid buffer was then added to facilitate the release of rhizosphere soil via a vortex shaker. The soil sediment was collected and centrifuged once again. Finally, the rhizosphere (RH) soil was stored at −80 °C in a refrigerator. Meanwhile, the root (R) was repeatedly washed with phosphate buffer solution until the solution turned clear. It was also stored at −80 °C in the refrigerator for the subsequent extraction of DNA.
2.5. Sample DNA Extraction, High-Throughput Sequencing, and Sequence Processing
Total DNA from RZ, RH, and R soil were extracted according to the DNA extraction kit MOBIO PowerSoil® DNA. The purity and concentration of DNA were determined using Thermo NanoDrop One (Thermo Fisher Scientific, Fitchburg, WI, USA). Genomic DNA was used as a template for PCR amplification using specific primers with barcode and TaKaRa Premix Taq® Version 2.0. Bacterial diversity was assessed using 16S V4 region primers (515F and 806R). The PCR amplification system comprised 25 μL of 2x Premix Taq, 1 μL of Primer-F (10 μM), 1 μL of Primer-R (10 μM), and 50 ng of DNA template; ddH2O was added to a final volume of 50 μL. The PCR amplification protocol involved initial denaturation at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 52 °C for 30 s, extension at 72 °C for 30 s, concluding with a final extension at 72 °C for 10 min. Each sample was subjected to three replicate reactions, and the resulting PCR products from the same sample were pooled. The PCR apparatus used was the BioRad S1000 (Bio-Rad Laboratories, Hercules, CA, USA). The fragment length and concentration of the PCR products were evaluated via 1% agarose gel electrophoresis. Samples exhibiting a main band within the expected size range were deemed suitable for subsequent experiments. Using GeneTools Analysis Software (Version 4.03.05.0), the concentration of PCR products was quantified, and the required volume of each sample was calculated based on the principle of equal mass, after which the PCR products were combined. The E.Z.N.A.® Gel Extraction Kit (Omega Bio-tek, Norcross, GA, USA) was employed to purify the PCR mixture, and the recovered target DNA fragments were eluted with TE buffer. The PE250 sequencing of the constructed amplicon libraries was conducted on the Illumina Nova 6000 platform. The DADA2-based bioinformatic pipeline was used to process the raw data [29]. The obtained amplicon sequence variants (ASV) were then classified and identified using the SILVA database v132 [30].
2.6. Root Exudate Collection
A total of 12 treatments were collected across 3 distinct Fe concentration gradients (Fe1, Fe2, Fe3) under 2 planting patterns (intercropping, monocropping) during the P2 period. For each treatment, complete roots were extracted from four distinct pots of plants, and rhizosphere soil was collected. They were placed in 10 mL centrifuge tubes, immediately placed into liquid nitrogen for quick freezing, and transferred to a −80 °C refrigerator. A 100 mg sample of liquid nitrogen-ground material was transferred to an EP tube, followed by the addition of 500 μL of an 80% methanolic aqueous solution. The mixture was vigorously vortexed and then incubated in an ice bath for 5 min. Subsequently, the sample was subjected to centrifugation at 15,000× g for 20 min at 4 °C. An aliquot of the supernatant was diluted with mass spectrometry-grade water to achieve a final methanol concentration of 53%. The diluted supernatant was then centrifuged again at 15,000× g for 20 min at 4 °C, and the resulting supernatant was collected for injection into the liquid chromatography-mass spectrometry (LC-MS) system for analysis.
2.7. Data Processing and Metabolite Identification
The off-machine data (.raw) file was imported into the CD 3.1 library search software for processing, and a simple screening of parameters, such as retention time and mass-to-charge ratio, was performed for each metabolite. The retention time deviation was set to 0.2 min, and the mass deviation was set to 5 ppm to align peaks for different samples to make identification more accurate. Then, the mass deviation was set to 5 ppm, the signal intensity deviation was set to 30%, and the signal-to-noise ratio was set to 3 for the minimum signal intensity, sum ion, and other information to perform peak extraction. Simultaneously, peak areas were quantified, target ions were integrated, and then molecular formulas were predicted using molecular ion peaks and fragment ions and compared with the mzCloud (https://www.mzcloud.org/), mzVault, and Masslist databases. The blank sample was used to remove background ions and normalize the original quantification results to obtain metabolite identification and relative quantification results. The data processing part is based on the Linux operating system (CentOS version 6.6) and software R software (Version 3.3.0) and Python (Version 2.7.12).
2.8. Statistical Analysis of Data
A three-way analysis of ANOVA was conducted to evaluate the effects of Time (P1, P2, and P3), crop type (monoculture vs. intercropping), and Fe levels (deficiency, sufficiency, and excess) on plant chlorophyll content, photosynthetic rate, and soil physicochemical properties. Post-hoc comparisons were performed using Tukey’s HSD tests for data on plant chlorophyll content, photosynthesis, dry weight, elemental composition, soil physicochemical properties, and microbial alpha diversity under the combined conditions of different iron concentrations and different planting patterns. The Alpha diversity Shannon index was calculated by the R software (Version 3.2.4) vegan package and the picante package, and the significant difference in the Shannon index was tested. Community similarity was calculated using the Bray–Curtis distance and then analyzed using the Principal Coordinate Analysis (PCoA), performed with the vegan package. Permutation multivariate analysis of variance (PPERMANOVA) analysis was performed using the “adonis” function in the vegan package. Species stacked bar graphs were drawn using the vegan and ggplot2 packages. Linear discriminant analysis (LDA) analysis was performed using the microeco package to explore the significant taxa in the different treatments [31]. Microbial co-occurrence networks were first filtered for microbial taxa present in less than half of the samples and used the igraph and Hmisc packages to calculate network topological properties, Spearman correlation, and significance P, with FDR correction for p-values. p-values were corrected for FDR, and the visual analysis was performed using Gephi 0.9.2. The topology parameters of each network graph were calculated, including average degree, average weighted degree, network diameter, graph density, modularity, average clustering coefficient, eigenvector centrality, and average path length. Canonical correspondence analysis (CCA) was performed using the vegan package (Version 2.5.6) to explore the correlation between microbial communities and plant physiological and soil physicochemical properties. Heat maps of correlations between environmental factors and major species abundances were created using the pheatmap package (Version 1.0.12).
3. Results
3.1. Effects of Planting Patterns and Fe Content on Plant Growth
Planting patterns and soil Fe content significantly influenced plant phenotype and photosynthetic performance (Figure 1). The optimal plant growth and greener leaf color were achieved when the soil Fe content was at an appropriate level, whereas both deficient and excessive levels of soil Fe content inhibited plant growth (Figure 1A). The chlorophyll content and photosynthetic rate of soybean and maize gradually increased with higher soil Fe availability, resulting in leaves becoming greener over time during all growth periods under both monocropping and intercropping systems (Figure 1B,C). In particular, the photosynthetic rate of intercropped soybeans was the highest when the Fe concentration was appropriate (Figure 1C). In parallel, soybean demonstrated greater dry weight in its roots, stems, leaves, nodules, pods, and seeds, while maize exhibited a similar trend in its roots, stems, and leaves under optimal soil Fe content throughout P1 to P3 stages (Figure 1D).
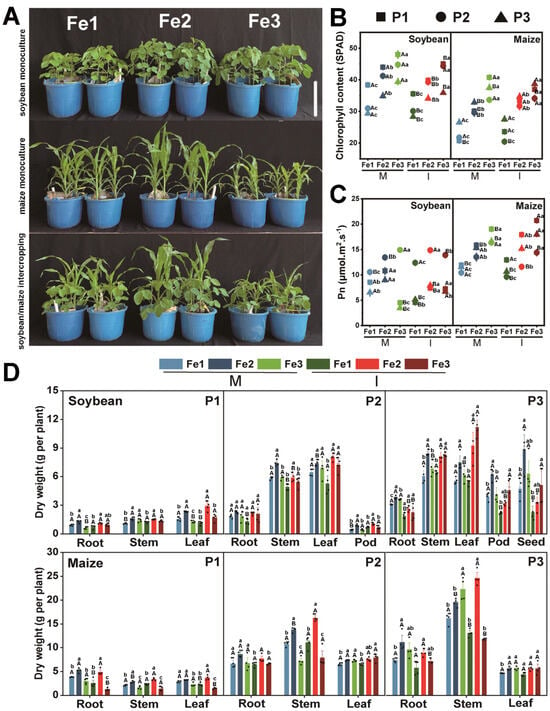
Figure 1.
Effects of planting patterns and Fe content on plant growth. (A) Phenotype of soybean and maize under monoculture and intercropping systems across different soil iron concentration gradients. Bar = 30 cm. (B) Plant chlorophyll content, (C) net photosynthetic rate, and (D) dry weight of soybean and maize during three growth stages. The determination time is based on the growth cycle of soybean and includes three phases: P1 (the flowering stage), P2 (the grain stage), and P3 (the maturity stage). The planting patterns consist of M (monoculture) and I (intercropping). The Fe levels are categorized as follows: Fe1 (soil-effective iron content of 3 mg·kg−1), Fe2 (soil-effective iron content of 5 mg·kg−1), and Fe3 (soil-effective iron content of 8 mg·kg−1). The data are the mean values ± SE. For the same sampling time (B,C) or the same part of the plant (D), capital letters represent significant differences (p < 0.05) among different planting patterns at the same Fe level, and lowercase letters represent significant differences (p < 0.05) among different Fe levels under the same planting pattern.
Planting patterns and soil Fe content also influenced plant element content (Figure 2). Soybean and maize exhibited the highest total Fe content when grown in soil with appropriate Fe levels (Figure 2A). The sufficient Fe levels increased the total nitrogen and organic carbon content of soybean and maize (Figure 2B,C). Excessive Fe hindered phosphorus uptake by soybeans during the P1 and P2 stages. (Figure 2D). On the other hand, intercropping increased the Fe content in soybean leaves, pods, and seeds at all growth stages, as well as the organic carbon content in roots and leaves (Figure 2A,C). It also enhanced nitrogen content in maize at all growth stages, phosphorus content in roots and stems at P1, and organic carbon content in roots and stems at P3 (Figure 2B–D).
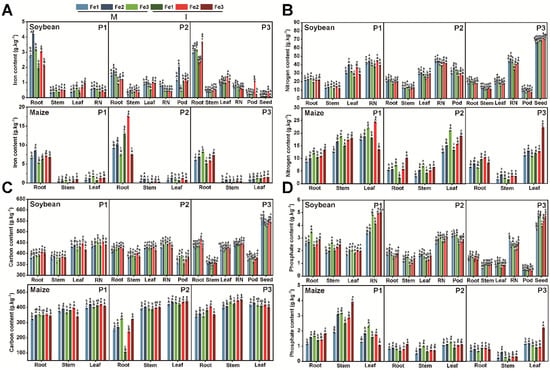
Figure 2.
Effect of planting pattern and soil iron content on plant elemental content. Total iron (A), total nitrogen (B), total organic carbon (C), and total phosphorus (D) of different tissues of soybean and maize. The determination time is based on the growth cycle of soybean and includes three phases: P1 (the flowering stage), P2 (the grain stage), and P3 (the maturity stage). The planting patterns consist of M (monoculture) and I (intercropping). The Fe levels are categorized as follows: Fe1 (soil-effective iron content of 3 mg·kg−1), Fe2 (soil-effective iron content of 5 mg·kg−1), and Fe3 (soil-effective iron content of 8 mg·kg−1). The data are the mean values ± SE. For the same part of the plant, capital letters represent significant differences (p < 0.05) among different planting patterns at the same Fe level, and lowercase letters represent significant differences (p < 0.05) among different Fe levels under the same planting pattern.
The diverse planting patterns and Fe content modified the soil’s physicochemical properties (Figure 3). In comparison to Fe deficiency, sufficient Fe levels led to an increase in soil pH, total nitrogen (TN), soil organic carbon (SOC), nitrate nitrogen (NO3−-N), soil urease (S-UE), as well as alkaline phosphatase (ALP) and β-glucosidase (β-GC) activities (Figure 3A,C–F,H,I). Intercropping resulted in a rise in TN, SOC, NO3−-N, ammonium nitrogen (NH4+-N), and β-GC activity for both soybean and maize throughout all three growth stages (Figure 3C,D,I). These results revealed that intercropping facilitates the uptake of essential nutrients and improves plant adaptability to Fe-deficient environments.
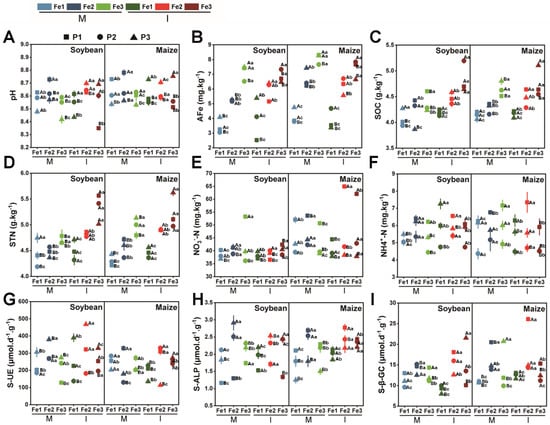
Figure 3.
Effects of planting patterns and soil iron content on soil physicochemical properties, including soil pH (A), available iron (AFe) (B), soil organic carbon (SOC) (C), total nitrogen (TN) (D), nitrate nitrogen (NO3−-N) (E), ammoniacal nitrogen (NH4+-N) (F), soil urease (S-UE) (G), soil alkaline phosphatase (S-ALP) (H), soil β-glucosidase (S-β-GC) (I). The determination time is based on the growth cycle of soybean and includes three phases: P1 (the flowering stage), P2 (the grain stage), and P3 (the maturity stage). The planting patterns consist of M (monoculture) and I (intercropping). The Fe levels are categorized as follows: Fe1 (soil-effective iron content of 3 mg·kg−1), Fe2 (soil-effective iron content of 5 mg·kg−1), and Fe3 (soil-effective iron content of 8 mg·kg−1). A three-way analysis of variance was conducted to examine the effects of time, crop type, and Fe level. The data are the mean values ± SE. For the same sampling time, capital letters are used to indicate significant differences (p < 0.05) among different planting patterns at the same Fe level, and lowercase letters indicate significant differences (p < 0.05) among different Fe levels under the same planting pattern.
3.2. Effects of Planting Patterns and Fe Content on Bacterial Diversity and Composition
We investigated the impact of planting patterns at varying Fe concentrations on the bacterial community diversity within the root (R), rhizosphere (RH), and root zone (RZ) (Figure 4). The Shannon index of the bacterial community in the root of soybean was not significantly affected by different treatments during the P1 and P2 periods, but it significantly increased with the improvement of soil Fe levels in the P3 period (Figure 4A). When the Fe content was optimal, the Shannon index was lower than when Fe was deficient in both the root zone and rhizosphere (Figure 4A). The influence of planting patterns on the Shannon index was not significant in the first two periods, but in the P3 period, the Shannon index was greater in intercropping than in monocropping (Figure 4A). On the other hand, the Shannon index in maize root was lower in monoculture compared to Fe deficiency but higher in intercropping than Fe deficiency (Figure 4B). In the root zone, the Shannon index is lower than that of Fe deficiency when the Fe content is appropriate (Figure 4B). During the P2 period of maize growth, the Shannon index was greater in intercropping than in monoculture (Figure 4B).
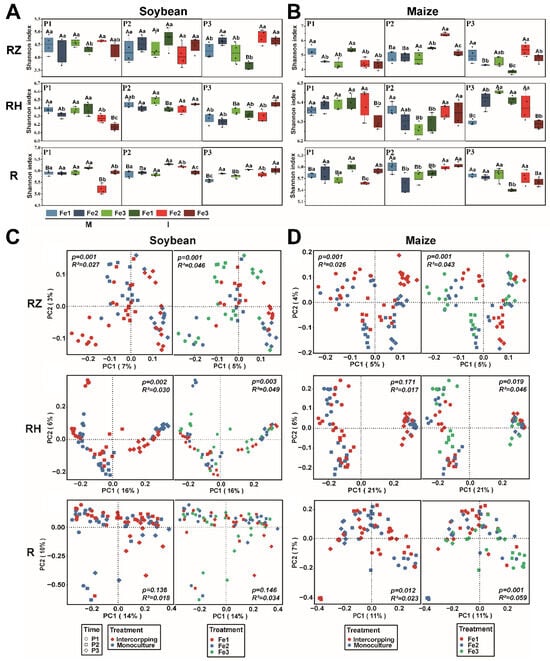
Figure 4.
The Shannon index of bacterial communities in the root (R), root zone (RZ), and rhizosphere (RH) of soybean (A) and maize (B). The determination time is based on the growth cycle of soybean and includes three phases: P1 (the flowering stage), P2 (the grain stage), and P3 (the maturity stage). The planting patterns consist of M (monoculture) and I (intercropping). The Fe levels are categorized as follows: Fe1 (soil-effective iron content of 3 mg·kg−1), Fe2 (soil-effective iron content of 5 mg·kg−1), and Fe3 (soil-effective iron content of 8 mg·kg−1). The data are the mean values ± SE. For the same sampling time, capital letters are used to indicate significant differences (p < 0.05) among different planting patterns at the same Fe level, and lowercase letters indicate significant differences (p < 0.05) among different Fe levels under the same planting pattern. The Principal Coordinate Analysis (PCoA) analysis is based on the Bray–Curtis distance of bacterial community in RZ, RH, and R of soybean (C) and maize (D) under different planting patterns or iron contents.
We further analyzed the differences in the bacterial community structure of the RZ, RH, and R in soybean and maize grown under different planting patterns and Fe contents by using PCoA analysis based on the Bray–Curtis distance (Figure 4C,D). PERMANOVA revealed significant differences in microbial communities associated with planting patterns and Fe content in both the RZ (P: p = 0.001, Fe: p = 0.001) and RH (P: p = 0.002, Fe: p = 0.003) of soybean (Figure 4C). However, no significant differences were observed in the R (Figure 4C). Significant differences were observed in maize for RZ (P: p = 0.001, Fe: p = 0.001), RH (Fe: p = 0.019), and R (P: p = 0.012, Fe: p = 0.001) (Figure 4D).
The rhizosphere is a hotspot for microbial activity, so it is important to focus on the composition of the microbial community in this area. The relative abundance distributions of the top 10 species in rhizosphere bacterial communities exhibited notable variations (Figure 5A,B). The dominant microorganisms in the rhizosphere of soybean and maize included Microbacterium, Sphingomonas, and Shinella (Figure 5A,B). The relative abundance of Microbacterium increased with elevated soil Fe content, exhibiting notably higher levels in intercropping as compared to monocropping (Figure 5A,B). LDA analysis revealed that Microbacterium and Rhizobium exhibited significantly higher abundance in the rhizosphere of intercropped soybean, while Microbacterium, Rhizobium, Nocardioides, and Sphingomonas showed significantly increased enrichment in the rhizosphere of intercropped maize (Figure 5A,B and Figure S1).
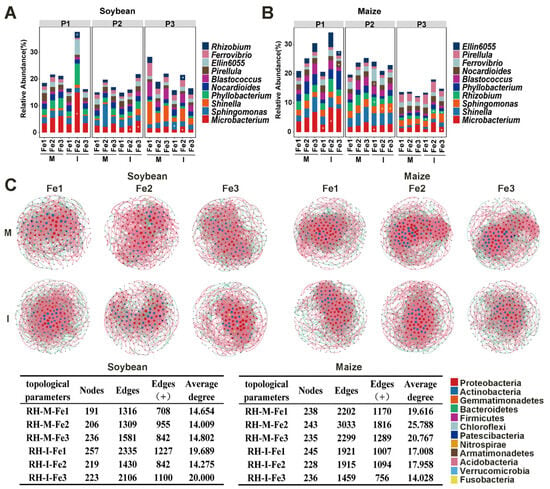
Figure 5.
Effects of planting patterns and Fe content on bacterial community composition. Classification of rhizosphere bacterial community in soybean (A) and in maize (B) at the generic level. P1, flowering period; P2, grain period; P3, maturity period; M, monoculture; I, intercropping; Fe1, soil effective iron content 3 mg·kg−1; Fe2: soil effective iron content 5 mg·kg−1; Fe3: soil effective iron content 8 mg·kg−1. The asterisks indicate the significantly enriched microorganisms determined through Linear Discriminant Analysis (LDA). (C) Co-occurrence network (phylum level) and network topological parameters of rhizosphere bacterial community in soybean and maize under different planting patterns and iron contents. Nodes are colored according to the different phyla, and edges are colored based on positive and negative correlations, with red representing positive correlation and green representing negative correlation.
The co-occurring network pattern observed in the rhizosphere microbial communities exhibited remarkable complexity. In the rhizosphere of soybean, the complexity of the intercropping pattern network is greater than that of the monoculture (Figure 5C). Under the monoculture pattern, the number of positive edges was the largest when the Fe content was appropriate, while under the intercropping pattern, the number of edges was the smallest when the Fe content was appropriate (Figure 5C). In the rhizosphere of maize, the complexity of the monoculture pattern network was greater than that of the intercropping (Figure 5C). Under the monoculture pattern, the number of positive edges was the largest when the Fe content was appropriate (Figure 5C). Under the intercropping pattern, excessive Fe significantly inhibited the complexity of the microbial network, resulting in fewer nodes and edges (Figure 5C). These results indicate that intercropping significantly increased the complexity of the microbial network and microbial interactions of soybean, while both excessively low and high Fe concentrations reduced the complexity of the microbial network in the rhizosphere of maize.
3.3. Effects of Planting Patterns and Fe Content on Root Exudates
Intercropping and Fe content influenced the composition and structure of root exudates in soybean and maize (Figure 6A,B). The PERMANOVA revealed a significant disparity in soybean root exudates between monocropping and intercropping (p = 0.001, R2 = 0.396) (Figure 6A). In maize, significant variations were observed in root exudates among different planting patterns (p = 0.012, R2 = 0.174) as well as different Fe content (p = 0.001, R2 = 0.319) (Figure 6B).
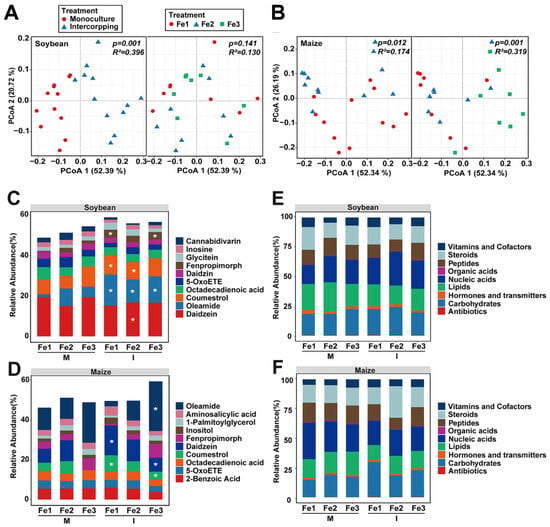
Figure 6.
Effects of planting patterns and Fe content on root exudates. The Principal Coordinate Analysis (PCoA) analysis is based on the Bray–Curtis distance of root exudates under different planting patterns and iron contents in soybean (A) and maize (B). Major root exudates composition under different planting patterns and iron contents in soybean (C) and maize (D). The asterisks indicate the significantly enriched root exudates determined through Linear Discriminant Analysis (LDA). Biological significance of root exudates under different planting patterns and iron contents in soybean (E) and maize (F). M, monoculture; I, intercropping; Fe1, soil effective iron content 3 mg·kg−1; Fe2: soil effective iron content 5 mg·kg−1; Fe3: soil effective iron content 8 mg·kg−1.
The distribution of the top 10 root exudates in soybean and maize varied under different planting patterns and Fe content (Figure 6C,D). In soybean, the levels of oleamide, coumestrol, and daidzein were notably higher in intercropping (Figure 6C and Figure S2). In the monocropping with sufficient Fe supply, the highest levels of oleamide, 5-oxoete, fenpropimorph, and cannabidivarin were observed, while the lowest levels of coumestrol, octadecadienoic acid, and daidzein were recorded (Figure 6C). In maize, the concentrations of coumestrol and daidzein were notably higher under low Fe conditions, while oleamide levels markedly increased with excess Fe content in intercropping systems (Figure 6D and Figure S2). Under monocropping with sufficient Fe, the levels of daidzein and 1-palmitoylglycerol were at their peak, while octadecadienoic acid and oleamide exhibited the lowest concentrations (Figure 6D).
Furthermore, the metabolites were annotated using the KEGG database, and the percentage content of the biological roles of metabolites was analyzed (Figure 6E,F). The levels of nucleic acids, vitamins, and cofactors in soybeans were higher in intercropping compared to monocropping (Figure 6E). In monoculture with appropriate Fe content, the highest contents were observed for lipids, nucleic acids, and peptides, while carbohydrates, steroids, and vitamins and cofactors had the lowest levels (Figure 6E). In the intercropping with appropriate Fe content, carbohydrates and nucleic acids exhibited the highest contents, whereas lipids, vitamins, and cofactors showed the lowest levels (Figure 6E). The levels of carbohydrates, steroids, and vitamins and cofactors of maize were markedly higher in intercropping compared to monocropping (Figure 6F). Under monocropping with appropriate Fe content, the level of carbohydrates was at its peak (Figure 6F). The level of vitamins and cofactors increased, but the level of nucleic acids decreased with rising Fe levels in monocropping (Figure 6F). In monocropping with suitable Fe content, steroids reached their highest level, while carbohydrate and peptide contents were at their lowest (Figure 6F). These findings suggested that intercropping markedly transformed the structure and composition of root exudates.
3.4. The Relationship Among Root Exudates, Soil Physicochemical Properties, and Bacterial Communities
The soil physicochemical properties reshaped the rhizosphere microbial community of soybean and maize (Figure 7). The soil available Fe (AFe), total nitrogen (TN), and soil organic carbon (SOC) significantly impacted the soybean rhizosphere microbial community (Figure 7A). In addition to these factors, soil AFe and soil pH also had a significant impact on the maize rhizosphere microbial community (Figure 7A). Moreover, the soil AFe was associated with the enrichment of Microbacterium, Phyllobacterium, Devosia, and Shinella in the rhizosphere of soybean (Figure 7A), whereas AFe content was related to the enrichment of Microbacterium, Phyllobacterium, Sphingomonas, and Ellin6055 in the rhizosphere of maize (Figure 7A). Both Microbacterium and Phyllobacterium were correlated with multiple physicochemical indicators in both soybean and maize rhizospheres (Figure 7A). These results revealed that soil physicochemical properties not only influence microbial community architecture but also exert a considerable effect on specific microorganisms.
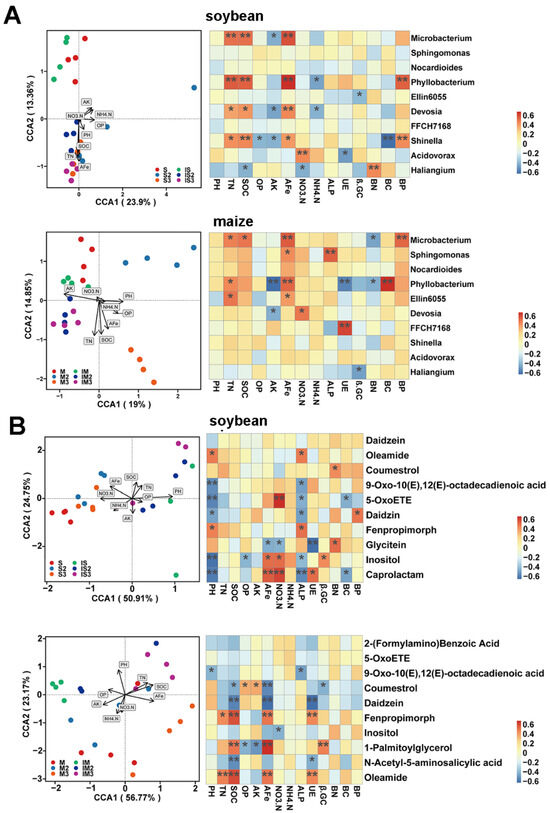
Figure 7.
Canonical Correspond Analysis (CCA) and correlation of heatmap between environmental factors and rhizosphere microbial community in soybean and in maize (A). CCA analysis and correlation of heatmap between environmental factors and root exudates of soybean and maize (B). S: soybean under Fe1 level and monocropping; S2: soybean under Fe2 level and monocropping; S3: soybean under Fe3 level and monocropping; IS: soybean under Fe1 level and intercropping; IS2: soybean under Fe2 level and intercropping; IS3: soybean under Fe3 level and intercropping; M: maize under Fe1 level and monocropping; M2: maize under Fe2 level and monocropping; M3: maize under Fe3 level and monocropping; IM: maize under Fe1 level and intercropping; IM2: maize under Fe2 level and intercropping; IM3: maize under Fe3 level and intercropping. The asterisk indicates a significant correlation (* < 0.05, ** < 0.01).
The soil’s physicochemical properties exerted a substantial influence on root exudates from soybean and maize (Figure 7B). Specifically, the pH level, AFe, NO3−-N, and NH4+-N in the soil played pivotal roles in shaping soybean root exudates (Figure 7B), while, apart from these factors, TN, SOC, and AFe significantly impacted maize root exudates (Figure 7B). The secretion of caprolactam, inositol, and glycitein by soybean, as well as the secretion of oleamide, 1-palmitoylglycerol, fenpropimorph, daidzein, and coumestrol by maize, showed a significant correlation with the AFe in soil (Figure 7B).
We further explored the correlation between microbial communities and root exudates (Figure 8). In the soybean rhizosphere, Ellin6055 and Haliangium were associated with a diverse range of root exudates (Figure 8A). Ellin6055 was linked to hexadecanamide, oleoyl ethylamide, α-lactose, fenpropimorph, daidzein, 9-oxo-10(e), 12(e)-octadecadienoic acid, oleamide and daidzein, and Haliangium were associated with hexadecanamide, oleoyl ethylamide, fenpropimorph, daidzein and oleamide (Figure 8A). In maize rhizosphere, Phyllobacterium, Ferrovibrio, and Nakamurella were associated with various root exudates, specifically daidzein, 1-palmitoylglycerol, and daidzein (Figure 8B). These findings suggested a more pronounced correlation between root exudates and microbial communities within the maize rhizosphere.
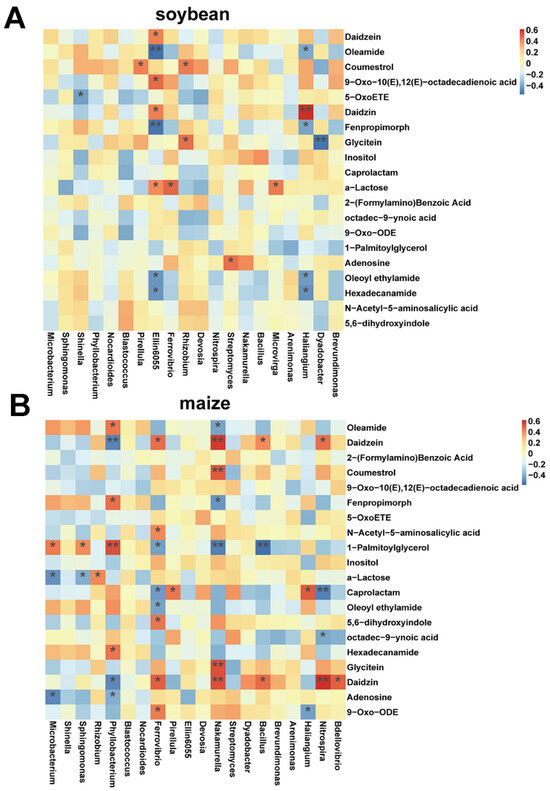
Figure 8.
Correlation of heatmap between root exudates and rhizosphere microbial community in soybean (A) and maize (B). The asterisk indicates a significant correlation (* < 0.05, ** < 0.01).
4. Discussion
4.1. Soybean and Maize Intercropping Enhances Nutrient Absorption Capacity
Intercropping is widely recognized as an ecologically sustainable approach that enhances crop productivity and optimizes the utilization of water and nutrients [32]. The intercropping of legumes and cereals has been extensively implemented in agriculture, and the mechanism of beneficial effects has been extensively studied [23]. In this study, soybean and maize intercropping led to an increase in the chlorophyll content and photosynthesis rate (Figure 1). This could be attributed to the complementary spatial and temporal niche and functional characteristics of C3 (soybean) and C4 (maize) crops when intercropped, thereby enhancing the light conversion efficiency of the system [33]. Furthermore, intercropping enhanced the levels of organic carbon and total nitrogen, along with a corresponding increase in the dry weight of maize (Figure 1D and Figure 2B,C). This might be attributed to the enhancement of photosynthetic product synthesis rate, which contributes to plant growth and development, thereby stimulating amino acid and protein synthesis in plants and directly augmenting carbon and nitrogen content within plants [34]. Intercropped legumes exhibit a 47% reduction in soil-derived nitrogen acquisition compared to monocultures, while grasses in intercropping systems demonstrate a significant increase of 61% in soil-derived nitrogen acquisition relative to monoculture [35]. These results revealed that intercropping maize exhibits a greater carbon and nitrogen uptake, thereby facilitating a substantial enhancement in dry weight.
Intercropping enhances plant nitrogen fixation and accumulation. Soybeans possess the ability of symbiotic nitrogen fixation, which allows them to convert a significant amount of atmospheric nitrogen into plant-available forms [36]. Conversely, maize primarily relies on soil nitrogen to fulfill its growth requirements and continues to absorb nitrogen throughout its entire growth cycle due to its significant demand. However, in an intercropping system, the symbiotic nitrogen fixation of soybeans not only satisfies their own needs but also releases a portion of nitrogen into the soil for the absorption and utilization of maize [37]. These findings revealed that a significant increase in the total nitrogen content of maize was observed through intercropping (Figure 2B) and a substantial enrichment of daidzein in the root exudates of intercropped maize (Figure 6D). Previous studies have shown that maize root exudates stimulate daidzein synthesis, enhance soybean nodulation, and facilitate symbiotic nitrogen fixation while acquiring nitrogen from the rhizosphere of soybean [38]. Legume crops complement the ecological niches of non-legume crops, promoting a more efficient nutrient cycling system resulting in significantly higher nitrogen utilization efficiency in intercropped maize compared to monocropped maize.
Intercropping impacts the phosphorus uptake of soybeans and maize. The bio-availability of soil phosphorus compounds is influenced by their chemical forms [39], and different plant species selectively acquire required nutrients from distinct types of soil phosphorus reserves. For instance, maize tends to rapidly consume inorganic phosphorus [40]. In contrast, soybeans utilize more organic phosphorus while consuming less inorganic phosphorus than maize [41]. Furthermore, intercropping maize with legumes can mobilize and utilize more soil phosphorus compared to monoculture maize [42]. Intercropping crops with high phosphorus activation ability alongside those with low phosphorus activation ability can enhance the phosphorus uptake of the latter, while soybean, known for its strong phosphorus activation ability, facilitates phosphorus absorption by maize [1]. Moreover, legume root systems release organic acids or hydrogen ions to compensate for calcium ion uptake, leading to soil acidification that promotes root nodule nitrogen fixation [23]. This acidification effect enhances the availability of phosphorus in calcareous soils and facilitates its absorption by the entire intercropping system [23].
4.2. Intercropping and Fe Content Affect the Rhizosphere Microbiome Assembly
Intercropping enhances soil organic matter content and provides abundant nutrient sources for microorganisms, thereby facilitating the enhancement of diversity and stability within the soil microbial community [43]. In this study, intercropping led to an increase in the Shannon index in the R, RZ, and RH of soybean at maturity, as well as of maize during the P2 period (Figure 4A,B). Moreover, we identified Microbacterium, Sphingomonas, and Rhizobium as the dominant genera inhabiting the rhizosphere of soybean and maize (Figure 5A,B). Microbacterium is the predominant group, and its relative abundance showed a positive correlation with increasing Fe concentration (Figure 5A,B). Microbacterium play a crucial role in Fe transport and exhibit the capability to suppress plant pathogens by producing Fe carriers [44,45]. Sphingomonas is a major contributor to functional redundancy in plant-associated microbial communities [46]. Sphingomonas also exhibits diverse functionalities, including environmental pollution remediation, promotion of plant growth, and strong competition for Fe resources, which can inhibit pathogenic bacteria [47]. Rhizobium is renowned for its pivotal role in nitrogen fixation as plant growth-promoting bacteria and has also demonstrated a robust capacity to synthesize Fe chelators [44]. These genera exhibited were positively correlated with available Fe concentration (Figure 7A,B), suggesting that they are likely to enhance plant tolerance to Fe-deficient environments by facilitating plant nutrient uptake and utilization.
More complex interactions among soil microorganisms in intercropping systems contribute to the diversity and stability of soil microbial communities. Microbial interactions tend to be simplified under resource scarcity, while they become more complex when resources are abundant [48,49]. Zhang, et al. [50] showed that soybean and sugarcane interactions increased the complexity of microbial networks. These results revealed that rhizosphere microbial networks of intercropped plants in low Fe concentrations exhibit greater intricacy compared to those in monocropping (Figure 5C), indicating that microorganisms prioritize survival strategies to ensure their own existence under limited Fe resources. Conversely, microorganisms exhibited a higher degree of negative correlations in Fe-rich environments (Figure 5C), suggesting that intense microbial competition occurs under nutrient-rich conditions [49]. Therefore, intercropping significantly augmented the complexity of the microbial network in soybeans, indicating that intercropping has the potential to enhance rhizosphere microbial niches and foster intricate microbial interactions.
The benefits of soybean–maize intercropping can be attributed to belowground interactions, specifically the enhancement of soil nutrient availability and microbial community [51]. The gramineous and leguminous crops intercropping affect the soil’s physiochemical properties, such as nutrient contents and enzyme activities [2]. Changes in soil nutrients resulting from intercropping are identified as crucial determinants of bacterial community structure and diversity [51]. The correlation between effective Fe and the soybean microbial community was found to be stronger compared to that of maize (Figure 7A). This discrepancy may be attributed to the distinct Fe absorption and utilization strategies [52,53]. Differences in Fe nutrition alter the root-related microbial community, and local variations in soil Fe content prompt plants to prefer specific root-associated microbial communities, particularly the distinctive inhibitory traits of root-associated microorganisms in competing for available Fe against pathogens [44,54]. In the rhizosphere of soybean and maize, Microbacterium, Phyllobacterium, and Shinella are positively correlated with the concentration of available Fe, indicating a preference for Fe by these microorganisms (Figure 7A). Numerous studies have documented the ability of Microbacterium and Shinella to utilize Fe for the production of Fe carriers [44,45], whereas no reports exist regarding the association between Phyllobacterium and Fe. Phyllobacterium has been demonstrated to play a crucial role in plant nitrogen fixation and promotion [55], as well as exhibiting phosphorus solubilization ability and promoting plant growth [56]. Moreover, soil pH plays a pivotal role in shaping the structure of microbial communities, and a decrease in soil pH is generally associated with reduced bacterial diversity [57]. This study demonstrated a significant influence of soil pH on the variation of plant rhizosphere microbial community (Figure 7A), consistent with previous findings indicating that pH is a key factor shaping turnover in rhizosphere microbial communities [58,59]. Soil total nitrogen, organic carbon, and available phosphorus contents also exerted effects on the rhizosphere microbial community (Figure 7A). These results revealed that the variations in soil physical and chemical properties induced by intercropping modify the microbial community composition, enhancing the abundance of Fe-absorbing microorganisms and promoting plant Fe tolerance.
4.3. Intercropping and Fe Content Change the Maize and Soybean Root Exudates
Root exudates encompass a diverse array of compounds, including monosaccharides, fatty acids, organic acids, amino acids, phytohormones, flavonoids, and phenolic acids [60]. Those compounds are released into the rhizosphere through the root system and are recognized for their diverse functions in ecological interactions with microbial soil communities [20]. Previous studies have supported that planting patterns can modify the root exudates. For instance, intercropping maize with peanuts results in a significant alteration of peanut root exudates by increasing the proportion of total isoflavones by 22.4% [43]. Additionally, intercropping maize with Lanzhou lily alleviates the continuous cropping barrier associated with Lanzhou lily through modulation of phenolic acid levels in root exudes [61]. Intercropping wheat with broad beans was found to reduce Fusarium abundance by modifying the composition of root exudates [62]. These findings demonstrated that intercropping significantly alters the structure and composition of root exudates in both soybean and maize, with a more pronounced effect observed on soybean root exudates (Figure 6A,B), possibly due to differential nutrient uptake responses between soybean and maize root.
The composition of maize root exudates was significantly influenced by varying Fe concentrations, while the effect on soybean root exudates was not statistically significant (Figure 6A,B). Specifically, the intercropping of soybean notably enhanced the accumulation of substances such as oleamide, fenpropimorph, and coumestrol under Fe deficiency (Figure 6C). Oleamide is a plant-derived lipid that plays a role in the interaction between plants and microorganisms [63]. Fenpropimorph can be utilized for the prevention of fungal pathogens in cereal and legume [64]. Notably, coumestrol has the potential to modify root-associated microorganisms, thereby enhancing plant growth in Fe-deficient soils [65,66]. Moreover, the rhizosphere of intercropping maize not only exhibited significant accumulations of oleamide and coumestrol but also demonstrated an accumulation of daidzein (Figure 6D). Daidzein not only increases the quantity and competitiveness of Bradyrhizobium diazoefficiens USDA11 [35], but also inhibits fungal growth and mycelial hydrolase activity, thereby reducing the abundance of Fusarium community in soils [67]. Furthermore, intercropping led to an enhanced secretion of carbohydrates and nucleic acid by soybean root under optimal soil Fe concentration (Figure 6E). Carbohydrates provide more nutrients to microorganisms, thus increasing their activity [68]. The root exudates of maize exhibited an enhanced enrichment of carbohydrates through intercropping, accompanied by a notable increase in the abundance of steroids, vitamins, and cofactors (Figure 6F). These substances have all been reported in the exudates of maize roots [69].
Plants employ various strategies to adapt to their environment, including releasing exudates into the soil. These exudates interact with the complex physical and chemical properties of the soil, which in turn influence their release and transformation [70]. This dynamic interplay allows plants to make adaptive adjustments in the composition of root exudates, thereby enhancing their capacity to respond to environmental changes in the soil [71]. This phenomenon of changes in soil characteristics caused by plants affecting plant performance is called plant-soil feedback [72]. There was a significant correlation between soil pH and the composition of root exudates from soybean and maize (Figure 7B), possibly attributed to the release of plant-derived organic acids. These organic acids can enhance soil carbon turnover by facilitating microbial-mediated utilization of available Fe [73]. Moreover, plant root secretions abundant in organic acids and ferriferous carriers produced by rhizosphere microorganisms can substitute for Fe within the complex, thereby enhancing its solubility [74]. In an intercropping system with Fe deficiency, maize roots significantly enrich daidzein and coumestrol secretions (Figure 6D). Oleamide, 1-palmitoylglycerol, fenpropimorph, daidzein, and coumestrol showed significant correlations with soil-available Fe (Figure 7B). Oleamide is a plant fatty acid amide that facilitates strong interactions between plants and microorganisms while stimulating nitrogen metabolism in rhizosphere bacteria [63]. Daidzein can increase the quantity and competitiveness of Bradyrhizobium diazoefficiens USDA11 [35]. Stringlis, Yu, Feussner, de Jonge, van Bentum, Van Verk, Berendsen, Bakker, Feussner, and Pieterse [66] reported that coumestrol enhances plant growth in Fe-limited soils by alleviating Fe deficiency. Therefore, soybean and maize enhance their adaptability to Fe deficiency conditions by dynamically interacting with the soil environment through root exudates.
4.4. The Interactions Among Microbial Communities and Root Exudates
Root exudates are regarded as the primary driver of root-specific microbial selection due to their inclusion of plant species-specific metabolites and signaling compounds [75,76]. Intercropping significantly enhanced the accumulation of specific metabolites in the rhizosphere of soybean and maize (Figure 6), and there was a significant association between different root exudates and distinct microbial communities (Figure 8), indicating that root exudates may contribute to belowground associational effects. The release of plant root exudates triggers the recruitment of specific microbial communities, which play a crucial role in assisting plants to adapt to changes in their external environment [71]. Increasing evidence suggested that alterations in rhizosphere metabolites recruit unique beneficial microbial communities, leading to pathogen suppression and improved nutrient availability in the soil, thereby promoting plant resilience and nutrient uptake [77,78]. In the intercropping system, soybean and maize roots secreted diverse organic substances containing sugars, amino acids, organic acids, etc., which not only served as carbon and nitrogen sources for microorganisms but also stimulated their growth and reproduction [43]. The correlation between root exudates and microbial community is more pronounced in maize (Figure 8B). The mixture of metabolites released from the roots of maize plants, including daidzein, 1-palmitoylglycerol, and daidzein, affected the composition of root-related microbiota composed of Phyllobacterium, Ferrovibrio, Nakamurella (Figure 8B). Nakamurella has been isolated from soil and animals [79,80], but there has been no further report on its function. Further experiments are required in the future to validate the role of Nakamurella in enhancing plant Fe uptake.
Microorganisms utilize root exudates as sources of carbon and nitrogen for their growth and reproduction while also modifying and regulating the production of root exudates [70]. For instance, the rhizosphere microbiome associated with tomato plants can directly impact both the chemical composition of roots and their exudation through a mechanism termed systemically induced root exudation of metabolites (SIREM) [70]. This process is exemplified by Bacillus subtilis, which triggers the secretion of acylsugars throughout the entire root system [70]. In this study, root exudates are associated with the root microbiome, and this relationship is more pronounced in maize (Figure 8). Taking into account the role of coumestrol in promoting plant Fe absorption, we observed a significant positive correlation between coumestrol and Nakamurella in the maize rhizosphere, as well as with Pirellula and Rhizobium in the soybean rhizosphere (Figure 8). These microorganisms may play a facilitative role in the exudation of coumestrol by plants. However, the knowledge of the colonization hotspots of most root microbial populations is limited, and the mechanisms by which metabolites are secreted in different root regions (such as the main root, lateral root, root hair, and root tip) have not been determined [81]. Whether these microorganisms can induce plants to generate the specific root exudates awaits further investigation.
In this study, we explored the mechanisms regulating adaptability to Fe deficiency in the intercropping system of soybean and maize through pot experiments, which provided us with certain basic data and preliminary insights. However, it must be acknowledged that pot experiments have certain limitations, as their environmental conditions differ from those in actual planting scenarios. For instance, the soil volume and root growth space in pots are restricted, and the supply patterns of water and nutrients are difficult to fully simulate the real conditions in nature. Therefore, we will conduct field experiments in the future to obtain more practical data and provide more solid support for theoretical development and practical application.
5. Conclusions
These results revealed the underlying mechanisms governing plant adaptation to Fe deficiency within the soybean and maize intercropping system (Figure 9). This investigation was conducted from four key perspectives: plant physiology, soil physicochemical properties, microbial community structure, and the distribution of root exudates. Soybean and maize secreted specific compounds, including oleamide, coumestrol, and daidzein, which served to attract microorganisms such as Microbacterium, Sphingomonas, and Rhizobium that are potentially capable of producing Fe carriers or facilitating plant Fe absorption. Consequently, this enhances the adaptability of plants to Fe-deficient environments while also promoting the uptake of other essential nutrients. Overall, these findings provide ideas and solutions for alleviating Fe deficiency in plants and provide a perspective and scientific foundation for plant regulation under varying soil Fe concentrations.
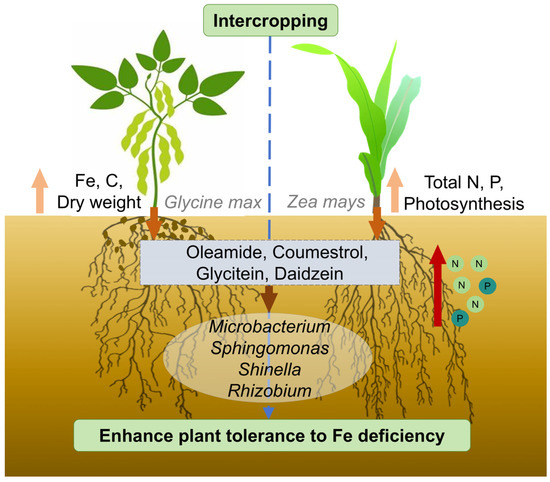
Figure 9.
Soybean and maize emit specific compounds, including oleamide, coumestrol, glycitein, and daidzein, which serve to attract microorganisms such as Microbacterium, Sphingomonas, Shinella, and Rhizobium. These microorganisms are potentially capable of producing Fe carriers or facilitating plant Fe absorption. Consequently, this enhances the adaptability of plants to Fe-deficient environments and promotes the uptake of other essential nutrients.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agronomy15020286/s1, Figure S1: The Linear Discriminant Analysis (LDA) analysis of bacterial communities in the rhizosphere of soybean and maize. P1, flowering period; P2, grain period; P3, maturity period. Fe1, soil effective iron content 3 mg·kg−1; Fe2: soil effective iron content 5 mg·kg−1; Fe3: soil effective iron content 8 mg·kg−1; Figure S2: Linear Discriminant Analysis (LDA) analysis of root exudates of different planting pattern with different iron content in soybean and maize. Fe1, soil effective iron content 3 mg·kg−1; Fe2: soil effective iron content 5 mg·kg−1; Fe3: soil effective iron content 8 mg·kg−1.
Author Contributions
Conceptualization, W.L. and Z.S.; methodology, G.W. (Guoqing Wang), S.W. and Y.Z.; software, W.L., G.W. (Guoqing Wang), S.W. and B.Y.; validation, Y.Z., Y.D. and Z.W.; formal analysis, S.W., Y.Z., B.Y. and Z.W.; investigation, G.W. (Guoqing Wang), S.W. and Y.D.; resources, D.S. and G.W. (Gehong Wei); data curation, D.S.; writing—original draft preparation, W.L.; writing—review and editing, W.L. and J.C.; visualization, G.W. (Guoqing Wang), Y.Z., Y.D. and B.Y.; supervision, D.S., G.W. (Gehong Wei), J.C. and Z.S.; project administration, G.W. (Gehong Wei), J.C. and Z.S.; funding acquisition, G.W. (Gehong Wei), J.C. and Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key Research and Development Program of China (2023yfd1900502) and the National Natural Science Foundation of China (42177329, 42477370).
Data Availability Statement
Data is contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F.S. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.D.; Zhou, L.; Chen, P.; Du, Q.; Pang, T.; Song, C.; Wang, X.C.; Liu, W.G.; Yang, W.Y.; Yong, T.W. Effects of maize-soybean relay intercropping on crop nutrient uptake and soil bacterial community. J. Integr. Agric. 2019, 18, 2006–2018. [Google Scholar] [CrossRef]
- Du, J.B.; Han, T.F.; Gai, J.Y.; Yong, T.W.; Sun, X.; Wang, X.C.; Yang, F.; Liu, J.; Shu, K.; Liu, W.G.; et al. Maize-soybean strip intercropping: Achieved a balance between high productivity and sustainability. J. Integr. Agric. 2018, 17, 747–754. [Google Scholar] [CrossRef]
- Dai, J.; Qiu, W.; Wang, N.; Wang, T.; Nakanishi, H.; Zuo, Y. From leguminosae/gramineae intercropping systems to see benefits of intercropping on iron nutrition. Front. Plant Sci. 2019, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, S.C.; Li, X.L.; Zhang, F.S.; Christie, P. Interspecific complementary and competitive interactions between intercropped maize and faba bean. Plant Soil 1999, 212, 105–114. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Wu, L.; Luo, X.; Li, N.; Arafat, Y.; Lin, S.; Lin, W. Belowground interactions impact the soil bacterial community, soil fertility, and crop yield in maize/peanut intercropping systems. Int. J. Mol. Sci. 2018, 19, 622. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Cantero, E.; Hernandez-Calderon, E.; Velazquez-Becerra, C.; Lopez-Meza, J.F.; Alfaro-Cuevas, R.; Lopez-Bucio, J. Role of dissimilatory fermentative iron-reducing bacteria in Fe uptake by common bean (Phaseolus vulgaris L.) plants grown in alkaline soil. Plant Soil 2007, 291, 263–273. [Google Scholar] [CrossRef]
- Jia, B.; Chang, X.; Fu, Y.; Heng, W.; Ye, Z.; Liu, P.; Liu, L.; Al Shoffe, Y.; Watkins, C.B.; Zhu, L. Metagenomic analysis of rhizosphere microbiome provides insights into occurrence of iron deficiency chlorosis in field of Asian pears. BMC Microbiol. 2022, 22, 18. [Google Scholar] [CrossRef]
- Mimmo, T.; Del Buono, D.; Terzano, R.; Tomasi, N.; Vigani, G.; Crecchio, C.; Pinton, R.; Zocchi, G.; Cesco, S. Rhizospheric organic compounds in the soil-microorganism-plant system: Their role in iron availability. Eur. J. Soil Sci. 2014, 65, 629–642. [Google Scholar] [CrossRef]
- Santi, S.; Schmidt, W. Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol. 2009, 183, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, V.; Oljaca, S.; Stojiljkovic, M.; Simic, M.; Dolijanovic, Z.; Kravic, N. Effect of the maize-soybean intercropping system on the potential bioavailability of magnesium, iron and zinc. Crop Pasture Sci. 2015, 66, 1118–1127. [Google Scholar] [CrossRef]
- Tian, P.; Razavi, B.S.; Zhang, X.; Wang, Q.; Blagodatskaya, E. Microbial growth and enzyme kinetics in rhizosphere hotspots are modulated by soil organics and nutrient availability. Soil Biol. Biochem. 2020, 141, 107662. [Google Scholar] [CrossRef]
- Panchal, P.; Preece, C.; Penuelas, J.; Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 2022, 27, 749–757. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial population and community dynamics on plant roots and their feedbacks on plant communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active microorganisms in soil: Critical review of estimation criteria and approaches. Soil Biol. Biochem. 2013, 67, 192–211. [Google Scholar] [CrossRef]
- Ma, Y.H.; Fu, S.L.; Zhang, X.P.; Zhao, K.; Chen, H.Y.H. Intercropping improves soil nutrient availability, soil enzyme activity and tea quantity and quality. Appl. Soil Ecol. 2017, 119, 171–178. [Google Scholar] [CrossRef]
- Nyawade, S.O.; Karanja, N.N.; Gachene, C.K.K.; Gitari, H.I.; Schulte-Geldermann, E.; Parker, M.L. Short-term dynamics of soil organic matter fractions and microbial activity in smallholder potato-legume intercropping systems. Appl. Soil Ecol. 2019, 142, 123–135. [Google Scholar] [CrossRef]
- Li, H.; Luo, L.; Tang, B.; Guo, H.; Cao, Z.; Zeng, Q.; Chen, S.; Chen, Z. Dynamic changes of rhizosphere soil bacterial community and nutrients in cadmium polluted soils with soybean-corn intercropping. BMC Microbiol. 2022, 22, 57. [Google Scholar] [CrossRef]
- Chamkhi, I.; Cheto, S.; Geistlinger, J.; Zeroual, Y.; Kouisni, L.; Bargaz, A.; Ghoulam, C. Legume-based intercropping systems promote beneficial rhizobacterial community and crop yield under stressing conditions. Ind. Crops Prod. 2022, 183, 114958. [Google Scholar] [CrossRef]
- Baetz, U.; Martinoia, E. Root exudates: The hidden part of plant defense. Trends Plant Sci. 2014, 19, 90–98. [Google Scholar] [CrossRef]
- López-Guerrero, M.G.; Ormeño-Orrillo, E.; Rosenblueth, M.; Martïnez-Romero, J.; Martïnez-Romero, E. Buffet hypothesis for microbial nutrition at the rhizosphere. Front. Plant Sci. 2013, 4, 188. [Google Scholar] [CrossRef]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do plant exudates shape the root microbiome? Trends Plant Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef]
- Xue, Y.; Xia, H.; Christie, P.; Zhang, Z.; Li, L.; Tang, C. Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: A critical review. Ann. Bot. 2016, 117, 363–377. [Google Scholar] [CrossRef]
- Hunt, E.R., Jr.; Daughtry, C.S.T. Chlorophyll meter calibrations for chlorophyll content using measured and simulated leaf transmittances. Agron. J. 2014, 106, 931–939. [Google Scholar] [CrossRef]
- Lobreaux, S.; Briat, J.F. Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem. J. 1991, 274, 601–606. [Google Scholar] [CrossRef]
- Ates, F.; Kaya, O. The relationship between iron and nitrogen concentrations based on kjeldahl method and SPAD-502 readings in grapevine (Vitis vinifera L. cv. “Sultana Seedless”). Erwerbs-Obstbau 2021, 63, 53–59. [Google Scholar] [CrossRef]
- Zhang, N.N.; Suo, B.Y.; Yao, L.L.; Ding, Y.X.; Zhang, J.H.; Wei, G.H.; Shangguan, Z.P.; Chen, J. H2S works synergistically with rhizobia to modify photosynthetic carbon assimilation and metabolism in nitrogen-deficient soybeans. Plant Cell Environ. 2023, 46, 2523–2541. [Google Scholar] [CrossRef] [PubMed]
- Iarmol’chuk, G.M. Method of quantitative analysis of phosphorus and stabilizing its content in biological and chemical assays. Ukr. Biokhimicheskii Zhurnal 1996, 68, 109436. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [PubMed]
- Cuartero, J.; Antonio Pascual, J.; Vivo, J.-M.; Ozbolat, O.; Sanchez-Navarro, V.; Egea-Cortines, M.; Zornoza, R.; Martinez Mena, M.; Garcia, E.; Ros, M. A first-year melon/cowpea intercropping system improves soil nutrients and changes the soil microbial community. Agric. Ecosyst. Environ. 2022, 328, 107856. [Google Scholar] [CrossRef]
- Stomph, T.; Dordas, C.; Baranger, A.; de Rijk, J.; Dong, B.; Evers, J.; Gu, C.F.; Li, L.; Simon, J.; Jensen, E.S.; et al. Designing intercrops for high yield, yield stability and efficient use of resources: Are there principles? Adv. Agron. 2020, 160, 1–50. [Google Scholar] [CrossRef]
- Walch-Liu, P.; Filleur, S.; Gan, Y.B.; Forde, B.G. Signaling mechanisms integrating root and shoot responses to changes in the nitrogen supply. Photosynth. Res. 2005, 83, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Ramongolalaina, C. Dynamics of symbiotic relationship of soybean with Bradyrhizobium diazoefficiens and involvements of root-secreted daidzein behind the continuous cropping. Eur. J. Soil Biol. 2019, 93, 103098. [Google Scholar] [CrossRef]
- Xu, P.; Wang, E. Diversity and regulation of symbiotic nitrogen fixation in plants. Curr. Biol. 2023, 33, 543–559. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, H. Maize-soybean intercropping improved maize growth traits by increasing soil nutrients and reducing plant pathogen abundance. Front. Microbiol. 2023, 14, 1290825. [Google Scholar] [CrossRef]
- Hu, H.Y.; Li, H.; Hao, M.M.; Ren, Y.N.; Zhang, M.K.; Liu, R.Y.; Zhang, Y.; Li, G.; Chen, J.S.; Ning, T.Y.; et al. Nitrogen fixation and crop productivity enhancements co-driven by intercrop root exudates and key rhizosphere bacteria. J. Appl. Ecol. 2021, 58, 2243–2255. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Cade-Menun, B.J.; Liang, X.; Hu, Y.; Liu, C.W.; Zhao, Y.; Li, L.; Shi, J. Complementary Phosphorus Speciation in Agricultural Soils by Sequential Fractionation, Solution 31P Nuclear Magnetic Resonance, and Phosphorus K-edge X-ray Absorption Near-Edge Structure Spectroscopy. J. Environ. Qual. 2013, 42, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.A.; Myint, K.; Steingrobe, B.; Stritsis, C.; Schulze, J.; Claassen, N. Phosphorus fractions depletion in the rhizosphere of young and adult maize and oilseed rape plants. J. Soil Sci. Plant Nutr. 2017, 17, 824–838. [Google Scholar] [CrossRef]
- Rubio, G.; Faggioli, V.; Scheiner, J.D.; Gutierrez-Boem, F.H. Rhizosphere phosphorus depletion by three crops differing in their phosphorus critical levels. J. Plant Nutr. Soil Sci. 2012, 175, 810–871. [Google Scholar] [CrossRef]
- Liao, D.; Zhang, C.; Li, H.; Lambers, H.; Zhang, F. Changes in soil phosphorus fractions following sole cropped and intercropped maize and faba bean grown on calcareous soil. Plant Soil 2020, 448, 587–601. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhang, Y.; Fei, J.; Rong, X.; Peng, J.; Yin, L.; Luo, G. Intercropping enhances maize growth and nutrient uptake by driving the link between rhizosphere metabolites and microbiomes. New Phytol. 2024, 243, 1506–1521. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Wei, Z.; Shao, Z.; Friman, V.; Cao, K.; Yang, T.; Kramer, J.; Wang, X.; Li, M.; Mei, X.; et al. Competition for iron drives phytopathogen control by natural rhizosphere microbiomes. Nat. Microbiol. 2020, 5, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Pesek, J.; Buechler, R.; Albrecht, R.; Boland, W.; Zeth, K. Structure and Mechanism of Iron Translocation by a Dps Protein from Microbacterium arborescens. J. Biol. Chem. 2011, 286, 34872–34882. [Google Scholar] [CrossRef]
- Louca, S.; Polz, M.F.; Mazel, F.; Albright, M.B.N.; Huber, J.A.; O’Connor, M.I.; Ackermann, M.; Hahn, A.S.; Srivastava, D.S.; Crowe, S.A.; et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2018, 2, 936–943. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. Isme J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- Ratzke, C.; Barrere, J.; Gore, J. Strength of species interactions determines biodiversity and stability in microbial communities. Nat. Ecol. Evol. 2020, 4, 376–383. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wei, B.L.; Wen, R.S.; Liu, Y.; Wang, Z.T. Genetically modified sugarcane intercropping soybean impact on rhizosphere bacterial communities and co-occurrence patterns. Front. Microbiol. 2021, 12, 742341. [Google Scholar] [CrossRef]
- Liu, R.; Zhou, G.p.; Chang, D.n.; Gao, S.j.; Han, M.; Zhang, J.d.; Sun, X.f.; Cao, W.d. Transfer characteristics of nitrogen fixed by leguminous green manure crops when intercropped with maize in northwestern China. J. Integr. Agric. 2022, 21, 1177–1187. [Google Scholar] [CrossRef]
- Curie, C.; Mari, S. New routes for plant iron mining. New Phytol. 2017, 214, 521–525. [Google Scholar] [CrossRef]
- Riaz, N.; Guerinot, M.L. All together now: Regulation of the iron deficiency response. J. Exp. Bot. 2021, 72, 2045–2055. [Google Scholar] [CrossRef] [PubMed]
- Tato, L.; Lattanzio, V.; Ercole, E.; Dell’Orto, M.; Sorgona, A.; Linsalata, V.; di Fossalunga, A.S.; Novero, M.; Astolfi, S.; Abenavoli, M.R.; et al. Plasticity, exudation and microbiome-association of the root system of Pellitory-of-the-wall plants grown in environments impaired in iron availability. Plant Physiol. Biochem. 2021, 168, 27–42. [Google Scholar] [CrossRef]
- Larcher, M.; Muller, B.; Mantelin, S.; Rapior, S.; Cleyet-Marel, J.C. Early modifications of Brassica napus root system architecture induced by a plant growth-promoting Phyllobacterium strain. New Phytol. 2003, 160, 119–125. [Google Scholar] [CrossRef]
- Chhetri, G.; Kim, I.; Kang, M.; Kim, J.; So, Y.; Seo, T. Devosia rhizoryzae sp. nov., and Devosia oryziradicis sp. nov., novel plant growth promoting members of the genus Devosia, isolated from the rhizosphere of rice plants. J. Microbiol. 2022, 60, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Jiang, X.; Wei, D.; Zhao, B.; Ma, M.; Chen, S.; Cao, F.; Shen, D.; Guan, D.; Li, J. Consistent effects of nitrogen fertilization on soil bacterial communities in black soils for two crop seasons in China. Sci. Rep. 2017, 7, 3267. [Google Scholar] [CrossRef]
- Zhong, Y.; Sorensen, P.O.; Zhu, G.; Jia, X.; Liu, J.; Shangguan, Z.; Wang, R.; Yan, W. Differential microbial assembly processes and co-occurrence networks in the soil-root continuum along an environmental gradient. iMeta 2022, 1, e18. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Liu, Y.; Guo, Y.; Shi, P.; Wei, G. Biogeography and ecological processes affecting root-associated bacterial communities in soybean fields across China. Sci. Total Environ. 2018, 627, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhou, C.; Wu, Y.; An, Q.; Zhang, J.; Fang, Y.; Li, J.; Pan, C. Nanoselenium integrates soil-pepper plant homeostasis by recruiting rhizosphere-beneficial microbiomes and allocating signaling molecule levels under Cd stress. J. Hazard. Mater. 2022, 432, 128763. [Google Scholar] [CrossRef]
- Hua, C.; Wang, Y.; Xie, Z.; Guo, Z.; Zhang, Y.; Qiu, Y.; Wang, L. Effects of intercropping on rhizosphere soil microorganisms and root exudates of Lanzhou lily (Lilium davidii var. Unicolor). J. Basic Microbiol. 2018, 10, 159–168. [Google Scholar]
- Lv, J.; Dong, Y.; Dong, K.; Zhao, Q.; Yang, Z.; Chen, L. Intercropping with wheat suppressed Fusarium wilt in faba bean and modulated the composition of root exudates. Plant Soil 2020, 448, 153–164. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Y.F.; Kronzucker, H.J.; Shi, W.M. Quantification and enzyme targets of fatty acid amides from duckweed root exudates involved in the stimulation of denitrification. J. Plant Physiol. 2016, 198, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Leistra, M.; Smelt, J.H.; van den Berg, F. Measured and computed volatilisation of the fungicide fenpropimorph from a sugar beet crop. Pest Manag. Sci. 2005, 61, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Schmid, N.B.; Giehl, R.F.H.; Doell, S.; Mock, H.; Strehmel, N.; Scheel, D.; Kong, X.; Hider, R.C.; von Wiren, N. Feruloyl-CoA 6′-hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol. 2014, 164, 160–172. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Yu, K.; Feussner, K.; de Jonge, R.; van Bentum, S.; Van Verk, M.C.; Berendsen, R.L.; Bakker, P.A.H.M.; Feussner, I.; Pieterse, C.M.J. MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc. Natl. Acad. Sci. USA 2018, 115, 5213–5222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, L.; Tantai, H.; Khan, M.U.; Letuma, P.; Wu, H.; Zhang, S.; Chen, T.; Lin, S.; Lin, W. Properties of bacterial community in the rhizosphere soils of Achyranthes bidentata tolerant to consecutive monoculture. Plant Growth Regul. 2019, 89, 167–178. [Google Scholar] [CrossRef]
- Guo, M.; Gong, Z.; Miao, R.; Su, D.; Li, X.; Jia, C.; Zhuang, J. The influence of root exudates of maize and soybean on polycyclic aromatic hydrocarbons degradation and soil bacterial community structure. Ecol. Eng. 2017, 99, 22–30. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Khan, M.U.; Lin, X.Q.; Lin, Z.M.; Lin, S.; Lin, W.X. Evaluation of maize/peanut intercropping effects on microbial assembly, root exudates and peanut nitrogen uptake. Plant Physiol. Biochem. 2022, 171, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Korenblum, E.; Massalha, H.; Aharoni, A. Plant-microbe interactions in the rhizosphere via a circular metabolic economy. Plant Cell 2022, 34, 3168–3182. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, J.; Yuan, J.; Hale, L.; Wen, T.; Huang, Q.; Vivanco, J.M.; Zhou, J.; Kowalchuk, G.A.; Shen, Q. Root exudates drive soil-microbe-nutrient feedbacks in response to plant growth. Plant Cell Environ. 2021, 44, 613–628. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interations with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Wen, T.; Yu, G.H.; Hong, W.D.; Yuan, J.; Niu, G.Q.; Xie, P.H.; Sun, F.S.; Guo, L.D.; Kuzyakov, Y.; Shen, Q.R. Root exudate chemistry affects soil carbon mobilization via microbial community reassembly. Fundam. Res. 2022, 2, 697–707. [Google Scholar] [CrossRef]
- Nuzzo, A.; De Martino, A.; Di Meo, V.; Piccolo, A. Potential alteration of iron-humate complexes by plant root exudates and microbial siderophores. Chem. Biol. Technol. Agric. 2018, 5, 19. [Google Scholar] [CrossRef]
- Rüger, L.; Feng, K.; Dumack, K.; Freudenthal, J.; Chen, Y.; Sun, R.; Wilson, M.; Yu, P.; Sun, B.; Deng, Y.; et al. Assembly patterns of the rhizosphere microbiome along the longitudinal root Axis of maize (Zea mays L.). Front. Microbiol. 2021, 12, 614501. [Google Scholar] [CrossRef]
- Ulbrich, T.C.; Rivas-Ubach, A.; Tiemann, L.K.; Friesen, M.L.; Evans, S.E. Plant root exudates and rhizosphere bacterial communities shift with neighbor context. Soil Biol. Biochem. 2022, 172, 108753. [Google Scholar] [CrossRef]
- Hu, L.; Robert, C.A.M.; Cadot, S.; Zhang, X.; Ye, M.; Li, B.; Manzo, D.; Chervet, N.; Steinger, T.; van der Heijden, M.G.A.; et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat. Commun. 2018, 9, 2738. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhao, J.; Wen, T.; Zhao, M.; Li, R.; Goossens, P.; Huang, Q.; Bai, Y.; Vivanco, J.M.; Kowalchuk, G.A.; et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome 2018, 6, 156. [Google Scholar] [CrossRef]
- Tuo, L.; Li, F.N.; Pan, Z.; Lou, I.; Guo, M.; Lee, S.M.Y.; Chen, L.; Hu, L.; Sun, C.H. Nakamurella endophytica sp nov., a novel endophytic actinobacterium isolated from the bark of Kandelia candel. Int. J. Syst. Evol. Microbiol. 2016, 66, 1577–1582. [Google Scholar] [CrossRef]
- Carro, L.; Nouioui, I. Taxonomy and systematics of plant probiotic bacteria in the genomic era. AIMS Microbiol. 2017, 3, 383–412. [Google Scholar] [CrossRef] [PubMed]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).