Non-Destructive Detection of pH Value During Secondary Fermentation of Maize Silage Using Colorimetric Sensor Array Combined with Hyperspectral Imaging Technology
Abstract
1. Introduction
2. Materials and Methods
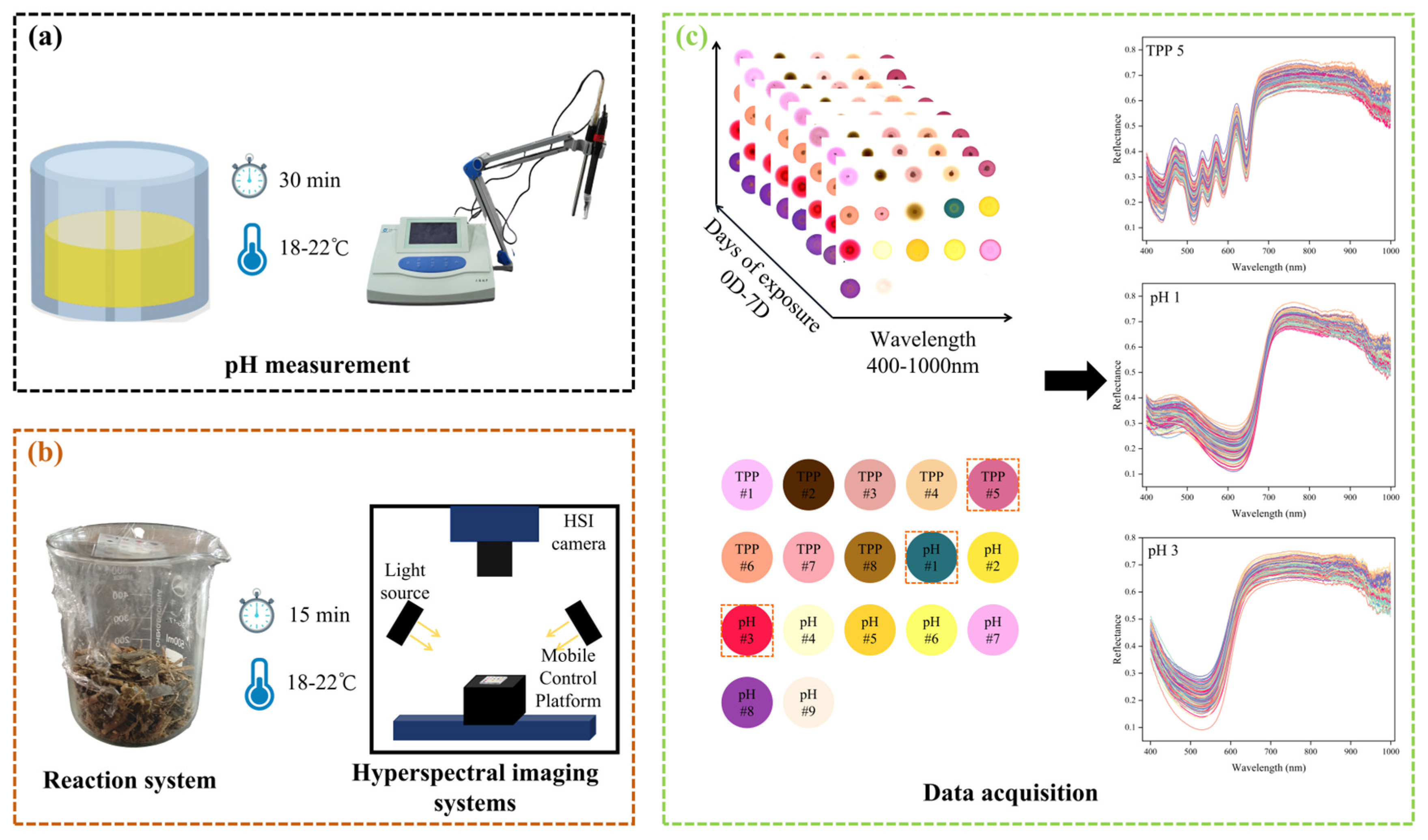
2.1. Preparation of Maize Silage Samples
2.2. Preparation of CSA
2.3. pH Determination
2.4. Hyperspectral Image Acquisition of CSA
2.5. Data Analysis Methods
2.5.1. Spectral Data Preprocessing
2.5.2. Feature Variable Screening
2.5.3. Adaptive Bacterial Foraging Optimization (ABFO)
2.5.4. Establishment of Models
2.5.5. Model Evaluation
3. Results
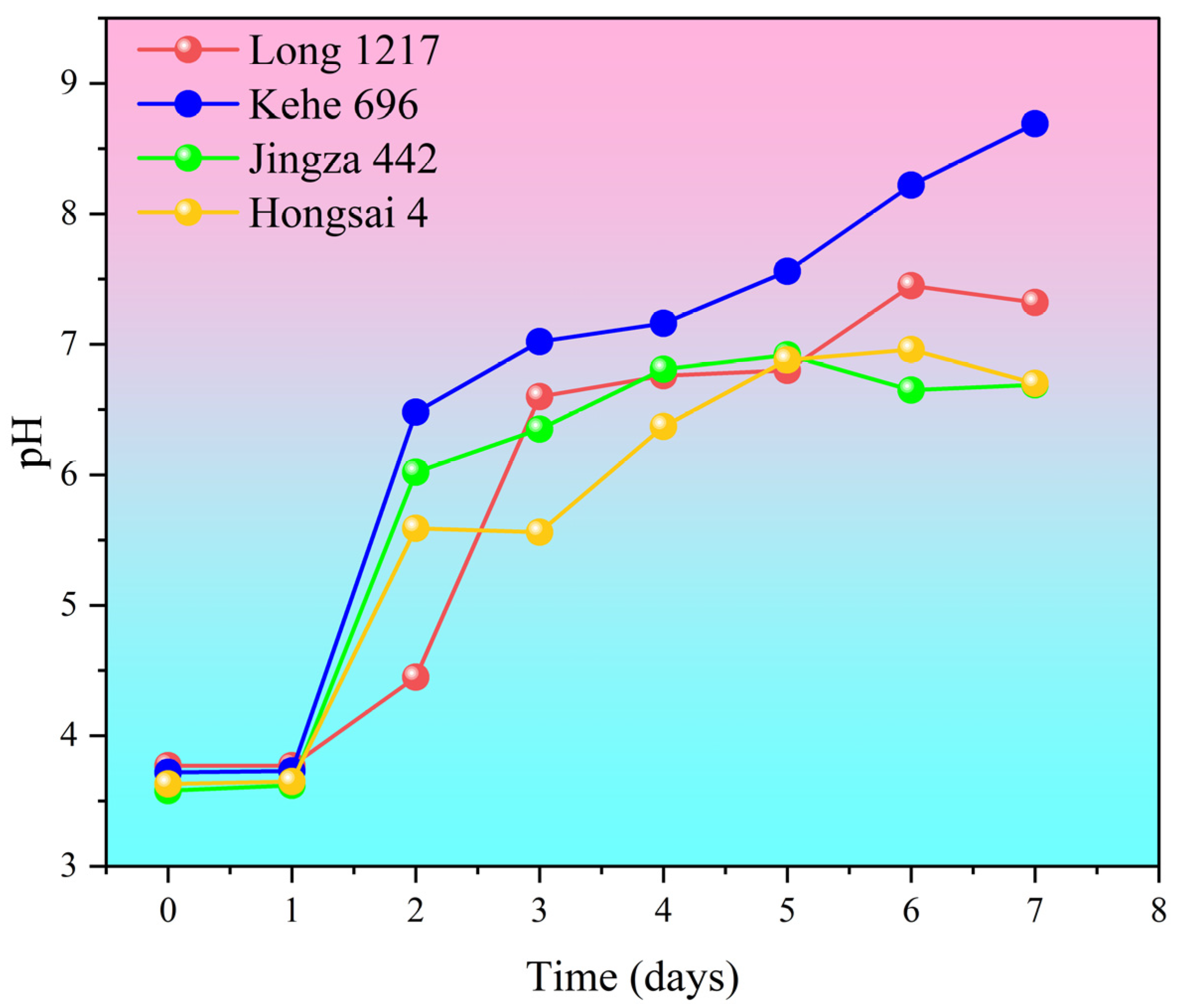
3.1. pH Changes During the Secondary Fermentation Process
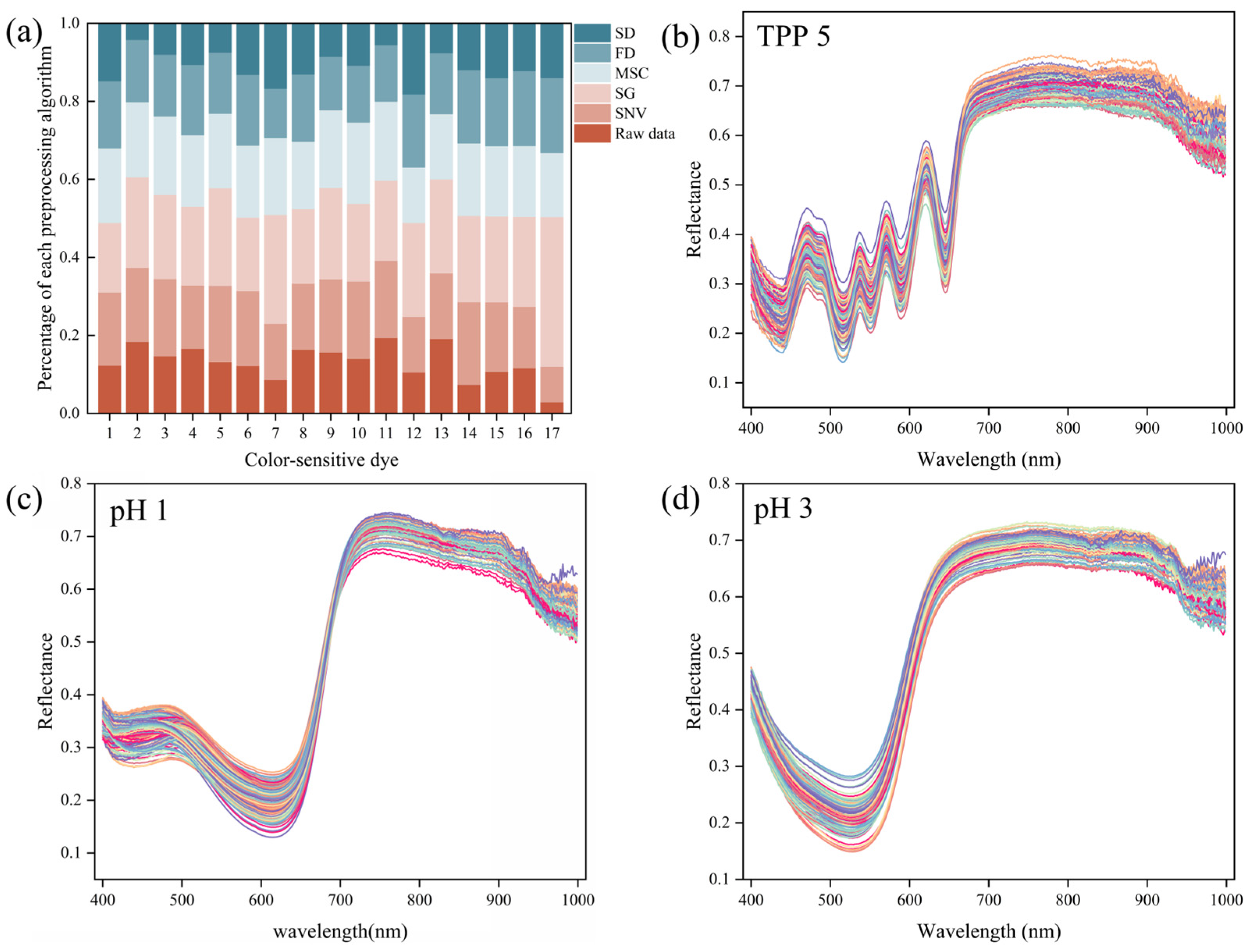
3.2. Data Preprocessing Results
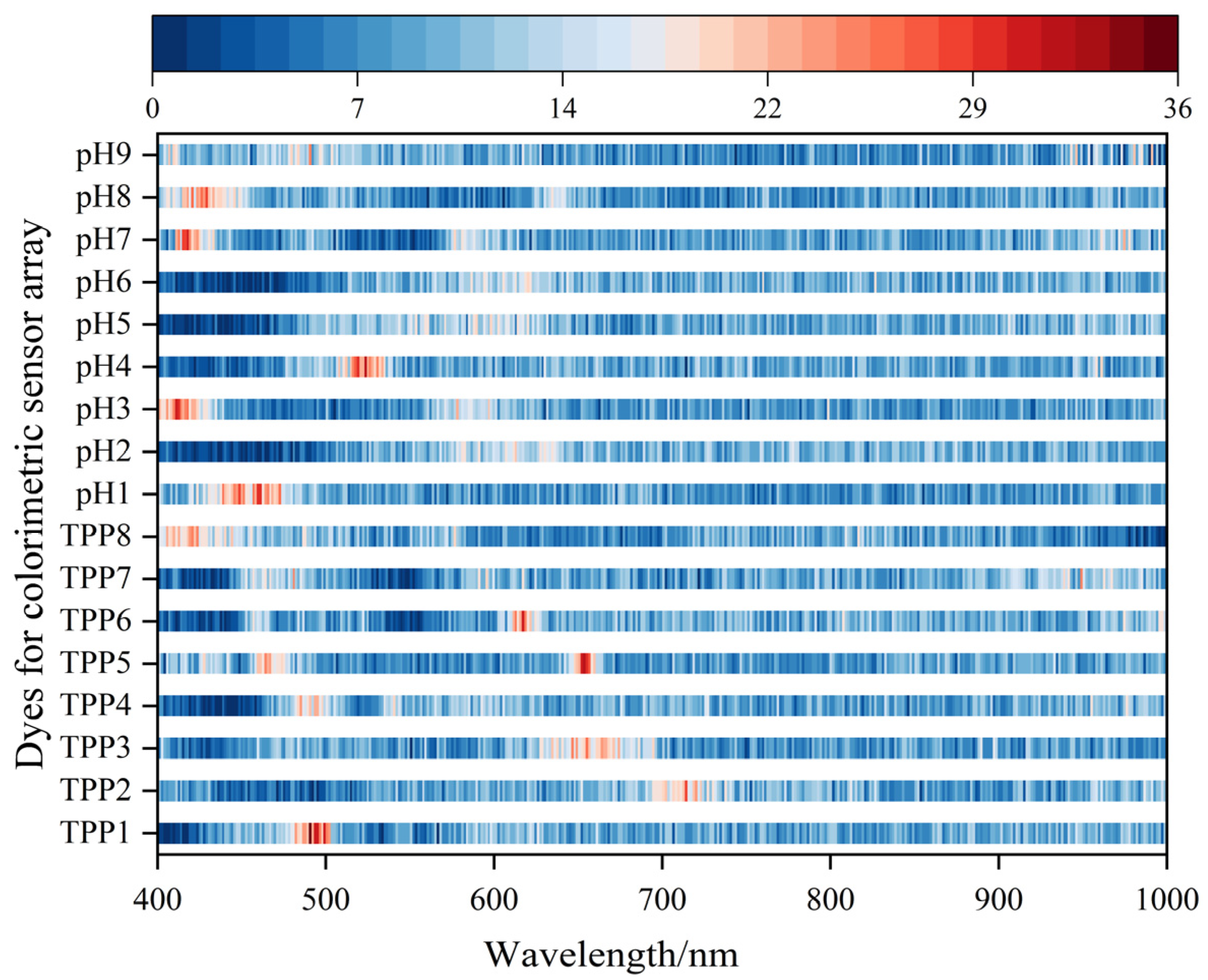
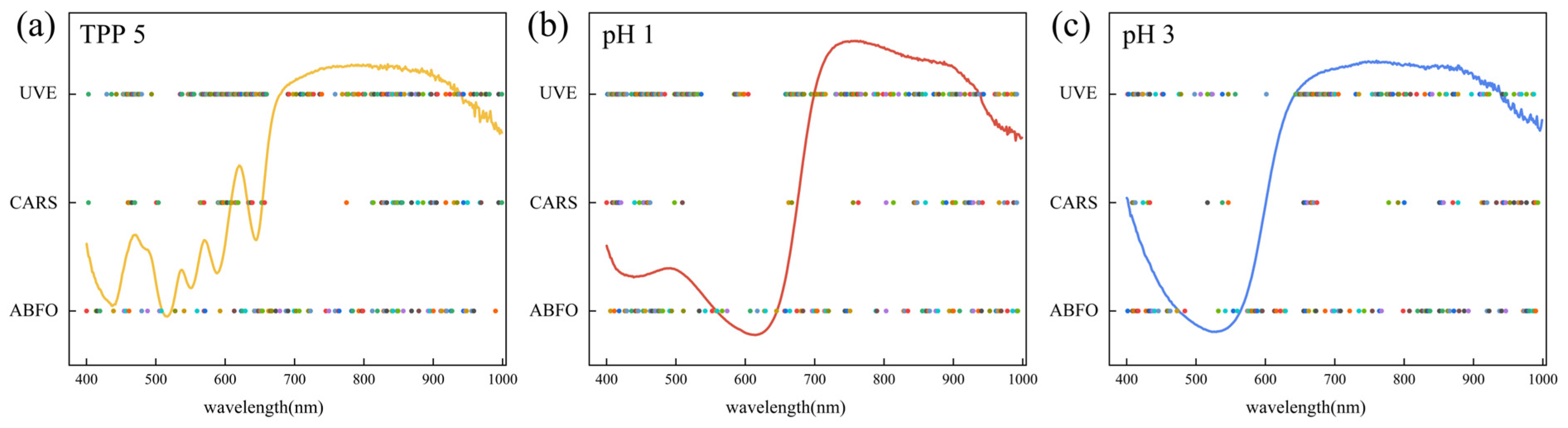
3.3. Results of ABFO Algorithm for Sensitive Dye Screening
3.4. Results of Feature Variable Screening
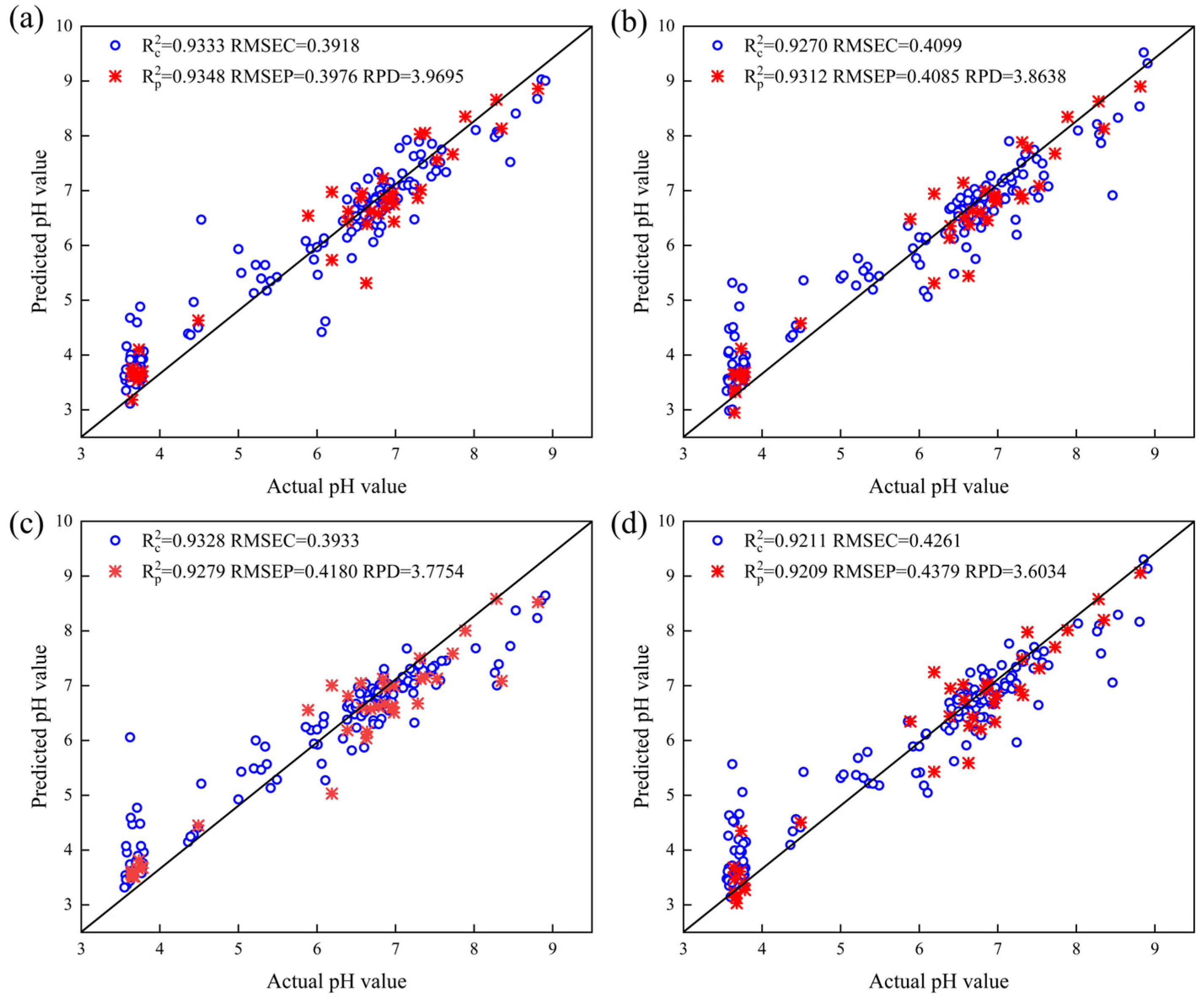
3.5. Results of the Two Regression Models
3.6. Model Comparison
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-Chávez, I.; Meraz-Romero, E.; Castelán-Ortega, O.; Zaragoza Esparza, J.; Osorio Avalos, J.; Robles Jimenez, L.E.; González-Ronquillo, M. Corn Silage, Meta-Analysis of The Quality and Yield of Different Regions in the World. 2020. Available online: https://www.preprints.org/manuscript/202010.0094 (accessed on 14 July 2024).
- Ma, X.; Sun, J.; Zhao, J.; Jv, X.; Dong, J.; Yang, W.; Jiang, Y.; Li, Y.; Yang, L.; Jiang, S. Effects of Different Dietary Inclusions of Whole-Plant Corn Silage on Growth Performance, Nutrient Availability and Jejunal Development in Growing-Finishing Pigs. Czech J. Anim. Sci. 2024, 69, 48–58. [Google Scholar] [CrossRef]
- Gallo, A.; Giuberti, G.; Bruschi, S.; Fortunati, P.; Masoero, F. Use of Principal Factor Analysis to Generate a Corn Silage Fermentative Quality Index to Rank Well- or Poorly Preserved Forages. J. Sci. Food Agric. 2016, 96, 1686–1696. [Google Scholar] [CrossRef]
- Borreani, G.; Tabacco, E.; Schmidt, R.J.; Holmes, B.J.; Muck, R.E. Silage Review: Factors Affecting Dry Matter and Quality Losses in Silages. J. Dairy Sci. 2018, 101, 3952–3979. [Google Scholar] [CrossRef]
- Ferraretto, L.F.; Shaver, R.D.; Luck, B.D. Silage Review: Recent Advances and Future Technologies for Whole-Plant and Fractionated Corn Silage Harvesting. J. Dairy Sci. 2018, 101, 3937–3951. [Google Scholar] [CrossRef]
- Yin, X.; Wang, P.; Yan, Z.; Yang, Q.; Huang, X.; Gun, S. Effects of Whole-Plant Corn Silage on Growth Performance, Serum Biochemical Indices, and Fecal Microorganisms in Hezuo Pigs. Animals 2024, 14, 662. [Google Scholar] [CrossRef]
- Jiang, F.; Cheng, H.; Liu, D.; Wei, C.; An, W.; Wang, Y.; Sun, H.; Song, E. Treatment of Whole-Plant Corn Silage with Lactic Acid Bacteria and Organic Acid Enhances Quality by Elevating Acid Content, Reducing pH, and Inhibiting Undesirable Microorganisms. Front. Microbiol. 2020, 11, 593088. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Gao, L.; Yu, J.; Yuan, X.; Zhu, W.; Wang, X.; Cui, Z. Aerobic Deterioration of Corn Stalk Silage and Its Effect on Methane Production and Microbial Community Dynamics in Anaerobic Digestion. Bioresour. Technol. 2018, 250, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Serva, L.; Andrighetto, I.; Segato, S.; Marchesini, G.; Chinello, M.; Magrin, L. Assessment of Maize Silage Quality under Different Pre-Ensiling Conditions. Data 2023, 8, 117. [Google Scholar] [CrossRef]
- Pereira-Crespo, S.; Sainz-Ramírez, A.; Plata-Reyes, D.A.; Gómez-Miranda, A.; González-Alcántara, F.; Botana, A.; González, L.; Veiga, M.; Resch, C.; Lorenzana, R.; et al. Predicción de la calidad fermentativa de ensilados de girasol mediante espectroscopía de reflectancia en el infrarrojo cercano (NIRS) sobre muestras secas. Rev. Mex. Cienc. Pecu. 2021, 12, 609–620. [Google Scholar] [CrossRef]
- Pastore, A.; Badocco, D.; Pastore, P. Determination of the Relevant Equilibrium Constants Working in pH Colorimetric Sensor Arrays (CSAs). Microchem. J. 2022, 177, 107288. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Wang, H.; Xiao, S.; Wang, J.; Feng, L. Sensitivity Enhancement of pH Indicator and Its Application in the Evaluation of Fish Freshness. Talanta 2015, 143, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Elrasheid Tahir, H.; Komla Mahunu, G.; Arslan, M.; Zhihua, L.; Wen, Z.; Xiaobo, Z.; Adam Mariod, A.; Jiyong, S. Feasibility Study for the Use of Colorimetric Sensor Arrays, NIR and FT-IR Spectroscopy in the Quantitative Analysis of Volatile Components in Honey. Microchem. J. 2021, 160, 105730. [Google Scholar] [CrossRef]
- Wojnowski, W.; Majchrzak, T.; Dymerski, T.; Gębicki, J.; Namieśnik, J. Electronic Noses: Powerful Tools in Meat Quality Assessment. Meat Sci. 2017, 131, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhai, X.; Huang, X.; Li, Z.; Shi, J.; Li, Q.; Zou, X.; Battino, M. Rapid Discrimination of Beer Based on Quantitative Aroma Determination Using Colorimetric Sensor Array. Food Chem. 2021, 363, 130297. [Google Scholar] [CrossRef]
- Lin, H.; Lin, J.; Man, Z.; Jin, H.; Kutsanedzie, F.Y.H.; Chen, Q. Development of Colorimetric Detection of 2,4,5-Trimethyloxazole in Volatile Organic Compounds Based on Porphyrin Complexes for Vinegar Storage Time Discrimination. Food Anal. Methods 2020, 13, 2192–2203. [Google Scholar] [CrossRef]
- Nie, W.; Chen, Y.; Zhang, H.; Liu, J.; Peng, Z.; Li, Y. A Novel Colorimetric Sensor Array for Real-Time and on-Site Monitoring of Meat Freshness. Anal. Bioanal. Chem. 2022, 414, 6017–6027. [Google Scholar] [CrossRef]
- Magnaghi, L.R.; Capone, F.; Zanoni, C.; Alberti, G.; Quadrelli, P.; Biesuz, R. Colorimetric Sensor Array for Monitoring, Modelling and Comparing Spoilage Processes of Different Meat and Fish Foods. Foods 2020, 9, 684. [Google Scholar] [CrossRef]
- Xu, W.; He, Y.; Li, J.; Deng, Y.; Xu, E.; Feng, J.; Ding, T.; Liu, D.; Wang, W. Non-Destructive Determination of Beef Freshness Based on Colorimetric Sensor Array and Multivariate Analysis. Sens. Actuators B Chem. 2022, 369, 132282. [Google Scholar] [CrossRef]
- Du, L.; Lao, Y.; Sasaki, Y.; Lyu, X.; Gao, P.; Wu, S.; Minami, T.; Liu, Y. Freshness Monitoring of Raw Fish by Detecting Biogenic Amines Using a Gold Nanoparticle-Based Colorimetric Sensor Array. RSC Adv. 2022, 12, 6803–6810. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Kang, W.; Jiang, H.; Zhou, M.; Lin, H. Freshness Identification of Oysters Based on Colorimetric Sensor Array Combined with Image Processing and Visible Near-Infrared Spectroscopy. Sensors 2022, 22, 683. [Google Scholar] [CrossRef]
- Wei, W.; Li, H.; Haruna, S.A.; Wu, J.; Chen, Q. Monitoring the Freshness of Pork during Storage via Near-Infrared Spectroscopy Based on Colorimetric Sensor Array Coupled with Efficient Multivariable Calibration. J. Food Compos. Anal. 2022, 113, 104726. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Q.; Han, Y.; Huang, S.; Wu, J.; Jiao, T.; Wei, J.; Chen, X.; Chen, Q. Non-Destructive Prediction of the Total Viable Count (TVC) in Fujian Oysters (Crassostrea Angulata) Based on the Colorimetric Sensor Array. Microchem. J. 2024, 197, 109911. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Zhao, J.; Wu, M. Nondestructive Detection of Total Volatile Basic Nitrogen (TVB-N) Content in Pork Meat by Integrating Hyperspectral Imaging and Colorimetric Sensor Combined with a Nonlinear Data Fusion. LWT—Food Sci. Technol. 2015, 63, 268–274. [Google Scholar] [CrossRef]
- An, T.; Li, Y.; Tian, X.; Fan, S.; Duan, D.; Zhao, C.; Huang, W.; Dong, C. Evaluation of Aroma Quality Using Multidimensional Olfactory Information during Black Tea Fermentation. Sens. Actuators B Chem. 2022, 371, 132518. [Google Scholar] [CrossRef]
- Ouyang, Q.; Rong, Y.; Wu, J.; Wang, Z.; Lin, H.; Chen, Q. Application of Colorimetric Sensor Array Combined with Visible Near-Infrared Spectroscopy for the Matcha Classification. Food Chem. 2023, 420, 136078. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Cui, Q.; Liu, Y.; Chen, Y.; Wang, Y.; Zhang, Z.; Chen, Q.; Ning, J. Rapid Monitoring of Black Tea Fermentation Quality Based on a Solution-Phase Sensor Array Combined with UV-Visible Spectroscopy. Food Chem. 2022, 377, 131974. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wang, Z.; Ahmad, W.; Man, Z.; Duan, Y. Identification of Rice Storage Time Based on Colorimetric Sensor Array Combined Hyperspectral Imaging Technology. J. Stored Prod. Res. 2020, 85, 101523. [Google Scholar] [CrossRef]
- Xu, F.; Huang, X.; Tian, X.; Yu, S.; Zhang, X.; Zareef, M. Application of Hyperspectral Imaging and Colorimetric Sensor Array Coupled with Multivariate Analysis for Quality Detection during Salted Duck Eggs Processing. J. Food Process Eng. 2024, 47, e14589. [Google Scholar] [CrossRef]
- GB/T 5009.237-2016; National Standard for Food Safety Determination of pH Value of Food. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016.
- DB15/T 1458-2018; Determination of pH Value, Organic Acid and Ammonium Nitrogen in Silage. Inner Mongolia Autonomous Region Bureau of Quality and Technical Supervision: Hohhot, China, 2018.
- He, H.-J. Simultaneous Quantifying and Visualizing Moisture, Ash and Protein Distribution in Sweet Potato [Ipomoea batatas (L.) Lam] by NIR Hyperspectral Imaging. Food Chem. 2023, 18, 100631. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.L.F.; Marcelo, M.C.A.; Porte, L.M.F.; Pontes, O.F.S.; Kaiser, S. Inline Simultaneous Quantitation of Tobacco Chemical Composition by Infrared Hyperspectral Image Associated with Chemometrics. Microchem. J. 2019, 151, 104225. [Google Scholar] [CrossRef]
- Ma, J.; Sun, D.-W. Prediction of Monounsaturated and Polyunsaturated Fatty Acids of Various Processed Pork Meats Using Improved Hyperspectral Imaging Technique. Food Chem. 2020, 321, 126695. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liang, Y.; Xu, Q.; Cao, D. Key Wavelengths Screening Using Competitive Adaptive Reweighted Sampling Method for Multivariate Calibration. Anal. Chim. Acta 2009, 648, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Centner, V.; Massart, D.-L.; De Noord, O.E.; De Jong, S.; Vandeginste, B.M.; Sterna, C. Elimination of Uninformative Variables for Multivariate Calibration. Anal. Chem. 1996, 68, 3851–3858. [Google Scholar] [CrossRef]
- Nguyen, T.; Nguyen, B.M.; Nguyen, G. Building Resource Auto-Scaler with Functional-Link Neural Network and Adaptive Bacterial Foraging Optimization. In Theory and Applications of Models of Computation; Gopal, T.V., Watada, J., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2019; Volume 11436, pp. 501–517. ISBN 978-3-030-14811-9. [Google Scholar]
- Wold, S.; Ruhe, A.; Wold, H.; Dunn, W.J., III. The Collinearity Problem in Linear Regression. The Partial Least Squares (PLS) Approach to Generalized Inverses. SIAM J. Sci. Stat. Comput. 1984, 5, 735–743. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, H.; Huang, L. A Joint Method of Spatial–Spectral Features and BP Neural Network for Hyperspectral Image Classification. Egypt. J. Remote Sens. Space Sci. 2023, 26, 107–115. [Google Scholar] [CrossRef]
- Díaz, E.O. Combined Analysis of Near-Infrared Spectra, Colour and Physicochemical Information of Brown Rice to Develop Accurate Calibration Models for Determining Amylose Content. Food Chem. 2019, 286, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Prieto, N.; Pawluczyk, O.; Dugan, M.E.R.; Aalhus, J.L. A Review of the Principles and Applications of Near-Infrared Spectroscopy to Characterize Meat, Fat, and Meat Products. Appl. Spectrosc. 2017, 71, 1403–1426. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, M.; Liu, Y.; Cui, Q.; Bi, K.; Jin, S.; Wang, Y.; Ning, J.; Zhang, Z. High-Sensitivity Hyperspectral Coupled Self-Assembled Nanoporphyrin Sensor for Monitoring Black Tea Fermentation. Sens. Actuators B Chem. 2021, 346, 130541. [Google Scholar] [CrossRef]
- Kang, W.; Lin, H.; Jiang, R.; Yan, Y.; Ahmad, W.; Ouyang, Q.; Chen, Q. Emerging Applications of Nano-Optical Sensors Combined with near-Infrared Spectroscopy for Detecting Tea Extract Fermentation Aroma under Ultrasound-Assisted Sonication. Ultrason. Sonochem. 2022, 88, 106095. [Google Scholar] [CrossRef]
- Wang, C.; Han, H.; Sun, L.; Na, N.; Xu, H.; Chang, S.; Jiang, Y.; Xue, Y. Bacterial Succession Pattern during the Fermentation Process in Whole-Plant Corn Silage Processed in Different Geographical Areas of Northern China. Processes 2021, 9, 900. [Google Scholar] [CrossRef]
- Brüning, D.; Gerlach, K.; Weiß, K.; Südekum, K.-H. Effect of Compaction, Delayed Sealing and Aerobic Exposure on Maize Silage Quality and on Formation of Volatile Organic Compounds. Grass Forage Sci. 2018, 73, 53–66. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Davies, D.R. The Aerobic Stability of Silage: Key Findings and Recent Developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
| Dye | Raw data | SNV | SG | MSC | FD | SD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TPP1 | 0.6805 | 0.5583 | 0.8883 | 0.8340 | 0.9471 | 0.8047 | 0.9061 | 0.8588 | 0.9230 | 0.7721 | 0.9671 | 0.6606 |
| TPP2 | 0.7178 | 0.5922 | 0.7548 | 0.6121 | 0.9333 | 0.7527 | 0.8331 | 0.6194 | 0.9755 | 0.5135 | 0.9882 | 0.1351 |
| TPP3 | 0.8717 | 0.5501 | 0.9231 | 0.7425 | 0.9414 | 0.8121 | 0.9016 | 0.7516 | 0.9679 | 0.5900 | 0.8625 | 0.2988 |
| TPP4 | 0.9292 | 0.6883 | 0.8500 | 0.6715 | 0.9241 | 0.8381 | 0.9529 | 0.7624 | 0.9194 | 0.7451 | 0.8444 | 0.4399 |
| TPP5 | 0.8615 | 0.4438 | 0.8314 | 0.6494 | 0.8887 | 0.8389 | 0.8761 | 0.6366 | 0.9157 | 0.5230 | 0.6689 | 0.2466 |
| TPP6 | 0.8084 | 0.4532 | 0.8324 | 0.7053 | 0.9102 | 0.6900 | 0.8435 | 0.6823 | 0.8494 | 0.6652 | 0.8330 | 0.4829 |
| TPP7 | 0.7026 | 0.2763 | 0.8767 | 0.4527 | 0.9304 | 0.8826 | 0.8103 | 0.6251 | 0.6913 | 0.3997 | 0.9603 | 0.5261 |
| TPP8 | 0.8610 | 0.6591 | 0.7522 | 0.6863 | 0.9284 | 0.7703 | 0.7526 | 0.6938 | 0.9363 | 0.6930 | 0.9231 | 0.5244 |
| pH1 | 0.7851 | 0.5525 | 0.9518 | 0.6646 | 0.8202 | 0.8290 | 0.9119 | 0.7021 | 0.8464 | 0.4831 | 0.9071 | 0.2994 |
| pH2 | 0.6996 | 0.5466 | 0.8501 | 0.7612 | 0.9571 | 0.7691 | 0.9053 | 0.8074 | 0.7342 | 0.5627 | 0.6319 | 0.4167 |
| pH3 | 0.8496 | 0.8108 | 0.8742 | 0.8226 | 0.9772 | 0.8613 | 0.8422 | 0.8420 | 0.8493 | 0.6049 | 0.9132 | 0.2286 |
| pH4 | 0.8430 | 0.3881 | 0.7611 | 0.5152 | 0.9391 | 0.8818 | 0.8145 | 0.5165 | 0.9011 | 0.6833 | 0.8478 | 0.6617 |
| pH5 | 0.7218 | 0.6365 | 0.8312 | 0.5648 | 0.8854 | 0.7996 | 0.7910 | 0.5562 | 0.8962 | 0.5217 | 0.6834 | 0.2517 |
| pH6 | 0.7217 | 0.2788 | 0.9024 | 0.8038 | 0.9110 | 0.8363 | 0.6514 | 0.6991 | 0.8067 | 0.7124 | 0.6408 | 0.4489 |
| pH7 | 0.8264 | 0.4230 | 0.7219 | 0.7025 | 0.9217 | 0.8681 | 0.9336 | 0.7058 | 0.8012 | 0.6872 | 0.8301 | 0.5485 |
| pH8 | 0.6928 | 0.3918 | 0.9761 | 0.5253 | 0.9548 | 0.7760 | 0.8845 | 0.6081 | 0.9586 | 0.6448 | 0.9065 | 0.4062 |
| pH9 | 0.6169 | 0.0585 | 0.6663 | 0.1829 | 0.9367 | 0.7722 | 0.8209 | 0.3301 | 0.7800 | 0.3862 | 0.6392 | 0.2796 |
| Dye | Extraction Method | BPNN | PLSR | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RMSEC | RMSEP | RPD | RMSEC | RMSEP | RPD | ||||||
| TPP5 | RAW | 0.8772 | 0.5316 | 0.8562 | 0.5906 | 2.6721 | 0.8887 | 0.5059 | 0.8389 | 0.6251 | 2.5249 |
| ABFO | 0.9253 | 0.4147 | 0.9056 | 0.4786 | 3.2975 | 0.9301 | 0.4012 | 0.8826 | 0.5335 | 2.9581 | |
| CARS | 0.9181 | 0.4342 | 0.8894 | 0.5179 | 3.0475 | 0.8789 | 0.5280 | 0.8636 | 0.5752 | 2.7437 | |
| UVE | 0.9009 | 0.4774 | 0.8788 | 0.5423 | 2.9104 | 0.9045 | 0.4688 | 0.8645 | 0.5734 | 2.7527 | |
| pH1 | RAW | 0.8910 | 0.5007 | 0.8696 | 0.5624 | 2.8065 | 0.8202 | 0.6432 | 0.8290 | 0.6439 | 2.4510 |
| ABFO | 0.9169 | 0.4371 | 0.8945 | 0.5059 | 3.1197 | 0.8911 | 0.5007 | 0.8576 | 0.5877 | 2.6856 | |
| CARS | 0.9007 | 0.4779 | 0.8878 | 0.5216 | 3.0256 | 0.9229 | 0.4212 | 0.9163 | 0.4506 | 3.5025 | |
| UVE | 0.9066 | 0.4635 | 0.8809 | 0.5376 | 2.9359 | 0.9164 | 0.4387 | 0.8937 | 0.5078 | 3.1080 | |
| pH3 | RAW | 0.9245 | 0.4169 | 0.8623 | 0.5778 | 2.7314 | 0.9772 | 0.2288 | 0.8613 | 0.5799 | 2.7212 |
| ABFO | 0.9328 | 0.3933 | 0.9279 | 0.4180 | 3.7754 | 0.9079 | 0.4602 | 0.8802 | 0.5391 | 2.9277 | |
| CARS | 0.9435 | 0.3605 | 0.9007 | 0.4907 | 3.2163 | 0.9211 | 0.4261 | 0.9209 | 0.4379 | 3.6034 | |
| UVE | 0.9214 | 0.4253 | 0.8911 | 0.5139 | 3.0709 | 0.9223 | 0.4228 | 0.8849 | 0.5283 | 2.9876 | |
| Combinatorial dyes | RAW | 0.9451 | 0.3555 | 0.8769 | 0.5463 | 2.8890 | 0.9411 | 0.3681 | 0.9063 | 0.4767 | 3.3111 |
| ABFO | 0.9333 | 0.3918 | 0.9348 | 0.3976 | 3.9695 | 0.9270 | 0.4099 | 0.9312 | 0.4085 | 3.8638 | |
| CARS | 0.9508 | 0.3364 | 0.9097 | 0.4679 | 3.3727 | 0.9306 | 0.3997 | 0.9301 | 0.4119 | 3.8317 | |
| UVE | 0.9436 | 0.3603 | 0.9029 | 0.4852 | 3.2529 | 0.9197 | 0.4299 | 0.9141 | 0.4565 | 3.4574 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Tian, H.; Zhao, K.; Yu, Y.; Zhuo, C.; Xiao, Z.; Wan, D. Non-Destructive Detection of pH Value During Secondary Fermentation of Maize Silage Using Colorimetric Sensor Array Combined with Hyperspectral Imaging Technology. Agronomy 2025, 15, 285. https://doi.org/10.3390/agronomy15020285
Xue X, Tian H, Zhao K, Yu Y, Zhuo C, Xiao Z, Wan D. Non-Destructive Detection of pH Value During Secondary Fermentation of Maize Silage Using Colorimetric Sensor Array Combined with Hyperspectral Imaging Technology. Agronomy. 2025; 15(2):285. https://doi.org/10.3390/agronomy15020285
Chicago/Turabian StyleXue, Xiaoyu, Haiqing Tian, Kai Zhao, Yang Yu, Chunxiang Zhuo, Ziqing Xiao, and Daqian Wan. 2025. "Non-Destructive Detection of pH Value During Secondary Fermentation of Maize Silage Using Colorimetric Sensor Array Combined with Hyperspectral Imaging Technology" Agronomy 15, no. 2: 285. https://doi.org/10.3390/agronomy15020285
APA StyleXue, X., Tian, H., Zhao, K., Yu, Y., Zhuo, C., Xiao, Z., & Wan, D. (2025). Non-Destructive Detection of pH Value During Secondary Fermentation of Maize Silage Using Colorimetric Sensor Array Combined with Hyperspectral Imaging Technology. Agronomy, 15(2), 285. https://doi.org/10.3390/agronomy15020285





