Biochar in the Bioremediation of Metal-Contaminated Soils
Abstract
1. Introduction
2. Production, General Characteristics and Potential Applications of Biochar
3. Biochar as the Adsorbent of Metals
| Ion exchange adsorption | The ions of the adsorbate concentrate on the active sites on the external surface of the adsorbent and/or in its pores (internal surface). The cations present in this site are exchanged for adsorbate cations in equivalent amounts [43,44,46,48,49,50,51]. |
| Chemisorption | The ions of the adsorbate form the bonds with the functional groups of the surface adsorbent. It is an irreversible or hardly reversible process. It involves the formation of insoluble salts on the adsorbent or surface complexation [43,44,46,48,49,50,51]. |
| Physisorption | It is the result of nonspecific Van der Waals’ forces without transfer of electrons between ions. It is an easily reversible process. Only molecules that have not dissociated are subject to this sorption [43,44,46,48,49,50,51]. |
| Mechanical (sieve) adsorption | It depends on the degree of porosity and the ability to trap molecules in the pores (pore filling). The porous material acts like a sieve, retaining larger molecules and allowing smaller ones to pass through [18,51,52]. |
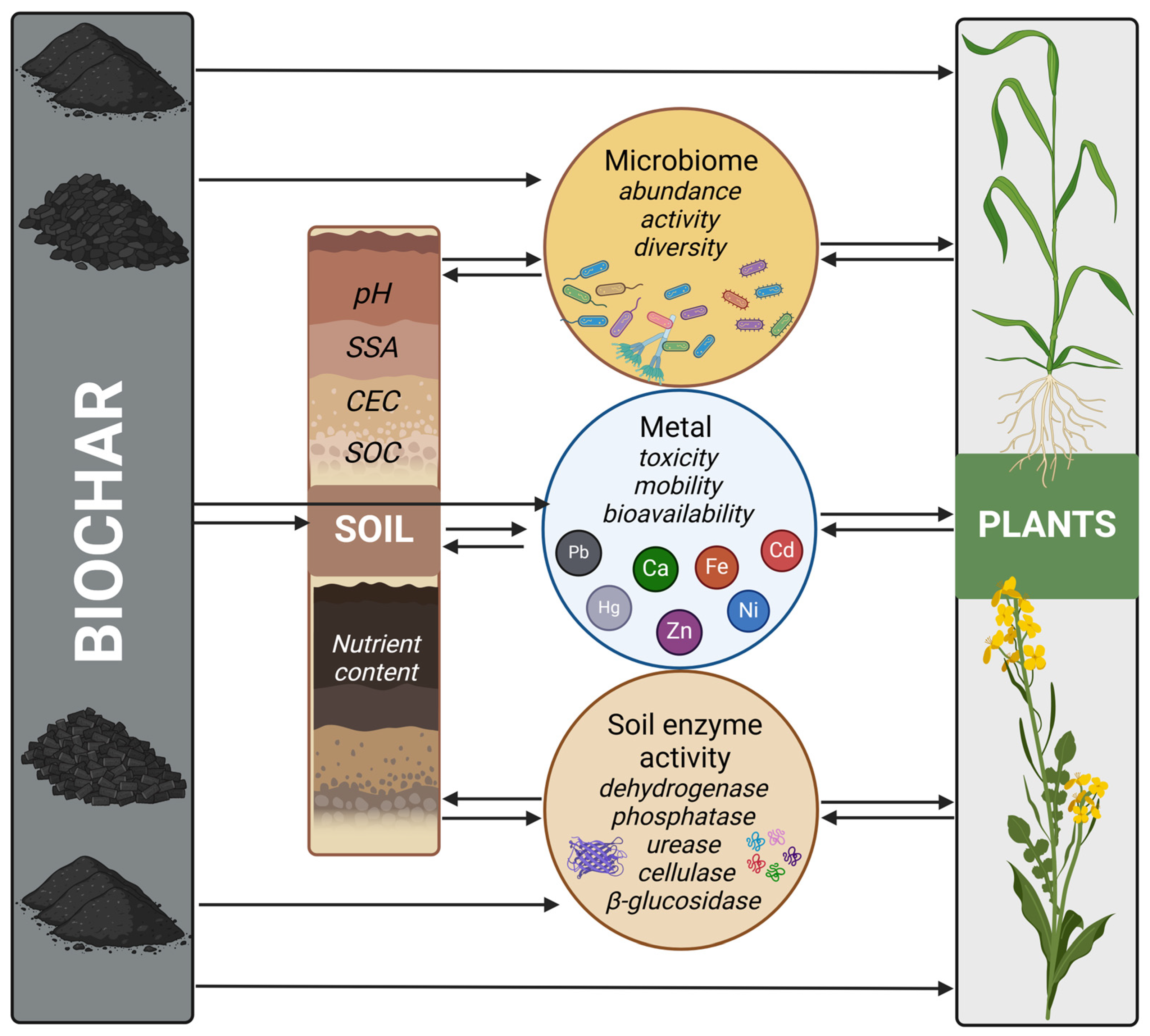
4. Biochar in Soil Bioremediation
4.1. Improvement of Soil Parameters
4.2. Modification of the Metal Mobility and Bioavailability
4.3. Modifiction of the Metal Bioaccumulation
4.4. Effect on the Soil Microbiota
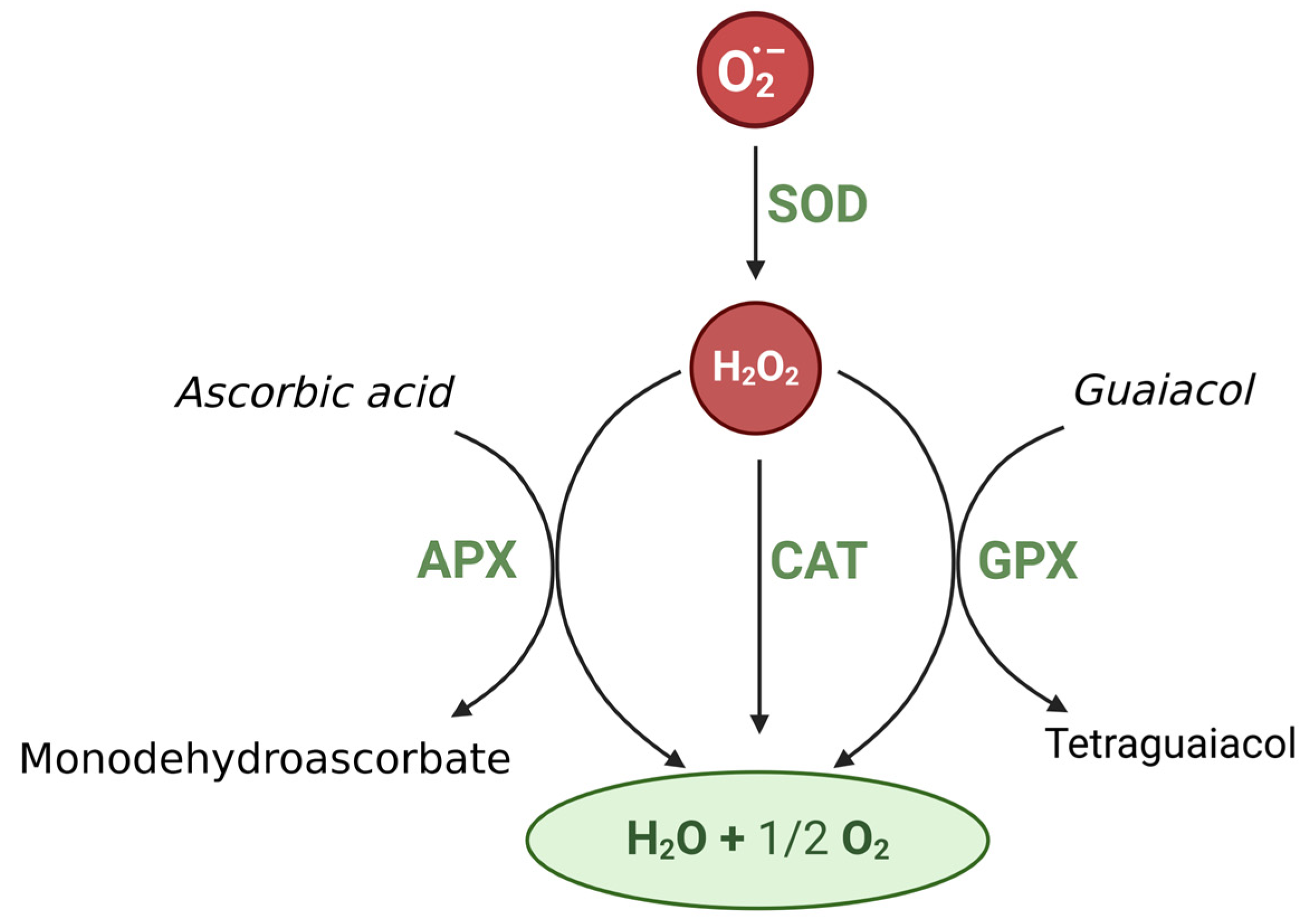
4.5. Activity of Enzymes in Biochar-Treaded Soils
4.6. Plant Growth Promotion and Stress Mitigation
5. Risks of Biochar Use
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lahori, A.H.; Guo, Z.; Zhang, Z.; Li, R.; Mahar, A.; Awasthi, M.K.; Shen, F.; Sial, T.A.; Kumbhar, F.; Wang, P.; et al. Use of biochar as an amendment for remediation of heavy metal-contaminated soils: Prospects and challenges. Pedosphere 2017, 27, 991–1014. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.-S.; Tang, C.-S.; Gu, K.; Shi, G. Remediation of heavy-metal-contaminated soils by biochar: A review. Environ. Geotech. 2019, 9, 135–148. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, T.; Xu, W.; Huang, J.; Jiang, S.; Yan, B. Application research of biochar for the remediation of soil heavy metals contamination: A review. Molecules 2020, 25, 3167. [Google Scholar] [CrossRef] [PubMed]
- Herliana, O.; Ahadiyat, Y.R.; Cahyani, W. Utilization of biochar and Trichoderma harzianum to promote growth of shallot and remediate lead-contaminated soil. J. Degrad. Min. Lands Manag. 2021, 8, 2743–2750. [Google Scholar] [CrossRef]
- Tan, H.; Lee, C.T.; Ong, P.Y.; Wong, K.Y.; Bong, C.P.C.; Li, C.; Gao, Y. A review on the comparison between slow pyrolysis and fast pyrolysis on the quality of lignocellulosic and lignin-based biochar. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1051, 012075. [Google Scholar] [CrossRef]
- Safarian, S. Performance analysis of sustainable technologies for biochar production: A comprehensive review. Energy Rep. 2023, 9, 4574–4593. [Google Scholar] [CrossRef]
- Dziok, T.; Strugała, A.; Olszewska, D. Valorization method for hard coal as fuel for nonindustrial combustion installations with special regard to reduction of mercury content. Energy Fuels 2020, 34, 2980–2988. [Google Scholar] [CrossRef]
- Saletnik, B.; Bajcar, M.; Saletnik, A.; Zaguła, G.; Puchalski, C. Effect of the pyrolysis process applied to waste branches biomass from fruit trees on the calorific value of the biochar and dust explosivity. Energies 2021, 14, 4898. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L.; Zhu, Y.; Fang, W.; Tan, Y.; He, Z.; Liao, H. Research status, trends, and mechanisms of biochar adsorption for wastewater treatment: A scientometric review. Environ. Sci. Eur. 2024, 36, 25. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K.; Mierzwa–Hersztek, M. Biological effects of biochar and zeolite used for remediation of soil contaminated with toxic heavy metals. Sci. Rep. 2021, 11, 6998. [Google Scholar] [CrossRef] [PubMed]
- Manori, S.; Shah, V.; Soni, V.; Dutta, K.; Daverey, A. Phytoremediation of cadmium-contaminated soil by Bidens pilosa L.: Impact of pine needle biochar amendment. Environ. Sci. Pollut. Res. 2021, 28, 58872–58884. [Google Scholar] [CrossRef] [PubMed]
- Simiele, M.; Lebrun, M.; Miard, F.; Trupiano, D.; Poupart, F.; Forestier, O.; Scippa, G.S.; Bourgerie, S.; Morabito, D. Assisted phytoremediation of a former mine soil using biochar and iron sulphate: Effects on As soil immobilization and accumulation in three Salicaceae species. Sci. Total Environ. 2020, 710, 136203. [Google Scholar] [CrossRef] [PubMed]
- Simiele, M.; Sferra, G.; Lebrun, M.; Renzone, G.; Bourgerie, S.; Scippa, G.S.; Morabito, D.; Scaloni, A.; Trupiano, D. In-depth study to decipher mechanisms underlying Arabidopsis thaliana tolerance to metal(loid) soil contamination in association with biochar and/or bacteria. Environ. Exp. Bot. 2021, 182, 104335. [Google Scholar] [CrossRef]
- Chen, C.; Yang, F.; Ma, Y.; Dai, L.; Zhang, Z.; Guo, H.; Ding, Y. Ball milling boosted magnetic cotton husk-derived biochar adsorptive removal of oxytetracycline and ciprofloxacin from water. Carbon Res. 2024, 3, 63. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M. Adsorption characteristics and mechanism of bisphenol A by magnetic biochar. Int. J. Environ. Res. Public Health 2020, 17, 1075. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of adsorption and functionalization of biochar for pesticides: A review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef] [PubMed]
- Edwin, T.; Mera, M.; Komala, P.S. Impact of pyrolysis temperature on the removal of nutrients using coarse rice-husk biochar. J. Ecol. Eng. 2023, 24, 247–257. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; van Hullebusch, E.D.; Amrane, A.; Rtimi, S. Mechanisms and adsorption capacities of biochar for the removal of organic and inorganic pollutants from industrial wastewater. Int. J. Environ. Sci. Technol. 2021, 18, 3273–3294. [Google Scholar] [CrossRef]
- Li, Z.; Niu, R.; Yu, J.; Yu, L.; Cao, D. Removal of cadmium from aqueous solution by magnetic biochar: Adsorption characteristics and mechanism. Environ. Sci. Pollut. Res. 2024, 31, 6543–6557. [Google Scholar] [CrossRef]
- Lopičić, Z.R.; Šoštarić, T.D.; Milojković, J.V.; Antanasković, A.V.; Milić, J.S.; Spasić, S.D.; Avdalović, J.S. Efficient removal of water soluble fraction of diesel oil by biochar sorption supported by microbiological degradation. Processes 2024, 12, 964. [Google Scholar] [CrossRef]
- Liang, M.; Lu, L.; He, H.; Li, J.; Zhu, Z.; Zhu, Y. Applications of biochar and modified biochar in heavy metal contaminated soil: A descriptive review. Sustainability 2021, 13, 14041. [Google Scholar] [CrossRef]
- Francis, J.C.; Nighojkar, A.; Kandasubramanian, B. Relevance of wood biochar on CO2 adsorption: A review. Hybrid Adv. 2023, 3, 100056. [Google Scholar] [CrossRef]
- Tayang, A.; Songachan, L.S. Microbial bioremediation of heavy metals. Curr. Sci. 2021, 120, 1013–1025. [Google Scholar] [CrossRef]
- Barton, L.L.; McLean, R.J.C. Environmental Microbiology and Microbial Ecology, 1st ed.; Willey Blackwell: Chichester, UK, 2019. [Google Scholar]
- Roy, R.; Samanta, S.; Pandit, S.; Naaz, T.; Banerjee, S.; Rawat, J.M.; Chaube, K.K.; Saha, R.P. An overview of bacteria-mediated heavy metal bioremediation strategies. Appl. Biochem. Biotechnol. 2024, 196, 1712–1751. [Google Scholar] [CrossRef] [PubMed]
- Sonker, A.B.; Fulke, A.; Monga, A. Recent trends on bioremediation of heavy metals; an insight with reference to the potential of marine microbes. Int. J. Environ. Sci. Technol. 2024, 21, 9763–9774. [Google Scholar] [CrossRef]
- Sur, I.M.; Micle, V.; Hegyi, A.; Lăzărescu, A.-V. Extraction of metals from polluted soils by bioleaching in relation to environmental risk assessment. Materials 2022, 15, 3973. [Google Scholar] [CrossRef]
- Deo, L.; Osborne, J.W.; Benjamin, L.K. Harnessing microbes for heavy metal remediation: Mechanisms and prospects. Environ. Monit. Assess. 2025, 197, 116. [Google Scholar] [CrossRef] [PubMed]
- Monga, Y.; Sharma, S.; Singh, S.; Gupta, A. Biochar, clay, zeolites, and microorganism-based methods for remediation of heavy metals. Curr. Green Chem. 2024, 11, 2–11. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. The combined rhizoremediation by a triad: Plant-microorganism-functional materials. Environ. Sci. Pollut. Res. 2023, 30, 90500–90521. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Zhang, W.; Zhou, J.; Luo, D.; Li, Z. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef]
- Ścisłowska, M.; Włodarczyk, R.; Kobyłecki, R.; Bis, Z. Biochar to improve the quality and productivity of soils. J. Ecol. Eng. 2015, 16, 31–35. [Google Scholar] [CrossRef]
- Saleem, A.; Ur Rahim, H.; Khan, U.; Irfan, M.; Akbar, W.A.; Akbar, Z.; Alatalo, J.M. Organic materials amendments can improve NPK availability and maize growth by reducing heavy metals stress in calcareous soil. Int. J. Environ. Sci. Technol. 2024, 21, 2533–2546. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the influence of compost and biochar amendments on the mobility and uptake of heavy metals by green leafy vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Mawof, A.; Prasher, S.O.; Bayen, S.; Anderson, E.C.; Nzediegwu, C.; Patel, R. Barley straw biochar and compost affect heavy metal transport in soil and uptake by potatoes grown under wastewater irrigation. Sustainability 2022, 14, 5665. [Google Scholar] [CrossRef]
- Jeffery, S.; van de Voorde, T.F.; Harris, W.E.; Mommer, L.; Van Groenigen, J.W.; De Deyn, G.B.; Ekelund, F.; Briones, M.J.I.; Bezemer, T.M. Biochar application differentially affects soil micro-, meso-macro-fauna and plant productivity within a nature restoration grassland. Soil Biol. Biochem. 2022, 174, 108789. [Google Scholar] [CrossRef]
- Sobik-Szołtysek, J.; Wystalska, K.; Malińska, K.; Meers, E. Influence of pyrolysis temperature on the heavy metal sorption capacity of biochar from poultry manure. Materials 2021, 14, 6566. [Google Scholar] [CrossRef] [PubMed]
- Man, K.Y.; Chow, K.L.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Use of biochar as feed supplements for animal farming. Crit. Rev. Environ. Sci. Technol. 2021, 51, 187–217. [Google Scholar] [CrossRef]
- Li, H.; Dong, H.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Lu, H.; Lonappan, L.; Brar, S.K.; He, L.; Chen, J.; Yang, S. Biochar application as a soil amendment for decreasing cadmium availability in soil and accumulation in Brassica chinensis. J. Soils Sediments 2018, 18, 2511–2519. [Google Scholar] [CrossRef]
- Mohamed, I.; Zhang, G.; Li, Z.; Liu, Y.; Chen, F.; Dai, K. Ecological restoration of an acidic Cd contaminated soil using bamboobiochar application. Ecol. Eng. 2025, 84, 67–76. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Feng, Y.; Qiao, J.; Zhao, H.; Xie, J.; Fang, Y.; Shen, S.; Liang, S. The effectiveness of nanobiochar for reducing phytotoxicity and improving soil remediation in cadmium-contaminated soil. Sci. Rep. 2020, 10, 858. [Google Scholar] [CrossRef] [PubMed]
- Strawn, D.G. Sorption mechanisms of chemicals in soils. Soil Syst. 2021, 5, 13. [Google Scholar] [CrossRef]
- Tan, W.T.; Zhou, H.; Tang, S.F.; Zeng, P.; Gu, J.F.; Liao, B.H. Enhancing Cd(II) adsorption on rice straw biochar by modification of iron and manganese oxides. Environ. Pollut. 2022, 300, 118899. [Google Scholar] [CrossRef]
- Li, Y.; Wang, B.; Shang, H.; Cao, Y.; Yang, C.; Hu, W.; Feng, Y.; Yu, Y. Influence of adsorption sites of biochar on its adsorption performance for sulfamethoxazole. Chemosphere 2023, 326, 138408. [Google Scholar] [CrossRef]
- Jagadeesh, N.; Sundaram, B. Adsorption of pollutants from wastewater by biochar: A review. J. Hazard. Mater. Adv. 2023, 9, 100226. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, W.; Wu, J.; Huang, Z.; Dai, J. Mechanism of Cu(II) adsorption inhibition on biochar by its aging process. J. Environ. Sci. 2014, 26, 2123–2130. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Huang, S.; Chen, H.; Wang, F. Binding of four heavy metals to hemicelluloses from rice bran. Food Res. Int. 2010, 43, 203–206. [Google Scholar] [CrossRef]
- Guo, J.; Knol, L.L.; Yang, X.; Kong, L. Dietary fiber intake is inversely related to serum heavy metal concentrations among US adults consuming recommended amounts of seafood: NHANES 2013–2014. Food Front. 2022, 3, 142–149. [Google Scholar] [CrossRef]
- Zhao, G.; Wu, X.; Tan, X.; Wang, X. Sorption of heavy metal ions from aqueous solutions: A review. Open Colloid Sci. J. 2010, 4, 19–31. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, S. Soil Science. Students’ Handbook, 4th ed.; PWRiL: Warsaw, Poland, 1999. (In Polish) [Google Scholar]
- Angelovičová, L.; Fazekašová, D. Contamination of the soil and water environment by heavy metals in the former mining area of Rudňany (Slovakia). Soil Water Res. 2014, 9, 18–24. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soil and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Van der Perk, M. Soil and Water Contamination from Molecular to Catchment Scale, 1st ed.; Taylor & Francis: London, UK, 2006. [Google Scholar]
- Majewska, M. Mobilization of cadmium from Festuca ovina roots and its distribution between operational fractions in soil. Pol. J. Soil Sci. 2017, 50, 141–151. [Google Scholar] [CrossRef][Green Version]
- Kumar, V.; Saxena, G. Microbe-Assisted Phytoremediation of Environmental Pollutants. Recent Advances and Challenges, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Huang, F.; Dong, F.; Chen, L.; Zeng, Y.; Zhou, L.; Sun, S.; Wang, Z.; Lai, J.; Fang, L. Biochar-mediated remediation of uranium-contaminated soils: Evidence, mechanisms, and perspectives. Biochar 2024, 6, 16. [Google Scholar] [CrossRef]
- Howell, C.R.; Jenkins, S.N.; Abbott, L.K.; Mickan, B.S. Amelioration of a saline-alkaline soil using biochar and compost: Impacts on plant growth, soil biological and chemical characteristics. Land Degrad. Dev. 2024, 35, 142–155. [Google Scholar] [CrossRef]
- Zaid, F.; Al-Awwal, N.; Yang, J.; Anderson, S.H.; Alsunuse, B.T. Effects of biochar-amended composts on selected enzyme activities in soils. Processes 2024, 12, 1678. [Google Scholar] [CrossRef]
- Radziemska, M.; Gusiatin, M.Z.; Cydzik-Kwiatkowska, A.; Blazejczyk, A.; Kumar, V.; Kintl, A.; Brtnicky, M. Effect of biochar on metal distribution and microbiome dynamic of a phytostabilized metalloid-contaminated soil following freeze–thaw cycles. Materials 2022, 15, 3801. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, J.L. Effects of biochar on soil microbial diversity and community structure in clay soil. Ann. Microbiol. 2022, 72, 35. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, R.; Zhao, Y.; Shi, H.; Liu, G. Effect of biochar on rhizosphere soil microbial diversity and metabolism in tobacco-growing soil. Ecologies 2022, 3, 539–556. [Google Scholar] [CrossRef]
- Singh, H.; Northup, B.K.; Rice, C.W.; Prasad, P.V. Biochar applications influence soil physical and chemical properties, microbial diversity, and crop productivity: A meta-analysis. Biochar 2022, 4, 8. [Google Scholar] [CrossRef]
- Bingari, H.S.; Gibson, A.; Teeuw, R. Sorption modelling of crude oil-contaminated soils using a derived spectral index. Geoderma 2024, 447, 116935. [Google Scholar] [CrossRef]
- Ali, J.; Tuzen, M.; Jatoi, W.B.; Feng, X.; Sun, G.; Saleh, T.A. A review of sequential extraction methods for fractionation analysis of toxic metals in solid environmental matrices. TrAC Trends Anal. Chem. 2024, 173, 117639. [Google Scholar] [CrossRef]
- Majewska, M.; Kurek, E.; Słomka, A. Effect sof plant growth on total concentrations of Zn, Pb, and Cd, and their distribution between operational fractions in the upper layer of a 100-year-old zinc-lead waste heap. Pol. J. Environ. Stud. 2011, 20, 591–597. [Google Scholar]
- Majewska, M.; Wdowiak-Wróbel, S.; Marek-Kozaczuk, M.; Nowak, A.; Tyśkiewicz, R. Cadmium-resistant Chryseobacterium sp. DEMBc1 strain: Characteristics and potential to assist phytoremediation and promote plant growth. Environ. Sci. Pollut. Res. 2022, 29, 83567–83579. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.M.; Galaon, T.; Pascu, L.F. Ultrasound-assisted Community Bureau of Reference (BCR) procedure for heavy metal removal in sewage sludge. Materials 2024, 17, 5452. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, L.; Bian, R.; Yan, J.; Quan, G.; Liu, Y.; Ippolito, J.A.; Wang, H. Short- and long-term biochar cadmium and lead immobilization mechanisms. Environments 2020, 7, 53. [Google Scholar] [CrossRef]
- Hanaka, A.; Plak, A.; Zagórski, P.; Ozimek, E.; Rysiak, A.; Majewska, M.; Jaroszuk-Ściseł, J. Relationships between the properties of Spitsbergen soil, number and biodiversity of rhizosphere microorganisms, and heavy metal concentration in selected plant species. Plant Soil 2019, 436, 49–69. [Google Scholar] [CrossRef]
- Zhu, Y.; An, M.; Anwar, T.; Wang, H. Differences in soil bacterial community structure during the remediation of Cd-polluted cotton fields by biochar and biofertilizer in Xinjiang, China. Front. Microbiol. 2024, 15, 1288526. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Vibhandik, R.; Agrahari, R.; Daverey, A.; Rani, R. Role of root exudates on the soil microbial diversity and biogeochemistry of heavy metals. Appl. Biochem. Biotechnol. 2024, 196, 2673–2693. [Google Scholar] [CrossRef]
- Reineke, W.; Schlömann, M. Environmental Microbiology, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Hanaka, A.; Ozimek, E.; Majewska, M.; Rysiak, A.; Jaroszuk-Ściseł, J. Physiological diversity of Spitsbergen soil microbial communities suggests their potential as plant growth-promoting bacteria. Int. J. Mol. Sci. 2019, 20, 1207. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Majewska, M.; Marzec-Grządziel, A.; Ozimek, E.; Przybyś, M.; Słomka, A.; Kutyrieva-Nowak, N.; Gałązka, A.; Jaroszuk-Ściseł, J. Effect of long-term radish (Raphanus sativus var. sativus) monoculture practice on physiological variability of microorganisms in cultivated soil. J. Environ. Manag. 2024, 367, 122007. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R.E. Deciphering diversity indices for a better understanding of microbial communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef]
- Xu, W.; Xu, H.; Delgado-Baquerizo, M.; Gundale, M.J.; Zou, X.; Ruan, H. Global meta-analysis reveals positive effects of biochar on soil microbial diversity. Geoderma 2023, 436, 116528. [Google Scholar] [CrossRef]
- Idbella, M.; Baronti, S.; Giagnoni, L.; Giancarlo Renella, G.; Becagli, M.; Cardelli, R.; Maienza, A.; Vaccari, F.P.; Bonanomi, G. Long-term effects of biochar on soil chemistry, biochemistry, and microbiota: Results from a 10-year field vineyard experiment. Appl. Soil Ecol. 2024, 95, 105217. [Google Scholar] [CrossRef]
- Nirukshan, G.S.; Ranasinghe, S.; Sleutel, S. The effect of biochar on mycorrhizal fungi mediated nutrient uptake by coconut (Cocos nucifera L.) seedlings grown on a Sandy Regosol. Biochar 2022, 4, 68. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biotechnology, 1st ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Kara, R.S.; Pazarlar, S.; Okur, B.; Almaz, C.; Okur, N.; Matula, S.; Miháliková, M. Texture and contamination-level dependent effects of calcium-rich deinking paper sludge biochar on soil Cd availability, enzymatic activity, and plant stress mitigation. Water Air Soil Pollut. 2024, 235, 454. [Google Scholar] [CrossRef]
- Ali, M.A.; Nafees, M.; Alomrani, S.O.; Li, Y.; Wang, Q.; Alshehri, M.A.; Al-Ghanim, K.A.; Ali, S.; Li, F. Novel nanocomposite and biochar insights to boost rice growth and alleviation of Cd toxicity. Sci. Rep. 2024, 14, 23158. [Google Scholar] [CrossRef]
- Hanaka, A.; Jaroszu-Ściseł, J.; Majewska, M. (Eds.) Study of the Influence of Abiotic and Biotic Stress Factors on Horticultural Plants; MDPI: Basel, Switzerland, 2022. [Google Scholar]
- Hanaka, A.; Majewska, M.; Hawrylak-Nowak, B. (Eds.) Horticultural Plants Facing Stressful Conditions—Ways of Stress Mitigation; MDPI: Basel, Switzerland, 2023. [Google Scholar]
- Gashi, B.; Buqaj, L.; Vataj, R.; Metin Tuna, M. Chlorophyll biosynthesis suppression, oxidative level and cell cycle arrest caused by Ni, Cr and Pb stress in maize exposed to treated soil from the Ferronikel smelter in Drenas, Kosovo. Plant Stress 2024, 11, 100379. [Google Scholar] [CrossRef]
- Hanaka, A.; Nowak, A.; Plak, A.; Dresler, S.; Ozimek, E.; Jaroszuk-Ściseł, J.; Wójciak-Kosior, M.; Sowa, I. Bacterial isolate inhabiting Spitsbergen soil modifies the physiological response of Phaseolus coccineus in control conditions and under exogenous application of methyl jasmonate and copper excess. Int. J. Mol. Sci. 2019, 20, 1909. [Google Scholar] [CrossRef]
- Hanaka, A.; Nowak, A.; Ozimek, E.; Dresler, S.; Plak, A.; Sujak, A.; Reszczyńska, E.; Strzemski, M. Effect of copper stress on Phaseolus coccineus in the presence of exogenous methyl jasmonate and/or Serratia plymuthica from the Spitsbergen soil. J. Hazard. Mater. 2022, 436, 129232. [Google Scholar] [CrossRef]
- da Cruz Ferreira, R.L.; Braga, D.G.; do Nascimento, V.R.; da Silva, C.B.; Alves, A.C.B.; da Costa Cabral, J.A.; Cruz, F.J.R.; de Araújo Brito, A.E.; dos Santos Nogueira, G.A.; Castro de Souza, L.; et al. Biochar improves growth and physiology of Swietenia macrophylla King in contaminated soil by copper. Sci. Rep. 2024, 14, 22546. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef]
- Nowak, A.; Tyśkiewicz, R.; Wiater, A.; Jaroszuk-Sciseł, J. (1→3)-α-D-glucooligosaccharides as elicitors influencing the activity of plant resistance pathways in wheat tissues. Agronomy 2022, 12, 1170. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Z.; Beiyuan, J.; Cui, Y.; Chen, L.; Chen, H.; Fang, F. A critical review of uranium in the soil-plant system: Distribution, bioavailability, toxicity, and bioremediation strategies. Crit. Rev. Environ. Sci. Technol. 2023, 53, 340–365. [Google Scholar] [CrossRef]
- Zheng, S.; Guo, D.; Yang, X.; Liu, Y.; Liu, S.; Tian, W.; Niu, Z.; Hua Huang, H. Full life cycle of uranium toward ore-soil-root-shoot system: A comprehensive review. Crit. Rev. Environ. Sci. Technol. 2024, 55, 123–145. [Google Scholar] [CrossRef]
- Ndirangu, S.M.; Liu, Y.; Xu, K.; Song, S. Risk evaluation of pyrolyzed biochar from multiple wastes. J. Chem. 2019, 1, 4506314. [Google Scholar] [CrossRef]
- Brtnicky, M.; Datta, R.; Holatko, J.; Bielska, J.; Gusiatin, Z.M.; Kucerik, J.; Hammerschmiedt, T.; Danish, S.; Radziemska, M.; Mravcova, L.; et al. A critical review of the possible adverse effects of biochar in the soil environment. Sci. Total Environ. 2021, 796, 148756. [Google Scholar] [CrossRef] [PubMed]
- Gelardi, D.L.; Li, C.; Parikh, S.J. An emerging environmental concern: Biochar-induced dust emissions and their potentially toxic properties. Sci. Total Environ. 2019, 678, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Zhao, J.; Qiu, C.; Fan, X.; Zheng, J.; Liu, N.; Wang, C.; Wang, D.; Wang, S. Chemical speciation and risk assessment of heavy metals in biochars derived from sewage sludge and anaerobically digested sludge. Water Sci. Technol. 2021, 84, 1079–1089. [Google Scholar] [CrossRef]
- Han, H.; Buss, W.; Zheng, Y.; Song, P.; Rafiq, M.K.; Liu, P.; Masek, O.; Li, X. Contaminants in biochar and suggested mitigation measure—A review. Chem. Eng. J. 2022, 429, 132287. [Google Scholar] [CrossRef]
- Feng, J.; Burke, I.T.; Chen, X.; Stewart, D.I. Assessing metal contamination and speciation in sewage sludge: Implications for soil application and environmental risk. Rev. Environ. Sci. Biotechnol. 2023, 22, 1037–1058. [Google Scholar] [CrossRef]
- Ishchenko, V. Heavy metals in municipal waste: The content and leaching ability by waste fraction. J. Environ. Sci. Health A 2019, 54, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Cowie, A.L.; Downie, A.E.; George, B.H.; Singh, B.P.; Van Zwieten, L.; O’Connell, D. Is sustainability certification for biochar the answer to environmental risks? Pesquisa Agropecuária Brasileira 2012, 47, 637–648. [Google Scholar] [CrossRef]
- He, P.; Liu, Y.; Shao, L.; Zhang, H.; Lü, F. Particle size dependence of the physicochemical properties of biochar. Chemosphere 2018, 212, 385–392. [Google Scholar] [CrossRef]
- Štrubelj, L. Waste, fertilising product, or something else? EU regulation of biochar. J. Environ. Law. 2022, 34, 529–540. [Google Scholar] [CrossRef]
- Rząsa, S.; Owczarzak, W. Methods for the granulometric analysis of soil for science and practice. Pol. J. Soil Sci. 2015, 46, 1. [Google Scholar]
- Izydorczyk, G.L.; Mikula, K.; Skrzypczak, D.; Trzaska, K.; Moustakas, K.; Witek-Krowiak, A.; Chojnacka, K. Agricultural and non-agricultural directions of bio-based sewage sludge valorization by chemical conditioning. Environ. Sci. Pollut. Res. 2021, 28, 47725–47740. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Schmidt, H.-P.; van Scholl, L.; Ruysschaert, G.; Nelissen, V.; Ibarrola, R.; O’Toole, A.; Shackley, S.; van Laer, T. The legality of biochar use: Regulatory requirements and risk assessment. In Biochar in European Soils and Agriculture: Science and Practice, 1st ed.; Shackley, S., Ruysschaert, G., Zwart, K., Glaser, B., Eds.; Routledge: Abingdon, UK, 2016; Chapter 10; pp. 227–252. [Google Scholar]
- The Global Market for Biochar 2025–2035, September 2024, Future Markets, Inc., ID: 5806837. Available online: https://www.researchandmarkets.com/report/biochar?srsltid=AfmBOorWC6XCzM2sGpIF0z4AUsnfgpNwroS9G0yE_YU4VWOh-R7PynCv (accessed on 15 January 2025).
- Biochar Market by Row Material, Feedstock, Technology, Application—Global Forecast 2025–2030, October 2024, 360iResearch™, ID: 6011346. Available online: https://www.researchandmarkets.com/reports/6011346/biochar-market-row-material-feedstock?srsltid=AfmBOoqN8ZL8Rwj6KTK-XfNJWedsCFb12RVe9T-HB6laUaQ8QPj458w5 (accessed on 15 January 2025).
- Commission Welcomes Political Agreement on EU-Wide Certification Scheme for Carbon Removals. Available online: https://ec.europa.eu/commission/presscorner/api/files/document/print/en/ip_24_885/IP_24_885_EN.pdf (accessed on 15 January 2025).
- Natural Resources Conservation Service Conservation Practice Standard Soil Carbon Amendment. Available online: https://www.nrcs.usda.gov/sites/default/files/2022-11/336-NHCP-CPS-Soil-Carbon-Amendment-2022.pdf (accessed on 15 January 2025).
- The Regulatory Landscape for Biochar: Highlights from 2024 and What to Expect in 2025. Available online: https://biochartoday.com/2024/12/11/the-regulatory-landscape-for-biochar-highlights-from-2024-and-what-to-expect-in-2025/ (accessed on 15 January 2025).


| Technology | Conditions | Products |
|---|---|---|
| Slow pyrolysis | Slow burning process in absence of O2 or under a limited O2 supply at 300–700 °C for about 5–6 h at a heating rate of less than 30 °C min−1; the feedstock must be heated [2,3,4,5,6] | biochar—35% bio-oil—30% syngas—35% |
| Intermediate pyrolysis | Moderate burning process at 300–500 °C for about 1–15 min at a 1–10 °C s−1 heating rate in the O2 free atmosphere [3,6,21] | biochar—20% bio-oil—50% syngas—30% |
| Fast pyrolysis | Fast burning process in absence of oxygen at 600–1000 °C for about 1–2 s at a 600–1000 °C s−1 heating rate in inert atmosphere (N2); the feedstock with low moisture content (<10%) [2,3,5,6,21] | biochar—12% bio-oil—75% syngas—13% |
| Torrefaction | Mild pyrolysis at 200–300 °C for 10–180 min at a heating rate of less than 50 °C min−1 under atmospheric pressure in inert atmosphere (N2) [3,6] | charcoal—70% syngas—30% |
| Microwave pyrolysis | Burning process at 350–650 °C for 1–60 min at a 25–50 °C min−1 heating rate; microwaves radiation penetrates the entire volume of the feedstock and generates a rapid and homogeneous temperature increase throughout the reactor [6,21] | biochar—15–80% bio-oil—8–70% syngas—12–60% |
| Gasification | Short burning process at 600–1500 °C for about 10–20 s at a 50–100 °C s−1 heating rate and oxygen-limited conditions; includes 5 steps: drying, pyrolysis, oxidation, reduction and cracking [2,3,21] | biochar—10% bio-oil—5% syngas—85% |
| Flash carbonization | Partial burning process at 300–600 °C for less than 30 min at a very fast heating rate in a pressurized reactor (1–2 MPa) under air supply [6,21] | charcoal—50% syngas—50% |
| Hydrothermal carbonization | Conversion of feedstock with high moisture content at 100–300 °C for 1–16 h at a 5–10 °C min−1 heating rate and elevated pressure (2–10 MPa) [2,3,21] | biochar—75% bio-oil—20% syngas—5% |
| Fractions | Extractants | Conditions |
|---|---|---|
| Mobile/Bioavailable exchangeable and acid-soluble | 40 mL of 0.11 M CH3COOH | shaking at room temperature for 16 h |
| Reducible bound to Fe and Mn oxides | 40 mL of 0.5 M NH2OH·HCl | shaking at room temperature for 16 h |
| Oxidizable bound to soil organic matter | step 1: 10 mL of 30% H2O2 step 2: 50 mL 1 M CH3COONH4 pH 2 | step 1: digestion at 85 °C for 1 h step 2: shaking for 16 h |
| Residual stable fraction | aqua regia: 7.5 mL 37% HCl and 2.5 mL 70% HNO3 | digestion for 2 h |
| Feedstock | Pyrolysis | Biochar Properties | Cd in Soil | Plant | Biochar in Soil | BAF | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Wheat straw | 550 °C, 30 s | pH SSA CEC | 9.86 239 m2 g−1 63 cmol(+) kg−1 | 6.2 mg kg−1 | Raphanus sativum L. | 0 5% 10% | 0.80 0.38 0.17 | [34] |
| Wheat straw | 550 °C, 30 s | pH SSA CEC | 9.86 239 m2 g−1 63 cmol(+) kg−1 | 6.2 mg kg−1 | Spinacia oleracea L. | 0 5% 10% | 0.62 0.37 0.35 | [34] |
| Wheat straw | 550 °C, 30 s | pH SSA CEC | 9.86 239 m2 g−1 63 cmol(+) kg−1 | 6.2 mg kg−1 | Lactuca sativa L. | 0 5% 10% | 0.73 0.91 0.81 | [34] |
| Pine needles | 500 °C, 3 h | pH SSA EC | 9.88 124 m2 g−1 5.82 µS cm−1 | 20 mg kg−1 | Bidens pilosa L. | 0 0.01% 0.02% | 3.46 5.14 4.72 | [11] |
| Rice straw | 600 °C, 1 h | pH SSA CEC TPV | 10.2 82 m2 g−1 45 cmol(+) kg−1 0.08 cm3 g−1 | 50 mg kg−1 | Brassica chinensis L. | 0 0.5% 1.0% 2.5% 5.0% | 0.83 0.83 0.63 0.59 0.49 | [40] |
| Bamboo chips | 600 °C, 1 h | pH SSA CEC TPV | 9.80 190 m2 g−1 15 cmol(+) kg−1 0.17 cm3 g−1 | 50 mg kg−1 | Brassica chinensis L. | 0 0.5% 1.0% 2.5% 5.0% | 0.83 0.71 0.60 0.54 0.41 | [40] |
| Bamboo residues | 400 °C, – | pH EC CEC | 9.16 3.24 µS cm−1 56 cmol(+) kg−1 | 50 mg kg−1 | Brassica rapa L. | 0 0.5% 1.0% 1.5% | 1.29 0.99 0.71 0.49 | [41] |
| Bamboo residues | 400 °C, – | pH EC CEC | 9.16 3.24 µS cm−1 56 cmol(+) kg−1 | 50 mg kg−1 | Zea mays L. | 0 0.5% 1.0% 1.5% | 0.77 0.57 0.48 0.33 | [41] |
| Gram Staining | Phylum | Genus |
|---|---|---|
| Gram-positive | Actinobacteria | Actinopolymorpha |
| Mycobacterium | ||
| Promicromonospora | ||
| Rhodococcus | ||
| Streptomyces | ||
| Thermobispora | ||
| Firmicutes | Lactococcus | |
| Thermobacillus | ||
| Gram-negative | Proteobacteria | Hyphomicrobium |
| Inquilinus | ||
| Ochrobactrum | ||
| Thermovum | ||
| Bacteroides | Prevotella |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, M.; Hanaka, A. Biochar in the Bioremediation of Metal-Contaminated Soils. Agronomy 2025, 15, 273. https://doi.org/10.3390/agronomy15020273
Majewska M, Hanaka A. Biochar in the Bioremediation of Metal-Contaminated Soils. Agronomy. 2025; 15(2):273. https://doi.org/10.3390/agronomy15020273
Chicago/Turabian StyleMajewska, Małgorzata, and Agnieszka Hanaka. 2025. "Biochar in the Bioremediation of Metal-Contaminated Soils" Agronomy 15, no. 2: 273. https://doi.org/10.3390/agronomy15020273
APA StyleMajewska, M., & Hanaka, A. (2025). Biochar in the Bioremediation of Metal-Contaminated Soils. Agronomy, 15(2), 273. https://doi.org/10.3390/agronomy15020273







