Seed Germination Ecology and Herbicide Sensitivity of Aeschynomene indica L.: Implications for Integrated Management in Paddy Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.1.1. Tested Weed Seeds
2.1.2. Test Herbicides
2.2. Test Methods
2.2.1. Seed Germination
- Experiment 1. Effect of Temperature on Germination
- Experiment 2. Effect of Light on Germination
- Experiment 3. Effect of pH on Germination
- Experiment 4. Effect of Salt Stress on Germination
- Experiment 5. Effect of Osmotic Potential on Germination
- Experiment 6. Effect of Burial Depth on Germination
2.2.2. Herbicidal Activity of Common Herbicides Against A. indica
2.3. Data Analysis and Processing
2.3.1. Seed Germination and Emergence Data Analysis
2.3.2. Herbicide Bioassay Data Analysis
3. Results
3.1. Effects of Temperature on Seed Germination
3.2. Effects of Light on Seed Germination
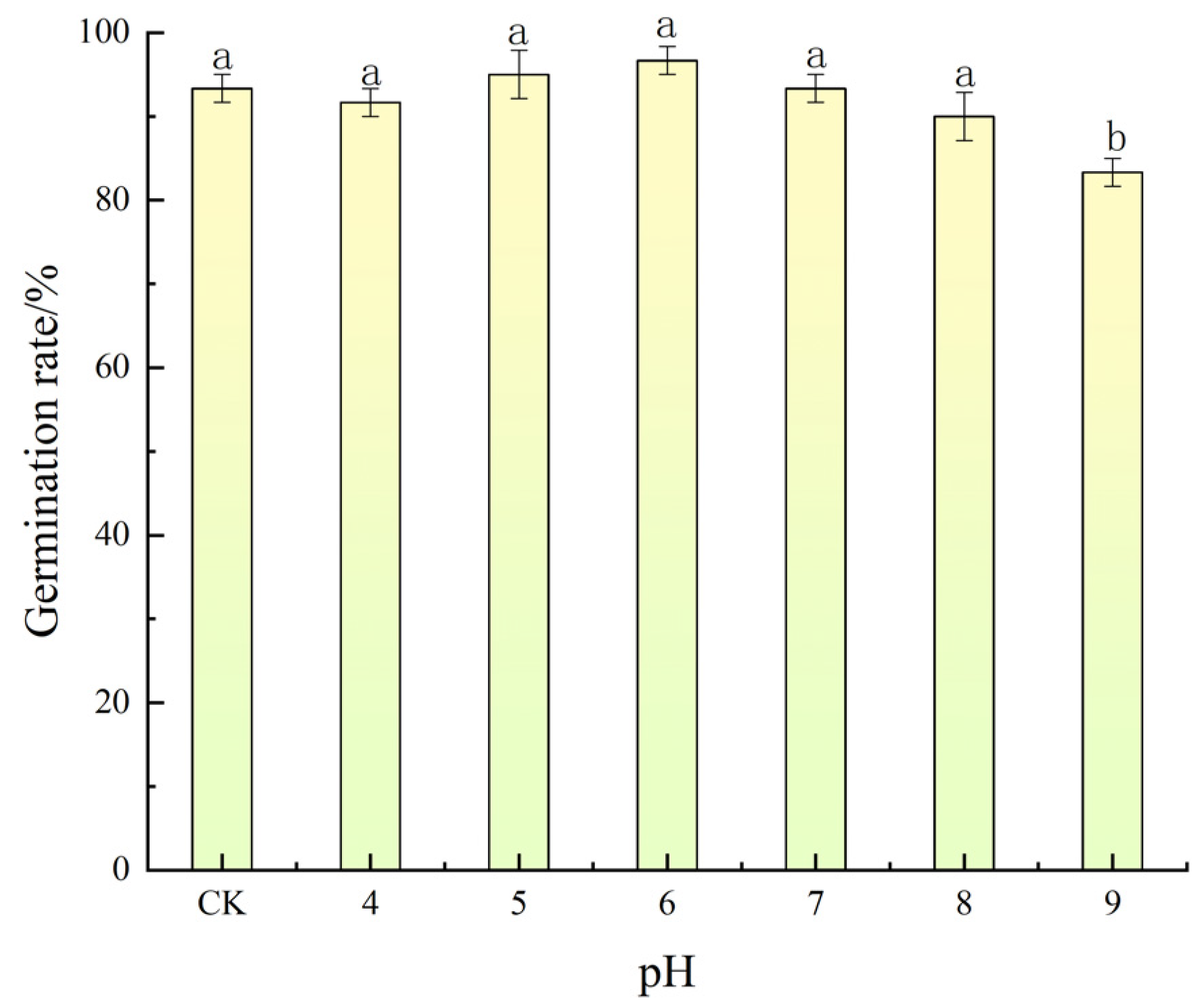
3.3. Effects of pH on Seed Germination
3.4. Effects of Salt Stress on Seed Germination
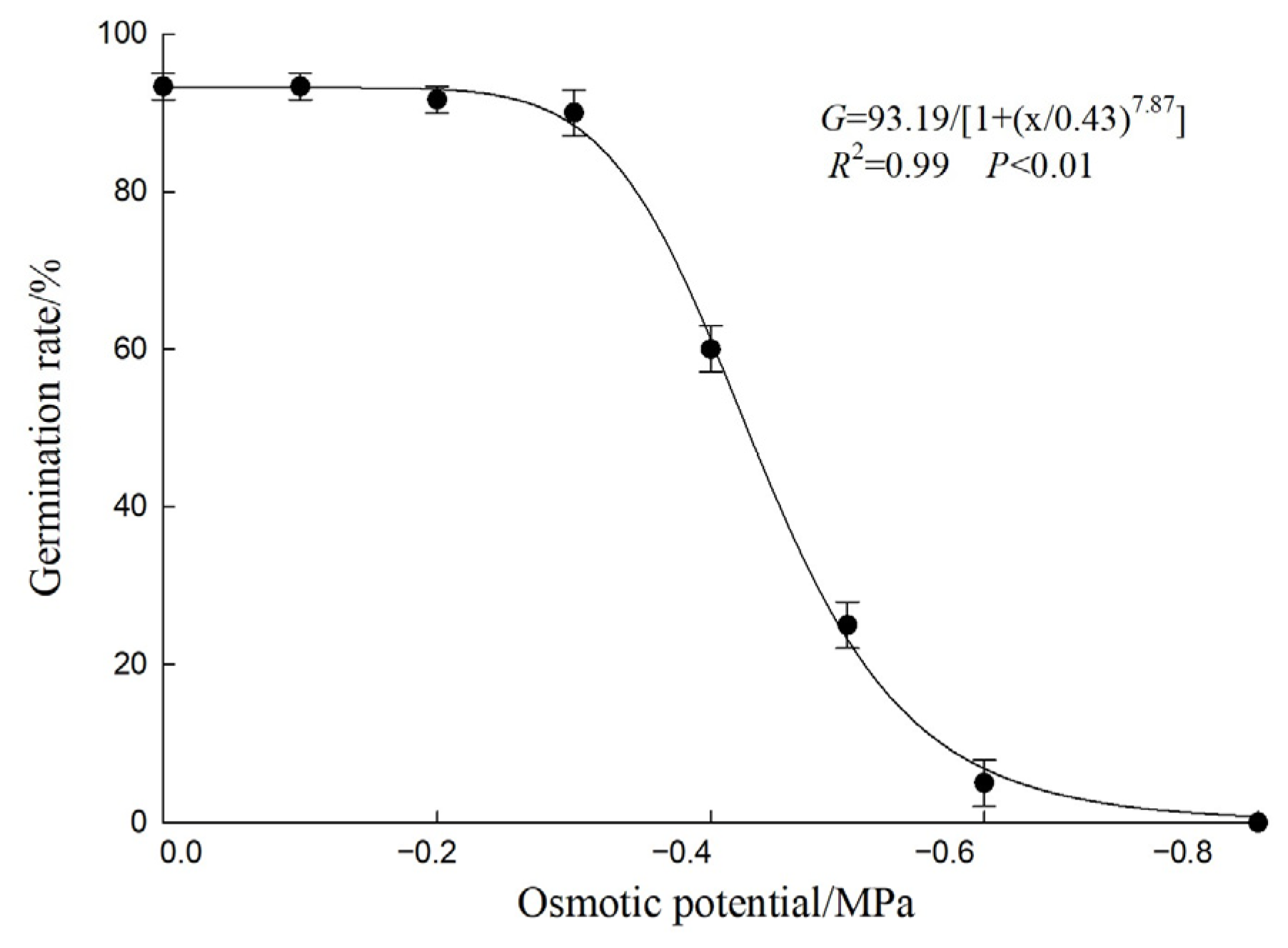
3.5. Effects of Osmotic Potential on Seed Germination
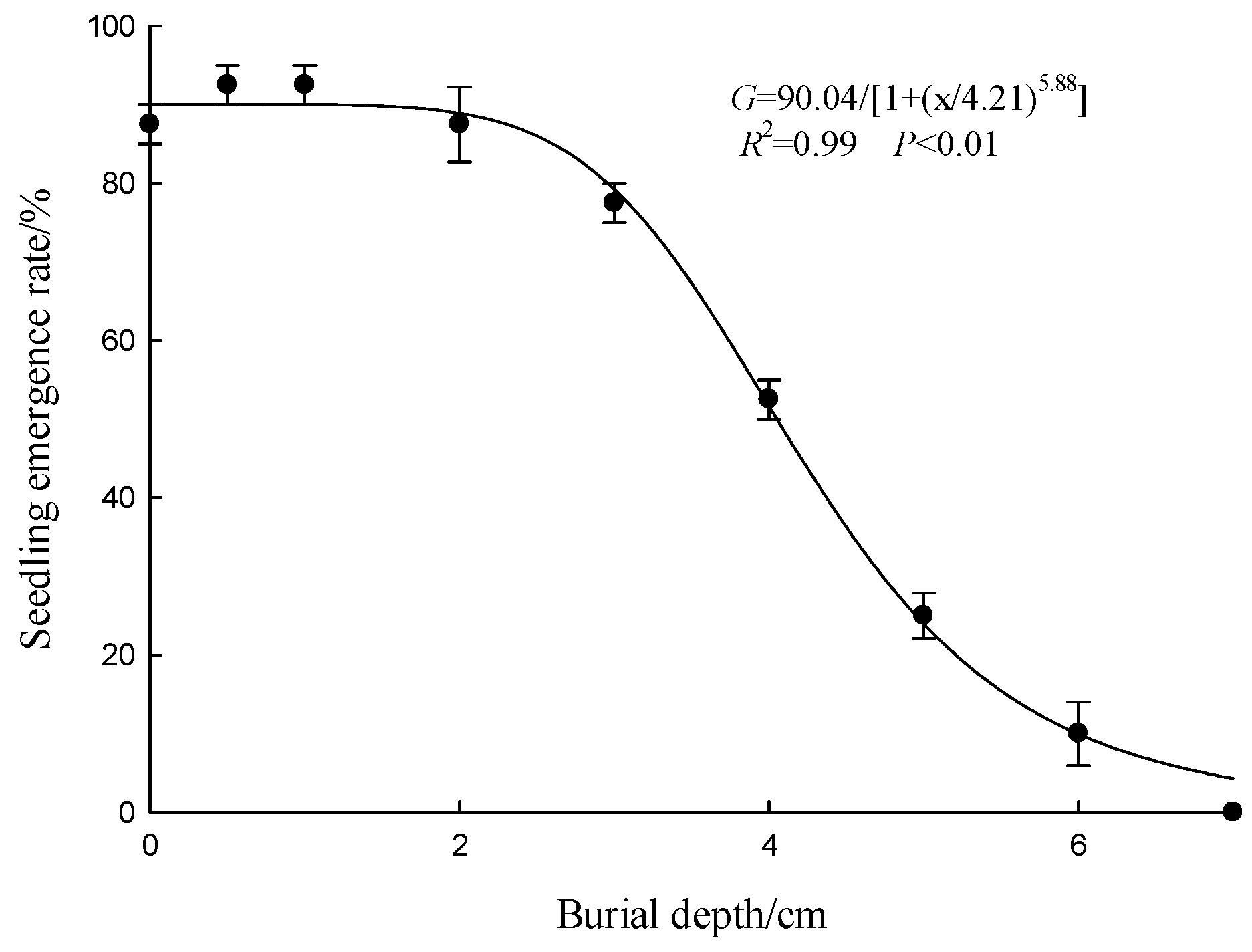
3.6. Effects of Burial Depth on Seed Emergence
3.7. Herbicidal Activity Against A. indica
4. Discussion
4.1. Influence of Environmental Factors on Seed Germination
4.2. Herbicide Efficacy and Integrated Management Recommendations
- Pre-Planting (Cultural Seedbank Depletion): Implement deep tillage (to depths > 7 cm) after harvest or before land preparation. This buries freshly shed seeds below their maximum emergence depth, providing long-term cultural suppression of the soil seed bank.
- Crop Establishment (Preventive Chemical Barrier): At rice planting, apply a residual pre-emergence herbicide (e.g., saflufenacil or mesotrione) to create a chemical barrier in the soil, controlling the first and most critical flush of seedlings.
- Early-Mid Season (Corrective Control, if needed): For any established weed escapes, use a high-efficacy post-emergence herbicide (e.g., florpyrauxifen-benzyl) for targeted rescue control.
- Long-Term Sustainability (Resistance Management): To preserve herbicide efficacy, rotate sites of action and use tank mixtures across seasons. This chemical strategy should be integrated with the periodic use of deep tillage in a multi-year management plan [47].
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, M.; Kukal, M.S.; Irmak, S.; Jhala, A.J. Water Use Characteristics of Weeds: A Global Review, Best Practices, and Future Directions. Front. Plant Sci. 2022, 12, 794090. [Google Scholar] [CrossRef] [PubMed]
- Dorner, Z.; Kovács, E.B.; Iványi, D.; Zalai, M. How the Management and Environmental Conditions Affect the Weed Vegetation in Canary Grass (Phalaris canariensis L.) Fields. Agronomy 2024, 14, 1169. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, T.; Long, J.; Shen, G.; Tian, Z. Complete Chloroplast Genome and Comparison of Herbicides Toxicity on Aeschynomene indica (Leguminosae) in Upland Direct-Seeding Paddy Field. BMC Genom. 2024, 25, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Korav, S.; Dhaka, A.; Singh, R.; Reddy, C. A Study on Crop Weed Competition in Field Crops. J. Pharmacogn. Phytochem. 2018, 7, 3235–3240. [Google Scholar]
- Martins, M.B.; Agostinetto, D.; Fogliatto, S.; Vidotto, F.; Andres, A. Aeschynomene spp. Identification and Weed Management in Rice Fields in Southern Brazil. Agronomy 2021, 11, 453. [Google Scholar] [CrossRef]
- Martins, M.B.; Munhos, T.F.; Schaedler, C.E.; Agostinetto, D.; Andres, A. Seed Dynamics of Aeschynomene denticulata and Aeschynomene indica. Weed Biol. Manag. 2021, 21, 172–180. [Google Scholar] [CrossRef]
- Shen, J.H.; Zeng, B.; Shi, M.F.; Liu, J.H.; Ayiqiaoli. Effects of Storage Condition and Duration on Seed Germination of Four Annual Species Growing in Water-Level-Fluctuation Zone of Three Gorges Reservoir. Acta Ecol. Sin. 2010, 30, 6571–6580. [Google Scholar]
- Wang, R.; Yang, Y.; Wang, X.; Li, J.; Gao, Y.; Huang, H.; Zhou, Z.; Wang, P.; Zhao, L. Response of Seed Germination and Seedling Growth of Perennial Ryegrass (Lolium perenne L.) to Drought, Salinity, and pH in Karst Regions. Sci. Rep. 2025, 15, 16874. [Google Scholar] [CrossRef]
- Nosratti, I.; Soltanabadi, S.; Honarmand, S.J.; Chauhan, B.S. Environmental Factors Affect Seed Germination and Seedling Emergence of Invasive Centaurea balsamita. Crop Pasture Sci. 2017, 68, 583–589. [Google Scholar] [CrossRef]
- Benvenuti, S.; Macchia, M.; Miele, S. Light, Temperature and Burial Depth Effects on Rumex obtusifolius Seed Germination and Emergence. Weed Res. 2001, 41, 177–186. [Google Scholar] [CrossRef]
- Kaya-Altop, E.; Uysal, M.S.; Haghnama, K.; Mennan, H. Environmental Factors on Seasonal Germination of Different Weedy Rice (Oryza sativa L.) Biotypes. Ciênc. Rural 2023, 53, e20210728. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Germination Ecology of Two Troublesome Asteraceae Species of Rainfed Rice: Siam Weed (Chromolaena odorata) and Coat Buttons (Tridax procumbens). Weed Sci. 2008, 56, 567–573. [Google Scholar] [CrossRef]
- Lim, C.A.A.; Awan, T.H.; Cruz, P.C.S.; Chauhan, B.S. Influence of Environmental Factors, Cultural Practices, and Herbicide Application on Seed Germination and Emergence Ecology of Ischaemum rugosum Salisb. PLoS ONE 2015, 10, e0137256. [Google Scholar] [CrossRef] [PubMed]
- Dyer, W.E. Exploiting Weed Seed Dormancy and Germination Requirements through Agronomic Practices. Weed Sci. 1995, 43, 498–503. [Google Scholar] [CrossRef]
- Hossain, M.; Begum, M.; Rahman, M.; Akanda, M. Weed Management on Direct-Seeded Rice System—A Review. Progress. Agric. 2016, 27, 1–8. [Google Scholar] [CrossRef]
- MacLaren, C.; Storkey, J.; Menegat, A.; Metcalfe, H.; Dehnen-Schmutz, K. An Ecological Future for Weed Science to Sustain Crop Production and the Environment. A Review. Agron. Sustain. Dev. 2020, 40, 24–53. [Google Scholar] [CrossRef]
- Silva, A.L.; Streck, N.A.; Zanon, A.J.; Ribas, G.G.; Fruet, B.L.; Ulguim, A.R. Surveys of Weed Management on Flooded Rice Yields in Southern Brazil. Weed Sci. 2022, 70, 249–258. [Google Scholar] [CrossRef]
- Benaragama, D.I.; Willenborg, C.J.; Shirtliffe, S.J.; Gulden, R.H. Revisiting Cropping Systems Research: An Ecological Framework towards Long-Term Weed Management. Agric. Syst. 2024, 213, 103811. [Google Scholar] [CrossRef]
- Jiang, L.; Chai, K.; Fida, M.; Fang, B.; Wang, K.; Bi, Y. Germination Biology of Three Cyperaceae Weeds and Their Response to Pre- and Post-Emergence Herbicides in Paddy Fields. Agronomy 2024, 14, 1592. [Google Scholar] [CrossRef]
- Chachalis, D.; Reddy, K.N. Factors Affecting Campsis Radicans Seed Germination and Seedling Emergence. Weed Sci. 2000, 48, 212–216. [Google Scholar] [CrossRef]
- Shrestha, A.; deSouza, L.L.; Yang, P.; Sosnoskie, L.; Hanson, B.D. Differential Tolerance of Glyphosate-Susceptible and Glyphosate-Resistant Biotypes of Junglerice (Echinochloa colona) to Environments during Germination, Growth, and Intraspecific Competition. Weed Sci. 2018, 66, 340–346. [Google Scholar] [CrossRef]
- Schaeffer, J.; Hembree, K.J.; Shrestha, A. Biology, Germination Ecology, and Shade Tolerance of Alkaliweed (Cressa truxillensis) and Its Response to Common Postemergence Herbicides. Plants 2023, 12, 2679. [Google Scholar] [CrossRef] [PubMed]
- Michel, B.E. Evaluation of the Water Potentials of Solutions of Polyethylene Glycol 8000 Both in the Absence and Presence of Other Solutes. Plant Physiol. 1983, 72, 66–70. [Google Scholar] [CrossRef] [PubMed]
- NY/T 1155.3-2006; Pesticides Guidelines for Laboratory Bioactivity Tests Part 3: Soil Spray Application Test for Herbicide Bioactivity. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006.
- NY/T 1155.4-2006; Pesticides Guidelines for Laboratory Bioactivity Tests Part 4: Foliar Spray Application Test for Herbicide Activity. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2006.
- Park, H.-H.; Lee, D.-J.; Kuk, Y.-I. Effects of Various Environmental Conditions on the Growth of Amaranthus patulus Bertol. and Changes of Herbicide Efficacy Caused by Increasing Temperatures. Agronomy 2021, 11, 1773. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Ma, H.; Wei, S.; Lan, Y.; Cao, Y.; Huang, H.; Huang, Z. First Report of the Molecular Mechanism of Resistance to Tribenuron-Methyl in Silene conoidea L. Plants 2022, 11, 3044. [Google Scholar] [CrossRef]
- Xu, H.; Leng, Q.; Su, W.; Sun, L.; Li, Q.; Wei, H.; Cheng, J.; Lu, C.; Wu, R. The Synergistic Effect and Mechanism of Different Adjuvants on Pinoxaden Efficacy against Lolium multiflorum Lam. Crop Prot. 2024, 184, 106844. [Google Scholar] [CrossRef]
- Chtourou, M.; Osuna, M.D.; Vázquez-García, J.G.; De Prado, R.; Lozano-Juste, J.; Marín, G.M.; Hada, Z.; Souissi, T.; Torra, J. Several Point Mutations and Metabolism Confer Cross-Resistance to ALS-Inhibiting Herbicides in Tunisian Wild Mustard. Plant Physiol. Biochem. 2025, 225, 110043. [Google Scholar] [CrossRef]
- Wang, H.; Gao, H.; Liu, Y.; Huang, Q.; Feng, Z.; Dong, L. Auxin Response Factor 3 (EcARF3) Regulates Ethylene and ABA Biosynthesis and Is Involved in Resistance to Synthetic Auxin Herbicides in Echinochloa crus-galli. Int. J. Biol. Macromol. 2025, 312, 144172. [Google Scholar] [CrossRef]
- Yuan, G.; Gao, Y.; Fang, J.; Shen, G.; Tian, Z. Environmental Influences on Seed Germination and Seedling Emergence in Four Echinochloa Taxa. Agronomy 2025, 15, 401. [Google Scholar] [CrossRef]
- Dhanda, S.; Sharma, K.; Chauhan, B.S. Germination Responses of Vipergrass (Dinebra retroflexa) to Environmental Factors and Herbicide Options for Its Control. Weed Sci. 2023, 71, 124–132. [Google Scholar] [CrossRef]
- Li, T.; Qian, H.; Yuan, G.; Fan, J.; Guo, S. Germination Ecology and Response to Herbicides of Ludwigia prostrata and Their Implication for Weed Control in Paddy Fields. Weed Technol. 2023, 37, 197–204. [Google Scholar] [CrossRef]
- Pan, J.; Wang, H.; You, Q.; Cao, R.; Sun, G.; Yu, D. Jasmonate-Regulated Seed Germination and Crosstalk with Other Phytohormones. J. Exp. Bot. 2023, 74, 1162–1175. [Google Scholar] [CrossRef] [PubMed]
- Ozden, E.; Light, M.E.; Demir, I. Alternating Temperatures Increase Germination and Emergence in Relation to Endogenous Hormones and Enzyme Activities in Aubergine Seeds. S. Afr. J. Bot. 2021, 139, 130–139. [Google Scholar] [CrossRef]
- Chen, Y.; Masoom, A.; Huang, Z.; Xue, J.; Chen, G. Interspecific and Intraspecific Differences in Seed Germination Response to Different Temperatures of Three Echinochloa Rice Weeds: A Case Study with 327 Populations. Weed Sci. 2025, 73, e23. [Google Scholar] [CrossRef]
- Li, W.; Lai, X.; Gu, T.; Wang, H.; Zhang, Z. Seed Germination Ecology of Eclipta (Eclipta prostrata) in Dry Direct-Seeded Rice Fields from China. Weed Sci. 2025, 73, e71. [Google Scholar] [CrossRef]
- Gautam, S.; Sarma, N.; Sarma, G.; Borthakur, U. Effects of Soil pH Stress on Plant Development: From Seed Germination to Early Seedling Growth. Afr. J. Biol. Sci. 2024, 6, 3461–3473. [Google Scholar]
- Aktaş, H.; Szpicer, A.; Strojny-Cieślak, B.; Borucki, W.; Schweiggert-Weisz, U.; Kurek, M.A. Molecular Interplay between Plant Proteins and Polyphenols: pH as a Switch for Structural and Functional Assembly. Foods 2025, 14, 3991. [Google Scholar] [CrossRef]
- Oreja, F.H.; De La Fuente, E.B.; Fernandez-Duvivier, M.E. Response of Digitaria insularis Seed Germination to Environmental Factors. Crop Pasture Sci. 2017, 68, 45–50. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G.D. How and Why to Measure the Germination Process? Rev. Bras. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Seed Germination Ecology of Portulaca oleracea L.: An Important Weed of Rice and Upland Crops. Ann. Appl. Biol. 2009, 155, 61–69. [Google Scholar] [CrossRef]
- Grossmann, K.; Hutzler, J.; Caspar, G.; Kwiatkowski, J.; Brommer, C.L. Saflufenacil (KixorTM): Biokinetic Properties and Mechanism of Selectivity of a New Protoporphyrinogen IX Oxidase Inhibiting Herbicide. Weed Sci. 2011, 59, 290–298. [Google Scholar] [CrossRef]
- Dayan, F.E.; Barker, A.; Tranel, P.J. Origins and Structure of Chloroplastic and Mitochondrial Plant Protoporphyrinogen Oxidases: Implications for the Evolution of Herbicide Resistance. Pest Manag. Sci. 2018, 74, 2226–2234. [Google Scholar] [CrossRef]
- Miller, M.R.; Norsworthy, J.K. Florpyrauxifen-Benzyl Weed Control Spectrum and Tank-Mix Compatibility with Other Commonly Applied Herbicides in Rice. Weed Technol. 2018, 32, 319–325. [Google Scholar] [CrossRef]
- Beckie, H.J.; Tardif, F.J. Herbicide Cross Resistance in Weeds. Crop Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Bradley, K.W.; Frisvold, G.; Powles, S.B.; Burgos, N.R.; et al. Reducing the Risks of Herbicide Resistance: Best Management Practices and Recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]



| Application Method | Tested Herbicides | Mode of Action (HRAC Group) | Manufacturer | Dose (g ai ha−1) |
|---|---|---|---|---|
| Pre-emergence | 50% Pretilachlor EW | 15 | Zhejiang Tianfeng Biological Science Co., Ltd., Jinhua, China | 28.13, 56.25, 112.50, 225.00, 450.00 |
| 24% Oxyfluorfen EC | 14 | Heze Maotairuinong Biotechnology Co., Ltd., Heze, China | 3.71, 7.43, 14.85, 29.70, 59.40 | |
| 20% Acetochlor WP | 15 | Chongqing Yiershuangfeng Technology Co., Ltd., Chongqing, China | 6.56, 13.13, 26.25, 52.50, 105.00 | |
| 70% Metolachlor EC | 15 | Jiangxi Heyi Chemical Co., Ltd., Jiujiang, China | 9.84, 19.69, 39.38, 78.75, 157.50 | |
| 15% Mesotrione SC | 27 | Shandong Tomple Crop Science Co., Ltd., Dezhou, China | 5.48, 10.97, 21.94, 43.88, 87.75 | |
| 70% Saflufenacil WG | 14 | BASF SE, Ludwigshafen, Germany | 2.46, 4.92, 9.84, 19.69, 39.38 | |
| 48% Butralin EC | 3 | Jiangxi Dunpai Chemical Co., Ltd., Fuzhou, China | 112.50, 225.00, 450.00, 900.00, 1800.00 | |
| 33% Pendimethalin EC | 3 | Zhejiang Tianfeng Biological Science Co., Ltd., Jinhua, China | 54.14, 108.28, 216.56, 433.13, 866.25 | |
| 8% Pentoxazone SC | 14 | Nippon Soda Co., Ltd., Tokyo, Japan | 16.50, 33.00, 66.00, 132.00, 264.00 | |
| 80% Oxadiargyl WG | 14 | Hefei Xingyu Chemical Co., Ltd., Hefei, China | 5.25, 10.50, 21.00, 42.00, 84.00 | |
| Post-emergence | 60% Bensulfuron-methyl WG | 2 | Zhejiang Tianfeng Biological Science Co., Ltd., Jinhua, China. | 2.11, 4.22, 8.44, 16.88, 33.75 |
| 22% Penoxsulam SC | 2 | Dezhou Luba Fine Chemical Co., Ltd., Dezhou, China | 1.65, 3.30, 6.60, 13.20, 26.40 | |
| 20% Bispyribac-sodium SC | 2 | Jinan Tianbang Chemical Co., Ltd., Jinan, China | 1.64, 3.28, 6.56, 13.13, 26.25 | |
| 15% Ethoxysulfuron WG | 2 | Zhejiang Taida Crop Science Technology Co., Ltd., Hangzhou, China | 0.98, 1.97, 3.94, 7.88, 15.75 | |
| 24.3% Pyriftalid SC | 2 | Syngenta Crop Protection AG, Basel, Switzerland | 14.81, 29.62, 59.23, 118.46, 236.93 | |
| 200 g L−1 Fluroxypyr-meptyl EC | 4 | Jilin Jinqiu Pesticide Co., Ltd., Jilin, China | 11.25, 22.50, 45.00, 90.00, 180.00 | |
| 3% Florpyrauxifen-benzyl EC | 4 | Corteva Agriscience LLC, Indianapolis, IN, USA | 0.11, 0.22, 0.43, 0.85, 1.69, 3.38 | |
| 56% MCPA-sodium SP | 4 | Jiangsu Jiangu Chemical Co., Ltd., Suqian, China | 52.50, 105.00, 210.00, 420.00, 840.00 | |
| 25% Bentazone AS | 6 | Heilongjiang Huanuo Biotechnology Co., Ltd., Harbin, China | 41.02, 82.03, 164.06, 328.13, 656.25 | |
| 15% Diflufenican OD | 12 | ADAMA Fengshun (Jiangsu) Co., Ltd., Nantong, China | 11.25, 22.50, 45.00, 90.00, 180.00 |
| Tested Herbicides | Dose (g ai ha−1) | Fresh Weight/g | Fresh Weight Control Efficacy/% |
|---|---|---|---|
| 50% Pretilachlor EW | 28.13 | 1.83 | 22.81 ± 4.85 d |
| 56.25 | 1.50 | 36.86 ± 1.32 c | |
| 112.50 | 1.38 | 41.71 ± 1.63 c | |
| 225.00 | 1.06 | 55.12 ± 1.56 b | |
| 450.00 | 0.74 | 68.85 ± 2.67 a | |
| 24% Oxyfluorfen EC | 3.71 | 1.78 | 24.71 ± 4.75 d |
| 7.43 | 1.42 | 39.92 ± 1.79 c | |
| 14.85 | 1.31 | 44.56 ± 0.36 bc | |
| 29.70 | 1.17 | 50.69 ± 1.68 b | |
| 59.40 | 0.85 | 64.10 ± 2.28 a | |
| 20% Acetochlor WP | 6.56 | 1.54 | 35.17 ± 0.74 d |
| 13.13 | 1.46 | 38.54 ± 0.66 cd | |
| 26.25 | 1.36 | 42.45 ± 1.25 c | |
| 52.50 | 1.10 | 53.43 ± 3.26 b | |
| 105.00 | 0.97 | 59.03 ± 1.18 a | |
| 70% Metolachlor EC | 9.84 | 1.89 | 20.07 ± 2.01 e |
| 19.69 | 1.61 | 31.99 ± 1.17 d | |
| 39.38 | 1.42 | 40.13 ± 2.85 c | |
| 78.75 | 0.99 | 58.29 ± 0.61 b | |
| 157.50 | 0.76 | 67.80 ± 1.30 a | |
| 15% Mesotrione SC | 5.48 | 1.64 | 30.83 ± 0.85 e |
| 10.97 | 1.28 | 46.04 ± 3.57 d | |
| 21.94 | 0.81 | 65.79 ± 2.87 c | |
| 43.88 | 0.54 | 77.19 ± 2.13 b | |
| 87.75 | 0.29 | 87.96 ± 1.36 a | |
| 70% Saflufenacil WG | 2.46 | 1.58 | 33.27 ± 2.93 e |
| 4.92 | 1.24 | 47.84 ± 2.96 d | |
| 9.84 | 0.89 | 62.41 ± 0.90 c | |
| 19.69 | 0.40 | 83.00 ± 2.87 b | |
| 39.38 | 0.07 | 97.04 ± 1.44 a | |
| 48% Butralin EC | 112.50 | 1.77 | 25.13 ± 1.09 d |
| 225.00 | 1.38 | 41.93 ± 2.34 c | |
| 450.00 | 1.13 | 52.48 ± 1.10 b | |
| 900.00 | 1.00 | 57.97 ± 2.04 b | |
| 1800.00 | 0.77 | 67.48 ± 2.13 a | |
| 33% Pendimethalin EC | 54.14 | 1.89 | 20.27 ± 3.63 e |
| 108.28 | 1.50 | 36.75 ± 1.45 d | |
| 216.56 | 1.31 | 44.77 ± 1.44 c | |
| 433.13 | 1.08 | 54.38 ± 1.37 b | |
| 866.25 | 0.88 | 62.73 ± 2.45 a | |
| 8% Pentoxazone SC | 16.50 | 1.86 | 21.33 ± 2.62 e |
| 33.00 | 1.60 | 32.42 ± 1.06 d | |
| 66.00 | 1.46 | 38.33 ± 0.62 c | |
| 132.00 | 1.20 | 49.53 ± 1.45 b | |
| 264.00 | 0.97 | 59.03 ± 1.56 a | |
| 80% Oxadiargyl WG | 5.25 | 1.64 | 30.94 ± 1.97 d |
| 10.50 | 1.36 | 42.56 ± 0.52 c | |
| 21.00 | 1.26 | 46.89 ± 0.74 c | |
| 42.00 | 1.05 | 55.65 ± 2.08 b | |
| 84.00 | 0.73 | 69.17 ± 3.69 a |
| Tested Herbicides | Dose (g ai ha−1) | Fresh Weight/g | Fresh Weight Control Efficacy/% |
|---|---|---|---|
| 60% Bensulfuron-methyl WG | 2.11 | 3.98 | 31.03 ± 3.91 d |
| 4.22 | 3.11 | 46.23 ± 1.37 c | |
| 8.44 | 2.59 | 55.11 ± 0.87 b | |
| 16.88 | 2.13 | 63.08 ± 0.64 a | |
| 33.75 | 2.07 | 64.20 ± 0.83 a | |
| 22% Penoxsulam SC | 1.65 | 3.95 | 31.59 ± 2.49 e |
| 3.30 | 2.60 | 54.96 ± 1.10 d | |
| 6.60 | 2.13 | 63.12 ± 0.75 c | |
| 13.20 | 1.83 | 68.31 ± 0.42 b | |
| 26.40 | 1.14 | 80.22 ± 0.59 a | |
| 20% Bispyribac-sodium SC | 1.64 | 4.47 | 22.69 ± 2.38 d |
| 3.28 | 3.3 | 42.86 ± 0.81 c | |
| 6.56 | 2.4 | 58.45 ± 0.89 b | |
| 13.13 | 2.17 | 62.43 ± 1.09 b | |
| 26.25 | 1.78 | 69.22 ± 1.19 a | |
| 15% Ethoxysulfuron WG | 0.98 | 4.35 | 24.64 ± 1.45 e |
| 1.97 | 2.86 | 50.57 ± 0.84 d | |
| 3.94 | 2.51 | 56.50 ± 0.46 c | |
| 7.88 | 2.35 | 59.28 ± 0.78 b | |
| 15.75 | 2.2 | 61.91 ± 0.37 a | |
| 24.3% Pyriftalid SC | 14.81 | 3.99 | 30.96 ± 5.40 d |
| 29.62 | 3.32 | 42.49 ± 1.14 c | |
| 59.23 | 2.8 | 51.52 ± 1.21 b | |
| 118.46 | 2.37 | 59.01 ± 0.61 ab | |
| 236.93 | 1.98 | 65.67 ± 0.95 a | |
| 200 g L−1 Fluroxypyr-meptyl EC | 11.25 | 4.09 | 29.27 ± 5.07 d |
| 22.50 | 2.54 | 55.97 ± 1.45 c | |
| 45.00 | 1.56 | 72.99 ± 0.82 b | |
| 90.00 | 1.22 | 78.88 ± 0.73 b | |
| 180.00 | 0.71 | 87.78 ± 1.12 a | |
| 3% Florpyrauxifen-benzyl EC | 0.11 | 4.57 | 20.92 ± 1.85 e |
| 0.22 | 2.31 | 60.02 ± 2.69 d | |
| 0.43 | 1.87 | 67.65 ± 0.86 c | |
| 0.85 | 1.63 | 71.75 ± 0.82 c | |
| 1.69 | 1.30 | 77.50 ± 0.72 b | |
| 3.38 | 0.54 | 90.59 ± 0.76 a | |
| 56% MCPA-sodium SP | 52.50 | 3.24 | 43.95 ± 0.89 e |
| 105.00 | 2.76 | 52.14 ± 0.82 d | |
| 210.00 | 2.6 | 54.98 ± 0.85 c | |
| 420.00 | 2.22 | 61.65 ± 0.76 b | |
| 840.00 | 2.03 | 64.89 ± 0.98 a | |
| 25% Bentazone AS | 41.02 | 4.08 | 29.31 ± 3.44 d |
| 82.03 | 2.64 | 54.38 ± 1.92 c | |
| 164.06 | 2.14 | 62.95 ± 0.51 b | |
| 328.13 | 1.87 | 67.67 ± 0.59 ab | |
| 656.25 | 1.55 | 73.21 ± 2.25 a | |
| 15% Diflufenican OD | 11.25 | 4.38 | 24.21 ± 3.49 d |
| 22.50 | 3.38 | 41.52 ± 2.15 c | |
| 45.00 | 2.69 | 53.38 ± 1.97 b | |
| 90.00 | 2.33 | 59.74 ± 1.48 ab | |
| 180.00 | 2.06 | 64.26 ± 0.59 a |
| Application Method | Tested Herbicides | GR50 (g ai ha−1) |
|---|---|---|
| Pre-emergence | 50% Pretilachlor EW | 165.23 |
| 24% Oxyfluorfen EC | 22.04 | |
| 20% Acetochlor WP | 44.71 | |
| 70% Metolachlor EC | 56.60 | |
| 15% Mesotrione SC | 12.02 | |
| 70% Saflufenacil WG | 5.38 | |
| 48% Butralin EC | 386.72 | |
| 33% Pendimethalin EC | 280.85 | |
| 8% Pentoxazone SC | 137.16 | |
| 80% Oxadiargyl WG | 25.27 | |
| Post-emergence | 60% Bensulfuron-methyl WG | 5.27 |
| 22% Penoxsulam SC | 3.09 | |
| 20% Bispyribac-sodium SC | 4.31 | |
| 15% Ethoxysulfuron WG | 1.97 | |
| 24.3% Pyriftalid SC | 51.83 | |
| 200 g L−1 Fluroxypyr-meptyl EC | 19.69 | |
| 3% Florpyrauxifen-benzyl EC | 0.20 | |
| 56% MCPA-sodium SP | 95.16 | |
| 25% Bentazone AS | 74.34 | |
| 15% Diflufenican OD | 35.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, K.; Cheng, R.; Shi, Y.; Fida, M.; Liu, W.; Wu, Z.; Bi, Y. Seed Germination Ecology and Herbicide Sensitivity of Aeschynomene indica L.: Implications for Integrated Management in Paddy Fields. Agronomy 2025, 15, 2908. https://doi.org/10.3390/agronomy15122908
Chai K, Cheng R, Shi Y, Fida M, Liu W, Wu Z, Bi Y. Seed Germination Ecology and Herbicide Sensitivity of Aeschynomene indica L.: Implications for Integrated Management in Paddy Fields. Agronomy. 2025; 15(12):2908. https://doi.org/10.3390/agronomy15122908
Chicago/Turabian StyleChai, Ke, Rui Cheng, Yueyue Shi, Mujeeba Fida, Weitang Liu, Zhiwen Wu, and Yaling Bi. 2025. "Seed Germination Ecology and Herbicide Sensitivity of Aeschynomene indica L.: Implications for Integrated Management in Paddy Fields" Agronomy 15, no. 12: 2908. https://doi.org/10.3390/agronomy15122908
APA StyleChai, K., Cheng, R., Shi, Y., Fida, M., Liu, W., Wu, Z., & Bi, Y. (2025). Seed Germination Ecology and Herbicide Sensitivity of Aeschynomene indica L.: Implications for Integrated Management in Paddy Fields. Agronomy, 15(12), 2908. https://doi.org/10.3390/agronomy15122908






