1. Introduction
Soil serves as the foundation of agricultural production, with its fertility directly influencing crop yield and quality. Amidst the growing global population and increasing demand for food, enhancing soil fertility through scientific fertilization practices has emerged as a critical research focus in agriculture to ensure food security. While the extensive use of chemical fertilizers in traditional agricultural practices has significantly boosted crop yields, it has also led to numerous environmental issues, including soil acidification, nutrient leaching, and microbial community imbalances [
1,
2]. These problems not only undermine the sustainable productivity of soil but also exert negative impacts on the ecological environment [
3]. Consequently, exploring more sustainable fertilization methods, reducing chemical fertilizer usage, and improving soil health have become prominent areas of interest in contemporary agricultural research.
Organic fertilizer, as a traditional soil amendment, has garnered significant attention in recent years due to its rich organic matter and diverse nutrient content, which enhance soil structure and improve water and nutrient retention capacities [
4,
5]. Compared to chemical fertilizers, organic fertilizers release nutrients gradually and provide long-lasting effects, ensuring sustained nutrient supply for crop growth. Furthermore, organic fertilizers stimulate soil microbial activity and enhance soil enzyme activity, thereby improving the availability of soil nutrients [
6]. However, relying solely on organic fertilizers often fails to meet the high-yield demands of crops. Consequently, the combined application of organic and chemical fertilizers has been recognized as a crucial approach to achieving sustainable agricultural development. In recent years, extensive research has been conducted by domestic and international scholars on the effects of combined organic and chemical fertilizer application on soil fertility, crop yield, and quality. Studies have demonstrated that this practice significantly increases soil organic matter content, improves soil structure, and enhances water and nutrient retention capacities [
6,
7,
8]. Additionally, the combined application promotes the diversity and activity of soil microbial communities, thereby enhancing the cycling and utilization efficiency of soil nutrients [
9]. For instance, the application of organic fertilizers increases the abundance of bacteria and fungi in the soil, particularly functional microorganisms involved in carbon, nitrogen, and phosphorus cycling, such as Gemmatimonadota and Pseudomonas, which facilitate the transformation and supply of soil nutrients [
10,
11,
12].
However, systematic research on the optimal substitution ratio of organic fertilizers for chemical fertilizers and their impact mechanisms on soil microbial communities and enzyme activities remains limited. In particular, the applicability of these practices under different soil types and climatic conditions has not been thoroughly validated. Although the combined application of organic and chemical fertilizers demonstrates significant advantages in enhancing soil fertility and crop yield, its effectiveness is influenced by multiple factors, including the type of organic fertilizer, application ratio, soil type, and climatic conditions [
13,
14,
15]. For instance, organic fertilizers derived from different sources, such as livestock manure, compost, and green manure, exhibit substantial variations in nutrient content and release characteristics, which may affect their synergistic effects with chemical fertilizers. Furthermore, the substitution ratio of organic fertilizers is a critical factor influencing fertilization outcomes. Excessive organic fertilizer proportions may lead to insufficient nutrient supply, adversely affecting crop growth, while insufficient proportions may fail to fully realize their soil-improving potential [
16,
17]. Therefore, further research is essential to determine the optimal substitution ratio of organic fertilizers to achieve the dual objectives of enhancing soil fertility and ensuring high crop yields. This study focuses on sugar beet as the research subject and systematically investigates the effects of organic fertilizer substitution on soil available nutrients, organic matter content, enzyme activity, and microbial community structure by establishing treatments with varying substitution ratios. Utilizing an equivalent nitrogen design, seven treatments were implemented. The effects of different fertilization practices were evaluated by measuring soil available nutrients (alkali-hydrolyzable nitrogen, available phosphorus, and available potassium), soil organic matter content, soil enzyme activities (such as urease and phosphatase), and soil microbial community structure (including the diversity and abundance of bacteria and fungi). The innovation of this study lies in its systematic exploration of the impact of different organic fertilizer substitution ratios on soil microbial communities and enzyme activities, as well as its elucidation of the relationship between these factors and soil nutrient cycling. The findings not only provide a scientific basis for optimizing fertilization ratios and reducing chemical fertilizer usage but also offer significant theoretical support for improving soil health and promoting sustainable agricultural development. Furthermore, this study lays the groundwork for further exploration of the mechanisms underlying the combined application of organic and chemical fertilizers and their applicability across diverse agricultural ecosystems.
In summary, this study systematically investigates the effects of substituting chemical fertilizers with organic fertilizers on soil fertility, microbial communities, and crop yield, aiming to provide theoretical foundations and practical guidance for scientific fertilization in agricultural production. The findings not only contribute to enhancing soil fertility and crop productivity but also mitigate the adverse environmental impacts associated with chemical fertilizer use, thereby promoting sustainable agricultural development. Future research should further explore the effects of different types of organic fertilizers, soil types, and climatic conditions on fertilization outcomes, offering more comprehensive scientific support for the global transition toward green agriculture.
2. Materials and Methods
2.1. Experiment Design
The experiment was conducted from 2021 to 2023 at the Manas Agricultural Experimental Station of the Xinjiang Academy of Agricultural Sciences, marking the third consecutive year of this continuous field trial. All data used in this study were obtained from the third year of the experiment. The experimental site featured gray desert soil with a sandy loam texture, and the preceding crop in 2021 was maize. The baseline physicochemical properties of the soil were as follows: organic matter content of 17.86 g·kg−1, available nitrogen of 76.9 mg·kg−1, available phosphorus of 34.89 mg·kg−1, and available potassium of 335.6 mg·kg−1.
This experiment comprised seven treatments: a no-fertilization control (CK), conventional farmer fertilization (CF), and organic fertilizer substitutions replacing 10% (SF1), 20% (SF2), 30% (SF3), 40% (SF4), and 50% (SF5) of chemical fertilizers. The experimental design followed an equivalent nitrogen substitution approach, with each treatment replicated three times in a randomized block arrangement. Each plot measured 10 m in length and 2 m in width, and drip irrigation was employed throughout the growing season, with a total irrigation volume of 5700 m3/hm2. Individual water meters were used for each treatment to ensure consistent irrigation volumes. Fertilizers were applied through fertigation, with a topdressing ratio of 5:3:2 during the rapid leaf growth stage, root and sugar accumulation stage, and sugar storage stage, respectively. All other cultivation management practices were consistent with local field farming practices. The chemical fertilizers used in the experiment Xinlianxin Brand urea (N ≥ 46.4%) supplied by Jiangxi Yuxin Environmental Protection Co., Ltd., located in Nanchang City, China, was selected as the nitrogen fertilizer for this experiment, Jingtai Brand Monoammonium Phosphate (MAP, N ≥ 12%, P2O5 ≥ 61%) produced in Shifang, Sichuan Province was used as the phosphorus source in the experiment and Luobupo Brand Potassium Sulfate (K2O ≥ 51.7%) produced in Luobupo, Xinjiang, was used as the potassium source in the experiment.From 2021 to 2022, the “Gen Shuai Te” humic acid water-soluble fertilizer developed by Xinjiang Kangzheng Agricultural Company of China was used in the experimental treatment. Its nutrient content is as follows: N ≥ 120 g/L, P2O5 ≥ 120 g/L, K2O ≥ 120 g/L. In 2023, due to unforeseen circumstances, the organic fertilizer was replaced with the “Heiyaoshi” microbial inoculant produced by Xinjiang Hei Yaoshi Acidic Fertilizer Co., Ltd., China (with nitrogen content ≥ 10%, phosphorus pentoxide content ≥ 30%, and potassium oxide content ≥ 10%).The crop samples were collected and evaluated in 2023. Moreover, soil samples (0–20 cm) from the experimental field were collected before beet harvest. The five-point method was used to collect soil samples near the drip irrigation belt in each district. The debris was removed, and the samples were air-dried, ground, and sifted for preservation before the soil nutrients were determined.
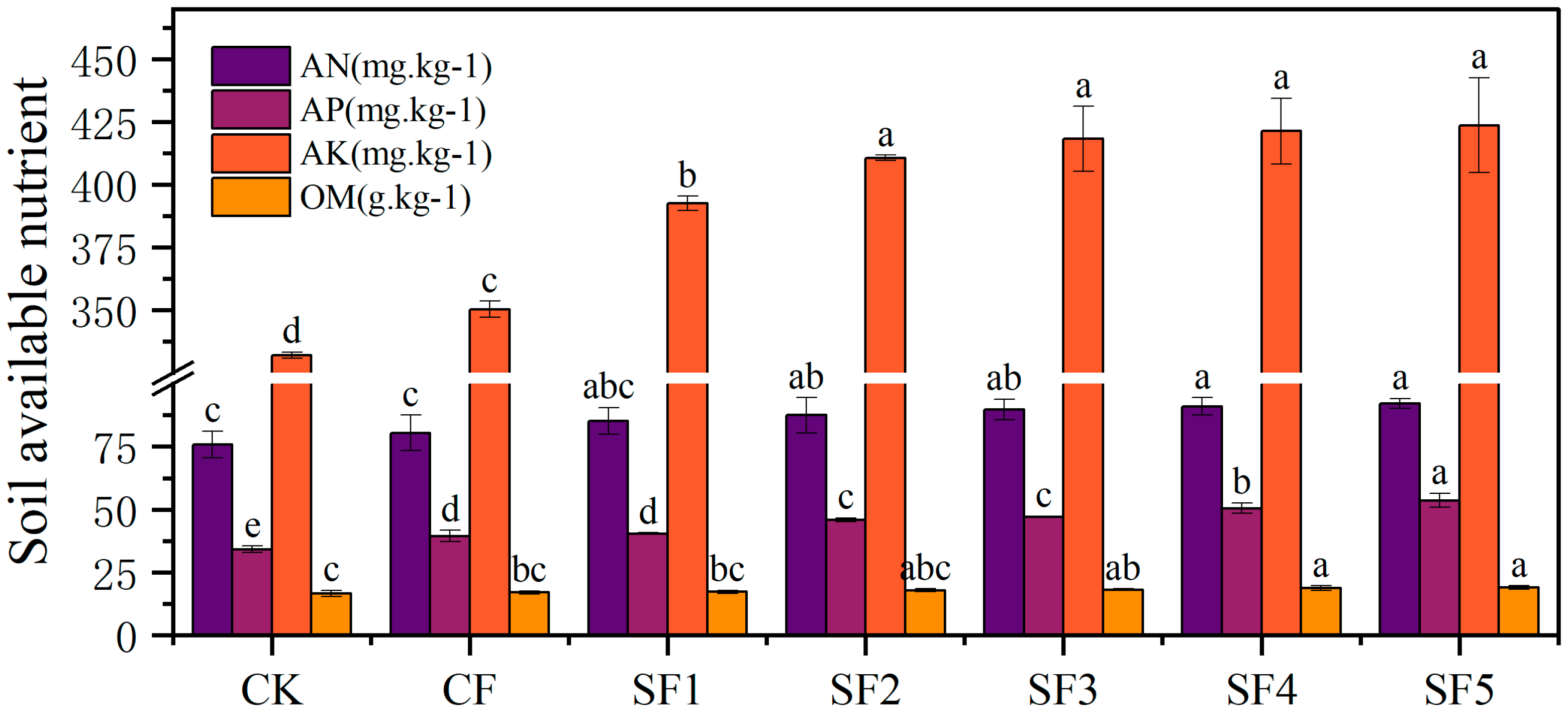
2.2. Soil Nutrient Contents
Soil-available nutrients refer to nutrients that can be directly absorbed and utilized by crops in the current season. The level of available nutrients is an indicator of the intensity of soil nutrient supply and a basic guarantee for crops to obtain a high yield [
15,
16]. For verification, soil alkaliolytic nitrogen (AN) was measured using the alkaliolytic diffusion method, and soil available phosphorus (AP) was extracted using the NaHCO3 and molybdenum-antimony resistance colorimetric method. Moreover, soil available potassium (AK) was determined using ammonium acetate extraction and flame spectrophotometry, and soil organic matter (OM) was determined using the potassium dichromate oxidation-external heating method [
11].
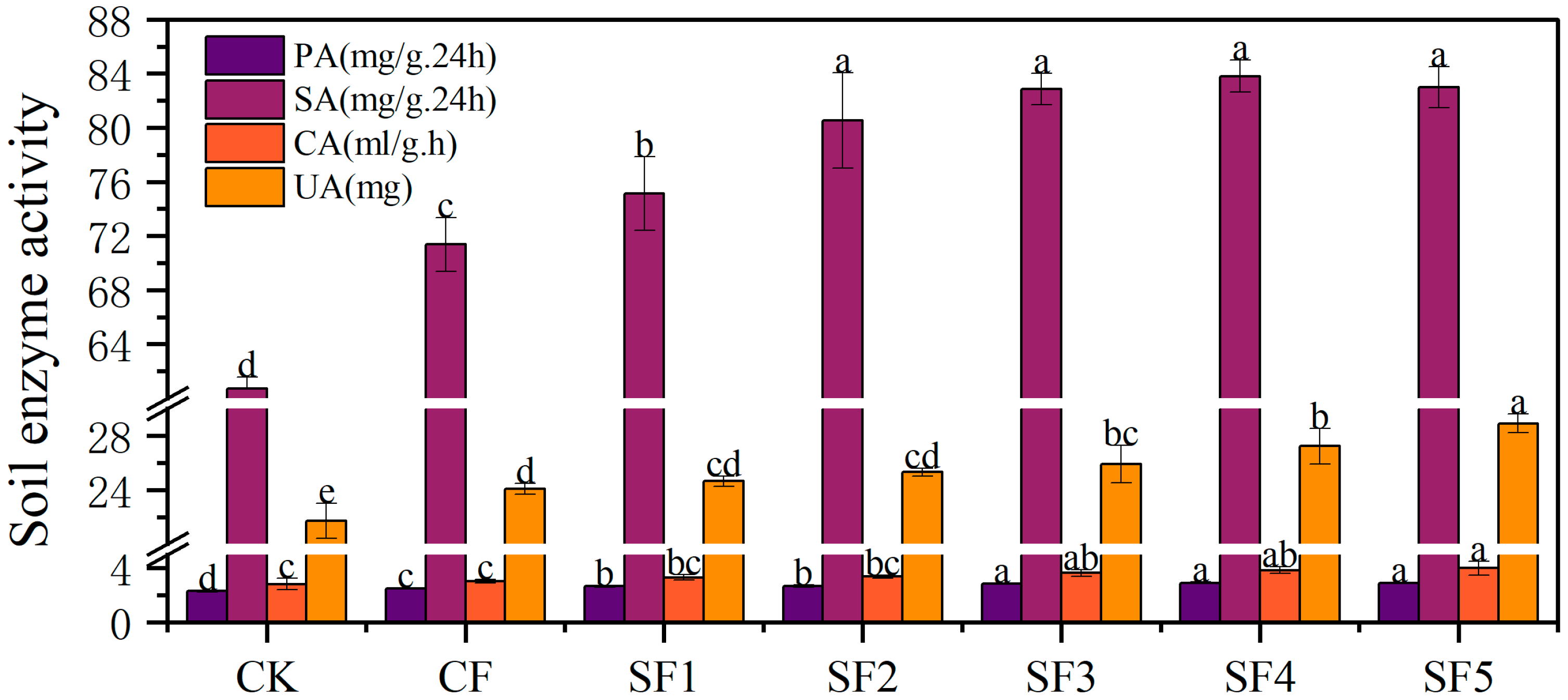
2.3. Soil Enzyme Activity
Soil enzyme activity refers to the capacity of enzymes in the soil to catalyze biochemical reactions, reflecting the intensity of soil biological activity and nutrient cycling. These enzymes, primarily derived from microorganisms, plant roots, and soil fauna, play crucial roles in key processes such as the decomposition of organic matter and the transformation and release of nutrients. Common soil enzymes include urease (involved in nitrogen cycling) and phosphatase (involved in phosphorus cycling), among others. Soil urease activity was determined using the phenol sodium-hypochlorite colorimetric method, while catalase activity was measured via potassium permanganate titration. Sucrase activity was assessed using the 3,5-dinitrosalicylic acid (DNS) colorimetric method, and alkaline phosphatase activity was quantified through the disodium phenyl phosphate colorimetric method.
2.4. Soil Microbial Community
The soil microbial community refers to the collective assemblage of various microorganisms (such as bacteria, fungi, actinomycetes, and archaea) inhabiting the soil, which play a crucial role in soil ecosystems. These microorganisms are involved in key processes, including the decomposition of organic matter, nutrient cycling (e.g., carbon, nitrogen, and phosphorus), the formation of soil structure, and the promotion of plant growth.
High-throughput 16S ribosomal RNA gene sequencing: The genomic DNA of the soil sample was extracted using TGuide S96 Magnetic Soil/Stool DNA Kit (Tiangen Biotech (Beijing.China) Co., Ltd.) according to manufacturer’s instructions. The V1-V9 hypervariable regions of the 16S rRNA gene were amplified using primers (27F: AGRGTTTGATYNTGGCTCAG; 1492R: TASGGHTACCTTGTTASGACTT). The amplicons were quantified, after which the normalized equimolar concentrations of amplicons were pooled and sequenced on the PacBio Sequel II platform (Beijing Biomarker Technologies Co., Ltd., Beijing, China).
2.5. Data Analysis
The precision (X ± SD) of the test results is expressed by arithmetic mean and standard error. Use Microsoft Excel 2010 software (Microsoft, Redmond, WA, USA) and SPSS 19.0 data analysis software (IBM Inc., Amok, NC, USA). The measurement data were first tested for normality through the Shapiro–Wilk test and for homogeneity of variance through the Levene test. For data that met the requirements of normality and homogeneity of variance, one-way analysis of variance (one-way ANOVA) was used to test the differences among treatments, and Duncan’s multiple range test was adopted for post hoc multiple comparisons. A randomized block design was employed in the experiment. In the statistical analysis, “block” was included in the model as a random effect to eliminate the impact of inter-block heterogeneity on the results. The data were then used for statistical calculation, statistical inspection, square difference of test data analysis, and other work. Origin 2018 (OriginLab Inc., Northampton, MA, USA) was used to make a columnar accumulation chart.
4. Discussion
The energy of soil organisms primarily derives from soil organic matter (SOM). The level of SOM directly influences the survival of various microbial communities in the soil. Moreover, higher organic matter content enhances soil fertility and buffering capacity. Increasing organic matter is essential for maintaining soil fertility in sugar beet fields. In this study, a comparison between conventional fertilization and partial replacement of nitrogen fertilizer with low or high amounts of organic fertilizer revealed that partial substitution with organic fertilizer can increase SOM. This effect becomes more pronounced with higher application rates of organic fertilizer, which is largely attributed to the high organic matter content in the fertilizer itself [
13]. Readily available nutrients in the soil, such as alkaline hydrolyzable nitrogen, available phosphorus, and available potassium, can be directly absorbed and utilized by crops during their growth and development. The levels of these available nutrients play a crucial role in the growth and development of sugar beet [
14]. This study found that partially replacing nitrogen fertilizer with organic fertilizer can increase the contents of alkaline hydrolyzable nitrogen, available phosphorus, and available potassium in the soil. However, the nutrient content does not increase proportionally with the amount of organic fertilizer applied. Beyond the SF3 treatment, further increases in organic fertilizer application had little effect on the levels of available nutrients. This finding is consistent with the results of a four-year organic fertilization experiment conducted by Zhang Peng et al. [
15], both demonstrating the positive effects of fertilization.
Numerous studies have shown that the application of organic fertilizer can enhance soil enzyme activity [
15,
16,
17,
18,
19]. Catalase, widely distributed in the soil matrix and organisms, effectively alleviates the potential toxicity of metabolically derived hydrogen peroxide to organisms. Its activity level is closely related to the abundance of soil organic matter and fertility status, making it an indicator of soil oxidation intensity [
16]. In this study, the application of organic fertilizer significantly increased soil catalase activity, and higher amounts of organic fertilizer further enhanced this effect. This suggests that organic fertilizer improves the soil micro-ecological environment and stimulates catalase activity to some extent, thereby reducing the toxic effect of hydrogen peroxide on sugar beets and enhancing their resistance to stress [
16,
17,
18]. Urease activity is influenced by multiple factors, including the content of soil organic matter, nitrogen supply and transformation efficiency, and the size of microbial populations [
19]. The application of organic fertilizer also increased soil urease activity, along with varying degrees of enhancement in phosphatase and sucrase activities. This may be attributed to the rich enzyme content in bio-organic fertilizer, which contributes to the increase in soil urease [
20] and promotes the growth and reproduction of enzyme-producing microorganisms, thereby indirectly enhancing soil enzyme activity. These findings are consistent with the results of Song Zhenzhen et al. [
21] and Luo Xinglu et al. [
22].
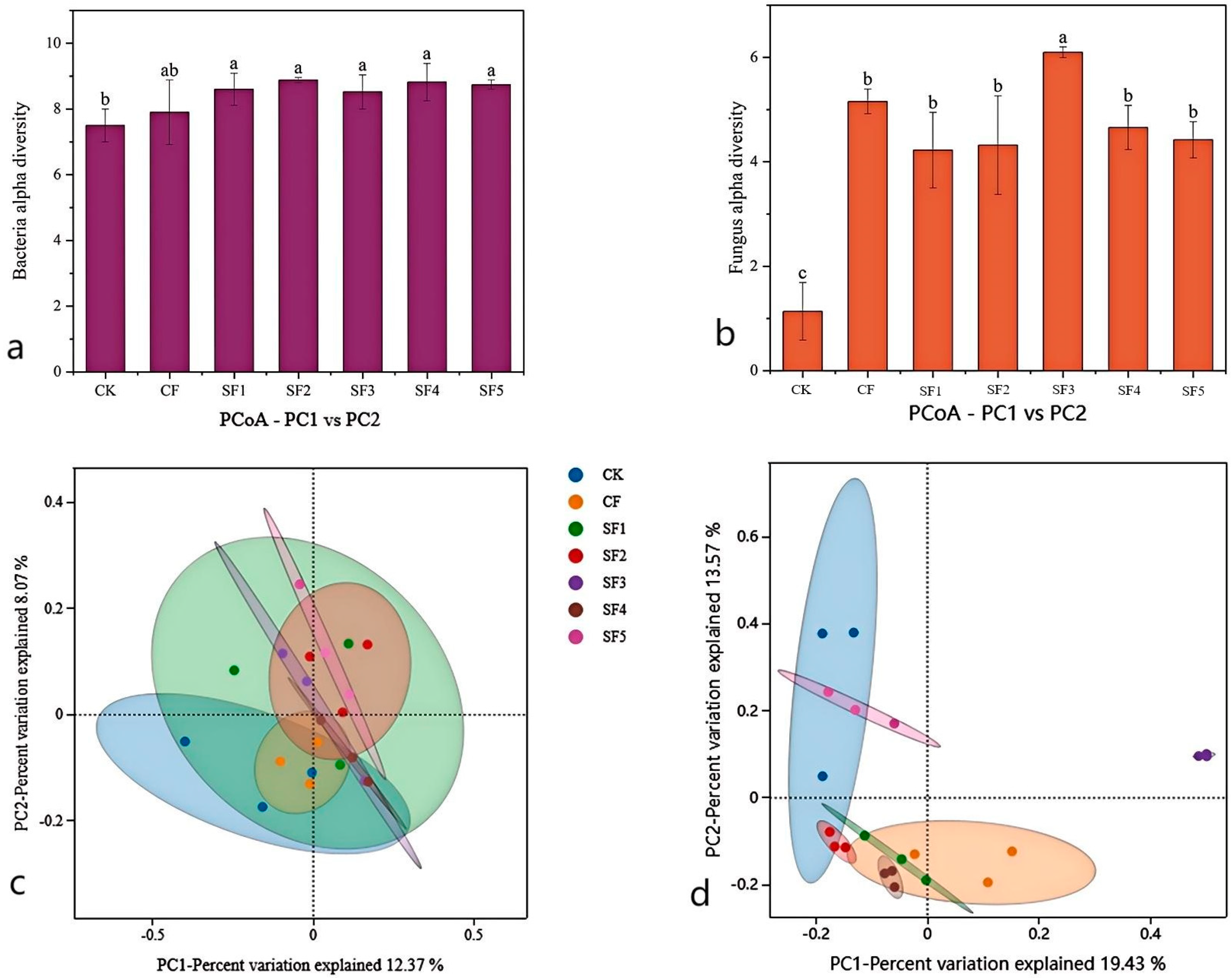
Soil microbial diversity is a key indicator for assessing soil health and ecosystem functioning. In this study, alpha diversity of soil bacteria and fungi under different fertilization treatments was characterized using the Shannon index. The results demonstrated that the application of organic fertilizer significantly increased the diversity of both soil bacteria and fungi, which is consistent with previous studies [
23,
24]. Organic fertilizer provides abundant organic matter and nutrients to soil microorganisms, promoting the growth and reproduction of microbial communities, thereby enhancing soil microbial diversity. The composition and function of soil microbial communities have a significant impact on soil fertility and ecosystem functioning [
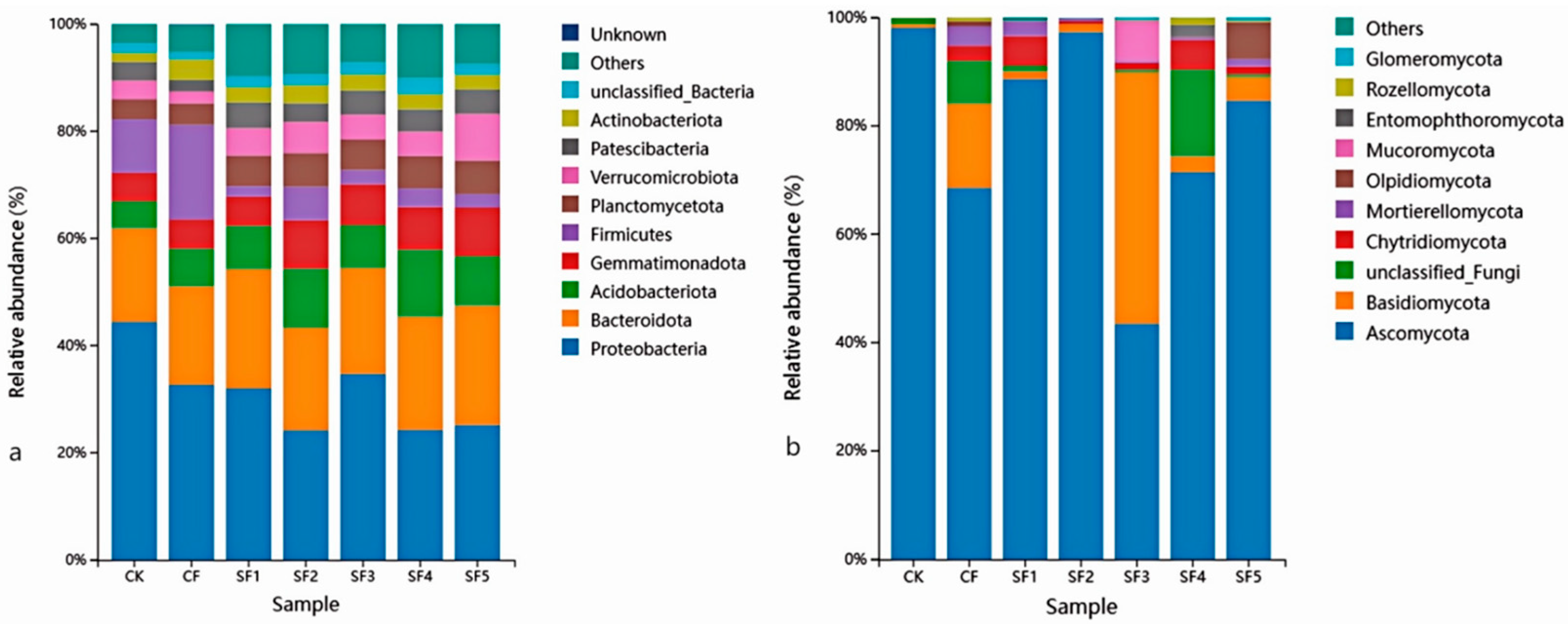
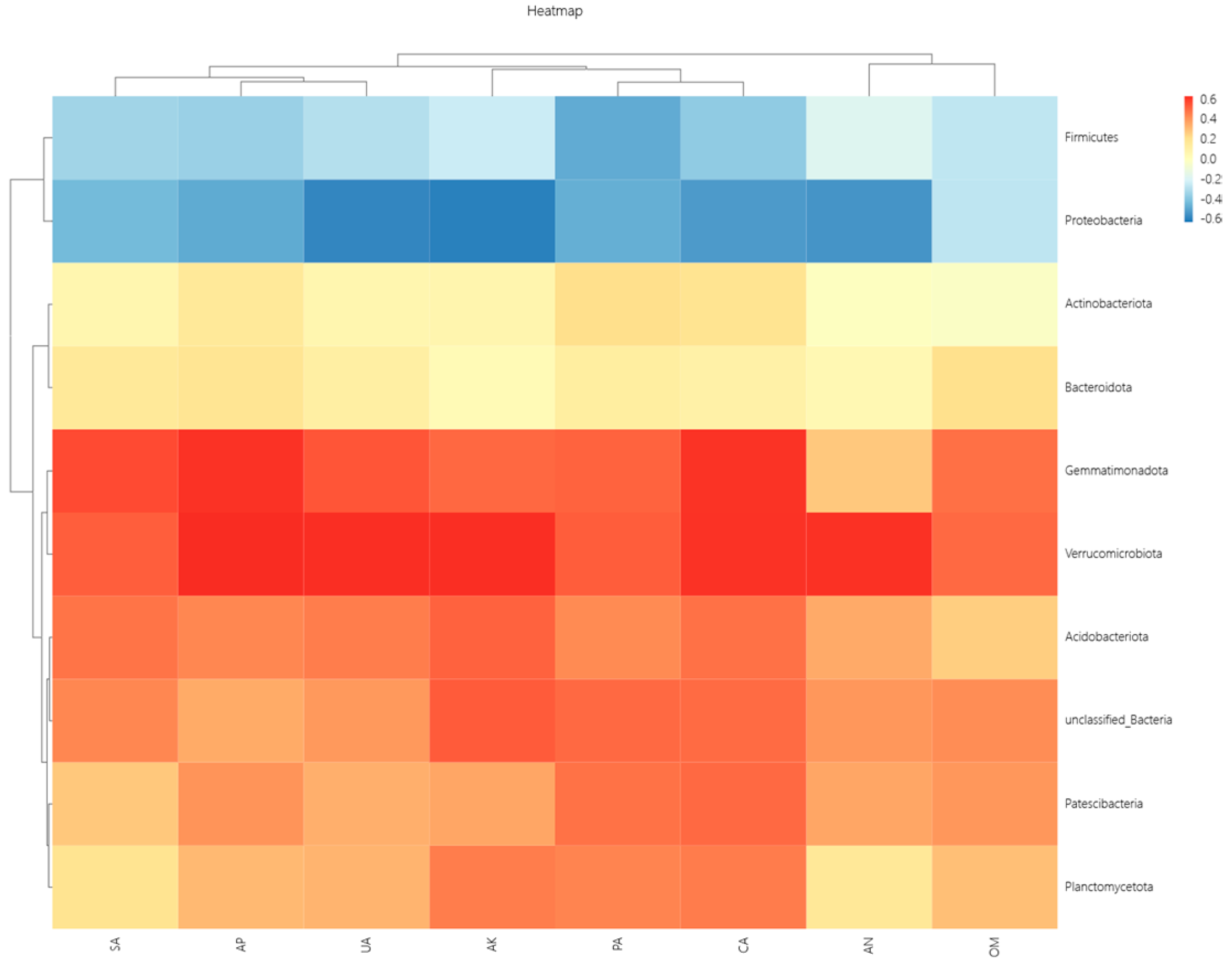
25]. In this study, organic fertilizer application promoted the growth of dominant bacterial groups such as Gemmatimonadota, Verrucomicrobiota, Planctomycota, and Acidobacteriota. Gemmatimonadota are bacteria that play an important role in soil ecosystems, capable of decomposing complex organic matter and participating in soil nutrient cycling [
26]. In this study, organic fertilizer application significantly increased the relative abundance of Gemmatimonadota, likely because the organic matter provided by the fertilizer created favorable conditions for their growth. Acidobacteriota is a common bacterial taxon in soil, involved in the formation of acidic environments and nutrient cycling. Organic fertilizer application significantly increased their relative abundance, possibly by altering soil pH balance and promoting the growth of this group. Yao Minna [
27] suggested that organic fertilizer application increases soil organic matter, thereby promoting the growth of prokaryotic functional groups associated with the degradation and fermentation of large molecules. The mineralization of organic matter in organic fertilizer involves nitrogen transformation, which can influence the abundance of bacterial functional groups related to the nitrogen cycle. These findings are consistent with the results of this study.
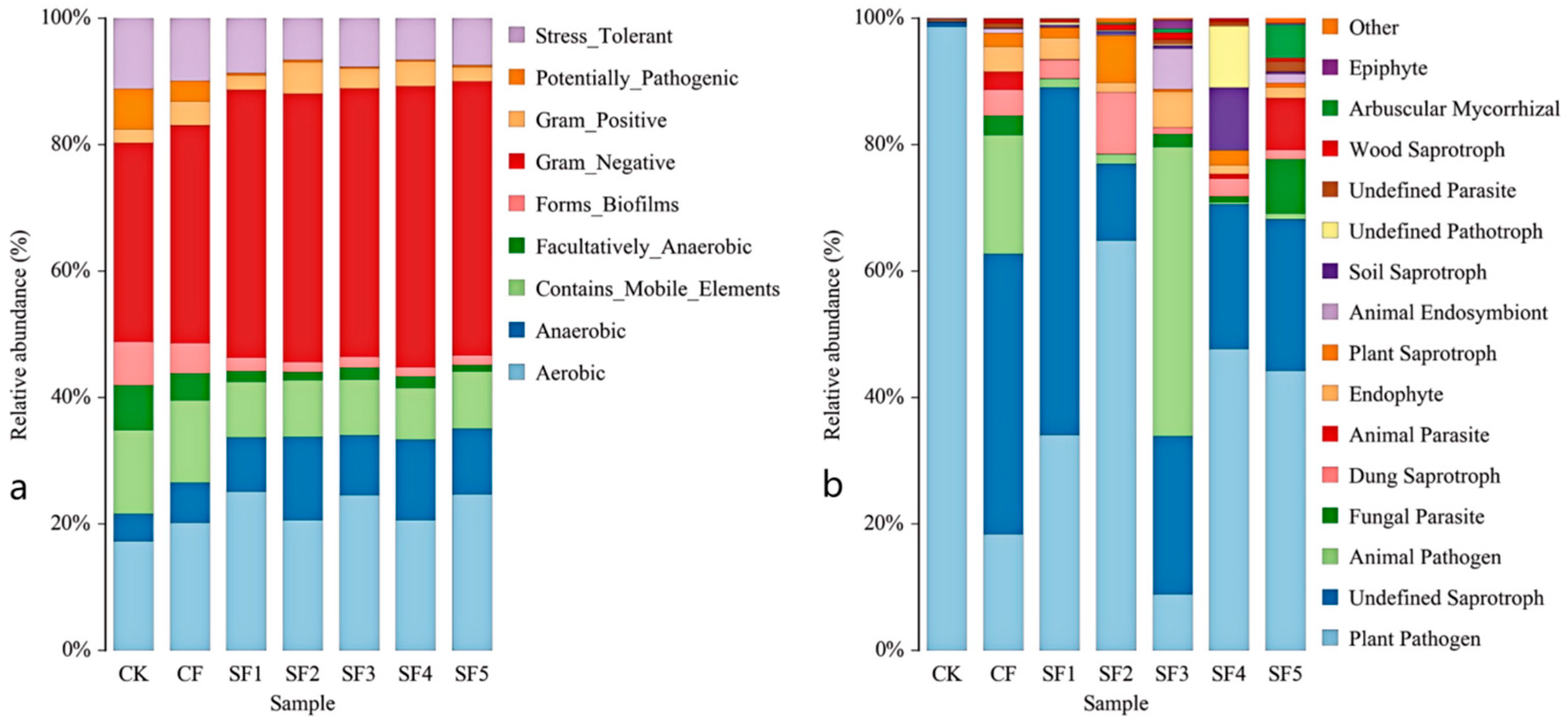
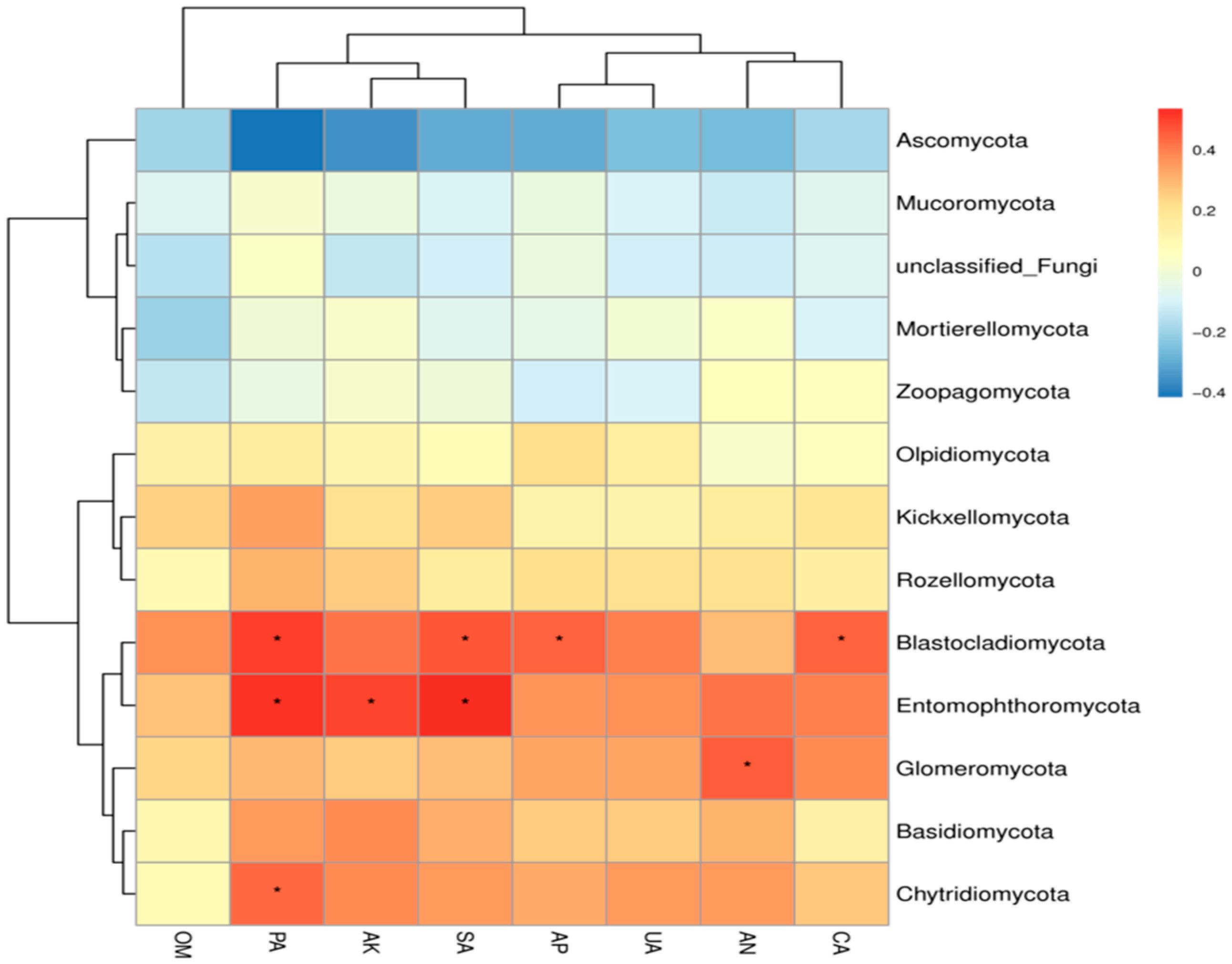
The application of organic fertilizer also significantly influenced the composition of the soil fungal community. Ascomycota, the most species-rich phylum in the fungal kingdom, plays a key role in decomposing organic matter by releasing locked-up nutrients such as carbon, nitrogen, and phosphorus into forms accessible to plants. This process is central to global carbon and nutrient cycling. However, Ascomycota also includes major pathogens responsible for sugar beet powdery mildew. In this study, the SF3 treatment significantly reduced the relative abundance of Ascomycota. BugBase-based prediction of high-level phenotypic functions of the soil bacterial community revealed that organic fertilizer application reduced the relative abundance of plant pathogens while increasing the proportions of endophytes and arbuscular mycorrhizal fungi, which may help enhance plant disease resistance and nutrient uptake capacity. The SF3 treatment also significantly increased the relative abundance of Basidiomycota and Mucoromycota, both of which play important roles in soil ecosystems by participating in the decomposition of organic matter and nutrient cycling. This may be attributed to the appropriate application rate of organic fertilizer, modifying the soil environment in a way that promotes the growth of these beneficial microbial groups. These results indicate that organic fertilizer application has the potential to increase the abundance of beneficial soil microorganisms. Previous studies have also confirmed that partial substitution of chemical fertilizers with organic fertilizers significantly affects soil microbial diversity and community structure. These results indicate that organic fertilizer application has the potential to increase the abundance of beneficial soil microorganisms. This study reveals the regulatory effects of four consecutive years of organic fertilizer substitution on soil microbial communities; however, there are several limitations in the research. For instance, soil pH, a key indicator, was not simultaneously measured, and this should be supplemented as a critical index in subsequent studies. Secondly, since the type of organic fertilizer was changed during the third year of the experiment, follow-up research will continue to monitor the impacts of this change. Additionally, the measured indicators were limited to soil nutrients, enzyme activities, and microbial community structure, failing to fully capture the specific changes in sugar beet rhizosphere soil. Therefore, future research should establish a multi-scale observation system and integrate multi-omics technologies such as metagenomics and metabolomics to systematically elucidate the dynamics of microbial communities and the long-term response mechanisms of soil functions under different organic fertilizer substitution ratios.