Synthesis, Bioevaluation and Structure-Activity Relationships of Novel N-Aryl Carbamate Derivatives as Potential Fungicidal Agents
Abstract
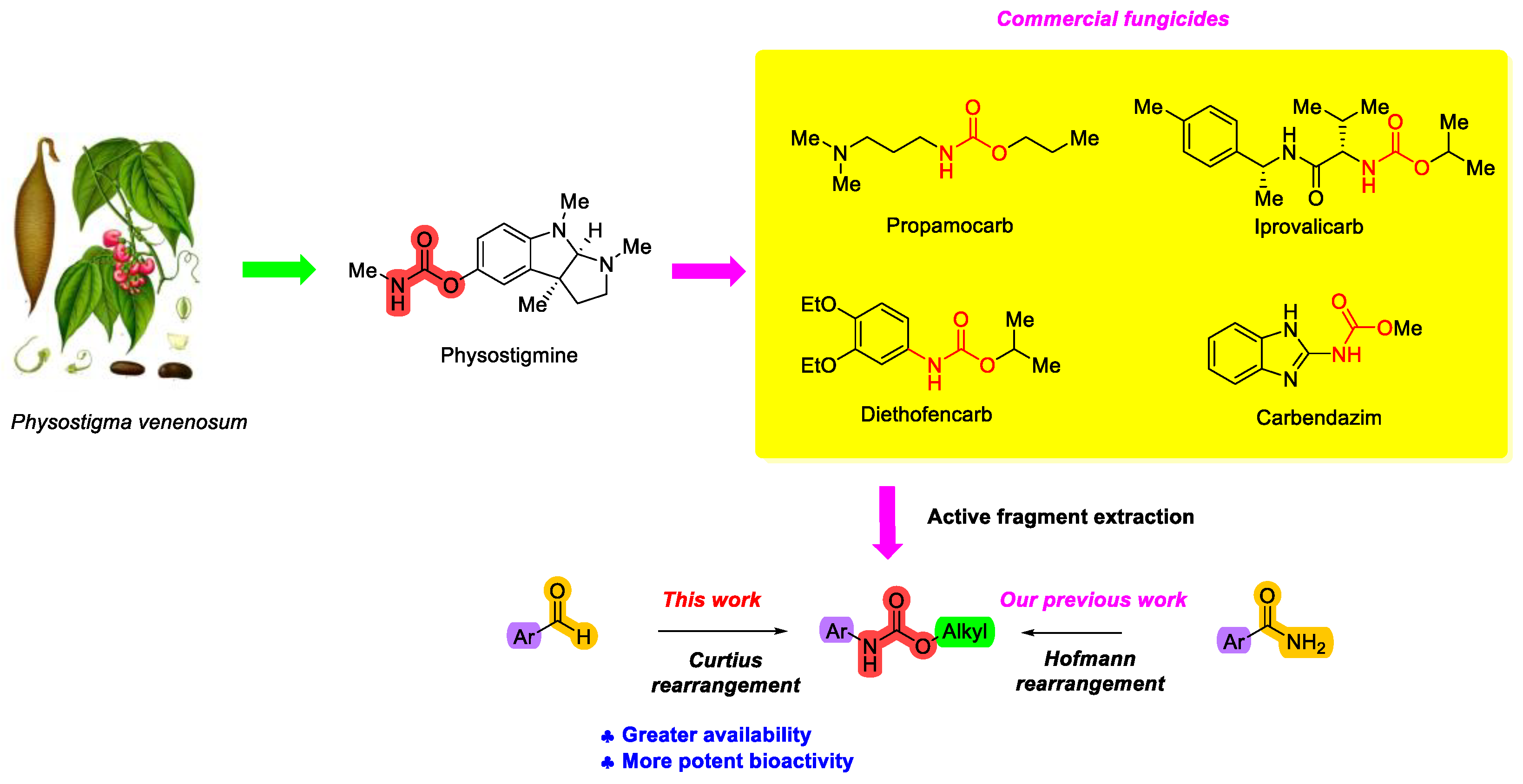
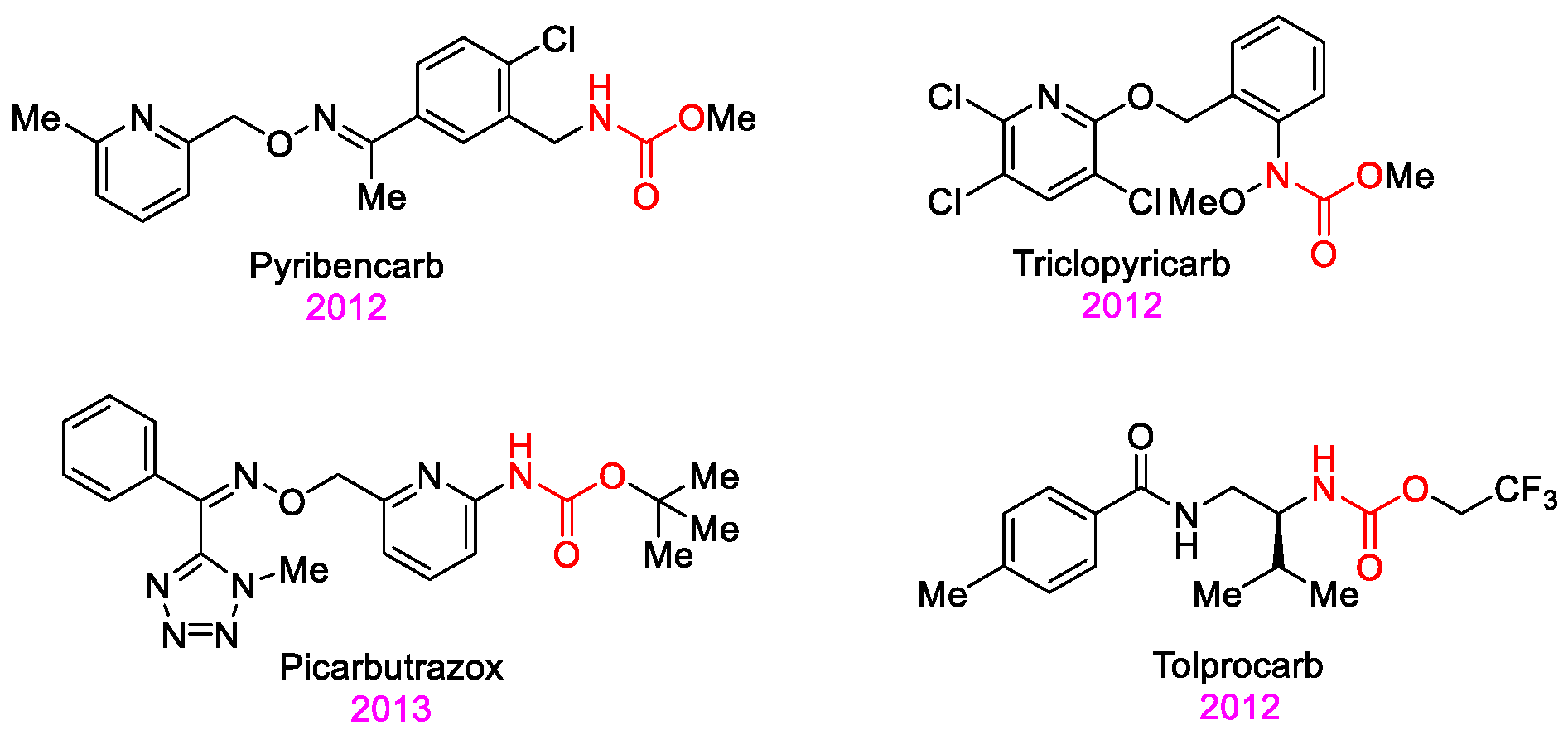
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Synthetic Procedures
2.3. Bioassays
3. Results
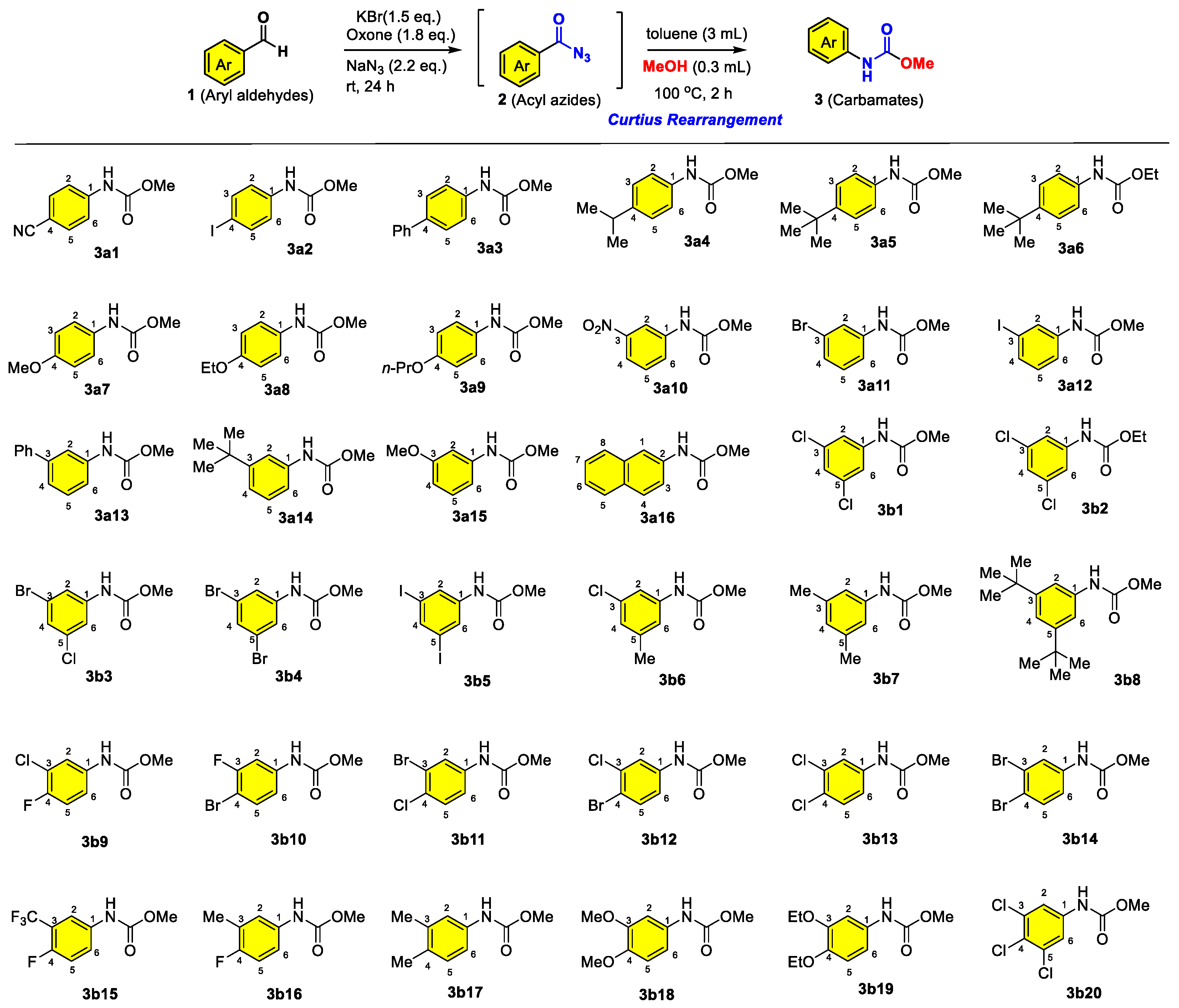
3.1. Chemistry
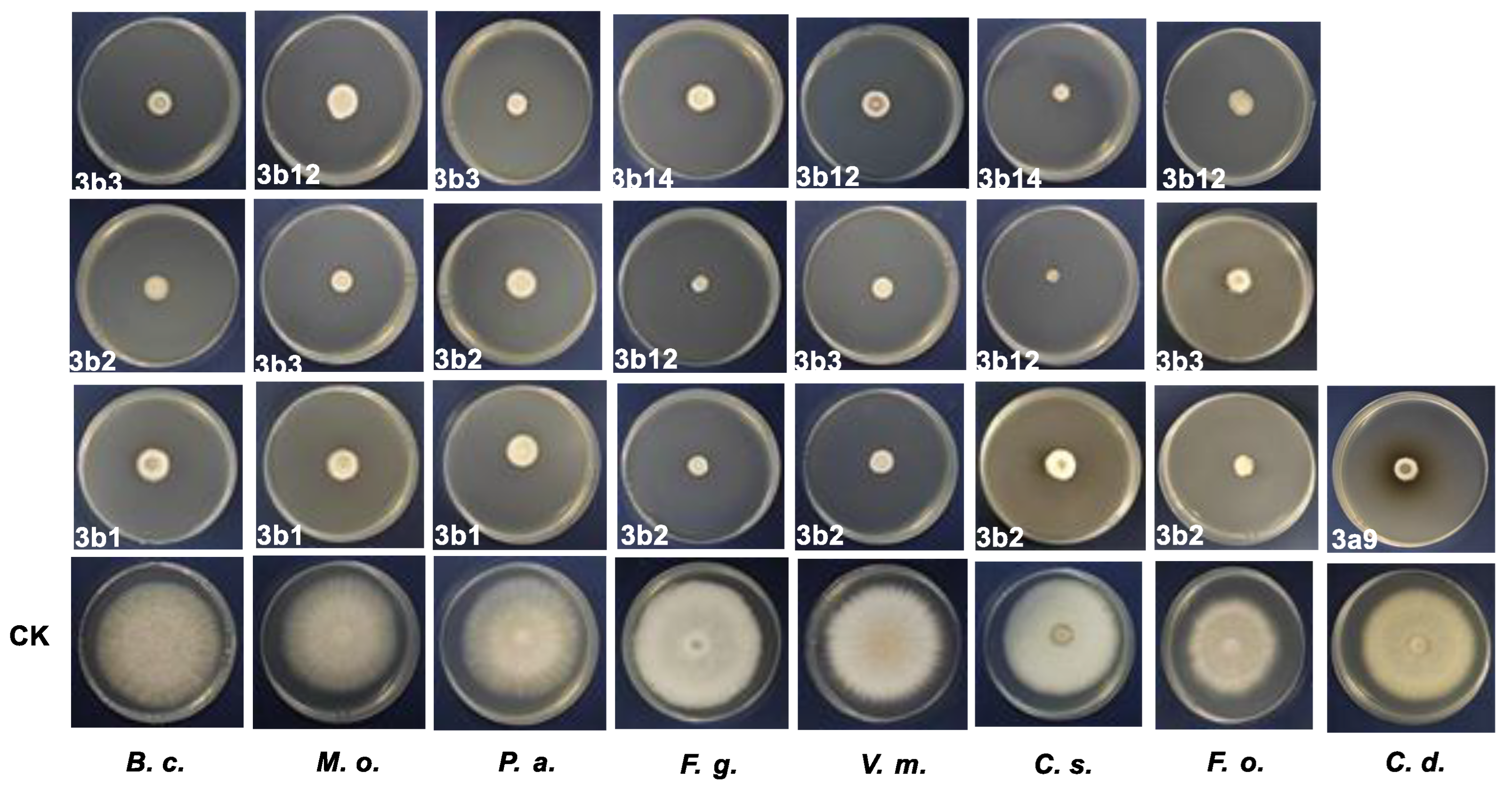
3.2. In Vitro Antifungal Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to Global Food Security from Emerging Fungal and Oomycete Crop Pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef]
- Lamberth, C.; Jeanmart, S.; Luksch, T.; Plant, A. Current Challenges and Trends in the Discovery of Agrochemicals. Science 2013, 341, 742–746. [Google Scholar] [CrossRef]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Yu, J.; Yang, M.; Han, J.; Pang, X. Fungal and mycotoxin occurrence, affecting factors, and prevention in herbal medicines: A review. Toxin Rev. 2022, 41, 976–994. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Brühl, C.A.; Imfeld, G.; Knäbel, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmüller, A.; et al. Fungicides: An overlooked pesticide class. Environ. Sci. Technol. 2019, 53, 3347–3365. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and prospects of botanical biopesticides in Europe and Mediterranean countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef]
- Jiang, M.; Wang, T.; Simal-Gandara, J.; Siddaiah, C.N.; Dai, X.F.; Chen, J.Y.; Wang, D.; Kong, Z.Q. Overview of the control of plant fungal pathogens by natural products derived from medicinal plants. Plant Prot. Sci. 2023, 59, 303–316. [Google Scholar] [CrossRef]
- Dong, F.-R.; Gao, L.; Wang, L.; Jiang, Y.-Y.; Jin, Y.-S. Natural Products as Antifungal Agents against Invasive Fungi. Curr. Top. Med. Chem. 2023, 23, 1859–1917. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef]
- Sparks, T.C.; Sparks, J.M.; Duke, S.O. Natural product-based crop protection compounds-origins and future prospects. J. Agric. Food Chem. 2023, 71, 2259–2269. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Alkazmi, L.M.; Nadwa, E.H.; Rashwan, E.K.; Rashwan, E.K.; Beshbishy, A.M.; Shaheen, H.; Wasef, L. Physostigmine: A Plant Alkaloid Isolated from Physostigma venenosum: A Review on Pharmacokinetics, Pharmacological and Toxicological Activities. J. Drug Deliv. Ther. 2020, 10, 187–190. [Google Scholar] [CrossRef]
- Şenocak, A.; Tümay, S.O.; Makhseed, S.; Demirbas, E.; Durmuş, M. A synergetic and sensitive physostigmine pesticide sensor using coppercomplex of 3D zinc (II) phthalocyanine-SWCNT hybrid material. Biosens. Bioelectron. 2021, 174, 112819. [Google Scholar] [CrossRef]
- Metcalf, R.L.; Fukuto, T.R.; Frederickson, M.; Peak, L. Insecticide Screening, Insecticidal Activity of Alkylthiophenyl N-Methylcarbamates. J. Agric. Food Chem. 1965, 13, 473–477. [Google Scholar] [CrossRef]
- Müller, F. Fungicides. In Agrochemicals: Composition, Production, Toxicology, Applications; Wiley-VCH: Weinheim, NY, USA, 2000; pp. 383–494. [Google Scholar]
- WHO. Carbamate Pesticides: A General Introduction; World Health Organization: Geneva, Switzerland, 1986. [Google Scholar]
- Takagaki, M.; Ozaki, M.; Fujimoto, S.; Fukumoto, S. Development of a novel fungicide, pyribencarb. J. Pestic. Sci. 2014, 39, 177–178. [Google Scholar] [CrossRef]
- Li, H.; Hu, S.; Sun, F.; Sun, Q.; Wang, N.; Li, B.; Zou, N.; Lin, J.; Mu, W.; Pang, X. Residual analysis of QoI fungicides in multiple (six) types of aquaticorganisms by UPLC-MS/MS under acutely toxic conditions. Environ. Sci. Pollut. Res. 2023, 30, 12075–12084. [Google Scholar] [CrossRef]
- Ichinari, D.; Nagaki, A.; Yoshida, J. Generation of hazardous methyl azide and its application to synthesis of a key-intermediate of picarbutrazox, a new potent pesticide in flow. Bioorganic Med. Chem. 2017, 25, 6224–6228. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, H.; Ezaki, R.; Hamada, T.; Tsuda, M.; Ebihara, K. Development of a novel fungicide, tolprocarb. J. Pestic. Sci. 2019, 44, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.-X.; Wang, J.-H.; Shi, Y.-H.; Niu, L.-Z.; Jiang, L. Design, Synthesis and Antifungal Activity of Novel Benzoylcarbamates Bearing a Pyridine Moiety. Appl. Sci. 2018, 8, 2577. [Google Scholar] [CrossRef]
- Zhao, F.-H.; Zhang, H.; Sun, C.-X.; Li, P.-H.; Jiang, L. Synthesis and fungicidal activity of 2-(methylthio)-4-methylpyrimidine carboxamides bearing a carbamate moiety. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 655–658. [Google Scholar]
- Li, Z.; Wu, Z.; Luo, F. Synthesis and Antifungal Activities of Alkyl N-(1,2,3-Thiadiazole-4-Carbonyl) Carbamates and S-Alkyl N-(1,2,3-Thiadiazole-4-Carbonyl) Carbamothioates. J. Agric. Food Chem. 2005, 53, 3872–3876. [Google Scholar] [CrossRef]
- Ning, L.; Wang, S.; Du, L.; Guo, B.; Zhang, J.; Lu, H.; Dong, Y. Synthesis, bioactivity and 3D-QSAR of azamacrolide compounds with a carbamate or urea moiety as potential fungicides and inhibitors of quorum sensing. New J. Chem. 2021, 45, 3048–3058. [Google Scholar] [CrossRef]
- Lu, Y.; Cui, Y.; Yang, W.; Meng, F. Design and synthesis of novel totarol derivatives bearing carbamate moiety as potential fungicides. Monatshefte Chem. 2023, 154, 915–923. [Google Scholar] [CrossRef]
- Jia, C.; Yang, D.; Che, C.; Ma, Y.; Rui, C.; Yan, X.; Qin, Z. Synthesis, Structural Characterization, Insecticidal and FungicidalActivity of (1H-1,2,4-Triazol-5-yl) carbamates. Chem. J. Chin. Univ. 2016, 37, 892–901. [Google Scholar]
- Liu, C. Synthesis and fungicidal activity of (2-chloropyridin-5-yl) methyl carbamates. Chin. J. Pestic. Sci. 2015, 17, 97–100. [Google Scholar]
- You, J.; Gao, Y.; Zhou, P.; Guo, Q.; Xu, Z. Synthesis and biological activity of N-substituted phenyl-1-1(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethylcarbamate. Chin. J. Pestic. Sci. 2022, 24, 723–731. [Google Scholar]
- Wang, S.; Lei, N.; Li, Z.; Dong, Y. Synthesis and bacteriostatic activity of ten-, twelve- and sixteen-membered azalactone compounds containing phenoxymethyl and chloromethyl group. Chin. J. Pestic. Sci. 2024, 26, 870–882. [Google Scholar]
- Chaturvedi, D.; Mishra, N.; Mishra, V. Various approaches for the synthesis of organic carbamates. Curr. Org. Synth. 2007, 4, 310–322. [Google Scholar] [CrossRef]
- Song, L.; Meng, Y.; Zhao, T.; Liu, L.; Pan, X.; Huang, B.; Yao, H.; Lin, R.; Tong, R. Unified and green oxidation of amides and aldehydes for the Hofmann and Curtius rearrangements. Green Chem. 2024, 26, 428–438. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Y.; Liu, L.; Duan, X.; You, S.; Yu, B.; Pan, X.; Guan, X.; Lin, R.; Song, L. Green synthesis and antifungal activities of novel N-aryl carbamate derivatives. Molecules 2024, 29, 3479. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, H.; Miyagi, M.; Nitta, S.; Takahashi, S. Production Method for Amidate Compound. U.S. Patent US 2020/0024237 A1, 23 January 2020. [Google Scholar]
- Karapetyan, V.; Mkrtchyan, S.; Schmidt, A.; Gütlein, J.-P.; Villinger, A.; Reinke, H.; Jiao, H.; Fisher, C.; Langer, P. Synthesis of 3,4-benzo-7-hydroxy-2,9-diazabicyclo [3.3.1]non-7-enes by cyclization of 1,3-bis(silyl enol ethers) with quinazolines. Org. Biomol. Chem. 2008, 6, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Reiffen, M.; Hoffmann, R.W. Zur Reaktion von Amidacetalen mit Heterocumulenen. Chem. Berichte 1977, 110, 37–48. [Google Scholar] [CrossRef]
- Guan, Z.-H.; Lei, H.; Chen, M.; Ren, Z.-H.; Bai, Y.; Wang, Y.-Y. Palladium-Catalyzed Carbonylation of Amines: Switchable Approaches to Carbamates and N,N’-Disubstituted Ureas. Adv. Synth. Catal. 2012, 354, 489–496. [Google Scholar] [CrossRef]
- Singh, A.S.; Kumar, D.; Mishra, N.; Tiwari, V.K. An efficient one-pot synthesis of N,N′-disubstituted ureas and carbamates from N-Acylbenzotriazoles. RSC Adv. 2016, 6, 84512–84522. [Google Scholar] [CrossRef]
- Ji, L.; Ablajan, K. Synthesis of N-Phenylcarbamate by C-N Coupling Reaction without Metal Participation. Synthesis 2023, 55, 3113–3120. [Google Scholar]
- Li, L.; Xue, M.; Yan, X.; Liu, W.; Xu, K.; Zhang, S. Electrochemical Hofmann rearrangement mediated by NaBr: Practical access to bioactive carbamates. Org. Biomol. Chem. 2018, 16, 4615–4618. [Google Scholar] [CrossRef]
- Nagai, Y.; Matsuo, M. The Hofmann Reaction of 3,5-Di-tert-butylbenzamide. J. Soc. Chem. Ind. 1967, 70, 931–934. [Google Scholar]
- Fra, L.; Millán, A.; Souto, J.A.; Muňiz, K. Indole Synthesis Based On A Modified Koser Reagent. Angew. Chem. Int. Ed. 2014, 53, 7349–7353. [Google Scholar] [CrossRef]
| Compounds | Average Inhibition Rate ± SD (%) (n = 3) a | |||||||
|---|---|---|---|---|---|---|---|---|
| No. | B. c. | M. o. | P. a. | F. g. | C. d. | V. m. | C. s. | F. o. |
| 3a1 | 6.50 ± 0.74 | 0.27 ± 0.87 | 3.75 ± 2.08 | 1.12 ± 3.22 | 7.11 ± 0.66 | 0 | 0 | 15.97 ± 0.67 |
| 3a2 | 58.97 ± 2.57 | 60.88 ± 2.13 | 56.30 ± 0.25 | 52.02 ± 3.47 | 53.60 ± 0.90 | 50.68 ± 0.66 | 34.06 ± 0.57 | 54.15 ± 0.82 |
| 3a3 | 35.70 ± 4.27 | 32.65 ± 0.70 | 42.91 ± 1.40 | 47.71 ± 1.42 | 30.19 ± 2.22 | 45.83 ± 1.34 | 48.37 ± 0.60 | 50.75 ± 1.20 |
| 3a4 | 58.93 ± 0.83 | 51.25 ± 1.00 | 61.69 ± 1.28 | 41.97 ± 0.98 | 48.11 ± 1.36 | 52.29 ± 0.76 | 19.44 ± 2.80 | 47.58 ± 1.37 |
| 3a5 | 60.10 ± 0.43 | 60.73 ± 0.53 | 63.45 ± 0.45 | 69.49 ± 0.64 | 39.22 ± 1.41 | 61.95 ± 0.76 | 21.67 ± 0.9 | 63.48 ± 0.63 |
| 3a6 | 45.55 ± 1.37 | 50.71 ± 0.61 | 51.86 ± 1.39 | 58.22 ± 1.12 | 8.50 ± 1.68 | 47.38 ± 1.13 | 50.34 ± 2.42 | 42.74 ± 1.23 |
| 3a7 | 0 | 0 | 3.43 ± 1.91 | 10.71 ± 0.31 | 8.35 ± 0.41 | 0.61 ± 0.91 | 7.66 ± 3.58 | 16.42 ± 0.92 |
| 3a8 | 20.05 ± 1.18 | 16.78 ± 1.25 | 13.36 ± 0.86 | 52.67 ± 1.48 | 73.86 ± 1.08 | 14.87 ± 3.20 | 14.00 ± 4.48 | 26.62 ± 0.73 |
| 3a9 | 67.58 ± 1.20 | 67.41 ± 1.36 | 68.79 ± 0.95 | 77.40 ± 1.07 | 83.65 ± 0.57 | 52.14 ± 2.03 | 50.67 ± 2.73 | 59.58 ± 0.71 |
| 3a10 | 0 | 0 | 0 | 12.07 ± 3.07 | 0 | 0.87 ± 1.76 | 11.46 ± 2.29 | 9.80 ± 0.83 |
| 3a11 | 35.66 ± 2.15 | 31.60 ± 2.05 | 35.74 ± 1.56 | 43.38 ± 0.57 | 22.64 ± 8.76 | 35.70 ± 1.37 | 48.57 ± 1.65 | 33.79 ± 0.73 |
| 3a12 | 44.7 ± 0.86 | 37.53 ± 2.80 | 36.66 ± 3.22 | 28.65 ± 1.59 | 33.19 ± 1.99 | 40.31 ± 0.87 | 40.54 ± 0.99 | 43.72 ± 0.79 |
| 3a13 | 75.20 ± 0.94 | 75.76 ± 0.73 | 77.52 ± 0.97 | 75.16 ± 0.58 | 63.42 ± 0.28 | 73.73 ± 1.46 | 86.51 ± 1.74 | 65.67 ± 0.38 |
| 3a14 | 52.83 ± 0.59 | 55.33 ± 0.92 | 56.61 ± 1.17 | 60.05 ± 1.79 | 45.86 ± 1.06 | 51.55 ± 1.17 | 18.04 ± 2.50 | 39.10 ± 0.95 |
| 3a15 | 9.58 ± 1.18 | 4.64 ± 1.56 | 15.16 ± 1.37 | 18.26 ± 3.83 | 10.73 ± 0.31 | 18.34 ± 5.42 | 15.94 ± 2.03 | 16.21 ± 0.80 |
| 3a16 | 69.06 ± 0.27 | 67.68 ± 0.36 | 68.08 ± 0.83 | 28.73 ± 1.52 | 44.35 ± 1.73 | 66.93 ± 0.65 | 62.23 ± 1.31 | 62.56 ± 0.97 |
| 3b1 | 82.40 ± 0.74 | 83.45 ± 0.61 | 80.28 ± 0.36 | 75.43 ± 1.94 | 60.03 ± 2.15 | 78.24 ± 1.31 | 60.89 ± 2.90 | 70.11 ± 1.15 |
| 3b2 | 85.79 ± 0.45 | 76.31 ± 0.37 | 82.95 ± 0.91 | 89.45 ± 2.10 | 61.97 ± 1.55 | 87.29 ± 0.86 | 81.24 ± 1.79 | 83.54 ± 0.61 |
| 3b3 | 87.36 ± 0.26 | 88.94 ± 0.3 | 85.61 ± 0.97 | 98.43 ± 1.14 | 72.33 ± 0.76 | 89.48 ± 0.60 | 86.89 ± 1.56 | 83.58 ± 0.77 |
| 3b4 | 77.66 ± 0.42 | 77.86 ± 0.59 | 78.24 ± 0.51 | 69.71 ± 3.06 | 53.34 ± 1.09 | 74.55 ± 1.04 | 56.96 ± 1.47 | 65.78 ± 0.48 |
| 3b5 | 28.63 ± 0.99 | 28.98 ± 1.29 | 36.83 ± 0.83 | 46.09 ± 1.10 | 4.38 ± 1.16 | 24.94 ± 0.98 | 19.65 ± 5.27 | 19.94 ± 0.83 |
| 3b6 | 68.11 ± 0.65 | 65.76 ± 1.07 | 73.39 ± 0.95 | 71.63 ± 0.81 | 62.26 ± 0.83 | 55.37 ± 1.80 | 57.00 ± 2.02 | 67.68 ± 1.54 |
| 3b7 | 28.78 ± 1.36 | 25.57 ± 0.91 | 30.07 ± 0.47 | 24.59 ± 0.10 | 2.63 ± 0.83 | 16.06 ± 1.30 | 21.88 ± 4.39 | 32.07 ± 2.34 |
| 3b8 | 8.85 ± 1.69 | 7.37 ± 0.97 | 9.53 ± 0.89 | 10.37 ± 1.52 | 0 | 5.32 ± 1.47 | 4.51 ± 3.48 | 12.39 ± 0.73 |
| 3b9 | 36.14 ± 5.51 | 35.52 ± 4.69 | 34.73 ± 1.78 | 38.64 ± 8.35 | 37.18 ± 2.83 | 39.00 ± 1.73 | 31.51 ± 0.46 | 37.37 ± 1.47 |
| 3b10 | 59.67 ± 1.11 | 57.35 ± 2.16 | 58.16 ± 2.11 | 56.67 ± 1.58 | 41.66 ± 1.25 | 56.12 ± 2.04 | 27.38 ± 5.31 | 51.26 ± 1.01 |
| 3b11 | 81.83 ± 0.66 | 78.55 ± 0.65 | 81.51 ± 1.45 | 83.13 ± 1.13 | 73.56 ± 0.98 | 74.21 ± 1.23 | 90.68 ± 0.84 | 78.31 ± 0.63 |
| 3b12 | 82.97 ± 0.26 | 80.04 ± 1.32 | 85.42 ± 0.98 | 85.78 ± 0.57 | 76.70 ± 0.53 | 84.32 ± 0.97 | 97.86 ± 0.28 | 83.50 ± 1.72 |
| 3b13 | 76.42 ± 0.44 | 76.45 ± 1.41 | 80.80 ± 0.48 | 94.12 ± 0.87 | 70.87 ± 0.41 | 78.41 ± 1.14 | 80.69 ± 1.66 | 77.54 ± 0.83 |
| 3b14 | 77.83 ± 0.50 | 76.86 ± 2.18 | 82.31 ± 1.26 | 87.87 ± 0.76 | 62.13 ± 0.48 | 79.01 ± 1.45 | 89.88 ± 1.80 | 78.66 ± 1.18 |
| 3b15 | 39.09 ± 1.05 | 35.33 ± 0.52 | 43.80 ± 1.54 | 59.13 ± 1.33 | 49.47 ± 0.48 | 40.63 ± 0.93 | 37.95 ± 1.52 | 42.31 ± 0.68 |
| 3b16 | 19.55 ± 2.26 | 9.93 ± 2.04 | 12.50 ± 0.92 | 14.58 ± 3.95 | 13.65 ± 1.01 | 23.23 ± 2.35 | 18.69 ± 1.69 | 17.66 ± 0.45 |
| 3b17 | 20.91 ± 1.06 | 27.94 ± 1.03 | 17.02 ± 0.44 | 1.81 ± 1.42 | 15.94 ± 1.46 | 12.74 ± 1.29 | 33.56 ± 1.15 | 18.65 ± 1.13 |
| 3b18 | 4.54 ± 2.23 | 0 | 4.26 ± 0.73 | 6.58 ± 1.95 | 6.63 ± 1.70 | 1.31 ± 1.11 | 1.30 ± 2.51 | 13.53 ± 0.97 |
| 3b19 | 12.24 ± 0.83 | 5.18 ± 1.06 | 12.45 ± 1.21 | 6.70 ± 1.12 | 15.28 ± 1.38 | 10.93 ± 1.79 | 9.44 ± 7.49 | 10.47 ± 1.27 |
| 3b20 | 62.24 ± 1.86 | 60.47 ± 1.15 | 59.87 ± 1.30 | 64.61 ± 1.44 | 57.14 ± 0.67 | 57.49 ± 0.68 | 71.12 ± 0.76 | 54.01 ± 1.17 |
| AZO | 54.39 ± 0.24 | 59.94 ± 0.24 | 56.40 ± 0.87 | 58.17 ± 0.22 | 53.46 ± 0.90 | 63.14 ± 0.66 | 54.79 ± 0.67 | 62.50 ± 2.14 |
 .
.| Compound | Regression Equation | R2 | EC50 (μg/mL, 95% CI) a,b |
|---|---|---|---|
| B. cinerea | |||
| 3b1 | y = 3.605x + 4.008 | 0.966 | 12.94 (8.56–17.74) |
| 3b2 | y = 3.826x + 4.728 | 0.993 | 17.21 (14.93–19.70) |
| 3b3 | y = 4.387x + 5.829 | 0.994 | 21.31 (18.80–24.11) |
| 3b11 | y = 4.810x + 6.903 | 0.985 | 27.24 (24.22–30.63) |
| 3b12 | y = 4.610x + 6.221 | 0.998 | 22.36 (19.81–25.20) |
| azoxystrobin | y = 0.608x + 0.764 | 0.916 | 18.06 (4.97–37.44) |
| M. oryzae | |||
| 3b1 | y = 4.028x + 4.698 | 0.971 | 14.67 (9.77–20.41) |
| 3b3 | y = 4.328x + 5.578 | 0.995 | 19.44 (17.11–22.02) |
| 3b12 | y = 4.601x + 6.167 | 0.989 | 21.90 (19.40–24.69) |
| azoxystrobin | y = 0.529x + 0.476 | 0.986 | 7.93 (0.08–17.84) |
| P. aphanidermatum | |||
| 3b1 | y = 3.996x + 4.732 | 0.983 | 15.28 (13.27–17.44) |
| 3b2 | y = 4.578x + 6.065 | 0.949 | 21.12 (14.48–30.25) |
| 3b3 | y = 4.791x + 6.384 | 0.990 | 21.50 (19.10–24.17) |
| 3b11 | y = 5.622x + 8.391 | 0.990 | 31.08 (27.94–34.64) |
| 3b12 | y = 4.202x + 5.753 | 0.998 | 23.41 (20.60–26.58) |
| 3b13 | y = 6.011x + 8.930 | 0.997 | 30.60 (27.60–33.95) |
| 3b14 | y = 4.500x + 6.375 | 0.996 | 26.12 (23.12–29.52) |
| azoxystrobin | y = 0.534x + 0.612 | 0.968 | 13.98 (1.08–30.50) |
| F. graminearum | |||
| 3b2 | y = 4.136 + 4.050 | 0.921 | 9.53 (5.03–13.92) |
| 3b3 | y = 4.674x + 5.835 | 0.913 | 17.72 (9.24–30.75) |
| 3b11 | y = 4.005 + 4.603 | 0.943 | 14.10 (7.65–22.06) |
| 3b12 | y = 4.983x + 5.645 | 0.902 | 13.58 (8.31–20.11) |
| 3b13 | y = 4.376x + 5.061 | 0.944 | 14.34 (8.74–21.22) |
| 3b14 | y = 3.268x + 3.736 | 0.993 | 13.90 (6.93–22.20) |
| azoxystrobin | y = 0.552x + 0.561 | 0.971 | 10.35 (0.48–21.80) |
| C. destructivum | |||
| 3a9 | y = 2.927x + 3.579 | 0.901 | 16.70 (5.73–33.15) |
| azoxystrobin | y = 0.673x + 0.996 | 0.998 | 30.14 (15.25–74.79) |
| V. mali | |||
| 3b2 | y = 3.871 + 4.767 | 0.997 | 17.04 (14.79–19.48) |
| 3b3 | y = 4.394x + 5.932 | 0.999 | 22.39 (19.76–25.33) |
| 3b12 | y = 3.996x + 5.351 | 0.985 | 21.83 (19.11–24.88) |
| azoxystrobin | y = 0.593 + 0.398 | 0.956 | 4.70 (0.06–11.32) |
| C. siamense | |||
| 3a13 | y = 3.439x + 4.522 | 0.987 | 20.66 (17.80–23.87) |
| 3b2 | y = 4.311x + 5.146 | 0.935 | 15.62 (8.76–24.75) |
| 3b3 | y = 4.005x + 5.044 | 0.934 | 18.18 (9.07–32.16) |
| 3b11 | y = 4.180x + 5.275 | 0.884 | 18.28 (7.03–38.57) |
| 3b12 | y = 4.528x + 5.387 | 0.999 | 15.48 (1.57–49.40) |
| 3b13 | y = 4.546x + 5.680 | 0.904 | 17.76 (8.08–33.78) |
| 3b14 | y = 3.538x + 4.325 | 0.942 | 16.70 (7.44–30.14) |
| azoxystrobin | y = 0.504x + 0.678 | 0.998 | 22.12 (4.01–73.56) |
| F. oxysporum | |||
| 3b2 | y = 3.464x + 4.218 | 0.996 | 16.51 (14.13–19.08) |
| 3b3 | y = 4.138x + 5.233 | 0.991 | 18.39 (16.10–20.90) |
| 3b12 | y = 4.401x + 5.971 | 0.985 | 22.73 (20.07–25.72) |
| azoxystrobin | y = 0.809x + 0.930 | 0.978 | 14.15 (5.66–23.54) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Han, Z.; Duan, X.; Zhang, J.; Cai, Y.; Gao, M.; Song, L.; Huang, B.; Lin, R. Synthesis, Bioevaluation and Structure-Activity Relationships of Novel N-Aryl Carbamate Derivatives as Potential Fungicidal Agents. Agronomy 2025, 15, 2741. https://doi.org/10.3390/agronomy15122741
Liu X, Han Z, Duan X, Zhang J, Cai Y, Gao M, Song L, Huang B, Lin R. Synthesis, Bioevaluation and Structure-Activity Relationships of Novel N-Aryl Carbamate Derivatives as Potential Fungicidal Agents. Agronomy. 2025; 15(12):2741. https://doi.org/10.3390/agronomy15122741
Chicago/Turabian StyleLiu, Xiyao, Zhonghao Han, Xufei Duan, Jiajun Zhang, Yanyan Cai, Meili Gao, Liyan Song, Binbin Huang, and Ran Lin. 2025. "Synthesis, Bioevaluation and Structure-Activity Relationships of Novel N-Aryl Carbamate Derivatives as Potential Fungicidal Agents" Agronomy 15, no. 12: 2741. https://doi.org/10.3390/agronomy15122741
APA StyleLiu, X., Han, Z., Duan, X., Zhang, J., Cai, Y., Gao, M., Song, L., Huang, B., & Lin, R. (2025). Synthesis, Bioevaluation and Structure-Activity Relationships of Novel N-Aryl Carbamate Derivatives as Potential Fungicidal Agents. Agronomy, 15(12), 2741. https://doi.org/10.3390/agronomy15122741








