Abstract
The dual benefit of wastewater and microalgal biomass is a major advantage of high-rate algal ponds, enabling the environmental valorization of these byproducts. This research explored the effect of treated wastewater on the agri-food species Hordeum vulgare (L.) and its associated weed, Emex spinosa (L.) Campd., along with the effects of algal biomass (primarily composed of Closterium, Chlorella, and Scenedesmus spp.) and Diplotaxis harra leaf powder. Initial pot trials applied microalgae and D. harra at 2, 4, and 6 g·kg−1 soil, also confirming that the treated wastewater met reuse standards and did not affect plant growth. The combined treatment at 4 g·kg−1 led to the highest H. vulgare increases in fresh weight (162.71%), root length (73.75%), and shoot length (72.87%), while reducing E. spinosa shoot and root lengths by 30.79% and 52.18%, and fresh weight by 68.24%. Subsequent field experiments using 1.26 t ha−1 of 0.5-cm-applied D. harra and microalgae powders enhanced H. vulgare growth, while reducing the growth of E. spinosa. The reduction in E. spinosa growth was associated with increased electrolyte leakage and malondialdehyde content. These results support the integration of high-rate algal ponds into agriculture, promoting water reuse and reducing reliance on synthetic fertilizers and herbicides in barley production.
1. Introduction
Water-related challenges are critical issues that must be addressed sustainably in ecosystems worldwide, with problems such as water scarcity and the consequences of climate change exerting increasing pressure on natural resources [1,2,3]. Agriculture, as the largest water-consuming sector, accounting for approximately 70% of global use, faces increasing competition from domestic and industrial demands [4]. At the same time, shifts in precipitation patterns, rising temperatures, and extreme events such as droughts and floods are reducing agricultural productivity. The reuse of treated wastewater has emerged as a viable strategy to expand water availability, improve crop yields, and generate socioeconomic benefits [5].
High-rate algal ponds represent a sustainable wastewater treatment option based on the symbiotic relationship between microalgae and bacteria. Microalgae assimilate nitrogen and phosphorus from effluents, fix carbon dioxide, and release oxygen that supports bacterial degradation of organic matter [6]. In addition to enabling the reuse of water in irrigation, this process produces algal biomass with potential as a renewable biofertilizer, improving soil fertility, nutrient uptake, pathogen resistance, and water-holding capacity [7,8]. Complementary to this approach, allelopathy offers natural means to promote crop growth and manage weeds. Allelopathic plants release bioactive secondary metabolites that can suppress weed growth, alleviate abiotic stress, and stimulate beneficial soil interactions [9,10,11,12,13]. Diplotaxis harra, which contains flavonoids, glucosinolates, phytosterols, and phenolic acids, has shown phytotoxic effects relevant to weed control [14,15].
Barley (Hordeum vulgare L.), ranked fourth in cultivation after wheat, rice, and maize, has historically been grown for human consumption, being currently extensively cultivated for multiple purposes, including forage, livestock feed, human nutrition, and malt production [16]. Despite its versatility and global significance, effective weed control in barley cultivation remains a major challenge. This is largely due to the growing issue of herbicide-resistant weed species and the aggressive dominance of competitors such as Emex spinosa (Devil’s thorn) [17]. The rapid reproductive and vegetative growth and persistent spiny seeds of E. spinosa facilitate its dominance in winter cereal systems, highlighting the need for integrated control strategies.
This study aimed to evaluate the effect of treated wastewater from a high-rate algal pond on the growth of H. vulgare and the associated weed E. spinosa, and develop a sustainable method to promote barley growth and control E. spinosa through the combined use of microalgal biomass and Diplotaxis harra leaf powder. Pot and field experiments were conducted to assess the efficiency of these treatments and investigate their modes of action.
2. Materials and Methods
2.1. Study Area, Microalgal Biomass, and Treated Wastewater Characterization
The study was conducted at the Higher Agronomic Institute of Chott Mariem, Sousse, Tunisia (latitude 35°56′8″ N, longitude 10°33′26″ E). This region is characterized by low and irregular precipitation, typically less than 361 mm per year, with two peak periods: one in winter (November-December) and another in spring (March). The average annual temperature is 18.3 °C. The main weather parameters were obtained from the meteorological station (WS-GP2 Advanced Weather Station, Delta-T Devices Ltd., Cambridge, UK) located at the university campus.
An agronomic assessment was conducted using treated effluent from the newly established pilot-scale wastewater treatment plant (WWTPP) at the Higher Institute of Agronomy in Chott Mariem. The plant is designed to treat a specific amount of the domestic wastewater generated on campus, originating from student housing, the campus restaurant, and the academic residence. It operates through a two-stage treatment process arranged in series: an aerated pond followed by a high-rate algal pond (HRAP), forming a compact and efficient secondary treatment system [18]. It was designed for 100 inhabitants with an average daily flow of 10 m3 d−1.
The treated effluent is collected in a dedicated storage tank designed for reuse within the campus site, particularly for agricultural applications. To minimize thermal fluctuations and inhibit potential algal regrowth or microbial degradation, the tank is strategically placed in a shaded area, ensuring more stable water quality prior to irrigation use.
During the experimental period, water irrigation was collected at the storage tank. A composite water sample was analyzed every fifteen days over a four-month period to assess its physicochemical characteristics and heavy metal content (from October to November 2023 for pot experiments, and from December 2023 to January 2024 for field trials). Water quality parameters included Biochemical Oxygen Demand (BOD5), Chemical Oxygen Demand (COD), Suspended Solids (SS), Electrical Conductivity (EC), pH, ammoniacal nitrogen (NH4-N), phosphate (PO4-P), and nitrate (NO3-N), which were performed in triplicate according to standard methods [19].
Heavy metals (Cd, Cr, Cu, Fe, Hg, Ni, Pb, and Zn) and nutrient (Ca, Mg, and Na) contents in treated wastewater were determined by inductively coupled plasma mass spectrometer (ICP-MS) (Thermo Scientific, iCAP RQ, Waltham, MA, USA) in accordance with the guidelines from ISO 17294-2 [20].
In order to assess biopesticide and biofertilizer potentials of microalgal biomass, those were separated from wastewater by centrifugation at 6000× g for 20 min and frozen at (−20 °C) until use. Prior to the experiments, the algal biomass was thawed and dried in the dark to preserve its integrity, then promptly used for the assays. The dried algal biomass, mainly composed by Closterium, Chlorella, and Scenedesmus spp., was characterized in terms of nitrogen according to the Kjeldahl method and heavy metals content (Cd, Cu, Ni, Pb, Zn, Fe, and Hg). For heavy metal analysis, algal biomass samples were pre-treated according to the EPA 3051A method [21] using a microwave oven system for digestion, followed by heavy metal measurements by ICP-MS (Thermo Scientific, iCAP RQ, Waltham, MA, USA). Characterization of the algal biomass and heavy metal analysis were conducted at the Department of Science and Environmental Management, SPHERES Research Unit, University of Liège. Nitrogen content was expressed as a percentage of fresh weight, while heavy metal content was expressed in milligrams per kilogram of dry weight (mg/kg DW).
2.2. Plant Material
The sampling of E. spinosa grains was conducted on 19 June 2023 from an infested field situated in the Higher Agronomic Institute of Chott Mariem. D. harra leaves (flowering stage) were collected on 23 March 2023 from the region of Sousse, Tunisia. Species were identified by Messaoud Mars, Professor at the Higher Agronomic Institute of Chott Mariem. D. harra leaves were dried in in darkness, and the resulting powder was stored in paper bags. H. vulgare seeds, variety “Imen”, were used for the experiments (registered in 2013; JORT NO. 12 of 8 December 2013; bred and maintained by INRAT).
2.3. Pot Experiments
One pre-germinated seed of H. vulgare was planted at a depth of 3 cm in each pot, which measured 16 cm in diameter. A single seed of E. spinosa was also included in every pot at a depth of 3.5 cm. A blend consisting of 20% perlite, 35% sand, and 45% Klasmann’s Potgrond-H peat was used to fill the pots. The experimental setup consisted of a series of treated pots, as Table 1 presents.

Table 1.
Experimental design of pot experiments with Hordeum vulgare and Emex spinosa.
The powders of D. harra leaf and microalgae were applied at a soil depth of 0.5 cm. Over the course of the two-month trial period, the treated pots were watered with treated wastewater every two days (300 mL per pot) to keep them moistened. Control pots contained powder-free soil and were irrigated with 300 mL of tap water every two days. The pots were maintained outdoors under natural environmental conditions at the Higher Agronomic Institute of Chott Mariem, Tunisia, starting from 1 October 2023. The irrigation volume and frequency were selected to maintain adequate soil moisture without causing waterlogging. The aim was to keep the pots moistened, that is, to maintain the soil at a moisture level sufficient to support plant growth and metabolic activity, without reaching saturation. This irrigation schedule was based on the small pot size, the composition of the soil mixture (which provides good drainage and moderate water retention), and the outdoor environmental conditions. These combined factors justified the use of 300 mL of treated wastewater every two days, which helped maintain a consistent soil moisture regime suitable for plant development throughout the experiment. Two months after sowing, root and shoot lengths, as well as fresh weights of both E. spinosa and H. vulgare, were measured. The measurements were carried out during the vegetative phase of H. vulgare, specifically at the tillering stage. Then, 10 pots were used as replicates for each treatment.
2.4. Field Experiments
To assess the effect of D. harra and microalgae on the coexisting plants, E. spinosa and H. vulgare, a tilled field was chosen. The field, located at the Higher Agronomic Institute of Chott-Mariem, featured soil composed of 24.34% clay, 63% sand, and 12.66% silt, with an organic matter content of 1.82% and a pH of 7.32. On 3 December 2023, the field sowed with E. spinosa grains and pregerminated H. vulgare seeds was sectioned into two plots, each measuring 40 m2. One plot was designated as the control, with E. spinosa weeds left untreated alongside H. vulgare. In the second plot, following sowing, a mixture of dried D. harra leaf and microalgae powders was distributed at a soil depth of 0.5 cm. The powders were used in equal proportions to achieve a total application rate of 1.26 t ha−1. Each 40 m2 plot was further subdivided into 10 4 m2 sub-plots, each one functioning as a replicate. Within each sub-plot, 20 grains of E. spinosa and 20 seeds of H. vulgare were planted. During the experiment, treated and control plants were irrigated with 9 L/m2 of water every two days, using treated wastewater and tap water, respectively. While irrigation in the pot trials were managed with 300 mL every two days to maintain adequate moisture in the pots, the field trial involved irrigation at a larger scale and under greater exposure to field-scale environmental variability. The irrigation schedule in the field was designed based on the sandy clay loam soil, which combines relatively high sand content with a moderate proportion of clay, resulting in moderate drainage and water retention capacity. The organic matter content (1.82%) provided limited but beneficial moisture retention, contributing to the decision to irrigate at this frequency and volume. Additional factors considered included the cool winter conditions during December and January, when precipitation is typically adequate but variable. Irrigation every two days with 9 L/m2 of water ensured consistent soil moisture levels throughout the experimental period. After two months, measurements of fresh weight and root and shoot length were taken for both E. spinosa and H. vulgare. Measurements were taken at the tillering stage of H. vulgare, in the vegetative phase.
2.5. Physiological Responses on Hordeum vulgare and Emex spinosa
H. vulgare and E. spinosa leaves and roots (harvested from pots treated with D. harra leaf powder and microalgae applied to the soil at a depth of 0.5 cm, at 4 g·kg−1 soil) were used in the subsequent assays after rinsing with distilled water. The samples were collected on 1 December 2023. For proline content and DPPH scavenging assay, the samples were ground into a fine powder after being dried in a well-ventilated dark room. The powder was kept in paper bags until needed. For the metabolic activity and electrolyte leakage assays, fresh leaves and roots were used immediately after collection. For lipid peroxidation analysis, samples were weighed and then stored at −20 °C until use.
2.5.1. Electrolyte Leakage
At room temperature and in the dark, roots and leaves from fresh H. vulgare and E. spinosa seedlings (both treated and control) were homogenized with 25 mL of distilled water. After 24 h, the initial electrical conductivity of the bathing solution (L1) was measured using a digital conductivity meter (model BCT-4308, Lutron Electronic Entreprise Co., Ltd., Taiwan). The samples were then autoclaved for 20 min at 121 °C and re-incubated in distilled water under the same conditions. A second conductivity measurement (L2) was taken after an additional 24 h [22]. The electrolyte leakage (EL) was calculated using Equation (1).
EL (%) = (L1/L2) × 100
2.5.2. Lipid Peroxidation
Using a mortar kept on ice, 250 mg of samples were triturated with 0.05 g of polyvinylpyrrolidone (PVP) (Shanghai Yuking Water Soluble Material Tech Co., Ltd., Shanghai, China) and 2.5 mL of 67 mM phosphate buffer (pH 7) (Thermo Fisher Scientific, Waltham, MA, USA). After centrifugation for 15 min at 4 °C and 2000× g, the supernatant was used to assess lipid peroxidation. To 750 µL of the prepared extract, 3 mL 0.5% thiobarbituric acid (ITW Reagents, Barcelona, Spain) (dissolved in 20% trichloroacetic acid (Merck KGaA, Darmstadt, Germany)) was added. The mixture was then heated at 90 °C for 10 min and quickly cooled in an ice-bath. Following this, the samples were centrifuged, and the absorbance of the supernatant was measured at 532 and 600 nm. The concentration of malondialdehyde (MDA) was determined using an extinction coefficient of 155 mM−1 cm−1 [23].
2.5.3. Proline Content
A total of 10 mg of dried roots and leaves of E. spinosa and H. vulgare were combined with 1.5 mL of 3% aqueous sulfosalicylic acid (w/v) (Merck KGaA, Darmstadt, Germany). The resulting homogenate was centrifuged at 14,000 rpm for 10 min. Subsequently, 1 mL of glacial acetic acid (Merck KGaA, Darmstadt, Germany) and 1 mL ninhydrin reagent (prepared by dissolving 1.25 g ninhydrin in 30 mL glacial acetic acid and 20 mL of 6 M H3PO4 (Merck KGaA, Darmstadt, Germany)) were added to 1 mL of each supernatant from the homogenates. This mixture was incubated for 1 h at 100 °C. The tubes were then cooled in an ice bath, and 2 mL of toluene (Merck KGaA, Darmstadt, Germany) was vigorously mixed with the reaction mixture for 20 s. The absorbance of the resulting upper pink-red phase was measured at 520 nm, with toluene serving as the blank [24]. Proline concentration was determined using a calibration curve established with a series of proline solutions (0–1 mg/mL).
2.5.4. DPPH Scavenging Assay
To prepare E. spinosa and H. vulgare leaves and roots samples for DPPH scavenging assay, distinct stock mixtures were formulated using microcrystalline cellulose (Roquette Frères., Lestrem, France) at 500 mg/g. These stock mixtures were subsequently diluted with additional cellulose following a series of solid dilutions to reach a concentration of 15 mg/g [10].
Next, 10 mg of cellulose (used as a control) or test samples were combined with 1 mL of DPPH methanolic solution (prepared at 30 mg/L and adjusted to an optical density between 0.750 and 0.8 nm) and 0.5 mL of methanol (Merck KGaA, Darmstadt, Germany). After incubation at room temperature for 2 h, the optical density was measured at 517 nm [25]. The percentage inhibition of DPPH free radicals (PI) was then calculated using Equation (2).
PI = (Acontrol − Aextract/Acontrol) × 100
- PI: percentage inhibition.
- DOcontrol: Absorbance of the control reaction.
- DOextract: Absorbance in the presence of E. spinosa or H. vulgare extract.
The variation in PI with respect to extract concentration enables the determination of the concentration required for 50% inhibition (IC50). A lower IC50 value (µg/mL) indicates a higher efficacy to the extract.
2.5.5. Metabolic Activity
Fresh leaves and roots (100 mg) were incubated in the dark at 37 °C for 4 h in 5 mL of a 0.2% TTC (2,3,5-triphenyltetrazolium chloride) solution (pH 7) (Merck KGaA, Darmstadt, Germany). Following incubation, 0.5 mL of 1 M sulfuric acid (Merck KGaA, Darmstadt, Germany) was added to each sample. After rinsing with distilled water, the samples were quickly dried between filter papers, then ground in a mortar with 3.5 mL of ethyl acetate (Merck KGaA, Darmstadt, Germany) on ice. The mixture was filtered through Whatman No. 1 paper, and the volume was adjusted to 7 mL with ethyl acetate. Absorbance was measured at 485 nm [26]. The formazan content was determined using Equation (3).
Formazan content (%) = DO485 treatment/DO485 control
2.6. Statistical Analysis
Experiments were conducted using a completely randomized design. Except for field and pot trials, where 10 plots (with 200 measurements per plot) and 10 pots were used, respectively, as replicates, the data were presented as mean ± standard error (S.E.) from three replicates. To evaluate differences between treatments, one-way ANOVA and Duncan tests were applied using IBM SPSS Statistics version 20.00 for Windows. Statistical significance was determined at the 5% level (p < 0.05). Normality of the data was assessed using the Shapiro–Wilk test at a 5% significance level (p < 0.05).
3. Results
3.1. Irrigation Water Composition and Microalgae Characterization
The composition of treated wastewater used for irrigation is shown in Table 2. The analyzed parameters, including heavy metals, comply with the requirements of the World Health Organization (WHO) [27] and the Tunisian standard (NT 106.03) [28] for the reuse of wastewater in agriculture with restricted crop irrigation. Therefore, the water is potentially suitable for irrigation.

Table 2.
Average quality of treated effluent and limit values for the reuse of treated wastewater [27,28].
Even if the limit for mercury was respected, since the detected concentration of 0.0002 mg/L is below the limit of 0.001 mg/L, its toxicity, persistence, and ability to bioaccumulate mean it could pose a long-term risk. Therefore, it is not an immediate food security threat, but it warrants precaution and ongoing monitoring to prevent future problems. To mitigate these risks, it is important to adopt solutions such as using alternate irrigation sources to prevent long-term accumulation of mercury in the soil. Additionally, implementing crop rotation and soil monitoring can help limit mercury buildup and reduce its potential impact on food safety and security.
Furthermore, the results indicated that the irrigation water contains low to moderate levels of nutrients, with a mean concentration of ammonium ion (NH4+) of 0.9 mg L−1, an average nitrate (NO3−) value of 2.7 mg L−1, and an average orthophosphate (PO4) content of 1 mg L−1 (Table 2).
Regarding microalgae characterization, the nitrogen accounted for 5% of the fresh weight. Moreover, the concentration of heavy metals in algae is in accordance with the European regulation (EC, 2019/1009) on fertilizer products [29] (Table 3). The concentrations measured in algal biomass followed this order: Cd < Hg < Pb < Ni< Cu < Zn < Fe. Iron is the most dominant heavy metal in algal biomass, with a mean concentration of 1858 mg/kg DW.

Table 3.
Biomass algae characterization [29].
3.2. Pot Experiments
Irrigation with treated wastewater had no significant effect on the growth of H. vulgare and E. spinosa, and neither stimulatory nor inhibitory effects were observed. Shoot and root lengths, as well as fresh weight of the species studied, were similar to those of the control samples (Figure 1).
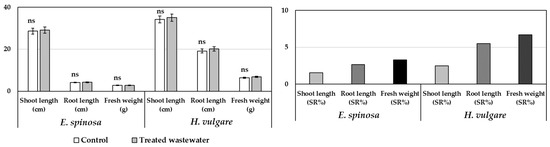
Figure 1.
Fresh weight (g) and shoot and root length (cm) of Emex spinosa and Hordeum vulgare (two-month-old seedlings), along with percentage stimulation or reduction in growth (SR%), following irrigation with treated wastewater in pot experiments. Measurements were taken on 1 December 2023. Error bars represent standard errors (SE). Data are means ± SE (n = 10). ns: There was no statistically significant difference between the treatment group and the control group (p > 0.05, Duncan test).
The response of E. spinosa (Figure 2) and H. vulgare (Figure 3) to the microalgae treatment, whether applied alone or in combination with D. harra, varied depending on the type of treatment, the plant species, and the applied dose.
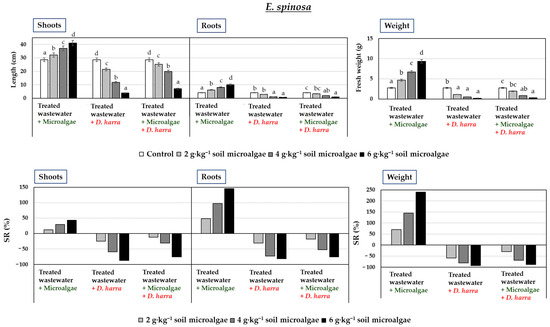
Figure 2.
Fresh weight (g) and shoot and root length (cm) of Emex spinosa (two-month-old seedlings), along with percentage stimulation or reduction in growth (SR%), following irrigation with treated wastewater and soil application of microalgae and Diplotaxis harra leaf powders at a depth of 0.5 cm (applied individually or in a 1:1 combination at 2, 4, and 6 g·kg−1 soil) in pot experiments. Pots irrigated with tap water and without powder served as controls. Measurements were taken on 1 December 2023. Error bars represent standard errors (SE). Data are means ± SE (n = 10). Means sharing one or more letters are not significantly different (p < 0.05, Duncan test).
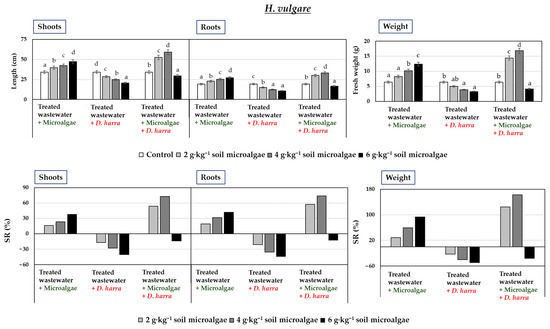
Figure 3.
Fresh weight (g) and shoot and root length (cm) of Hordeum vulgare (two-month-old seedlings), along with percentage stimulation or reduction in growth (SR%), following irrigation with treated wastewater and soil application of microalgae and Diplotaxis harra leaf powders at a depth of 0.5 cm (applied individually or in a 1:1 combination at 2, 4, and 6 g·kg−1 soil) in pot experiments. Pots irrigated with tap water and without powder served as controls. Measurements were taken on 1 December 2023. Error bars represent standard errors (SE). Data are means ± SE (n = 10). Means sharing one or more letters are not significantly different (p < 0.05, Duncan test).
Results indicated that treatments with only microalgae was not an effective control strategy against E. spinosa, as its growth notably increased in a dose-dependent way, with each dose applied for all the parameters measured (Figure 2). In the case of H. vulgare, dose-dependent increases in all the parameters were also observed (Figure 3).
Treatments with D. harra alone had the most significant negative impact on E. spinosa and H. vulgare growth, leading to reductions in both shoot and root length, as well as fresh weight, at all doses (Figure 2 and Figure 3). At 6 g·kg−1 soil, percentages of reduction in these parameters were of 86.43%, 82.03%, and 92.33% for E. spinosa, and 39.95%, 43.80%, and 48.98% for H. vulgare.
The combined application of microalgae with D. harra resulted in simultaneous promotion of the growth of H. vulgare and reduction of E. spinosa when tested at 2 g·kg−1 soil and 4 g·kg−1 soil (Figure 2 and Figure 3). In fact, at 4 g·kg−1 soil, the treatment produced the greatest increase in H. vulgare shoot length (72.87%), root length (73.75%), and fresh weight (162.71%). This treatment also had a potential negative influence on the target weed E. spinosa, causing reductions of 30.79% in shoot length, 52.18% in root length, and 68.24% in fresh weight. Interestingly, the highest tested dose of the treatment (6 g·kg−1 soil) led to a decrease in H. vulgare growth, with reductions of 13.70% in shoot length, 12.49% in root length, and 35.41% in fresh weight.
3.3. Field Experiments
To assess the effects on H. vulgare and E. spinosa growth in field trials (with irrigation using treated wastewater), D. harra leaves and microalgae powders were applied to the soil at a 0.5 cm depth, at 1.26 t ha−1 soil. Consistent with the results from pot trials, this treatment enhanced H. vulgare growth. The stimulations were 76.66% for shoot length, 80.43% for root length, and 170.89% for fresh weight (Table 4). Additionally, the treatment demonstrated its ability to diminish E. spinosa growth, resulting in reductions of 38.25%, 61.77%, and 72.37% in shoot length, root length, and fresh weight, respectively (Table 4).

Table 4.
Biological parameters of Emex spinosa and Hordeum vulgare (two-month-old seedlings) measured after irrigation with treated wastewater and the application of microalgae and Diplotaxis harra leaf powders to the soil at a 0.5 cm depth (applied in combination in equal ratio to have a total of 1.26 t ha−1). Nonpowdered and tap water irrigated seedlings were used as the control. The measured parameters were determined on 3 February 2024.
3.4. E. spinosa Physiological Response to D. harra Leaves and Microalgae Powders
3.4.1. Electrolyte Leakage and Lipid Peroxidation
Electrolyte leakage increased by 163.66% in treated weed roots and by 92.66% in treated leaves (Figure 4). The measured values were 480.95% and 303.79% for the treated samples compared to 182.41% and 157.68% in the control group.
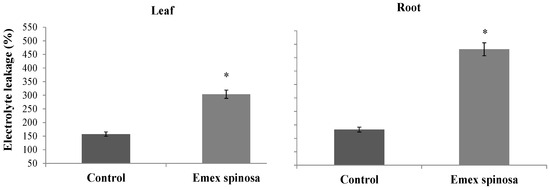
Figure 4.
Electrolyte leakage (%) from roots and leaves of Emex spinosa after irrigation with treated wastewater and application of a 1:1 mixture of microalgae and Diplotaxis harra leaf powders to the soil at a depth of 0.5 cm (total dose: 4 g·kg−1 soil) in pot trials. Pots irrigated with tap water and without powder served as controls. Samples were collected from two-month-old seedlings on 1 December 2023. Error bars represent standard error. Values are means ± SE (n = 3). Statistical analysis was performed using ANOVA; * indicates significant differences (p < 0.01).
MDA content was improved by 94.67% and 134.70% for leaves and roots, respectively, after E. spinosa treatment. The treated samples showed values of 145.48 and 100.43 nmol MDA/g FW, in contrast to 74.73 and 42.79 nmol MDA/g FW for the control (Figure 5).
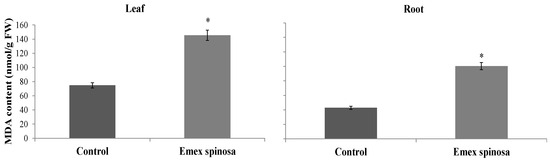
Figure 5.
Malondialdehyde (MDA) content (μmol·g−1 FW) in roots and leaves of Emex spinosa following irrigation with treated wastewater and application of a 1:1 mixture of microalgae and Diplotaxis harra leaf powders to the soil at a depth of 0.5 cm (total dose: 4 g·kg−1 soil) in pot trials. Pots irrigated with tap water and without powder served as controls. Samples were collected from two-month-old seedlings on 1 December 2023. Error bars represent standard error. Values are means ± SE (n = 3). Statistical analysis was performed using ANOVA; * indicates significant differences (p < 0.01).
3.4.2. Content of Proline
E. spinosa seedlings cultivated in soil containing microalgae and D. harra leaf powders showed increased proline levels in both roots and leaves (Figure 6). Specifically, root proline content increased by 32.07% compared to the control, reaching 90.16 µmol/g DW and 68.27 µmol/g DW. In leaves, proline accumulation was enhanced by 16.71% relative to the control. Values were, respectively, 103.08 µmol/g DW and 88.32 µmol/g DW (Figure 6).
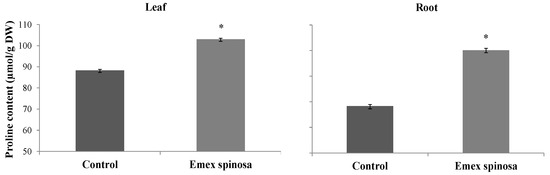
Figure 6.
Proline content (μmol·g−1 DW) in roots and leaves of Emex spinosa following irrigation with treated wastewater and application of a 1:1 mixture of microalgae and Diplotaxis harra leaf powders to the soil at a depth of 0.5 cm (total dose: 4 g·kg−1 soil) in pot trials. Pots irrigated with tap water and without powder served as controls. Samples were collected from two-month-old seedlings on 1 December 2023. Error bars represent standard error. Values are means ± SE (n = 3). Statistical analysis was performed using ANOVA; * indicates significant differences (p < 0.01).
3.4.3. DPPH Free Radical-Scavenging
Table 5 presents the results of radical scavenging assays for weed samples, which are expressed as the extract concentration required to neutralize 50% of the DPPH free radicals in the test solutions (μg/mL). The data indicated that treated E. spinosa samples exhibited a greater free radical scavenging capacity compared to the control samples. Specifically, the values were 288.4 µg/mL for treated roots and 375.8 µg/mL for treated leaves, whereas the control samples had values of 456.76 µg/mL and 445.93 µg/mL, respectively.

Table 5.
DPPH free radical-scavenging activity in leaves and roots of Emex spinosa and Hordeum vulgare following irrigation with treated wastewater and application of a 1:1 mixture of microalgae and Diplotaxis harra leaf powders to the soil at a depth of 0.5 cm (total dose: 4 g·kg−1 soil) in pot trials. Pots irrigated with tap water and without powder served as controls. Samples were collected from two-month-old seedlings on 1 December 2023.
3.4.4. Metabolic Activity
Table 6 shows the content of formazan in treated E. spinosa leaves and roots, expressed as a percentage of the control. The findings showed a decline in formazan levels, with a 44.07% reduction in treated leaves and a 68.24% reduction in treated roots, in comparison to the control samples.

Table 6.
Formazan content (expressed as % of control) in Emex spinosa and Hordeum vulgare following irrigation with treated wastewater and application of a 1:1 mixture of microalgae and Diplotaxis harra leaf powders to the soil at a depth of 0.5 cm (total dose: 4 g·kg−1 soil) in pot trials. Pots irrigated with tap water and without powder served as controls. Samples were collected from two-month-old seedlings on 1 December 2023.
3.5. H. vulgare Physiological Response to D. harra Leaf and Microalgae Powders
3.5.1. Electrolyte Leakage and Lipid Peroxidation
H. vulgare treatment with microalgae and D. harra powders resulted in a decrease in electrolyte leakage, with reductions of 44.69% for roots and 27.89% for leaves compared to the control. The treatment led to electrolyte leakage levels of 165.07% for roots and 137.76% for leaves, whereas the control values were 98.47% and 191.06%, respectively (Figure 7). Additionally, there was a decrease in MDA content, with reductions of 50.28% for roots and 37.90% for leaves. The MDA levels were 18.92 nmol MDA/g FW for roots and 38.92 nmol MDA/g FW for leaves in the treated samples, compared to 38.06 nmol MDA/g FW and 62.68 nmol MDA/g FW in the control samples (Figure 8).
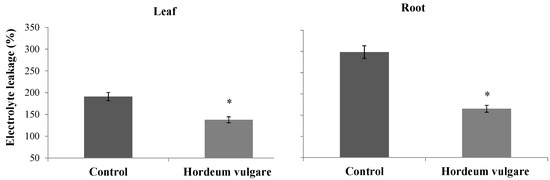
Figure 7.
Electrolyte leakage (%) from roots and leaves of Hordeum vulgare following irrigation with treated wastewater and application of a 1:1 mixture of microalgae and Diplotaxis harra leaf powders to the soil at a depth of 0.5 cm (total dose: 4 g·kg−1 soil) in pot trials. Pots irrigated with tap water and without powder served as controls. Samples were collected from two-month-old seedlings on 1 December 2023. Error bars represent standard error. Values are means ± SE (n = 3). Statistical analysis was performed using ANOVA; * indicates significant differences (p < 0.01).
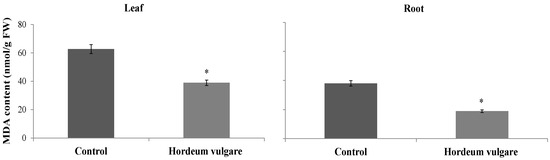
Figure 8.
Malondialdehyde (MDA) content (μmol·g−1 FW) in roots and leaves of Hordeum vulgare following irrigation with treated wastewater and application of a 1:1 mixture of microalgae and Diplotaxis harra leaf powders to the soil at a depth of 0.5 cm (total dose: 4 g·kg−1 soil) in pot trials. Pots irrigated with tap water and without powder served as controls. Samples were collected from two-month-old seedlings on 1 December 2023. Error bars represent standard error. Values are means ± SE (n = 3). Statistical analysis was performed using ANOVA; * indicates significant differences (p < 0.01).
3.5.2. Content of Proline
The impact of microalgae and D. harra leaf powders on proline content in H. vulgare roots and leaves is represented in Figure 9. Results indicated that there was no significant variation in proline content between treated and control samples. For treated roots, the proline content was comparable to that of the control. The values were 51.04 µmol/g DW for treated roots and 50.18 µmol/g DW for the control.
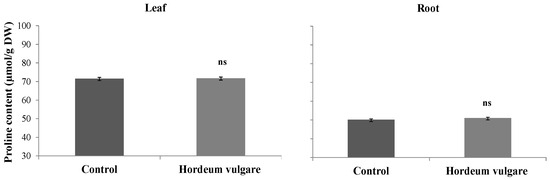
Figure 9.
Proline content (μmol·g−1 DW) in roots and leaves of Hordeum vulgare following irrigation with treated wastewater and application of a 1:1 mixture of microalgae and Diplotaxis harra leaf powders to the soil at a depth of 0.5 cm (total dose: 4 g·kg−1 soil) in pot trials. Pots irrigated with tap water and without powder served as controls. Samples were collected from two-month-old seedlings on 1 December 2023. Error bars represent standard error. Values are means ± SE (n = 3). Statistical analysis was performed using ANOVA; ns indicates no significant differences (p < 0.01).
For the treated leaves, the proline content was 71.71 µmol/g DW, while it was 71.53 µmol/g DW in the control leaves.
3.5.3. DPPH Free Radical-Scavenging
Results showed that the presence of microalgae and D. harra powders enhanced the free radical scavenging ability of H. vulgare treated organs. In fact, the values were 300.57 µg/mL and 283.68 µg/mL for treated roots and leaves, respectively, compared to 395.87 µg/mL and 333.57 µg/mL for controls (Table 5).
3.5.4. Metabolic Activity
Treated H. vulgare leaves and roots showed formazan levels that increased by 35.86% and 59.05%, respectively (Table 6).
4. Discussion
The effect of treated wastewater, microalgae, and D. harra leaf powders (applied in combination or separately) was evaluated on two coexisting species (H. vulgare and E. spinosa). The results in Table 2 suggest that the reuse of treated wastewater is unlikely to have a negative impact on plant growth, but they also indicate that its fertilizing potential is likely to be limited. Since irrigation water quality plays an important role in crop productivity, the levels of macro and micronutrients in wastewater are critical factors determining its impact on plant development [8]. The low nutrient content observed in the effluent from the WWTPP at the Chott Mariem university campus appears to stem from the initially low nutrient content of the HRAP influent [18]. Nonetheless, the comparable growth observed between the control and treated plants suggest that the treated wastewater could serve as a viable alternative to conventional water sources. It seems to provide sufficient nutrients to support plant growth without causing overstimulation or nutrient imbalances. Therefore, these results demonstrate that the effects on the weed and H. vulgare are due to the presence of D. harra and microalgae powders.
When applied individually, the powders produced contrasting effects on the growth of the target species, with microalgae powder stimulating growth while powdered leaves of D. harra caused significant inhibition. (Figure 1).
The impact of research with D. harra indicated allelopathic potentialities of this plant [14]. These potentialities play a key role in its invasive nature, which could explain its phytotoxicity on neighboring species. In fact, it can prevent their seed germination and their seedling growth [14].
Regarding microalgae characterization, the algae’s heavy metals concentration is in accordance with the European regulation on fertilizer products [29] (Table 3). Therefore, algal biomass could be an alternative to organo-mineral fertilizer due to its abundance in nitrogen, phosphorus, and micronutrients [30]. It was demonstrated that the amino acids furnish beneficial effects to plants [31]. Even in small concentrations, these amino acids are greatly beneficial for plants, enhancing their growth. Additionally, microalgae incorporate other appreciated molecules including carbohydrates and phytohormones (auxin-like and cytokinin-like). Phytohormones promote vegetal growth. Auxins, for example, considerably improve root development, thus enhancing the plant’s ability to absorb water and nutrients and leading to better tolerance to harmful stress conditions. In addition, microalgae are able to act as biopesticides, thereby protecting plants from pathogens (like bacteria and fungi) [31].
It is important that, despite the stimulating effect of microalgae, its mixed application caused reduction in the growth of E. spinosa (at all doses) and H. vulgare (at the highest dose, 6 g·kg−1 soil) (Figure 1). Nevertheless, treatment with D. harra resulted in a reduction in the growth of H. vulgare and weeds. However, this reduction was still greater compared to the effects observed when its leaf powder was mixed with microalgae (Figure 1). These results could be explained by the differential sensitivity of the target species. In fact, H. vulgare and E. spinosa may have different responses to substances released by microalgae and D. harra. H. vulgare was less sensitive to the inhibitory substances released by D. harra, which could explain why its growth was stimulated at low doses of microalgae, while E. spinosa is more strongly affected by these substances, showing inhibited growth at all doses. Such findings align with previous reports, suggesting that plants exhibit selectivity in their responses to phytotoxic compounds, which may account for the differences observed in our study [32].
The leaves of D. harra have been reported to be rich in phenolic compounds, with flavonoids (predominantly flavones and flavonols) comprising more than 50% of the total phenols [33]. Thus, this category of secondary metabolic products may be the source of the detrimental impact on the receptor plants. Potential ways in which flavonoids can participate in allelopathy can involve ATP production disruption, hindering auxins’ proper functioning and cell growth inhibition [34]. Furthermore, it was shown that many phytotoxins (such as phenolic compounds) could be generated through the breakdown of allelopathic plant materials present in the soil. These phenols have the ability to engender chemical and physiological effects on crop and weed growth [35].
The obtained results suggest that even if microalgae produce compounds that stimulate the growth of H. vulgare, the effects of these microalgae and of D. harra on this target plant could be specific to each combination of applied doses. Thus, the combination of D. harra powder and microalgae modified the effect on the growth of H. vulgare, possibly leading to a negative effect at high doses. Dose-dependent stimulation or inhibition of allelochemicals on plant growth has been consistently interpreted as evidence for allelopathic interactions [36].
Despite the phytotoxicity of D. harra, its combination with microalgae was more effective in enhancing H. vulgare growth than application of microalgae separately (only at 2 and 4 g·kg−1 soil) (Figure 1). These results indicate that D. harra contains secondary metabolites that enhance the stimulatory effect of microalgae on H. vulgare, consistent with evidence that combined application of natural products can improve plant growth through additive or synergistic effects [35]. The beneficial effect was not observed when D. harra leaf powder was applied alone, possibly due to the low concentration of active secondary metabolites in the leaves. For example, previous analyses have identified the presence of dihydroflavonols and flavanones (types of flavonoids) in D. harra leaves, although at relatively low amounts [33]. The combination of these compounds with others appears to amplify the beneficial effect of microalgae on crop growth, even though each compound alone is present at levels too low to elicit a significant response. Hence, a combination treatment may elevate the levels of bioactive compounds as well as the number of sites susceptible to these compounds [35].
To ensure that weed treatment does not harm crops, combining microalgae and D. harra powders at low doses proved more effective in promoting H. vulgare growth and suppressing E. spinosa compared to using the powders individually. Consequently, results from pot experiments revealed that applying the treatment at 4 g·kg−1 soil had the most substantial negative impact on E. spinosa and significantly enhanced the shoot and root length as well as the fresh weight of H. vulgare.
While pot trials conducted in natural settings can provide valuable insights into the effect of the 4 g·kg−1 soil treatment on target species, they may not entirely replicate the complex and dynamic conditions experienced by plants in the field. As a result, a combination of pot experiments and field trials was used to validate the effectiveness of the applied treatment. In fact, pots can create microclimates around the plants that differ from the surrounding field environment. Additionally, in the field, plants interact with neighboring vegetation, competing for resources such as light, water, and nutrients. Pots may not capture these competitive interactions accurately as they isolate individual plants from surrounding vegetation. Pots may offer some protection against certain pests and diseases compared to field-grown plants, leading to differences in plant performance. In our work, field trial confirmed pot experiment results. The treatment applied at 1.26 t ha−1 (4 g·kg−1 soil) exerted a negative effect on E. spinosa. It also resulted in H. vulgare growth enhancement (Table 4).
The powders of microalgae and D. harra were applied at a rate of 1.26 t ha−1 (4 g·Kg−1 soil) in the field as this dose provided the best overall results in the pot trial. At this concentration, the treatment significantly promoted the growth of H. vulgare, as shown by increased shoot length, root length, and fresh weight, indicating optimal crop vigor. At the same time, it effectively suppressed the growth of E. spinosa, thereby achieving the dual objective of enhancing barley performance and controlling weed infestation. In contrast, the highest dose of 6 g·kg−1 soil (1.89 t ha−1) resulted in reduced H. vulgare growth, even though weed suppression continued. For this reason, the 1.26 t ha−1 rate was selected for field application as it represented the optimal compromise, maximizing barley growth, minimizing weed presence, and maintaining effectiveness with a lower, more efficient dose. This rate stood out as the most effective and appropriate option based on the experimental outcomes.
Treated E. spinosa seedlings had improved proline content in roots and leaves (Figure 4). For H. vulgare, the contents were like those in the control (Figure 7). Consequently, it appears that E. spinosa refuged to defense mechanisms through proline accumulation to alleviate the negative effect of phytotoxic substances produced by D. harra. In fact, the proline content increase in plants is part of their adaptive responses to environmental stresses, helping them to survive and thrive under adverse conditions [37]. Proline protects macromolecules and cellular structures from damage caused by induced oxidative stress. It acts as a reactive oxygen species scavenger. It helps to stabilize proteins and membranes, thereby mitigating the detrimental effects of stress on cellular integrity [37].
Otherwise, results showed that treated organs of H. vulgare and E. spinosa are better equipped to accumulate and produce antioxidants, resulting in scavenging potential against DPPH radicals’ enhancement (Table 5). DPPH radical quenching effectiveness increase in treated weed organs could be another part of their adaptive response, added to proline content enhancement, to counteract cellular oxidative disturbances and prevent deterioration from free radicals. The beneficial effect of the 4 g·kg−1 soil treatment on H. vulgare growth is reflected in its improved DPPH free radical neutralizing capability, which signifies the plant’s enhanced ability to maintain cellular balance and manage oxidative stress. This heightened antioxidant capacity is a hallmark of plant growth stimulation. In this context, it was well established that effective regulation of oxidative stress in plants relies on a fine balance between the production and detoxification of reactive oxygen species (ROS) [38]. Hence, the intricate interplay between ROS generation and elimination is critical for plants to withstand oxidative stress and maintain cellular redox balance. A finely tuned antioxidant defense system, coupled with precise regulation of ROS production and signaling, enables plants to adapt to the environmental conditions changes and ensure their survival and growth [38].
In addition, results showed that H. vulgare treated organs exhibited strong structural and membrane integrity (demonstrated by electrolyte leakage and MDA content decrease) which is essential for cellular functions and ROS management (Figure 5 and Figure 6). This preservation of membrane integrity contributes to the overall antioxidant capacity of this plant, including DPPH radical scavenging activity. Previous studies have highlighted that both electrolyte leakage and MDA levels serve as key indicators of oxidative stress and membrane damage in plants [39]. There is a positive correlation between these two parameters under oxidative stress conditions. As membrane lipids undergo peroxidation, leading to membrane damage, the extent of electrolyte leakage increases due to compromised membrane integrity. Concurrently, the accumulation of MDA, a byproduct of lipid peroxidation, also increases. Therefore, higher MDA content is associated with elevated electrolyte leakage, indicating membrane damage [39]. Consequently, the treatment that increased DPPH radical scavenging activity in weed organs may not guarantee effective protection against oxidative stress, where multiple ROS species and oxidative pathways may be involved, leading to oxidative damage despite the apparent improvement in antioxidant capacity. E. spinosa growth decrease could be explained by its inability to resist the oxidative stress due to an imbalance between ROS generation and elimination.
Contrary to H. vulgare, the combination of D. harra and microalgae powders at 4 g·kg−1 soil affected the weed respiration (Table 6). Given that cellular respiration is the main pathway for ATP production (the universal energy currency), the rate of formazan formation serves as a useful proxy for assessing cellular metabolic activity and energy status [40]. Hence, formazan content decrease may indicate reduced cellular respiration [40]. It seems that the presence of the powders in soil can disrupt mitochondrial function, impairing the electron transport chain and reducing ATP production. As a result, respiration decrease could be among the causes of E. spinosa growth reduction.
To conclude, D. harra and microalgae may have complementary mechanisms of action. D. harra could provide allelopathic compounds that inhibit weed growth, while microalgae powder may contribute nutrients and organic matter to the soil, promoting better crop growth. Together, applied at 4 g·kg−1 soil (1.26 t ha−1) in equal ratio, they can create a more favorable environment for H. vulgare while controlling E. spinosa.
5. Conclusions
The study showed that microalgae-based wastewater treatment could provide an alternative water resource for irrigation but also a valuable source of microalgae that can be valorized in bio-agriculture. Proper management practices, including monitoring water quality and plant responses, are essential to minimize potential risks and optimize the benefits of treated wastewater irrigation for crop production.
Combining D. harra and microalgae powders (at 4 g·kg−1 soil) for E. spinosa control and H. vulgare growth enhancement may reduce the need for synthetic herbicides and fertilizers, thus minimizing the environmental impact associated with conventional agricultural practices. This combination could offer selective broad-leaf weed control. The specific mechanism appears to involve oxidative stress and mitochondrial dysfunction, as evidenced by elevated MDA levels, increased electrolyte leakage (indicating membrane damage), and reduced formazan content (suggesting impaired mitochondrial respiration). Bioactive compounds present in the treatment may selectively affect dicotyledonous species more than monocotyledonous species due to differences in cellular structure, metabolism, or detoxification pathways.
To confirm the potential for selective weed control, it would be essential to test this combination against a broader range of broad-leaf weeds and crops. Additionally, comparative trials with locally used broad-leaf herbicides would help determine its relative efficacy and potential for integration into existing weed management programs.
By integrating treated wastewater management with microalgae cultivation or recuperation, communities could achieve multiple benefits including water conservation and sustainable agriculture.
Author Contributions
Conceptualization, G.J. and C.K.; methodology, G.J. and C.K.; software, G.J. and J.G.Z.; validation, G.J., C.K., J.G.Z., F.Z., H.J., A.M. and B.T.; formal analysis, G.J., C.K. and J.G.Z.; investigation, G.J.; resources, C.K.; data curation, G.J., C.K. and J.G.Z.; writing—original draft preparation, G.J.; writing—review and editing, G.J., C.K. and J.G.Z.; visualization, G.J. and J.G.Z.; supervision, C.K., J.G.Z., H.J. and B.T.; project administration, G.J., C.K., F.Z., H.J. and B.T.; funding acquisition, C.K., F.Z. and B.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received a specific grant from Wallonie Bruxelles International agency, Belgium, under the project “Traitement des eaux usées II” (ref 1.1.5) between Higher Agronomic Institute of Chott Meriem and Liège University.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
Authors would like to thank Wallonie Bruxelles International agency and the University of Cadiz for their valuable support and collaboration.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BOD5 | Biochemical oxygen demand |
| COD | Chemical oxygen demand |
| DW | Dry weight |
| HRAP | High-rate algal pond |
| MDA | Malondialdehyde |
| PE | Population equivalent |
| ROS | Reactive oxygen species |
| WHO | World health organization |
| WWTPP | Pilot-scale wastewater treatment plant |
References
- Jones, E.R.; Bierkens, M.F.; van Vliet, M.T. Current and future global water scarcity intensifies when accounting for surface water quality. Nat. Clim. Change 2024, 14, 629–635. [Google Scholar] [CrossRef]
- Zorrilla, J.G.; Siciliano, A.; Petraretti, M.; Saviano, L.; Spampinato, M.; Cimmino, A.; Guida, M.; Pollio, A.; Bravi, S.; Masi, M. Ecotoxicological assessment of cyclic peptides produced by a Planktothrix rubescens bloom: Impact on aquatic model organisms. Environ. Res. 2024, 257, 119394. [Google Scholar] [CrossRef] [PubMed]
- Karimidastenaei, Z.; Avellán, T.; Sadegh, M.; Kløve, B.; Haghighi, A.T. Unconventional water resources: Global opportunities and challenges. Sci. Total Environ. 2022, 827, 154429. [Google Scholar] [CrossRef]
- Mojid, M.A.; Mainuddin, M.; Murad, K.F.I.; Mac Kirby, J. Water usage trends under intensive groundwater-irrigated agricultural development in a changing climate–Evidence from Bangladesh. Agric. Water Manag. 2021, 251, 106873. [Google Scholar] [CrossRef]
- Lopes, A.R.; Becerra-Castro, C.; Vaz-Moreira, I.; Silva, M.E.F.; Nunes, O.C.; Manaia, C.M. Irrigation with treated wastewater: Potential impacts on microbial function and diversity in agricultural soils. In Wastewater Reuse and Current Challenges, 1st ed.; Fatta-Kassinos, D., Dionysiou, D., Kümmerer, K., Eds.; The Handbook of Environmental Chemistry Series; Springer International Publishing: Cham, Switzerland, 2015; pp. 105–128. [Google Scholar] [CrossRef]
- Ortiz, A.; Díez-Montero, R.; García, J.; Khalil, N.; Uggetti, E. Advanced biokinetic and hydrodynamic modelling to support and optimize the design of full-scale high rate algal ponds. Comput. Struct. Biotechnol. J. 2022, 20, 386–398. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, soil and plants: A critical review of microalgae as renewable resources for agriculture. Algal. Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Akbar, R.; Sun, J.; Bo, Y.; Khattak, W.A.; Khan, A.A.; Jin, C.; Zeb, U.; Ullah, N.; Abbas, A.D.; Kiu, W.; et al. Understanding the influence of secondary metabolites in plant invasion strategies: A comprehensive review. Plants 2024, 13, 3162. [Google Scholar] [CrossRef] [PubMed]
- Jmii, G.; Khadhri, A.; Haouala, R. Thapsia garganica allelopathic potentialities explored for lettuce growth enhancement and associated weed control. Sci. Hortic. 2020, 262, 109068. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Chinchilla, D.; Simonet, A.M.; Molinillo, J.M. Isolation and synthesis of allelochemicals from gramineae: Benzoxazinones and related compounds. J. Agric. Food Chem. 2006, 54, 991–1000. [Google Scholar] [CrossRef]
- Islam, A.M.; Suttiyut, T.; Anwar, M.P.; Juraimi, A.S.; Kato-Noguchi, H. Allelopathic properties of Lamiaceae species: Prospects and challenges to use in agriculture. Plants 2022, 11, 1478. [Google Scholar] [CrossRef]
- Jmii, G.; Zorrilla, J.G.; Haouala, R. Allelochemicals from Thapsia garganica leaves for Lolium perenne L. control: The magic of mixtures. Chemoecology 2022, 32, 81–87. [Google Scholar] [CrossRef]
- Tlig, T.; Gorai, M.; Neffati, M. Étude expérimentale de la compétition entre l’adventice Diplotaxis harra (Forssk.) Boiss. et l’orge (Hordeum vulgare var. Ardhaoui). Ecol. Mediterr. 2012, 38, 89–95. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Wen, Z.H.; Bakheit, A.H.; Basudan, O.A.; Ghabbour, H.A.; Al-Ahmari, A.; Feng, C.W. A major Diplotaxis harra-derived bioflavonoid glycoside as a protective agent against chemically induced neurotoxicity and parkinson’s models; in silico target prediction; and biphasic HPTLC-based quantification. Plants 2022, 11, 648. [Google Scholar] [CrossRef]
- Lukinac, J.; Jukić, M. Barley in the production of cereal-based products. Plants 2022, 11, 3519. [Google Scholar] [CrossRef] [PubMed]
- Javaid, M.M.; Tanveer, A. Germination ecology of Emex spinosa and Emex australis, invasive weeds of winter crops. Weed Res. 2014, 54, 565–575. [Google Scholar] [CrossRef]
- Keffala, C.; Jmii, G.; Mokhtar, A.; Zouhir, F.; Liady, N.D.; Tychon, B.; Jupsin, H. Diagnosis and assessment of a combined oxylag and high rate algal pond (COHRAP) for sustainable water reuse: Case study of the University Campus in Tunisia. Water 2025, 17, 1326. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- ISO 17294-2: 2023; Water Quality, Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes. ISO: Geneva, Switzerland, 2003.
- USEPA. Sw-846 EPA method 3051A. Microwave assisted acid digestion of sediments, sludges, soils and oils. In Test Methods for Evaluating Solid Waste, 2nd ed.; US Environmental Protection Agency: Washington, DC, USA, 1998; pp. 1–30. [Google Scholar]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Doblinski, P.M.F.; Ferrarese, M.L.L.; Huber, D.A.; Scapim, C.A.; Braccini, A.L.; Ferrarese-Filho, O. Peroxidase and lipid peroxidation of soybean roots in response to p-coumaric and p-hydroxybenzoic acids. Braz. Arch. Biol. Technol. 2003, 46, 193–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Sampietro, D.A.; Vattuone, M.A.; Isla, M.I. Plant growth inhibitors isolated from sugarcane (Saccharum officinarum) Straw. J. Plant Physiol. 2006, 163, 837–846. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines for the Safe Use of Wastewater, Excreta and Greywater, 3rd ed.; World Health Organization: Geneva, Switzerland, 2006; Volume 2, pp. 1–196. [Google Scholar]
- Tunisian Standards NT 106.03; Reuse of Treated Wastewater in Agriculture—Physicochemical and Biological Specifications. National Institute for Standardization and Industrial Properties-INNORPI, Tunisia: Tunis, Tunisia, 1989. (In French)
- European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying Down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003; European Union: Luxembourg, 2019. [Google Scholar]
- Álvarez-González, A.; Uggetti, E.; Serrano, L.; Gorchs, G.; Escolà Casas, M.; Matamoros, V.; Gonzalez-Flo, E.; Díez-Montero, R. The potential of wastewater grown microalgae for agricultural purposes: Contaminants of emerging concern, heavy metals and pathogens assessment. Environ. Pollut. 2023, 324, 121399. [Google Scholar] [CrossRef]
- Dagnaisser, L.S.; Dos Santos, M.G.B.; Rita, A.V.S.; Chaves Cardoso, J.; De Carvalho, D.F.; De Mendonça, H.V. Microalgae as bio-fertilizer: A new strategy for advancing modern agriculture, wastewater bioremediation, and atmospheric carbon mitigation. Water Air Soil Pollut. 2022, 233, 477. [Google Scholar] [CrossRef]
- Alshahrani, T.S.; Verlinden, S.; Siedel, G.E. Effects of leaf extracts of Ziziphus spina-christi and Prosopis juliflora on each others seedlings roots. Allelopath. J 2009, 23, 111–118. [Google Scholar]
- Bahloul, N.; Bellili, S.; Aazza, S.; Chérif, A.; Faleiro, M.L.; Antunes, M.D.; Miguel, M.G.; Mnif, W. Aqueous extracts from Tunisian diplotaxis: Phenol content, antioxidant and anti-acetylcholinesterase activities, and impact of exposure to simulated gastrointestinal fluids. Antioxidants 2016, 5, 12. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Khaliq, A.; Matloob, A.; Farooq, M.; Mushtaq, M.N.; Khan, M.B. Effect of crop residues applied isolated or in combination on the germination and seedling growth of horse purslane (Trianthema portulacastrum). Planta Daninha 2011, 29, 121–128. [Google Scholar] [CrossRef]
- Mamude, C.; Asfaw, Z. Allelopathic effects of Oldeania alpina (K. Schum.) Stapleton leaf aqueous extract on seed germination and initial seedling growth of two selected crops. Adv. Bamboo Sci. 2023, 4, 100034. [Google Scholar] [CrossRef]
- Mohammadrezakhani, S.; Hajilou, J.; Rezanejad, F.; Zaare-Nahandi, F. Assessment of exogenous application of proline on antioxidant compounds in three Citrus species under low temperature stress. Plant-Environ. Interact. 2019, 14, 347–358. [Google Scholar] [CrossRef]
- Cheng, F.; Cheng, Z. Research progress on the use of plant allelopathy in agriculture and the physiological and ecological mechanisms of allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Kong-ngern, K.; Bunnag, S.; Theerakulpisut, P. Proline, hydrogen peroxide, membrane stability and antioxidant enzyme activity as potential indicators for salt tolerance in Rice (Oryza sativa L.). Int. J. Bot. 2012, 8, 54–65. [Google Scholar] [CrossRef]
- Van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).