Synergistic Strategy Against the Effects of Climate Change Using Non-Positioned Vegetation Training Systems and the Application of Kaolin in a Vineyard in a Semi-Arid Climate: Agronomic and Oenological Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments Description
2.2. Climate Data Measurements
2.3. Agronomic Measurements
2.4. Winemaking and Oenological General Parameters
2.5. Volatile Compounds Determination
2.5.1. Major Volatile Compounds
2.5.2. Minor Volatile Compounds
2.5.3. Aromatic Series Calculation
2.6. Statistical Analysis
3. Results and Discussion
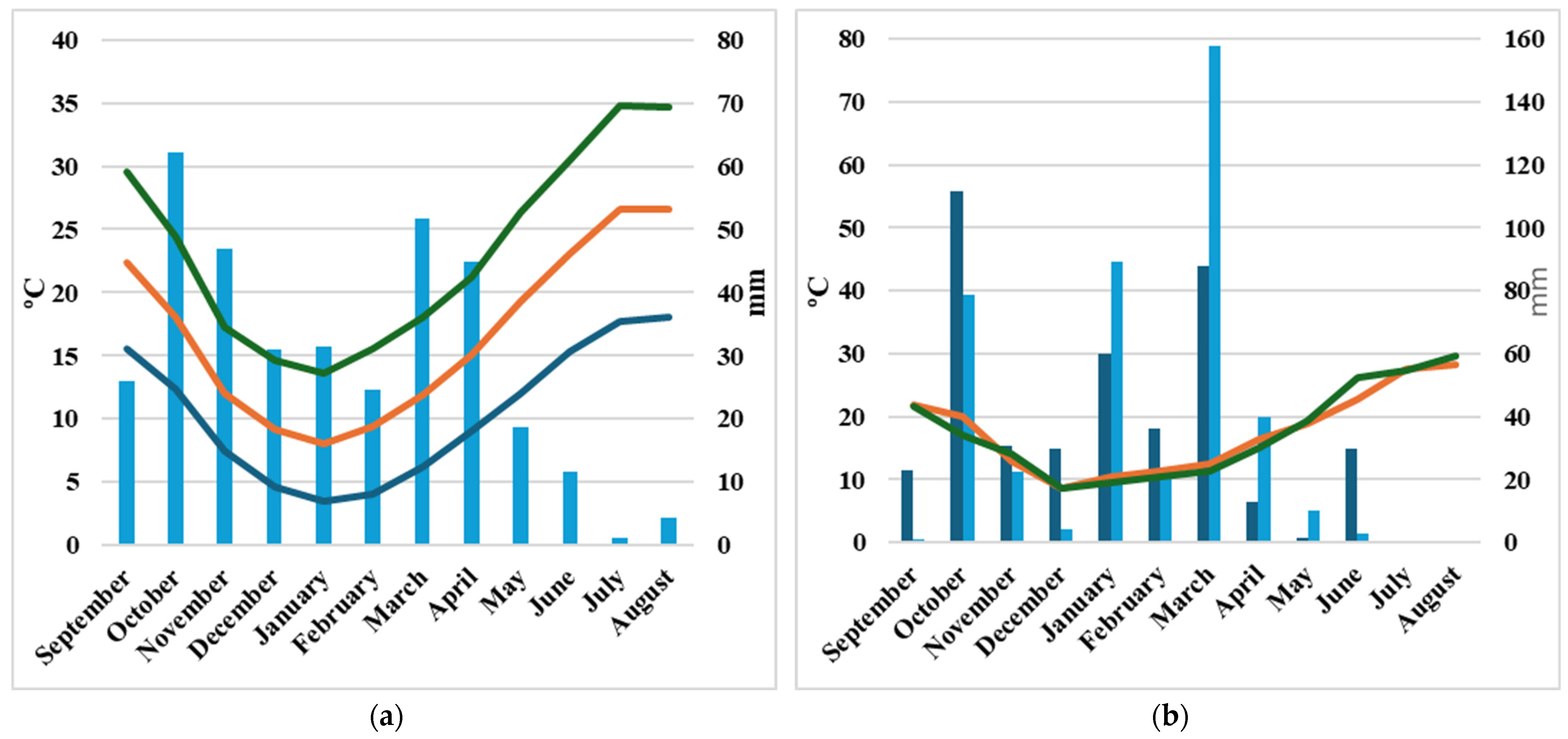
3.1. Climate Data
3.2. Agronomic Parameters
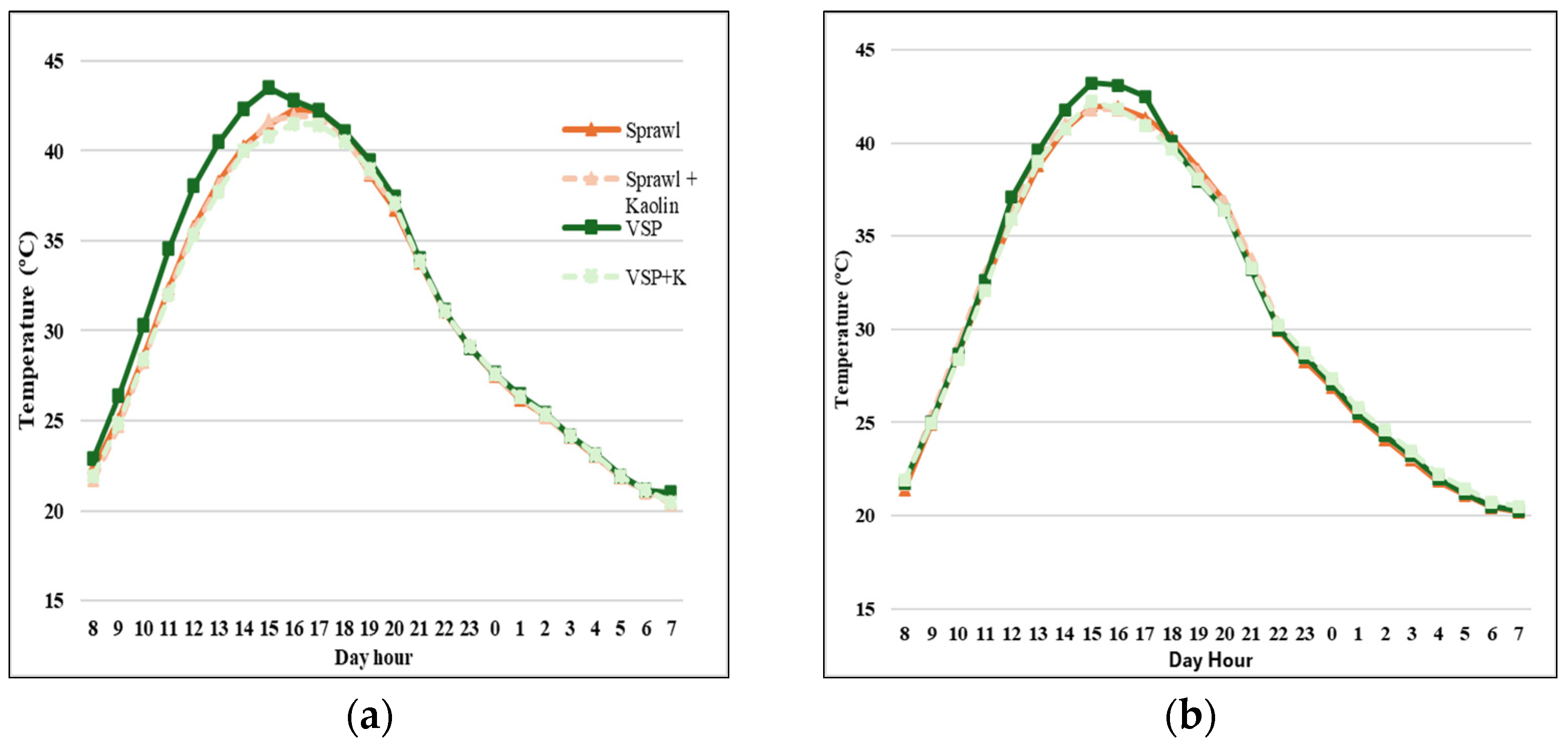
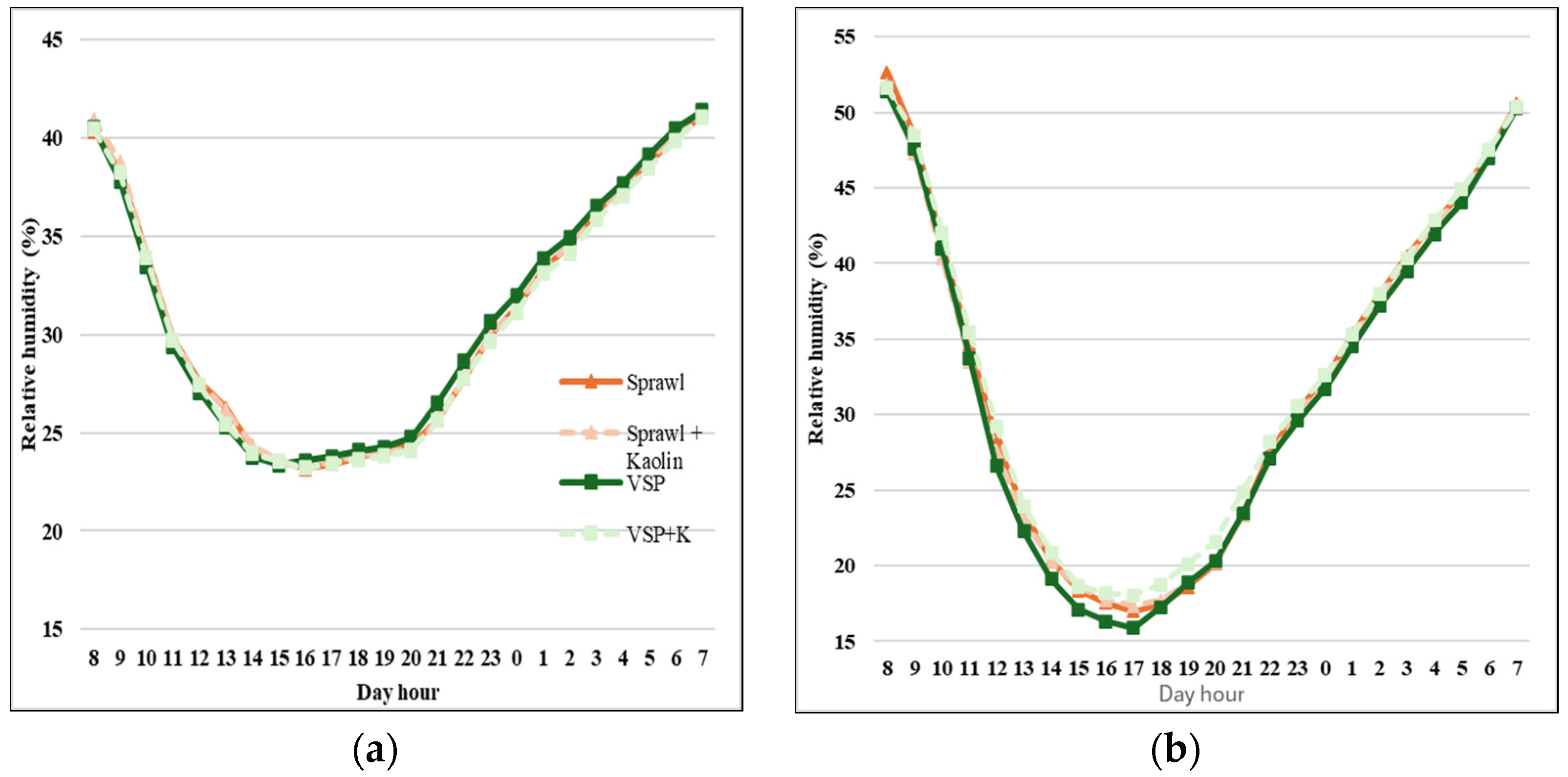
3.3. Bunches Microclimate
3.4. Oenological Parameters of the Obtained Wines
3.5. Volatile Wine Composition
3.5.1. Chemical Families
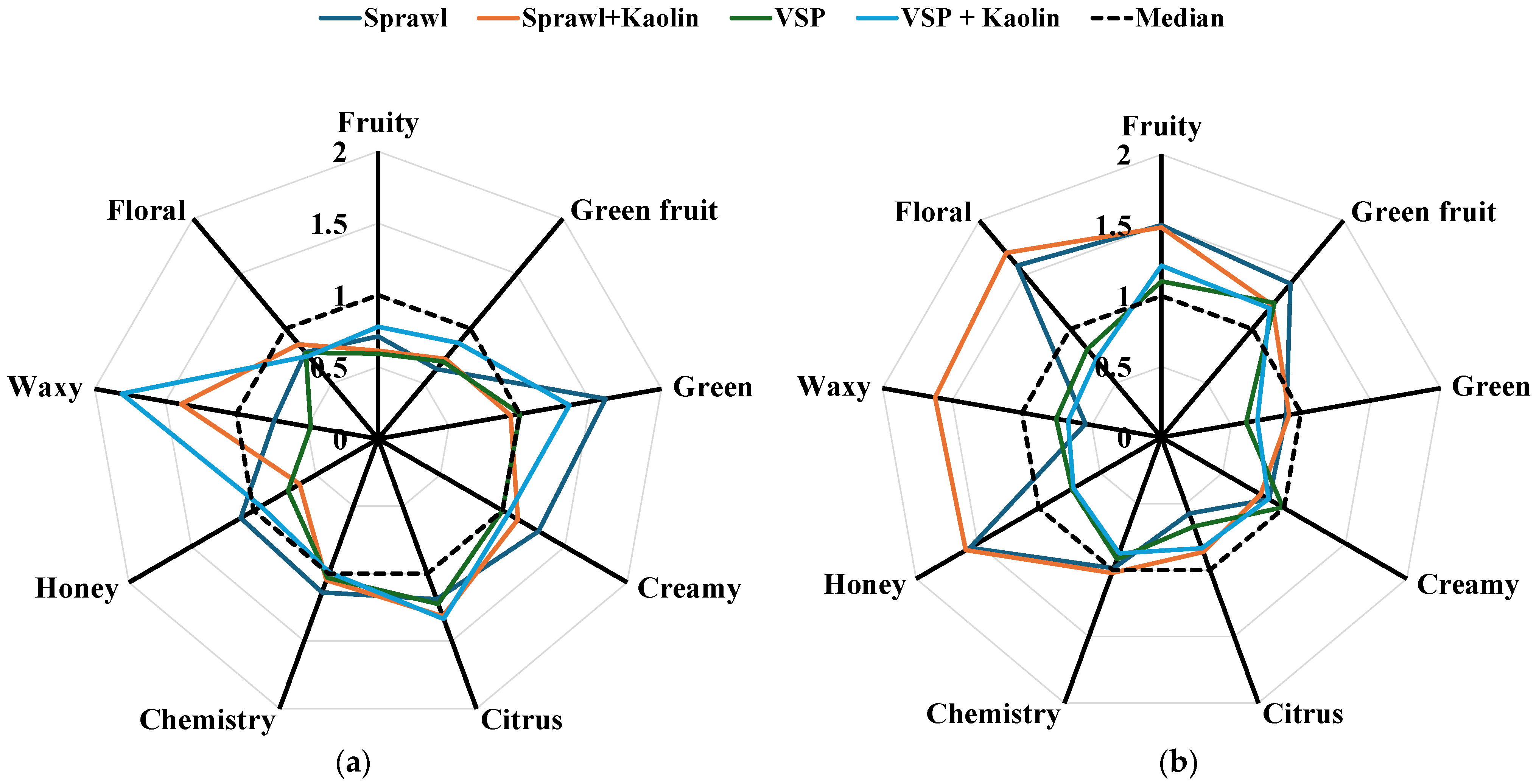
3.5.2. Aromatic Series Values
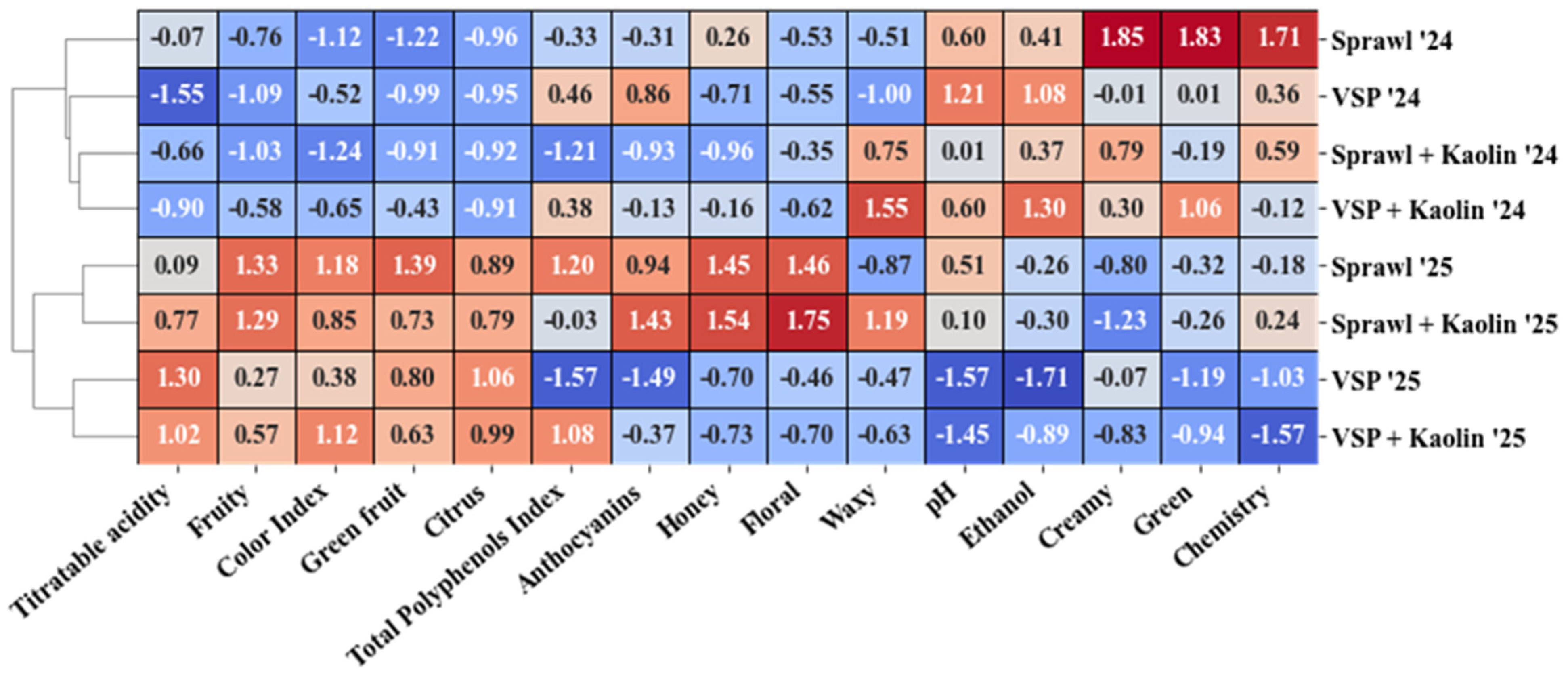
3.6. Multivariate Analysis
3.7. Cluster Heatmap
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC-FID | Gas chromatography flame ionisation detector |
| GC-MS | Gas chromatography mass spectrum detector |
| K | Kaolin application |
| OAV | Odor activity value |
| T | Training system |
| VSP | Vertical shoot positioning training system |
References
- Droulia, F.; Charalampopoulos, I. Future Climate Change Impacts on European Viticulture: A Review on Recent Scientific Advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- OIV State of the World Vine and Wine Sector in 2024. Available online: https://www.oiv.int/sites/default/files/2025-04/OIV-State_of_the_World_Vine-and-Wine-Sector-in-2024.pdf (accessed on 21 April 2025).
- Santillán, D.; Sotés, V.; Iglesias, A.; Garrote, L. Adapting Viticulture to Climate Change in the Mediterranean Region: Evaluations Accounting for Spatial Differences in the Producers-Climate Interactions. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2019; Volume 12. [Google Scholar] [CrossRef]
- Bucur, G.M.; Dejeu, L. Research on Adaptation Measures of Viticulture to Climate Change: Overview. Sci. Pap.-Ser. B-Hortic. 2022, 66, 177–190. [Google Scholar]
- Giorgi, F.; Lionello, P. Climate Change Projections for the Mediterranean Region. Glob. Planet. Change 2008, 63, 90–104. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Bernardo, S.; Yang, C.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. Mediterranean Viticulture in the Context of Climate Change. Cienc. Tec. Vitivinic. 2022, 37, 139–158. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Sgubin, G.; Bois, B.; Ollat, N.; Swingedouw, D.; Zito, S.; Gambetta, G.A. Climate Change Impacts and Adaptations of Wine Production. Nat. Rev. Earth Environ. 2024, 5, 258–275. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Darriet, P. The Impact of Climate Change on Viticulture and Wine Quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Sánchez-Suárez, F.; Martínez-García, R.; Peinado, R.A. Climate Change Adaptation in Winemaking: Combined Use of Non-Saccharomyces Yeasts to Improve the Quality of Pedro Ximénez Wines. Microorganisms 2025, 13, 1908. [Google Scholar] [CrossRef]
- Sgubin, G.; Swingedouw, D.; Mignot, J.; Gambetta, G.A.; Bois, B.; Loukos, H.; Noël, T.; Pieri, P.; García de Cortázar-Atauri, I.; Ollat, N.; et al. Non-linear Loss of Suitable Wine Regions over Europe in Response to Increasing Global Warming. Glob. Change Biol. 2023, 29, 808–826. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gamboa, G.; Zheng, W.; de Toda, F. Current Viticultural Techniques to Mitigate the Effects of Global Warming on Grape and Wine Quality: A Comprehensive Review. Food Res. Int. 2021, 139, 109946. [Google Scholar] [CrossRef] [PubMed]
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Magarelli, A.; Mazzeo, A.; Ferrara, G. Exploring the Grape Agrivoltaic System: Climate Modulation and Vine Benefits in the Puglia Region, Southeastern Italy. Horticulturae 2025, 11, 160. [Google Scholar] [CrossRef]
- Del Zozzo, F.; Poni, S. Climate Change Affects Choice and Management of Training Systems in the Grapevine. Aust. J. Grape Wine Res. 2024, 2024, 7834357. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Vanden Heuvel, J.E. Influence of Grapevine Training Systems on Vine Growth and Fruit Composition: A Review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar] [CrossRef]
- Intrieri, C.; Poni, S.; Rebucci, B.; Magnanini, E. Effects of Canopy Manipulations on Whole-Vine Photosynthesis: Results from Pot and Field Experiments. Vitis 1997, 36, 167–174. [Google Scholar]
- Zurowietz, A.; Lehr, P.P.; Kleb, M.; Merkt, N.; Gödde, V.; Bednarz, H.; Niehaus, K.; Zörb, C. Training Grapevines Generates a Metabolomic Signature of Wine. Food Chem. 2022, 368, 130665. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; Tombesi, S.; Luciani, E.; Sabbatini, P.; Berrios, J.G.; Palliotti, A. Kaolin Treatments on Pinot Noir Grapevines for the Control of Heat Stress Damages. In Proceedings of the CO.NA.VI. 2018—7 Convegno Nazionale di Viticoltura, Piacenza, Italy, 9–11 July 2018; Poni, S., Ed.; EDP Sciences: Les Ulis, France, 2019; Volume 13. [Google Scholar]
- Hosseinabad, A.; Khadivi, A. Foliar Application of Kaolin Reduces the Incidence of Sunburn in ‘Thompson Seedless’ Grapevine. Eur. J. Hortic. Sci. 2019, 84, 171–176. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Bernardo, S.; Matos, C.; Malheiro, A.; Flores, R.; Alves, S.; Costa, C.; Rocha, S.; Correia, C.; Luzio, A.; et al. Overview of Kaolin Outcomes from Vine to Wine: Cerceal White Variety Case Study. Agronomy 2020, 10, 1422. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Goncalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of Grapevine Physiology and Yield under Summer Stress By Kaolin-Foliar Application: Water Relations, Photosynthesis and Oxidative Damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin-Based, Foliar Reflective Film Protects Photosystem II structure and Function in Grapevine Leaves Exposed to Heat and High Solar Radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Yu, R.; Torres, N.; Tanner, J.D.; Kacur, S.M.; Marigliano, L.E.; Zumkeller, M.; Gilmer, J.C.; Gambetta, G.A.; Kurtural, S.K. Adapting Wine Grape Production to Climate Change through Canopy Architecture Manipulation and Irrigation in Warm Climates. Front. Vine Sci. 2022, 13, 1015574. [Google Scholar] [CrossRef]
- Szmania, C.; Waber, J.; Bogs, J.; Fischer, U. Sensory and Aroma Impact of Mitigation Strategies against Sunburn in Riesling. OENO One 2023, 57, 127–140. [Google Scholar] [CrossRef]
- Luzio, A.; Bernardo, S.; Correia, C.; Moutinho-Pereira, J.; Dinis, L.-T. Phytochemical Screening and Antioxidant Activity on Berry, Skin, pulp and Seed from Seven Red Mediterranean Grapevine Varieties (Vitis vinifera L.) Treated with Kaolin Foliar Sunscreen. Sci. Hortic. 2021, 281, 109962. [Google Scholar] [CrossRef]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing Kaolin and Pinolene to Improve Sustainable Grapevine Production During Drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef]
- Red de Asesoramiento al Regante de Extremadura (REDAREX). Available online: https://redarexplus.juntaex.es/REDAREX_plus/index.php?modulo=portada (accessed on 10 October 2025).
- Sánchez-de-Miguel, P.; Baeza, P.; Junquera, P.; Lissarrague, J.R. Vegetative Development: Total Leaf Area and Surface Area Indexes. In Methodologies and Results in Grapevine Research; Springer: Dordrecht, The Netherlands, 2010; pp. 31–44. [Google Scholar]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Dijon, France, 2023; ISBN 9782850380686. [Google Scholar]
- Peinado, R.A.; Moreno, J.A.; Muñoz, D.; Medina, M.; Moreno, J. Gas Chromatographic Quantification of Major Volatile Compounds and Polyols in Wine by Direct Injection. J. Agric. Food Chem. 2004, 52, 6389–6393. [Google Scholar] [CrossRef]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of Two Yeast Strains in Free, Bioimmobilized or Immobilized with Alginate Forms on the Aromatic Profile of Long Aged Sparkling Wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef]
- Aguilera, E.; Díaz-Gaona, C.; García-Laureano, R.; Reyes-Palomo, C.; Guzmán, G.I.; Ortolani, L.; Sánchez-Rodríguez, M.; Rodríguez-Estévez, V. Agroecology for Adaptation to Climate Change and Resource Depletion in the Mediterranean Region. A Review. Agric. Syst. 2020, 181, 102809. [Google Scholar] [CrossRef]
- Reynier, A. Manual de Viticultura, 11th ed.; Omega: Jackson, MI, USA, 2013. [Google Scholar]
- Hidalgo Fernández-Cano, L.; Hidalgo Togores, J. Tratado de Viticultura; Mundi Prensa: Madrid, Spain, 2019; Volumes I and II. [Google Scholar]
- Martínez-Vidaurre, J.M.; Pérez-Álvarez, E.P.; García-Escudero, E.; Peregrina, F. Effects of Soil Water-Holding Capacity and Soil N-NO3 and K on the Nutrient Content, Vigour and Yield of cv. Tempranillo Vine and the Composition of Its Must and Wine. OENO One 2023, 57, 447–466. [Google Scholar] [CrossRef]
- Keller, M. The Science of Grapevines; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128163658. [Google Scholar]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on Vegetative Growth and ‘Merlot’ Grapevine Yield in a Semi-Arid Mediterranean Climate. Horticulturae 2020, 6, 95. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Kurtural, S.K. Same Season and Carry-Over Effects of Source-Sink Adjustments on Grapevine Yields and Non-Structural Carbohydrates. Front. Vine Sci. 2021, 12, 695319. [Google Scholar] [CrossRef]
- Verdenal, T.; Spangenberg, J.E.; Zufferey, V.; Lorenzini, F.; Dienes-Nagy, A.; Gindro, K.; Spring, J.-L.; Viret, O. Leaf-to-Fruit Ratio Affects the Impact of Foliar-Applied Nitrogen on N Accumulation in the Grape Must. OENO One 2016, 50, 23–33. [Google Scholar] [CrossRef]
- Cataldo, E.; Eichmeier, A.; Mattii, G.B. Effects of Global Warming on Grapevine Berries Phenolic Compounds—A Review. Agronomy 2023, 13, 2192. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Friedel, M.; Holzapfel, B.P.; Stoll, M. Sunburn in Grapes: A Review. Front. Vine Sci. 2021, 11, 604691. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.J.; Peinado, R.A. Enological Chemistry; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Deloire, A.; Rogiers, S.; Šuklje, K.; Antalick, G.; Zeyu, X.; Pellegrino, A. Grapevine Berry Shrivelling, Water Loss and Cell Death: An Increasing Challenge for Growers in the Context of Climate Change. IVES Tech. Rev. Vine Wine 2021. [Google Scholar] [CrossRef]
- Hidalgo Togores, J. Tratado de Enología; Mundi-Prensa Libros: Madrid, Spain, 2018; Volumes I and II. [Google Scholar]
- Campos-Arguedas, F.; Sarrailhé, G.; Nicolle, P.; Dorais, M.; Brereton, N.J.B.; Pitre, F.E.; Pedneault, K. Different Temperature and UV Patterns Modulate Berry Maturation and Volatile Compounds Accumulation in Vitis sp. Front. Vine Sci. 2022, 13, 862259. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate Change Associated Effects on Grape and Wine Quality and Production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; Wiley: Hoboken, NJ, USA, 2016; ISBN 9781118627808. [Google Scholar]
- Wang, H.; Yang, M.; Martinez-Luscher, J.; Hilbert-Masson, G.; Gomès, E.; Pascual, I.; Fan, P.; Kong, J.; Liang, Z.; Xu, Z.; et al. Effects of Elevated Temperature and Shaded UV-B Radiation Exclusion on Berry Biochemical Composition in Four Grape (Vitis Vinifera L.) Cultivars with Distinct Anthocyanin Profiles. Food Res. Int. 2025, 218, 116823. [Google Scholar] [CrossRef]
- Trujillo, M.; Bely, M.; Albertin, W.; Masneuf-Pomarède, I.; Colonna-Ceccaldi, B.; Marullo, P.; Barbe, J.-C. Impact of Grape Maturity on Ester Composition and Sensory Properties of Merlot and Tempranillo Wines. J. Agric. Food Chem. 2022, 70, 11520–11530. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine Aroma Compounds in Grapes: A Critical Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Bindon, K.; Varela, C.; Kennedy, J.; Holt, H.; Herderich, M. Relationships between Harvest Time and Wine Composition in Vitis Vinifera L. cv. Cabernet Sauvignon 1. Grape and Wine Chemistry. Food Chem. 2013, 138, 1696–1705. [Google Scholar] [CrossRef]
- Martin, D.M.; Chiang, A.; Lund, S.T.; Bohlmann, J. Biosynthesis of Wine Aroma: Transcript Profiles of Hydroxymethylbutenyl Diphosphate Reductase, Geranyl Diphosphate Synthase, and Linalool/Nerolidol Synthase Parallel Monoterpenol Glycoside Accumulation in Gewürztraminer Grapes. Vinea 2012, 236, 919–929. [Google Scholar] [CrossRef]
- Talaverano, I.; Ubeda, C.; Cáceres-Mella, A.; Valdés, M.E.; Pastenes, C.; Peña-Neira, Á. Water Stress and Ripeness Effects on the Volatile Composition of Cabernet Sauvignon Wines. J. Sci. Food Agric. 2018, 98, 1140–1152. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining Wine Aroma from Compositional Data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Dumitriu (Gabur), G.-D.; Peinado, R.A.; Cotea, V.V.; López de Lerma, N. Volatilome Fingerprint of Red Wines Aged with Chips or Staves: Influence of the Aging Time and Toasting Degree. Food Chem. 2020, 310, 125801. [Google Scholar] [CrossRef]
- Sánchez-Suárez, F.; Palenzuela, M.d.V.; Rosal, A.; Peinado, R.A. Innovative Fermentation Approach Employing Lachancea Thermotolerans for the Selective Production of High-Acidity Wines, Designed for Blending with Low-Acidity Counterparts to Achieve Chemically and Organoleptically Balanced Final Compositions. Foods 2025, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Hein, K.; Ebeler, S.E.; Heymann, H. Perception of Fruity and Vegetative Aromas in Red Wine. J. Sens. Stud. 2009, 24, 441–455. [Google Scholar] [CrossRef]
- Moukarzel, R.; Parker, A.K.; Schelezki, O.J.; Gregan, S.M.; Jordan, B. Bunch Microclimate Influence Amino Acids and Phenolic Profiles of Pinot Noir Grape Berries. Front. Vine Sci. 2023, 14, 1162062. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Basile, B.; Lamorte, S.A.; Lisanti, M.T.; Corrado, G.; Lecce, L.; Strollo, D.; Moio, L.; Gambuti, A. Influence of Berry Ripening Stages over Phenolics and Volatile Compounds in Aged Aglianico Wine. Horticulturae 2021, 7, 184. [Google Scholar] [CrossRef]
- Chambers, J. Graphical Methods for Data Analysis; Wadsworth & Brooks: Belmont, CA, USA, 1983. [Google Scholar]


| Shoots/Vine | Bunches/Vine | Yield | Bunches Weight | Fertility | Surface Area | Surface Area/Yield | |||
|---|---|---|---|---|---|---|---|---|---|
| kg/Vine | g | Bunches/Shoot | m2/Vine | m2/kg | |||||
| 2024 Vintage | Sprawl | 12 ± 2 | 16 ± 4 | 1.4 ± 0.5 | 92 ± 22 | 1.3 ± 0.4 | 2.5 ± 0.2 | 2.0 ± 0.9 | |
| Sprawl + Kaolin | 11 ± 2 | 17 ± 3 | 1.9 ± 0.6 | 108 ± 30 | 1.5 ± 0.3 | 2.3 ± 0.2 | 1.4 ± 0.5 | ||
| VSP | 9 ± 1 | 15 ± 3 | 1.4 ± 0.3 | 93 ± 12 | 1.6 ± 0.4 | 2.7 ± 0.3 | 2.2 ± 0.7 | ||
| VSP + Kaolin | 11 ± 2 | 16 ± 4 | 1.5 ± 0.5 | 88 ± 16 | 1.5 ± 0.3 | 2.8 ± 0.3 | 2.1 ± 0.6 | ||
| MANOVA | Training System | *** | ns | ns | ns | ns | * | * | |
| Kaolin | ns | ns | ns | ns | ns | ns | ns | ||
| TS × Kaolin | ** | ns | ns | ns | ns | ns | ns | ||
| 2025 Vintage | Sprawl | 14 ± 1 | 21 ± 5 | 2.7 ± 0.6 | 132 ± 41 | 1.5 ± 0.3 | 3.2 ± 0.2 | 0.8 ± 0.2 | |
| Sprawl + Kaolin | 15 ± 2 | 25 ± 7 | 3.3 ± 1.3 | 126 ± 31 | 1.7 ± 0.3 | 3.4 ± 0.4 | 0.8 ± 0.2 | ||
| VSP | 16 ± 3 | 27 ± 6 | 3.1 ± 0.9 | 116 ± 27 | 1.7 ± 0.3 | 3.2 ± 0.2 | 0.7 ± 0.2 | ||
| VSP + Kaolin | 16 ± 2 | 25 ± 5 | 2.7 ± 0.7 | 111 ± 22 | 1.6 ± 0.3 | 3.3 ± 0.3 | 0.8 ± 0.2 | ||
| MANOVA | Training System | * | ns | ns | ns | ns | ns | ns | |
| Kaolin | ns | ns | ns | ns | ns | * | ns | ||
| TS × Kaolin | ns | ns | ns | ns | ns | ns | ns | ||
| Sugars ¥ | pH | Titratable Acidity | Ethanol | Volatile Acidity | Anthocyanins | Colour Index | TPI | |||
|---|---|---|---|---|---|---|---|---|---|---|
| g/L | g/L TH2 | % v/v | g/L AcH | mg/L | ||||||
| 2024 Vintage | Sprawl | 244 ± 3 | 3.44 ± 0.01 | 6.86 ± 0.09 | 14.2 ± 0.1 | 0.33 ± 0.06 | 468 ± 15 | 28.2 ± 0.8 | 32.8 ± 0.6 | |
| Sprawl + Kaolin | 240 ± 4 | 3.37 ± 0.03 | 6.4 ± 0.2 | 14.1 ± 0.2 | 0.42 ± 0.03 | 434 ± 68 | 27.1 ± 0.6 | 32.0 ± 1.0 | ||
| VSP | 254 ± 2 | 3.51 ± 0.03 | 5.79 ± 0.08 | 14.8 ± 0.1 | 0.44 ± 0.04 | 521 ± 24 | 33.0 ± 1.0 | 33.5 ± 0.5 | ||
| VSP + Kaolin | 258 ± 6 | 3.44 ± 0.02 | 6.25 ± 0.04 | 15.0 ± 0.1 | 0.45 ± 0.05 | 478 ± 32 | 32.0 ± 1.1 | 33.4 ± 0.4 | ||
| MANOVA | Training System | *** | ** | *** | *** | * | * | *** | * | |
| Kaolin | ns | ** | ns | ns | ns | ns | ns | ns | ||
| TS × Kaolin | ns | ns | *** | ns | ns | ns | ns | ns | ||
| 2025 Vintage | Sprawl | 233 ± 4 | 3.43 ± 0.04 | 7 ± 0.1 | 13.6 ± 0.1 | 0.36 ± 0.03 | 537 ± 40 | 46.5 ± 0.6 | 30.8 ± 0.4 | |
| Sprawl + Kaolin | 235 ± 5 | 3.38 ± 0.03 | 7.46 ± 0.06 | 13.5 ± 0.1 | 0.32 ± 0.02 | 564 ± 39 | 43.9 ± 0.3 | 33.1 ± 0.2 | ||
| VSP | 220 ± 5 | 3.19 ± 0.04 | 7.8 ± 0.3 | 12.6 ± 0.2 | 0.32 ±0.02 | 404 ± 22 | 40.2 ± 0.3 | 31.7 ± 0.2 | ||
| VSP + Kaolin | 230 ± 3 | 3.21 ± 0.03 | 7.6 ± 0.3 | 13 ± 0.1 | 0.36 ± 0.03 | 465 ± 38 | 46.1 ± 0.6 | 34.1 ± 0.4 | ||
| MANOVA | Training System | ns | ** | *** | ns | *** | *** | *** | ** | |
| Kaolin | ns | ns | * | ns | * | *** | *** | ** | ||
| TS × Kaolin | ns | * | *** | ns | ns | *** | *** | *** | ||
| 2024 Vintage | 2025 Vintage | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MANOVA | MANOVA | |||||||||||||
| Sprawl | Sprawl + K | VSP | VSP + K | TS | K | TS × K | Sprawl | Sprawl + K | VSP | VSP + K | TS | K | TS × K | |
| Major alcohols. | 891 ± 8 | 889 ± 4 | 740 ± 7 | 712 ± 8 | ** | * | ** | 651 ± 2 | 708 ± 3 | 579 ± 7 | 534 ± 2 | ** | ns | ** |
| Methanol | 259 ± 16 | 359 ± 13 | 235 ± 19 | 242 ± 3 | *** | *** | *** | 96 ± 4 | 120 ± 9 | 108 ± 11 | 93 ± 3 | ns | ns | ** |
| Propanol | 66.7 ± 0.6 | 81 ± 3 | 76 ± 5 | 77 ± 10 | ns | * | ns | 67 ± 3 | 64 ± 2 | 79 ± 2 | 88 ± 4 | *** | ns | ** |
| Isobutanol | 67 ± 3 | 44.4 ± 0.9 | 45 ± 4 | 46 ± 4 | *** | *** | *** | 45 ± 5 | 51 ± 2 | 43 ± 3 | 40 ± 1 | ** | ns | * |
| 2-methylbutanol | 66 ± 6 | 46 ± 5 | 56 ± 7 | 44 ± 6 | ns | ** | ns | 59 ± 2 | 64 ± 2 | 41 ± 2 | 30 ± 6 | *** | ns | * |
| 3-methylbutanol | 373 ± 23 | 277 ± 7 | 263 ± 21 | 244 ± 25 | *** | ** | * | 309 ± 8 | 323 ± 7 | 265 ± 22 | 246 ± 6 | *** | ns | ns |
| 2-Phenylethanol | 58 ± 9 | 80 ± 8 | 65 ± 5 | 58 ± 5 | ns | ns | ** | 74 ± 2 | 86 ± 2 | 44 ± 3 | 37 ± 1 | *** | * | *** |
| Minor alcohols. | 2576 ± 18 | 1580 ± 45 | 2355 ± 80 | 1984 ± 141 | ns | *** | *** | 2196 ± 131 | 2391 ± 113 | 2357 ± 164 | 2012 ± 54 | ns | ns | ** |
| Hexanol | 2319 ± 16 | 1326 ± 39 | 2007 ± 75 | 1774 ± 149 | ns | *** | *** | 2079 ± 141 | 2289 ± 109 | 2250 ± 171 | 1910 ± 51 | ns | ns | ** |
| 2-ethyl-1-hexanol | 39 ± 2 | 47 ± 1 | 53 ± 5 | 51 ± 5 | ** | ns | ns | 21.7 ± 0.5 | 24.4 ± 0.9 | 22 ± 2 | 21 ± 2 | ns | ns | ns |
| Octanol | 215 ± 20 | 203 ± 11 | 207 ± 23 | 154 ± 4 | * | ** | ns | 94 ± 11 | 75 ± 6 | 83 ± 9 | 79 ± 6 | ns | *- | ns |
| Dodecanol | 0.8 ± 0.06 | 2.8 ± 0.3 | 86 ± 6 | 2.9 ± 0.2 | *** | *** | *** | 1.13 ± 0.03 | 1.7 ± 0.1 | 1.5 ± 0.2 | 1.1 ± 0.2 | ns | ns | *** |
| Farnesol | 1.6 ± 0.1 | 0.18 ± 0.01 | 1.35 ± 0.08 | 2 ± 0.2 | *** | *** | *** | 0.29 ± 0.04 | 1.1 ± 0.2 | 0.14 ± 0.02 | 0.12 ± 0.02 | *** | *** | *** |
| Major esters | 175.5 ± 0.7 | 170.1 ± 0.4 | 171 ± 3 | 174 ± 1 | ns | ns | ns | 148.3 ± 0.8 | 136.2 ± 0.6 | 144 ± 1 | 155 ± 1 | * | ns | * |
| Ethyl acetate | 63 ± 2 | 61 ± 3 | 78 ± 2 | 76 ± 5 | *** | ns | ns | 78 ± 3 | 74 ± 3 | 78 ± 3 | 83 ± 2 | * | ns | * |
| Ethyl lactate | 48 ± 2 | 32 ± 2 | 35.8 ± 0.9 | 33 ± 7 | * | ** | * | 24.4 ± 0.9 | 25 ± 1 | 28.8 ± 0.8 | 30 ± 2 | *** | ns | ns |
| Diethyl succinate | 65 ± 4 | 78 ± 2 | 57 ± 8 | 66 ± 5 | * | ** | ns | 46 ± 1 | 38 ± 2 | 37 ± 1 | 42 ± 4 | ns | ns | ** |
| Minor esters: | 3966 ± 140 | 2571 ± 28 | 3267 ± 47 | 3327 ± 80 | ns | *** | *** | 10,246 ± 410 | 9559 ± 473 | 6367 ± 241 | 6971 ± 131 | *** | ns | ns |
| Ethyl propanoate | nd | nd | nd | nd | 62 ± 6 | 59 ± 3 | 45 ± 1 | 43 ± 2 | *** | ns | ns | |||
| Ethyl isobutanoate | 26 ± 1 | 26 ± 1 | 31.1 ± 0.1 | 22.7 ± 0.9 | ns | *** | *** | nd | nd | nd | nd | |||
| Ethyl butanoate | 198 ± 10 | 140 ± 4 | 153 ± 2 | 161 ± 7 | * | *** | *** | 237 ± 21 | 207 ± 10 | 157 ± 4 | 180 ± 4 | *** | ns | ** |
| Butyl acetate | 0.45 ± 0.05 | 0.57 ± 0.02 | 0.73 ± 0.08 | 0.7 ± 0.06 | *** | ns | ns | 2.2 ± 0.2 | 1.5 ± 0.2 | 1.6 ± 0.1 | 2.65 ± 0.05 | * | ns | *** |
| Ethyl 2-methylbutanoate | 1.2 ± 0.07 | 1.7 ± 0.2 | 2.1 ± 0.2 | 1.6 ± 0.2 | ** | ns | ** | 2.3 ± 0.2 | 2.2 ± 0.3 | 1.2 ± 0.1 | 1.76 ± 0.06 | *** | ns | ** |
| Ethyl 3-methylbutanoate | 3.7 ± 0.2 | 4.1 ± 0.1 | 5.1 ± 0.3 | 4.4 ± 0.3 | *** | ns | ** | 2.1 ± 0.2 | 2.72 ± 0.03 | 2.1 ± 0.2 | 2 ± 0.2 | ** | * | * |
| Isoamyl acetate | 2408 ± 74 | 1254 ± 31 | 1982 ± 15 | 1652 ± 38 | ns | *** | *** | 5834 ± 122 | 5069 ± 263 | 3808 ± 121 | 4580 ± 132 | *** | ns | *** |
| Ethyl hexanoate | 284 ± 13 | 326 ± 2 | 310 ± 10 | 392 ± 27 | ** | *** | ns | 661 ± 36 | 562 ± 12 | 577 ± 16 | 553 ± 13 | ** | ** | * |
| Hexyl acetate | 28.2 ± 0.6 | 25.1 ± 0.8 | 18.3 ± 0.6 | 27 ± 2 | *** | ** | *** | 253 ± 9 | 230 ± 4 | 272 ± 8 | 252 ± 6 | ** | ** | ns |
| Ethyl heptanoate | 0.13 ± 0.01 | 0.19 ± 0.02 | 0.15 ± 0.01 | 0.48 ± 0.03 | *** | *** | *** | 0.23 ± 0.01 | 0.33 ± 0.01 | 0.23 ± 0.03 | 0.22 ± 0.01 | *** | ** | *** |
| Ethyl octanoate | 77 ± 2 | 175 ± 9 | 39 ± 2 | 236 ± 27 | ns | *** | *** | 67 ± 4 | 222 ± 4 | 98 ± 5 | 76 ± 2 | *** | *** | *** |
| Ethyl phenylacetate | 6.4 ± 0.3 | 2.2 ± 0.1 | 1.6 ± 0.1 | 2.1 ± 0.2 | *** | *** | *** | 317 ± 14 | 326 ± 19 | 168 ± 9 | 139 ± 3 | *** | ns | * |
| 2-Phenylethyl acetate | 729 ± 85 | 435 ± 10 | 570 ± 13 | 641 ± 37 | ns | ** | *** | 2549 ± 268 | 2602 ± 181 | 1049 ± 94 | 902 ± 37 | *** | ns | * |
| Ethyl decanoate | 106 ± 8 | 93 ± 3 | 64 ± 2 | 108 ± 10 | ** | ** | *** | 199 ± 5 | 213 ± 8 | 145 ± 5 | 183 ± 2 | *** | *** | ** |
| 2-Phenylethyl butanoate | 17 ± 1 | 5.6 ± 0.2 | 11.1 ± 0.6 | 12.8 ± 0.9 | ns | *** | *** | 25 ± 0.1 | 25 ± 1 | 9 ± 0.4 | 7 ± 0.4 | *** | ns | ns |
| Ethyl tetradecanoate | 24 ± 2 | 27 ± 1 | nd | 20 ± 3 | ns | ns | ** | 11.7 ± 0.3 | 12.3 ± 0.5 | 12 ± 0.5 | 17 ± 1 | *** | *** | ** |
| Phenethyl benzoate | 2.67 ± 0.03 | 2.7 ± 0.1 | 3.4 ± 0.1 | 3.3 ± 0.1 | *** | ns | ns | 1.4 ± 0.03 | 1.38 ± 0.04 | 1.4 ± 0.1 | 1.47 ± 0.05 | ns | ns | ns |
| Ethyl hexadecanoate | 54 ± 6 | 53 ± 4 | 56 ± 4 | 41 ± 7 | ns | ns | ns | 22.5 ± 0.5 | 23 ± 1 | 22 ± 1 | 30 ± 3 | ** | ** | ** |
| Major aldehydes: | 139 ± 14 | 142 ± 11 | 138 ± 15 | 132 ± 5 | ns | ns | ns | 98 ± 3 | 98 ± 3 | 105 ± 6 | 108 ± 7 | ns | ns | ns |
| Acetaldehyde | 139 ± 14 | 142 ± 11 | 138 ± 15 | 132 ± 5 | ns | ns | ns | 98 ± 3 | 98 ± 3 | 105 ± 6 | 108 ± 7 | ns | ns | ns |
| Minor aldehydes: | 49 ± 3 | 36 ± 2 | 37 ± 1 | 45.7 ± 0.8 | ns | ns | *** | 24 ± 1 | 28 ± 2 | 19.9 ± 0.4 | 24.1 ± 0.3 | * | * | ns |
| Benzaldehyde | 2 ± 0.2 | 0.79 ± 0.08 | 2.11 ± 0.08 | 1.8 ± 0.1 | *** | *** | *** | 2.1 ± 0.3 | 2.4 ± 0.2 | 2.35 ± 0.09 | 1.7 ± 0.1 | ns | ns | ** |
| Heptanal | 1.1 ± 0.1 | 1.13 ± 0.09 | 0.7 ± 0.1 | 0.82 ± 0.09 | *** | ns | ns | 0.13 ± 0.01 | 0.15 ± 0.03 | 0.58 ± 0.03 | 0.22 ± 0.03 | *** | *** | *** |
| Octanal | 1.46 ± 0.07 | 1.9 ± 0.2 | 1.8 ± 0.1 | 1.9 ± 0.1 | ns | * | ns | 4.0 ± 0.1 | 3.64 ± 0.08 | 4.3 ± 0.1 | 4.0 ± 0.1 | ** | ** | ns |
| Nonanal | 4.8 ± 0.6 | 6.3 ± 0.4 | 5.6 ± 0.2 | 5.5 ± 0.2 | ns | * | * | 1.9 ± 0.3 | 3.6 ± 0.4 | 2.7 ± 0.3 | 3.7 ± 0.3 | * | *** | ns |
| Decanal | 7.3 ± 0.6 | 7.6 ± 0.9 | 7 ± 1 | 8.3 ± 0.9 | ns | ns | ns | 2.3 ± 0.2 | 4.1 ± 0.3 | 3 ± 0.3 | 5 ± 0.6 | ** | *** | ns |
| Phenylacetaldehyde | 32 ± 2 | 18.2 ± 0.8 | 19.9 ± 0.6 | 27 ± 2 | ns | ** | *** | 17 ± 2 | 17 ± 2 | 9.8 ± 0.9 | 12 ± 1 | *** | ns | ns |
| Major ketones. | 68 ± 3 | 69 ± 3 | 42 ± 1 | 51 ± 5 | *** | * | ns | 35 ± 1 | 29 ± 2 | 51 ± 2 | 59 ± 6 | *** | ns | ** |
| Acetoin | 68 ± 3 | 69 ± 3 | 42 ± 1 | 51 ± 5 | *** | * | ns | 35 ± 1 | 29 ± 2 | 51 ± 2 | 59 ± 6 | *** | ns | ** |
| Minor ketones: | 3.4 ± 0.2 | 2.4 ± 0.2 | 3.08 ± 0.06 | 3.1 ± 0.3 | ns | ** | ** | 7 ± 0.4 | 8.3 ± 0.3 | 7.9 ± 0.4 | 6.8 ± 0.2 | ns | ns | *** |
| Benzophenone | 0.86 ± 0.06 | 0.77 ± 0.07 | 0.69 ± 0.05 | 0.78 ± 0.07 | ns | ns | * | 2.3 ± 0.2 | 2.5 ± 0.1 | 2.1 ± 0.1 | 1.5 ± 0.2 | *** | * | ** |
| 3-heptanone | 0.15 ± 0.02 | 0.07 ± 0.01 | 0.27 ± 0.05 | 0.24 ± 0.02 | *** | * | ns | 2.9 ± 0.2 | 2.86 ± 0.07 | 2.9 ± 0.3 | 3 ± 0.3 | ns | ns | ns |
| Acetophenone | 2.4 ± 0.2 | 1.6 ± 0.1 | 2.12 ± 0.09 | 2.1 ± 0.3 | ns | ** | * | 1.8 ± 0.2 | 2.9 ± 0.3 | 2.9 ± 0.3 | 2.3 ± 0.3 | ns | ns | ** |
| Lactones | 10 ± 0.7 | 8.8 ± 0.6 | 10 ± 1 | 10.2 ± 0.7 | ns | ns | ns | 10.9 ± 0.8 | 10.1 ± 0.8 | 9.4 ± 0.8 | 4.5 ± 0.4 | *** | *** | ** |
| γ-nonalactone | 8.7 ± 0.6 | 7.3 ± 0.5 | 9 ± 1 | 8.9 ± 0.8 | ns | ns | ns | 9.4 ± 0.7 | 8.9 ± 0.9 | 8.7 ± 0.8 | 3.4 ± 0.3 | *** | *** | *** |
| γ-decalactone | 1.33 ± 0.09 | 1.5 ± 0.1 | 1.48 ± 0.09 | 1.3 ± 0.1 | ns | ns | * | 1.5 ± 0.1 | 1.1 ± 0.1 | 0.7 ± 0.1 | 1.1 ± 0.1 | ** | ns | ** |
| Terpenes and norisoprenoids | 51 ± 3 | 50 ± 2 | 54 ± 4 | 50.06 ± 0.09 | ns | ns | ns | 50 ± 2 | 55 ± 1 | 38 ± 4 | 33 ± 2 | *** | ns | ** |
| Limonene | 16.8 ± 0.8 | 17 ± 1 | 15 ± 1 | 16 ± 1 | ns | ns | ns | 17 ± 1 | 21 ± 1 | 16 ± 2 | 10 ± 1 | *** | ns | ** |
| β-citronellol | 25 ± 3 | 22 ± 2 | 30 ± 3 | 23 ± 2 | ns | ** | ns | 23 ± 2 | 23.9 ± 0.6 | 12 ± 1 | 11.8 ± 0.9 | *** | ns | ns |
| β-damascenone | 4.1 ± 0.2 | 4.6 ± 0.4 | 2.74 ± 0.05 | 3.6 ± 0.3 | *** | * | ** | 6.7 ± 0.2 | 7 ± 0.2 | 6.2 ± 0.4 | 7.2 ± 0.1 | ns | ** | ns |
| Z-geranyl acetone | 2.3 ± 0.2 | 2.09 ± 0.09 | 2.1 ± 0.1 | 2.23 ± 0.03 | ns | ns | ns | 2.02 ± 0.03 | 2.2 ± 0.2 | 2.11 ± 0.08 | 2.1 ± 0.1 | ns | ns | ns |
| E-methyldihydrojasmonate | 3.2 ± 0.3 | 3.8 ± 0.2 | 4.1 ± 0.4 | 4.7 ± 0.5 | ** | * | ns | 1.3 ± 0.1 | 0.94 ± 0.07 | 1.6 ± 0.2 | 1.4 ± 0.2 | ** | ** | ns |
| Fruity | Green Fruit | Green | Creamy | Citrus | Chemistry | Honey | Waxy | Floral | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024 Vintage | Sprawl | 132 ± 1 | 22 ± 1 | 8.8 ± 0.5 | 1.1 ± 0.1 | 10.0 ± 0.7 | 32.9 ± 1 | 11.0 ± 0.5 | 22.1 ± 0.5 | 10.2 ± 1.1 | |
| Sprawl + Kaolin | 113 ± 2 | 25 ± 2 | 5.1 ± 0.2 | 1.0 ± 0.2 | 11.1 ± 0.8 | 30.3 ± 0.5 | 6.3 ± 0.2 | 42 ± 1 | 11.1 ± 0.8 | ||
| VSP | 110 ± 2 | 24 ± 1 | 5.5 ± 0.1 | 0.8 ± 0.1 | 10 ± 1.3 | 29.8 ± 1.2 | 7.3 ± 0.1 | 14.3 ± 1.2 | 10.1 ± 0.5 | ||
| VSP + Kaolin | 144 ± 6 | 30 ± 2 | 7.4 ± 0.4 | 0.9 ± 0.1 | 11.2 ± 1 | 28.6 ± 0.5 | 9.4 ± 0.4 | 55 ± 5 | 9.7 ± 0.4 | ||
| MANOVA | TS | ns | ** | * | ** | ns | ** | ns | ns | ns | |
| Kaolin | ** | *** | ** | ns | ns | ** | *** | *** | ns | ||
| TS × Kaolin | *** | ns | *** | * | ns | ns | *** | *** | ns | ||
| 2025 Vintage | Sprawl | 277 ± 9 | 49 ± 3 | 4.9 ± 0.4 | 0.7 ± 0 | 5.9 ± 0.4 | 28.5 ± 0.9 | 51 ± 2 | 16.4 ± 1 | 20.5 ± 1.2 | |
| Sprawl + Kaolin | 274 ± 10 | 42 ± 1 | 5 ± 0.5 | 0.7 ± 0 | 8.2 ± 0.1 | 29.5 ± 0.9 | 53 ± 3 | 48.9 ± 0.8 | 22 ± 0.9 | ||
| VSP | 204 ± 6 | 42 ± 1 | 3.3 ± 0.2 | 0.8 ± 0 | 6.8 ± 0.2 | 26.5 ± 1.0 | 27 ± 1 | 22.7 ± 0.7 | 10.5 ± 0.4 | ||
| VSP + Kaolin | 224 ± 5 | 41 ± 1 | 3.8 ± 0.3 | 0.7 ± 0.1 | 8.1 ± 0.5 | 25.2 ± 0.7 | 24 ± 1 | 20.2 ± 0.9 | 9.3 ± 0.2 | ||
| MANOVA | TS | *** | ** | *** | ** | ** | *** | *** | *** | *** | |
| Kaolin | ns | ** | ns | ** | ns | ns | ns | *** | ns | ||
| TS × Kaolin | ns | * | ns | ns | ns | ns | ns | *** | * | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Suárez, F.; Martínez-García, R.; de Lerma, N.L.; Peinado, R.A. Synergistic Strategy Against the Effects of Climate Change Using Non-Positioned Vegetation Training Systems and the Application of Kaolin in a Vineyard in a Semi-Arid Climate: Agronomic and Oenological Effects. Agronomy 2025, 15, 2730. https://doi.org/10.3390/agronomy15122730
Sánchez-Suárez F, Martínez-García R, de Lerma NL, Peinado RA. Synergistic Strategy Against the Effects of Climate Change Using Non-Positioned Vegetation Training Systems and the Application of Kaolin in a Vineyard in a Semi-Arid Climate: Agronomic and Oenological Effects. Agronomy. 2025; 15(12):2730. https://doi.org/10.3390/agronomy15122730
Chicago/Turabian StyleSánchez-Suárez, Fernando, Rafael Martínez-García, Nieves López de Lerma, and Rafael A. Peinado. 2025. "Synergistic Strategy Against the Effects of Climate Change Using Non-Positioned Vegetation Training Systems and the Application of Kaolin in a Vineyard in a Semi-Arid Climate: Agronomic and Oenological Effects" Agronomy 15, no. 12: 2730. https://doi.org/10.3390/agronomy15122730
APA StyleSánchez-Suárez, F., Martínez-García, R., de Lerma, N. L., & Peinado, R. A. (2025). Synergistic Strategy Against the Effects of Climate Change Using Non-Positioned Vegetation Training Systems and the Application of Kaolin in a Vineyard in a Semi-Arid Climate: Agronomic and Oenological Effects. Agronomy, 15(12), 2730. https://doi.org/10.3390/agronomy15122730






