Biostimulants as a Tool for Mitigating Water Deficit Stress in Strawberry Cultivation
Abstract
1. Introduction
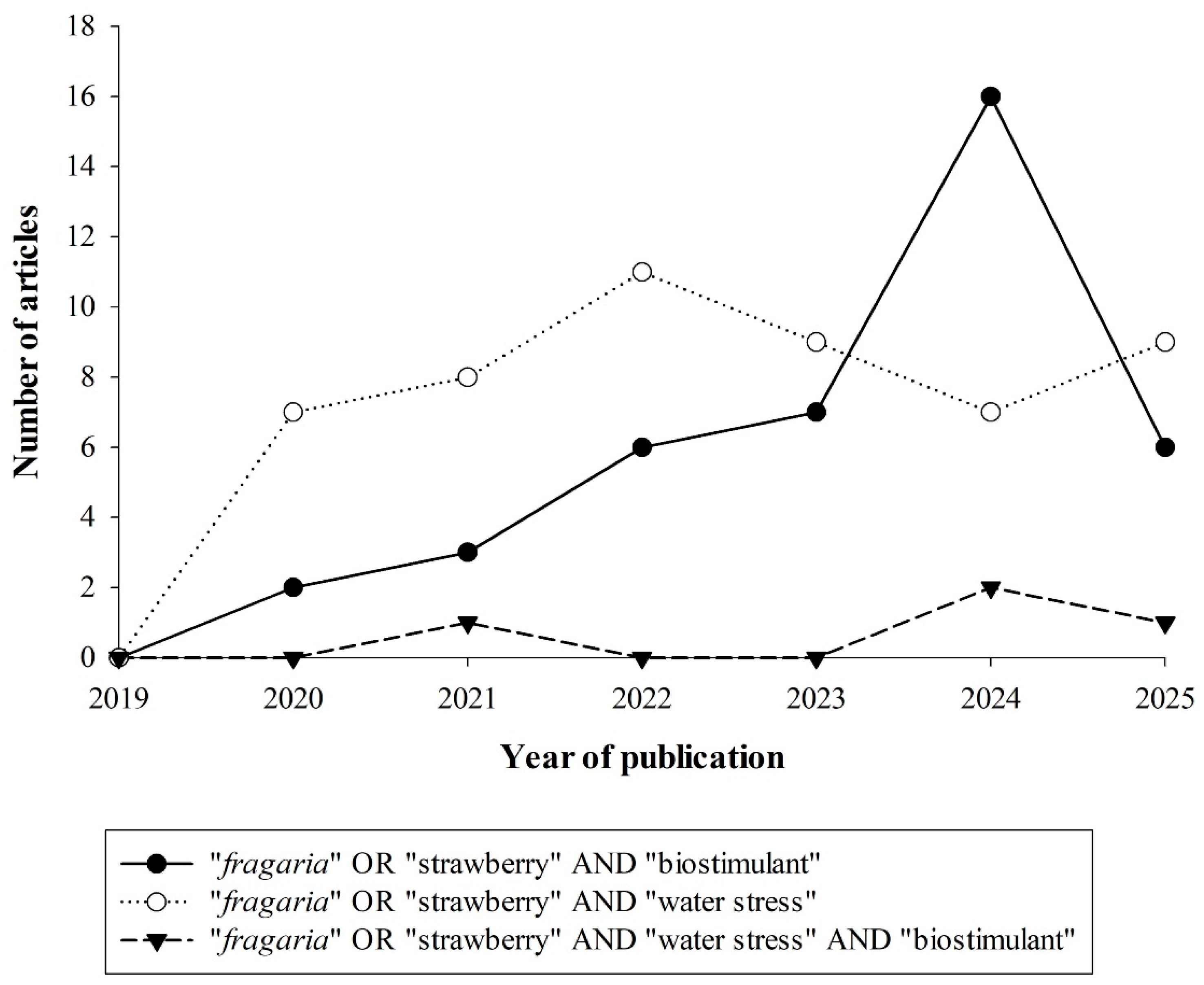
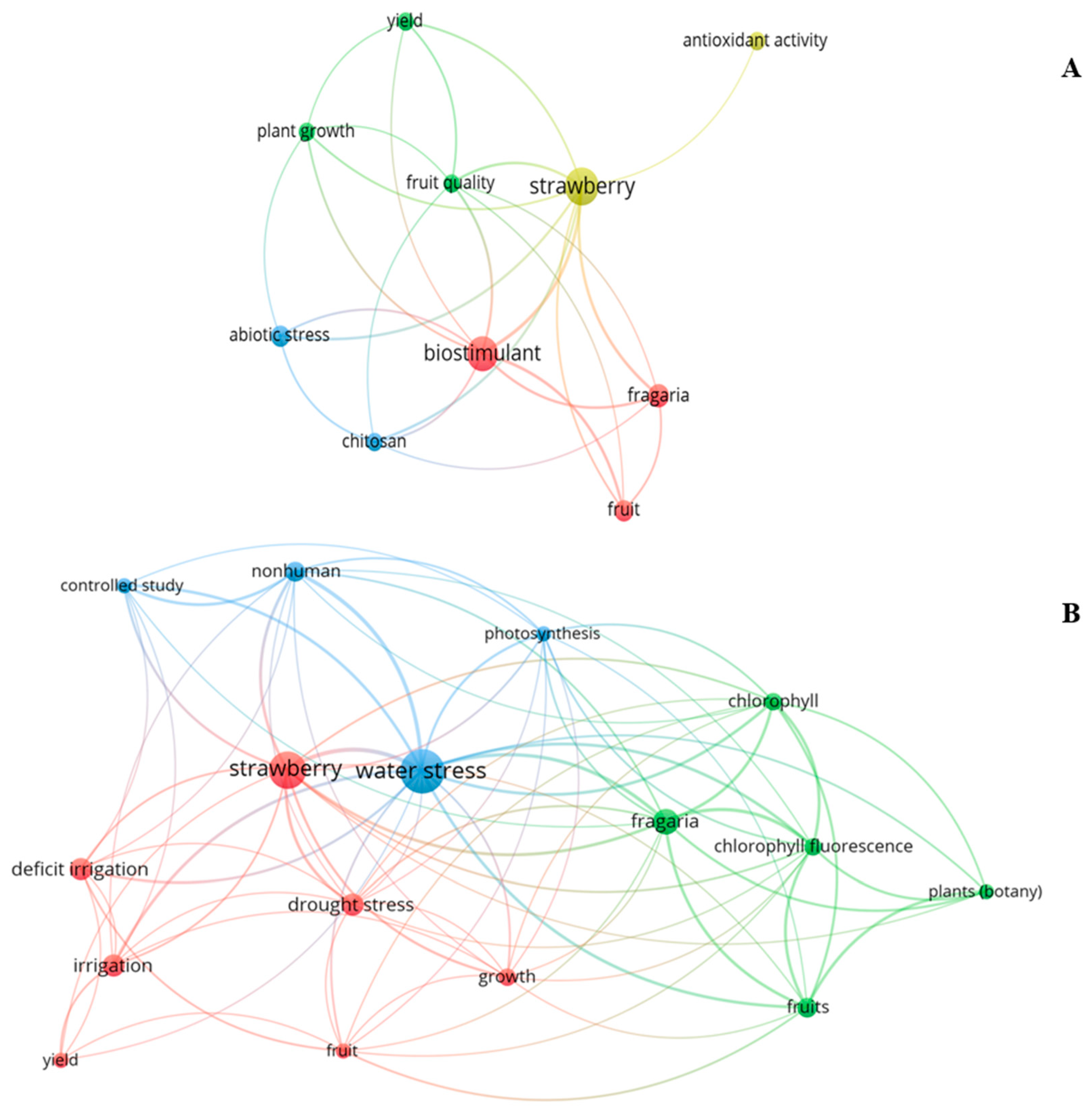
2. Bibliometric Review
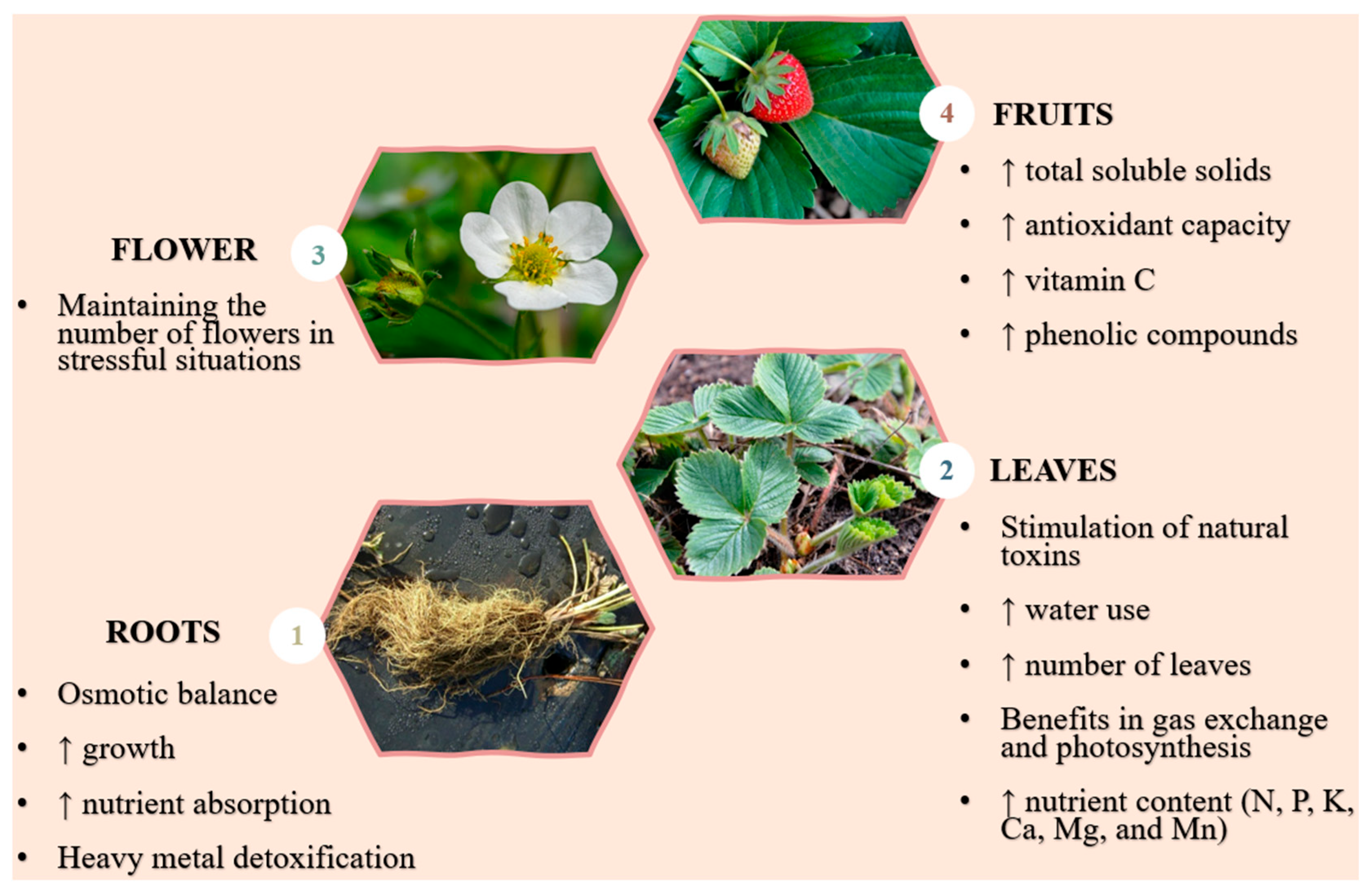
3. Strawberry Cultivation Associated with Water Stress
| Parts of a Plant | Effects of Water Regimes on Strawberry Morphophysiology | References |
|---|---|---|
| Root |
| [25,40,41] |
| [42] | |
| [43] | |
| [44] | |
| [45] | |
| [40] | |
| Leaf |
| [46,47] |
| [43] | |
| [36,48,49] | |
| [23,50] | |
| [34] | |
| [38] | |
| [20,28,51] | |
| [52] | |
| Flower |
| [53] |
| [36] | |
| [54] | |
| Fruit |
| [55,56] |
| [34] | |
| [18] | |
| [16,36] | |
| [40] | |
| [37] | |
| [20,49,57] | |
| [39] | |
| [58] | |
| [38] | |
| [35] | |
| Stolon |
| [32] |
| [59] | |
| Hormonal dynamics |
| [25] |
| Gene regulation |
| [48] |
| [25] | |
| [60] |
4. Biostimulants and Perspectives in the Attenuation of Water Deficit in Strawberry
4.1. Overview of the Use of Biostimulants
4.2. Effects of Biostimulants on Morphophysiological Processes
| Biostimulants | Effects | References |
|---|---|---|
| Silicon |
| [78,79] |
| Potassium silicate and potassium phosphite compound |
| [9] |
| Nanoselenium |
| [80] |
| Zeolite, kaolin, and chitosan |
| [81,82] |
| Seaweed extracts associated with microorganisms |
| [11,14,77] |
| Seaweed extracts (Durvillaea potatorum and Ascophyllum nodosum) |
| [69,83] |
| Ascophyllum nodosum |
| [70,84] |
| Ecklonia maxima |
| [85] |
| Scenedesmus sp. |
| [86] |
| Thymus capitatus essential oil and microalgae consortium (Chlorella sp., Scenedesmus sp., Spirulina sp., and Synechocystis sp.) |
| [87] |
| Amino acids and yeast extracts |
| [88] |
| Polyamines |
| [89] |
| Humic acid |
| [72] |
| Fulvic acid |
| [73] |
| Salicylic acid, glutamic acid, and cysteine |
| [2,3,90] |
| Citric acid |
| [63] |
| Fermented kiwi |
| [91] |
| Hydroalcoholic extracts of Calendula officinalis, Salvia officinalis, Tagetes sp., and Taraxacum officinalis |
| [92] |
| Extract of Moringa oleifera L. |
| [93] |
| Trichoderma atroviride and vegetable protein hydrolysate |
| [94] |
| Trichoderma harzianum strain T22 |
| [63] |
| Azospirillum brasilense, Gluconacetobacter diazotrophicus, and Bacillus amyloliquefaciens |
| [63] |
| Bacillus pumilis and Ampelomyces |
| [95] |
| Arthrobacter agilis UMCV2 and Bacillus methylotrophicus M4-96 |
| [96] |
| Methylobacterium symbioticum |
| [74] |
| Beauveria bassiana |
| [75] |
| Metarhizium brunneum |
| [76] |
4.3. State-of-the-Art on Biostimulants as Mitigators of Water Deficit in Strawberry Plants
5. Final Considerations and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiomento, J.L.T.; De Nardi, F.S.; Kujawa, S.C.; Deggerone, Y.S.; Fante, R.; Kaspary, I.J.; Dornelles, A.G.; Huzar-Novakowiski, J.; Trentin, T.S. Multivariate contrasts of seven strawberry cultivars in soilless cultivation and greenhouse in southern Brazil. Adv. Chem. Res. 2023, 2, 62–76. [Google Scholar] [CrossRef]
- Almutairi, K.F.; Alharbi, A.R.; Abdelaziz, M.E.; Mosa, W.F.A. Salicylic acid and chitosan effects on fruit quality when applied to fresh strawberry or during different periods of cold storage. BioResources 2024, 19, 6057–6075. [Google Scholar] [CrossRef]
- Javalera-Rincón, M.D.L.; González-Fuentes, J.A.; Benavides-Mendoza, A.; Robledo-Olivo, A.; Lara-Reimers, E.A.; Morelos-Moreno, Á. Efecto de bioestimulantes en producción y calidad de fresa (Fragaria ananassa cv. Albión) bajo estrés hídrico. Terra Latinoam. 2024, 42, 1937. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Chiomento, J.L.T.; De Nardi, F.S.; Fante, R.; Dal Pizzol, E.; Trentin, T.S.; Borba, A.C.; Basílio, L.S.P.; Garcia, V.A.S.; Lima, G.P.P. Building the microbiota in strawberry soilless cultivation systems with on-farm AMF inoculants: Roles in yield, phytochemical profile, and root morphology. S. Afr. J. Bot. 2025, 178, 226–234. [Google Scholar] [CrossRef]
- Dal Magro, S.Z.; Chiomento, J.L.T.; Werner, H.A.; Bortoluzzi, E.C.; Bortoluzzi, M.P. Enhancing greenhouse strawberry irrigation: Integrating IoT technologies and low-cost moisture sensors in substrate. Cad. Pedagóg. 2024, 21, 7258. [Google Scholar] [CrossRef]
- Kaman, H.; Gubbuk, H.; Tezcan, A.; Can, M.; Özbek, Ö. Water-yield relationship of greenhouse-grown strawberry under limited irrigation. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13235. [Google Scholar] [CrossRef]
- Klamkowski, K.; Treder, W. Morphological and physiological responses of strawberry plants to water stress. Agric. Conspec. Sci. 2006, 71, 159–165. [Google Scholar]
- Wise, K.; Selby-Pham, J. Strawberry field trial in Australia demonstrates improvements to fruit yield and quality control conformity, from application of two biostimulant complexes. N. Z. J. Crop Hortic. Sci. 2024, 53, 3124–3139. [Google Scholar] [CrossRef]
- Ranasingha, R.; Perera, A.; Tabugbo, K.; Vasilev, V. Enhancing plant growth and yield under reduced water and nutrient conditions: The role of biostimulants in improving irrigation efficiency and drought resilience in soilless strawberry cultivation under glasshouse conditions. J. Sustain. Agric. Environ. 2025, 4, 70082. [Google Scholar] [CrossRef]
- Ciriello, M.; Pannico, A.; Rouphael, Y.; Basile, B. Enhancing yield, physiological, and quality traits of strawberry cultivated under organic management by applying different non-microbial biostimulants. Plants 2025, 14, 712. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Treviño, S.; Manzano-Camarillo, M.G.F. Review of water scarcity assessments: Highlights of Mexico’s water situation. WIREs Water 2024, 11, 1721. [Google Scholar] [CrossRef]
- Leogrande, R.; El Chami, D.; Fumarola, G.; Di Carolo, M.; Piegari, G.; Elefante, M.; Perrelli, D.; Dongiovanni, C. Biostimulants for resilient agriculture: A preliminary assessment in Italy. Sustainability 2022, 14, 6816. [Google Scholar] [CrossRef]
- Kapur, B.; Karaca, C.; Sarıdaş, M.A.; Ağçam, E.; Çeliktopuz, E.; Kargı, S.P. Enhancing secondary compounds in strawberry fruit through optimized irrigation and seaweed application. Sci. Hortic. 2024, 324, 112609. [Google Scholar] [CrossRef]
- Díaz-Galián, M.V.; Torres, M.; Sanchez-Pagán, J.D.; Navarro, P.J.; Weiss, J.; Egea-Cortines, M. Enhancement of strawberry production and fruit quality by blue and red LED lights in research and commercial greenhouses. S. Afr. J. Bot. 2021, 140, 269–275. [Google Scholar] [CrossRef]
- Çeliktopuz, E.; Sarıdaş, M.A.; Kapur, B.; Ağçam, E.; Koyuncu, H.C. The impact of irrigation levels and abscisic acid application on the biochemical profiles of strawberries. Food Chem. 2025, 482, 144077. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, B.; Tang, G.; Chen, Y.; Deng, M.; Lin, Y.; Li, M.; He, W.; Wang, Y.; Zhang, Y.; et al. Application of γ-aminobutyric acid improves the postharvest marketability of strawberry by maintaining fruit quality and enhancing antioxidant system. Food Chem. 2024, 21, 101252. [Google Scholar] [CrossRef]
- Raffaelli, D.; Qaderi, R.; Mazzoni, L.; Mezzetti, B.; Capocasa, F. Yield and sensorial and nutritional quality of strawberry (Fragaria × ananassa Duch.) fruits from plants grown under different amounts of irrigation in soilless cultivation. Plants 2025, 14, 286. [Google Scholar] [CrossRef]
- Zhong, Y.; Wei, X.; Zhang, J.; Wang, L. Transcriptome sequencing reveals jasmonate playing a key role in ALA-induced osmotic stress tolerance in strawberry. BMC Plant Biol. 2025, 25, 41. [Google Scholar] [CrossRef]
- Kapur, B.; Saridaş, M.A.; Çelİktopuz, E.; Kargi, S.P. Evaluation of the yield of strawberry genotypes by morpho-physiological parameters under deficit irrigation in the Mediterranean environment. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13079. [Google Scholar] [CrossRef]
- Appezzato-da-Glória, B.; Miranda-Stalder, S.H.G. Anatomia foliar e do pedúnculo floral de plantas de morangueiro (Fragaria × ananassa) “Sequoia” tratadas com fitorreguladores. An. ESALQ 1991, 48, 127–154. [Google Scholar]
- Bortolozzo, A.R.; Sanhueza, R.M.V.; Melo, G.W.B.; Kovaleski, A.; Bernardi, J.; Botton, M.; Freire, J.M.; Braghini, L.C.; Vargas, L.; Calegario, F.F.; et al. Produção de Morangos no Sistema Semi-Hidropônico, 2nd ed.; Embrapa Uva e Vinho: Bento Gonçalves, Brasil, 2007; p. 24. [Google Scholar]
- Gao, F.; Zheng, R.; Guo, J.; Li, Y.; Dong, S.; Shen, Y.; Li, X. Physiological response of strawberry to water stress under different deficit irrigations. J. Irrig. Drain. Eng. 2021, 40, 1–6. [Google Scholar]
- Garrido, A.; Conde, A.; Serôdio, J.; De Vos, R.C.H.; Cunha, A. Fruit photosynthesis: More to know about where, how and why. Plants 2023, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Song, R.; Wang, X.; Wang, J.; Wu, C. Transcriptomic and metabolomic analyses provide new insights into the response of strawberry (Fragaria × ananassa Duch.) to drought stress. Horticulturae 2024, 10, 734. [Google Scholar] [CrossRef]
- Martínez-Ferri, E.; Soria, C.; Ariza, M.T.; Medina, J.J.; Miranda, L.; Domíguez, P.; Muriel, J.L. Water relations, growth and physiological response of seven strawberry cultivars (Fragaria × ananassa Duch.) to different water availability. Agric. Water Manag. 2016, 164, 73–82. [Google Scholar] [CrossRef]
- Schattman, R.E.; Smart, A.; Birkel, S.; Jean, H.; Barai, K.; Zhang, Y.J. Strawberry growth under current and future rainfall scenarios. Water 2022, 14, 313. [Google Scholar] [CrossRef]
- Shokaeva, D.B. Drought tolerance of strawberries as related to reaction type and water content and redistribution in leaves. Acta Hortic. 2021, 1309, 253–260. [Google Scholar] [CrossRef]
- Wamser, A.F.; Valmorbida, J. Evolução do consumo hídrico do morangueiro em cultivo com substrato. Agropecu. Catarin. 2022, 35, 24–26. [Google Scholar] [CrossRef]
- Ariza, M.T.; Miranda, L.; Gómez-Mora, J.A.; Medina, J.J.; Lozano, D.; Gavilán, P.; Soria, C.; Martínez-Ferri, E. Yield and fruit quality of strawberry cultivars under different irrigation regimes. Agronomy 2021, 11, 261. [Google Scholar] [CrossRef]
- Amini, A.; Karami, F.; Sedri, M.H.; Khaledi, V. Determination of water requirement and crop coefficient for strawberry using lysimeter experiment in a semi-arid climate. H2Open J. 2022, 5, 642–655. [Google Scholar] [CrossRef]
- Erdem, S.Ö.; Mutluay, M.K.; Karaer, M.; Gültaş, H.T. The effect of Fe3O4 nanoparticle applications on seedling development in water-stressed strawberry (Fragaria × ananassa ‘Albion’) plants. Appl. Fruit Sci. 2025, 67, 73. [Google Scholar] [CrossRef]
- Yamanaka, R.; Yano, T.; Hikawa-Endo, M.; Yoshikoshi, H.; Kawashima, H.; Tojo, M.; Wada, T. Relationship between vapor pressure deficit condition and water-stress status in strawberry. Hortic. J. 2025, 94, 73–80. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Nulit, R.; Sakimin, S.Z. Influence of drought stress on growth, biochemical changes and leaf gas exchange of strawberry (Fragaria × ananassa Duch.) in Indonesia. AIMS Agric. Food 2022, 7, 37–60. [Google Scholar] [CrossRef]
- Atakan, E.; Saridaş, M.A.; Pehlivan, S.; Achiri, T.D.; Çeliktopuz, E.; Kapur, B. Influence of irrigation regimes on yield, pomological parameters and population development of Tetranychus cinnabarinus Boisd. (Acari: Tetranychidae) in strawberry. Syst. Appl. Acarol. 2021, 26, 1241–1253. [Google Scholar] [CrossRef]
- Marcellini, M.; Mazzoni, L.; Raffaelli, D.; Pergolotti, V.; Balducci, F.; Capocasa, F.; Mezzetti, B. Evaluation of single-cropping under reduced water supply in strawberry cultivation. Agronomy 2022, 12, 1396. [Google Scholar] [CrossRef]
- Yang, P.; Drohan, P.J.; Zhang, X.; Long, H.; Soulis, K.X.; Shi, X. Impacts of deficit irrigation on strawberry physiology, water productivity, quality, and yield. Sustainability 2025, 17, 675. [Google Scholar] [CrossRef]
- Çeliktopuz, E. Determination of drought tolerance of different strawberry genotypes. PeerJ 2023, 11, 14972. [Google Scholar] [CrossRef]
- Erdem, S.Ö.; Karakoyun, M.; Karaer, M. Impact of sodium selenate foliar application under water stress conditions on yield, quality, and mineral composition in strawberry cultivation. N. Z. J. Crop Hortic. Sci. 2025, 53, 2353–2366. [Google Scholar] [CrossRef]
- Javan, M.; Selahvarzi, Y.; Sayyad-Amin, P.; Rastegar, S. Potential application of TiO2 nanoparticles to improve the nutritional quality of strawberry cv. Camarosa under drought stress. Sci. Hortic. 2024, 330, 113055. [Google Scholar] [CrossRef]
- Korkmaz, K.; Bolat, I.; Karakas, S.; Dikilitas, M. Responses to single and combined application of humic acid and silicon under water stress on strawberry. Erwerbs-Obstbau 2022, 64, 523–533. [Google Scholar] [CrossRef]
- Şimşek, Ö. Machine learning offers insights into the impact of in vitro drought stress on strawberry cultivars. Agriculture 2024, 14, 294. [Google Scholar] [CrossRef]
- Yosefi, A.; Mozafari, A.; Javadi, T. In vitro assessment of strawberry (Fragaria × ananassa Duch.) plant responses to water shortage stress under nano-iron application. Vitr. Cell. Dev. Biol. Plant. 2022, 58, 499–510. [Google Scholar]
- Gil-Marín, J.A.; Zermeño-González, A.; Moreno-Ibarra, L.A.; Hernández–Pérez, A.; Ramírez-Rodríguez, H.; Gaspar-Ramírez, O. Effect of substrate water consumption and fertilization levels in the yield of strawberry (Fragaria × ananassa Duch.). Agrociencia 2025, 59, 246–261. [Google Scholar]
- Lone, J.M.; Agehara, S.; Abd-Elrahman, A. Intermittent sprinkler irrigation during the establishment of strawberry (Fragaria × ananassa Duch.) bare-root transplants conserves water without loss of yield and fruit quality. Agric. Water Manag. 2024, 306, 109169. [Google Scholar] [CrossRef]
- Sekhi, Y.S.; Kadhim, Z.K.; Hamad, A.H. Effect of sodium azide on some vegetative and biochemical properties of strawberry under polyethylene glycol of Albion variety in vitro. Ann. Agric. Bio. Res. 2023, 28, 121–128. [Google Scholar]
- Yosefi, A.; Mozafari, A.; Javadi, T. Jasmonic acid improved in vitro strawberry (Fragaria × ananassa Duch.) resistance to PEG-induced water stress. Plant Cell Tissue Organ Cult. 2020, 142, 549–558. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, T.; Wang, X.; Zong, X.; Wu, C. Functional physiological phenotyping and transcriptome analysis provide new insight into strawberry growth and water consumption. Front. Plant Sci. 2022, 13, 1074132. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Nulit, R.; Sakimin, S.Z. The interactive effects of fertilizer and water stress on plant growth, leaf gas exchange and nutrient uptake on strawberry (Fragaria × ananassa, Duch). AIMS Environ. Sci. 2021, 8, 597–618. [Google Scholar]
- Si, B.; Jing, M.; Jiang, N.; He, Z.; Han, H.; Chen, M. Predictive analysis of effects of water stress on strawberry seedlings using fluorescent image channel components. Rev. Sci. Instrum. 2023, 94, 125108. [Google Scholar] [CrossRef]
- Poobalasubramanian, M.; Park, E.S.; Faqeerzada, M.A.; Kim, T.; Kim, M.S.; Baek, I.; Cho, B.K. Identification of early heat and water stress in strawberry plants using chlorophyll-fluorescence indices extracted via hyperspectral images. Sensors 2022, 22, 8706. [Google Scholar] [CrossRef]
- Zeid, I.M.A.; Mohamed, F.H.; Metwali, E.M. Zinc and silicon nanomolecules application enhances tolerance to PEG-induced drought stress in strawberry cultured in vitro. Int. J. Agric. Biol. 2021, 26, 469–478. [Google Scholar]
- Shakya, R.; Capilla, E.; Torres-Pagán, N.; Muñoz, M.; Boscaiu, M.; Lupuţ, I.; Vicente, O.; Verdeguer, M. Effect of two biostimulants, based on Ascophyllum nodosum extracts, on strawberry performance under mild drought stress. Agriculture 2023, 13, 2108. [Google Scholar] [CrossRef]
- Radhi, I.M.; Abudl-Hasan, M.M. Effect of spraying with proline acid and potassium on chemical traits and yield of strawberry under water stress. Plant Arch. 2020, 20, 75–83. [Google Scholar]
- Furio, R.N.; Salazar, S.M.; Mariotti-Martínez, J.A.; Martínez-Zamora, G.M.; Coll, Y.; Díaz-Ricci, J.C. Brassinosteroid applications enhance the tolerance to abiotic stresses, production and quality of strawberry fruits. Horticulturae 2022, 8, 572. [Google Scholar] [CrossRef]
- Rokosa, M.; Mikiciuk, M. Assessment of physiological and morphological traits of plants of the genus Fragaria under conditions of water deficit—A study review. Acta Sci. Pol. Hortorum Cultus 2020, 19, 21–40. [Google Scholar] [CrossRef]
- Çeliktopuz, E.; Kapur, B.; Sarıdas, M.A.; Kargı, S.P. Response of strawberry fruit and leaf nutrient concentrations to the application of irrigation levels and a biostimulant. J. Plant Nutr. 2021, 44, 153–165. [Google Scholar] [CrossRef]
- Yamanaka, R.; Yano, T.; Hikawa-Endo, M.; Yoshikoshi, H.; Kawashima, H.; Tojo, M.; Wada, T. Effects of humidification based on vapor pressure deficit (VPD) on plant growth, fruit yield, and fruit quality traits in june-bearing strawberry. Hortic. J. 2024, 93, 377–388. [Google Scholar] [CrossRef]
- Wei, H.; Liu, C.; Ryong Jeong, B. An optimal combination of the propagation medium and fogging duration enhances the survival, rooting and early growth of strawberry daughter plants. Agronomy 2020, 10, 557. [Google Scholar] [CrossRef]
- Kesici, M.; Ipek, A.; Ersoy, F.; Ergin, S.; Gülen, H. Genotype-dependent gene expression in strawberry (Fragaria × ananassa) plants under high temperature stress. Biochem. Genet. 2020, 58, 848–866. [Google Scholar] [CrossRef]
- Cayuela, M.L.; Sánchez-Monedero, M.A.; Roig, A.; Hanley, K.; Enders, A.; Lehmann, J. Biochar and denitrification in soils: When, how much and why does biochar reduce N2O emissions? Sci. Rep. 2013, 3, 1732. [Google Scholar] [CrossRef]
- Saavedra, T.; Gama, F.; Correia, P.J.; Silva, J.P.; Miguel, M.G.; Varennes, A.; Pestana, M. A novel plant extract as a biostimulant to recover strawberry plants from iron chlorosis. J. Plant Nutr. 2020, 43, 2054–2066. [Google Scholar] [CrossRef]
- Soltaniband, V.; Brégard, A.; Gaudreau, L.; Dorais, M. Biostimulants promote plant development, crop productivity, and fruit quality of protected strawberries. Agronomy 2022, 12, 1684. [Google Scholar] [CrossRef]
- Garza-Alonso, C.A.; Olivares-Sáenz, E.; González-Morales, S.; Cabrera-De la Fuente, M.; Juárez-Maldonado, A.; González-Fuentes, J.A.; Tortella, G.; Valdés-Caballero, M.V.; Benavides-Mendoza, A. Strawberry biostimulation: From mechanisms of action to plant growth and fruit quality. Plants 2022, 11, 3463. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.B.; Petry, C.; Silveira, D.C.; Trentin, T.S.; Turmina, A.P.F.L.; Chiomento, J.L.T. Use of biostimulants in fruiting crops’ sustainable management: A narrative review. LADEE 2023, 4, 29–48. [Google Scholar] [CrossRef]
- Berrie, A.; Passey, T.; Xu, X. Integrating management of powdery mildew with Botrytis in protected strawberries in the UK. Crop Prot. 2022, 154, 105902. [Google Scholar] [CrossRef]
- Martinez-Alonso, A.; Fermoso, J.; Verdugo, F.; Carvajal, M. Benefit from biomass boiler emissions to increase greenhouse CO2 levels for optimal growth and yield in tomato, cucumber, and strawberry. Adv. Energy Sustain. Res. 2025, 6, 2400395. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W. The importance of salicylic acid, humic acid and fulvic acid on crop production. Lett. Drug Des. Discov. 2024, 21, 1465–1480. [Google Scholar] [CrossRef]
- Rana, V.S.; Lingwal, K.; Sharma, S.; Rana, N.; Pawar, R.; Kumar, V.; Sharma, U. Enhancement in growth, yield and nutritive characteristics of strawberry (Fragaria × ananassa Duch.) by the application of biostimulant: Seaweed extract. Acta Physiol. Plant. 2023, 45, 122. [Google Scholar] [CrossRef]
- Pereira, S.; Rodrigues, J.; Sujeeth, N.; Guinan, K.J.; Gonçalves, B. Optimizing strawberry growth: Impact of irrigation and biostimulant application on physiology and fruit quality. Plant Stress 2025, 15, 100715. [Google Scholar] [CrossRef]
- de Oliveira, E.; Nadal, M.C.; Rodrigues, F.A.; dos Santos, H.O.; de Sousa, L.F.; Martins, A.D.; Sepúlveda, A.M.G.; Dória, J.; Pasqual, M. Use of organic acids in micropropagation helps the production of salinity tolerant strawberry. Plant Cell Tissue Organ Cult. 2024, 156, 71. [Google Scholar] [CrossRef]
- Kilic, N. Improvement in plant growth, yield, and fruit quality with biostimulant treatment in organic strawberry cultivation. HortScience 2024, 59, 1165–1171. [Google Scholar] [CrossRef]
- Cruz, S.M.D.; Gonzálezfuentes, J.A.; Robledo-Olivo, A.; Mendozavillarreal, R.; Hernández-Pérez, A.; Dávila-Medina, M.D.; Alvarado-Camarillo, D. Humic substances and rhizobacteria enhance the yield, physiology and quality of strawberries. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12578. [Google Scholar] [CrossRef]
- Torres Vera, R.; Bernabé García, A.J.; Carmona Álvarez, F.J.; Martínez Ruiz, J.; Fernández Martín, F. Application and effectiveness of Methylobacterium symbioticum as a biological inoculant in maize and strawberry crops. Folia Microbiol. 2024, 69, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Mantzoukas, S.; Daskalaki, E.; Kitsiou, F.; Papantzikos, V.; Servis, D.; Bitivanos, S.; Patakioutas, G.; Eliopoulos, P.A. Dual action of Beauveria bassiana (Hypocreales; Cordycipitaceae) endophytic stains as biocontrol agents against sucking pests and plant growth biostimulants on melon and strawberry field plants. Microorganisms 2022, 10, 2306. [Google Scholar] [CrossRef]
- Wood, M.J.; Kortsinoglou, A.M.; Khoja, S.; Kouvelis, V.N.; Myrta, A.; Midthassel, A.; Loveridge, E.J.; Butt, T.M. Metarhizium brunneum (Hypocreales: Clavicipitaceae) and its derived volatile organic compounds as biostimulants of commercially valuable angiosperms and gymnosperms. J. Fungi 2022, 8, 1052. [Google Scholar] [CrossRef]
- Li, J.; Brecht, J.K.; Kim, J.; Bailey, L.S.; Kamat, M.N.; Basso, K.B.; Colee, J.C.; Zhao, X. Seaweed extract and microbial biostimulants show synergistic effects on improving organic strawberry production. HortScience 2024, 59, 1114–1126. [Google Scholar] [CrossRef]
- Ambros, E.; Kotsupiy, O.; Karpova, E.; Panova, U.; Chernonosov, A.; Trofimova, E.; Goldenberg, B. A biostimulant based on silicon chelates enhances growth and modulates physiological responses of in-vitro-derived strawberry plants to in vivo conditions. Plants 2023, 12, 4193. [Google Scholar] [CrossRef]
- Goldenberg, B.G.; Gusev, I.S.; Kopalkin, I.P.; Ambros, E.V. Upgrowth of a method for identification of light elements in plants by the SR-XRF method at VEPP-4M storage ring. Russ. Phys. J. 2024, 67, 2349–2354. [Google Scholar] [CrossRef]
- Dong, Q.; Chen, G.; Zhou, Y.; Wu, Y.; Zhou, C.; Lin, Y.; Liu, D.; Pan, C. Combined application of tank-mix adjuvants, mist sprayer and nano-selenium promoted pesticide reduction and enhanced strawberry quality. Bull. Environ. Contam. Toxicol. 2023, 111, 11. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Almutairi, K.F.; Górnik, K.; Sas-Paszt, L. Influence of zeolite, kaolin, and chitosan on the growth and productivity of strawberry. BioResources 2025, 20, 1771–1793. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable agriculture systems in vegetable production using chitin and chitosan as plant biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Mattner, S.W.; Villalta, O.N.; McFarlane, D.J.; Islam, M.T.; Arioli, T.; Cahill, D.M. The biostimulant effect of an extract from Durvillaea potatorum and Ascophyllum nodosum is associated with the priming of reactive oxygen species in strawberry in south-eastern Australia. J. Appl. Phycol. 2023, 35, 1789–1800. [Google Scholar] [CrossRef]
- Kazakov, P.; Alseekh, S.; Ivanova, V.; Gechev, T. Biostimulant-based molecular priming improves crop quality and enhances yield of raspberry and strawberry fruits. Metabolites 2024, 14, 594. [Google Scholar] [CrossRef]
- Consentino, B.B.; Vultaggio, L.; Iacuzzi, N.; La Bella, S.; De Pasquale, C.; Rouphael, Y.; Ntatsi, G.; Virga, G.; Sabatino, L. Iodine biofortification and seaweed extract-based biostimulant supply interactively drive the yield, quality, and functional traits in strawberry fruits. Plants 2023, 12, 245. [Google Scholar] [CrossRef]
- Cho, G.; Jo, G.S.; Lee, Y.; Kwak, Y.S. Effect of Scenedesmus sp. CHK0059 on strawberry microbiota community. J. Microbiol. Biotechnol. 2022, 32, 862–868. [Google Scholar] [CrossRef]
- Chaouch, R.; Kthiri, Z.; Soufi, S.; Ben Jabeur, M.; Bettaieb, T. Assessing the biostimulant effect of micro-algae and thyme essential oil during in-vitro and ex-vitro rooting of strawberry. S Afr. J. Bot. 2023, 162, 120–128. [Google Scholar] [CrossRef]
- Ranasingha, R.; Perera, A.; Baghalian, K.; Gerofotis, C. Amino acid-based biostimulants and microbial biostimulants promote the growth, yield and resilience of strawberries in soilless glasshouse cultivation. J. Sustain. Agric. Environ. 2024, 3, 12113. [Google Scholar] [CrossRef]
- Adak, N.; Nasircilar, A.; Ulukapi, K. The effects of putrescine on pomology, yield and biochemical characteristics of strawberry plants under deficit irrigation. Acta Hortic. 2021, 1308, 189–195. [Google Scholar] [CrossRef]
- Kim, D.R.; Jeon, C.W.; Cho, G.; Thomashow, L.S.; Weller, D.M.; Paik, M.J.; Lee, Y.B.; Kwak, Y.S. Glutamic acid reshapes the plant microbiota to protect plants against pathogens. Microbiome 2021, 9, 244. [Google Scholar] [CrossRef]
- Nazeer, S.; Agosti, A.; Del Vecchio, L.; Leto, L.; Di Fazio, A.; Hadj Saadoun, J.; Levante, A.; Lazzi, C.; Cirlini, M.; Chiancone, B. Assessment of fermented kiwifruit on morpho-physiological and productive performances of Fragaria spp. plants, grown under hydroponic conditions. J. Sustain. Agric. Environ. 2024, 3, 1–12. [Google Scholar] [CrossRef]
- Furmańczyk, E.M.; Tartanus, M.; Malusà, E. The influence of plant extracts on root biostimulation in different strawberry (Fragaria × ananassa Duchense) cultivars. Acta Sci. Pol. Hortorum Cultus 2023, 22, 43–54. [Google Scholar] [CrossRef]
- Yuniati, N.; Kusumiyati, K.; Mubarok, S.; Nurhadi, B. The role of moringa leaf extract as a plant biostimulant in improving the quality of agricultural products. Plants 2022, 11, 2186. [Google Scholar] [CrossRef]
- Vultaggio, L.; Allevato, E.; Consentino, B.B.; Bellitto, P.; Napoli, S.; Cannata, C.; Ntatsi, G.; Vasto, S.; Baldassano, S.; La Bella, S.; et al. Joint action of Trichoderma atroviride and a vegetal derived-protein hydrolysate improves performances of woodland strawberry in Italy. Horticulturae 2024, 10, 459. [Google Scholar] [CrossRef]
- Berrie, A.; Xu, X. Developing biopesticide-based programmes for managing powdery mildew in protected strawberries in the UK. Crop Prot. 2021, 149, 105766. [Google Scholar] [CrossRef]
- Hernández-Soberano, C.; Ruíz-Herrera, L.F.; Valencia-Cantero, E. Endophytic bacteria Arthrobacter agilis UMCV2 and Bacillus methylotrophicus M4-96 stimulate achene germination, in vitro growth, and greenhouse yield of strawberry (Fragaria × ananassa). Sci. Hortic. 2020, 261, 109005. [Google Scholar] [CrossRef]
- Paliwoda, D.; Mikiciuk, G.; Mikiciuk, M.; Kisiel, A.; Sas-Paszt, L.; Miller, T. Effects of rhizosphere bacteria on strawberry plants (Fragaria × ananassa Duch.) under water deficit. Int. J. Mol. Sci. 2022, 23, 10449. [Google Scholar] [CrossRef] [PubMed]
- Marcellini, M.; Raffaelli, D.; Mazzoni, L.; Pergolotti, V.; Balducci, F.; Armas Diaz, Y.; Mezzetti, B.; Capocasa, F. Effects of different irrigation rates on remontant strawberry cultivars grown in soil. Horticulturae 2023, 9, 1026. [Google Scholar] [CrossRef]
- Danilov, I.; Vlajkov, V.; Šumić, Z.; Milić, A.; Horecki, A.T.; Dujković, T.; Živanović, N.; Simin, N.; Lesjak, M.; Grahovac, J. Valorization of strawberry juice production wastewater: Possibilities for polyphenols recovery and plant biostimulant production. Foods 2024, 13, 3224. [Google Scholar] [CrossRef]
- Daghari, I.; Abouaziza, F.B.; Daghari, H. Rethinking water and crop management in the irrigated district of Diyar-Al-Hujjej (Tunisia). Environ. Sci. Pollut. Res. 2023, 30, 71689–71700. [Google Scholar] [CrossRef]
- Zheng, C.; Abd-elrahman, A.; Whitaker, V. Remote sensing and machine learning in crop phenotyping and management, with an emphasis on applications in strawberry farming. Remote Sens. 2021, 13, 531. [Google Scholar] [CrossRef]
- Sýs, V.; Fošumpaur, P.; Kašpar, T. The impact of climate change on the reliability of water resources. Climate 2021, 9, 153. [Google Scholar] [CrossRef]
- Seddon, N.; Smith, A.; Smith, P.; Key, I.; Chausson, A.; Girardin, C.; House, J.; Srivastava, S.; Turner, B. Getting the message right on nature-based solutions to climate change. Glob. Change Biol. 2021, 27, 1518–1546. [Google Scholar] [CrossRef]
- Webb, P.; Benton, T.G.; Beddington, J.; Flynn, D.; Kelly, N.M.; Thomas, S.M. The urgency of food system transformation is now irrefutable. Nature Food 2020, 1, 584–585. [Google Scholar] [CrossRef]
- Galanakis, C.M. The future of food. Foods 2024, 13, 506. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.M.; Gad, D.A.M. Irrigation management for strawberry plants (Fragaria × ananassa Duch.) under greenhouse conditions. Egypt. J. Agric. Res. 2022, 100, 581–590. [Google Scholar] [CrossRef]
- Melo, B.P.; Carpinetti, P.A.; Fraga, O.T.; Rodrigues-Silva, P.L.; Fioresi, V.S.; Camargos, L.F.; Ferreira, M.F.S. Abiotic stresses in plants and their markers: A practice view of plant stress responses and programmed cell death mechanisms. Plants 2022, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Fedoreyeva, L.I. ROS as signaling molecules to initiate the process of plant acclimatization to abiotic stress. Int. J. Mol. Sci. 2024, 25, 11820. [Google Scholar] [CrossRef]
- Drobek, M.; Cybulska, M.F.J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2020, 10, 433. [Google Scholar] [CrossRef]
- Lau, S.E.; Lim, L.W.T.; Hamdan, M.F.; Chan, C.; Saidi, N.B.; Ong-Abdullah, J.; Tan, B.C. Enhancing plant resilience to abiotic stress: The power of biostimulants. Phyton-Int. J. Exp. Bot. 2025, 94, 1–31. [Google Scholar]
- Mannino, G. Plant biostimulants interaction: Scientific trends, markets dynamics, and real-world implication. J. Plant Interact. 2025, 20, 2572668. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassel, J.L.; Maldaner, L.V.C.; Bortoluzzi, M.P.; Colla, L.M.; Reichert Junior, F.W.; Palencia, P.; Chiomento, J.L.T. Biostimulants as a Tool for Mitigating Water Deficit Stress in Strawberry Cultivation. Agronomy 2025, 15, 2643. https://doi.org/10.3390/agronomy15112643
Cassel JL, Maldaner LVC, Bortoluzzi MP, Colla LM, Reichert Junior FW, Palencia P, Chiomento JLT. Biostimulants as a Tool for Mitigating Water Deficit Stress in Strawberry Cultivation. Agronomy. 2025; 15(11):2643. https://doi.org/10.3390/agronomy15112643
Chicago/Turabian StyleCassel, Júlia Letícia, Laura Valentina Caus Maldaner, Mateus Possebon Bortoluzzi, Luciane Maria Colla, Francisco Wilson Reichert Junior, Pedro Palencia, and José Luís Trevizan Chiomento. 2025. "Biostimulants as a Tool for Mitigating Water Deficit Stress in Strawberry Cultivation" Agronomy 15, no. 11: 2643. https://doi.org/10.3390/agronomy15112643
APA StyleCassel, J. L., Maldaner, L. V. C., Bortoluzzi, M. P., Colla, L. M., Reichert Junior, F. W., Palencia, P., & Chiomento, J. L. T. (2025). Biostimulants as a Tool for Mitigating Water Deficit Stress in Strawberry Cultivation. Agronomy, 15(11), 2643. https://doi.org/10.3390/agronomy15112643









