Dynamics of Soil Organic Carbon Mineralization Under Straw Addition: Evidence from a Controlled Incubation Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Preparation
2.2. Experimental Design
2.3. Incubation Experiment
2.4. Soil Analysis
2.5. Soil Extracellular Enzyme Activity
2.6. Statistical Analysis
3. Results
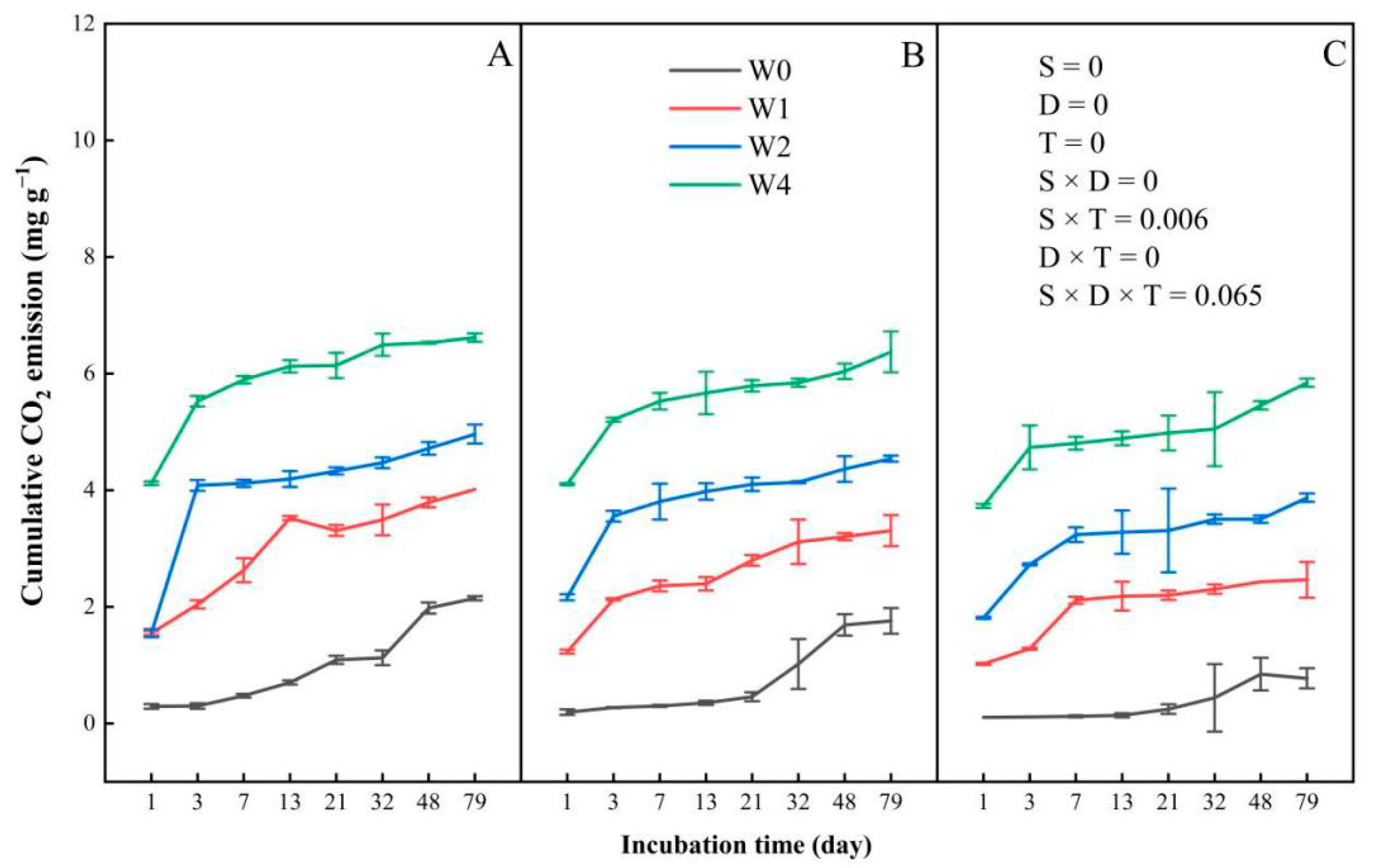
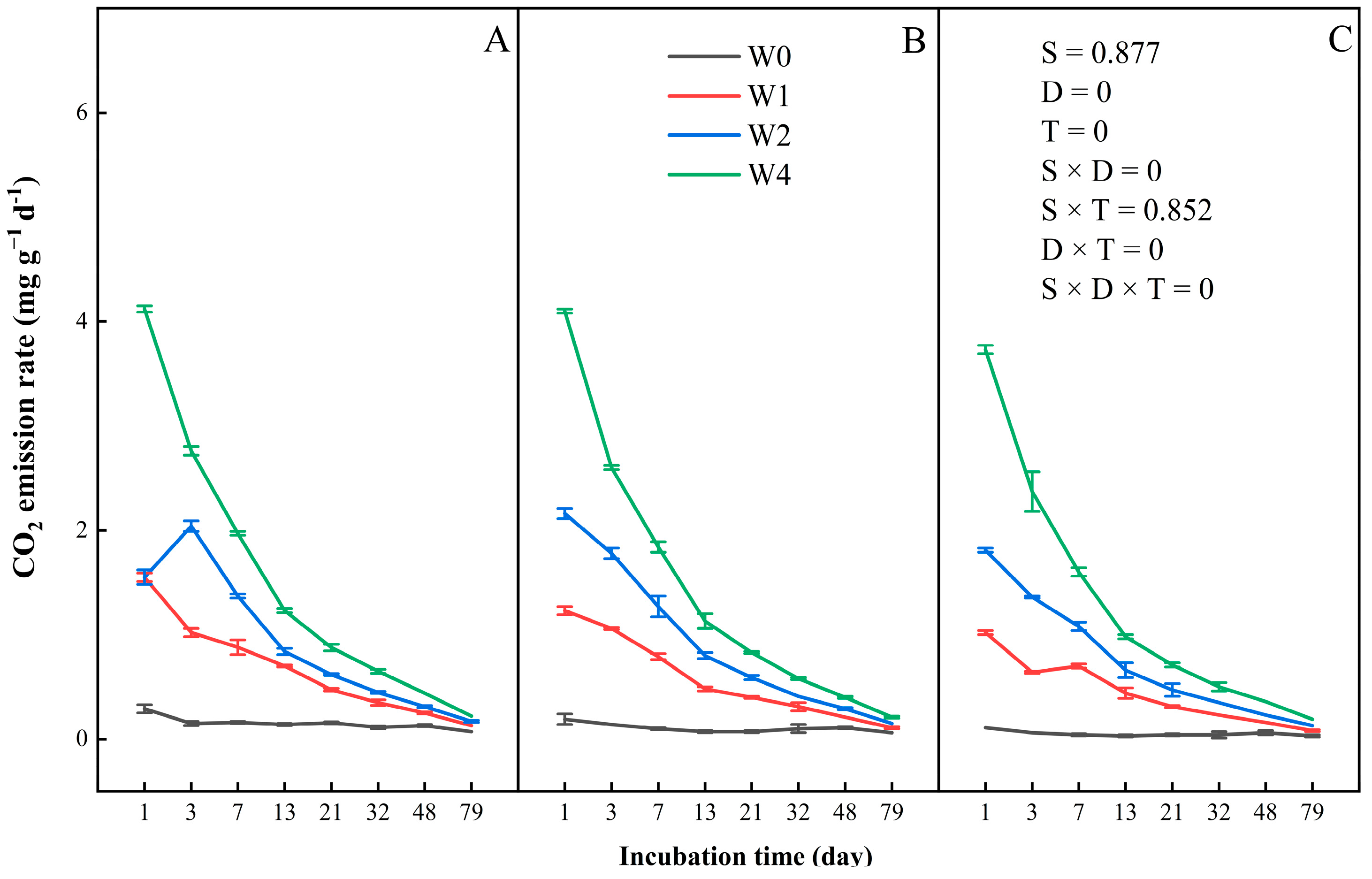
3.1. CO2 Emission
3.1.1. Cumulative CO2 Emission
3.1.2. CO2 Emission Rate
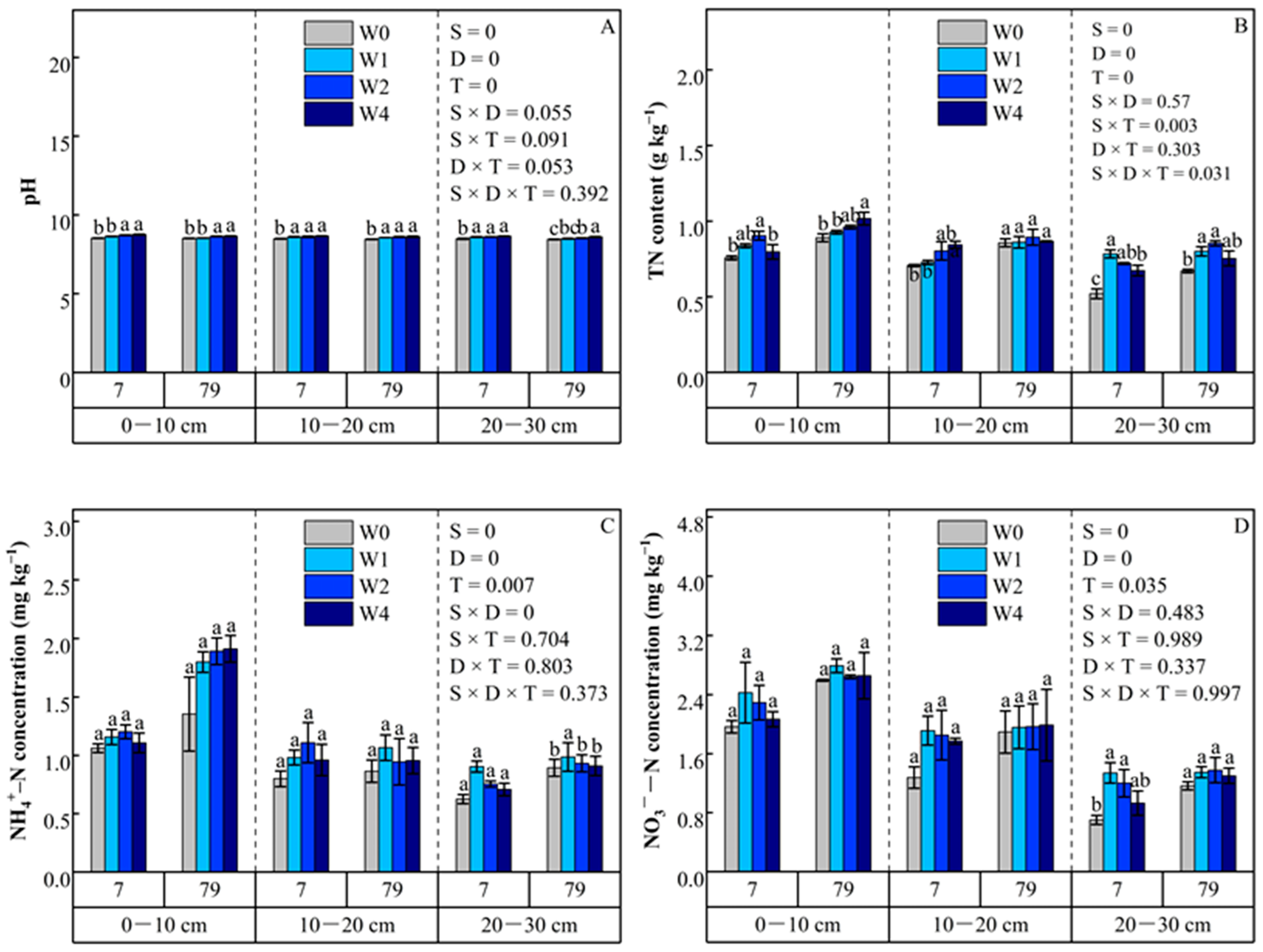
3.2. Soil Physicochemical Properties
3.2.1. pH
3.2.2. Total Nitrogen
3.2.3. Ammonium Nitrogen
3.2.4. Nitrate Nitrogen
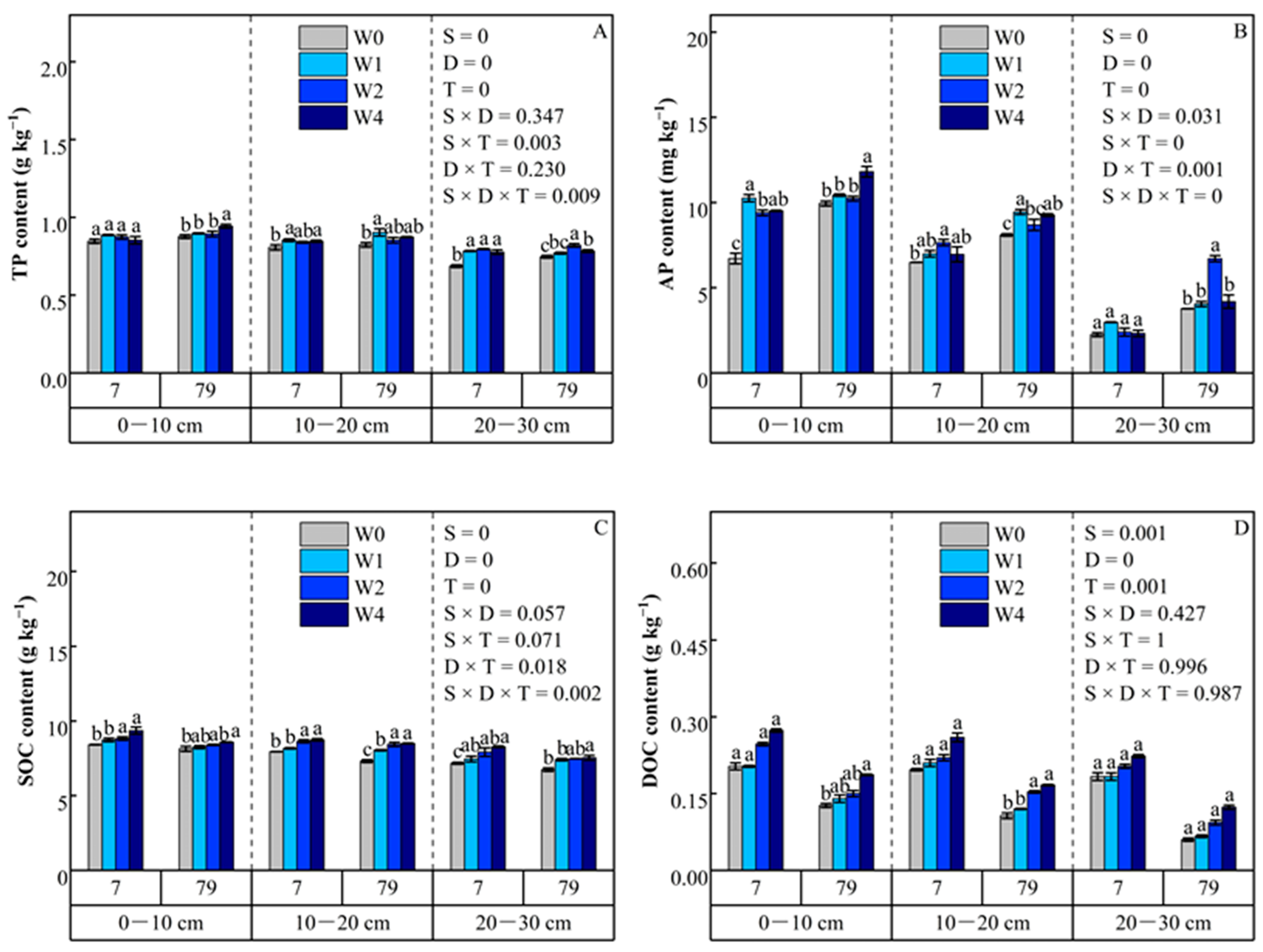
3.2.5. Total Phosphorus
3.2.6. Available Phosphorus
3.3. Soil Organic Carbon and Dissolved Organic Carbon
3.3.1. Soil Organic Carbon
3.3.2. Dissolved Organic Carbon
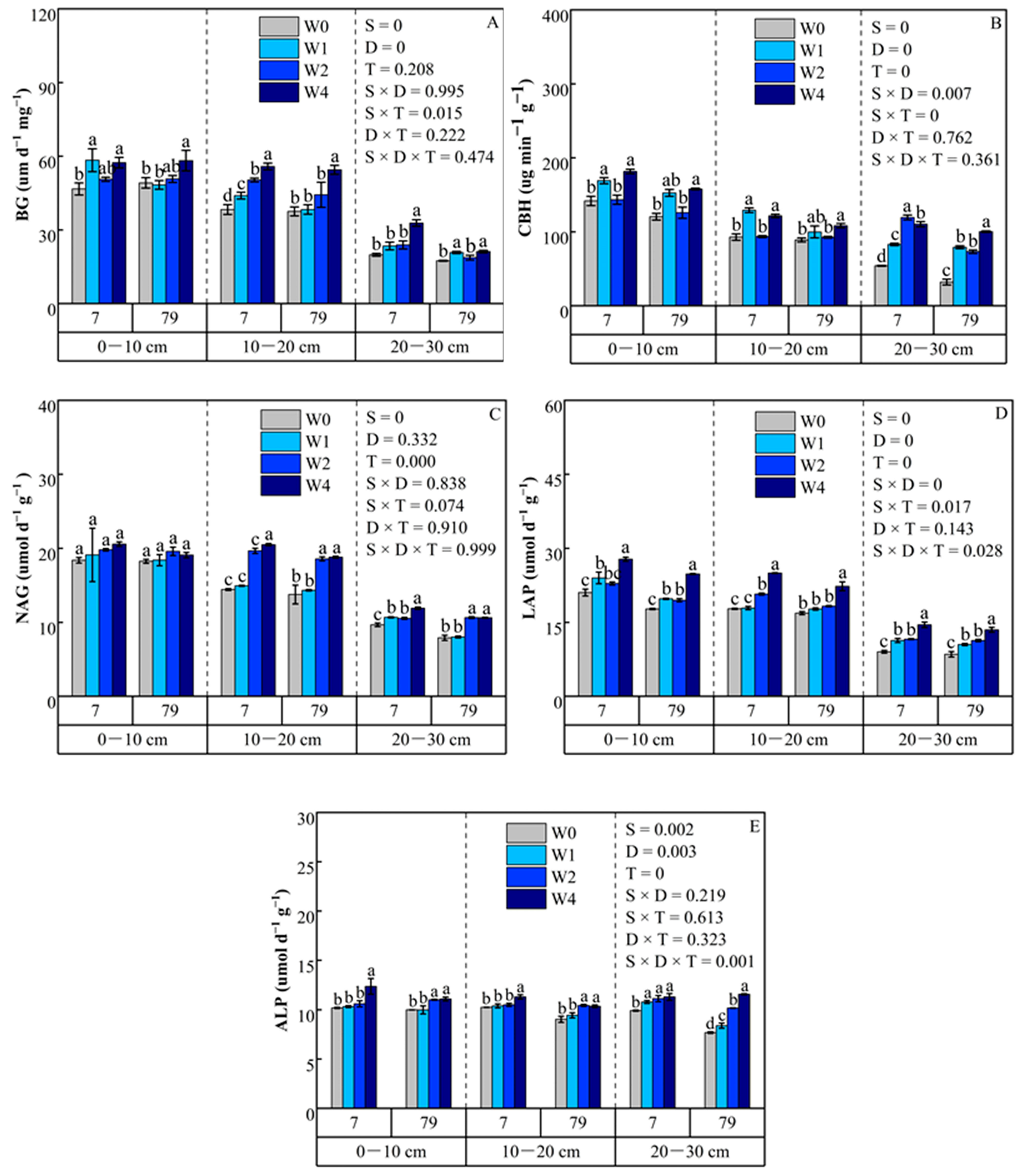
3.4. Extracellular Enzyme Activities (EEAs)
3.4.1. β-Glucosidase
3.4.2. β-Cellobiosidase
3.4.3. β-1, 4-N-Acetylglucosaminidase
3.4.4. L-Leucine Aminopeptidase
3.4.5. Alkaline Phosphatase
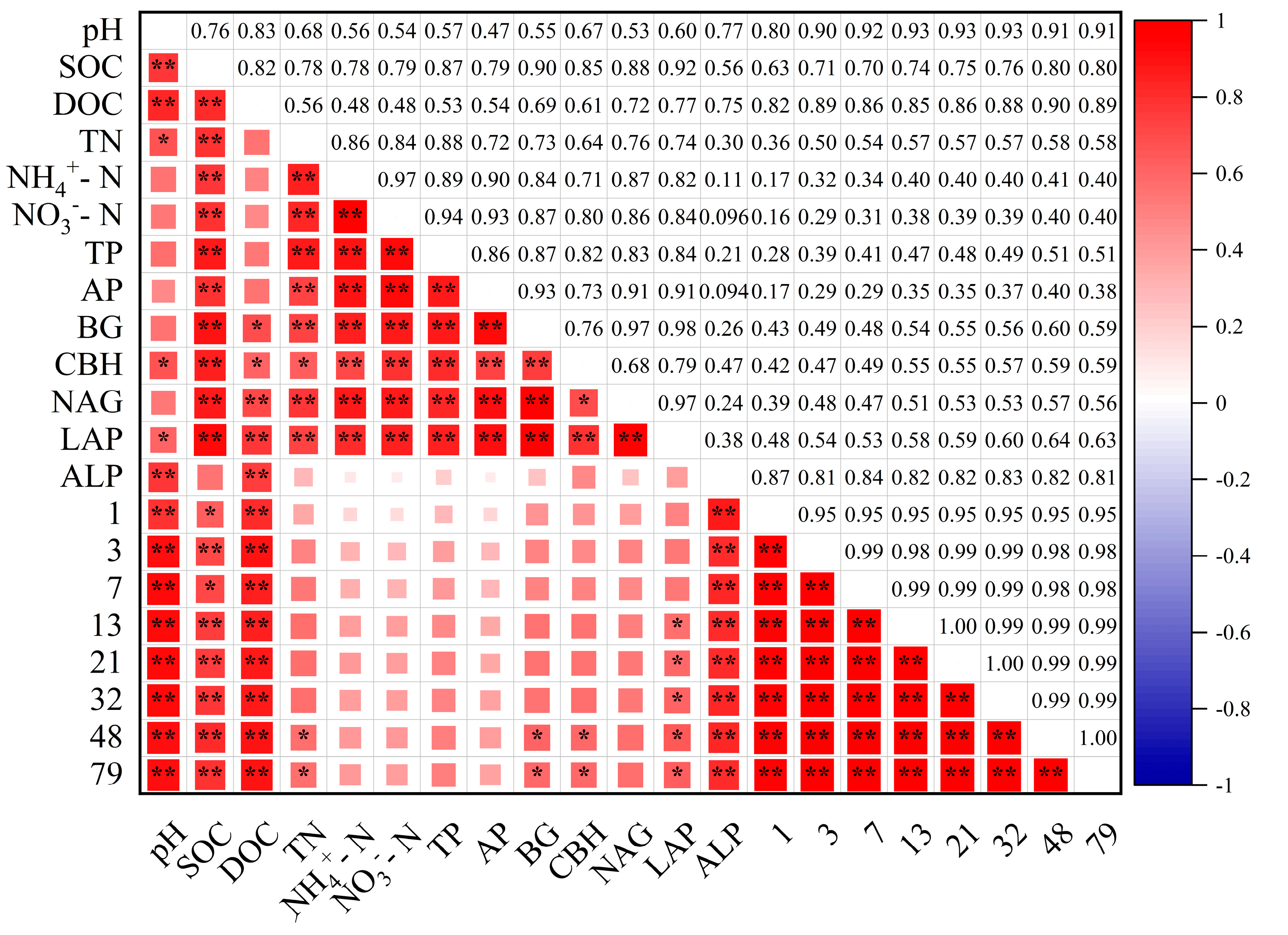
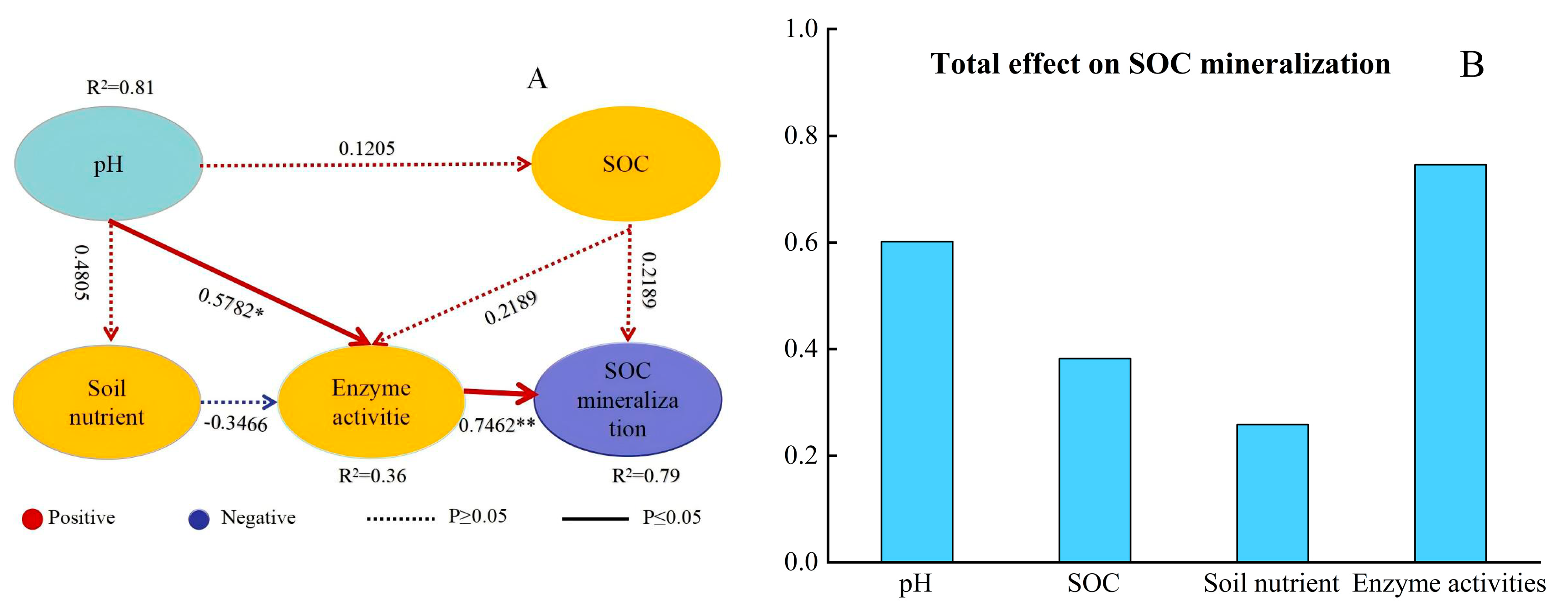
3.5. Contribution of Abiotic and Biotic Factors to SOC Mineralization
3.5.1. Correlation Analysis
3.5.2. Structural Equation Modeling
4. Discussion
4.1. Effects of Straw Addition on Carbon Dioxide Emission
4.2. Effects of Straw Addition on Soil Physicochemical Properties
4.3. Effects of Straw Addition on Soil Organic Carbon and Dissolved Organic Carbon
4.3.1. Soil Organic Carbon
4.3.2. Dissolved Organic Carbon
4.4. Effects of Straw Addition on Enzyme Activities and Microbial Processes
4.5. Effects of Straw Addition on SOC Mineralization
4.6. Limitations, Future Strategies, and Prospects
- (1)
- Experimental Design Considerations
- (2)
- Environmental and Soil Context
- (3)
- Methodological Approaches for Future Research
- (4)
- Management Practices and Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, H. Evaluation on the Production of Food Crop Straw in China from 2006 to 2014. BioEnergy Res. 2017, 10, 949–957. [Google Scholar] [CrossRef]
- Zhao, X.; Li, R.C.; Liu, W.X.; Liu, W.S.; Xue, Y.H.; Sun, R.H.; Wei, Y.X.; Chen, Z.; Lal, R.; Dang, Y.P. Estimation of crop residue production and its contribution to carbon neutrality in China. Resour. Conserv. Recycl. 2024, 203, 107450. [Google Scholar] [CrossRef]
- Liang, Z.; Cao, B.; Jiao, Y.; Liu, C.; Li, X.; Meng, X.; Shi, J.; Tian, X. Effect of the combined addition of mineral nitrogen and crop residue on soil respiration, organic carbon sequestration, and exogenous nitrogen in stable organic matter. Appl. Soil Ecol. 2022, 171, 104324. [Google Scholar] [CrossRef]
- Blagodatsky, S.; Blagodatskaya, E.; Yuyukina, T.; Kuzyakov, Y. Model of apparent and real priming effects: Linking microbial activity with soil organic matter decomposition. Soil Biol. Biochem. 2010, 42, 1275–1283. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Wang, S.; Wang, J.; Xu, L. Combined application of a straw layer and flue gas desulphurization gypsum to reduce soil salinity and alkalinity. Pedosphere 2020, 30, 226–235. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, S.; Ma, R.; Chen, M.; Wei, W.; Ding, X. Carbon sequestration under different organic amendments in saline-alkaline soils. Catena 2021, 196, 104882. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Nierop, K.G.J.; Simpson, M.J. Plant- or microbial-derived? A review on the molecular composition of stabilized soil organic matter. Soil Biol. Biochem. 2021, 156, 108189. [Google Scholar] [CrossRef]
- Wu, H.; Sun, W.; Zhu, F.; Jiang, Y.; Huang, S.; Goloran, J.; Xue, S. Straw addition increases enzyme activities and microbial carbon metabolism activities in bauxite residue. J. Environ. Sci. 2024, 1, 332–344. [Google Scholar] [CrossRef]
- Sun, N.; Gao, C.; Ding, Y.; Bi, Y.; Seglah, P.A.; Wang, Y. Five-Dimensional Straw Utilization Model and Its Impact on Carbon Emission Reduction in China. Sustainability 2022, 14, 16722. [Google Scholar] [CrossRef]
- Zhang, H.; Cao, Y.; Lyu, J. Decomposition of different crop straws and variation in straw-associated microbial communities in a peach orchard, China. J. Arid Land 2021, 13, 152–164. [Google Scholar] [CrossRef]
- Li, N.; Teng, P.J.; Lei, W.Y.; Long, J.H.; Li, L.J. Effects and mechanisms of addition of different types of exogenous organic materials on priming effect of organic carbon in arable black soils. Chin. J. Eco-Agric. 2023, 31, 1588–1601. [Google Scholar] [CrossRef]
- Kan, Z.; Virk, A.L.; Wu, G.; Qi, J.; Ma, S.T.; Wang, X.; Zhao, X.; Lal, R. Priming effect intensity of soil organic carbon mineralization under no-till and residue retention. Appl. Soil Ecol. 2020, 147, 103445. [Google Scholar] [CrossRef]
- Li, S.; Cui, Y.; Xia, Z.; Zhang, X.; Zhu, M.; Gao, Y.; An, S.; Yu, W.; Ma, Q. The mechanism of the dose effect of straw on soil respiration: Evidence from enzymatic stoichiometry and functional genes. Soil Biol. Biochem. 2022, 168, 108636. [Google Scholar] [CrossRef]
- Lang, D.; Zhou, R.; Hao, Z.Z.P. Effect of Wheat Straw Addition on Organic Carbon Mineralisation and Bacterial Community in Orchard Soil. J. Soil Sci. Plant Nutr. 2023, 23, 4328–4341. [Google Scholar] [CrossRef]
- Fontaine, S.; Mariotti, A.; Abbadie, L. The priming effect of organic matter: A question of microbial competition? Soil Biol. Biochem. 2003, 35, 837–843. [Google Scholar] [CrossRef]
- Fontaine, S.; Henault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Gan, Z.; Wang, H.; Ding, C.; Lei, M.; Yang, X.G.; Cai, J.; Qiu, Q.; Hu, Y. Effects of dissolved organic matter derived from different plant and tissues in a subtropical forest on soil priming effect and the underlying mechanisms. Acta Phytoecol. Sin. 2022, 46, 797–810. [Google Scholar] [CrossRef]
- Mendoza, O.; De Neve, S.; Deroo, H.; Li, H.; Françoys, A.; Sleutel, S. Soil organic carbon mineralization is controlled by the application dose of exogenous organic matter. Soil 2025, 11, 105–119. [Google Scholar] [CrossRef]
- Luo, Z.K.; Wang, E.L.; Sun, O.J. A meta-analysis of the temporal dynamics of priming soil carbon decomposition by fresh carbon inputs across ecosystems. Soil Biol. Biochem. 2016, 101, 96–103. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Zhang, T.; Zhao, X.; Chen, S.; Wang, Q. Priming of soil organic carbon decomposition induced by exogenous organic carbon input: A meta-analysis. Plant Soil 2019, 443, 463–471. [Google Scholar] [CrossRef]
- Ren, C.; Mo, F.; Zhou, Z.; Bastida, F.; Delgado-Baquerizo, M.; Wang, J.; Zhang, X.; Luo, Y.; Griffis, T.J.; Han, X.; et al. The global biogeography of soil priming effect intensity. Glob. Ecol. Biogeogr. 2022, 31, 1679–1687. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Yuan, S.; Wang, W.; Feng, Y.; Bo, Q. Effects of Long-term Conservation Tillage on Soil Nutrients in Sloping Fields in Regions Characterized by Water and Wind Erosion. Sci. Rep. 2015, 5, 17592. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhu, J.; Shi, L.; Fu, Q.; Hu, H.; Huang, Q. Influence mechanisms of iron, aluminum and manganese oxides on the mineralization of organic matter in paddy soil. J. Environ. Manag. 2022, 301, 113916. [Google Scholar] [CrossRef]
- Jing, Z.; Chen, R.; Wei, S.; Feng, Y.; Zhang, J.; Lin, X. Response and feedback of C mineralization to P availability driven by soil microorganisms. Soil Biol. Biochem. 2017, 105, 111–120. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, Z.; Liu, Y.; Luo, Y.; Deng, Y.; Xu, X.; Liu, S.; Richter, A.; Shibistova, O.; Guggenberger, G.; et al. C:N:P stoichiometry regulates soil organic carbon mineralization and concomitant shifts in microbial community composition in paddy soil. Biol. Fertil. Soils 2020, 56, 1093–1107. [Google Scholar] [CrossRef]
- Peixoto, L.; Elsgaard, L.; Rasmussen, J.; Olesen, J.E. Nitrogen and phosphorus co-limit mineralization of labile carbon in deep subsoil. Eur. J. Soil Sci. 2021, 72, 1879–1884. [Google Scholar] [CrossRef]
- Wang, C.; Morrissey, E.M.; Mau, R.L.; Hayer, M.; Pieiro, J.; Mack, M.C.; Marks, J.C.; Bell, S.L.; Miller, S.N.; Schwartz, E.; et al. The temperature sensitivity of soil: Microbial biodiversity, growth, and carbon mineralization. ISME J. 2021, 15, 2738–2747. [Google Scholar] [CrossRef]
- Burns, R.G.; Deforest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Mganga, K.Z.; Razavi, B.S.; Kuzyakov, Y. Microbial and enzymes response to nutrient additions in soils of Mt. Kilimanjaro region depending on land use. Eur. J. Soil Biol. 2015, 69, 33–40. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Shah, J.J.F. Ecoenzymatic stoichiometry of recalcitrant organic matter decomposition: The growth rate hypothesis in reverse. Biogeochemistry 2011, 102, 31–43. [Google Scholar] [CrossRef]
- Sanaullah, M.; Blagodatskaya, E.; Chabbi, A.; Rumpel, C.; Kuzyakov, Y. Drought effects on microbial biomass and enzyme activities in the rhizosphere of grasses depend on plant community composition. Appl. Soil Ecol. 2011, 48, 38–44. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Carreiro, M.M.; Repert, D.A. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 2002, 60, 1–24. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Wang, S. Addition of External Organic Carbon and Native Soil Organic Carbon Decomposition: A Meta-Analysis. PLoS ONE 2013, 8, e54779. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Hu, Z.; Hui, G. CO2 emissions from a forest soil as influenced by amendments of different crop straws: Implications for priming effects. Catena 2015, 131, 56–63. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, S.; Paz-Ferreiro, J. Linkages between straw decomposition rate and the change in microbial fractions and extracellular enzyme activities in soils under different long-term fertilization treatments. PLoS ONE 2018, 13, e0202660. [Google Scholar] [CrossRef]
- Haider, F.U.; Virk, A.L.; Nian, L.; Farooq, M.; Liu, J.; Yang, M.; Huang, W.; Li, Y. Soil Organic Carbon and Aggregate Associated Changes in Three Subtropical Evergreen Forest Ecosystems of China. J. Soil Sci. Plant Nutr. 2024, 24, 7480–7490. [Google Scholar] [CrossRef]
- Carter, M.; Gregorich, E. Soil Sampling and Methods of Analysis, 2nd ed.; Soil Sampling Designs; CRC Press: Boca Raton, FL, USA, 2007; Volume 10, p. 1201/9781420005271. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Willis, R.B.; Montgomery, M.E.; Allen, P.R. Improved Method for Manual, Colorimetric De-termination of Total Kjeldahl Nitrogen Using Salicylate. J. Agric. Food Chem. 1996, 44, 1804–1807. [Google Scholar] [CrossRef]
- Cavagnaro, T.R.; Jackson, L.E.; Six, J.; Ferris, H.; Goyal, S.; Asami, D.; Scow, K.M. Arbuscu-lar Mycorrhizas, Microbial Communities, Nutri-ent Availability, and Soil Aggregates in Organic Tomato Production. Plant Soil 2006, 282, 209–225. [Google Scholar] [CrossRef]
- Watanabe, F.S.; Olsen, S.R. Test of an Ascorbic Acid Method for Determining Phosphorus in Water and NaHCO3 Extracts from Soil. Soil Sci. Soc. Am. J. 1965, 291, 677–678. [Google Scholar] [CrossRef]
- Marx, M.C.; Wood, M.; Jarvis, S.C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Shar, A.G.; Peng, Y.; Tian, X.; Siyal, T.A.; Hessini, K. Contrasting effects of maize residue, coal gas residue and their biochars on nutrient mineralization, enzyme activities and CO2 emissions in sandy loess soil. Saudi J. Biol. Sci. 2021, 28, 4155–4163. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Carbon sequestration in agricultural soils via cultivation of cover crops—A meta-analysis. Agric. Ecosyst. Environ. 2015, 200, 33–41. [Google Scholar] [CrossRef]
- Sanz-Cobena, A.; Garda-Marco, S.; Quemada, M.; Gabriel, J.L.; Almendros, P.; Vallejo, A. Do cover crops enhance N2O, CO2 or CH4 emissions from soil in Mediterranean arable systems? Sci. Total Environ. 2014, 466–467, 164–174. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: Critical review. Biol. Fertil. Soils 2008, 45, 115–131. [Google Scholar] [CrossRef]
- Cao, Y.F.; Zhang, H.; Liu, K.; Dai, Y.C. Organic acids variation in plant residues and soils among agricultural treatments. Agron. J. 2015, 107, 2171–2180. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Miao, F.; Li, Z.; Tang, W.; Sun, J. Assessing the effect of soil salinization on soil microbial respiration and diversities under incubation conditions. Appl. Soil Ecol. 2020, 155, 103671. [Google Scholar] [CrossRef]
- Qin, S.; Kou, D.; Mao, C.; Chen, Y.; Chen, L.; Yang, Y. Temperature sensitivity of permafrost carbon release mediated by mineral and microbial properties. Sci. Adv. 2021, 7, eabe3596. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, M.; Nobili, M.D.; Lin, Q.; Brookes, P.C. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Fontaine, S.; Barot, S.; Barré, P.; Bdioui, N.; Mary, B.; Rumpel, C. Stability of organic carbon in deep soil layers controlled by fresh carbon supply. Nature 2007, 450, 277–280. [Google Scholar] [CrossRef]
- Shen, C.; Wang, J.; Jing, Z. Plant diversity enhances soil fungal network stability indirectly through the increase of soil carbon and fungal keystone taxa richness. Sci. Total Environ. 2022, 8, 151737. [Google Scholar] [CrossRef]
- Tamura, M.; Harayil, N. Plant litter chemistry and microbial priming regulate the accrual, composition and stability of soil carbon in invaded ecosystems. New Phytol. 2014, 203, 110–124. [Google Scholar] [CrossRef]
- Cao, H.; Jia, M.; Song, J.; Xun, M.; Yang, H. Rice-straw mat mulching improves the soil integrated fertility index of apple orchards on cinnamon soil and fluvo-aquic soil. Sci. Hortic. 2020, 278, 109837. [Google Scholar] [CrossRef]
- Shao, Y.; Xie, Y.; Wang, C.; Yue, J.; Yao, Y.; Li, X.; Liu, W.; Zhu, Y.; Guo, T. Effects of different soil conservation tillage approaches on soil nutrients, water use and wheat maize yield in rain fed dry-land regions of North China. Eur. J. Agron. 2016, 81, 37–45. [Google Scholar] [CrossRef]
- Xu, J.M.; Tang, C.; Chen, Z.L. The role of plant residues in pH change of acid soils differing in initial pH. Soil Biol. Biochem. 2006, 38, 709–719. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Yang, G. Changes in soil enzymes, soil properties, and maize crop productivity under wheat straw mulching in Guanzhong, China. Soil Tillage Res. 2018, 182, 94–102. [Google Scholar] [CrossRef]
- Wieder, W.R.; Grandy, A.S.; Kallenbach, C.M.; Bonan, G.B. Integrating microbial physiology and physio-chemical principles in soils with the MIcrobial-MIneral Carbon Stabilization (MIMICS) model. Biogeosciences 2014, 11, 3899–3917. [Google Scholar] [CrossRef]
- Wang, B.; An, S.; Liang, C.; Liu, Y.; Kuzyakov, Y. Microbial necromass as the source of soil organic carbon in global ecosystems. Soil Biol. Biochem. 2021, 162, 108422. [Google Scholar] [CrossRef]
- Zhu, E.; Cao, Z.; Jia, J.; Liu, C.; Feng, X. Inactive and inefficient: Warming and drought effect on microbial carbon processing in alpine grassland at depth. Glob. Change Biol. 2021, 27, 2241–2253. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, H.; Mao, Z.; Bao, X.; He, H.; Zhang, X.; Liang, C. Fungi determine increased soil organic carbon more than bacteria through their necromass inputs in conservation tillage croplands. Soil Biol. Biochem. 2022, 167, 108587. [Google Scholar] [CrossRef]
- West, T.O.; Marland, G. A synthesis of carbon sequestration, carbon emissions, and net carbon flux in agriculture: Comparing tillage practices in the United States. Agric. Ecosyst. Environ. 2002, 91, 217–232. [Google Scholar] [CrossRef]
- Stewart, C.E.; Paustian, K.; Conant, R.T.; Plante, A.F.; Six, J. Soil carbon saturation: Concept, evidence and evaluation. Biogeochemistry 2007, 86, 19–31. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol. Biochem. 2005, 37, 937–944. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Dai, Y.; Tian, H.; Zhou, W.; Lv, J. Soil organic carbon transformation and dynamics of microorganisms under different organic amendments. Sci. Total Environ. 2021, 750, 141719. [Google Scholar] [CrossRef]
- Liu, C.; Lu, M.; Cui, J.; Li, B.; Fang, C. Effects of straw carbon input on carbon dynamics in agricultural soils: A meta-analysis. Glob. Change Biol. 2014, 20, 1366–1381. [Google Scholar] [CrossRef]
- Shu, X.; Hu, Y.; Liu, W.; Xia, L.; Zhang, Y.; Zhou, W.; Liu, W.; Zhang, Y. Linking between soil properties, bacterial communities, enzyme activities, and soil organic carbon mineralization under ecological restoration in an alpine degraded grassland. Front. Microbiol. 2023, 14, 1131836. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.; Zha, T.; Zhang, X. Vegetation Restoration Alleviated the Soil Surface Organic Carbon Redistribution in the Hillslope Scale on the Loess Plateau, China. Front. Environ. Sci. 2021, 8, 614761. [Google Scholar] [CrossRef]
- Novara, A.; Mantia, L.T.; Rühl, J.; Badalucco, L.; Kuzyakov, Y.; Gristina, L.; Laudicina, V.A. Dynamics of soil organic carbon pools after agricultural abandonment. Geoderma 2014, 235–236, 191–198. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; Zhou, J.; Xu, X.; Zhu, Y. Long-term straw mulching with nitrogen fertilization increases nutrient and microbial determinants of soil quality in a maize–wheat rotation on China’s Loess Plateau. Sci. Total Environ. 2021, 775, 145930. [Google Scholar] [CrossRef]
- Shahbaz, M.; Kuzyakov, Y.; Sanaullah, M.; Heitkamp, F.; Zelenev, V.; Kumar, A.; Blagodatskaya, E. Microbial decomposition of soil organic matter is mediated by quality and quantity of crop residues: Mechanisms and thresholds. Biol. Fertil. Soils 2017, 53, 287–301. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Khomyakov, N.; Myachina, O.; Bogomolova, I.; Blagodatsky, S.; Kuzyakov, Y. Microbial interactions affect sources of priming induced by cellulose. Soil Biol. Biochem. 2014, 74, 39–49. [Google Scholar] [CrossRef]
- Xiao, C.; Guenet, B.; Zhou, Y.; Su, J.; Janssens, I.A. Priming of soil organic matter decomposition scales linearly with microbial biomass response to litter input in steppe vegetation. Oikos 2015, 124, 649–657. [Google Scholar] [CrossRef]
- Zhang, C.; Tayyab, M.; Abubakar, A.Y.; Yang, Z.; Pang, Z.; Islam, W.; Lin, Z.; Li, S.; Luo, J.; Fan, X.; et al. Bacteria with Different Assemblages in the Soil Profile Drive the Diverse Nutrient Cycles in the Sugarcane Straw Retention Ecosystem. Diversity 2019, 11, 194. [Google Scholar] [CrossRef]
- Pan, Z.; Cai, X.; Cai, L.; Dong, B.; Haider, F.U.; Bo, Y.; Hu, Z.; Li, A.; Xue, Q. Soil and Microbial Biomass Response to Land-Use Changes in the Loess Plateau. Sustainability 2024, 16, 10496. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Cruz, L.; Sotomayor-Ramírez, D.; Pérez-Alegría, L. Enzyme activities as affected by soil properties and land use in a tropical watershed. Appl. Soil Ecol. 2007, 35, 35–45. [Google Scholar] [CrossRef]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Change 2007, 80, 5–23. [Google Scholar] [CrossRef]
- Korai, P.; Sial, T.; Pan, G.; Abdelrahman, H.; Sikdar, A.; Kumbhar, F.; Channa, S.; Ali, E.; Zhang, J.; Jörg, R.; et al. Wheat and maize-derived water-washed and unwashed biochar improved the nutrients phytoavailability and the grain and straw yield of rice and wheat: A field trial for sustainable management of paddy soils. J. Environ. Manag. 2021, 297, 113250. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Virk, A.L.; Zhou, S.; Ul Ain, N.; Aguila, L.C.R.; Siddique, K.H.; Farooq, M.; Li, Y. Impact of silicon nitride nanoparticles on soil organic carbon dynamics in subtropical evergreen forest ecosystems of China: An incubation study. Sci. Total Environ. 2025, 965, 178682. [Google Scholar] [CrossRef]
- Pan, Z.; Cai, X.; Bo, Y.; Guan, C.; Cai, L.; Haider, F.U.; Li, X.; Yu, H. Response of soil organic carbon and soil aggregate stability to changes in land use patterns on the Loess Plateau. Sci. Rep. 2024, 14, 31775. [Google Scholar] [CrossRef]
- Bertrand, I.; Delfosse, O.; Mary, B. Carbon and nitrogen mineralization in acidic, limed and calcareous agricultural soils: Apparent and actual effects. Soil Biol. Biochem. 2007, 39, 276–288. [Google Scholar] [CrossRef]
- Zhang, J.; Sayer, E.J.; Zhou, J.; Li, Y.; Li, Y.; Li, Z.; Wang, F. Long-term fertilization modifies the mineralization of soil organic matter in response to added substrate. Sci. Total Environ. 2021, 798, 149341. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.A.; Ritz, K.; Paterson, E.; Kirk, G.J.D. Effects of soil type and composition of rhizodeposits on rhizosphere priming phenomena. Soil Biol. Biochem. 2016, 103, 512–521. [Google Scholar] [CrossRef]
- Razanamalala, K.; Razafimbelo, T.; Maron, P.A.; Ranjard, L.; Chemidlin, N.; Lelièvre, M.; Dequiedt, S.; Ramaroson, V.H.; Marsden, C.; Becquer, T. Soil microbial diversity drives the priming effect along climate gradients: A case study in Madagascar. ISME J. 2017, 12, 451–462. [Google Scholar] [CrossRef]
- Hopkins, F.M.; Filley, T.R.; Gleixner, G.; Lange, M.; Top, S.M.; Trumbore, S.E. Increased belowground carbon inputs and warming promote loss of soil organic carbon through complementary microbial responses. Soil Biol. Biochem. 2014, 76, 57–69. [Google Scholar] [CrossRef]
- Saviozzi, A.; Cardelli, R.; Puccio, R.D. Impact of Salinity on Soil Biological Activities: A Laboratory Experiment. Commun. Soil Sci. Plant Anal. 2011, 42, 358–367. [Google Scholar] [CrossRef]
- Swift, M.J.; Heal, O.W.; Anderson, J.M. Decomposition in terrestrial ecosystems. Stud. Ecol. 1979, 5, 2772–2774. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Wang, S.; He, T.; Liu, L. Fresh carbon and nitrogen inputs alter organic carbon mineralization and microbial community in forest deep soil layers. Soil Biol. Biochem. 2014, 72, 145–151. [Google Scholar] [CrossRef]
- Blagodatskaya, E.V.; Blagodatsky, S.A.; Anderson, T.H.; Kuzyakov, Y. Priming effects in Chernozem induced by glucose and N in relation to microbial growth strategies. Appl. Soil Ecol. 2007, 37, 95–105. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, Y.; Shi, L.; Marshall, M.R.; Zang, H. Strong priming of soil organic matter induced by frequent input of labile carbon. Soil Biol. Biochem. 2021, 152, 108069. [Google Scholar] [CrossRef]
| Tillage Modes | Treatments | Straw Input Level (kg ha−1) | Soil Depth (cm) |
|---|---|---|---|
| Wheat | |||
| Traditional tillage with straw return | Control (W0) | 0 | 0–10 cm, 10–20 cm, and 20–30 cm |
| 1 times treatment (W1) | 3500 | ||
| 2 times treatment (W2) | 7000 | ||
| 4 times treatment (W4) | 14,000 |
| Enzyme | Substrate | Compounds |
|---|---|---|
| β-glucosidase (BG) | 4-MUF-β-d-glucopyranoside | Cellulose, cellobiose |
| β-cellobiosidase (CBH) | MUF-β-d-cellobioside | Cellulose |
| β-1, 4-N-acetylglucosaminidase (NAG) | 4-methylum-belliferyl N-acetyl-b-D-glucosaminide | Chitin |
| L-leucine aminopeptidase (LAP) | L-Leucine-7-amino-4-methyl coumarin | Proteins |
| Alkaline phosphatase (ALP) | 4-MUB-phosphate | Organic P mineralization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Cai, L.; Wu, J.; Ahmad, M.K.; Haider, F.U. Dynamics of Soil Organic Carbon Mineralization Under Straw Addition: Evidence from a Controlled Incubation Experiment. Agronomy 2025, 15, 2642. https://doi.org/10.3390/agronomy15112642
Ren X, Cai L, Wu J, Ahmad MK, Haider FU. Dynamics of Soil Organic Carbon Mineralization Under Straw Addition: Evidence from a Controlled Incubation Experiment. Agronomy. 2025; 15(11):2642. https://doi.org/10.3390/agronomy15112642
Chicago/Turabian StyleRen, Xiaoyan, Liqun Cai, Jun Wu, Muhammad Kashif Ahmad, and Fasih Ullah Haider. 2025. "Dynamics of Soil Organic Carbon Mineralization Under Straw Addition: Evidence from a Controlled Incubation Experiment" Agronomy 15, no. 11: 2642. https://doi.org/10.3390/agronomy15112642
APA StyleRen, X., Cai, L., Wu, J., Ahmad, M. K., & Haider, F. U. (2025). Dynamics of Soil Organic Carbon Mineralization Under Straw Addition: Evidence from a Controlled Incubation Experiment. Agronomy, 15(11), 2642. https://doi.org/10.3390/agronomy15112642






