Abstract
Wheat blast, caused by Pyricularia oryzae Triticum lineage (PoTl), is one of the most destructive and significant fungal diseases affecting wheat crops. The stability of the G143A mutation in the cytB gene, which confers resistance to Quinone outside inhibitor fungicides (QoIs) in PoTl isolates, has not been extensively studied. This study was conducted to evaluate the stability of fungicide resistance, fitness, and competitive ability of the QoI-resistant (R) PoTl isolates group over nine and five consecutive transfer cycles in vitro and in vivo, respectively, without fungicide exposure. No changes in azoxystrobin sensitivity were observed in either the QoI-resistant or sensitive (S) PoTl isolate groups after the successive transfer cycles in vitro and in vivo. The mycelial growth of the QoI-R PoTl isolate group remained stable, while the conidial germination capacity increased over time. For the QoI-resistant isolates, leaf and head disease, conidial production, and the latent period on wheat leaves did not change between the first and fifth infection cycles. In each transfer cycle, the highest levels of leaf and head disease, as well as the largest quantities of conidia collected from wheat leaves, were observed in isolate mixtures. Also, the G143A mutation responsible for QoI resistance remained stable after five transfer cycles of the QoI-resistant (0S:100R) isolate on wheat leaves. Our findings indicate that the G143A mutation remains stable, and there are adaptive benefits in QoI-R PoTl isolates. We discuss the ecological implications of the wheat blast population’s adaptation and PoTl QoIs resistance stability in wheat-cropping areas in Brazil.
1. Introduction
Wheat blast, caused by Pyricularia oryzae Triticum lineage (PoTl), also known as P. graminis-tritici [1,2], affects several important cereal crops, including wheat, rice, barley, millet, and oats [1]. In regions where wheat blast occurs, PoTl can cause grain losses ranging from 10% to 100% [1,3,4]. In 2022, Brazilian wheat production reached 10.3 million tons, accounting for 1.3% of the 808 million tons produced globally [5]. Due to the prevalence of various diseases, Brazilian wheat quality is compromised, resulting in low domestic production and supply. Consequently, Brazil continues to import 4 million tons of wheat grain each year [1]. Wheat blast was first reported in 1986 in the state of Paraná, Brazil [6]. Following this, the destructive pathogen rapidly spread to south-central Brazil and neighboring countries including Argentina, Bolivia, and Paraguay [1,2]. Up until April 2016, wheat blast was confined to South America [1]. However, in 2016 and 2017, the first occurrences of this disease were reported in Bangladesh, southeast Asia, and Zambia, southern Africa, respectively [7,8]. In Brazil, the use of fungicides has become common for controlling wheat blast, although their effectiveness seldom exceeds 60% in managing the disease [1]. The limited effectiveness of molecules with specific modes of action, combined with the lack of varietal resistance, makes managing wheat blast in wheat crops challenging.
In 2015, the first report emerged of selection for resistance to Quinone outside inhibitor fungicides (QoI) in Brazilian populations of PoTl. The mutation G143A in the cytochrome b (cytB) gene was linked to QoI resistance in PoTl populations [9]. Besides target-site-based mechanisms of fungicide resistance, non-target site mechanisms, including the overexpression of drug efflux pumps, insufficient conversion into active components, and detoxification processes may also play roles in QoI resistance among phytopathogens [10,11,12]. Recently, PoTl populations have been found to exhibit resistance to two additional groups of chemicals: both triazoles or demethylation inhibitors (DMIs) and succinate dehydrogenase inhibitors (SDHIs) [13,14].
Fungicide resistance may confer fitness advantages to phytopathogens, potentially through an evolutionary compensatory process [10]. However, mutations associated with fungicide resistance can also impose fitness costs, leading to evolutionary trade-offs [10]. Fitness is defined as the survival and reproductive success of an allele, individual, or group carrying a specific fungicide resistance trait, measured by their contribution of progeny to the next generation [15]. Fitness can likewise be utilized to illustrate all calculated metrics for an individual isolate, including mycelial growth, conidial production, germination, and virulence [16]. The compensatory process can sometimes reduce the fitness cost associated with a point mutation in the target gene that confers fungicide resistance [17]. For instance, Zymoseptoria tritici isolates resistant to cyproconazole with no CYP51 mutations exhibit higher virulence and fitness advantages compared to wild-type isolates, likely due to an efflux pump mechanism against fungicides [18]. Similarly, the QoI-resistant (QoI-R) group of PoTl isolates exhibited fitness advantages, including enhanced mycelial growth, virulence, conidial production, and competitive ability, compared to the sensitive (QoI-S) isolates [19]. Furthermore, PoTl isolates showed increased disease severity over five disease cycles on wheat leaves and heads when tested under growth chamber conditions [20].
Despite the widespread presence of QoIs resistance in PoTl populations in south-central Brazil for seven years, from 2012 to 2019 [13], there is limited understanding of the stability of fungicide resistance, the persistence of fitness advantages, and the fitness costs connected with the G143A mutation in QoI-R PoTl populations. In addition to previous studies that focused on the resistance frequency of QoI-R PoTl isolates, the present study is the first to validate the resistance stability of QoI-R PoTl isolates through successive transfer experiments. Resistance stability refers to the capacity of the pathogen to maintain a consistent level of insensitivity to fungicides after multiple generations, regardless of whether they have been exposed to the target fungicide [21]. Few studies have examined whether the fitness advantage associated with QoI-R PoTl populations bearing the G143A mutation remains stable over successive transfer cycles in the wheat blast pathogen. In contrast, previous experiments with the related rice blast pathogen (Pyricularia oryzae) demonstrated a fitness cost linked to QoI fungicide resistance after four disease cycles, alongside stable QoI resistance in vitro, and unstable QoI resistance in vivo [22]. Therefore, investigating the stability of fungicide resistance associated with the G143A mutation and fitness advantage stability in PoTl populations resistant to QoIs may be valuable for developing management strategies to reduce the decline in sensitivity of PoTl populations to QoIs in wheat fields.
For other phytopathogenic fungi species that possess the G143A mutation, such as Colletotrichum acutatum from strawberries, the resistance to QoIs persisted even after four cycles in vitro in the absence of fungicide [23]. In Erysiphe necator, the fungus causing grape powdery mildew, genotypes exhibited stable QoI resistance for up to four years after the last fungicide exposure, while isolates of Plasmopara viticola from grapes remained resistant to QoIs for four consecutive cycles [24,25]. Furthermore, G143A mutants of Pyricularia oryzae on barley did not revert to sensitivity throughout four successive cycles [26]. Similarly, Botrytis cinerea on apple maintained stable resistance even after four consecutive cycles [27]. Conversely, a loss of QoI resistance was recently observed in P. oryzae isolates on rice [22] and Monilinia fructicola on peach [28], where resistance to QoIs was not stable.
Therefore, the objective of this study were to (i) determine whether the resistance of QoI-R PoTl field isolates remained stable after nine and five successive transfer cycles in vitro and in vivo, respectively, without fungicide; (ii) verify whether the fitness variables and competitive abilities of the QoI-R PoTl field isolates remain stable (that is, show no decrease) after successive transfer cycles in vitro and in vivo in the absence of fungicide selection pressure; and (iii) confirm whether the G143A mutation in the cytB gene of QoI-R PoTl field isolates remains stable after successive transfer cycles in the absence of fungicide selection pressure.
2. Materials and Methods
2.1. Fungal Material, Plant Material and Fungicides
Four QoIs resistant (QoI-R) and four sensitive (QoI-S) Pyricularia oryzae Triticum lineage isolates characterized in previous studies were used in this present study [9,19]. The PoTl isolates were collected from wheat fields in Amambaí, Mato Grosso do Sul (MS), in 2012, Goiânia, Goiás (GO), in 2006, Londrina, Parana (PR), in 2018 and Itapetininga, in the São Paulo (SP) state, Brazil, in 2018, from leaves and heads with typical blast symptoms (Supplementary Table S1). Sampling was conducted following the procedure described by [9]. These isolates were deposited in the Molecular Plant Pathology fungal collection from São Paulo State University, Ilha Solteira, SP, Brazil.
For conidial production, mycelial discs (7 mm diameter) of PoTl isolate groups were transferred from 7-day-old growth cultures to 15 plates (90 mm diameter) containing PDA medium (42 g/L potato-dextrose-agar, KASVI, Mumbai, India) and 15 plates with oatmeal agar medium (OA, 60 g of oatmeal flour, 12 g of agar). In each plate, 50 μg/mL streptomycin sulfate and chloramphenicol were added. These plates were incubated at 25 °C under constant light for fifteen days using 1060-lumen (Osram® fluorescent lamps, Munich, Germany). Four mL of distilled water with the surfactant Tween 80 (10 μL/L) were added to the culture medium to facilitate the release of conidia. The conidial suspensions were obtained by scraping the mycelia produced in the plates using a sterilized spatula. For QoI-R and QoI-S PoTl isolate groups, the adjustment of the conidia concentration, 2.2 × 104 conidia/mL, was performed using a Neubauer chamber.
Simultaneously, for conidial production, three wheat ‘Anahuac 75’ plants were grown in the same plastic pot containing 770 mL of the plant substrate Tropstrato HT potting mix (Vida Verde). The wheat growth was conducted in a greenhouse, and a 0.84 g dose of N-P2O5-K2O (10-10-10) was applied per pot every 15 days. The wheat plants were watered daily. PoTl isolates were inoculated on wheat leaves with 1-month-old plants at growth stage 14 [29]. Separately from the leaves assay, the PoTl isolates were inoculated on wheat heads at the beginning of anthesis, at growth stage 60, on 2-month-old immature heads [29].
For fungicide sensitivity assays, a stock solution of the QoIs fungicide azoxystrobin (250 g/L active ingredient, Syngenta, Basel, Switzerland) was prepared at a concentration of 1000 μg/mL by diluting it in deionized water.
2.2. Resistance Stability
The effect of successive transfer cycles, both cycles in vitro and in vivo, on the resistance stability of the QoI-R PoTl isolate group to QoI fungicides was investigated. The procedure for the number of transfer cycles in vitro and in vivo for PoTl isolates followed the method described by refs. [20,30]. Nine consecutive in vitro transfer cycles were conducted for both QoI-R and QoI-S PoTl isolate groups without applying fungicide pressure. The sensitivity of PoTl isolates to azoxystrobin was assessed after the 1st, 5th, and 9th transfer cycles. Between infection cycles, mycelial discs (7 mm in diameter) were taken from the growing edge of the colony and transferred to 90 mm Petri dishes containing PDA medium. These plates were incubated at 25 °C with a 12 h light cycle, using 1060-lumen Osram® fluorescent lamps.
Five successive transfer cycles of the QoI-R and QoI-S PoTl isolate groups on wheat leaves and heads were conducted in the absence of fungicide pressure. The inoculated plants were maintained in a growth chamber at 25 °C under a 12 h photoperiod using 33.354-lumen Osram® sodium vapor lamps (400 W, model HQI-T NDL E40 5200K, Osram, Munich, Germany). Each transfer cycle of the PoTl isolates on leaves and heads lasted 21 days. Temperature and relative humidity in the growth chamber were monitored using an Even® digital thermo-hygrometer (Even Ltd., Moscow, Russia). To prevent cross-infection, wheat plants were removed from the growth chamber before initiating the next infection cycle. The sensitivity of the PoTl isolates to azoxystrobin was assessed at the 1st, 3rd, and 5th transfer cycles. Every 21 days, between infection cycles, conidia produced by the QoI-resistant and -sensitive PoTl isolate groups on wheat leaves were inoculated onto healthy plants for the subsequent cycle. Preparation of both the wheat plants and the PoTl inoculum followed the procedures outlined in Section 2.1.
2.2.1. Sensitivity of QoIs-Resistant and -Sensitive PoTl Isolate Groups to Azoxystrobin
The resistance stability of PoTl isolates to QoIs was evaluated using the effective concentration that inhibits 50% of mycelial growth (EC50) in both in vitro and in vivo assays. Portions of a stock solution of the QoIs fungicide azoxystrobin were added to autoclaved PDA medium, which was then cooled to 45 °C, to achieve final azoxystrobin concentrations of 0, 0.08, 0.16, 0.32, 0.63, 1.25, 2.5, 5.0, 7.0, and 10.0 μg/mL. Also, 0.5 mM salicyl hydroxamic acid (SHAM) was included in all QoIs concentrations to inhibit the fungal alternative oxidase pathway (AOX) [31]. For each isolate group, 7 mm diameter mycelial discs taken from a 7-day-old colony were placed onto plates containing PDA medium with varying concentrations of azoxystrobin. The mycelial growth of PoTl isolates was assessed by measuring the diameter of the fungal colonies. Measurements were taken with a caliper seven days after incubation at 25 °C under a 12 h light cycle. For each dose, the EC50 values of PoTl isolates were calculated based on the fungi’s relative growth (RG) compared to the control without fungicide. These EC50 values were determined using the RG and the final azoxystrobin concentrations, which were converted to log10 using the ED50plus v1.0 software [32].
2.2.2. Stability of G143A Mutation in Cytochrome b from QoIs Resistant PoTl Isolate
The presence of the G143A mutation in the cytochrome b (cytB) gene was evaluated for PoTl 121146 alone (QoI-R) (0S:100R) and PoTl Py6038 alone (QoI-S) (100S:0R) isolates from the 1st and 5th transfer cycles collected from wheat leaves exhibiting classic blast symptoms at 7 days after inoculation (d.a.i). The procedure for inoculating the PoTl mixtures on wheat leaves was carried out as detailed in Section 2.3. For each treatment, twenty single cultures were isolated from typical blast lesions on twelve wheat plants, which were distributed across four pots containing three plants each. Genomic DNA was extracted from the mycelium of each single culture grown on PDA at 25 °C under a 12 h photoperiod. DNA extraction was carried out using the Wizard® Genomic DNA Purification kit (Promega, Madison, WI, USA). The DNA concentration was measured with a NanoDrop® Lite spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) and diluted to a final concentration of 25 ng/mL. For the QoI-R isolates, PCR amplification of a 245 bp fragment of the cytB gene was performed using the primers PoTl (5′-ATGAGAGATGTTAATAACGGGTGAT-3′) and PoTlR (5′-TTAGTAATAACTGTAGCAG-3′). For the QoI-S isolates, the primers PoTl and PoTlS (5′-TTAGTAATAACTGTAGCAC-3′) were used to amplify a similar fragment. The specific primers for QoI-R and QoI-S isolates were used for frequency analysis. Polymerase chain reactions (PCR) were performed to amplify the cytochrome b (cytB) gene [9]. The PCR products were visualized under UV light following electrophoresis on a 0.8% agarose gel prepared with 1× Tris-acetate-EDTA buffer and stained with GelRed. These PCR products were then sequenced to determine the frequency of the G143A mutation and to evaluate its stability after five transfer cycles. All sequences obtained in this study were submitted to GenBank (see Table 1). Amplification and sequencing of the cytB gene served to monitor any potential changes in mutation.

Table 1.
Detection of the G143A mutation in the cytochrome b (cytb) gene of Pyricularia oryzae Triticum lineage (PoTl), after successive transfer cycles in vivo u.
2.3. Fitness Variables of the QoI-Resistant and -Sensitive PoTl Isolate Groups
For fitness variables, the QoI-R and QoI-S PoTl isolate groups were evaluated in both in vitro and in vivo experiments. The variables studied in vitro included mycelial growth, conidial production, and germination ability. For the in vivo assays, incubation period, latent period, disease severity on wheat leaves and heads, conidial production, and germination ability were also determined. The evaluations of transfer cycles in both in vitro and in vivo settings were conducted following the method outlined in Section 2.2.
Mycelial growth on PDA. The PoTl isolates were cultured on PDA medium at 25 °C with continuous light for seven days. To evaluate the mycelial growth, the colony diameter (mm) was measured using a caliper.
In vitro conidial production. The conidial production of either QoI-R or QoI-S isolate group of PoTl isolates was carried out following the same procedures described in Section 2.1.
In vitro germination ability. Conidial suspensions of QoI-R and QoI-S isolate groups of PoTl were prepared as described in Section 2.1. A mixture of 200 μL of PD medium (potato dextrose, Sigma-Aldrich, St. Louis, MO, USA) and 200 μL of conidial suspension supplemented with 0.5 mM SHAM was transferred to each well of a 24-well culture plate. The plates were incubated on a shaker at 110 rpm and 25 °C for 12 h in the dark. The germination percentage for each isolate group was determined by counting 50 conidia per replicate. Conidia were considered germinated when the germ tube length was at least twice the length of the conidium [33].
Incubation period and latent period. The incubation and latent periods were determined by daily monitoring of wheat leaves inoculated with PoTl isolates until the appearance of the first symptoms and fungal reproductive structures, conducted following the procedure described by [19,20]. The incubation period is defined as the time from inoculation to the appearance of the first symptoms. The latent period is the time from inoculation to the emergence of the first conidia on the inoculated wheat leaves.
Disease severity. After preparing both wheat plants and conidial suspensions from QoI-R and QoI-S isolate groups PoTl as described in Section 2.1, the wheat plants were inoculated with the fungal isolates. For each isolate group, the conidial suspension was adjusted to 2.2 × 104 conidia/mL. Inoculations were performed using a manual sprayer until runoff, with each transfer cycle tested simultaneously. For inoculation of wheat leaves, 25 mL of the conidial suspension of each isolate was applied to twelve wheat plants distributed across four pots. For wheat heads, 25 mL of the conidial suspension of each isolate was inoculated in twelve wheat heads distributed on four pots, each containing three plants. Subsequently, the wheat plants were maintained in a growth chamber under nebulization at 25 °C, with 90% relative humidity, and kept in the dark for the first 24 h. After this period, the wheat plants were incubated under a 12 h photoperiod as described in Section 2.3. An Even® digital thermo-hygrometer was used to monitor temperature and relative humidity. Wheat leaves and heads showing typical blast symptoms were photographed using a Canon® digital camera (EOS Rebel T1i model). The digital images were analyzed with the software Asses 2.0 (APS, St. Paul, MN, USA) to calculate the percentage of leaf area affected by lesions relative to the total leaf size, representing the blast severity on leaves or heads at 7 days after inoculation (d.a.i). For the heads, two sides of each head were photographed, and the severity score was calculated as the average of the two images.
In vivo conidial production. The conidial production of QoI-R and QoI-S isolate groups PoTl was determined on wheat leaves at 21 d.a.i on typical blast lesions. Three sporulated wheat leaves were placed into plastic tubes containing 10 mL of distilled water. The tubes were then vortexed for 20 s to detach conidia from the leaf fragments with blast lesions. The number of conidia collected from the lesions was counted using a Neubauer chamber. For each isolate group, conidial suspensions were prepared from plastic tubes containing three wheat leaves.
In vivo germination ability. The in vivo germination percentage of the QoI-R and QoI-S isolate groups PoTl was determined as outlined in Section 2.3.
2.4. Competitive Abilities of QoI-Resistant and QoI-Sensitive PoTl Isolates
Two competitive ability experiments were conducted: one in vitro and one in vivo. Mixtures of conidia from the QoI-resistant PoTl isolate 121146 (R) and the QoI-sensitive PoTl isolate Py6038 (S) were used without applying fungicide pressure. The conidial suspensions of the R and S isolates adjusted to 2.2 × 104 conidia mL−1 were mixed to obtain the following proportions: 100S:0R, 80S:20R, 50S:50R, 20S:80R and 0S:100R.
In vitro competitive ability. Aliquots of each mixture (20 μL) were transferred to 15 plates containing either PDA or oatmeal agar medium. The plates were incubated at 25 °C under constant light for 15 days. Nine successive transfer cycles were performed following the procedures described in Section 2.2. For each cycle, a 20 μL aliquot of conidial suspension from each treatment was used to initiate the subsequent cycle. Conidial production for each treatment was quantified using a Neubauer chamber.
In vivo competitive ability. The wheat plants were inoculated following the procedures described in Section 2.3. For each mixture, 12 wheat plants, distributed across four pots, were inoculated. For each treatment, wheat blast severity and conidial production on wheat leaves were evaluated at 7 and 21 d.a.i, respectively. Five successive transfer cycles were carried out as described in Section 2.2.
2.5. Frequency of QoI-Resistant Conidia in Mixtures of PoTl Isolates
For each mixture of QoI-R and QoI-S isolates, the frequency of QoI-R conidia was determined both in vitro using PDA or oatmeal agar medium at 15 days after inoculation (d.a.i.), and in vivo from wheat blast lesions at 21 d.a.i. The frequency of QoI-R PoTl conidia was measured in PD medium containing 10 μg mL−1 of azoxystrobin, following the procedures described in Section 2.4. The frequency was calculated based on [34] using the formula: =[(A/B)/(C/D)] × 100, where A = number of conidia germinated in PD medium when in 10 μg mL−1 of azoxystrobin; B = total number of conidia counted in PD medium when 10 μg mL−1 of azoxystrobin; C = the number of conidia germinated in PD medium without fungicide; and D = the total number of conidia counted in PD medium without fungicide. (A/B) represents the proportion of germinated QoI-R conidia among all conidia on the azoxystrobin-containing medium, which allows only QoI-R conidia to germinate. Meanwhile, (C/D) represents the germination frequency of all conidia on fungicide-free medium, where both QoI-R and QoI-S conidia germinate equally well. Therefore, [(A/B) ÷ (C/D)] × 100 represents the frequency of QoI-R conidia within the total conidia population.
2.6. Data Analysis
The data from the in vitro and in vivo experiments, repeated over time, were subjected to a combined analysis of two repetitions. The Shapiro–Wilk and Bartlett tests were used to evaluate the assumptions of analysis of variance (ANOVA) for fitness variables and the competitive ability of the QoI-R and QoI-S PoTl isolates (p = 0.05). For both in vitro and in vivo assays, differences in fitness variables and competitive ability of PoTl isolates on wheat leaves and heads were evaluated using ANOVA’s F test. A factorial design was employed, considering the PoTl isolate groups and transfer cycles, allowing comparisons of isolate groups within each cycle and between nine transfer cycles in vitro and five transfer cycles in vivo. Within each cycle, the QoI-R and QoI-S PoTl isolate groups were compared by ANOVA’s F test, and groups between them were assessed with the Scott-Knott test (p ≤ 0.05). For both in vitro and in vivo assays, Pearson correlation analysis was conducted using data on competitive ability to explore relationships among transfer cycles and mixtures, conidia production, disease severity, and germination frequency of harvested QoI-R conidia. The t-test was used to verify the significance of these correlations. All analyses were performed using the ExpDes.pt package for the statistical software R Studio version 1.2.5033 [35].
3. Results
3.1. Sensitivity of the QoI-Resistant and -Sensitive PoTl Isolate Groups to Azoxystrobin and cytB Genotyping
The sensitivity of QoI-R and QoI-S PoTl isolate groups to azoxystrobin was tested over nine and five successive transfer cycles in vitro and in vivo, respectively. In both experiments, the EC50 value of the QoI-R PoTl isolate group exceeded 10 μg/mL, indicating that the resistance of PoTl isolates to azoxystrobin remained stable (Figure 1). For the QoI-S PoTl isolate group, no changes in sensitivity to azoxystrobin were observed after the 9th and 5th successive transfer cycles in vitro and in vivo, respectively (Figure 1).
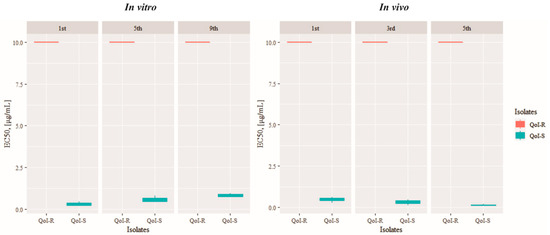
Figure 1.
Boxplot showing the distribution of EC50 of Pyricularia oryzae Triticum lineage isolate group resistant (R) and sensitive (S) to quinone outside inhibitor (QoI) fungicide after successive transfer cycles in the absence of azoxystrobin.
A comparison of the sequences generated from the QoI-resistant isolate (0S:100R) and the QoI-sensitive isolate (100S:0R) confirmed the presence of the G143A mutation. In both the 1st and 5th transfer cycles, the G143A mutation was detected in 100% of the QoI-resistant isolates, indicating that the G143A mutation of the PoTl QoI-resistant isolates remains stable (Table 1, Figure 2A,C). The G143A mutation was not detected in the sequences of the QoI-sensitive isolates (Table 1, Figure 2B,D).
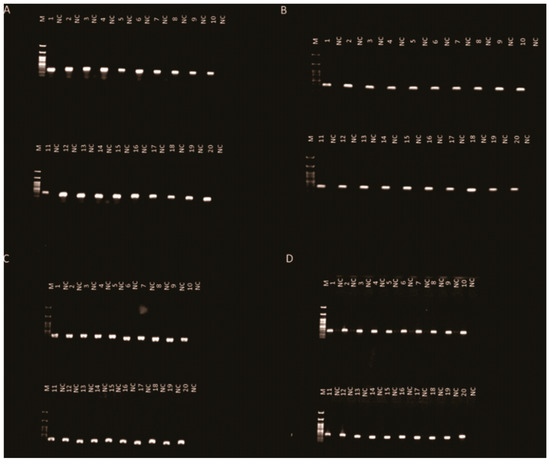
Figure 2.
Detection of azoxystrobin-sensitive and resistant isolates of Pyricularia oryzae Triticum lineage after successive transfer cycles in the absence of azoxystrobin by polymerase chain reaction with the primers used to amplify a product similar to QoI-R (0S:100R) isolates, PoTl/PoTlR and QoI-S (100S:0R) isolates, PoTl/PoTlS, respectively, amplifying a 245 bp product. Twenty single QoI-R (0S:100R) isolates were obtained from typical blast lesions at the 1st infection cycle (A) and 5th infection cycle (C). Twenty single QoI-S (100S:0R) isolates were obtained from typical blast lesions at the 1st infection cycle (B) and 5th infection cycle (D). The negative control (NC) primers were PoTl/PoTlS and PoTl/PoTlR for QoI-R and QoI-S isolates, respectively.
3.2. Fitness Variables of the QoI-Resistant and -Sensitive PoTl Isolate Groups
Within each infection cycle for in vitro fitness variables, significant differences were observed between QoI-R and QoI-S PoTl isolates in mycelial growth (p ≤ 0.001), conidial production (p ≤ 0.001), and germination ability (p ≤ 0.001). Across the transfer cycles, an interaction between cycles and PoTl isolate groups was detected for mycelial growth (p ≤ 0.001), conidial production (p ≤ 0.001), and germination ability (p ≤ 0.05). Between the 1st and 9th transfer cycles, mycelial growth remained stable in the QoI-R PoTl isolate group, whereas the QoI-S PoTl isolate group showed an increase from 40.4 to 51.2 mm (p ≤ 0.001) (Table 2). Similarly, germination ability increased from 50.7 to 63.6% in the QoI-R PoTl isolate group and from 38.1 to 51.8% in the QoI-S PoTl isolate group (p ≤ 0.001) (Table 2). In contrast, conidial production decreased in both QoI-R and QoI-S PoTl isolate groups between the 5th and 9th transfer cycles (p ≤ 0.001) (Table 2).

Table 2.
Fitness variables (mycelial growth, conidial production and conidial germination) of Pyricularia oryzae Triticum lineage isolate group resistant (R) and sensitive (S) to Quinone outside inhibitor (QoI) fungicide, and effects of successive transfer cycles in vitro.
Within each infection cycle for in vivo fitness variables, significant differences were observed between QoI-R and QoI-S PoTl isolates in incubation and latent periods (p ≤ 0.001), disease severity (p ≤ 0.001), conidial production (p ≤ 0.001), and germination ability (p ≤ 0.001). Across transfer cycles, there was a significant interaction between cycles and PoTl isolate groups for incubation period on wheat heads (p ≤ 0.01), latent period (p ≤ 0.05), conidial production (p ≤ 0.001), and disease severity on wheat leaves (p ≤ 0.001) and heads (p ≤ 0.05). No significant interaction was found between transfer cycles and germination ability (p = 0.1074) or incubation period on leaves (p = 0.1163). At the end of the 5th infection cycle, the incubation period of the QoI-R and QoI-S PoTl isolate groups on wheat leaves increased from 2.1 to 2.5 days and from 2.9 to 3.1 days, respectively (p ≤ 0.001) (Table 3). In contrast, the transfer cycles had no effect on the incubation period on wheat heads for either the QoI-R and QoI-S PoTl isolate groups (p ≤ 0.001) (Table 3). On wheat leaves, the transfer cycles did not affect the time required for conidial production in the QoI-R and QoI-S PoTl isolate groups (p ≤ 0.001) (Table 4). Similarly, disease severity on wheat leaves and heads at 7 days after inoculation (d.a.i.) was unaffected by the transfer cycles, indicating that the QoI-R and QoI-S PoTl isolate groups did not exhibit changes in disease severity between the 1st and the 5th transfer cycles (p ≤ 0.001) (Table 3; Figure 3).

Table 3.
Fitness variables (incubation period and disease severity on wheat leaves and heads) of Pyricularia oryzae Triticum lineage isolates resistant (R) and sensitive (S) to Quinone outside inhibitor (QoI) fungicide, and effects of successive transfer cycles in vivo.

Table 4.
Fitness variables (latent period, conidial production and conidial germination) of Pyricularia oryzae Triticum lineage isolates resistant (R) and sensitive (S) to Quinone outside inhibitor (QoI) fungicide, and effects of successive transfer cycles in vivo.
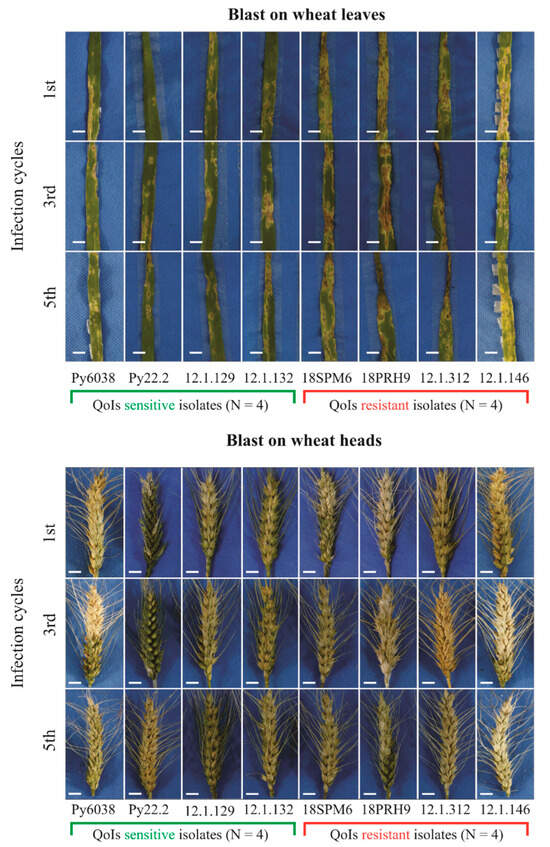
Figure 3.
Photographs of blast symptoms on the leaves and heads of wheat cv. Anahuac 75 at 7 days after inoculation (d.a.i) with Pyricularia oryzae Triticum lineage isolate group resistant (R) and sensitive (S) to quinone outside inhibitor (QoI) fungicide after successive transfer cycles in the absence of azoxystrobin. Bars: 10 mm.
On wheat leaves at 21 d.a.i, the conidial production increased significantly from 1.5 × 105 to 6.5 × 105 conidia mL−1 in the QoI-resistant (QoI-R) PoTl isolate group, and from 7.7 × 104 to 2.4 × 105 conidia mL−1 for QoI-S PoTl isolate group between the 1st and the 5th infection cycles (p ≤ 0.001) (Table 4). No changes were observed in the germination ability of the QoI-R isolate group between the 1st and the 5th infection cycles. However, a significant reduction from 57.0 to 49.3 was detected in the QoI-S isolate group (p ≤ 0.001) (Table 4).
3.3. Competitive Abilities of the QoI-Resistant and -Sensitive PoTl Isolates
In vitro competitive ability. Within each transfer cycle, significant differences were observed between mixtures of PoTl 121146 (QoI-R) and Py6038 (QoI-S) isolates in conidial production on both media at 15 d.a.i. (p ≤ 0.001). Across the transfer cycles, there was a significant interaction between the transfer cycles and conidial production of mixtures of the QoI-R and QoI-S isolates (p ≤ 0.001). After nine successive transfer cycles, conidial production capacity increased significantly for both PoTl 121146 alone (QoI-R) (0S:100R) and Py6038 alone (QoI-S) (100S:0R) isolates (p ≤ 0.001).
Within each infection cycle for in vivo competitive ability, there were differences between mixtures of QoI-R and QoI-S isolates in disease severity at 7 d.a.i and conidial production from wheat blast lesions at 21 d.a.i. (p ≤ 0.001). Across the transfer cycles, significant interactions were observed between transfer cycles and disease severity on wheat leaves and heads inoculated with mixtures of QoI-R and QoI-S isolates (p ≤ 0.001) (Figure 4). By the end of the 5th infection cycle, a decrease in conidial production capacity and disease severity on wheat leaves and heads was observed for both PoTl 121146 alone (QoI-R) (0S:100R) and PoTl Py6038 alone (QoI-S) (100S:0R) isolates (p ≤ 0.001) (Figure 4 and Figure 5). A positive and significant correlation was detected between transfer cycles and conidial production of mixtures in vitro (r = 0.35, p ≤ 0.01) (Figure 6). On the other hand, a negative correlation was found between transfer cycles and conidial production (r = −0.88, p ≤ 0.001), as well as between transfer cycles and disease severity (r = −0.36, p ≤ 0.01) (Figure 6). A significant positive correlation was observed between conidial production and disease severity (r = 0.57, p ≤ 0.001) (Figure 6).
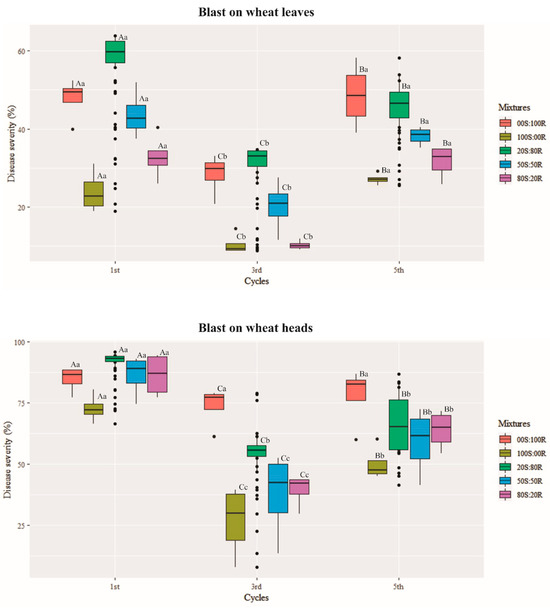
Figure 4.
Boxplot distribution of wheat blast severity on the leaves and heads at 7 days after inoculation (d.a.i.) with Pyricularia oryzae Triticum lineage conidia mixtures of conidial resistant (R) and sensitive (S) to quinone outside inhibitor (QoI) over the course of five successive transfer cycles. Five mixtures of QoI-resistant (R) and QoI-sensitive (S) isolates were used in the following proportions: 0S:100R, 20S:80R, 50S:50R, 80S:20R, 100S:0R and 0S:0R, in which R corresponds to the QoI-resistant isolate 121146 and S corresponds to the QoI-sensitive isolate Py6038. Means followed by the same letter are not significantly different according to the Scott–Knott test (p ≤ 0.001). The capital letter compares the severity values among the transfer cycles, while lowercase letters compare the severity values of the Pyricularia oryzae Triticum lineage mixtures. The error bars in each box represent the average standard error of the severity values.
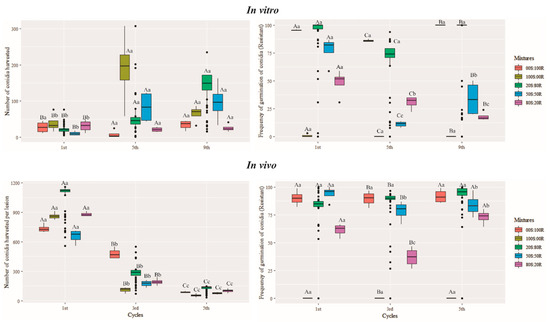
Figure 5.
Boxplot showing the number of conidia and frequency of germination of conidia (resistant) of the Pyricularia oryzae Triticum lineage obtained in vitro at 15 d.a.i. and in vivo at 21 d.a.i. for mixtures of conidial resistant (R) and sensitive (S) to quinone outside inhibitor (QoI) throughout nine successive transfer cycles in vitro and five successive transfer cycles in vivo. Five mixtures of QoI-resistant (R) and QoI-sensitive (S) isolates were used in the following proportions: 0S:100R, 20S:80R, 50S:50R, 80S:20R, 100S:0R and 0S:0R, in which R corresponds to the QoI-resistant isolate 121146 and S corresponds to the QoI-sensitive isolate Py6038. The germination was tested in a PD medium with 10 μg mL−1 of azoxystrobin. Means followed by the same letter are not significantly different according to the Scott–Knott test (p ≤ 0.001). The capital letter compares the number of conidia harvested and the frequency of germination of conidia (resistant) among the transfer cycles, while lowercase letters compare the number of conidia harvested and the frequency of germination of conidia (resistant) of the Pyricularia oryzae Triticum lineage mixtures. The error bars in each box represent the average standard error from the number of conidia harvested and the frequency of germination of conidia (resistant) in vitro and in vivo.
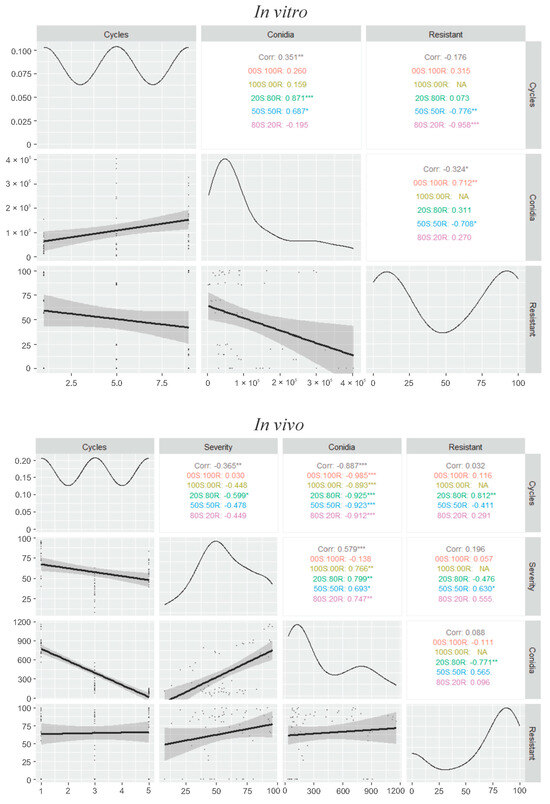
Figure 6.
Pearson correlation analysis among the transfer cycles and disease severity values, conidial production, and frequency of germination of conidia (resistant) obtained in vitro at 15 d.a.i. and in vivo at 21 d.a.i. for Pyricularia oryzae Triticum lineage (PoTl) conidia mixtures of conidial resistant (R) and sensitive (S) to quinone outside inhibitor (QoI). Diagonal frames represent the distribution of the transfer cycles, mixtures of isolates at different ratios, disease severity values, conidial production and frequency of germination of conidia (resistant) throughout nine successive transfer cycles in vitro and five successive transfers cycles in vivo; frames below, the bivariate dispersion; frames above, the correlations values and the significance levels. Significance of the correlation was determined by t-tests. p values were represented by: *** = 0.001; ** = 0.01; * = 0.1.
3.4. Frequency of QoIs Resistant Conidia of Mixtures of the PoTl Isolates
In each infection cycle, there were differences in the frequency of germinated QoI-resistant (QoI-R) conidia between the mixtures of QoI-R and QoI-sensitive (QoI-S) isolates, both in vitro at 15 d.a.i. and in vivo at 21 d.a.i. (p ≤ 0.001). Across the transfer cycles, significant interactions were observed between transfer cycles and the frequency of germinated QoI-R conidia both in vitro and in vivo (p ≤ 0.001). At 10 μg/mL azoxystrobin, no changes in the frequency of germinated QoI-R conidia were observed for the 80S:20R, 20S:80R, and 0S:100R (100.0%) mixtures after nine successive transfer cycles in vitro (p ≤ 0.001) (Figure 5). However, when grown in 10 μg/mL azoxystrobin in vivo, a decreasing frequency of germinated QoI-R conidia was observed between the 1st and 5th infection cycles (p ≤ 0.001) (Figure 5). For all transfer cycles, the frequency of QoI-S conidia in the 100S:0R mixture remained at 100.0% both in vitro and in vivo (Figure 5).
4. Discussion
For over 30 years, wheat blast has been identified as one of the major obstacles to expanding wheat production in Brazil. Control of wheat blast depends on the use of fungicides, but their efficacy rarely exceeds 60% [1]. So far, PoTl populations have also been associated with resistance to three different chemical groups, including QoI, DMI, and SDHI fungicides [13]. However, when comparing PoTl populations from Brazil collected in 2012 and 2018, no differences in sensitivity to QoI fungicides were observed. We hypothesize that the persistence of QoI-R PoTl populations with higher fitness and adaptive advantages driven by the intensive use of fungicides on wheat crops, which imposes strong selection pressure on these populations [19]. In the present study, we evaluated the stability of fungicide resistance, fitness advantages, and competitive ability of the QoI-R PoTl isolates group over successive transfer cycles. Understanding the biological and ecological characteristics of the QoI-R PoTl isolate could help in developing anti-resistance strategies. In the present study, the QoI-resistant isolates of PoTl show no evidence of a fitness cost associated with the G143A mutation. These resistant isolates maintain high fitness levels and do not revert to QoI sensitivity, even after undergoing multiple cycles of passage in the absence of QoI fungicides. This indicates that once the G143A mutation emerges, QoI fungicides will no longer be effective against PoTl, even if used in a mixture or reintroduced to a region where QoI resistance has first occurred. Once the fungicide fails, there is no way to reverse the evolutionary changes. This scenario reinforces an urgent need to develop and implement effective anti-resistance strategies against QoI-R PoTl populations in Brazil.
In the present study, no changes in the sensitivity of PoTl to azoxystrobin were detected after successive transfer cycles both in vitro and in vivo. This finding is in line with previous studies on certain fungal species that exhibited stable QoI-resistance. These include B. cinerea on apple [27], Cercospora beticola on sugar beet [36], C. acutatum on strawberries [23], E. necator on grapes [24], Lasiodiplodia theobromae on mango [37], P. viticola on grapes [25], P. oryzae on barley [26]. Contrary to the above, QoI-resistant isolates of P. oryzae on rice [22] and Monilinia fructicola on peach [28] exhibited unstable resistance to QoIs. Furthermore, we observed that the frequency of 0S:100R isolates harboring the G143A mutation remained at 100%, even after five transfer cycles (Table 1). This result confirms that PoTl resistance to QoI fungicides, associated with the G143A mutation, remains stable even in the absence of selection pressure. Similar findings have been reported in several fungal species carrying the G143A mutation, where QoI-R was stable. These include Alternaria alternata pathotype tangerine on citrus [21], A. alternata on potato [38], B. cinerea on apple [27], C. acutatum on strawberries [23], E. necator on grapes [24], P. oryzae on barley [26], P. vitícola on grapes [25], and Z. tritici on wheat [39]. These species exhibit stable QoI-R and no fitness costs. Conversely, P. oryzae on perennial ryegrass [34] and P. oryzae on rice [22] do not exhibit stable QoI-R, which is associated with fitness costs.
Initially, we determined the fitness variables of QoI-R and QoI-S PoTl isolate groups in vitro after nine transfer cycles without azoxystrobin. Unexpectedly, between the 1st and the 9th transfer cycles, the QoI-R and QoI-S PoTl isolate groups showed an increase in mycelial growth and germination ability, except for conidial production. For the variables in vivo, no changes were found for disease severity on wheat leaves and heads, incubation period, latent period, conidial production, and the germination ability of the QoI-R PoTl isolates group. Our findings for most variables showed that the fitness advantage of the QoI-R PoTl isolate group remained stable and was higher than that of the QoI-S PoTl isolate group throughout nine and five transfer cycles in vitro and in vivo, respectively. Conversely, G143A mutants of P. oryzae on barley, tested under saprophytic and infection conditions, did not show differences in fitness variables compared to the QoI-S isolate throughout four infection cycles [26].
Subsequently, we compared the competitive abilities of the QoI-R and QoI-S PoTl isolates on wheat leaves and heads. From the 1st to 5th infection cycle, disease severity and conidial production on wheat leaves decreased for both the QoI-R 121146 isolate alone (0S:100R) and the QoI-S Py6038 isolate alone (100S:0R). Similarly, as observed for QoI-R and QoI-S PoTl isolates, some PoTl isolates showed a significant decrease in their area under the disease progress curve (AUDPC) between the 1st and 5th infection cycles [20]. However, during all transfer cycles, the values of disease severity and conidial production on wheat leaves of the 0S:100R mixture were higher than those from the 100S:0R mixture (Figure 4 and Figure 5). At discriminatory doses of the fungicide, both in vitro and in vivo, the frequency of conidia germination (resistant) in the 20S:80R and 0S:100R mixtures remained resistant after successive transfer cycles. This indicates that there was no change in QoI-R or in the competitive advantage of the QoI-R PoTl isolate (Figure 5). These findings are in line with observations in Erysiphe graminis f. sp. tritici, where some QoI-resistant isolates competed better than sensitive ones [40]. In the present study, this may be explained by the fitness advantage and greater competitive ability of the QoI-R PoTl isolates compared to their wild-type isolates, possibly due to an evolutionary compensatory process [10].
In general, our findings support and build upon previous research showing that resistance to QoIs in PoTl populations remains stable. This indicates that the spread of resistant mutants may persist after successive transfer cycles under field conditions. Moreover, there was no change in the fitness advantage and competitive ability of the QoI-R PoTl isolates, even after planta transfer cycles, indicating their persistence in wheat fields over time. These results are in line with the observations of absence of fitness costs associated with QoI resistance in C. acutatum on strawberries and Phakopsora pachyrhizi on soybeans [23,41]. Our findings could have significant implications for the integrated management of wheat blast. Consequently, strategies such as using fungicides from different chemical classes or applying QoIs in combination with multi-site fungicides that have a low risk of resistance development have been adopted to limit the spread of QoI-R PoTl populations [42]. Although both approaches are practiced, fungicide mixtures have become a routine resistance management strategy when resistance is not associated with a fitness cost [43]. Among site-specific fungicides, tebuconazole and benzovindiflupyr remain effective for managing wheat blast, especially when co-formulated with multi-site fungicides [44]. Mixing of QoIs with multi-site fungicides such as Azonil 56 SC (Azoxystrobin 6% + Chlorothalonil 50%) has been used against wheat blast, with dose rate of 0.1 mL L−1 [45]. Implementing these anti-resistance strategies can help prevent the selection of PoTl populations with enhanced fitness, adaptive advantages, and fungicide resistance in Brazilian wheat fields.
5. Conclusions
Our study demonstrates the stability of PoTl resistance to QoI fungicides, associated with the G143A mutation, even in the absence of selection pressure. Furthermore, QoI-R PoTl populations maintained higher fitness and competitive advantage over time, indicating the need for further investigations to gain more insights into wheat crop field management, such as rotating or mixing fungicides with different modes of action, to delay the development of resistance. Future research should investigate whether the higher fitness of QoI-R PoT populations increase or decrease under fungicide selection pressure.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15112599/s1, Table S1: Description of Pyricularia oryzae Triticum lineage isolates resistant (R) and sensitive (S) to quinone outside inhibitor (QoI) obtained from wheat plants that were used in the fungicide resistance stability and fitness study.
Author Contributions
Conceptualization, A.F.D. and E.A.P.; methodology, A.F.D., P.R.d.S., I.C.L.P. and R.L.A.; software, A.F.D., S.d.S.C.G. and R.L.A.; validation, A.F.D., E.A.P., R.L.A., I.C.L.P., P.R.d.S., S.I.M. and E.A.; formal analysis, A.F.D. and E.A.P.; investigation, A.F.D., resources, A.F.D. and E.A.P.; data curation, A.F.D.; writing—original draft preparation, A.F.D.; writing—review and editing, A.F.D., E.A.P., S.d.S.C.G., R.L.A., I.C.L.P., P.R.d.S., S.I.M. and E.A.; visualization, E.A.; supervision, E.A.; project administration, E.A.; funding acquisition, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Foundation to Support Research in the state of Minas Gerais, Brazil (Fapemig CAG-APQ01975-15). E.A. is supported by a productivity grant from the National Council for Scientific and Technological Development (CNPq 305482/2017-3, 432445/2018-8, 313825/2018-1, 306133/2021-0). Dorigan A.F. was supported by a doctoral scholarship from CAPES (Coordination for the Improvement of Higher Education Personnel, Brazil).
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Ceresini, P.C.; Castroagudín, V.L.; Rodrigues, F.Á.; Rios, J.A.; Eduardo Aucique-Pérez, C.; Moreira, S.I.; Alves, E.; Croll, D.; Maciel, J.L.N. Wheat blast: Past, present, and future. Annu. Rev. Phytopathol. 2018, 56, 427–456. [Google Scholar] [CrossRef]
- Gladieux, P.; Condon, B.; Ravel, S.; Soanes, D.; Maciel, J.L.N.; Nhani, A.; Chen, L.; Terauchi, R.; Lebrun, M.-H.; Tharreau, D.; et al. Gene flow between divergent cereal- and grass-specific lineages of the rice blast fungus Magnaporthe oryzae. mBio 2018, 9, e01219-17. [Google Scholar] [CrossRef] [PubMed]
- Duveiller, E.A. History of Wheat. In World Wheat Book; Academic Press: Cambridge, MA, USA, 2016; Volume 3, p. 1107. [Google Scholar]
- Bonjean, A.P.; Angus, W.J.; van Ginkel, M. The world wheat book:a history of wheat breeding. Cereal Res. Ann. Bot. 2001, 88, 953–955. [Google Scholar]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 22 May 2024).
- Igarashi, S. Ocorrência de Pyricularia spp. no estado do Paraná. Fitopatol. Bras. 1986, 11, 351–352. [Google Scholar]
- Islam, M.T.; Croll, D.; Gladieux, P.; Soanes, D.M.; Persoons, A.; Bhattacharjee, P.; Hossain, M.S.; Gupta, D.R.; Rahman, M.M.; Mahboob, M.G.; et al. Emergence of wheat blast in Bangladesh was caused by a south american lineage of Magnaporthe oryzae. BMC Biol. 2016, 14, 84. [Google Scholar] [CrossRef]
- Tembo, B.; Mulenga, R.M.; Sichilima, S.; M’siska, K.K.; Mwale, M.; Chikoti, P.C.; Singh, P.K.; He, X.; Pedley, K.F.; Peterson, G.L.; et al. Detection and characterization of fungus (Magnaporthe oryzae Pathotype Triticum) causing wheat blast disease on rain-fed grown wheat (Triticum aestivum L.) in Zambia. PLoS ONE 2020, 15, e0238724. [Google Scholar] [CrossRef]
- Castroagudín, V.L.; Ceresini, P.C.; de Oliveira, S.C.; Reges, J.T.A.; Maciel, J.L.N.; Bonato, A.L.V.; Dorigan, A.F.; McDonald, B.A. Resistance to QoI fungicides is widespread in Brazilian populations of the wheat blast pathogen Magnaporthe oryzae. Phytopathology 2015, 105, 284–294. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Fraaije, B.A. Fitness penalties in the evolution of fungicide resistance. Annu. Rev. Phytopathol. 2018, 56, 339–360. [Google Scholar] [CrossRef]
- Hu, M.; Chen, S. Non-target site mechanisms of fungicide resistance in crop pathogens: A review. Microorganisms 2021, 9, 502. [Google Scholar] [CrossRef]
- Alkemade, J.A.; Hawkins, N.J.; Baraldi, E.; Buddie, A.G.; Cockerton, H.M.; Corkley, I.; Fraaije, B.A.; Gaya, E.; Gifford, D.R.; Hartig, F.; et al. Learning from fungicide resistance: Evolutionary insights to guide RNAi-based control of fungal crop pathogens. Fungal Biol. Rev. 2025, 53, 100443. [Google Scholar] [CrossRef]
- Ceresini, P.C.; Silva, T.C.; Vicentini, S.N.C.; Júnior, R.P.L.; Moreira, S.I.; Castro-Ríos, K.; Garcés-Fiallos, F.R.; Krug, L.D.; Moura, S.S.; Silva, A.G.; et al. Strategies for managing fungicide resistance in the Brazilian tropical agroecosystem: Safeguarding food safety, health, and the environmental quality. Trop. Plant Pathol. 2024, 49, 36–70. [Google Scholar] [CrossRef]
- Poloni, N.M.; Carvalho, G.; Vicentini, S.N.C.; Dorigan, A.F.; Maciel, J.L.N.; McDonald, B.A.; Moreira, S.I.; Hawkins, N.J.; Fraaije, B.A.; Kelly, D.E.; et al. Widespread distribution of resistance to triazole fungicides in Brazilian populations of the wheat blast pathogen. Plant Pathol. 2021, 70, 436–448. [Google Scholar] [CrossRef]
- Pringle, A.; Taylor, J.W. The fitness of filamentous fungi. Trends Microbiol. 2002, 10, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Karaoglanidis, G.S.; Thanassoulopoulos, C.C.; Ioannidis, P.M. Fitness of Cercospora beticola field isolates resistant and sensitive to demethylation inhibitor fungicides. Eur. J. Plant Pathol. 2001, 107, 337–347. [Google Scholar] [CrossRef]
- Zur Wiesch, P.A.; Kouyos, R.; Engelstädter, J.; Regoes, R.R.; Bonhoeffer, S. Population biological principles of drug-resistance evolution in infectious diseases. Lancet Infect. Dis. 2011, 11, 236–247. [Google Scholar] [CrossRef]
- Yang, L.; Gao, F.; Shang, L.; Zhan, J.; McDonald, B.A. Association between virulence and triazole tolerance in the phytopathogenic fungus Mycosphaerella graminicola. PLoS ONE 2013, 8, e59568. [Google Scholar] [CrossRef]
- Dorigan, A.F.; Moreira, S.I.; Ceresini, P.C.; Pozza, E.A.; Belan, L.L.; da Silveira, R.S.; Alves, E. Higher fitness and competitive advantage of Pyricularia oryzae Triticum lineage resistant to QoI fungicides. Pest Manag. Sci. 2022, 78, 5251–5258. [Google Scholar] [CrossRef]
- Dorigan, A.F.; Pozza, E.A.; Pereira, R.C.M.; Moreira, S.I.; Ceresini, P.C.; Silva, H.R.; Alves, E. Temporal dynamics of wheat blast caused by Pyricularia oryzae Triticum lineage throughout the successive wheat cycles. Eur. J. Plant Pathol. 2024, 169, 755–770. [Google Scholar] [CrossRef]
- Vega, B.; Dewdney, M.M. QoI-resistance stability in relation to pathogenic and saprophytic fitness components of Alternaria alternata from citrus. Plant Dis. 2014, 98, 1371–1378. [Google Scholar] [CrossRef]
- D’Ávila, L.S.; De Filippi, M.C.C.; Café-Filho, A.C. Fungicide resistance in Pyricularia oryzae populations from southern and northern Brazil and evidence of fitness costs for QoI-resistant isolates. Crop Prot. 2022, 153, 105887. [Google Scholar] [CrossRef]
- Forcelini, B.B.; Rebello, C.S.; Wang, N.Y.; Peres, N.A. Fitness, competitive ability, and mutation stability of isolates of Colletotrichum acutatum from strawberry resistant to QoI fungicides. Dis. Control Pest Manag. 2018, 108, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Rallos, L.E.E.; Johnson, N.G.; Schmale, D.G.; Prussin, A.J.; Baudoin, A.B. Fitness of Erysiphe necator with G143A based resistance to quinone outside inhibitors. Plant Dis. 2014, 98, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Genet, J.L.; Jaworska, G.; Deparis, F. Effect of dose rate and mixtures of fungicides on selection for QoI resistance in populations of Plasmopara viticola. Pest Manag. Sci. 2006, 62, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Avila-Adame, C.; Koller, W. Characterization of spontaneous mutants of Magnaporthe grisea expressing stable resistance to the Qo-inhibiting fungicide azoxystrobin. Curr. Genet. 2003, 42, 332–338. [Google Scholar] [CrossRef]
- Chen, S.N.; Luo, C.X.; Hu, M.J.; Schnabel, G. Fitness and competitive ability of Botrytis cinerea isolates with resistance to multiple chemical classes of fungicides. Phytopathology 2016, 106, 997–1005. [Google Scholar] [CrossRef]
- Chen, S.N.; Shang, Y.; Wang, Y.; Schnabel, G.; Lin, Y.; Yin, L.F.; Luo, C.X. Sensitivity of Monilinia fructicola from peach farms in China to four fungicides and characterization of isolates resistant to carbendazim and azoxystrobin. Plant Dis. 2014, 98, 1555–1560. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stage of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Lichtemberg, P.S.F.; Michailides, T.J.; Puckett, R.D.; Zeviani, W.M.; May De Mio, L.L. Fitness costs associated with G461S mutants of Monilinia fructicola could favor the management of tebuconazole resistance in Brazil. Trop. Plant Pathol. 2019, 44, 140–150. [Google Scholar] [CrossRef]
- Ma, Z.; Felts, D.; Michailides, T.J. Resistance to azoxystrobin in Alternaria isolates from pistachio in California. Pestic. Biochem. Physiol. 2003, 77, 66–74. [Google Scholar] [CrossRef]
- Vargas, M.H. ED50plus, version 1.0; Instituto Nacional de Enfermedades Respiratorias (Computer Software): Mexico City, Mexico, 2000.
- Olaya, G.; Zheng, D.; Koller, W. Differential responses of germinating Venturia inaequalis conidia to Kresoxim-methyl. Pestic. Sci. 1998, 54, 230–236. [Google Scholar] [CrossRef]
- Ma, B.; Uddin, W. Fitness and competitive ability of an azoxystrobin-resistant G143A mutant of Magnaporthe oryzae from Perennial ryegrass. Plant Dis. 2009, 93, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.B.; Cavalcant, P.P. Função R para analisar experimentos em DBC com fatorial duplo e um tratamento adicional, em uma só rodada. In Proceedings of the Reunião Anual da Região Brasileira da Sociedade Internacional de Biometria, São Paulo, Brazil, 21–24 July 2009; Volume 13, p. 5. [Google Scholar]
- Malandrakis, A.A.; Markoglou, A.N.; Nikou, D.C.; Vontas, J.C.; Ziogas, B.N. Biological and molecular characterization of laboratory mutants of Cercospora beticola resistant to Qo inhibitors. Eur. J. Plant Pathol. 2006, 116, 155–166. [Google Scholar] [CrossRef]
- He, R.; Yang, Y.; Hu, Z.; Xue, R.; Hu, Y. Resistance mechanisms and fitness of pyraclostrobin-resistant isolates of Lasiodiplodia theobromae from mango orchards. PLoS ONE 2021, 16, e0253659. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Halterman, A.D.; Meinholz, K.; Gevens, A.J. Distribution and stability of Quinone Outside Inhibitor fungicide resistance in populations of potato pathogenic Alternaria spp. in Wisconsin. Plant Dis. 2019, 103, 2033–2040. [Google Scholar] [CrossRef]
- Fouché, G.; Michel, T.; Lalève, A.; Wang, N.X.; Young, D.H.; Meunier, B.; Debieu, D.; Fillinger, S.; Walker, A. Directed evolution predicts cytochrome b G37V target site modification as probable adaptive mechanism towards the QiI fungicide fenpicoxamid in Zymoseptoria tritici. Environ. Microbiol. 2022, 24, 1117–1132. [Google Scholar] [CrossRef]
- Chin, K.M.; Chavaillaz, D.; Kaesbohrer, M.; Staub, T.; Felsenstein, F. Characterizing resistance risk of Erysiphe graminis f. sp. tritici to strobilurins. Crop Prot. 2001, 20, 87–96. [Google Scholar] [CrossRef]
- Klosowski, A.C.; Brahm, L.; Stammler, G.; De Mio, L.L.M. Competitive fitness of Phakopsora pachyrhizi isolates with mutations in the CYP51 and CYTB genes. Phytopathology 2016, 106, 1278–1284. [Google Scholar] [CrossRef]
- van den Bosch, F.; Paveley, N.; van den Berg, F.; Hobbelen, P.; Oliver, R. Mixtures as a fungicide resistance management tactic. Phytopathology 2014, 104, 1264–1273. [Google Scholar] [CrossRef]
- Bardas, G.A.; Myresiotis, C.K.; Karaoglanidis, G.S. Stability and fitness of anilinopyrimidine-resistant strains of Botrytis cinerea. Phytopathology 2008, 98, 443–450. [Google Scholar] [CrossRef]
- Cazón, L.I.; Ascari, J.P.; Dos Santos, G.B.; Del Ponte, E.M. Differential response to DMI, QoI and SDHI fungicides in wheat and signal grass blast populations from Minas Gerais, Brazil. Plant Pathol. 2023, 72, 449–467. [Google Scholar] [CrossRef]
- Farthouse, J.; Kashem, M.A.; Hasna, M.K.; Khalil, M.I.; Haque, M.M.; Nabi, K.M.E. Screening of different fungicides in controlling wheat blast in Bangladesh. J. Agrofor. Environ. 2021, 14, 21–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).