Transcription Factor AcMYB5 Activates Flavonoid Biosynthesis and Enhances Resistance of Kiwifruit to Bacterial Canker
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. In Vitro Infection Assay and Bacterial Growth Counting
2.3. Kiwifruit Stable Transformation
2.4. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.5. Dual-Luciferase Assay
2.6. SA and JA Quantification
2.7. Determination of Flavonoid Content
2.8. Statistical Analysis
3. Results
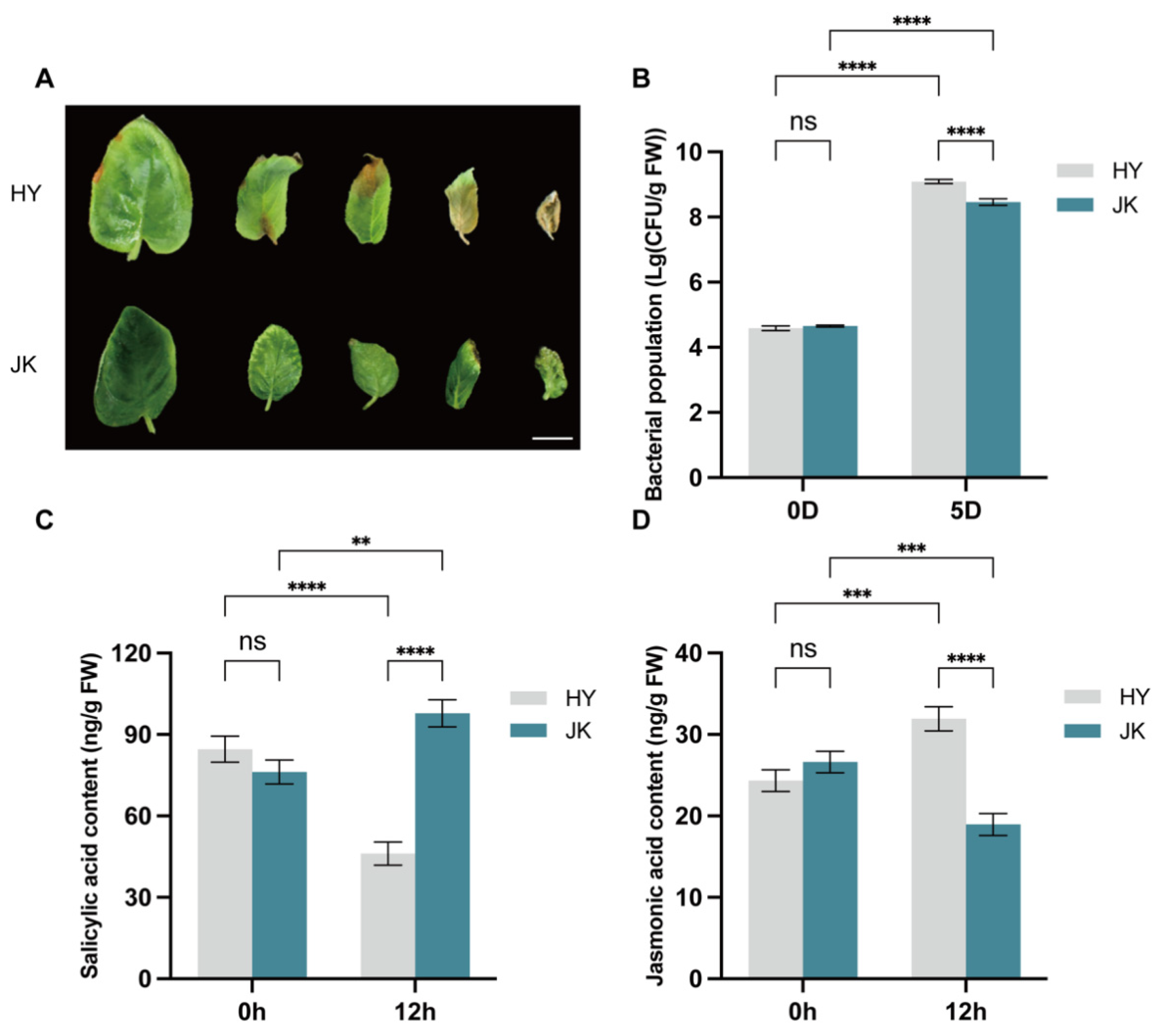
3.1. Hormone Signal Changes in Resistant and Susceptible Varieties After Infection with Psa
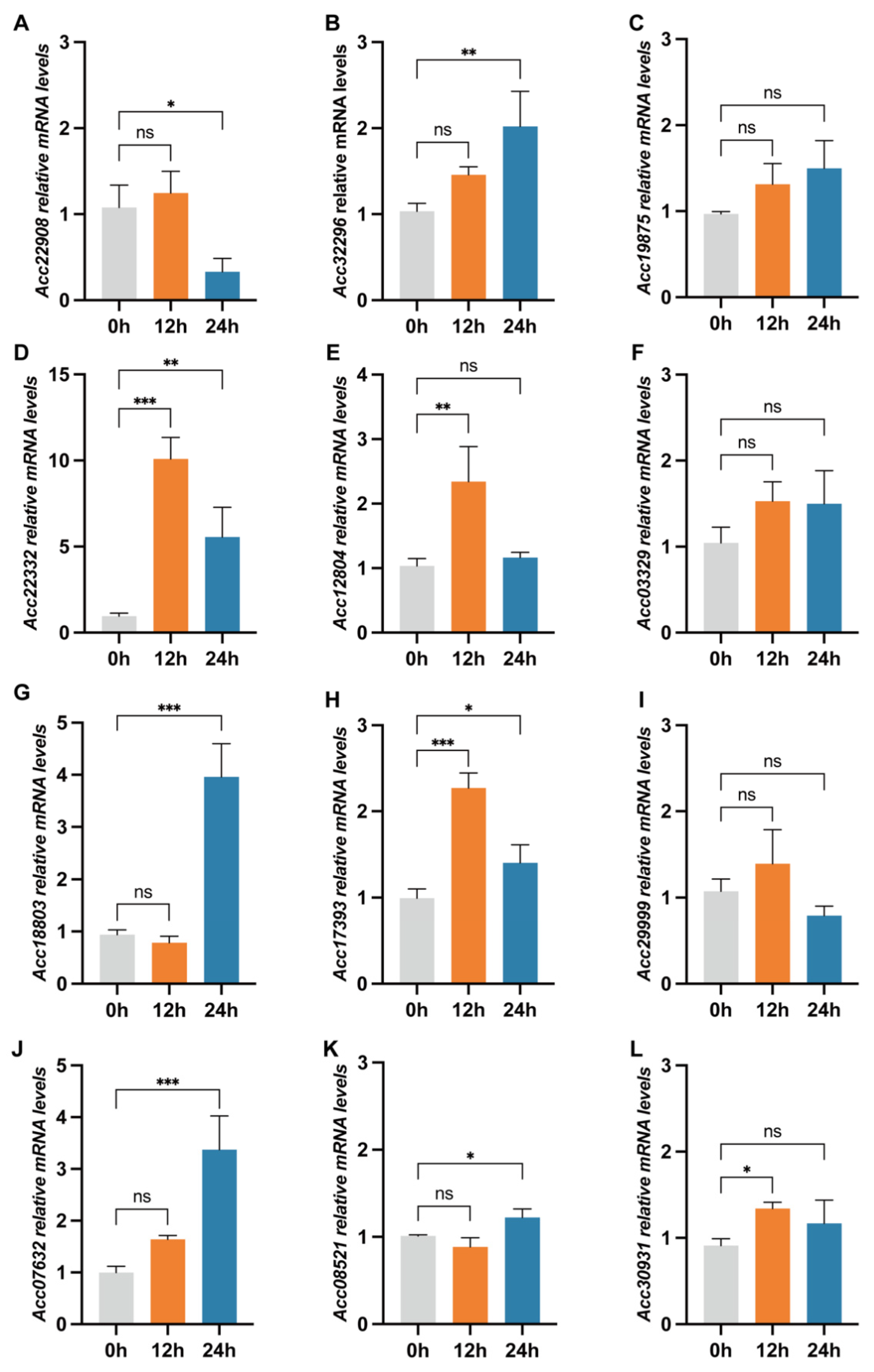
3.2. Screening of Genes Responding to Salicylic Acid Induction
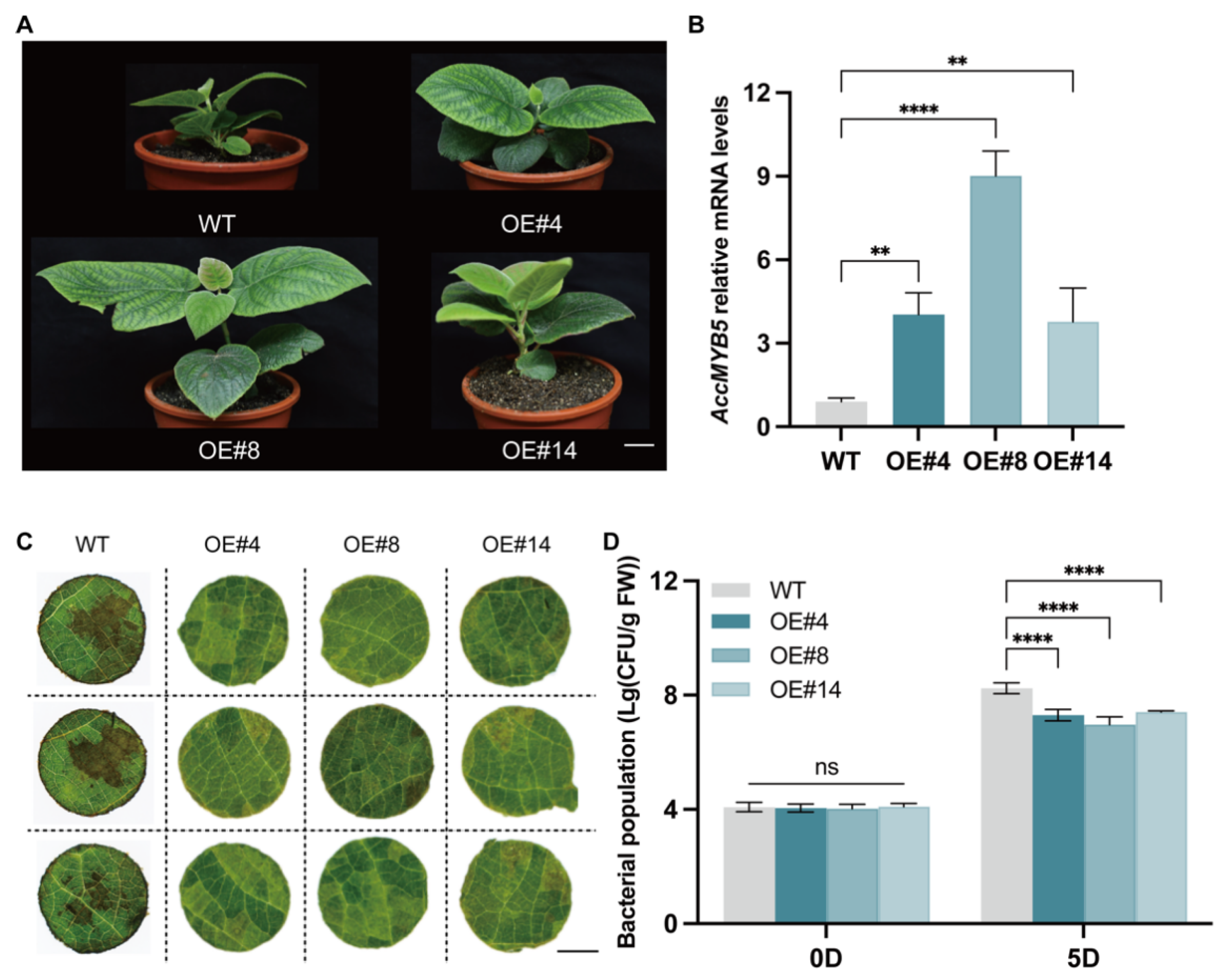
3.3. Overexpression of AcMYB5 Enhances Kiwifruit Resistance to Psa
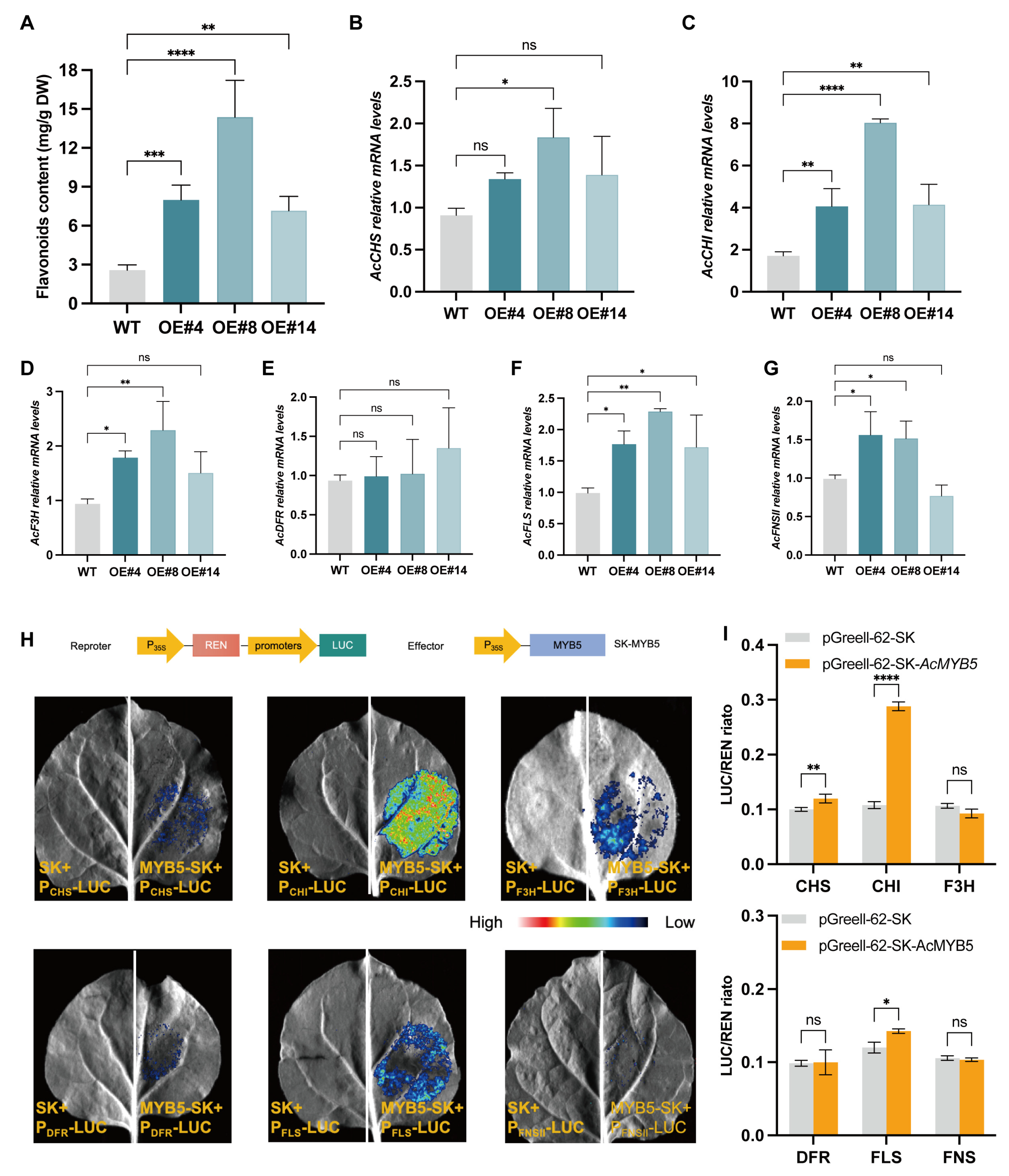
3.4. AcMYB5 Transcriptionally Activates AcCHI to Promote Flavonoid Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, R.; Shu, P.; Zhang, C.; Zhang, J.; Chen, Y.; Zhang, Y.; Du, K.; Xie, Y.; Li, M.; Ma, T.; et al. Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis). New Phytol. 2022, 233, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, D.; Wang, X.; Bai, F.; Li, R.; Zhou, R.; Wu, S.; Fang, Z.; Liu, W.; Huang, L.; et al. LACCASE35 enhances lignification and resistance against Pseudomonas syringae pv. actinidiae infection in kiwifruit. Plant Physiol. 2025, 197, kiaf040. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Tian, Y.; Dang, Q.; Zhang, H.; Huang, L. Vascular Network-mediated Systemic Spread of Pseudomonas syringae pv. actinidiae Causes the Bacterial Canker of Kiwifruit. Hortic. Plant J. 2024, 7. [Google Scholar] [CrossRef]
- Scortichini, M. Occurrence of Pseudomonas syringae pv. actinidiae on kiwifruit in Italy. Plant Pathol. 2010, 43, 1035–1038. [Google Scholar] [CrossRef]
- Balestra, G.M.; Renzi, M.; Mazzaglia, A. First report of bacterial canker of Actinidia deliciosa caused by Pseudomonas syringae pv. actinidiae in Portugal. New Dis. Rep. 2010, 22, 2510–2513. [Google Scholar] [CrossRef]
- Vanneste, J.L.; Poliakoff, F.; Audusseau, C.; Cornish, D.A.; Paillard, S.; Rivoal, C.; Yu, J. First report of Pseudomonas syringae pv. actinidiae the causal agent of bacterial canker of kiwifruit on Actinidia deliciosa in France. Plant Dis. J. 2011, 96, 169–175. [Google Scholar]
- Everett, K.R.; Taylor, R.K.; Romberg, M.K.; Rees-George, J.; Fullerton, R.A.; Vanneste, J.L.; Manning, M.A. First report of Pseudomonas syringae pv. actinidiae causing kiwifruit bacterial canker in New Zealand. Australas. Plant Dis. Notes 2011, 6, 67–71. [Google Scholar] [CrossRef]
- Lu, L.; Fang, J.; Xia, N.; Zhang, J.; Diao, Z.; Wang, X.; Liu, Y.; Tang, D.; Li, S. Phosphorylation of the transcription factor OsNAC29 by OsMAPK3 activates diterpenoid genes to promote rice immunity. Plant Cell 2025, 37, koae320. [Google Scholar] [CrossRef]
- Qiu, X.; Kong, L.; Chen, H.; Lin, Y.; Tu, S.; Wang, L.; Chen, Z.; Zeng, M.; Xiao, J.; Yuan, P. The Phytophthora sojae nuclear effector PsAvh110 targets a host transcriptional complex to modulate plant immunity. Plant Cell 2023, 35, 574–597. [Google Scholar] [CrossRef]
- Yeh, S.M.; Yoon, M.; Scott, S.; Chatterjee, A.; Hemara, L.M.; Chen, R.K.; Wang, T.; Templeton, K.; Rikkerink, E.H.; Jayaraman, J. NbPTR1 confers resistance against Pseudomonas syringae pv. actinidiae in kiwifruit. Plant Cell Environ. 2024, 47, 4101–4115. [Google Scholar] [CrossRef]
- Kvitko, B.H.; Collmer, A. Discovery of the Hrp Type III Secretion System in Phytopathogenic Bacteria: How Investigation of Hypersensitive Cell Death in Plants Led to a Novel Protein Injector System and a World of Inter-Organismal Molecular Interactions Within Plant Cells. Phytopathology® 2023, 113, 626–636. [Google Scholar] [CrossRef]
- Rui, L.; Yang, S.-Q.; Zhou, X.-H.; Wang, W. The important role of chloroplasts in plant immunity. Plant Commun. 2025, 6, 101420. [Google Scholar] [CrossRef]
- Katsir, L.; Schilmiller, A.L.; Staswick, P.E.; He, S.Y.; Howe, G.A. COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc. Natl. Acad. Sci. USA 2008, 105, 7100–7105. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Niu, G.; Fan, Y.; Liu, W.; Wu, Q.; Yu, C.; Wang, J.; Xiao, Y.; Hou, L.; Jin, D.; et al. Synthetic dual hormone-responsive promoters enable engineering of plants with broad-spectrum resistance. Plant Commun. 2023, 4, 100596. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Ke, Y.; Chen, D.; Wang, L.; Li, Q.; Yuan, M. Rice microRNA156/529-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE7/14/17 modules regulate defenses against bacteria. Plant Physiol. 2023, 192, 2537–2553. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, W.; Zhang, Y.; Li, Y.; Ma, C.; Tian, R.; Li, R.; Li, M.; Huang, L. Two transcription factors, AcREM14 and AcC3H1, enhance the resistance of kiwifruit Actinidia chinensis var. chinensis to Pseudomonas syringae pv. actinidiae. Hortic. Res. 2024, 11, uhad242. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, C.; Chen, C.; Su, K.; Lin, H.; Zhao, Y.; Guo, Y. VvWRKY5 enhances white rot resistance in grape by promoting the jasmonic acid pathway. Hortic. Res. 2023, 10, uhad172. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, W.; Yao, C.; Zhang, Y.; Du, X.; Ma, C.; Li, R.; Wang, H.; Huang, L. AcNAC10, regulated by AcTGA07, enhances kiwifruit resistance to Pseudomonas syringae pv. actinidiae via inhibiting jasmonic acid pathway. Mol. Hortic. 2025, 5, 21. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Z.; Pan, H.; Li, W.; Huang, L.; Wang, F.; Zhang, Q.; Yu, X.; Li, D.; Li, L.; et al. AcJAZ2L2 Confers Resistance to Kiwifruit Bacterial Canker via Regulation of JA Signaling and Stomatal Immunity. Hortic. Res. 2025, 12, uhaf215. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Xu, C.; Wang, Q. WRKYs as regulatory hubs of secondary metabolic networks: Diverse inducers and distinct responses. Plant Commun. 2025, 6, 101438. [Google Scholar] [CrossRef]
- Song, M.; Liu, Y.; Li, T.; Liu, X.; Hao, Z.; Ding, S.; Panichayupakaranant, P.; Zhu, K.; Shen, J. Plant Natural Flavonoids Against Multidrug Resistant Pathogens. Adv. Sci. 2021, 8, 2100749. [Google Scholar] [CrossRef] [PubMed]
- Su, D.; Wu, M.; Wang, H.; Shu, P.; Song, H.; Deng, H.; Yu, S.; Garcia-Caparros, P.; Bouzayen, M.; Zhang, Y.; et al. Bi-functional transcription factor SlbHLH95 regulates fruits flavonoid metabolism and grey mould resistance in tomato. Plant Biotechnol. J. 2025, 23, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, D.; Zhao, G.; Wang, J.; Zhang, S.; Wang, C.; Guo, X. Group IIc WRKY transcription factors regulate cotton resistance to Fusarium oxysporum by promoting GhMKK2-mediated flavonoid biosynthesis. New Phytol. 2022, 236, 249–265. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Qu, J.; Zeng, Y.; Xiao, W.; Fang, T.; Wang, Y.; Zeng, X.; Li, C.; Liu, J. The Transcription Factors NFYA1 and GBF3 Jointly Regulate CHS2 to Promote Tangeretin Accumulation and Cold Tolerance in Citrus. Plant Biotechnol. J. 2025. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, X.; Cao, B.; Feng, S.; Bin, T.; Zhang, Y.; Gao, P.; Lu, Y.; Li, X.; Liu, L.; et al. The Transcription Factor MYB8 Positively Regulates Flavonoid Biosynthesis of Scutellaria baicalensis in Response to Drought Stress. Plant Cell Environ. 2025, 48, 8898–8914. [Google Scholar] [CrossRef]
- Su, L.; Lv, A.; Wen, W.; Fan, N.; You, X.; Gao, L.; Zhou, P.; Shi, F.; An, Y. MsMYB206-MsMYB450-MsHY5 complex regulates alfalfa tolerance to salt stress via regulating flavonoid biosynthesis during the day and night cycles. Plant J. 2025, 121, e17216. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, R.-Y.; Li, X.; Zheng, X.-Q.; Lu, J.-L.; Liang, Y.-R.; Wei, C.-L.; Xu, Y.-Q.; Ye, J.-H. CsMYB67 participates in the flavonoid biosynthesis of summer tea leaves. Hortic. Res. 2024, 11, uhad231. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Zeng, Y.; Liu, P. Metabolic profiling reveals local and systemic responses of kiwifruit to Pseudomonas syringae pv. actinidiae. Plant Direct 2020, 4, e00297. [Google Scholar] [CrossRef]
- Gao, X.; Huang, Q.; Zhao, Z.; Han, Q.; Ke, X.; Qin, H.; Huang, L. Studies on the Infection, Colonization, and Movement of Pseudomonas syringae pv. actinidiae in Kiwifruit Tissues Using a GFPuv-Labeled Strain. PLoS ONE 2016, 11, e0151169. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Liu, Y.; Zhang, D.; Ni, M.; Jia, B.; Heng, W.; Fang, Z.; Zhu, L.-W.; Liu, P. Transcriptomic and Proteomic Profiling Reveal the Key Role of AcMYB16 in the Response of Pseudomonas syringae pv. actinidiae in Kiwifruit. Front. Plant Sci. 2021, 12, 756330. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Chen, Y.; Liu, Y.-X.; Chang, X.; Zhang, L.; Yang, X.; Li, L.; Zhang, L. Genotype-associated core bacteria enhance host resistance against kiwifruit bacterial canker. Hortic. Res. 2024, 11, uhae236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Y.; Gao, R.; Wu, Z.; Zhang, W.; Zhang, C.; Zhang, P.; Ye, C.; Yao, L.; Jin, Y.; et al. Complete biosynthesis of salicylic acid from phenylalanine in plants. Nature 2025, 645, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Ullah, C.; Schmidt, A.; Reichelt, M.; Tsai, C.; Gershenzon, J. Lack of antagonism between salicylic acid and jasmonate signalling pathways in poplar. New Phytol. 2022, 235, 701–717. [Google Scholar] [CrossRef]
- Jia, H.; Hewitt, N.; Jordá, L.; Abramyan, T.M.; Tolliver, J.; Jones, J.L.; Nomura, K.; Yang, J.; He, S.-Y.; Tropsha, A.; et al. Phosphorylation-activated G protein signaling stabilizes TCP14 and JAZ3 to repress JA signaling and enhance plant immunity. Mol. Plant 2025, 18, 1171–1192. [Google Scholar] [CrossRef]
- Zhu, F.; Li, K.; Cao, M.; Zhang, Q.; Zhou, Y.; Chen, H.; AlKhazindar, M.; Ji, Z. NbNAC1 enhances plant immunity against TMV by regulating isochorismate synthase 1 expression and the SA pathway. Plant J. 2025, 121, e17242. [Google Scholar] [CrossRef]
- Lee, H.G.; Seo, P.J. MYB96 recruits the HDA15 protein to suppress negative regulators of ABA signaling in Arabidopsis. Nat. Commun. 2019, 10, 1713. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Yuan, D.; Duan, M.; Liu, Y.; Shen, Z.; Yang, C.; Qiu, Z.; Liu, D.; Wen, P.; et al. An R2R3 MYB transcription factor confers brown planthopper resistance by regulating the phenylalanine ammonia-lyase pathway in rice. Proc. Natl. Acad. Sci. USA 2020, 117, 271–277. [Google Scholar] [CrossRef]
- Jian, W.; Cao, H.; Yuan, S.; Liu, Y.; Lu, J.; Lu, W.; Li, N.; Wang, J.; Zou, J.; Tang, N.; et al. SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic. Res. 2019, 6, 22. [Google Scholar] [CrossRef]
- An, X.-H.; Tian, Y.; Chen, K.-Q.; Liu, X.-J.; Liu, D.-D.; Xie, X.-B.; Cheng, C.-G.; Cong, P.-H.; Hao, Y.-J. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef]
- Wang, C.; Wei, X.; Wang, Y.; Wu, C.; Jiao, P.; Jiang, Z.; Liu, S.; Ma, Y.; Guan, S. Metabolomics and Transcriptomic Analysis Revealed the Response Mechanism of Maize to Saline-Alkali Stress. Plant Biotechnol. J. 2025. [Google Scholar] [CrossRef]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.-M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, J.; Luo, W.; Niu, S.; Qu, L.; Li, J.; Chen, Y.; Li, G.; Yang, H.; Lu, D. Salicylic Acid Cooperates with Lignin and Sucrose Signals to Alleviate Waxy Maize Leaf Senescence under Heat Stress. Plant Cell Environ. 2025, 48, 4341–4355. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, Z.; Huang, W.; Li, D.; Zeng, Q.; Chen, L.; Li, B.; Zhang, E. Integrated metabolome and transcriptome analysis reveals salicylic acid and flavonoid pathways’ key roles in cabbage’s defense responses to Xanthomonas campestris pv. campestris. Front. Plant Sci. 2022, 13, 1005764. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Dai, R.; Yue, W.; Guo, G.; Liu, J.; Huang, Y.; Liu, P. Transcription Factor AcMYB5 Activates Flavonoid Biosynthesis and Enhances Resistance of Kiwifruit to Bacterial Canker. Agronomy 2025, 15, 2598. https://doi.org/10.3390/agronomy15112598
Wu S, Dai R, Yue W, Guo G, Liu J, Huang Y, Liu P. Transcription Factor AcMYB5 Activates Flavonoid Biosynthesis and Enhances Resistance of Kiwifruit to Bacterial Canker. Agronomy. 2025; 15(11):2598. https://doi.org/10.3390/agronomy15112598
Chicago/Turabian StyleWu, Shunyuan, Rundong Dai, Wenli Yue, Ge Guo, Jiawei Liu, Yue Huang, and Pu Liu. 2025. "Transcription Factor AcMYB5 Activates Flavonoid Biosynthesis and Enhances Resistance of Kiwifruit to Bacterial Canker" Agronomy 15, no. 11: 2598. https://doi.org/10.3390/agronomy15112598
APA StyleWu, S., Dai, R., Yue, W., Guo, G., Liu, J., Huang, Y., & Liu, P. (2025). Transcription Factor AcMYB5 Activates Flavonoid Biosynthesis and Enhances Resistance of Kiwifruit to Bacterial Canker. Agronomy, 15(11), 2598. https://doi.org/10.3390/agronomy15112598





